Abstract
Dendritic cell (DC)-mediated vaccination against Leishmania major induces a parasite-specific T helper 1 (Th1) response and long-lasting protective immunity in susceptible mice. As the cytokine interleukin-12 required for induction of this Th1 response is not derived from the transferred DC, but has to be produced by the vaccinated host, we examined cross-presentation of transferred DC via resident DC of the host and cross-activation with natural killer (NK) cells as mechanisms supporting the induction of protective immunity after DC-mediated vaccination. Co-culture with DC that had been conditioned ex vivo by loading with L. major lysate and stimulation with CpG-containing oligodeoxynucleotides did not result in the activation of naive DC in vitro. Furthermore, L. major antigen from conditioned DC was not cross-presented to a significant extent in vivo. In contrast, co-culture of DC with NK cells led to cross-activation of both cell populations with induction of interferon-γ, which was dependent on the activation status of the conditioned DC. Transient depletion of NK cells during vaccination of L. major-susceptible mice with conditioned DC resulted in reduced protection. Our findings indicate that cross-presentation of conditioned DC after DC-based vaccination against L. major plays a minor role in the induction of protective immunity. However, we demonstrated for the first time that the capacity of DC to mediate protection against L. major is supported by cross-activation with NK cells of the host and NK-cell-derived interferon-γ.
Keywords: cross-activation, dendritic cells, Leishmania major, natural killer cells, vaccination
Introduction
Dendritic cells (DC) are critical decision-making cells in the immune response. They direct diverse and sometimes opposing functions of the immune system, including initiation and regulation of adaptive immune responses as well as tolerance and anergy.1 Acting at the interface of innate and adaptive immunity, DC play a key role in the activation of naive T cells and the development of T helper type 1 (Th1) or type 2 (Th2) T-cell responses during the immune defence against pathogens. This makes DC attractive tools for immune intervention strategies, whether to reduce allergic reactions or as candidates for novel immune intervention strategies in the field of infectious diseases, where conventional vaccination regimes so far have elicited only insufficient immune responses, e.g. towards human immunodeficiency virus and protozoan parasites.
Studies in our laboratory had given the first proof of principle that several distinct DC populations are able to induce complete protection against microbial pathogens.2–4 Using murine leishmaniasis as one of the best characterized immunological models, we showed that a single vaccination with bone-marrow-derived DC (BMDC) that had been conditioned ex vivo by loading with Leishmania major lysate and stimulation with synthetic oligodeoxynucleotides containing a CpG motif (CpG ODN) confers complete protection to susceptible BALB/c mice against an otherwise lethal challenge infection with L. major. According to the current paradigm, protective immunity against L. major in mice is dependent on the development of a Th1 immune response with production of the cytokines interleukin-12 (IL-12) and interferon-γ (IFN-γ) and expression of inducible nitric oxide synthase. We previously demonstrated that DC-mediated protection against L. major is associated with the induction of a Th1 immune response in susceptible BALB/c mice, whereas unprotected BALB/c mice develop a Th2 response accompanied by progressive disease and finally succumb to the infection. Depending on the population of antigen-loaded conditioned DC used for vaccination, Th1 induction is observed systemically or only locally at the site of infection.2,3,5,6 As IL-12 is a key cytokine for triggering Th1 polarization, it was a surprising finding that conditioned BMDC do not need to secrete IL-12 for successful vaccination. In contrast, it is the vaccinated host that needs to be competent to produce IL-12.3 These unexpected findings suggest that conditioned BMDC, in contrast to the early models of DC-based immune intervention, may exert their protective capacity not only by direct activation of naive T cells, but also via interaction with a population of cells in the immunized host. One possible mechanism of this interaction is the cross-presentation of conditioned BMDC by cells in the vaccinated recipient similar to what has been demonstrated for the DC-dependent induction of protective immunity during viral infection.7 According to our current understanding, resident DC in spleen and lymph nodes (LN) of the recipient are best suited to cross-present conditioned BMDC after vaccination and secrete protective IL-12 in response to contact with the transferred BMDC.
Another mechanism to be considered is the cross-activation of vaccinating donor DC and additional cell populations in the recipient. Prime candidates for the induction of protective immunity via cross-activation are natural killer (NK) cells. Emerging data demonstrate that NK cells are involved not only in immune responses against viral infections, but, like DC, play an important role at the interface between innate and adaptive immunity in the initiation and modulation of immune responses against a wide range of pathogens. Increasing amounts of data show that NK cells interact closely with DC in the defence against many infectious agents, including parasites, resulting in the cross-activation of both cell populations, cytokine production and cell maturation or cytolytic activity.8,9 Interestingly, NK cells can be directly stimulated by Leishmania lipophosphoglycan, one of the major surface molecules of this parasite, through Toll-like receptor 2.10 Recent work in this field documented that during the early phase of infection with Leishmania parasites, the interaction of NK cells and DC plays a key role in the local containment of infection and the production of protective cytokines.11,12 However, all published work so far addressed the interaction of DC and NK cells during the resolution of primary infection. The role of NK cells during vaccination and, in particular, the in vivo interaction of NK cells with antigen-loaded DC adoptively transferred as a vaccine has not yet been investigated.
Together, the new insights demonstrate the complexity of the mechanisms underlying the immunomodulatory properties of DC. The use of DC for vaccination strategies is still hampered by our limited understanding of these mechanisms, so it is important to identify and characterize the multifaceted cell interactions leading to the induction of protective immunity after DC-based vaccination. In the present study, we examined the cross-presentation of conditioned BMDC and the cross-activation of DC and NK cells as mechanisms of DC-mediated immunoprotection.
Materials and methods
Mice, parasites and reagents
Female wild-type BALB/c mice were obtained from Charles River Breeding Laboratories (Sulzfeld, Germany) and were 10–12 weeks old at the onset of the experiments. Congenic C.B10-H2b mice with the major histocompatibility complex (MHC) haplotype H-2b (formerly termed BALB/b) were obtained from Harlan (Harlan Winkelmann GmbH, Borchen, Germany). Interleukin-12 p40−/− mice on a BALB/c background were originally provided by Dr G. Alber (University of Leipzig, Leipzig, Germany) and further maintained in our breeding facility. Housing and handling of the animals followed the guidelines of the animal welfare committee of the German government. All animal experiments were approved by the government of Lower Franconia. The cloned virulent L. major isolate MHOM/IL/81/FE/BNI was maintained by passage in BALB/c mice. Promastigotes were grown in blood agar cultures. For in vivo monitoring of the parasite burden after infection, parasites stably expressing the firefly luciferase gene (luc2/SV40 from Promega GmbH, Mannheim, Germany) were generated, using the pLEXSY-hyg plasmid (Jena Bioscience, Jena, Germany) essentially as described by Lecoeur et al.13 For the preparation of parasite lysate, stationary-phase promastigotes were subjected to three cycles of rapid freezing and thawing. The CpG ODN 1668 (5′-TCCATGACGTTCCTGATGCT-3′) was obtained from Qiagen Operon (Cologne, Germany) and was not phosphorothioate modified.
Preparation of BMDC and NK cells
Murine BMDC were generated from bone marrow progenitors as described previously.14 Briefly, bone marrow cells were cultured in RPMI-1640 medium (GIBCO Invitrogen, Karlsruhe, Germany), supplemented with 10% heat-inactivated fetal calf serum, 2 mm l-glutamine, 10 mm HEPES buffer, 60 μg/ml penicillin and 20 μg/ml gentamycin in the presence of 200 U/ml recombinant murine granulocyte–macrophage colony-stimulating factor (GM-CSF; PeproTech, London, UK). Fresh GM-CSF was added to the cultures at days 3 and 6. After 8–10 days, non-adherent cells were collected and used for further experimentation. These cells were shown to have typical myeloid DC morphology.3 Cells were resuspended at a density of 1 × 106/ml in RPMI-1640 medium and loaded overnight with L. major lysate (equivalent to 30 parasites per cell) in the absence or presence of CpG ODN (25 μg/ml).
Natural killer cells were isolated from spleens of 12-week-old BALB/c mice by magnetic bead separation according to the manufacturer's instructions (NK cell Isolation kit, Miltenyi Biotec, Bergisch Gladbach, Germany) and resuspended at a density of 1 × 106 cells/ml in RPMI-1640 medium for co-culture experiments.
Co-cultures and cross-presentation studies in vitro
To examine a direct cross-activation of naive BMDC by antigen-loaded BMDC in vitro, BMDC were prepared from the bone marrow of IL-12 p40−/− mice and loaded with L. major lysate with or without additional CpG ODN stimulation or were mock-treated with phosphate-buffered saline (PBS) only. After overnight culture, IL-12 p40−/− BMDC were harvested, washed and resuspended in RPMI-1640 medium at a density of 1 × 106/ml. Conditioned IL-12 p40−/− BMDC were then cultured with equal numbers of naive wild-type BALB/c BMDC. After 48 hr, supernatants were collected and stored at − 80° until analysis of cytokine secretion by enzyme-linked immunosorbent assay (ELISA).
The expression of co-stimulatory molecules on DC was analysed by flow cytometry. To this end, IL-12 p40−/− BMDC were conditioned overnight by loading with L. major lysate and activation by CpG ODN. These pre-conditioned IL-12 p40−/− BMDC were then cultured for 24 hr with naive congenic wild-type BALB/c BMDC. For discrimination between pre-conditioned BMDC and BMDC activated by cross-presentation, the pre-conditioned BMDC population was labelled with 1 μm of the fluorescent dye carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes, Invitrogen, Karlsruhe, Germany) before co-culture. For the last 5 hr of co-culture, the protein transport inhibitor monensin (GolgiStop™; BD Biosciences, Heidelberg, Germany) was added to the medium. After 5 hr of culture in the presence of monensin, cells were washed and fixed with 4% paraformaldehyde in PBS, pH 7·4, and labelled with fluorochrome-conjugated antibodies against MHC class II, CD80 and CD86 (all from BD Pharmingen, Heidelberg, Germany). For intracellular detection of IL-12, cells were treated with 0·1% saponin in PBS, pH 7·6, and labelled with fluorochrome-conjugated antibodies against IL-12 p40/p70 (BD Pharmingen). To demonstrate the uptake of particles derived from CFSE-labelled L. major lysate-loaded, CpG ODN-activated IL-12 p40−/− BMDC by naive BMDC, wild-type BALB/c BMDC were labelled with 2 μm of the fluorescence dye PKH26 (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) and, after overnight co-culture, were examined for the presence of double-positive cells by flow cytometry with a FACSCalibur (BD Biosciences). For data analysis, the CellQuest software (BD Biosciences) was used.
T-cell proliferation after cross-presentation in vivo
To detect cross-presentation of BMDC in vivo, BMDC generated from the bone marrow of BALB/c mice were loaded with L. major lysate in vitro and stimulated with CpG ODN. Cells were resuspended in PBS and 5 × 105 cells per animal were injected into the tail vein of F1 mice of a BALB/c × C.B10-H2b background. After 10 days, animals were killed and spleens were harvested aseptically for T-cell isolation by magnetic bead depletion with a Pan T-cell Isolation kit from Miltenyi Biotec according to the manufacturer's instructions. T cells from F1 mice that had received antigen-loaded BALB/c BMDC or from naive mice without previous BMDC treatment were plated at a density of 2 × 106/ml in RPMI-1640 medium in 96-well microtitre plates and either left unstimulated or were re-stimulated with BMDC of BALB/c or C.B10-H2b mice loaded with L. major lysate and stimulated with CpG ODN at decreasing concentrations ranging from 2 × 105 to 2 × 102 cells/ml. After 24 hr, 100 μl/ml of the dye Alamar Blue™ (Trinova, Giessen, Germany) were added and cell proliferation was measured colorimetrically after a total incubation time of 48 and 72 hr in an ELISA reader with Multiskan Ascent software (Thermo Electronic Corporation, Dreieich, Germany). Culture supernatants were stored at − 80° for additional analysis of IL-2 and IFN-γ secretion by ELISA.
Cross-activation of BMDC and NK cells
For cross-activation of NK cells and BMDC, freshly isolated NK cells from naive BALB/c mice were resuspended at a density of 1 × 106/ml in RPMI-1640 medium with or without additional activation by 1 μg/ml of phorbol 12-myristate 13-acetate (PMA) and 1 μg/ml ionomycin (both from Sigma-Aldrich Chemie GmbH). Equal numbers of wild-type BALB/c BMDC loaded with L. major lysate and activated by CpG ODN, or left untreated, were added to the NK cells. Supernatants were harvested after 48 hr and stored at − 80° until analysis of cytokine secretion. Activation of NK cells was further analysed by flow cytometry using fluorochrome-conjugated antibodies against CD49b and CD69 (both from BD Pharmingen).
IFN-γ production by splenic NK cells after transfer of conditioned BMDC
To study the interaction of NK cells and DC ex vivo, spleens from naive BALB/c mice were harvested aseptically and single-cell suspensions were prepared. Cells were resuspended at a density of 1 × 107/ml in RPMI-1640 medium and stimulated with L. major lysate plus CpG ODN, with CpG ODN alone or with a combination of CpG ODN plus PMA and ionomycin for maximum activation. Splenocytes without any activation were used as controls. To analyse the contribution of DC to the activation of NK cells in this setting, splenic DC were depleted from the total splenocyte preparation by magnetic bead separation with anti-CD11c microbeads (Miltenyi Biotec) according to the manufacturer's instructions. After 20 hr of culture, 2 μm monensin (GolgiStop, BD Biosciences) were added to the splenocyte preparation for the last 4 hr, the NK cells were enriched by magnetic bead separation using anti-CD49b microbeads (Miltenyi Biotec) according to the manufacturer's instructions and isolated cells were analysed by flow cytometry for the presence of CD49b, CD69 and IFN-γ after staining with fluorochrome-conjugated antibodies (all from BD Pharmingen).
Interferon-γ production by NK cells upon vaccination with BMDC was studied directly in the spleen. After overnight conditioning with L. major lysate and CpG ODN, BMDC were harvested, washed and resuspended in PBS, and 5 × 106 cells per animal were injected into the tail vein of naive BALB/c mice. Four hours after BMDC injection, animals were killed, spleens were harvested aseptically and single cell suspensions were prepared. Interferon-γ-secreting cells were enriched from the total splenocytes by magnetic bead treatment (IFN-γ Enrichment and Detection kit, Miltenyi Biotec) according to the manufacturer's instructions and IFN-γ induction in NK cells was analysed by flow cytometry using fluorochrome-conjugated antibodies against CD49b and IFN-γ (both from BD Pharmingen). For some experiments, total splenocytes were cultured overnight in 12-well plates in the presence of 2 μm monensin, and PMA and ionomycin were added to the wells as positive controls. The next day, NK cells were enriched by a NK cell Isolation kit (Miltenyi Biotec) according to the manufacturer's instructions and IFN-γ induction was subsequently analysed by flow cytometry.
Vaccination of mice and analysis of the course of disease
After in vitro generation of wild-type BALB/c BMDC and loading with L. major lysate in the presence of CpG ODN, cells were washed and resuspended in PBS. Then, 5 × 105 cells per animal were injected into the tail vein of naive BALB/c mice. To study the contribution of NK cells to the induction of protective immunity after DC-based vaccination, the mice were transiently depleted of NK cells during the vaccination phase by two intravenous (i.v.) injections of anti-asialo antiserum 3 days before and on the day of vaccination in the concentrations recommended by the manufacturer for total NK cell depletion (BIOZOL Diagnostica, Eching Germany). Control mice were treated either with anti-asialo antiserum alone, without BMDC, or with PBS only. Ten days after DC transfer, mice were infected subcutaneously with 2 × 105 stationary-phase L. major promastigotes into one hind footpad. The course of the infection was monitored weekly by measuring the increase in footpad size of the infected versus the non-infected footpad.
Analysis of parasite burden
The amount of viable parasites in the infected footpads was determined by bioluminescence. Six weeks after infection, mice were anaesthetized and received a single intraperitoneal injection of luciferin (Luciferin-D potassium salt from Synchem OHG, Felsberg/Altenburg, Germany). The activity of firefly luciferase, correlating with the presence of viable parasites, in the infected footpads was monitored using a charge-coupled device camera with WinLight software (NightOwl, Berthold, Bad Wildbad, Germany) and was expressed as average radiance in photons per second per cm2 per steradian.
Analysis of cytokine production
Cytokine levels in the culture supernatants were measured by sandwich ELISA as described previously.4 Briefly, microtitre plates were coated with purified rat antibodies directed against murine IFN-γ, IL-2, IL-4, IL-10, IL-12 p40 or IL-12 p70, followed by overnight incubation with the culture supernatants and serial dilutions of recombinant cytokines for the generation of standard curves. After washing, bound cytokines were detected by incubation with the appropriate biotin-conjugated rat anti-cytokine antibodies (all antibody pairs and recombinant cytokine standards from BD Pharmingen), followed by addition of a streptavidin–alkaline phosphatase complex (BD Pharmingen). After washing, the enzyme activity was visualized using the substrate p-nitrophenylphosphate (Sigma-Aldrich Chemie GmbH). Samples were set up in duplicates and the absorbance was measured using an ELISA reader with Multiskan Ascent software (Thermo Electronic Corporation). Cytokine detection thresholds were 39 pg/ml for IFN-γ, IL-2 and IL-12 p40, 16 pg/ml for IL-4 and IL-12 p70 and 20 pg/ml for IL-10.
Statistical analysis
Data of the vaccination experiments were analysed using the GraphPad Prism 4.00 software (GraphPad Software Inc., La Jolla, CA). For determining statistical significance of the vaccination experiments, multiple group comparisons were performed by non-parametric one-way analysis of variance (Kruskal–Wallis test). Selected groups were compared using the unpaired Student's t-test.
Results
Direct cross-presentation in vitro does not induce IL-12 in naive BMDC
We previously demonstrated that conditioned BMDC adoptively transferred for vaccination against L. major do not need to secrete IL-12 for successful induction of protective immunity, but that the IL-12 is induced in cells of the vaccinated recipient mice.3 The best candidates for production of the protective IL-12 in the recipients are resident DC in spleen and LN, which become activated by cross-presentation after contact with the transferred conditioned BMDC.
In a first approach, we studied whether contact with antigen-loaded BMDC can induce IL-12 in naive BMDC by cross-activation in vitro. To exclude the directly antigen-loaded BMDC as the source of IL-12 in this set-up, we used congenic IL-12 p40−/− BMDC (deficient in IL-12 secretion) as the antigen-loaded BMDC population, which was then co-cultured with naive BMDC for cross-activation. In this setting, any IL-12 observed in the co-culture supernatants would be derived from cross-activated naive BMDC. The treatment groups for co-culture included IL-12 p40−/− BMDC loaded with L. major lysate alone or additionally activated with CpG ODN as used for the previously described vaccination protocol.3 The IL-12 p40−/− BMDC stimulated with CpG ODN in the absence of antigen and unstimulated naive IL-12 p40−/− BMDC were used as control groups for the cross-activation of naive BMDC. Wild-type BMDC directly activated with CpG ODN served as a positive control for IL-12 secretion and untreated naive BMDC without any co-culture served as negative control. In this in vitro set-up of DC–DC cross-activation, ELISA measurement of IL-12 in the supernatants of co-cultures did not detect any induction of IL-12 secretion in naive wild-type BMDC upon culture with any treatment group of the IL-12 p40−/− BMDC (Fig. 2b).
Figure 2.
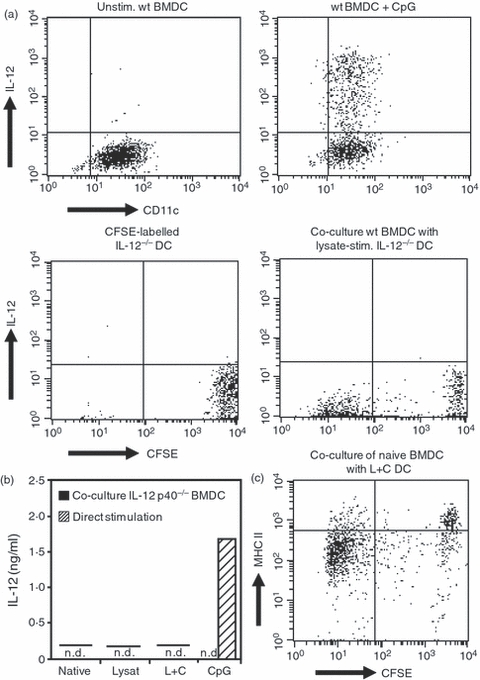
Induction of interleukin-12 (IL-12) and activation markers after co-culture of naive bone-marrow-derived dendritic cells (BMDC) with IL-12 p40−/− BMDC. (a) Fluorescence staining of intracellular IL-12 after direct stimulation of BMDC with CpG oligodeoxynucleotides (ODN) or after co-culture of naive BMDC with CpG ODN-stimulated IL-12 p40−/− BMDC. Upper panels: IL-12 production by unstimulated wild-type (wt) BMDC and after direct stimulation with CpG ODN. Lower panels: CFSE-labelled naive IL-12 p40−/− BMDC and co-culture of wild-type BMDC with CFSE-labelled IL-12 p40−/− BMDC loaded with Leishmania major lysate. Representative dot blots of three independent experiments. (b) Production of IL-12 after direct stimulation of BMDC and after co-culture of naive BMDC with stimulated IL-12 p40−/− BMDC. Native, unstimulated BMDC; Lysate, stimulation with L. major lysate; L+C, stimulation with L. major lysate and CpG ODN; CpG, stimulation with CpG ODN; n.d., no cytokine secretion detected. Representative enzyme-linked immunosorbent assay of three independent experiments. (c) Expression of major histocompatibility complex (MHC) II on unlabelled naive BMDC after co-culture with CFSE-labelled BMDC stimulated with L. major lysate and CpG ODN. The CFSE-intermediate population demonstrates the uptake of particles from CFSE-labelled stimulated BMDC by naive BMDC. L+C, stimulation with L. major lysate and CpG ODN. Representative dot blots of three independent experiments.
The uptake of pre-conditioned IL-12 p40−/− BMDC or particles derived from these cells by naive wild-type BMDC was examined by fluorescence-activated cell sorting. Co-culture of naive wild-type BMDC labelled with the fluorescent dye PKH26 together with CFSE-labelled IL-12 p40−/− BMDC, pre-cultured in the absence or presence of L. major lysate and/or CpG ODN, demonstrated that the congenic IL-12 p40−/− BMDC were taken up by naive wild-type BMDC, regardless of any previous activation of the IL-12 p40−/− BMDC (Fig. 1). The cells were gated on the population of single DC according to forward/sideward scatter to exclude any cell doublets. This result was further confirmed by fluorescence microscopy, revealing the presence of particles of IL-12 p40−/− BMDC in naive wild-type BMDC after co-culture (data not shown).
Figure 1.
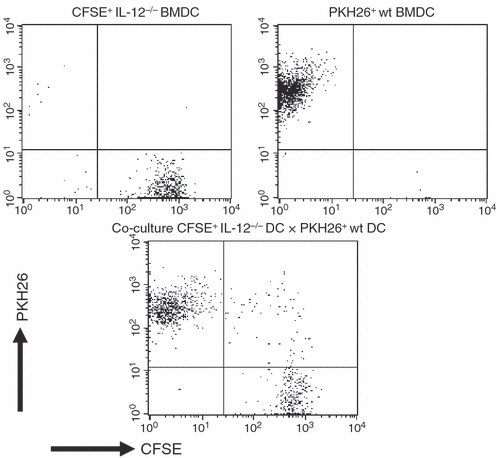
Uptake of particles derived from CFSE-labelled interleukin-12 (IL-12) p40−/− bone-marrow-derived dendritic cells (BMDC) by PKH26-labelled congenic BALB/c BMDC after co-culture. Naive congenic BMDC were labelled with PKH26 and cultured overnight with CFSE-labelled Leishmania major lysate-loaded, CpG-activated IL-12 p40−/− BMDC. Upper panel: fluorescence signals of CFSE-labelled IL-12 p40−/− BMDC and PKH26-labelled BALB/c BMDC. Lower panel: co-culture of pre-conditioned CFSE-labelled IL-12 p40−/− BMDC with PKH26-labelled BMDC. Representative data of three independent experiments.
However, analysis of intracellular IL-12 production by flow cytometry did not detect any significant cytokine induction upon cross-presentation, neither in BMDC with intermediate CFSE fluorescence, indicating that these cells had taken up particles of CFSE-labelled IL-12 p40−/− BMDC, in contrast to the strong CFSE signal after ingestion of intact IL-12 p40−/− BMDC, nor in bystander BMDC without CFSE signalling (Fig. 2a, lower panels). As expected, direct stimulation with CpG ODN induced robust IL-12 production in wild-type BMDC controls (Fig. 2a, upper panels).
In a next step, we examined the up-regulation of activation markers after co-culture of L. major lysate-loaded and CpG ODN-activated IL-12 p40−/− BMDC with CFSE-labelled naive wild-type BMDC by flow cytometry. For this purpose, either congenic BALB/c BMDC as in the vaccination protocols were used as the naive BMDC population, or MHC class II-heterologous C.B10-H2b BMDC, to rule out an autotolerance as the cause of the lack of stimulatory capacity. Flow cytometric analysis of the cell surface activation markers CD86, CD80 and MHC class II revealed that co-culture of conditioned IL-12 p40−/− BMDC with both BMDC populations also failed to induce a maturation or activation of the naive BMDC regardless of previous activation and antigen-loading of the conditioned IL-12 p40−/− BMDC (Fig. 2c and data not shown).
Together, these findings clearly demonstrate that the uptake of particles derived from conditioned BMDC by naive BMDC, regardless of whether they had been conditioned with L. major lysate and/or CpG ODN, does not induce an activation of the naive BMDC as indicated by IL-12 production or up-regulation of surface activation markers.
Low level of cross-presentation of antigen-loaded BMDC in vivo
Vaccination with a single dose of L. major lysate-loaded and CpG ODN-stimulated IL-12 p40−/− BMDC induces a highly protective Th1-mediated immune response,3 so we next sought to analyse the contribution of cross-presentation of the transferred conditioned BMDC to induction of L. major-specific T cells.
To detect cross-presentation of transferred BMDC in an in vivo setting, BALB/c × C.B10-H2b F1 mice were vaccinated with L. major lysate-loaded and CpG ODN-activated BALB/c BMDC. The T cells were prepared from the spleens of these mice 7 days after transfer of conditioned BMDC, because this period of time has been shown to be sufficient for the induction of antigen-specific T cells after BMDC-mediated vaccination. BALB/c × C.B10-H2b F1 T cells were then re-stimulated either with lysate-loaded and CpG ODN-activated BALB/c BMDC homologous to the BMDC used for initial vaccination or with heterologous C.B10-H2b-BMDC. Analysis of the T-cell proliferation by Alamar Blue™ colour conversion indicated that co-culture of T cells from vaccinated F1 mice with lysate plus CpG ODN-treated homologous BALB/c BMDC or heterologous C.B10-H2b BMDC resulted in only a very moderate T-cell activation in both groups (data not shown). This activation was sufficient for T cells from F1 mice to secrete large amounts of IL-2 in response to re-stimulation with lysate plus CpG ODN-treated homologous BALB/c BMDC (Fig. 3a and Fig. S1). In contrast, heterologous C.B10-H2b BMDC elicited only negligible IL-2 secretion at the highest ratio of BMDC to T cells. At this ratio, naive T cells also showed proliferation and weak IL-2 secretion in response to re-stimulation with conditioned BMDC. This indicates that an unspecific T-cell activation caused by the high BMDC numbers was responsible for the IL-2 secretion elicited by heterologous C.B10-H2b BMDC at the highest BMDC to T-cell ratio. These results were further corroborated by the finding that co-cultures of lysate plus CpG ODN-treated homologous BALB/c BMDC and heterologous C.B10-H2b-BMDC with naive T cells from untreated F1 mice did not elicit a detectable IL-2 secretion at any of the BMDC to T-cell ratios that induced a specific T-cell proliferation (Fig. 3a). Surprisingly, upon re-stimulation with conditioned BMDC, T cells from mice vaccinated with homologous BALB/c BMDC as well as those vaccinated with heterologous C.B10-H2b-BMDC secreted large amounts of IFN-γ, whereas naive T cells from untreated mice secreted only small amounts of IFN-γ at the highest ratio of BMDC to T cells (Fig. 3b).
Figure 3.
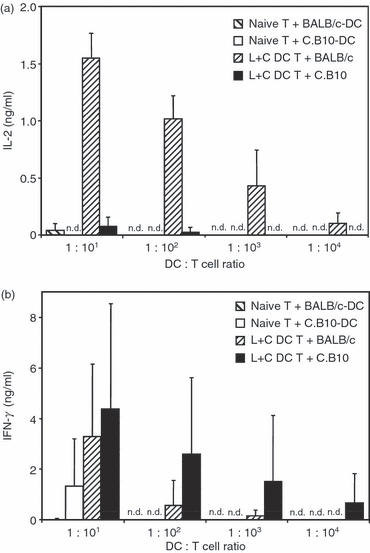
Interleukin-2 (IL-2) production after cross-presentation of conditioned bone-marrow-derived dendritic cells (BMDC) in F1 mice. F1 mice of a mixed BALB/c × C.B10H-2b background were vaccinated with BALB/c BMDC that had been conditioned with Leishmania major lysate and CpG oligodeoxynucleotides (ODN; L+C DC). Untreated naive mice were used as negative controls. T cells from spleens of the mice were harvested 7 days after immunization and re-stimulated with either BALB/c or C.B10H-2b BMDC that had been conditioned with L. major lysate and CpG ODN. The results are representative of three independent experiments. (a) IL-2 in the supernatants of T cells after 72 hr of co-culture with conditioned BMDC. Mean ± SD of three animals, all samples were measured in duplicates. Wide hatched bars, naive T cells restimulated with BALB/c BMDC; open bars, naive T cells re-stimulated with C.B10H-2b BMDC; fine hatched bars, T cells from vaccinated mice re-stimulated with BALB/c BMDC; filled bars, T cells from vaccinated mice re-stimulated with C.B10H-2b BMDC. (b) Interferon-γ (IFN-γ) in the supernatants of T cells after 72 hr of co-culture with conditioned BMDC. Mean ± SD of three animals, all samples were measured in duplicates. Wide hatched bars, naive T cells re-stimulated with BALB/c BMDC; open bars, naive T cells re-stimulated with C.B10H-2b BMDC; fine hatched bars, T cells from vaccinated mice re-stimulated with BALB/c BMDC; filled bars, T cells from vaccinated mice re-stimulated with C.B10H-2b BMDC; n.d., no cytokine secretion detected.
Together, these results support the conclusion that cross-presentation of transferred BMDC contributes only to a minor extent to the efficient protection against leishmaniasis induced by DC-based vaccination, and confirm the central role of IFN-γ for induction of protective immunity.
Cross-activation of BMDC and NK cells
The results of the cross-presentation experiments showed that a direct interaction of antigen-loaded BMDC with naive BMDC in vitro failed to induce the key cytokine IL-12 or any other detectable activation of naive BMDC in terms of DC-specific activation markers. Furthermore, re-stimulation of T cells from vaccinated F1 mice with homologous or heterologous BMDC indicated that also in vivo only a very limited cross-presentation of the conditioned BMDC used for vaccination can be detected. Therefore, we examined whether an additional cell population may support the induction of protective immunity after vaccination with BMDC. Natural killer cells are the prime candidate for this.
In a first step, we analysed the interaction of NK cells and conditioned BMDC in vitro. Co-culture of CpG ODN-activated BMDC with NK cells resulted in cross-activation of both cell populations as indicated by secretion of IL-2 and IFN-γ by BMDC and NK cells, respectively. In contrast, naive BMDC failed to induce any cross-activation with NK cells, and co-culture of L. major lysate-loaded BMDC and NK cells in the absence of additional activation also induced only background levels of IL-2 and IFN-γ. Additional stimulation of L. major lysate-loaded BMDC with CpG ODN during co-culture with NK cells resulted in the induction of a low IL-2 level (Fig. 4a). These findings are in accordance with our observation that the induction of protective immunity by BMDC-mediated vaccination requires CpG ODN stimulation in addition to L. major lysate loading of the BMDC.3 Cytokine secretion was strongly enhanced when L. major lysate-loaded and CpG ODN-activated BMDC were cultured with NK cells that had also been stimulated with PMA and ionomycin, which alone did not elicit any detectable cytokine secretion by NK cells or BMDC (Fig. 4a).
Figure 4.
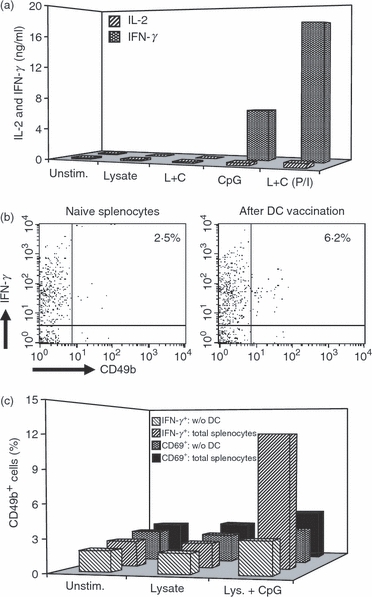
Cross-activation of bone-marrow-derived dendritic cells (BMDC) and natural killer (NK) cells. (a) Interferon-γ (IFN-γ) and interleukin-2 (IL-2) secretion after co-culture of either naive NK cells or NK cells that had been pre-activated with phorbol 12-myristate 13-acetate (PMA) and ionomycin, with BMDC that had been stimulated with Leishmania major lysate and/or CpG oligodeoxynucleotides (ODN). Supernatants were collected after 48 hr of culture and assayed for IFN-γ and IL-2 by enzyme-linked immunosorbent assay. The results are representative of three independent experiments. Hatched bars, IL-2 secretion; dotted bars, IFN-γ secretion; L+C, stimulation of DC with L. major lysate plus CpG ODN; P/I, pre-activation of NK cells with PMA and ionomycin. (b) Increase of IFN-γ-secreting NK cells in the spleen 4 hr after vaccination with BMDC that had been stimulated with L. major lysate and CpG ODN. Representative data of three animals per treatment group and three independent experiments. (c) Presence of IFN-γ and the activation marker CD69 on CD49+ NK cells after in vitro stimulation of total splenocytes and after depletion of DC from the splenocytes. The data are representative of three independent experiments. Wide hatched bars, IFN-γ in total splenocytes; narrow hatched bars, IFN-γ after depletion of DC; dotted bars, CD69-expression in total splenocytes; black bars, CD69 expression after depletion of DC.
During vaccination, conditioned BMDC reach the spleen via the blood shortly after i.v. transfer. Here, they get in close contact with not only the resident antigen-presenting cells, but also NK cells of the host. To demonstrate the cross-activation of NK cells and DC in vivo, we analysed the production of IFN-γ by NK cells isolated from the spleen shortly after vaccination with conditioned BMDC. Although NK cells represent only a very small population of the total IFN-γ-secreting cells in the spleen, vaccination of mice with lysate-loaded and CpG ODN-stimulated BMDC resulted in a more than twofold increase of the IFN-γ-secreting NK cell population in the spleen. Furthermore, additional activation with PMA and ionomycin during overnight culture resulted in a marked increase of IFN-γ-producing NK cells in the spleens of mice that had received conditioned DC, but not in the spleens of mice that had not been vaccinated or had received naive DC only (Fig. 4b, and data not shown).
To further confirm the role of DC–NK cell cross-activation for IFN-γ production in the spleen after BMDC-based vaccination, we prepared total splenocytes from naive mice, which comprise not only NK cells and resident DC, but also macrophages and several lymphocyte populations that are also capable of IL-2 and IFN-γ production. Flow cytometric analysis showed that ex vivo stimulation of naive splenocytes with either L. major lysate plus CpG or CpG alone indeed resulted in IFN-γ expression in a subset of cells that was positive for the NK cell marker CD49b and the NK cell activation marker CD69. Selective depletion of DC from the splenocytes before the addition of the stimuli markedly reduced subsequent IFN-γ expression and activation of NK cells (Fig. 4c). This finding supports our hypothesis that cross-activation of DC and NK cells is involved in the induction of protective immunity after BMDC-mediated vaccination.
To analyse the in vitro cross-activation of NK cells and conditioned BMDC, the expression of activation markers upon co-culture of BMDC and NK cells was determined by flow cytometry. Analysis for the up-regulation of the activation marker CD69 on CD49b+ NK cells showed that NK cells, upon co-culture with naive BMDC, have only background levels of CD69 similar to naive NK cells alone. Despite the failure to induce marked IFN-γ secretion, co-culture with lysate-conditioned and lysate plus CpG-conditioned BMDC induced a twofold to threefold increase of CD69 expression on NK cells, whereas the increase of CD69 levels upon co-culture with CpG-conditioned BMDC reflected the level of cytokine induction in this treatment group (Fig. 5a). On the DC side, co-culture of unstimulated naive BMDC with naive NK cells already induced an up-regulation of the activation markers MHC class II and CD86 on BMDC, which was in the range of that observed with BMDC directly stimulated with CpG ODN. Conditioning of the BMDC with L. major lysate and, even more pronounced, with L. major lysate plus CpG ODN further enhanced this up-regulation to levels of MHC class II and CD86 higher than those induced by direct stimulation of BMDC with CpG ODN (Fig 5b,c). Surprisingly, co-culture of BMDC stimulated with CpG ODN alone and naive NK cells resulted in lower levels of MHC class II and CD86 than co-cultures of BMDC loaded with L. major lysate (Fig 5b,c).
Figure 5.
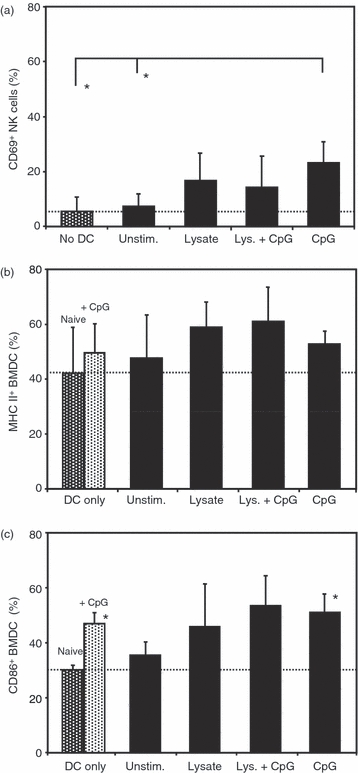
Up-regulation of activation markers after co-culture of bone-marrow-derived dendritic cells (BMDC) and natural killer (NK) cells. Expression of the activation markers CD69 on NK cells or major histocompatibility complex (MHC) class II and CD86 on BMDC after co-culture of BMDC that had been stimulated with Leishmania major lysate and/or CpG oligodeoxynucleotides (ODN) together with NK cells. Each bar represents the mean ± SD of three independent experiments. (a) Expression of CD69 on NK cells: dotted bar, only NK cells without addition of BMDC; * indicates significant differences (P < 0.05). (b) Expression of MHC class II on BMDC: dotted bars, only BMDC without addition of NK cells; black bars, co-cultures with NK cells. (c) Expression of CD86 on BMDC: dotted bars, only BMDC without addition of NK cells; black bars, co-cultures with NK cells; * indicates significant differences to the naive control group (P < 0.05). Dotted line, threshold of basal activation of untreated naive control group.
Taken together, these findings show that co-culture of NK cells and BMDC that had been conditioned with L. major lysate and CpG ODN results in a reciprocal activation of both cell populations that has the potential to support the induction of protective immunity after BMDC-mediated vaccination.
Role of NK cells in DC-mediated induction of protective immunity
To assess the contribution of NK cells to the induction of protective immunity after DC-mediated vaccination in vivo, mice were transiently depleted of NK cells by two injections of anti-asialo antiserum before vaccination with L. major lysate-loaded and CpG ODN-activated BMDC. Two weeks after NK cell depletion and treatment with conditioned BMDC, the mice were infected with L. major promastigotes.
While animals that had been immunized with antigen-loaded and CpG ODN-activated BMDC were fully protected against the challenge infection, the previous treatment with anti-asialo antiserum resulted in insufficient protection. The difference in footpad swelling in animals of the anti-asialo-treated group compared with that in the fully protected group was already significant at 4 weeks post-infection (Fig. 6a). These differences in lesion size were reflected in the parasite load. The average radiance, which correlates with the presence of viable parasites, from the infection site of mice treated with asialo-antiserum was half an order of magnitude higher than that from mice of the protected group (Fig. 6b).
Figure 6.
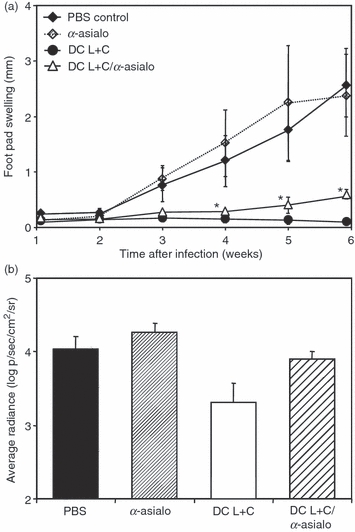
Contribution of natural killer (NK) cells to dendritic cell (DC) -mediated vaccination against Leishmania major. (a) Lesion development in mice infected with L. major after vaccination with conditioned bone-marrow-derived dendritic cells (BMDC) in the absence or presence of NK cells during the vaccination phase. NK cells were depleted by two intravenous (i.v.) injections of anti-asialo-serum. Mice were subsequently immunized i.v. with 5 × 105 BMDC conditioned in vitro with L. major lysate and CpG oligodeoxynucleotides (ODN) and were challenged with 2 × 105 L. major promastigotes 14 days later. Control mice were mock-treated with phosphate-buffered saline (PBS) or received only anti-asialo antiserum without BMDC. The increase in size of the infected compared with the non-infected footpad was measured weekly. Each symbol represents the mean ± SD of a minimum of four animals. Where error bars are not visible the bars lie within the confines of the symbol. Differences between the groups are significant (*P < 0.05). (b) Log numbers of average radiance in photons per second per cm2 per steradian (p/s/cm2/sr) of luciferase-transgenic L. major parasites in the footpads of infected mice after vaccination with conditioned BMDC with or without NK-cell depletion during the vaccination phase. Better protection results in lower bioluminescence because of lower numbers of parasites in the infected footpad. Parasite burden was determined 6 weeks after challenge infection with L. major. Each bar represents the mean ± SD of a minimum of three animals. Black bar, mock treatment with PBS only; narrow hatched bar, anti-asialo antiserum without vaccination; open bar, vaccination with BMDC conditioned with L. major lysate plus CpG ODN; wide hatched bar, depletion of NK cells during vaccination by anti-asialo antiserum. The data are representative of two independent experiments.
Discussion
According to the current understanding of DC-mediated vaccination, adoptively transferred DC that had been conditioned ex vivo for the presentation of an antigen of interest in the context of co-stimulatory molecules and cytokine secretion directly interact with naive T cells in the recipient to induce protective immunity. This model has been questioned by our previous observation that the key cytokine IL-12 required for the induction of a protective Th1-type immune response against L. major parasites does not have to be secreted by the transferred DC, but is produced by an unknown cell population in the vaccinated recipient mice.3 As the application of DC-mediated vaccination against pathogens in humans is still hampered by our limited understanding of the complex mechanisms underlying the immunomodulatory properties of DC, it was the aim of the present study to investigate the impact of cross-presentation and cross-activation in DC-mediated protection against L. major. We showed that cross-presentation of the transferred BMDC via resident antigen-presenting cells of the host does not contribute significantly to the induction of a protective immune response. Instead, our findings indicate that DC-mediated vaccination is supported by cross-activation with NK cells of the host, resulting in IFN-γ induction.
For DC-mediated vaccination of mice against L. major, a single dose of parasite antigen-loaded and CpG ODN-activated BMDC is sufficient to induce robust and long-lasting immunity.3 Shortly after i.v. transfer in vivo, the conditioned BMDC appear in the spleen of the recipients and make contact with resident antigen-presenting cells in this organ.15 The uptake of apoptotic infected DC by immature DC has been demonstrated to be a key mechanism of T-cell priming, e.g. during infection with herpes simplex virus,7 so we analysed the cross-presentation of conditioned BMDC via immature recipient DC as a potential mechanism for induction of immunity in DC-mediated vaccination against L. major. Indeed, our findings show that particles of conditioned BMDC are taken up by immature BMDC in vitro. However, we could not detect any induction of IL-12 secretion or up-regulation of activation markers in the immature BMDC after co-culture with conditioned BMDC. Furthermore, even co-culture with MHC class II-heterologous conditioned BMDC, which would be recognized as non-self and therefore treated as an antigen, did not result in detectable activation of immature BMDC. This may be explained by the fact that the uptake of apoptotic particles in the absence of strong additional immunostimulatory signals leads to the induction of an anti-inflammatory state in the phagocytosing cells to prevent autoimmunity.16 Nevertheless, uptake of particles from transferred conditioned BMDC in vivo might still induce cytokine secretion and activation of resident antigen-presenting cells. Different from the in vitro approach with co-cultures of defined purified cell populations, additional cell types, such as NK cells and NK T cells, might contribute to the activation of resident antigen-presenting cells in vivo via secretion of IFN-γ, as shown by our cross-activation studies.
Previous results have demonstrated that the highly protective immune response induced by conditioned DC is mediated by L. major-specific T cells;4 for this reason, we now analysed the contribution of cross-presentation of the transferred conditioned BMDC to priming of L. major-specific T cell in vivo. To this end, mice of a mixed BALB/c × C.B10H-2b background were vaccinated with conditioned BALB/c BMDC carrying the I-Ad alloantigen of MHC class II. As BALB/c × C.B10H-2b F1 mice have both the I-Ad type of MHC class II molecules from the BALB/c parent as well as the I-Ab type of MHC class II molecules from the C.B10H-2b parent, cross-presentation of the conditioned I-Ad BMDC in vivo should result in the priming of T cells which recognize antigen presented on both I-Ad and I-Ab MHC class II molecules. However, findings from this experimental setting showed that the ex vivo re-stimulation of I-Ad × I-Ab T cells with conditioned BMDC resulted in only weak T-cell activation as detected by the Alamar Blue™ colour conversion assay, regardless of whether the MHC class II type of the BMDC used for re-stimulation had been homologous or heterologous to the BALB/c BMDC used for the initial priming. This weak T-cell activation in response to re-stimulation could be explained by the low amount of antigen presented on the transferred BMDC during initial priming of the T cells in vivo. Although further booster immunizations might well enhance the T-cell response, it is important to note that we were interested in the mechanisms underlying the induction of a protective T-cell response in our model of BMDC-mediated vaccination against leishmaniasis, where a single vaccination with conditioned BMDC is absolutely sufficient to induce complete protection.
As a highly sensitive parameter indicating T-cell priming in vivo, we further analysed the secretion of the cytokine IL-2 after re-stimulation of T cells ex vivo. Surprisingly, we observed pronounced differences in the levels of IL-2 secretion after re-stimulation with homologous I-Ad BMDC or heterologous I-Ab BMDC, which were not detected by Alamar Blue™ colour conversion.17 Although homologous BMDC induced the secretion of high amounts of IL-2 by T cells, heterologous BMDC elicited only background levels, comparable to unspecific IL-2 secretion after stimulation of naive T cells. These differences between IL-2 secretion as indicator of specific T-cell priming and colorimetric detection of T-cell proliferation demonstrate that only measurement of IL-2 secretion is sensitive enough to detect the small population of L. major-specific T cells that is activated by BMDC-mediated vaccination. The observed differences in the amount of IL-2 secreted by proliferating T cells after re-stimulation with homologous I-Ad BALB/c BMDC versus heterologous I-Ab C.B10H-2b BMDC indicate that there is only a very limited level of cross-presentation of conditioned BMDC transferred for vaccination. In contrast to IL-2 as an indicator of proliferation of antigen-specific T cells, T cells were primed for the secretion of IFN-γ by vaccination with both homologous as well as heterologous BMDC. As IFN-γ is the key Th1 effector cytokine associated with protection against L. major infection, this finding points to mechanisms other than only cross-presentation being involved in the induction of protective immunity by transferred BMDC.
The obvious discrepancy between the low level of T-cell proliferation observed after re-stimulation of purified T cells, which may be attributed to cross-presentation of conditioned BMDC, and the highly effective protection against L. major infection in vivo prompted us to address further mechanisms that may support the induction of a protective immune response after BMDC-mediated vaccination. An increasing number of reports about the ability of NK cells to interact with DC in the modulation of immune responses against invading pathogens makes them prime candidates for contributing to efficient T-cell priming in our model.18–20 The results of the in vitro experiments show that co-culture of NK cells and conditioned BMDC indeed induced a cross-activation of both cell populations. Co-culture resulted in the up-regulation of the activation marker CD69 and secretion of high levels of IFN-γ by NK cells. Whereas NK cells readily respond to any stimulation with the up-regulation of CD69, as demonstrated by high levels of CD69 after co-culture with conditioned BMDC of all treatment groups, induction of IFN-γ in NK cells requires more specific signalling, here provided only by the CpG ODN-conditioned BMDC. The crucial role of CpG ODN in DC-mediated induction of protective immunity against L. major has also been demonstrated in a model of leishmanization in naturally self-healing C57BL/6 mice, in which locally applied CpG ODN contributed to the reduction of disease-promoting regulatory T cells, probably via cross-talk of stimulated local dermal DC with NK cells recruited to the site of infection.21 Our observation is also in line with reports that only the interaction of NK cells with fully activated DC leads to a maximal T-cell stimulatory potential of the NK cells.22 Cross-activation of conditioned BMDC upon co-culture with NK cells was further demonstrated by induction of the cytokine IL-2. Although L. major lysate-conditioned BMDC secreted only low levels of cytokine upon co-culture with naive NK cells, compared with BMDC that had been stimulated with CpG ODN, cytokine secretion could be massively augmented by co-culture with NK cells that had been additionally stimulated by PMA/ionomycin. This mimics the situation during infection in vivo, where NK cells receive such an activating stimulus by cross-talk with NK T cells. The observation that L. major lysate-conditioned BMDC, even with additional CpG ODN activation, elicit only lower levels of cytokine production than BMDC that had been stimulated with CpG ODN, can be explained by the peculiar property of L. major lysate to selectively inhibit cytokine secretion of DC and macrophages,4,23,24 although treatment of BMDC with L. major lysate readily induced an up-regulation of the activation markers MHC class II and CD86 upon co-culture with NK cells. This would also help to explain why in our model of BMDC-mediated vaccination against L. major, both parasite lysate and an additional activation by CpG ODN are required for BMDC-induced protection.3 Interferon-γ secretion induced by the cross-activation of NK cells and L. major lysate plus CpG ODN-conditioned BMDC during vaccination may activate macrophages for the elimination of Leishmania and may direct the development of protective Th1 cells, while IL-2 might support the further expansion of those low numbers of antigen-specific T cells primed by the cross-presentation of conditioned BMDC. To test this hypothesis, we depleted mice of NK cells during the vaccination phase with lysate-pulsed and CpG ODN-activated BMDC and challenged the animals with a lethal dose of L. major promastigotes after replenishment of the NK cell pool. Indeed, NK cell-depleted mice showed significantly increased footpad swelling and a parasite load in the draining LN that was two orders of magnitude higher than that observed in fully protected mice, demonstrating the contribution of NK cells to induction of protective immunity after BMDC-mediated vaccination. In the light of a very recent report by Sun et al.25 about adaptive immune features of NK cells, it now needs to be examined whether cross-activation with conditioned BMDC induces a population of L. major-specific memory NK cells, and whether cross-activated NK cells support the development of a type 1-dominated T-cell response.22
Taken together, the results of the present study demonstrate that during DC-mediated vaccination, conditioned DC confer protection not only by direct interaction with naive T cells, but also via involvement of additional cell populations of the vaccinated host. Our data show that there is only a minor contribution of cross-presentation of conditioned BMDC after vaccination. Instead, conditioned BMDC interact with NK cells of the host. This results in a cross-activation of both cell populations with secretion of the key cytokine IFN-γ, which supports the induction of a protective immune response after BMDC-based vaccination. To the best of our knowledge, this is the first report demonstrating an involvement of NK cells in DC-mediated vaccination.
Acknowledgments
This work was supported by grant MO418/9-3 by the Deutsche Forschungsgemeinschaft (DFG), Germany, to H.M. The authors thank Drs A. Ponte-Sucre, T. Herrmann and T. Hünig for helpful discussions.
Glossary
Abbreviations
- BMDC
bone marrow-derived dendritic cells
- CFSE
carboxyfluorescein succinimidyl ester
- CpG ODN
CpG oligodeoxynucleotides
- DC
dendritic cell
- ELISA
enzyme-linked immunosorbent assay
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- IFN
interferon
- IL
interleukin
- LN
lymph node
- MHC
major histocompatibility complex
- NK cell
natural killer cell
- PMA
phorbol 12-myristate 13-acetate
- PBS
phosphate-buffered saline
- Th1
T helper type 1
- Th2
T helper type 2
Disclosures
The authors have no conflicts of interest to disclose.
Supporting Information
Additional Supporting information may be found in the online version of this article:
Figure S1. Comparison of interleukin-2 secretion by T cells after cross-presentation of conditioned bone-marrow-derived dendritic cells (BMDC) in vivo, upon re-stimulation for 24 hr or 72 hr with homologous or heterologous BMDC, which had been conditioned with Leishmania major lysate and CpG.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Flohé SB, Bauer C, Flohé S, Moll H. Antigen-pulsed epidermal Langerhans cells protect susceptible mice from infection with the intracellular parasite Leishmania major. Eur J Immunol. 1998;28:3800–11. doi: 10.1002/(SICI)1521-4141(199811)28:11<3800::AID-IMMU3800>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 3.Ramírez-Pineda JR, Fröhlich A, Berberich C, Moll H. Dendritic cells (DC) activated by CpG DNA ex vivo are potent inducers of host resistance to an intracellular pathogen that is independent of IL-12 derived from the immunizing DC. J Immunol. 2004;172:6281–9. doi: 10.4049/jimmunol.172.10.6281. [DOI] [PubMed] [Google Scholar]
- 4.Remer KA, Apetrei C, Schwarz T, Linden C, Moll H. Vaccination with plasmacytoid dendritic cells induces protection against infection with Leishmania major in mice. Eur J Immunol. 2007;37:2463–73. doi: 10.1002/eji.200636780. [DOI] [PubMed] [Google Scholar]
- 5.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–58. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 6.Berberich C, Ramírez-Pineda JR, Hambrecht C, Alber G, Skeiky YA, Moll H. Dendritic cell (DC)-based protection against an intracellular pathogen is dependent upon DC-derived IL-12 and can be induced by molecularly defined antigens. J Immunol. 2003;170:3171–9. doi: 10.4049/jimmunol.170.6.3171. [DOI] [PubMed] [Google Scholar]
- 7.Bosnjak L, Miranda-Saksena M, Koelle DM, Boadle RA, Jones CA, Cunningham AL. Herpes simplex virus infection of human dendritic cells induces apoptosis and allows cross-presentation via uninfected dendritic cells. J Immunol. 2005;174:2220–7. doi: 10.4049/jimmunol.174.4.2220. [DOI] [PubMed] [Google Scholar]
- 8.Adam C, King S, Allgeier T, et al. DC-NK cell cross talk as a novel CD4+ T-cell-independent pathway for antitumor CTL induction. Blood. 2005;106:338–44. doi: 10.1182/blood-2004-09-3775. [DOI] [PubMed] [Google Scholar]
- 9.Brilot F, Strowig T, Münz C. NK cells interactions with dendritic cells shape innate and adaptive immunity. Front Biosci. 2008;13:6443–54. doi: 10.2741/3165. [DOI] [PubMed] [Google Scholar]
- 10.Becker I, Salaiza N, Aguirre M, et al. Leishmania lipophosphoglycan (LPG) activates NK cells through toll-like receptor-2. Mol Biochem Parasitol. 2003;130:65–74. doi: 10.1016/s0166-6851(03)00160-9. [DOI] [PubMed] [Google Scholar]
- 11.Liese J, Schleicher U, Bogdan C. TLR9 signaling is essential for the innate NK cell response in murine cutaneous leishmaniasis. Eur J Immunol. 2007;37:3424–34. doi: 10.1002/eji.200737182. [DOI] [PubMed] [Google Scholar]
- 12.Schleicher U, Liese J, Knippertz I, et al. NK cell activation in visceral leishmaniasis requires TLR9, myeloid DCs, and IL-12, but is independent of plasmacytoid DCs. J Exp Med. 2007;204:893–906. doi: 10.1084/jem.20061293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lecoeur H, Buffet P, Morizot G, Goyard S, Guigon G, Milon G, Lang T. Optimization of topical therapy for Leishmania major localized cutaneous leishmaniasis using a reliable C57BL/6 model. PLoS Negl Trop Dis. 2007;1:e34. doi: 10.1371/journal.pntd.0000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lutz MB, Kukutsch N, Ogilvie AL, Rößner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 15.Schnitzer JK, Berzel S, Fajardo-Moser M, Remer KA, Moll H. Fragments of antigen-loaded dendritic cells (DC) and DC-derived exosomes induce protective immunity against Leishmania major. Vaccine. 2010 doi: 10.1016/j.vaccine.2010.06.077. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Chung EY, Kim SJ, Ma XJ. Regulation of cytokine production during phagocytosis of apoptotic cells. Cell Res. 2006;16:154–61. doi: 10.1038/sj.cr.7310021. [DOI] [PubMed] [Google Scholar]
- 17.Zhi-Jun Y, Sriranganathan N, Vaught T, Arastu SK, Ahmed SA. A dye-based lymphocyte proliferation assay that permits multiple immunological analyses: mRNA, cytogenetic, apoptosis, and immunophenotyping studies. J Immunol Methods. 1997;210:25–39. doi: 10.1016/s0022-1759(97)00171-3. [DOI] [PubMed] [Google Scholar]
- 18.Buendía AJ, Martínez CM, Ortega N, et al. Natural killer (NK) cells play a critical role in the early innate immune response to Chlamydophila abortus infection in mice. J Comp Pathol. 2004;130:48–57. doi: 10.1016/s0021-9975(03)00069-0. [DOI] [PubMed] [Google Scholar]
- 19.Byrne P, McGuirk P, Todryk S, Mills KH. Depletion of NK cells results in disseminating lethal infection with Bordetella pertussis associated with a reduction of antigen-specific Th1 and enhancement of Th2, but not Tr1 cells. Eur J Immunol. 2004;34:2579–88. doi: 10.1002/eji.200425092. [DOI] [PubMed] [Google Scholar]
- 20.Sanabria MX, Vargas-Inchaustegui DA, Xin L, Soong L. Role of natural killer cells in modulating dendritic cell responses to Leishmania amazonensis infection. Infect Immun. 2008;76:5100–9. doi: 10.1128/IAI.00438-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu W, Weigand L, Belkaid Y, Mendez S. Immunomodulatory effects associated with a live vaccine against Leishmania major containig clG oligodeoxynucleotides. Eur J Immunol. 2006;36:3238–47. doi: 10.1002/eji.200636472. [DOI] [PubMed] [Google Scholar]
- 22.Mailliard RB, Son YI, Redlinger R, Coates PT, Giermasz A, Morel PA, Storkus WJ, Kalinski P. Dendritic cells mediate NK cell help for Th1 and CTL responses: two-signal requirement for the induction of NK cell helper function. J Immunol. 2003;171:2366–73. doi: 10.4049/jimmunol.171.5.2366. [DOI] [PubMed] [Google Scholar]
- 23.Jayakumar A, Widenmaier R, Ma X, McDowell MA. Transcriptional inhibition of interleukin-12 promoter activity in Leishmania spp.-infected macrophages. J Parasitol. 2008;94:84–93. doi: 10.1645/GE-1153.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carrera L, Gazzinelli RT, Badolato R, Hieny S, Müller W, Kühn R, Sacks DL. Leishmania promastigotes selectively inhibit interleukin-12 induction in bone marrow-derived macrophages from susceptible and resistant mice. J Exp Med. 1996;183:515–26. doi: 10.1084/jem.183.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–61. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


