Abstract
Conditional responding during simple Pavlovian conditioning is often characterized as a form of implicit memory. The extent to which this type of associative learning is independent of awareness is an issue of continuing debate. Previous studies have demonstrated conditioning in the absence of awareness. However, their results have been questioned based on methodological concerns with postexperimental questionnaires. In the present study, skin conductance response (SCR) and unconditioned stimulus (UCS) expectancy were measured concurrently as participants were exposed to a differential delay fear conditioning procedure in which one tone (CS+) predicted a loud white noise, whereas a second tone (CS-) was presented alone. UCS predictability was varied on a trial-by-trial basis by presenting conditioned stimuli (CSs) at volumes just above or below the perceptual threshold. Differential UCS expectancy (awareness) was observed only on perceived trials, whereas differential SCR developed on both perceived and unperceived trials. Although perceived stimuli elicited larger SCRs, the magnitude of conditioning, indexed by differential conditioned response expression (conditioned SCR to CS+ minus the SCR to CS-), was not influenced by stimulus perception. These data indicate that conditional fear can be expressed when individuals are unaware of fear-eliciting stimuli and suggest that the degree of conditioning is independent of awareness during differential Pavlovian fear conditioning.
In Pavlovian fear conditioning, the presentation of a neutral stimulus (conditioned stimulus, CS) predicts an aversive event (unconditioned stimulus, UCS), such as electric shock or loud white noise. Expression of a conditional response (CR) to the CS is taken as evidence that an association between the CS and UCS has been learned. Development of the CR during Pavlovian conditioning reflects a type of associative learning that is often classified as an implicit or nondeclarative memory (a skill, habit, or other behavior that is unconsciously performed). Implicit memory is frequently contrasted with explicit or declarative memory (awareness or consciously recalled facts and events; refs. 1 and 2).
The relationship between awareness and Pavlovian conditioning is an issue of continuing debate (3-5). Convincing evidence of conditioning without awareness comes from studies of the conditioned eye blink response. Eye blink conditioning with amnestic and normal volunteers indicates that simple single-cue and differential delay procedures support learning in the absence of awareness (6-8). Similar results have been demonstrated during fear conditioning (9, 10). However, the methodology of many of these studies has recently been questioned (3). The postexperimental questionnaires frequently used as measures of awareness are of particular concern. Specifically, such measures do not assess awareness of CS-UCS contingencies on a trial-by-trial basis, may be insensitive to subtle evidence of CS discrimination, and can be susceptible to forgetting, interference, and reconstruction of events before the assessment of awareness. Concurrent measures of awareness address many of these concerns (3, 11).
Concurrent awareness measures have been previously used to demonstrate a relationship between awareness and conditional fear as subjects performed a distraction task (12, 13). Distraction tasks are often used in such studies to delay the onset of contingency awareness, because most subjects would quickly become aware of stimulus relationships otherwise. However, the role of awareness may vary with the processing demands of the conditioning procedure (11, 14-16). Therefore, conditional responding may require an awareness of CS-UCS contingencies as a consequence of increasing task complexity.
An alternative to increasing task difficulty is to study conditioned responding to stimuli presented below the perceptual threshold. Subthreshold stimuli can be presented within a conditioning session to evaluate conditional responding to unperceived CSs without distracting participants or increasing the complexity of the task (10, 17). Prior subconscious perception studies have demonstrated behavioral/autonomic responses and functional brain activation to unperceived auditory, visual, somatosensory, and visceral stimuli (18-25). These studies indicate that subthreshold stimuli can induce physiological changes within the brain and influence behavior even when stimuli are not perceived.
Previous studies investigating subconscious perception have generally presented stimuli at a set level below a separately established perceptual threshold (10, 17, 23). However, perceptual thresholds often vary across trials and may reduce the power to detect subliminal effects (26). Alternatively, peri-threshold presentations of auditory stimuli can be individually tailored to each participant's perceptual threshold on a trial-by-trial basis such that subthreshold and suprathreshold presentations of a CS can be presented within the same conditioning session (27). Thus, expression of conditional fear can be explored when an individual is aware and unaware of CSs.
The present study was designed to determine the relationship between awareness and Pavlovian fear conditioning by using concurrent measurement of UCS expectancy and skin conductance. Participants were exposed to a differential fear conditioning procedure in which their ability to predict the UCS was varied on a trial-by-trial basis by presenting CSs at volumes just above or below their perceptual detection threshold. Participants were expected to demonstrate differential UCS expectancies on perceived trials only. If conditional fear can be expressed without awareness, then learning-related changes in skin conductance response (SCR) should be observed with and without awareness of CS presentation.
Materials and Methods
Participants. Eleven volunteers (three women, eight men; mean age: 32 ± 3 SEM; range 22-51 years) gave informed consent and participated in this study, which was conducted under a protocol approved by the National Institute of Mental Health Institutional Review Board.
CS and UCS. Two pure tones (700 and 1,300 Hz) were presented as CSs (10-s duration, 20-s intertrial interval) during the training session. The CS+ coterminated with a 500-ms loud (100 dB) white noise UCS, and the CS- was presented alone. CSs were counterbalanced and presented in a pseudorandom order such that no more than two trials of the same CS were consecutively presented. The volumes of the CS+ and CS- were modulated on a trial-by-trial basis by using an adaptive threshold estimation procedure (27, 28) as described below.
UCS Expectancy. A computer mouse was used to monitor subjects' perception of CSs and expectancy of receiving the UCS. Perception of CSs was monitored by instructing subjects to push the left mouse button immediately upon hearing either tone. In addition, the mouse controlled a rating bar presented throughout training at the bottom of the visual display (see Fig. 1). Subjects were instructed to rate their UCS expectancy on a continuous scale from 0 to 100 (0 = certain that the UCS will not be presented, 50 = uncertain whether the UCS will be presented, 100 = certain that the UCS will be presented) and to continuously update (sampled at 40 Hz) their rating to reflect their current UCS expectancy.
Fig. 1.
UCS expectancy rating bar. Subjects were instructed to rate their UCS expectancy on a continuous scale from 0 to 100 (0 = certain that the UCS will not be presented, 50 = uncertain whether the UCS will be presented, 100 = certain that the UCS will be presented) and directed to continuously update their rating to reflect their current UCS expectancy.
SCR. A Contact Precision Instruments (Cambridge, MA) skin conductance monitoring system was used to monitor SCR throughout the assessment. SCR was sampled (40 Hz) with a pair of surface gel cup electrodes [silver/silver chloride, 6 mm diameter, BIOPAC (Goleta, CA) model TSD203] attached to the distal phalanx of the middle and ring fingers of the non-dominant hand.
Procedure. Subjects were informed that two tones would be repeatedly presented and that the volume of each tone would vary above and below their perceptual threshold. Subjects were instructed to push the left mouse button immediately upon hearing a tone and to update their UCS expectancy accordingly. Unknown to the subjects, the volume of each CS was controlled by their button press responses, such that the volume of the CS was decreased by 5 dB after perceived trials (i.e., when a button press was made). CS volume was increased by 5 dB after unperceived trials (i.e., when a button press was not made). The volumes of the CS+ and CS- were modulated independently. Subjects perceived 29.36 ± 0.68 (mean ± SEM) trials, whereas 30.63 ± 0.68 (mean ± SEM) trials were unperceived.
Data Analysis. UCS expectancy was calculated as the average response during the last second of each CS. In addition, the magnitude of learning was calculated as the UCS expectancy during the CS+ minus that during the CS-.
First and second interval SCRs were monitored during the conditioning session. SCRs were calculated by subtracting the average skin conductance measurement during the baseline period (5 s immediately before CS presentation) from the first interval response (FIR, peak response during the 5 s after CS onset) and second interval response (SIR, peak response during the 5 s before CS termination). The FIR is often interpreted as an orienting response to CS presentation, whereas the SIR is generally considered an emotional response, elicited by UCS anticipation, that reflects learning the CS-UCS association (29-31).
Paired t test comparisons of UCS expectancy and SCR data for CS+ versus CS- presentations were completed for perceived and unperceived trials. In addition, the magnitude of learning was calculated as the response (UCS expectancy and SCR) amplitude elicited by the CS+ minus that evoked by the CS- and compared for perceived versus unperceived trials.
Results
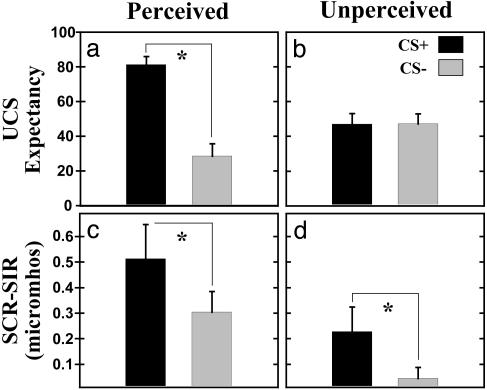
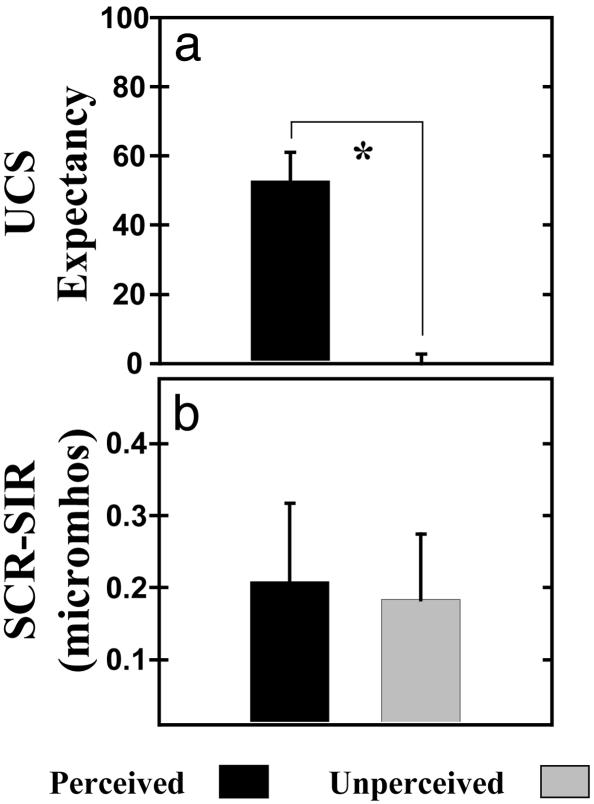
UCS Expectancy. Button-press responses were used to index perception of CSs. Similar numbers of perceived (mean ± SEM = CS+: 14.82 ± 0.30; CS-: 14.55 ± 0.46) and unperceived (mean ± SEM = CS+: 15.18 ± 0.30; CS-: 15.45 ± 0.46) trials were presented over the conditioning session [t(10) < 1.00]. On perceived trials, subjects demonstrated an awareness of CS-UCS contingencies with greater UCS expectancy during CS+ (mean ± SEM = 81.22 ± 4.81) than CS- (mean ± SEM = 28.52 ± 7.15) presentations [t(10) = 6.32, P < 0.05; see Fig. 2a]. In contrast, subjects were uncertain whether the UCS would be presented during the CS+ (mean ± SEM = 47.06 ± 6.01) and CS- (mean ± SEM = 47.27 ± 5.76) on unperceived trials [t(10) < 1.00; see Fig. 2b]. The magnitude of the learned UCS expectancy response is shown in Fig. 3a. As expected, the magnitude of learning as indexed by differential UCS expectancy (UCS expectancy during CS+ trials minus CS- trials) was larger on perceived (mean ± SEM = 52.70 ± 8.35) than unperceived (mean ± SEM = -0.21 ± 2.89) trials [t(10) = 6.57, P < 0.05].
Fig. 2.
UCS expectancy (a and b) and second interval SCR (c and d) data for perceived (a and c) and unperceived (b and d) trials. Awareness was demonstrated only on perceived trials as demonstrated by differential UCS expectancy to CSs. However, differential SCR was observed on perceived and unperceived trials. The asterisk indicates significant learning at P < 0.05.
Fig. 3.
Magnitude of learning (response to the CS+ minus the response to the CS-). (a) The magnitude of learning expressed through UCS expectancy was significantly greater on perceived than unperceived trials. (b) In contrast, the magnitude of SCR conditioning was similar on perceived and unperceived trials. These results demonstrate equivalent CR expression with and without awareness. The asterisk indicates significance at P < 0.05.
SCR. Learning-related SIR changes were demonstrated during the conditioning session. Significant conditional SIRs were expressed on perceived and unperceived trials (see Fig. 2 c and d). On perceived trials, SIRs evoked by the CS+ (mean ± SEM = 0.51 ± 0.14) were greater than those elicited by CS- (mean ± SEM = 0.30 ± 0.08) presentations [t(10) = 1.90, P < 0.05]. On unperceived trials, similar learning-related responses were observed such that the SIR on CS+ (mean ± SEM = 0.22 ± 0.10) trials was larger than those during CS- (mean ± SEM = 0.04 ± 0.04) presentations [t(10) = 2.00, P < 0.05]. Although, the SIR was larger during perceived (mean ± SEM = 0.41 ± 0.08) than unperceived (mean ± SEM = 0.14 ± 0.06) stimuli [t(21) = 3.98, P < 0.05], the magnitude of conditioning (the CR to the CS+ minus the response to the CS-) was similar for perceived (mean ± SEM = 0.21 ± 0.11) and unperceived (mean ± SEM = 0.18 ± 0.09) trials [t(10) < 1.00; see Fig. 3b]. In contrast, FIRs did not show learning-related changes [aware: t(10) < 1.00; unaware: t(10) < 1.35] even though significant FIRs were evoked by both perceived (mean ± SEM = CS+: 0.41 ± 0.08; CS-: 0.36 ± 0.07) and unperceived (mean ± SEM = CS+: 0.26 ± 0.07; CS-: 0.15 ± 0.07) CSs. Perception of CSs influenced FIR amplitude such that perceived (mean ± SEM = 0.39 ± 0.05) CSs elicited larger responses than unperceived (mean ± SEM = 0.20 ± 0.05) stimuli [t(21) = 3.65, P < 0.05].
Discussion
The present study explored the role of awareness in Pavlovian fear conditioning. SCR and UCS expectancy were measured concurrently as participants were exposed to a differential fear conditioning procedure in which the ability of subjects to predict the UCS was varied on a trial-by-trial basis by presenting CSs at peri-threshold volumes. Thus, subjects were exposed to perceptible and imperceptible trials of the CS+ and CS-. Differential UCS expectancy was demonstrated only on perceived trials. However, consistent with the view that conditioning can develop without awareness, learning-related SCRs developed on both perceived and unperceived trials. Although perceived stimuli evoked larger SCRs than unperceived CSs, the magnitude of conditioning, reflected by differential CR expression, was not influenced by stimulus perception. These results demonstrate equivalent CR expression with and without awareness.
The present results can be understood in terms of explicit and implicit memory systems. Explicit knowledge of stimulus relationships often develops during training as demonstrated by the differential UCS expectancy on perceived trials in the current study. However, this explicit knowledge appears to be unnecessary for the expression of implicit memory as demonstrated by differential conditioned SCR production on unperceived trials. The neural pathways supporting conditional fear have been well characterized and indicate the amygdala is critical for acquisition, consolidation, and expression of fear (32, 33). Auditory fear conditioning can be mediated through cortical or subcortical projections to the amygdala (32). CS perception likely occurs along the pathway projecting to auditory cortex and extending to the amygdala (32, 34). In addition, explicit memory formation appears to rely on the interaction of the auditory cortex and medial temporal lobe (2). Even though explicit memory processes supported by the medial temporal lobe may typically be engaged during fear conditioning, simple forms of conditional fear can develop and be expressed through independent subcortical circuitry that projects directly from the auditory thalamus to the lateral amygdala (2, 32, 33). This pathway may provide circuitry for CS input to reach the amygdala and elicit fear responses without passing through cortical regions and evoking physiological changes necessary for CS perception and awareness of CS-UCS relationships. An alternative possibility is that subthreshold auditory stimuli elicit cortical activation that falls below the level required for stimulus perception (22). This subthreshold cortical activation may in turn be projected to the amygdala where CRs are evoked.
Overall, the current data support previous findings of conditioning without awareness (6-8, 10, 17, 35). However, many of these studies have been challenged because awareness was assessed postexperimentally (3). Studies using concurrent awareness measures during fear conditioning have previously shown a relationship between awareness and conditioning (12, 13). However, these studies relied on distraction tasks to delay the onset of contingency awareness. Such tasks increase processing demands and interfere with conditional responding (11, 14-16). Because the role of awareness may vary with the complexity of the conditioning procedure, conditional responding may require an awareness of CS-UCS contingencies as a result of increasing task complexity (4, 11, 15, 16). In the present study, processing demands extraneous to the conditioning task were minimized to promote CR expression in the absence of awareness. In sum, the current results support the view that conditional fear can be expressed without awareness and suggest that the role of awareness in Pavlovian conditioning be further assessed with procedures that do not divide attention.
The current results are generally consistent with a recent study that monitored UCS expectancy during presentation of masked visual CSs (36). Learning-related SCR changes were observed even though subjects did not recognize masked CS+ and CS- presentations. However, several subjects were able to predict the UCS even though they were apparently unaware of the CS-UCS relationships. Interestingly, these subjects were more sensitive to visceral cues than those who were unable to predict the UCS, suggesting that visceral sensations may signal impending aversive events without awareness of stimulus contingencies (36). None of the subjects in the present study showed CS discrimination on unperceived trials. Although subject sensitivity to visceral cues was not measured, it seems likely that at least some participants would have shown reliable differential UCS expectancies on unperceived trials had they used this strategy. The strategy used to rate UCS expectancy may have been influenced by methodological differences between the previous (36) and present studies. Specifically, subjects were instructed to make a response even when unsure of their decision in the previous study (36). In contrast, subjects were instructed to indicate uncertainty (i.e., a rating of 50) when unsure whether the UCS would be presented in the present study. Encouraging subjects to guess even though unsure of their response may lead them to use alternative strategies such as relying on visceral cues to guide their decisions, while the option to indicate uncertainty may make the use of such cues unnecessary. Further exploration of the impact visceral sensations have on UCS expectancy may be an important contribution to the conditioning and awareness literature.
Although the current results are consistent with other delay conditioning studies, trace fear conditioning procedures may yield different results. Delay and trace conditioning differ in the temporal relationship between the CS and UCS. In delay conditioning, stimuli are arranged such that the CS and UCS coterminate, whereas in trace conditioning, an interval of time passes between CS termination and UCS onset. Previous research has shown a relationship between UCS expectancy and trace, but not delay eye blink conditioning (37). Further, systematic investigations of the role of awareness in delay and trace eye blink conditioning indicate awareness is necessary for learning in trace, but not delay procedures (6, 7, 35, 38). Although these eye blink conditioning results have yet to be fully replicated with a conditional fear paradigm, a recent study has examined awareness during delay and trace fear conditioning by using single-cue and differential training procedures (14). A relationship was demonstrated between awareness and the extinction of differential trace fear conditioning. However, no relationship was demonstrated between awareness and delay conditioning or other trace conditioning phases (14). Similar systematic investigations of the role of awareness during delay and trace fear conditioning would be a significant contribution.
The present study used a concurrent measure of awareness that addresses many concerns with postexperimental questionnaires (3, 11). However, monitoring awareness simultaneously with conditioning may alter task demands such that CRs do not reflect learning-related processes in the absence of such measures (11). In the current study, perceived stimuli elicited larger first and second interval SCRs than unperceived stimuli. One explanation is that CS perception elicits processes associated with conscious orienting toward these stimuli. However, subjects are also required to make button-press responses to indicate CS perception and must select and execute the appropriate motor response to indicate their UCS expectancy on perceived trials. Similar responses may not be present on unperceived trials. Although cognitive and motor processes may be differentially engaged by perceived and unperceived stimuli in this study, they do not adversely affect the learning-related SCRs observed on unperceived trials.
The present study explored the role of awareness during Pavlovian fear conditioning and suggests that awareness is unnecessary for the expression of conditional fear. These results further demonstrate the independence of explicit and implicit forms of memory. Although prior research has demonstrated that separate brain systems subserve explicit and implicit memory processes (2), the functional overlap and specificity of this circuitry within the healthy human brain remains unclear. Functional MRI (fMRI) offers a noninvasive means to study these systems, and the methodology described in the present study is easily adapted to fMRI investigations (39, 40). Further, Pavlovian conditioning is particularly well suited for exploring the relationship between brain regions that support explicit and implicit processes because both forms of memory are expressed during conditioning. Exploring the neural substrates that support conditional fear with and without awareness by using fMRI may provide a means to further characterize the neural circuitry supporting explicit and implicit memory.
Acknowledgments
This study was supported by the National Institute of Mental Health Intramural Research Program.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CS, conditioned stimulus; UCS, unconditioned stimulus; CR, conditioned response; SCR, skin conductance response; FIR, first interval response; SIR, second interval response.
References
- 1.Clark, R. E., Manns, J. R. & Squire, L. R. (2002) Trends Cognit. Sci. 6, 524-531. [DOI] [PubMed] [Google Scholar]
- 2.Milner, B., Squire, L. R. & Kandel, E. R. (1998) Neuron 20, 445-468. [DOI] [PubMed] [Google Scholar]
- 3.Lovibond, P. F. & Shanks, D. R. (2002) J. Exp. Psychol. Anim. Behav. Process 28, 3-26. [PubMed] [Google Scholar]
- 4.Manns, J. R., Clark, R. E. & Squire, L. R. (2002) J. Exp. Psychol. Anim. Behav. Process. 28, 32-37. [PubMed] [Google Scholar]
- 5.Wiens, S. & Öhmon, A. (2002) J. Exp. Psychol. Anim. Behav. Process. 28, 27-31. [PubMed] [Google Scholar]
- 6.Clark, R. E. & Squire, L. R. (1998) Science 280, 77-81. [DOI] [PubMed] [Google Scholar]
- 7.Gabrieli, J. D., McGlinchey-Berroth, R., Carrillo, M. C., Gluck, M. A., Cermak, L. S. & Disterhoft, J. F. (1995) Behav. Neurosci. 109, 819-827. [DOI] [PubMed] [Google Scholar]
- 8.Manns, J. R., Clark, R. E. & Squire, L. R. (2001) Cognit. Affect. Behav. Neurosci. 1, 192-198. [DOI] [PubMed] [Google Scholar]
- 9.Bechara, A., Tranel, D., Damasio, H., Adolphs, R., Rockland, C. & Damasio, A. (1995) Science 269, 1115-1118. [DOI] [PubMed] [Google Scholar]
- 10.Esteves, F., Parra, C., Dimberg, U. & Öhmon, A. (1994) Psychophysiology 31, 375-385. [DOI] [PubMed] [Google Scholar]
- 11.LaBar, K. S. & Disterhoft, J. F. (1998) Hippocampus 8, 620-626. [DOI] [PubMed] [Google Scholar]
- 12.Biferno, M. A. & Dawson, M. E. (1977) Psychophysiology 14, 164-171. [DOI] [PubMed] [Google Scholar]
- 13.Dawson, M. E. & Biferno, M. A. (1973) J. Exp. Psychol. 101, 55-62. [DOI] [PubMed] [Google Scholar]
- 14.Carter, R. M., Hofstotter, C., Tsuchiya, N. & Koch, C. (2003) Proc. Natl. Acad. Sci. USA 100, 1399-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carrillo, M. C., Gabrieli, J. D. E. & Disterhoft, J. F. (2000) Psychobiology 28, 293-302. [Google Scholar]
- 16.Knuttinen, M. G., Power, J. M., Preston, A. R. & Disterhoft, J. F. (2001) Behav. Neurosci. 115, 747-757. [DOI] [PubMed] [Google Scholar]
- 17.Bunce, S. C., Bernat, E., Wong, P. S. & Shevrin, H. (1999) J. Psychiatr. Res. 33, 341-347. [DOI] [PubMed] [Google Scholar]
- 18.Merikle, P. M., Smilek, D. & Eastwood, J. D. (2001) Cognition 79, 115-134. [DOI] [PubMed] [Google Scholar]
- 19.Öhman, A. & Soares, J. J. F. (1994) J. Abnorm. Psychol. 103, 231-240. [DOI] [PubMed] [Google Scholar]
- 20.Shevrin, H. (2001) Int. J. Psychophysiol. 42, 209-218. [DOI] [PubMed] [Google Scholar]
- 21.Borgeat, F., Elie, R., Chaloult, L. & Chabot, R. (1985) Can. J. Psychiatry 30, 22-27. [DOI] [PubMed] [Google Scholar]
- 22.Colder, B. W. & Tanenbaum, L. (1999) Brain Res. Cognit. Brain Res. 8, 177-184. [DOI] [PubMed] [Google Scholar]
- 23.Kotzé, H. F. & Möller, A. T. (1990) Psychol. Rep. 67, 931-934. [DOI] [PubMed] [Google Scholar]
- 24.Blankenburg, F., Taskin, B., Ruben, J., Moosmann, M., Ritter, P., Curio, G. & Villringer, A. (2003) Science 299, 1864. [DOI] [PubMed] [Google Scholar]
- 25.Kern, M. K. & Shaker, R. (2002) Gastroenterology 122, 290-298. [DOI] [PubMed] [Google Scholar]
- 26.Miller, J. (1991) J. Exp. Psychol. Hum. Percept Perform. 17, 841-851. [DOI] [PubMed] [Google Scholar]
- 27.Kaernbach, C. (2001) Percept. Psychophys. 63, 1377-1388. [DOI] [PubMed] [Google Scholar]
- 28.Treutwein, B. (1995) Vision Res. 35, 2503-2522. [PubMed] [Google Scholar]
- 29.Boucsein, W. (1992) in Electrodermal Activity, ed. Ray, W. J. (Plenum, New York), pp. 232-239.
- 30.Prokasy, W. F. & Raskin, D. C. (1973) Electrodermal Activity in Psychological Research (Academic, New York).
- 31.Wolter, J. & Lachnit, H. (1993) Integr. Physiol. Behav. Sci. 28, 163-166. [DOI] [PubMed] [Google Scholar]
- 32.LeDoux, J. E. (2000) Annu. Rev. Neurosci. 23, 155-184. [DOI] [PubMed] [Google Scholar]
- 33.Maren, S. (2001) Annu. Rev. Neurosci. 24, 897-931. [DOI] [PubMed] [Google Scholar]
- 34.Näätänen, R. & Winkler, I. (1999) Psychol. Bull. 125, 826-859. [DOI] [PubMed] [Google Scholar]
- 35.Clark, R. E. & Squire, L. R. (1999) Psychol. Sci. 10, 14-18. [Google Scholar]
- 36.Katkin, E. S., Weins, S. & Öhman, A. (2001) Psychol. Sci. 12, 366-370. [DOI] [PubMed] [Google Scholar]
- 37.Clark, R. E., Manns, J. R. & Squire, L. R. (2001) Psychol. Sci. 12, 304-308. [DOI] [PubMed] [Google Scholar]
- 38.Manns, J. R., Clark, R. E. & Squire, L. R. (2000) Learn. Mem. 7, 267-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng, D. T., Knight, D. C., Smith, C. N., Stein, E. A. & Helmstetter, F. J. (2003) Behav. Neurosci. 117, 3-10. [DOI] [PubMed] [Google Scholar]
- 40.Knight, D. C., Smith, C. N., Stein, E. A. & Helmstetter, F. J. (1999) NeuroReport 10, 3665-3670. [DOI] [PubMed] [Google Scholar]