Graphical abstract
Keywords: Error, Response monitoring, Autism, Children, fMRI
Abstract
Evidence exists for deficits in error monitoring in autism. These deficits may be particularly important because they may contribute to excessive perseveration and repetitive behavior in autism. We examined the neural correlates of error monitoring using functional magnetic resonance imaging (fMRI) in 8–12-year-old children with high functioning autism (HFA, n = 11) and typically developing children (TD, n = 15) during performance of a Go/No-Go task by comparing the neural correlates of commission errors versus correct response inhibition trials. Compared to TD children, children with HFA showed increased BOLD fMRI signal in the anterior medial prefrontal cortex (amPFC) and the left superior temporal gyrus (STempG) during commission error (versus correct inhibition) trials. A follow-up region-of-interest analysis also showed increased BOLD signal in the right insula in HFA compared to TD controls. Our findings of increased amPFC and STempG activity in HFA, together with the increased activity in the insula, suggest a greater attention towards the internally driven emotional state associated with making an error in children with HFA. Since error monitoring occurs across different cognitive tasks throughout daily life, an increased emotional reaction to errors may have important consequences for early learning processes.
1. Introduction
Autism is a neurodevelopmental disorder characterized by qualitative impairments in social interaction and communication and by the presence of restricted repetitive and stereotyped patterns of behavior, interests and activities (APA, 1994). Individuals with autism are often reported to have impairments in executive function, including deficits in response monitoring (e.g., Bogte et al., 2007, Happe et al., 2006, Henderson et al., 2006, Robinson et al., 2009, Russell and Jarrold, 1998, Thakkar et al., 2008, Vlamings et al., 2008; but see Russell and Hill, 2001), a process that involves the ability to evaluate, monitor, and adjust one's own behavior if it does not match a desired goal. Impairments in adjusting behavioral strategies according to the response outcome may be particularly important in autism because failure to do so may contribute to the perseverative and repetitive behaviors often observed (Thakkar et al., 2008).
Response monitoring involves evaluating correct and incorrect outcomes. When healthy adults and children are engaged in a fast reaction time task, error monitoring is thought to be reflected behaviorally by longer reaction times (RT) on trials immediately following an error (post-error) (Rabbitt, 1966, Sergeant and van der Meere, 1988). This post-error slowing is interpreted as a sign of ongoing cognitive control processes and allows for modification of subsequent behaviors and improvement in task performance. Behavioral analyses have revealed that, unlike controls, children (Vlamings et al., 2008) and adults (Bogte et al., 2007) with high functioning autism (HFA) do not show slowing in RT on trials following an error, suggesting deficits in error monitoring.
Event-related potential (ERP) studies in healthy adults of error processing have identified an electrophysiological component, called error-related negativity (ERN), localized in dorsal anterior cingulate cortex (dACC) and considered to represent error detection, correction or both (Kiehl et al., 2000, Garavan et al., 2002, Taylor et al., 2007). Electrophysiological data in autism examining ERN show contradictory results. In a study involving a probabilistic learning paradigm, Groen et al. (2008) found no ERN differences in children with HFA compared to typically developing (TD) children for correct and incorrect responses. However, using a flanker task, Henderson et al. (2006) found significantly larger ERN amplitudes following error versus correct trials in children with HFA with higher verbal ability (but not lower verbal ability), compared to TD children. Moreover, Vlamings et al. (2008) using an auditory decision task found that unlike TD children, children with autism spectrum disorder (ASD) did not show a significant difference in ERN between correct and incorrect trials. This was due to smaller ERN activity in ASD on incorrect trials, compared to TD children. Taken together the reported behavioral and ERP findings suggest that deficits in error monitoring, are present in autism and may be related to functional abnormalities in the dACC.
The involvement of the dACC in error monitoring is also supported by functional magnetic resonance imaging (fMRI) studies. Using various tasks, including a fast reaction time task such as the Go/No-Go task, fMRI findings indicate a network of regions involved/associated with error monitoring. These regions include the dACC and the adjoining medial frontal cortex, the bilateral insula, the rostral ACC, the lateral prefrontal cortex (PFC), and the inferior parietal cortex (see for a review Taylor et al., 2007) (Hester et al., 2004; Menon et al., 2001).
FMRI evidence investigating error monitoring in autism, however, is very limited. To our knowledge, there is only one study in the literature that has used fMRI to examine the neural correlates of response monitoring in individuals with autism (Thakkar et al., 2008). Thakkar et al. (2008) examined response monitoring during the performance of an antisaccade task in adults with ASD. Results showed that responses in ASD were relatively less differentiated to antisaccade errors versus correct antisaccades compared to controls in the right rostral anterior cingulate cortex (rACC). These findings, however, were attributed to increased activation in bilateral rACC in ASD during correct antisaccades compared to controls, rather than to significant group differences in antisaccade errors.
The rACC is considered the ‘affective’ subdivision of the ACC important for the regulation of emotional responses (Bush et al., 2000). As suggested by Thakkar et al. (2008) the abnormal rACC activity in autism may suggest a reduced affective discrimination to positive outcomes. However, it is possible that functional abnormalities of the rACC also affect error processes related to the emotional valence of an incorrect response. Moreover, functional and structural abnormalities in both, the dACC and rACC, have been previously reported in autism (Haznedar et al., 2000, Mundy, 2003).
In the present study, we used fMRI and a simple Go/No-Go task with low cognitive demand to examine the neural correlates of error monitoring in 8-12 year old children with HFA compared with TD children. Based on behavioral and ERP findings, we predicted that children with autism would show differences in neural activation in the dACC during commission error versus correct inhibition trials.
2. Methods
2.1. Participants
Eleven children with high functioning autism (72.73% Caucasian,) and 15 typically developing controls (73.33% Caucasian) were included in the final analyses for this study. All children had full-scale IQ scores greater than 80, as measured by the Wechsler Intelligence Scale for Children (WISC) third or fourth edition (Wechsler, 1991, Wechsler, 2003). The groups were matched on Perceptual Reasoning Index (PRI, WISC-IV) or Performance IQ (PIQ, WISC-III) scores (HFA = 104.6 ± 15.6; TD = 106.3 ± 13.5, reported as mean ± standard deviation) since the task in the present study was performance-based rather than verbal. The groups were matched for gender (three females in each group), age (HFA = 10.4 ± 1.6; TD = 10.5 ± 1.2) and performance on the Go/No-Go task in terms of % commission error (HFA = 35.65 ± 3.61; TD = 29.47 ± 2.83).
Only children with a commission error rate of at least 14% were included in order to ensure sufficient statistical power for fMRI analyses. One child with HFA was excluded from the HFA group because of low commission error rate (2%). Participants in both groups were predominantly right handed with the exception that in the HFA group there was one participant who was left handed and two who had mixed handedness (Edinburgh Handedness Inventory, Oldfield, 1971). In the TD group, there was one participant with mixed handedness. The two groups were similar in socio-economic status (Hollingshead, 1975, HFA = 58.56 ± 5.5; TD = 52.13 ± 10.2).
This study was conducted at the Kennedy Krieger Institute in Baltimore, MD. Participants were recruited from community-wide service groups, local schools, area medical institutions, Autism Society of America chapters, and outpatient clinics at KKI. Exclusion criteria for participants in both groups consisted of speech/language disorder, reading disability, seizures, traumatic brain injury, mental retardation, or any other neurological condition. Children with significant visual, hearing, or medical problems that would interfere with their ability to perform the behavioral task were also excluded.
2.1.1. High functioning autism group
Participants were included in the HFA group if they met DSM-IV-R criteria for autism. All subjects met criteria for autism on the Autism Diagnostic Interview-Revised (ADI-R, Le Couteur et al., 2003, Lord et al., 1994) and the Autism Diagnostic Observation Schedule – Generic, Module 3 (ADOS-G, Lord et al., 2000); in addition, all were judged clinically by a child neurologist (S.H.M.) to have autism. The HFA group included only those with idiopathic autism (e.g., no history of Fragile X, encephalitis, or other known medical conditions associated with autism).
The clinician-administered Diagnostic Interview for Children and Adolescents – IV (DICA-IV, Reich et al., 1997) was used to assess the presence of comorbid psychiatric symptoms in all but one child with HFA. Six children with HFA had comorbidities on the DICA: One child with HFA met criteria for attention-deficit hyperactivity disorder (ADHD), oppositional-defiant disorder (ODD) and obsessive-compulsive disorder (OCD). One child had comorbid ADHD and ODD, one child met criteria for simple phobia, one for OCD, one for ODD, and one for social phobia and OCD. Children were excluded from the HFA group if they met criteria on the DICA for conduct disorder, mood disorder, generalized anxiety disorder, separation anxiety disorder, or post-traumatic stress disorder.
Six children with HFA were taking medications: one was taking Adderall, one was taking Concerta, one was taking Concerta and Buspar, one was taking Adderall and Zoloft, one was taking Dexedrine and Strattera, and one was taking Depakote, Risperdal, and Dexedrine. For children treated with stimulant medication, their parents were asked to withhold the medication on the day prior to and the day of testing. Withholding of medication was confirmed for both children by parent report on the day of testing.
2.1.2. Typically developing group
TD children were selected from larger group of children who participated in neuroimaging studies in our laboratory (see Suskauer et al., 2008a, Suskauer et al., 2008b). Children included in the TD group showed no evidence of psychopathology on the DICA-IV. In addition, children in the TD control group were free of ADHD based on the Conners Parent Rating Scale-Revised (CPRS-R, Conners, 1997), Conners Teacher Rating Scale-Revised CTRS-R (Conners, 1997), and the Attention Deficit Disorder-Hyperactivity Disorder Rating Scale (DuPaul, 1991). None of the children in the TD control group had a history or current use of any psychoactive medication or an immediate family member (sibling or parent) with autism or another pervasive developmental disorder.
Written consent was provided by a parent/guardian for each subject, and assent was obtained from the participating child. All study procedures were approved by the Johns Hopkins Medicine Institutional Review Board.
2.2. Go/No-Go paradigm
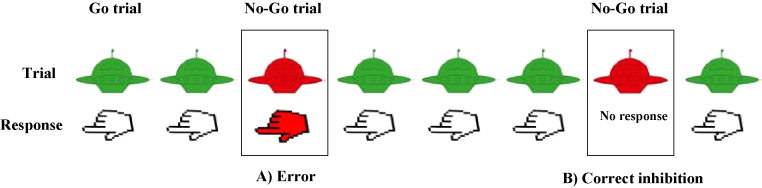
All children performed a Go/No-Go task fMRI scanning (see Suskauer et al., 2008a, Suskauer et al., 2008b for detailed description). In the Go/No-Go task, either a green or a red spaceship was presented on the screen one at a time; subjects were asked to push a button with their right index finger as quickly as possible each time a green spaceship appeared (Go trials), and to refrain from pushing the button when a red spaceship appeared (No-Go trials) (Fig. 1). Stimuli were presented for 300 ms, followed by a fixation cross that was displayed for 1500 ms, which allowed subjects sufficient time to respond until the next stimulus appeared. Go trials (green space ships) were presented in 3–7 consecutive trials, while No-Go trials never appeared more than twice in a row. Thus, No-Go stimuli were jittered, with a varying number of preceding Go stimuli. The task was divided into two 5-min runs, each with 95 Go and 32 No-Go trials. Each run began and ended with a 10 s rest period; four 10-s rest periods also occurred at irregular intervals during each run. Ten practice trials preceded the onset of the task. Stimuli were projected onto a screen at the rear of the scanner and were viewed through a mirror mounted on the head coil at a 45° angle. Stimulus presentation and recording of behavioral responses were carried out using EPrime (Psychology Software Tools, Pittsburgh, PA, USA).
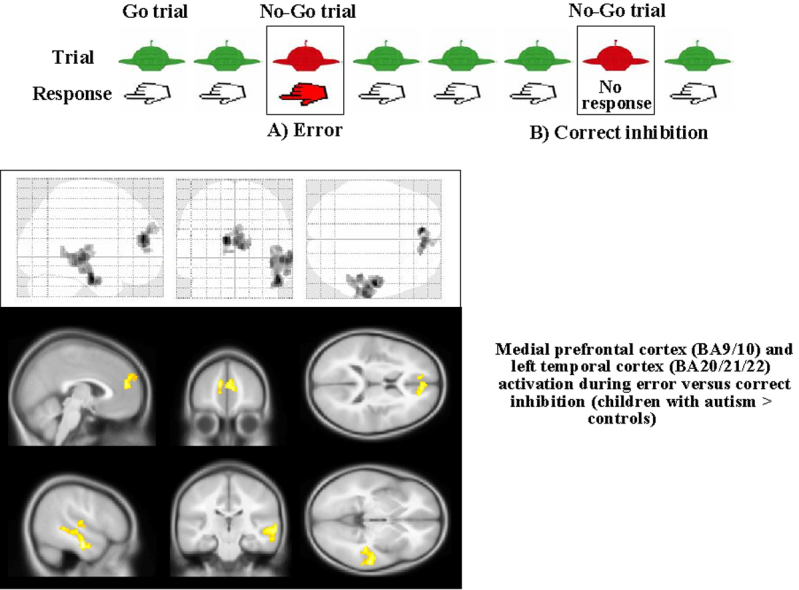
Fig. 1.
Example sequence of trials highlighting the two trial types of interest: (A) commission error and (B) correct inhibition.
Commission errors were defined as the failure to inhibit responding to “No-Go” stimuli, omission responses as the failure to respond to “Go” stimuli, and anticipatory responses as responses occurring less than 200 ms after stimulus presentation.
2.3. Scanning procedures
Images were acquired on a 1.5 T ACS-NT Powertrack 6000 MRI scanner (Philips Medical Systems) using a body coil transmission and quadrature end-capped head-coil reception. T1-weighted high-resolution images were acquired for each participant and used in the creation of cost function masks. Functional images were acquired every 2.5 s in coronally oriented volumes using single-shot echo-planar imaging (EPI) with the following parameters: 64 × 64 voxel matrix, 3.59 mm × 3.59 mm × 4.5 mm voxels, TE 40 ms, flip angle 90°. Each volume was composed of 41 4 mm thick interleaved slices with a 0.5 mm interslice gap. For a subset of participants (1 TD, 3 HFA), initial scanning parameters did not permit full coverage of the brain during EPI acquisition; for these subjects, each volume was composed of 34 4.5 mm slices which captured all brain regions except for the posterior occipital lobe and a small region of the posterior cerebellum.
Image processing was carried out using SPM2 (Wellcome Department of Imaging Neuroscience). To reduce the effects of susceptibility artifacts in EPI acquisition, cost function masking was used to optimize normalization to the EPI template (Brett et al., 2001). Functional volumes were corrected for slice timing, and spatially realigned to the location of the first image in the time series. Head movement was measured using the root mean square of the realignment parameters from each subject as reported by SPM2. No subjects demonstrated greater than 3.5 mm of motion in any direction (less than the size of one voxel). For each subject, a mean image was created and coregistered to the subject's T1 anatomical image to facilitate identification of areas susceptible to signal dropout. Masks were created by hand using MRIcro (http://www.sph.sc.edu/comd/rorden/mricro.html). Images were normalized to the MNI EPI template using a 12-parameter affine transformation and 16 nonlinear deformations. Smoothing was performed using a Gaussian kernel of 6 mm × 6 mm × 6 mm.
3. Calculation/data analysis
Behavioral data were examined using independent sample t-tests or repeated measures analysis of variance (RM-ANOVA) with diagnostic status as a between subject factor and task variables as within subject factors. Analyses were conducted using StatView 5.0.1 (SAS Institute, Inc., Cary, NC). All data were reported as mean ± S.E.M., and significance was set at p < 0.05 two-tailed alternatives.
Functional MRI data were analyzed using SPM5 to construct and examine the fit of the voxelwise time course data to a general linear model (GLM). Instead of using the canonical hemodynamic response functional (HRF), an optimized hemodynamic impulse response latency was estimated for each subject, in order to more accurately account for inter-subject variation in hemodynamic response latency. The GLM coefficients were estimated for multiple HRF latencies (time to peak 2.5 s post-stimulus to 9.5 s post-stimulus in increments of 250 ms). The optimal HRF latency was estimated as the latency at which the activation amplitude was highest for the “correct Go” trial in the left primary motor cortex (BA 4). For three children (one in the HFA group; two in the TD group), optimal latency could not be estimated and the SPM default (canonical HRF) was used. Optimal latency was not significantly different between groups (TD: 4.85 ± 0.23 s; HFA: 4.47 ± 0.14 s; t(24) = 0.88, p > 0.3).
Using the optimized latency for each subject, event-related response amplitudes were estimated using the GLM. Five regressors were modeled: correct No-Go trials (correct inhibition), commission error trials (error), correct response on Go trials, failure to respond on Go trials or omission trials and anticipatory trials. Motion parameters obtained from motion correction were also added as nuisance regressors in the GLM.
In order to examine brain activity during commission error trials compared to correct No-Go trials, a contrast map depicting areas of greater activation on “error versus correct inhibition” was created for each subject. Individual subject contrasts were entered into a second level analysis to estimate the differences in activation between HFA and TD subjects (two-sample t-test). Whole-brain random effects analyses was performed using a spatial extent cluster size threshold to achieve a corrected statistical threshold of p = 0.05, based on the number of voxels included in the ROI and the spatial smoothness of the data. The cluster size threshold was determined using a script provided by Thomas Nichols (CorrClusTh; https://www.jiscmail.ac.uk/cgi-bin/webadmin?A2=ind05&L=SPM&P=R103693&D=0).
Percent signal change in each functional ROI was calculated using Marsbar from the SPM toolbox (http://marsbar.sourceforge.net/) by computing mean voxel values within the specified ROI.
Location of voxels significantly associated with contrasts of interest was determined by summarizing local maxima separated by at least 8 mm and converting maxima from MNI to Talaraich coordinate space using a script provided by Matthew Brett (Medical Research Council – Cognition and Brain Sciences Unit; www.mrc-cbu.cam.ac.uk/Imaging/Common/mnispace.shtml).
4. Results
4.1. Behavioral data
There were no significant differences between groups in performance on the Go/No-Go task in terms of percent omission errors and groups were matched for percent commission errors. RT was examined separately for three Go trial categories: post-error trials (the Go trials after commission error), post-correct inhibition trials (the Go trials after correct response inhibition) and Go trials (the “remaining” Go trials). Participants in both groups showed slower RTs on post-error and post-correct inhibition trials compared to RT for Go trials (RM-ANOVA; main effect of trial category: F(2,48) = 10.02, p < 0.001), but no effect of diagnosis or diagnosis × trial category interaction (Table 1).
Table 1.
Behavioral performance on the Go/No-Go task.
| Behavioral measures | TD children | Children with HFA |
|---|---|---|
| % Commission errorsa | 29.5 ± 2.8 | 35.7 ± 3.6 |
| % Omissions | 2.7 ± 1.1 | 4.9 ± 1.8 |
| RT Go trials | 381.4 ± 71.0 | 405.1 ± 63.2 |
| RT post-error trials | 417.1 ± 82.8 | 464.5 ± 109.2 |
| RT post-correct inhibition trials | 430.2 ± 84.8 | 455.8 ± 65.3 |
RT: reaction time (ms). Mean ± S.E.M.
Groups were matched for task performance.
4.2. BOLD signal during commission errors minus correct response inhibition
4.2.1. Within-group analyses
For the HFA group, the one-sample t-tests examining BOLD signal during error versus correct inhibition trials showed 8 suprathresholded activation clusters at a corrected p = 0.05 (Table 2).
Table 2.
Error versus correct inhibition contrast in children with HFA.
| Extent (voxels) | t (peak voxel) | Region | BA | x | y | z | Hem |
|---|---|---|---|---|---|---|---|
| 471 | 8.85 | Insula | 13 | 40 | −24 | 16 | R |
| Claustrum | 34 | −14 | 8 | R | |||
| Postcentral G | 40 | 66 | −22 | 16 | R | ||
| 289 | 8.24 | Middle temporal G | 21 | −54 | −8 | −24 | L |
| Insula | 13 | −42 | −18 | −16 | L | ||
| Middle temporal G | 21 | −62 | −18 | −18 | L | ||
| 1140 | 7.93 | Anterior cingulate | 24 | −4 | 40 | 8 | L |
| Anterior cingulate | 24 | −8 | 36 | 2 | L | ||
| Anterior cingulate | 32 | 8 | 36 | 24 | R | ||
| 874 | 7.86 | Cingulate G | 24 | −6 | −6 | 40 | L |
| Cingulate G | 23 | 0 | −14 | 28 | B | ||
| Cingulate G | 23 | 0 | −28 | 34 | B | ||
| 245 | 7.41 | Insula | 13 | −48 | −20 | 16 | L |
| Superior temporal G | 22 | −58 | −10 | 6 | L | ||
| Insula | 13 | −42 | −16 | 12 | L | ||
| 162 | 6.90 | Cerebellum (nodule) | −14 | −56 | −38 | L | |
| Cerebellum (pyramis) | −16 | −70 | −40 | L | |||
| 1052 | 6.23 | Posterior cingulate | 23 | 2 | −48 | 20 | R |
| Cingulate G | 31 | 6 | −40 | 28 | R | ||
| Precuneus | 7 | 2 | −62 | 34 | R | ||
| 163 | 5.04 | Inferior parietal lobule | 40 | −42 | −60 | 42 | L |
| Inferior parietal lobule | 39 | −48 | −66 | 42 | L | ||
| Superior parietal lobule | 7 | −34 | −66 | 46 | L |
G: gyrus; Hem: hemisphere, B: bilateral, R: right, L: left. Coordinates are in MNI space.
For the TD group, the one-sample t-tests examining BOLD signal during error versus correct inhibition trials showed no suprathresholded activation clusters at a corrected p = 0.05.
4.2.2. Between-group analyses
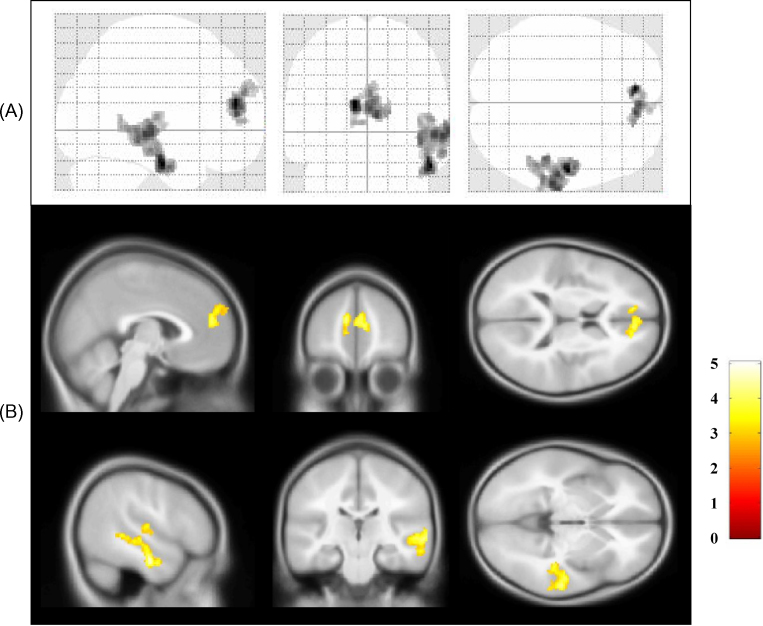
The two-sample t-tests examining for group differences in the error versus correct inhibition contrast showed two suprathresholded activation clusters at a corrected p = 0.05. The anterior medial prefrontal cortex (amPFC, BA10/9) and the left superior temporal gyrus (STempG, BA20/21/22; Fig. 2, Table 3) showed increased BOLD signal in the HFA group compared to the TD group. No suprathresholded activation clusters where TD controls activated more than children with HFA were found.
Fig. 2.
(A) Glass brain and (B) sectional maps representation of the areas of greater activation in children with HFA than in TD controls during commission error versus correct inhibition trials. Shown in radiological convention.
Table 3.
Between-group contrast associated with error versus correct inhibition trials (HFA > TD).
| Extent (voxels) | t (peak voxel) | Region | BA | x | y | z | Hem |
|---|---|---|---|---|---|---|---|
| 451 | 5.05 | Medial frontal G | 9 | 10 | 48 | 18 | R |
| Medial frontal G | 10 | −8 | 48 | 12 | L | ||
| Medial frontal G | 10 | −2 | 52 | 16 | L | ||
| 1072 | 4.96 | Sub-gyral | 20 | −52 | −12 | −24 | L |
| Superior temporal G | 22 | −52 | −26 | 2 | L | ||
| Superior temporal G | 21 | −56 | −20 | −2 | L |
G: gyrus; Hem: hemisphere, R: right, L: left. Coordinates are in MNI space.
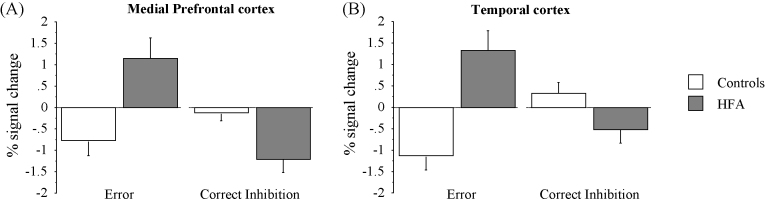
To identify if the between-group results reported above were related to changes in BOLD signal during error or correct inhibition trials, we extracted the mean percent signal change of each cluster for error trials and correct inhibition trials separately. In both clusters during error trials, children with HFA showed a relative increase in percent signal change as opposed to TD children, who showed a relative decrease (Fig. 3A). In contrast, during correct response inhibition trials, children with HFA showed a relative decrease in both, the amPFC and the STempG, clusters, compared to the TD group (Fig. 3B).
Fig. 3.
Percent signal change in medial prefrontal cortex (A) and the temporal cortex (B) during commission error and correct inhibition trials in typically developing children (controls) and children with HFA.
4.2.3. Follow-up region-of-interest (ROI) analyses
In the context of error processing, the findings of increased activity in the amPFC and the STempG in children with HFA compared to controls may suggest an exaggerated processing of negative information and the possibility of an abnormal processing of one's own emotional state (Frith and Frith, 2003, Gusnard et al., 2001, Ochsner et al., 2004).
The insula is considered to be part of the neural system mediating internal somatic states, and is believed to be necessary but may not be sufficient for feelings of emotion to occur (Bechara and Damasio, 2005). Activation of the insula, associated with a negative somatic state during a commission error on a No-Go trial (compared to correctly inhibiting a response on a No-Go trial), is commonly reported during error processing using the Go/No-Go task (Hester et al., 2004, Menon et al., 2001). To understand whether there were any between-group differences related to somatic markers of emotional state and cognitive integration in the brain associated with committing incorrect responses on No-Go trials, we conducted a ROI analysis of the bilateral insula.
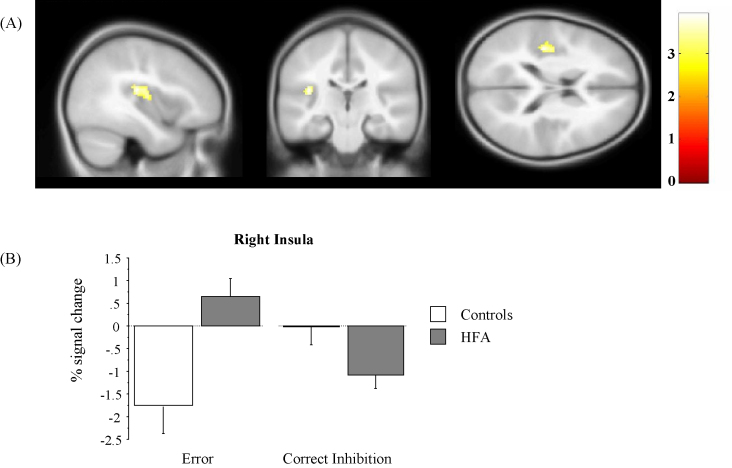
The left and right insula were anatomically defined and were selected from the WFU Pickatlas from the SPM Toolbox. The same insula ROIs were applied to all subjects. Examination of the commission error versus correct inhibition contrast revealed a significant effect of diagnosis within the right insula, with children with HFA showing increased activation compared with controls at a corrected p = 0.05 (cluster size: 165 voxels, uncorrected p < 0.005, t = 3.92, x = 38, y = −24, z = 18; x = 36, y = −14, z = 12, MNI coordinates). This result was mainly driven by between-group differences during error trials, where the HFA group showed a relative increase in percent signal change while the TD group showed a relative decrease (Fig. 4). In contrast, during correct response inhibition children with HFA showed a relative decrease in BOLD signal compared to TD children.
Fig. 4.
(A). Sectional map representation of the region-of-interest analysis in the bilateral insula showing the area of greater activation in children with HFA than in TD controls during commission error versus correct inhibition trials. (B) Percent signal change in the right insula during commission error and correct inhibition trials in typically developing children (controls) and children with HFA.
5. Discussion
The aim of the present study was to increase our understanding about the neural correlates of error monitoring in a sample of 8–12-year-old children with HFA during the performance of a simple Go/No-Go task. Participants in the HFA group and the TD group did not exhibit behavioral differences in terms of post-error RT (and were matched on commission error rate), indicating that post-error behavioral adjustment was not impaired in autism. However, fMRI analysis of commission error versus correct inhibition trials showed a different pattern of neural activation in the amPFC and the left STempG between the two groups. Specifically, during error trials, children with HFA displayed a relative increase in BOLD signal in the amPFC and STempG, while TD children showed a relative decrease in both clusters.
The PFC region differentially activated in the autism group versus the TD group included the anterior medial PFC/fronto-polar cortex. Structural and functional abnormalities of the amPFC have been widely reported in autism (Castelli et al., 2002, Gilbert et al., 2008, Schmitz et al., 2006) and, more recently, it has been suggested that an abnormal functional specialization of the amPFC may occur in autism (Gilbert et al., 2009). This medial region has been implicated in controlling attention to emotional information (Gilbert et al., 2006), consistent with previous studies demonstrating interconnections between this region and subcortical structures involved in emotional processing (Ongür et al., 2003, Ongür and Price, 2000, Porrino et al., 1981). fMRI studies in neurotypical adults have implicated the amPFC in the processing of mentalizing (Gilbert et al., 2006) and affective conflict (Ochsner et al., 2009). Specifically it has been suggested that it may be important “in attending to one's own emotional states” (Frith and Frith, 2003, Gusnard et al., 2001, Ochsner et al., 2004).
Similarly, in healthy control subjects, activity in the left temporal area during the Go/No-Go task has been linked to awareness and emotional reaction to errors (Hester et al., 2005). Moreover, increased BOLD signal in the amPFC and the superior temporal cortex (BA21/22) has been consistently reported when subjects are involved in self-reflective thought, and these regions are considered to play an important role in tasks that involve mentalizing, the ability to understand the intentions and beliefs of others (or theory of mind) (Amodio and Frith, 2006, Hooker et al., 2008, Spreng et al., 2009). Functional abnormalities in the amPFC in particular have been proposed to be related to the pattern of “missing the forest for the trees or weak central coherence evident in persons with autism” (Solomon et al., 2009).
In the context of error processing in autism, the increased activation in the amPFC and the STempG during error trials may indicate an abnormal/exaggerated processing of negative information relevant to one's own emotional state. It is difficult to substantiate this interpretation given that the behavioral data do not reveal increased post-error slowing in the children with ASD. We would not, however, necessarily expect an exaggerated emotional processing of emotional state to result in post-error slowing. Previous studies involving both children (Vlamings et al., 2008) and adults (Bogte et al., 2007), with autism have shown decreased post-error slowing compared to control subjects. The lack of behavioral differences in our study taken together with findings of increased activity in amPFC and the STempG, brain regions involved in self-reflective thought could alternatively be interpreted to suggest that children with HFA were less emotionally burdened by errors compared to controls.
The abnormal processing of emotionally negative information in children with HFA, however, is supported by our ROI follow-up analysis demonstrating increased BOLD signal in the insula in children with HFA compared to TD controls. The insula is considered to play an important role in decision-making processes by integrating information about internal emotional/arousal states and the risk or uncertainty associated with current decisions (Paulus and Stein, 2006). Activation of the insula has been widely reported during error processing and, it has usually been associated with a negative emotional state (Hester et al., 2004, Menon et al., 2001), supporting the hypothesis of an exaggerated, rather than reduced, error processing in children with HFA. Simultaneous use of methods for measuring emotional state, such as use of galvanic skin response (GSR), would help with interpretation of these findings.
It should be noticed that we employed emotionally “neutral” stimuli consisting of red and green spaceship rather than emotional and/or social stimuli, such as angry/happy faces. Moreover, each subject was instructed to ignore errors and keep performing the task, which may explain the lower activity in TD children; perhaps because TD children are less emotionally burdened by errors or negative events.
Overall our findings of increased amPFC, STempG and insula activity in children with HFA compared to TD controls suggest a greater attention towards self-generated information, e.g., the internally driven emotional state associated with making an error. Increased reliance on self- or internally generated information in autism may affect learning across different task conditions. Recently, it has been reported that compared to TD controls, children with HFA relied more on proprioceptive, rather than visuo-spatial, information during a motor learning task (Haswell et al., 2009). However, several studies have shown that children with autism have anomalous and impaired patterns of learning (Mostofsky et al., 2000, Gidley Larson and Mostofsky, 2008). Error monitoring occurs across different cognitive tasks and throughout our every day life, therefore, understanding differences in error monitoring in autism may have implications for developing interventions to help improve self-monitoring skills related to executive function and social–emotional abilities in autism (see also Henderson et al., 2006).
Although our results were mainly driven by between-group differences during error trials, we found that children with HFA also showed a relative decrease in BOLD signal in the amPFC and STempG during correct response inhibition. In a previous fMRI study in adults with autism, higher ACC activity has been reported during correct response monitoring compared to controls (Thakkar et al., 2008). The present study did not reveal differences in ACC signal change in children with HFA. A limitation of our study was the small sample size included in the analysis. Therefore, it is possible that differences in ACC activity were present in children with HFA compared to controls but we were unable to detect them. Further studies are warranted, since repetitive behaviors associated with autism might reflect a wider dysfunction within executive circuitries.
6. Concluding remarks
Replication studies are needed as differences between studies could be attributed to tasks differences, sample population (adults versus children), and/or the limited number of children participating in our study. Future fMRI research is needed on error monitoring in children with autism as well as in TD children in order to add to the small literature and to provide replication across studies. This research has implications for improving our understanding about error monitoring deficits in autism and elucidating the neural mechanisms that give rise to/contribute to these impairments in autism.
Acknowledgments
This work was supported by the National Institute of Health grants: K01 MH01824 (MCG), R01NS048527 (SHM), K02 NS044850 (SHM), the Developmental Disabilities Research Center (HD-24061), the Johns Hopkins General Clinical Research Center (GCRC M01 RR00052), Johns Hopkins University School of Medicine Institute for Clinical and Translational Research, and the NIH/NCRR CTSA Program (UL1-RR025005).
Biographies

Melissa C. Goldberg, Ph.D., is a research scientist at the Kennedy Krieger Institute and Assistant Professor in the Department of Psychiatry and Behavioral Sciences at the Johns Hopkins School of Medicine, Baltimore MD. She earned a B.A. in Psychology from Washington University in St. Louis, Missouri and a Master's degree in Counseling Psychology from Harvard University. She completed her Ph.D. in Developmental Psychology from McMaster University, Hamilton, Ontario, Canada, and a postdoctoral fellowship at the Johns Hopkins University School of Medicine. The overall focus of Dr. Goldberg's research is to advance understanding about cognitive neuropsychological mechanisms underlying autism and other neurodevelopmental disorders.

Simona Spinelli, Ph.D., completed her Ph.D. at the Swiss Federal Institute of Technology in Zurich, Switzerland. She worked as a postdoctoral fellow first at the National Institute of Alcohol Abuse and Alcoholism, and then at the Kennedy Krieger Institute/Johns Hopkins University School of Medicine. She is currently a Research Associate in the Clinic for Affective Disorders and General Psychiatry at the Psychiatric University Hospital in Zurich, Switzerland. The focus of Dr. Spinelli's research is to advance understanding of the possible endophenotypes of anxiety and depression.

Suresh Joel, Ph.D., completed his M.Sc. in Biological Sciences and BE in Electronics and Instrumentation from Birla Institute of Technology and Science, Pilani, India. He completed his Ph.D. in Biomedical Engineering in 2005 from Virginia Commonwealth University, Richmond VA. He currently is a Research Associate Faculty at Johns Hopkins University and Kennedy Krieger Institute where he develops novel analysis methods for functional brain imaging.

James J. Pekar, Ph.D., is associate professor of radiology at Johns Hopkins University and Manager and Research Coordinator of the F.M. Kirby Research Center for Functional Brain Imaging at Kennedy Krieger Institute. He holds a B.S. in physics from the Massachusetts Institute of Technology, a Ph.D. in biophysics from the University of Pennsylvania, and completed a postdoctoral fellowship at the National Institutes of Health. He was assistant professor of neurology at Georgetown University before joining Johns Hopkins. His research focuses on the development of methods for the acquisition and analysis of magnetic resonance data reporting on brain function; he has authored over 60 peer-reviewed scientific publications.

Martha B. Denckla, M.D. is Batza Family Endowed Chair and Director of Developmental Cognitive Neurology at the Kennedy Krieger Institute and Professor of Neurology, Pediatrics and Psychiatry at the Johns Hopkins University School of Medicine. She graduated from Bryn Mawr College and Harvard Medical School. She has combined clinical practice and research, both focused on developmental cognitive/academic disorders, at Columbia's Neurological Institute and Harvard's Children's Hospital. She served as Chief of the Section on Autism & Related Disorders at the Developmental Neurology Branch of the Neurological Disorders Program at the National Institute of Neurological and Communicative Disorders and Stroke (NIH). Her research focuses on the biological bases for learning disabilities and ADHD.

Stewart H. Mostofsky, M.D. is a child neurologist and research scientist at the Kennedy Krieger Institute and Associate Professor in the Departments of Neurology and Psychiatry at the Johns Hopkins University School of Medicine. He is the Director of the Laboratory for Neurocognitive and Imaging Research and is the Medical Director of the Center for Autism and Related Disorders at Kennedy Krieger Institute. The overall focus of Dr. Mostofsky's research is on using experimental behavioral, neuroimaging, and electrophysiologic methods to advance understanding of the neural mechanisms underlying the brain basis of neurodevelopmental disorders, including autism and attention-deficit hyperactivity disorder (ADHD) and the neural mechanisms underlying effective treatments of those disorders.
Contributor Information
Melissa C. Goldberg, Email: goldbergm@kennedykrieger.org.
Simona Spinelli, Email: spinellisimona@gmail.com.
References
- American Psychiatric Association, 1994. Desk Reference to the Diagnostic Criteria from DSM-IV. American Psychiatric Association, Washington D.C.
- Amodio D.M., Frith C.D. Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Bechara A., Damasio A.R. The somatic marker hypothesis: a neural theory of economic decision. Games Econ. Behav. 2005;52:336–372. [Google Scholar]
- Bogte H., Flamma B., van der Meere J., van England H. Post-error adaptation in adults with high functioning autism. Neuropsychologia. 2007;45:1707–1714. doi: 10.1016/j.neuropsychologia.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Brett M., Leff A.P., Rorden C., Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage. 2001;14:486–500. doi: 10.1006/nimg.2001.0845. [DOI] [PubMed] [Google Scholar]
- Bush G., Luu P., Posner M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends Neurosci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Castelli F., Frith C., Happé F., Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Conners C.K. Multi-Health Systems, Inc.; North Tonawanda, New York: 1997. Conners’ Rating Scales-Revised. [Google Scholar]
- DuPaul G.J. Parent and teacher ratings of ADHD symptoms: psychometric properties in a community based sample. J. Clin. Child Psychol. 1991;20:243–253. [Google Scholar]
- Frith U., Frith C.D. Development and neurophysiology of mentalizing. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H., Ross T.J., Murphy K., Roche R.A., Stein E.A. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. Neuroimage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Gidley Larson J.C., Mostofsky S.H. Evidence that the pattern of visuomotor sequence learning is altered in children with autism. Autism Res. 2008;1:341–353. doi: 10.1002/aur.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert S.J., Bird G., Brindley R., Frith C.D., Burgess P.W. Atypical recruitment of medial prefrontal cortex in autism spectrum disorders: an fMRI study of two executive function tasks. Neuropsychologia. 2008;46:2281–2291. doi: 10.1016/j.neuropsychologia.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert S.J., Meuwese J.D., Towgood K.J., Frith C.D., Burgess P.W. Abnormal functional specialization within medial prefrontal cortex in high-functioning autism: a multi-voxel similarity analysis. Brain. 2009;132:869–878. doi: 10.1093/brain/awn365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert S.J., Spengler S., Simons J.S., Steele J.D., Lawrie S.M., Frith C.D., Burgess P.W. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. J. Cogn. Neurosci. 2006;18:932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Groen Y., Wijers A.A., Mulder L.J.M., Waggerveld B., Minderaa R.B., Altaus M. Error and feedback processing in children with ADHD and children with autistic spectrum disorder: an EEG event-related potential study. Clin. Neurophysiol. 2008;119:2476–2493. doi: 10.1016/j.clinph.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Gusnard D.A., Akbudak E., Shulman G.L., Raichle M.E. Medial prefrontal cortexand self-referential mental activity: relation to a default mode of brain function. Proc. Natl. Acad. Sci. U.S.A. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happe F., Booth R., Charlton R., Hughes C. Executive function deficits in autism spectrum disorders and attention-deficit/hyperactivity disorder: examining profiles across domains and ages. Brain Cogn. 2006;61:25–39. doi: 10.1016/j.bandc.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Haswell C.C., Izawa J., Dowell L.R., Mostofsky S.H., Shadmehr R. Representation of internal models of action in the autistic brain. Nat. Neurosci. 2009;12:970–972. doi: 10.1038/nn.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haznedar M.M., Buchsbaum M.S., Wei T.C., Hof P.R., Cartwright C., Bienstock C.A., Hollander E. Am. J. Psychiatry. 2000;157:1994–2001. doi: 10.1176/appi.ajp.157.12.1994. [DOI] [PubMed] [Google Scholar]
- Henderson H., Schwartz C., Mundy P., Burnett C., Sutton S. Response monitoring, the error-related negativity, and differences in social behavior in autism. Brain Cogn. 2006;61:96–109. doi: 10.1016/j.bandc.2005.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R., Fassenbender C., Garavan H. Individual differences in error processing: A review and reanalysis of three event-related fMRI studies using the GO/NOGO task. Cereb. Cortex. 2004;14:986–994. doi: 10.1093/cercor/bhh059. [DOI] [PubMed] [Google Scholar]
- Hester R., Foxe J.J., Molholm S., Shpaner M., Garavan H. Neural mechanisms involved in error processing: a comparison of errors made with and without awareness. Neuroimage. 2005;27:602–608. doi: 10.1016/j.neuroimage.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Hollingshead A.B. Yale University, Department of Sociology; New Haven, CT: 1975. Four Factor Index of Social Status. [Google Scholar]
- Hooker C.I., Verosky S.C., Germine L.T., Knight R.T., D’Esposito M. Mentalizing about emotion and its relationship to empathy. Soc. Cogn. Affect Neurosci. 2008;3:204–217. doi: 10.1093/scan/nsn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl K.A., Liddle P.F., Hopfinger J.B. Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiology. 2000;37:216–223. [PubMed] [Google Scholar]
- Le Couteur A., Lord C., Rutter M. Western Psychological Services; Los Angeles, CA: 2003. The Autism Diagnostic Interview - Revised (ADI-R) [Google Scholar]
- Lord C., Risi S., Lambrecht L., Cook E.H., Jr., Leventhal B.L., DiLavore P.C., Pickles A., Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C., Rutter M., LeCouteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Menon V., Adleman N.E., White C.D., Glover G.H., Reiss A.L. Error-related brain activation during a Go/NoGo response inhibition task. Hum. Brain Mapp. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky S.H., Goldberg M.C., Landa R.J., Denckla M.B. Evidence for a deficit in procedural learning in children and adolescents with autism: implications for cerebellar contribution. J. Int. Neuropsychol. Soc. 2000;6:752–759. doi: 10.1017/s1355617700677020. [DOI] [PubMed] [Google Scholar]
- Mundy P. Annotation: the neural basis of social impairments in autism: the role of the dorsal medial-frontal cortex and anterior cingulate system. J. Child Psychol. Psychiatry. 2003;44:793–809. doi: 10.1111/1469-7610.00165. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Hughes B., Robertson E.R., Cooper J.C., Gabrielli J.D. Neural systems supporting the control of affective and cognitive conflicts. J. Cogn. Neurosci. 2009;21:1842–1855. doi: 10.1162/jocn.2009.21129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Knierim K., Ludlow D.H., Hanelin J., Ramachandran T., Glover G., Mackey S.C. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J. Cogn. Neurosci. 2004;16:1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ongür D., Ferry A.T., Price J.L. Architectonic subdivision of the human orbital and medial prefrontal cortex. J. Comp. Neurol. 2003;460:425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Ongür D., Price J.L. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb. Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Paulus M.P., Stein M.B. An insular view of anxiety. Biol. Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Porrino L.J., Crane A.M., Goldman-Rakic P.S. Direct and indirect pathways from the amygdala to the frontal lobe in rhesus monkeys. J. Comp. Neurol. 1981;198:121–136. doi: 10.1002/cne.901980111. [DOI] [PubMed] [Google Scholar]
- Rabbitt P.M. Errors and error correction in choice-response tasks. J. Exp. Psychol. 1966;71:264–272. doi: 10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- Reich W., Welner Z., Herjanic B. Multi-Health Systems; North Tonawanda, New York: 1997. The Diagnostic Interview for Children and Adolescents-IV. [Google Scholar]
- Robinson S., Goddard L., Dritschel B., Wisley M., Howlin P. Executive functions in children with autism spectrum disorders. Brain Cogn. 2009;71:362–368. doi: 10.1016/j.bandc.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Russell J., Hill E.L. Action-monitoring and intention reporting in children with autism. J. Child Psychol. Psychiatry. 2001;42:317–328. [PubMed] [Google Scholar]
- Russell J., Jarrold C. Error-correction problems in autism: evidence for a monitoring impairment? J. Autism Dev. Disord. 1998;28:177–188. doi: 10.1023/a:1026009203333. [DOI] [PubMed] [Google Scholar]
- Schmitz N., Rubia K., Daly E., Smith A., Williams S., Murphy D.S. Neural correlates of executive function in autistic spectrum disorders. Biol. Psychiatry. 2006;59:7–16. doi: 10.1016/j.biopsych.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Sergeant J.A., van der Meere J. What happens after a hyperactive child commits an error? Psychiatry Res. 1988;24:157–164. doi: 10.1016/0165-1781(88)90058-3. [DOI] [PubMed] [Google Scholar]
- Solomon M., Ozonoff S.J., Ursu S., Ravizza S., Cummings N., Ly S., Carter C.S. The neural substrates of cognitive control deficits in autism spectrum disorders. Neuropsychologia. 2009;47:2515–2526. doi: 10.1016/j.neuropsychologia.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng R.N., Mar R.A., Kim A.S. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J. Cogn. Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Suskauer S.J., Simmonds D.S., Caffo B.S., Denckla M.B., Pekar J.J., Mostofsky S.H. fMRI of intrasubject variability in ADHD: anomalous premotor activity with prefrontal compensation. J. Am. Acad. Child Adolesc. Psychiatry. 2008;47(10):1141–1150. doi: 10.1097/CHI.0b013e3181825b1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suskauer S.J., Simmonds D.J., Fotedar S., Blankner J.G., Pekar J.J., Denckla M.B., Mostofsky S.H. Functional magnetic resonance imaging evidence for abnormalities in response selection in attention deficit hyperactivity disorder: differences in activation associated with response inhibition but not habitual motor response. J. Cogn. Neurosci. 2008;20:478–493. doi: 10.1162/jocn.2008.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S.F., Stern E.R., Gehring W.J. Neural systems for error monitoring: recent findings and theoretical perspectives. Neuroscientist. 2007;13:160–172. doi: 10.1177/1073858406298184. [DOI] [PubMed] [Google Scholar]
- Thakkar K.N., Polli F.E., Joseph R.M., Tuch D.S., Hadjikhani N., Barton J.J.S., Manoach D.S. Response monitoring, repetitive behavior and anterior cingulate abnormalities in autism spectrum disorders (ASD) Brain. 2008;131:2464–2478. doi: 10.1093/brain/awn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlamings P.H.J.M., Jonkman L.M., Hoeksma M.R., van Engeland H., Kemner C. Reduced error monitoring in children with autism spectrum disorder: an ERP study. Eur. J. Neurosci. 2008;28:399–406. doi: 10.1111/j.1460-9568.2008.06336.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. third ed. The Psychological Corporation; San Antonio, TX: 1991. Manual for the Wechsler Intelligence Scale for Children. [Google Scholar]
- Wechsler D. fourth ed. The Psychological Corporation; San Antonio, TX: 2003. Manual for the Wechsler Intelligence Scale for Children. [Google Scholar]