Abstract
Symptoms of diseases such as rheumatoid arthritis, which is T helper 1 (Th1) dependent, and asthma, which is T helper 2 (Th2) dependent, are influenced by diurnal rhythms and natural regulatory T cells (nTreg). However, the mechanisms responsible for the diurnal rhythm of disease activity have not been identified and it is unclear whether nTreg activity is diurnal rhythm-dependent. We therefore investigated whether a 24-hr diurnal cycle affected the ability of various helper T-cell populations to generate immunomodulatory and pro-inflammatory cytokines, as well as its suppression by nTreg cells. Using a within-subject crossover design, sleep versus continuous wakefulness was compared over a 24-hr period in healthy young volunteers under defined environmental conditions. Venous blood was drawn periodically every 5 hr and the function of T cells was explored in vitro. We demonstrated that interleukin (IL)-2, interferon-γ (IFN-γ), tumour necrosis factor-α (TNF-α) and IL-10 secretion by naïve CD4+ T cells follows a diurnal rhythm. Furthermore, multiple regression analysis, as well as subsequent in vitro experiments, suggested that serum levels of cortisol and prolactin are part of the underlying mechanism. Additionally, we observed that nTreg suppressed the secretion of IFN-γ, IL-2 and TNF-α, but not the secretion of IL-4, IL-6, IL-10 and IL-17A. However, the abrogation of IL-2 release was reversed upon inhibiting CD25 on nTreg. Highly purified nTreg secreted IL-6, IL-10 and IL-17A, but not IL-2, IL-4, IFN-γ or TNF-α. Taken together, our results demonstrate that hormones and nTreg modulate the diurnal rhythm of T helper cell activity.
Keywords: CD4+ CD25+, circadian, cytokines, hormones, T cells
Introduction
Several studies suggest that immune responses in autoimmunity, allergy and following vaccinations are dependent on diurnal rhythms.1–6 A diurnal rhythm is an endogenous rhythm that is entrained by external timing signals and usually has a 24 hr period.
An underlying mechanism may be associated with a diurnal shift in the balance of T helper 1/T helper 2/T helper 17 (Th1/Th2/Th17) cells. A diurnal sleep-dependent shift towards Th1 immune responses was suggested by previous studies that analyzed the migration of cytokine-producing T cells in unseparated peripheral blood lymphocytes.7,8 The aim of our study was to extend these findings and to elucidate whether the demonstrated Th1/Th2 shift is caused by diurnal changes in the leucocyte composition of peripheral blood or by functional rhythms of T helper cells.8–12 Therefore, we isolated and stimulated naïve T cells (CD4+ CD45RA+ CD25−) and natural regulatory T cells (nTreg) in order to investigate functional changes at the cellular level. Understanding the cellular rhythm of immune cells is vital in order to unravel the mechanism of diurnal immune responses.13
Other potentially important regulators of the T helper cell balance are nTreg, which were first described by Sakaguchi et al.14 nTreg are naturally occurring regulatory T cells that express the transcription factor forkhead box P3 (FOXP3) as well as high levels of the interleukin (IL)-2 receptor alpha chain (CD25). These cells suppress the activity of T helper cells in vitro and have been shown to control autoimmune diseases (which are Th1- and Th17-dependent) and asthma (which is Th2-dependent) in vivo.15,16 The suppressive activities of nTreg have been shown to be crucially dependent on IL-6 and IL-2.17–19 However, several studies suggest that nTreg do not universally suppress all T helper cell subsets to the same extent. In newborns, human thymus-derived nTreg strongly suppress Th1 cells but not Th2 cells, and similar properties have been ascribed to nTreg in mice.20,21 Additionally, nTreg isolated from peripheral human blood have been shown to strongly suppress the production and secretion of interferon-γ (IFN-γ), IL-2 and IL-4, but not that of IL-10, in an allogenic model.22 Thus, diurnal changes in the Th1/Th2 balance could also be regulated by the diurnal rhythm of nTreg-suppressive activity. We previously demonstrated that the suppression of CD4+ CD25− T-cell proliferation by nTreg followed a sleep-dependent rhythm.23 However, whether this suppressive rhythm of nTreg affects the proliferation and cytokine secretion of Th1, Th2 and Th17 cells to the same extent is not yet clear. Furthermore, the signal-transduction mechanisms by which nTreg mediate their suppressive function in responder T cells (Tres) are largely unknown in humans. One possible mechanism of diurnal changes in the Th1/Th2/Th17 balance could be the hormonal priming of T cells and/or nTreg in vivo through the diurnal secretion of hormones with known immunomodulatory effects, such as prolactin, growth hormone, cortisol, noradrenalin and melatonin.8,24–31
To address the vital question of whether nTreg or hormones regulate diurnal changes in the Th1/Th2/Th17 balance, and whether Th1, Th2 and Th17 cell activity follows a diurnal rhythm, we investigated the activity of the Th1/Th2/Th17 cells and their regulation by nTreg. We were able to demonstrate that nTreg suppressed IFN-γ, IL-2 and tumour necrosis factor-α (TNF-α), but not IL-4, IL-6, IL-10, or IL-17A. The suppression of IL-2 was reduced if nTreg-associated CD25 was inhibited. Highly purified nTreg secreted IL-6, IL-10 and IL-17, but not IL-2, IL-4, IFN-γ or TNF-α. Furthermore, we observed that secretion of the cytokines IL-2, IFN-γ, TNF-α and IL-10 by naïve CD4+ T cells follows a diurnal rhythm. Multiple regression analysis, as well as subsequent in vitro experiments, suggested that serum levels of cortisol and prolactin contribute to the underlying mechanisms. Taken together, our findings imply that hormones and nTreg contribute to the diurnal secretion of cytokines from T helper cells.
Materials and methods
Experimental design, procedure and subjects
Cytokine secretion, and suppression of cytokine secretion by nTreg, was analyzed for Th1 (IFN-γ), Th2 (IL-4, IL-6) and Th17 (IL-17) cytokines, as well as for the cytokines IL-2, IL-10 and TNF-α. Furthermore, the proliferation of cytokine (IL-2, IL-4, IL-10, IL-17A, IFN-γ, TNF-α)-producing CD4+ CD25− Tres was investigated. For these analyses, T cells were isolated from blood samples taken from healthy male donors at 08:30 hr.
Diurnal cytokine secretions were analyzed in the peripheral blood cells collected from male subjects (21–32 years of age) during a within-subject crossover study with two conditions (sleep and continuous wakefulness), as previously published.8 The continuous wakefulness condition was performed in order to distinguish sleep-dependent and diurnal variations in T-cell responses. Inclusion criteria for volunteers were as follows: mental and physical health (determined from medical history, physical examination and routine laboratory testing); a body mass index between 18 and 26 kg/m2; no sleep disturbances; non-smoker; and not taking medication. Each subject participated in two experimental sessions, each covering 24 hr and starting at 20:00 hr. Each subject spent an adaptation night in the sleep laboratory, where sleep was determined offline from polysomnographic recordings according to standard criteria.32 All subjects received standardized meals and blood samples were processed immediately. An intravenous forearm catheter (Braun, Melsungen, Germany) was connected to a long thin tube, allowing blood collection from an adjacent room without disturbing the subject's sleep. Blood samples, taken at five time-points (20:00, 02:00, 07:00, 15:00 and 20:00 hr) into heparin anticoagulant, were used for isolation and functional analyses of CD4+ CD25high nTreg and CD4+ CD25− Tres. Hormone levels were measured periodically every 3 hr. The protocol was approved by the local ethics committee and all subjects signed informed consent forms.
Peripheral blood mononuclear cells and plasma isolation
Peripheral blood mononuclear cells (PBMC) were isolated from whole blood applying into CPT® Vacutainer (BD Biosciences, Heidelberg, Germany), according to the manufacturer's instructions. Plasma was collected, inactivated by heating at 56° for 30 min and then centrifuged at 4500 g. The supernatant was designated as autologous inactivated plasma.
T-cell isolation
T cells were isolated from PBMC and separated into nTreg and Tres populations using the CD4+ CD25+ Regulatory T Cell Isolation Kit® (Miltenyi Biotec, Bergisch-Gladbach, Germany), according to the manufacturer's instructions, in combination with an autoMacs® Separator (Miltenyi Biotec). We subsequently refer to this isolation protocol as MACS®. For logistical reasons we performed this protocol for the diurnal analysis. Cell purities were examined using flow cytometry. As a control for the results obtained with MACS-isolated Tres and nTreg we also performed an isolation protocol where negatively MACS isolated CD4+ T cells were sorted in CD25− and CD25high T cells by fluorescence-activated cell sorting (FACS), using MoFlo® (DakoCytomation, Hamburg, Germany). We will refer to this isolation protocol as MACS + Sort. The CD4− cells were enriched for monocytes by plastic adherence for 2·5 hr and, after harvesting, were irradiated with 60 Gy using a cobalt source. For proliferation assays, half of the Tres obtained were stained with carboxyfluorescein diacetate (CFSE) and the other half were left unstained for control purposes.
Functional assays
For analysis of the suppressive activity of nTreg on Tres, we employed a procedure described previously33 with minor modifications. Briefly, 4 × 104 Tres were co-cultured with either 2 × 104 Tres or 2 × 104 nTreg in the presence of 1 × 105 adherent cells for 62 hr in 200 μl of X-VIVO 15 medium (Lonza, Cologne, Germany). The medium was enriched with 1% inactivated autologous plasma, and cell cultures were stimulated with 0·5 μg/ml of αCD3 monoclonal antibody (mAb) (clone Okt3; eBioscience, San Diego, CA). For analysis of cytokine production by nTreg, 6 × 104 nTreg were polyclonally stimulated (as described above) and cultured for 62 hr. To verify that isolated nTreg did not proliferate, which would have indicated contamination with other T helper cells, we stained nTreg with CFSE, co-cultured them with Tres and measured CFSE dilution in nTreg using FACS, as described above.
Cytokine analysis
Culture supernatants were collected and the amounts of IL-2, IL-4, IL-6, IL-10, IL-17A, IFN-γ and TNF-α were assessed using the Bio-Plex™ Cytokine Assay (Bio-Rad, Munich, Germany) on the Bio-Plex™ Protein Array System (BioRad), following the manufacturer's instructions. To analyse the nTreg-mediated suppression of cytokine secretion we calculated the suppression ratio as: supernatant cytokine concentration (assay without nTreg)/supernatant cytokine concentration (assay with nTreg).
Intracellular cytokine staining in CD4+ CD25− T cells
To analyze possible differences in the suppressive activity of nTreg on the proliferation of Tres subpopulations, we investigated the percentage of IL-2-, IL-4-, IL-10-, IL-17A-, IFN-γ- and TNF-α-producing cells within the proliferated Tres in representative blood samples. All samples were collected at 08:30 hr, purified as described above and cultured for 62 hr before being restimulated with 5 ng/ml of phorbol myristate acetate (PMA; Sigma-Aldrich, Munich, Germany) and 500 ng/ml of ionomycin (Sigma-Aldrich) for 4 hr (IL-2, IFN-γ or TNF-α), 6 hr (IL-17A) or 8 hr (IL-4 or IL-10); 1 μg/ml of brefeldin A (BD Biosciences) was added to the cells after 1 hr of restimulation. Cells were then stained with αCD4-mAb labelled with allophycocyanin (clone M-T466; Miltenyi Biotec) and co-stained with αIL-2-mAb (clone N7.48A; Miltenyi Biotec), αIL-4-mAb (clone 8D4-8; BD Pharmingen, Heidelberg, Germany), αIL-10-mAb (clone B-T10; Miltenyi Biotec), αIL-17A (clone eBio64DEC17; eBiosciences), αIFN-γ-mAb (clone 45–15; Miltenyi Biotec), or αTNF-α-mAb (clone MAb11; BD Pharmigen) labelled with phycoerythrin or allophycocyanin. The percentage of cytokine-producing cells was determined by gating on the proliferated CD4+ CFSE-stained T cells (see Fig. 2a) applying the Cellquestpro®Software (BD Biosciences).
Figure 2.

Suppression of T helper 1 cell proliferation, but not of T helper 2 cell proliferation, by CD4+ CD25high natural regulatory T cells (nTreg). CD4+ CD25− responder T cells (Tres) [mean purity (MACS®): 96·7 ± 0·2%] and CD4+ CD25high natural regulatory T cells [mean purity (MACS®): 89·6 ± 0·9%] were isolated from the peripheral blood of healthy young men which was sampled at 08:30 hr. Monoclonal αCD3-stimulated cultures of CD4+ CD25− Tres labelled with carboxyfluorescein diacetate (CFSE), and with or without nTreg, were restimulated after 62 hr with phorbol 12-myristate 13-acetate (PMA)/ionomycin and the intracellular cytokine production was analyzed applying flow cytometry by gating on living cells and acquiring 20 000 counts. (a) CFSE-labelled Tres, with (thin line) or without nTreg (thick line), and unstimulated controls, are shown (dashed line). The rectangle named gate marks the gate with the proliferated Tres, which were analyzed to determine intracellular cytokine production, as shown for interleukin (IL)-2 (right panels). (b) The percentage of IL-2-, IL-4-, IL-10-, IL-17A-, interferon-γ (IFN-γ)- and tumour necrosis factor-α (TNF-α)-producing Tres in the absence or presence of nTreg is depicted. (c) For IL-2-, IFN-γ- and TNF-α-producing Tres, for which the absolute number is reduced by the addition of nTreg, we analyzed the percentage of proliferation with (w, dotted line) and without (w/o, solid line) nTreg in the culture by gating on CD4+ Cytokine+ (IL-2, IFN-γ, or TNF-α) T cells and measuring the reduction in CFSE fluorescence. The percentage of proliferated Tres is depicted in the tables below the graphs (one representative experiment is shown). Data represent mean values ± standard error of the mean (n = 6). *P < 0·05.
Phenotyping of Tres and nTreg
The aim of our study was to characterize the diurnal activity of T-cell subsets. We therefore analyzed whether the expression of CD126 (IL-6R alpha chainl; BD Bioscience), CD25 (IL-2R alpha chain; Miltenyi Biotec), or FOXP3 (clone PCH101; eBioscience) on/in nTreg or Tres, changed over a diurnal cycle. Additionally, we assessed whether the isolated T-cell subsets contained the same amount of FOXP3−, CD45RA+ and CD25− T cells. The expression of these markers was analyzed using FACS.
To investigate if the expression of CD25 on nTreg could change the nTreg-mediated suppression of cytokine secretion, we blocked CD25 on nTreg before performing a functional assay (see below). In brief, nTreg were isolated, using the MACS® protocol (see above), from peripheral blood samples taken at 08:30 hr, which were then incubated at 37° in 5% CO2 for 30 min with 1 μg/ml of Simulect® (Novartis, Basel, Switzerland), a CD25-neutralizing antibody. nTreg were then washed twice with phosphate-buffered saline (PBS) and used for functional assays as described above.
Hormone analysis
To analyze whether hormone levels at the time of T-cell isolation influenced Tres and nTreg activities, we measured cortisol, melatonin, prolactin, growth hormone and noradrenalin levels in serum or plasma using commercially available assays. For cortisol and growth hormone analysis the Immulite® system was used (Immulite; DPC-Biermann GmbH, Bad Nauheim, Germany). Prolactin was measured using an immunoradiometric assay (Prolactin IRMA; DPC-Biermann GmbH) and melatonin was measured using a radioimmunoassay (Bühlmann Laboratories AG, Schönenbuch, Switzerland). Noradrenalin was analysed using standard high-performance liquid chromatography with subsequent electrochemical detection (Chromsystems, Munich, Germany).34
In vitro analysis of hormone influence on T-cell cytokine production
In order to investigate whether the correlational data obtained regarding the influence of hormones on Tres cytokine secretion can be proven in an in vitro system, we isolated Tres, using the MACS protocol (see above), from peripheral blood collected at 08:30 hr. These purified Tres were then incubated (37°, 5% CO2) for 2 hr with physiological serum levels of cortisol (12 μg/dl; Sigma-Aldrich, Munich, Germany), melatonin (50 pg/ml; Sigma-Aldrich), or prolactin (20 ng/ml, R&D, Munich, Germany) in X-VIVO 15. After incubation, cells were washed twice, cultured as described above and the supernatants collected for analysis of cytokine concentrations.
Sleep quality
To ensure that the subjects slept well in the sleep condition, sleep quality was monitored using polysomnographic electroencephalogram (EEG) recordings. EEG measurements were analyzed according to previously published standards.32 The mean time for sleep onset was 22·6 ± 5·6 min. Sleep time was 451 ± 6·2 min: time in stage 1 sleep was 26·3 ± 4·1 min; time in stage 2 sleep was 236 ± 23·1 min; time in slow wave sleep (SWS) was 77·8 ± 10·5 min; and time in rapid eye movement (REM) sleep was 76·8 ± 9·8 min. Latencies (with reference to sleep onset) were 19·3 ± 5·2 min for SWS and 172·1 ± 36·8 min for REM sleep. In all six subjects, SWS predominated during the first half of the night (49·3 ± 5·5 min versus 28·5 ± 9·6 min for the first half of the night and the second half of the night, respectively), while REM sleep dominated during the second half of the night (7·9 ± 2·6 min versus 70·3 ± 8·5 min for the first half of the night and the second half of the night, respectively). Hence, all subjects slept normally during the night of the experiment.
Statistics
Statistical analysis was based on a mixed linear model using Z-transformed values, including the factors ‘sleep/sleep deprivation’ (reflecting the condition) and ‘time’ (reflecting the different time-points of measurement). If a significant time effect was found we described this as a diurnal rhythm.
The nTreg-mediated suppression of cytokine synthesis was analyzed using a paired t-test comparing cytokine concentrations in culture supernatants with versus without nTreg.
To assess temporal relationships between serum/plasma levels of hormones and cytokine secretion by CD4+ CD25− T cells and their suppression by nTreg, a backward multiple linear regression analysis was calculated. For these analyses individual data were normalized by Z-transformation.
Results
Cytokine analysis in T-cell assays with and without nTreg
Before we analyzed the diurnal Tres and nTreg activities we compared whether T cells, isolated and sorted using MACS, would give the same results. We observed that MACS-isolated nTreg (Fig. 1), as well as MACS-sorted nTreg (Fig. S1), significantly suppressed IL-2, IFN-γ and TNF-α secretion by polyclonally stimulated CD4+ CD25− Tres. By contrast, the secretion of IL-4, IL-6, IL-10 and IL-17 was not suppressed. For IL-10 and IL-17A, we detected an increase in supernatant levels only if sorted nTreg were added (Figs 1 and S1). Because the assays with MACS-isolated and MACS-sorted T cells produced strikingly similar results, we chose the MACS isolation protocol (which for logistical reasons was more appropriate for the diurnal approach) for diurnal Tres and nTreg activity analyses.
Figure 1.

Suppression of cytokine secretion from CD4+ CD25− responder T cells (Tres) by CD4+ CD25high natural regulatory T cells (nTreg). CD4+ CD25− responder T cells [mean purity (MACS®): 96·7 ± 0·2%] and CD4+ CD25high natural regulatory T cells [mean purity (MACS®): 89·6 ± 0·9%] were isolated from the peripheral blood of healthy young men which was sampled at 08:30 hr. A high proportion (91·5 ± 1%) of the CD4+ CD25high T cells were positive for forkhead box P3 (FOXP3). Cultures of Tres with or without nTreg, or cultures of nTreg alone, were stimulated with monoclonal αCD3, supernatants were collected after 62 hr and the concentrations of the cytokines interferon-γ (IFN-γ), tumour necrosis factor-α (TNF-α), interleukin (IL)-4, IL-6, IL-10 and IL-17A were determined. Data represent mean values ± standard error of the mean (n = 6). *P < 0·005; **P < 0·001; ***P < 0·00000001.
We also investigated whether αCD3-activated nTreg secrete cytokines and discovered substantial amounts of IL-6, IL-10 and IL-17A, but almost no IL-2, IL-4, IFN-γ or TNF-α, in the culture supernatants (Figs 1 and S1). Negative controls included adherent cells that were stimulated with αCD3-mAb. None of the analyzed cytokines were detected in these controls (data not shown). These data show that nTreg are suppressors of IL-2, IFN-γ and TNF-α secretion, but not of IL-4, IL-6, IL-10, or IL-17A secretion. Furthermore, our results suggest that nTreg are selective producers of IL-6, IL-10 and IL-17A. To rule out the possibility that cultured nTreg were contaminated with other T cells we cultured CFSE-stained nTreg in co-culture with unstained Tres and measured nTreg proliferation after 62 hr of stimulation with αCD3-mAb in the presence of adherent cells. We did not, however, observe any proliferation of nTreg (Fig. S2).
nTreg-mediated suppression of Th1/Th2/Th17 cell proliferation
To confirm the nTreg-mediated suppression of cytokine secretion by Tres (shown above), we investigated the reduced proliferation of cytokine-producing Tres through the addition of nTreg, at a single-cell level, using flow cytometry. After culturing Tres in the presence or absence of nTreg, we restimulated the cultures and then co-stained them with αCD4-mAb and αIL-2-, αIL-4-, αIL-10-, αIL-17A, αIFN-γ-, or αTNF-α-mAb. We then quantified the percentage of proliferating, cytokine-producing Tres (Fig. 2a). Figure 2(b,c) shows that the proliferation as well as the percentage of IL-2-, IFN-γ- and TNF-α-producing Tres was significantly reduced by the addition of nTreg (Fig. 2b, P < 0·05). By contrast, the proliferation (data not shown) as well as the percentage of IL-4-, IL-10- and IL-17A-producing Tres was not affected by the addition of nTreg.
Phenotyping of Tres and nTreg
To investigate whether isolated Tres and nTreg express receptors and FOXP3, which are relevant to their function, either constantly or with a diurnal rhythm, we performed FACS analysis for these markers. Tres did not show any diurnal or sleep-dependent changes with respect to CD126 (IL-6R alpha chain) expression, measured using the geometrical mean. Furthermore, these cells also failed to show any diurnal changes in terms of the percentage of CD45RA+ (naive) Tres (76·4 ± 1·9%). nTreg showed no diurnal rhythm in the expression of either FOXP3 or CD126 (IL-6R alpha chain) measured using the geometrical mean and no change in the percentage of FOXP3+ (91·2 ± 1%) cells. Interestingly, we observed a diurnal rhythm in the expression of CD25 [F(1,4) = 5·7, P = 0·01, Fig. 3a]. Blocking CD25 (IL-2R alpha chain) on nTreg decreased the nTreg-suppressive activity of the secretion of IL-2 and TNF-α by Tres (Fig. 3b,d) and increased the secretion of IL-17A (Fig. 3c). The suppression of cytokine secretion from Tres by nTreg did not correlate with CD25 expression (Table S1).
Figure 3.

CD25 expression on natural regulatory T cells (nTreg) and its influence on the suppressive activity of nTreg. (a) Peripheral blood mononuclear cells (PBMC) from the peripheral blood of healthy young men were collected over a 24-hr period at five time-points (20:00, 02:00, 07:00, 15:00 and 20:00 hr). PBMC were stained with monoclonal αCD4 labelled with fluorescein, monoclonal forkhead box P3 (FOXP3) labelled with allophycocyanin and monoclonal αCD25 labelled with phycoerythrin. CD25 expression was analyzed by flow cytometry applying the geometrical mean (geo mean). As no effect of sleep was found, data were collapsed (data from both conditions are depicted in one graph) from the sleep and the continuous wakefulness conditions and represent mean values ± standard error of the mean (n = 6 in each condition). (b–d) CD4+ CD25− responder T cells (Tres) [mean purity (MACS® + Sort): 98·2%] and CD4+ CD25high nTreg [mean purity (MACS® + Sort): 99·1%] were isolated from the peripheral blood of healthy young men which was sampled at 08:30 hr. Cultures of Tres, with or without nTreg, were stimulated with monoclonal αCD3, supernatants were collected after 62 hr and the concentrations of the cytokines interferon-γ (IFN-γ), tumour necrosis factor-α (TNF-α), interleukin (IL)-2 and IL-17A were determined and are shown (panel b, IL-2; panel c, IL-17; panel d, TNF-α). Control: Tres without nTreg; w CD4+ CD25high: Tres with nTreg; w CD4+ CD25high (+Simulect®): Tres with nTreg where CD25 (IL-2R alpha chain) was blocked with Simulect®. Panels b–d show one representative experiment out of two.
Diurnal cytokine secretion of CD4+ CD25− T cells and their suppression by nTreg
Because we discovered that nTreg suppress Th1 cells, but not Th2 or Th17 cells, we investigated whether nTreg activity changes over a diurnal cycle. First, we analyzed the secretion of IL-2, IL-4, IL-6, IL-10 IL-17A, IFN-γ, or TNF-α by Tres over a diurnal cycle at five time-points (20:00, 02:00, 07:00, 15:00 and 20:00 hr) in the culture supernatant. We found that the Tres-mediated secretion of IL-2 [F(1,4) = 8·1, P = 0·001], IFN-γ [F(1,4) = 14·4, P = 0·0001], TNF-α [F(1,4) = 5·8, P = 0·006] and IL-10 [F(1,4) = 3·8, P = 0·045] followed a significant diurnal rhythm, peaking at 02:00 hr (Fig. 4). By contrast, IL-4, IL-6 and IL-17A secretion did not follow a significant diurnal rhythm (Fig. 4). The addition of nTreg to the Tres culture significantly decreased the concentrations of IL-2, IFN-γ and TNF-α but not those of IL-4, IL-6, IL-10 and IL-17A (Fig. 4). However, the diurnal rhythm of IL-2 [F(1,4) = 7·1, P = 0·003], IFN-γ [F(1,4) = 6·3, P = 0·005], TNF-α [F(1,4) = 6·4, P = 0·003] and IL-10 [F(1,4) = 4·2, P = 0·04] secretion by Tres in the presence of nTreg was still evident (Fig. 4). Maximum IL-2, IL-10, IFN-γ and TNF-α release still occurred at 02:00 hr. The suppression ratio [suppression ratio = supernatant cytokine concentration (assay without nTreg)/supernatant cytokine concentration (assay with nTreg)], which is a value for nTreg mediated inhibition of cytokine secretion by Tres as a result of the addition of nTreg, showed a diurnal rhythm for IL-2 [F(1,4) = 3·3; P = 0·037], with a peak at 07:00 hr, but not for IFN-γ [F(1,4) = 1·34; P = 0·29] and TNF-α [F(1,4) = 0·74; P = 0·591]. We found no significant differences between the sleep and wake conditions (data not shown).
Figure 4.
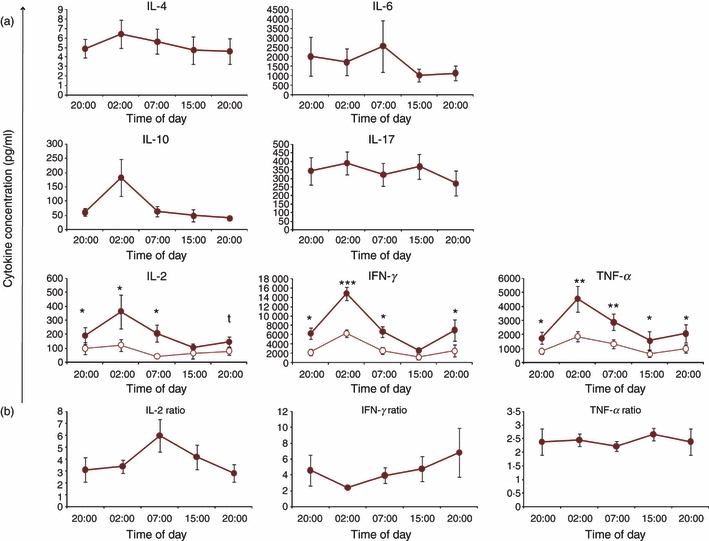
Diurnal rhythm of cytokine secretion by CD4+ CD25− responder T cells (Tres) with and without CD4+ CD25high natural regulatory T cells (nTreg): CD4+ CD25− Tres were isolated at five time-points (20:00, 02:00, 07:00, 15:00 and 20:00 hr), using MACS technology, from the peripheral blood of healthy young men over a 24-hr period. The mean purity of Tres was 96·9 ± 0·7% and the mean purity of CD4+ CD25high nTreg was 78·3 ± 8·2%. Cultures of Tres with (open circles) and without (closed circles) nTreg were stimulated with monoclonal αCD3, supernatants were collected after 62 hr and (a) the concentrations of the cytokines interleukin (IL)-2, IL-4, IL-6, IL-10, IL-17A, interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α) were measured. (b) For IL-2, IFN-γ and TNF-α, for which the secretion is significantly suppressed through the addition of nTreg, we calculated the suppression ratio as described above. As no effect of sleep was found, data were collapsed from the sleep and the continuous wakefulness conditions and represent mean values ± standard error of the mean (n = 6 in each condition). *P < 0·05; **P < 0·01; ***P < 0·005.
Hormone analysis
Analysis of the levels of cortisol, melatonin, prolactin, growth hormone and noradrenalin in plasma/serum revealed that the subjects had a normal diurnal hormonal rhythm (data for the sleep condition are shown inFig. 5) and that at least some of the hormones influenced T-cell activity. As expected from in vitro data, cortisol levels from the time of T-cell isolation negatively correlated with Tres cytokine secretion (Table 1). By contrast, melatonin and prolactin levels showed a positive correlation with Tres cytokine secretion (Table 1). The levels of growth hormone and noradrenalin generally did not correlate with the secretion of cytokines (Table 1). The suppression of Tres cytokine secretion by nTreg did not correlate with any of the investigated hormones (Table S1).
Figure 5.

Diurnal rhythm of immunomodulatory hormones in plasma/serum. The plasma/serum levels of prolactin, melatonin, cortisol, noradrenalin and growth hormone were analyzed every 3 hr. The measurements from the sleep condition are depicted. Data represent mean values ± standard error of the mean (n = 6).
Table 1.
Correlation between hormone levels and T-cell cytokine secretion
| Cytokine/hormone correlation | IL-2 | IL-4 | IL-6 | IL-10 | IFN-γ | TNF-α | IL-17 |
|---|---|---|---|---|---|---|---|
| R2 | 0·26 | 0·064 | 0·35 | 0·198 | 0·211 | 0·168 | 0·086 |
| Significance of the model | P = 0·001 | P = 0·067 | P = 0·09 | P = 0·007 | P = 0·002 | P = 0·003 | P = 0·017 |
| Included hormones | Cortisol, melatonin, prolactin, growth hormone | Cortisol, noradrenaline | Melatonin | Cortisol, melatonin, prolactin, growth hormone | Cortisol, melatonin, prolactin | Cortisol, melatonin | – |
| Beta (Cortisol) | −0·472, P < 0·0001 | −0·248, P = 0·065 | – | −0·284, r = 0·041 | −0·412 r = 0·003 | −0·191, P = 0·133 | – |
| Beta (Melatonin) | 0·285, P = 0·028 | – | 0·231, r = 0·09 | 0·44, r = 0·005 | 0·346, r = 0·008 | 0·433, P = 0·001 | – |
| Beta (Prolactin) | 0·1, P = 0·09 | – | – | 0·358, r = 0·065 | 0·186, r = 0·174 | – | – |
| Beta (Growth hormone) | 0·124, P = 0·246 | – | – | 0·279, r = 0·042 | – | – | – |
| Beta (Noradrenalin) | – | −0·182, r = 0·173 | – | – | – | – | – |
Correlations between plasma/serum levels of cortisol, melatonin, prolactin, growth hormone and noradrenaline and the secretion of interleukin (IL)-2, IL-4, IL-6, IL-10, IL-17A, interferon-γ (IFN-γ) and tumour necrosis-α (TNF-α) by responder T cells (Tres). Results were calculated by applying a backward multiple linear regression analysis. R2 is the percentage variance that can be explained by the model (e.g. R2 = 0·35 explains 35% of data variance). Beta values are only shown if they are significant or show a trend. n = 6.
In vitro analysis of hormonal influence on T-cell cytokine production
To investigate whether cortisol, melatonin and prolactin influence diurnal cytokine secretion from Tres, we incubated Tresin vitro with cortisol, melatonin, or prolactin for 2 hr and measured the levels of IL-2, IL-10, IFN-γ and TNF-α (for which we found a diurnal rhythm – see above) after 62 hr of polyclonal stimulation. We chose cortisol, melatonin and prolactin because the serum levels of these hormones correlated with Tres cytokine secretion (Table 1). The prediction, from our multiple linear regression analysis, was that cortisol would suppress the secretion of IL-2, IL-10, IFN-γ and TNF-α, whereas melatonin and prolactin would increase the secretion of IL-2, IL-10, IFN-γ and TNF-α. The influence of growth hormone and noradrenalin in the multiple linear regression analysis was only minor and we therefore did not test these hormones in vitro. As depicted in Fig. 6, 2 hr of incubation with cortisol at physiological daytime levels suppressed the secretion of IL-2 and IL-10, but not that of IFN-γ and TNF-α. While incubation of Tres for 2 hr with physiological night-time levels of prolactin increased IL-10 release and reduced IL-2 secretion, the generation of IFN-γ and TNF-α was not significantly changed. In contrast to our statistical findings, 2 hr of incubation with physiological night-time levels of melatonin did not increase the secretion of IL-2, IL-10, IFN-γ or TNF-α from Tres.
Figure 6.
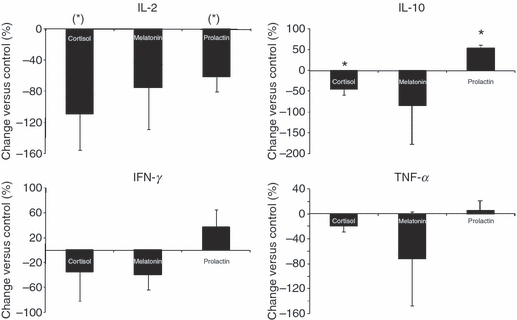
In vitro analysis of the effects of hormones on CD4+ CD25− responder T cell (Tres) cytokine secretion. CD4+ CD25− Tres [mean purity (MACS®): 96·7 ± 0·2%] were isolated from the peripheral blood of healthy young men which was sampled at 08:30 hr, incubated for 2 hr with cortisol (12 μg/dl), melatonin (50 pg/ml), or prolactin (20 ng/ml) and then stimulated with monoclonal αCD3. After 62 hr, culture supernatants were collected and the concentrations of interleukin (IL)-2, IL-10, interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α) were determined. The percentage change in cytokine secretions are shown compared with controls where cells were pre-incubated with medium alone. n = 3; (*)P < 0·1; *P < 0·05.
Discussion
In this study, we investigated T helper cell activity and its diurnal regulation by hormones and nTreg. We showed that nTreg suppress the secretion of IL-2, IFN-γ and TNF-α, but not that of IL-4, IL-6, IL-10 and IL-17A, by CD4+ CD25− Tres. Interestingly, we found that nTreg secrete IL-6, IL-10 and IL-17A. Furthermore, we demonstrated that nTreg selectively suppress the proliferation of Tres which produce IL-2, IFN-γ and TNF-α, but not of Tres which produce IL-4, IL-10, or IL-17A. We could also show that the secretion of IL-2, IL-10, IFN-γ and TNF-α by Tres followed a diurnal rhythm, peaking at 02:00 hr. In addition, we found a diurnal rhythm of nTreg-mediated suppression of IL-2 secretion by Tres with a peak at 07:00 hr.
Our findings are in line with previous work, where it was shown that CD4+ CD25high regulatory T-cell clones from the human thymus of neonates suppress Th1 clones but have a lesser effect on Th2 clones.21 In mice, it was demonstrated that freshly isolated nTreg were unable to suppress Th2 cells.20 Oberle et al.22 showed in human that IL-2 and IFN-γ secretion, but not that of IL-10, was suppressed through the addition of nTreg. In contrast to our findings, however, these researchers reported that nTreg suppress IL-4 secretion. The reason for this conflicting data may be a result of the different assay conditions employed, where we used nTreg and Tres from the same donor rather than nTreg from HLA-A2+ donors and Tres from HLA-A2− donors. Therefore, allogenic effects are likely to be responsible for these different findings. In mice, the induction of Foxp3 in Tres has been implicated as a mechanism for the suppression of Th2 cytokines by pre-activated nTreg.20 However, in human cells this could not be shown and candidate factors, such as ‘Suppressor of Cytokine Secretion 1 and 3’, as well as many other factors, could be excluded as relevant to the suppression of cytokine production.22 A mechanism for the higher resistance of Th2 cell proliferation to suppression by nTreg was identified by Cosmi and co-workers. They found that thymic Th2 cell clones are less susceptible to nTreg-mediated suppression because they were able to produce and respond to growth factors distinct from IL-2, such as IL-4 and IL-9.21 These findings from thymocyte clones are in line with our current findings of peripheral blood nTreg. Interestingly, we discovered that nTreg did not suppress IL-17A secretion by Tres and that nTreg actually secrete IL-17A. IL-17A has been shown to be a detrimental cytokine in autoimmune diseases such as experimental autoimmune encephalitis.35,36 Recently published studies indicate that nTreg are able to convert into IL-17A-secreting cells.37–40 Hence, our finding that nTreg secrete IL-17A might be caused by the conversion of nTreg into IL-17A-secreting cells. Taken together, we showed that human nTreg mainly suppress Th1 cell proliferation and cytokine secretion.
Previous studies have shown that either non-adherent leucocytes or T cells within whole blood samples produced or secreted cytokines in a diurnal manner.8,10,11 To dissect whether these changes in cytokine production are caused by functional changes of the single cell or if diurnal changes of the leucocyte composition are responsible for this observation (as described in9–11), we addressed whether T-cell function follows a diurnal rhythm. This was achieved by stimulating highly purified Tres which were isolated at five time-points over a 24 hr period. We controlled surface markers and confirmed that there were no diurnal changes in the composition of the analyzed Tres subsets in terms of CD25, CD45RA, FOXP3 and CD126 (IL-6 receptor alpha chain) expression. We could clearly demonstrate that the secretion of IL-2, IL-10, IFN-γ and TNF-α by Tres followed a diurnal rhythm with a peak at 02:00 hr. A similar pattern has previously been shown for the proliferation of Tres.23 This clearly implies that T-cell functions follow a diurnal rhythm. The rhythm in cytokine secretion by Tres was sustained if we added nTreg from the same time (when Tres were isolated) to the Tres cultures. nTreg suppressed the secretion of IL-2 with a diurnal rhythm and this was independent of sleep. We previously demonstrated that nTreg suppress the proliferation of Tres in a sleep-dependent rhythm.23 The differential nTreg-mediated suppression of cytokine secretion by, and proliferation of, Tres by nTreg may reflect different mechanisms of suppression.
Different mechanisms of nTreg-mediated suppression have been suggested by Stockinger et al.36 Numerous suppressive mechanisms of nTreg have been described (reviewed in ref. 15) but the distinction between mechanisms by which nTreg suppress cytokine secretion or proliferation of Tres remain elusive.15,22 To elucidate the underlying mechanism of nTreg-mediated suppression, we investigated the diurnal secretion of IL-6, a cytokine that substantially modulates nTreg-mediated suppression,17,18,41 as well as the expression of the membrane-bound IL-6 receptor (CD126). However, IL-6 secretion by Tres and CD126 expression on Tres and nTreg did not show a diurnal rhythm at the time-points analyzed. Therefore, it is unlikely that IL-6, known to reduce nTreg-mediated suppression, contributes to the diurnal rhythm of nTreg suppressive activity. Besides IL-6, we also investigated CD25 expression on nTreg because it was shown in mice that nTreg consume IL-2 with their highly expressed IL-2 receptor alpha chain (CD25), thereby suppressing Tres proliferation.19,42 To investigate whether CD25 expression on nTreg contributes to nTreg-mediated suppression, we blocked CD25 on nTreg and this resulted in a decreased nTreg-mediated suppression of IL-2 secretion. Analyzing the diurnal expression of CD25 on CD4+ FOXP3+ T cells (nTreg) we observed a diurnal rhythm with a peak at 20:00 hr and a nadir at 07:00 hr. Hence, CD25 expression on nTreg is lowest when the suppression of IL-2 secretion is highest. This makes the IL-2 consumption by nTreg an unlikely mechanism for the diurnal rhythm of nTreg-mediated IL-2 suppression. Furthermore, multiple linear regression analysis did not reveal any correlation between IL-2 secretion in co-culture assays of Tres/nTreg and the expression of CD25 on nTreg. Nevertheless, the diurnal rhythm of CD25 expression on nTreg is interesting in itself, although the underlying mechanism is unknown. A candidate for this mechanism might be the cellular circadian clock. Recently, it was shown that the transcription factor retinoid-related orphan receptor-alpha (RORA), which is part of the cellular circadian clock, interacts with FOXP3.43 Furthermore, it was shown that natural killer (NK) cells and macrophages harbour a cellular circadian clock that modulates circadian immune functions of these cells.3–5,44 Hence, the diurnal suppression of Tres cytokine secretion by nTreg might, in part, be driven by the cellular circadian clock of nTreg via yet-unknown pathways. Therefore, the analysis of the circadian clock in T cells should be addressed in future studies.
Besides the cellular circadian clock, the hormonal priming of T cells in vivo could be another mechanism for the diurnal rhythm of cytokine secretion by Tres.13 To investigate this possible mechanism we analyzed the hormone levels from all subjects and performed a multiple linear regression analysis. We found a negative correlation between cortisol serum levels and T-cell cytokine secretion. Furthermore, we demonstrated in vitro that a 2 hr pre-incubation with physiological daytime levels of cortisol decreased cytokine secretion. This is in line with in vitro data published by other investigators demonstrating an immunosuppressive effect of cortisol.8,26,30,45–47 A positive correlation was found between melatonin and prolactin serum levels and T-cell cytokine secretion. Whereas we could show in vitro that pre-incubation of Tres with prolactin increased the secretion of IL-10 but decreased that of IL-2 by Tres, we were unable to demonstrate this effect for melatonin. Prolactin was described to display immune-stimulatory functions in vitro, whereas conflicting data are published for melatonin.27,30,48,49 We also observed increased IFN-γ after prolactin pre-incubation but this effect was not significant, as previously described by Matera et al. and Dimitrov et al.29,30 However, Matera et al. investigated unstimulated T cells while we used polyclonally stimulated Tres. Dimitrov et al. studied the percentage of IFN-γ-producing T cells in whole blood which were stimulated with PMA/ionomycin in the presence of prolactin. By contrast, we pre-incubated T cells with prolactin, performed the assays (αCD3 stimulated) without prolactin and measured the concentration of IFN-γ in the supernatant. Despite these different approaches, our observations are broadly similar to these other reports.29,30 Our findings on the effect of melatonin are in line with other investigators who did not observe stimulatory effects of melatonin in vitro.49 We could not confirm the proposed Th1-enhancing effect of melatonin in vitro but these published data are from in vivo experiments in mice and conflicting data have also been published.50 In any case, one can speculate, from the effects of cortisol and prolactin, that the hormonal milieu could be one mechanism of the diurnal rhythm of cytokine secretion by Tres. The suppressive activity of nTreg on cytokine secretion by Tres did not correlate with the serum levels of any of the hormones. However, this does not exclude an influence of the hormones analyzed in the present study, and there are, of course, many more hormones with diurnal rhythms that may affect nTreg-suppressive activity. Hence, further studies are needed to characterize the influence of hormones on nTreg.
Taken together, we could demonstrate that nTreg isolated from peripheral blood distinctly suppress Th1 cells, but not Th2 or Th17 cells. We also showed that nTreg secrete IL-10 and IL-17A but almost no IL-2, IL-4, IFN-γ or TNF-α. Additionally, nTreg produced IL-6, which is known as a critical factor in breaking nTreg-mediated tolerance and in the development of nTreg and Th17 cells.17,18,41 Furthermore, we discovered the presence of a diurnal cycle dynamic that affects the abilities of Tres to generate cytokines and nTreg to suppress cytokine secretion. Additionally, our data indicate that the diurnal rhythm of cytokine secretion by Tres might be partially regulated by cortisol and prolactin. In conclusion, our data demonstrate that not only does the migration of leucocytes in the peripheral blood change over a diurnal cycle but also the function of defined T-cell subsets. This finding is novel and it will be interesting to study the effect of the diurnal rhythm of T-cell function on diurnal immune responses in relation to autoimmunity, allergy and vaccination.
Acknowledgments
We are grateful to Susanne Diekelmann, Stojan Dimitrov, and Ines Wilhelm, Dept. of Neuroendocrinology, University of Luebeck for helping us with the planning of the study design and the sleep lab protocol and Monika Bajtus for lab work. We thank Dr. Andreas Katopodis at the Novartis Institutes of Biomedical Research for providing basiliximab (Simulect®). We also thank Jochen Hühn (Helmholtz Center, Braunschweig, Germany), Nina Oberle (Deutsches Krebsforschungszentrum, Heidelberg) and Antje Müller (Rheumatology, University of Luebeck) for helpful scientific discussions. We also thank Bernhard Gibbs (Medway School of Pharmacy, University of Kent) for editing our manuscript. This work was funded by the DFG, SFB 654, project C6 and C8, SFB/TR 22, and the E37-2008 grant of the University of Luebeck.
Disclosures
We declare that none of the authors has any financial conflict of interest.
Supporting Information
Additional Supporting Information may be found in theonline version of this article:
Figure S1. Suppression of cytokine secretion of CD4+ CD25− responder T cells by CD4+ CD25high natural regulatory T cells. CD4+ CD25− responder T cells (Tres, mean purity (MACS® + Sort): 99·2 ± 0·5%) and CD4+ CD25high natural regulatory T cells (nTreg, mean purity (MACS® + Sort): 98·5 ± 0·6%) were isolated from peripheral blood of healthy young men which was sampled at 8:30 hr. Cultures of Tres with or without nTreg or cultures of only nTreg were stimulated with αCD3-mAb, supernatants were collected after 62 hr and the cytokine concentration of IFN-γ, TNF-α, IL-2, IL-4, IL-6, IL10, and IL-17A was analyzed. Data represent mean values ± standard error of the mean (n = 6). P < 0·05*
Figure S2. Proliferation of CFSE stained CD4+ CD25high natural regulatory T cells in co-culture with CD4+ CD25− responder T cells. CD4+ CD25− responder T cells (Tres, purity (MACS® + Sort): 99·1%) and CD4+ CD25high natural regulatory T cells (nTreg, purity (MACS® + Sort): 99·2%) were isolated from peripheral blood of healthy young men which was sampled at 8:30 hr. Cultures of aCD3-mAb stimulated 4 × 104 Tres with either 2 × 104 CFSE stained Tres (green line) or nTreg (black line). Unstimulated control is shown as a red line. One representative out of two experiments is shown.
Table S1. Correlation between hormone levels and nTreg suppression ratio. The correlations between the plasma/serum levels of cortisol, melatonin, prolactin, growth hormone, and noradrenaline and the suppression ratio (see ‘Results’) are depicted and were calculated applying a backward multiple linear regression analysis. R2 is the percent of variance which can be explained by the model (e.g. R2 = 0·35 explains 35% of data variance). Beta values are not shown because none of the calculated models were significant. n = 6.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Poellman L, Poellmann B. Tagesrhythmische Unterschiede bei der Antikörperbildung nach Hepatitis-B-Schutzimpfung [Circadian differences of antibody response to hepatitis B vaccination] In: Hoffmann F, Stoessel U, editors. Arbeitsmedizin im Gesundheitsdienst [Occupational medicine in medical service] Stuttgart: Gentner Verlag; 1988. [Google Scholar]
- 2.Boivin DB, James FO, Wu A, Cho-Park PF, Xiong H, Sun ZS. Circadian clock genes oscillate in human peripheral blood mononuclear cells. Blood. 2003;102:4143–5. doi: 10.1182/blood-2003-03-0779. [DOI] [PubMed] [Google Scholar]
- 3.Keller M, Mazuch J, Abraham U, et al. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci U S A. 2009;106:21407–12. doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayashi M, Shimba S, Tezuka M. Characterization of the molecular clock in mouse peritoneal macrophages. Biol Pharm Bull. 2007;30:621–6. doi: 10.1248/bpb.30.621. [DOI] [PubMed] [Google Scholar]
- 5.Arjona A, Sarkar DK. Evidence supporting a circadian control of natural killer cell function. Brain Behav Immun. 2006;20:469–76. doi: 10.1016/j.bbi.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Phillips AC, Gallagher S, Carroll D, Drayson M. Preliminary evidence that morning vaccination is associated with an enhanced antibody response in men. Psychophysiology. 2008;45:663–6. doi: 10.1111/j.1469-8986.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 7.Petrovsky N, McNair P, Harrison LC. Diurnal rhythms of pro-inflammatory cytokines: regulation by plasma cortisol and therapeutic implications. Cytokine. 1998;10:307–12. doi: 10.1006/cyto.1997.0289. [DOI] [PubMed] [Google Scholar]
- 8.Dimitrov S, Lange T, Tieken S, Fehm HL, Born J. Sleep associated regulation of T helper 1/T helper 2 cytokine balance in humans. Brain Behav Immun. 2004;18:341–8. doi: 10.1016/j.bbi.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Dinges DF, Douglas SD, Zaugg L, et al. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest. 1994;93:1930–9. doi: 10.1172/JCI117184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irwin M, McClintick J, Costlow C, Fortner M, White J, Gillin JC. Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. FASEB J. 1996;10:643–53. doi: 10.1096/fasebj.10.5.8621064. [DOI] [PubMed] [Google Scholar]
- 11.Born J, Lange T, Hansen K, Molle M, Fehm HL. Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol. 1997;158:4454–64. [PubMed] [Google Scholar]
- 12.Dimitrov S, Benedict C, Heutling D, Westermann J, Born J, Lange T. Cortisol and epinephrine control opposing circadian rhythms in T cell subsets. Blood. 2009;113:5134–43. doi: 10.1182/blood-2008-11-190769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bollinger T, Bollinger A, Oster H, Solbach W. Sleep, immunity, and circadian clocks: a mechanistic model. Gerontology. 2010 doi: 10.1159/000281827. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 15.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–44. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corthay A. How do regulatory T cells work? Scand J Immunol. 2009;70:326–36. doi: 10.1111/j.1365-3083.2009.02308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–6. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 18.Doganci A, Eigenbrod T, Krug N, et al. The IL-6R alpha chain controls lung CD4+CD25+ Treg development and function during allergic airway inflammation in vivo. J Clin Invest. 2005;115:313–25. doi: 10.1172/JCI22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353–62. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 20.Stassen M, Jonuleit H, Muller C, et al. Differential regulatory capacity of CD25+ T regulatory cells and preactivated CD25+ T regulatory cells on development, functional activation, and proliferation of Th2 cells. J Immunol. 2004;173:267–74. doi: 10.4049/jimmunol.173.1.267. [DOI] [PubMed] [Google Scholar]
- 21.Cosmi L, Liotta F, Angeli R, et al. Th2 cells are less susceptible than Th1 cells to the suppressive activity of CD25+ regulatory thymocytes because of their responsiveness to different cytokines. Blood. 2004;103:3117–21. doi: 10.1182/blood-2003-09-3302. [DOI] [PubMed] [Google Scholar]
- 22.Oberle N, Eberhardt N, Falk CS, Krammer PH, Suri-Payer E. Rapid suppression of cytokine transcription in human CD4+CD25 T cells by CD4+Foxp3+ regulatory T cells: independence of IL-2 consumption, TGF-beta, and various inhibitors of TCR signaling. J Immunol. 2007;179:3578–87. doi: 10.4049/jimmunol.179.6.3578. [DOI] [PubMed] [Google Scholar]
- 23.Bollinger T, Bollinger A, Skrum L, Dimitrov S, Lange T, Solbach W. Sleep-dependent activity of T cells and regulatory T cells. Clin Exp Immunol. 2009;155:231–8. doi: 10.1111/j.1365-2249.2008.03822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gala RR. Prolactin and growth hormone in the regulation of the immune system. Proc Soc Exp Biol Med. 1991;198:513–27. doi: 10.3181/00379727-198-43286b. [DOI] [PubMed] [Google Scholar]
- 25.Anisman H, Baines MG, Berczi I, et al. Neuroimmune mechanisms in health and disease: 1. Health. CMAJ. 1996;155:867–74. [PMC free article] [PubMed] [Google Scholar]
- 26.Elenkov IJ, Chrousos GP, Wilder RL. Neuroendocrine regulation of IL-12 and TNF-alpha/IL-10 balance. Clinical implications. Ann N Y Acad Sci. 2000;917:94–105. doi: 10.1111/j.1749-6632.2000.tb05374.x. [DOI] [PubMed] [Google Scholar]
- 27.Chikanza IC. Prolactin and neuroimmunomodulation: in vitro and in vivo observations. Ann N Y Acad Sci. 1999;876:119–30. doi: 10.1111/j.1749-6632.1999.tb07629.x. [DOI] [PubMed] [Google Scholar]
- 28.Matera L, Mori M, Galetto A. Effect of prolactin on the antigen presenting function of monocyte-derived dendritic cells. Lupus. 2001;10:728–34. doi: 10.1191/096120301717164967. [DOI] [PubMed] [Google Scholar]
- 29.Matera L, Mori M. Cooperation of pituitary hormone prolactin with interleukin-2 and interleukin-12 on production of interferon-gamma by natural killer and T cells. Ann N Y Acad Sci. 2000;917:505–13. doi: 10.1111/j.1749-6632.2000.tb05415.x. [DOI] [PubMed] [Google Scholar]
- 30.Dimitrov S, Lange T, Fehm HL, Born J. A regulatory role of prolactin, growth hormone, and corticosteroids for human T-cell production of cytokines. Brain Behav Immun. 2004;18:368–74. doi: 10.1016/j.bbi.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Lange T, Dimitrov S, Fehm HL, Westermann J, Born J. Shift of monocyte function toward cellular immunity during sleep. Arch Intern Med. 2006;166:1695–700. doi: 10.1001/archinte.166.16.1695. [DOI] [PubMed] [Google Scholar]
- 32.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep of human subjects. Washington, DC: US Government Printing Office; 1992. [Google Scholar]
- 33.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–94. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldstein DS, Feuerstein G, Izzo JL, Jr, Kopin IJ, Keiser HR. Validity and reliability of liquid chromatography with electrochemical detection for measuring plasma levels of norepinephrine and epinephrine in man. Life Sci. 1981;28:467–75. doi: 10.1016/0024-3205(81)90139-9. [DOI] [PubMed] [Google Scholar]
- 35.Weaver CT, Murphy KM. The central role of the Th17 lineage in regulating the inflammatory/autoimmune axis. Semin Immunol. 2007;19:351–2. doi: 10.1016/j.smim.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–6. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Ayyoub M, Deknuydt F, Raimbaud I, et al. Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORgamma t. Proc Natl Acad Sci U S A. 2009;106:8635–40. doi: 10.1073/pnas.0900621106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koenen HJ, Smeets RL, Vink PM, van RE, Boots AM, Joosten I. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112:2340–52. doi: 10.1182/blood-2008-01-133967. [DOI] [PubMed] [Google Scholar]
- 39.Zhou L, Lopes JE, Chong MM, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–40. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beriou G, Costantino CM, Ashley CW, et al. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood. 2009;113:4240–9. doi: 10.1182/blood-2008-10-183251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doganci A, Sauer K, Karwot R, Finotto S. Pathological role of IL-6 in the experimental allergic bronchial asthma in mice. Clin Rev Allergy Immunol. 2005;28:257–70. doi: 10.1385/CRIAI:28:3:257. [DOI] [PubMed] [Google Scholar]
- 42.Scheffold A, Murphy KM, Hofer T. Competition for cytokines: T(reg) cells take all. Nat Immunol. 2007;8:1285–7. doi: 10.1038/ni1207-1285. [DOI] [PubMed] [Google Scholar]
- 43.Du J, Huang C, Zhou B, Ziegler SF. Isoform-specific inhibition of ROR alpha-mediated transcriptional activation by human FOXP3. J Immunol. 2008;180:4785–92. doi: 10.4049/jimmunol.180.7.4785. [DOI] [PubMed] [Google Scholar]
- 44.Arjona A, Sarkar DK. The circadian gene mPer2 regulates the daily rhythm of IFN-gamma. J Interferon Cytokine Res. 2006;26:645–9. doi: 10.1089/jir.2006.26.645. [DOI] [PubMed] [Google Scholar]
- 45.Blotta MH, DeKruyff RH, Umetsu DT. Corticosteroids inhibit IL-12 production in human monocytes and enhance their capacity to induce IL-4 synthesis in CD4+ lymphocytes. J Immunol. 1997;158:5589–95. [PubMed] [Google Scholar]
- 46.Panina-Bordignon P, Mazzeo D, Lucia PD, et al. Beta2-agonists prevent Th1 development by selective inhibition of interleukin 12. J Clin Invest. 1997;100:1513–9. doi: 10.1172/JCI119674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brunetti M, Mascetra N, Martelli N, Ranelletti FO, Musiani P, Aiello FB. Synergistic inhibitory activities of interleukin-10 and dexamethasone on human CD4+ T cells. Transplantation. 2002;74:1152–8. doi: 10.1097/00007890-200210270-00017. [DOI] [PubMed] [Google Scholar]
- 48.Matera L, Mori M, Geuna M, Buttiglieri S, Palestro G. Prolactin in autoimmunity and antitumor defence. J Neuroimmunol. 2000;109:47–55. doi: 10.1016/s0165-5728(00)00302-7. [DOI] [PubMed] [Google Scholar]
- 49.Srinivasan V, Maestroni GJ, Cardinali DP, Esquifino AI, Perumal SR, Miller SC. Melatonin, immune function and aging. Immun Ageing. 2005;2:17. doi: 10.1186/1742-4933-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin GJ, Huang SH, Chen YW, et al. Melatonin prolongs islet graft survival in diabetic NOD mice. J Pineal Res. 2009;47:284–92. doi: 10.1111/j.1600-079X.2009.00712.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


