Abstract
Bipolar disorder (BPD) is characterized by vulnerability to episodic depression and mania and spontaneous cycling. Because of marked advances in candidate-gene and genome-wide association studies, the list of risk genes for BPD is growing rapidly, creating an unprecedented opportunity to understand the pathophysiology of BPD and to develop novel therapeutics for its treatment. However, genetic findings are associated with major unresolved issues, including whether and how risk variance leads to behavioral abnormalities. Although animal studies are key to resolving these issues, consensus is needed regarding how to define and monitor phenotypes related to mania, depression and mood swing vulnerability in genetically manipulated rodents. In this study we discuss multiple facets of this challenging area, including theoretical considerations, available tests, limitations associated with rodent behavioral modeling and promising molecular–behavioral findings. These include CLOCK, glycogen synthase kinase 3β (GSK-3β), glutamate receptor 6 (GluR6), extracellular signal-regulated kinase-1 (ERK1), p11 (or S100A10), vesicular monoamine transporter 2 (VMAT2 or SLC18A2), glucocorticoid receptors (GRs), Bcl-2-associated athanogene-1 (BAG1) and mitochondrial DNA polymerase-γ (POLG). Some mutant rodent strains show behavioral clusters or activity patterns that cross-species phenocopy objective/observable facets of mood syndromes, and changes in these clustered behaviors can be used as outcome measures in genetic–behavioral research in BPD.
Keywords: mania, depression, bipolar disorder, animal model, lithium
Introduction
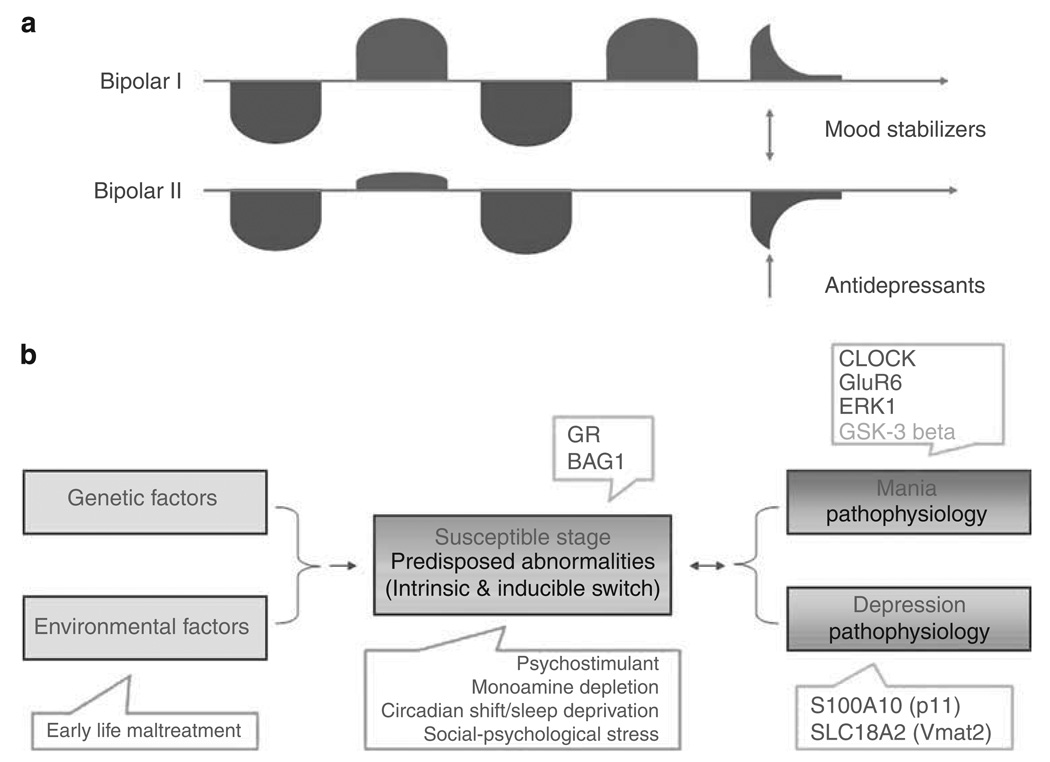
Bipolar disorder (BPD) is a severe, disabling and often life-threatening psychiatric disorder that affects approximately 1–2% of the general population.1,2 Phenotypically, BPD is a very complex disease in which patients alternate between episodes of mania and depression1,2 (Figure 1). Accumulating data support the theory that BPD arises from the complex inheritance of multiple genetic variants3–5 (Figure 1). In susceptible subjects, physical and psychosocial stressors can facilitate the progress of BPD into a stage of vulnerability and trigger mood episodes.6 Such episodes can also be induced by psychostimulants, monoamine depletion and corticosteroids; an intrinsic biological trigger is also suspected for ‘spontaneous’ mood episodes.1,2
Figure 1.
(a) BPD has unique episodic and dual-directional features in which mood states can be either elevated (red, mania), suppressed (blue, depression) or rapidly alternate between the two extremes (mixed episodes). Euthymic/remitted phases occur between the episodes in which patients, especially those with residual mood symptoms, remain vulnerable to the recurrence of full-blown mood episodes. Mood stabilizers, such as lithium, valproate, carbamazepine, lamotrigine, as well as atypical antipsychotics, are effective therapeutic agents that gradually relieve the symptoms of mania and/or depression. They also prevent the recurrence of mood episodes. Recent data from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) study found that approximately 60% of patients recover after appropriate treatments, and approximately 50% of recovered patients experience a recurrence within 2 years; recurrence is highly associated with residual mood symptoms at initial recovery. BPD-I: patient has one or more manic episodes or mixed episodes. Often individuals have also had one or more major depressive episodes. BPD-II: patient has at least one hypomanic episode and at least one major depressive episode. (b) Conceptually, the course of BPD can be divided into two major parts: vulnerability and episode stages; the illness is further characterized by a transition period from vulnerability to episode stage. Genetic variants and environmental stressors, and the interaction between the two (G × E), contribute to the predisposed abnormalities underlying the vulnerability stage. These two factors and their interaction may also contribute to the intrinsic (or spontaneous) switch mechanism. Psychosocial and biological stressors can trigger mood episodes in susceptible individuals. The use of psychostimulants and experimental monoamine depletion can cause mania or depressive relapses in subjects with a personal or family history of mood disorders, or with certain genetic variants. Unique pathophysiological mechanisms are believed to underlie depression, mania and mixed episodes. The color reproduction of this figure is available on the html full text version of the manuscript.
Recently, large-scale candidate and genome-wide association studies have generated a rapidly growing list of risk genes for BPD, including ANK3 and CACNA1A.5,7 More key findings are expected from copy-number variance and in-depth sequencing studies of thousands of individuals. Advances in genetic studies create an unprecedented opportunity to finally understand the pathophysiology of this devastating illness at the molecular level, and to develop novel therapeutics capable of relieving mood symptoms as well as preventing recurrences. However, both existing and future association studies have at least three key issues that remain unresolved: (1) whether, and to what extent, risk variance causes gene dysfunction; (2) whether risk genes are causally linked to behavioral abnormalities in BPD and (3) if so, what are the precise mechanisms that lead to behavioral abnormalities.
Animal studies have long had a pivotal role in attempting to resolve these issues. Currently, two types of behavioral approaches are used to analyze the pathogenesis of BPD; broadly, these can be thought of as the animal model and model animal approaches.8 The animal model approach begins by identifying a behavioral stress or chemical treatment that induces specific behavior(s) thought to cross-species phenocopy one or a few mood symptoms (for example, immobility in the forced swim or tail suspension tests, escaping deficit in the learned helplessness paradigm9 or social avoidance or reduced sweet solution preference in the social defeat paradigm10). The biological basis of induced behaviors can then be studied. In contrast, the initial step of the model animal approach is to introduce specific gene or pathway alterations implicated in BPD into animals. These animals are then evaluated using batteries of behavioral tests to examine whether the abnormality is causally linked to behaviors or to behavior clusters that cross-species phenocopy mood symptoms, mood syndromes or mood swing vulnerability observed in euthymic BPD patients. Once these causal relationships are established, the implicated biological alterations to behavior can be further analyzed. Although both animal models and model animals can be used to evaluate novel therapeutics, the increased use of model animals is expected, given the unique ability of such animals to evaluate pathophysiology driven target treatments.
The term ‘cross-species phenocopy’, which we use throughout this review, is used to emphasize apparent phenomenological similarities that can result from the same or different underlying mechanism(s) in humans and rodents.11 To be cautious with regard to animal research, investigators have used the term ‘cross-species phenocopy of depression or mania’ rather than ‘model of depression or mania’ to reflect the fact that true behavioral models for a given illness stem from established pathology, and that the pathology of BPD is still largely unknown.
Below, we discuss the strengths and limitations of each animal approach and describe recent promising findings from several mutant strains. Specifically, we discuss (1) the advantages and drawbacks associated with current batteries of tests that characterize behavioral clusters or activity patterns related to the three major components of BPD: mania, depression and vulnerability to mood swings; (2) the relevance of behavioral clusters or patterns to depression and mania, and how these can be further validated through treatment with antidepressants or mood stabilizers; and (3) how these behavioral clusters or patterns can be used as major behavioral outcome measures in studies that use the model animal approach to address issues related to genetic–behavioral relationships in BPD.
It is worth noting that although rodents are nocturnal animals, the behavioral tests or measures discussed in this review are currently conducted during the light phase (with the exception of home cage wheel running12 and home cage activity11 scans). Furthermore, animals are bred in the laboratory for experimental purposes, and these conditions are far from natural. This adds a further dimension to our ability to extrapolate the results of one or a few tests to the overall state of rodent activity, and from rodents to humans. Future studies should address alternative behavioral tests and measures, as well as the logistical problems of animal holding and behavioral facilities for experiments that are more in line with the nocturnal nature of rodents.
The animal model approach
A comprehensive list of current animal models for depression or mania can be found in several recent, well-written reviews.13–16 These animal models are valid for evaluating mood stabilizers as well as ‘metoo’ and novel types of antidepressants.13,14 For instance, the forced swim test is sensitive to most direct monoamine manipulating antidepressants13,14 as well as other clinically effective antidepressant agents such as ketamine,9 lamotrigine17,18 and lithium.19 Because the outcome measures of these models are sensitive to treatment with mood stabilizers or antidepressants,13–16 they are often collectively referred to as antidepressant- or mood stabilizer-sensitive behavioral paradigms. Two terms describe experimental outcomes from these paradigms: (1) mood-stabilizer-like or antidepressant-like behavioral actions and (2) the behavioral effects of mood stabilizers or antidepressants. It should be noted that these terms are not directly equivocal to mood stabilizing or antidepressant actions. Presently, it remains unknown whether or not the administration of mood stabilizers or antidepressants elicits different behavioral actions through the same mechanisms. These animal models are associated with other advantages and weaknesses, and these have been well addressed in previous reviews.13–16
The model animal approach
The ‘reverse translation’ model animal approach is being used in the study of BPD with increasing frequency. Loosely defined, reverse translation shifts the focus from creating animal behaviors that phenotypically resemble aspects of mental disorders (that is, animal models) to instead using what we have learned about the potential pathological changes found in BPD from human genetic, post-mortem and other studies (that is, creating model animals).8 Potential pathological changes include genetic, epigenetic and other biological alterations. This subtle distinction shifts the focus from creating animal models to understanding the role of those specific changes already implicated in BPD. Thus, instead of approaching this issue from the perspective of creating an ‘animal model of BPD’, researchers can focus on understanding whether and how specific changes contribute to a ‘mania-like phenotype’, ‘depression-like phenotype’ or ‘vulnerability-like phenotype’. Studies using this approach can ultimately provide fundamental information for designing novel therapeutics that are able to target causally linked changes, or the biological consequences of the changes. The animals created from this type of research can, in turn, serve as tools for the comprehensive evaluation of the effectiveness of novel therapeutic methods for BPD at both the neurochemical and behavioral levels.
In the model animal approach, biologically predisposed animals are generated using genetic (for example, gene transgenic, knockout or mutation–knock-in manipulation) or other biological means (such as viral vector-based gene overexpression or knockdown). These predisposed animals are then studied using batteries of behavioral tests for phenocopies related to different components of BPD. Some of these tests are highlighted in Tables 1–3.
Table 1.
Clinical features of mania and rodent phenocopies of components of excessive behavioral excitement
| Domains | Clinical diagnostic features | Suitable animal tests |
Expected outcomes |
mCLOCK | GluR6 −/− |
ERK1 −/− |
|---|---|---|---|---|---|---|
| Mood | Elevated, expansive or irritable mood | Unknown | NA | NA | NA | NA |
| Thought | Inflated self-esteem or grandiosity | Unknown | NA | NA | NA | NA |
| Flight of ideas or racing thoughts | Unknown | NA | NA | NA | NA | |
| Behavior | More talkative than usual or pressure to keep talking | Unknown | NA | NA | NA | NA |
| Increase in goal-directed activity (either socially, at work or school or sexually) or psychomotor agitation | Open field test Novel object exploration | Increased investigation | Yes | Yes | Yes | |
| Social interaction test | Increased social interaction, constructive or destructive | No data | Yes | No data | ||
| Resident–intruder test | ||||||
| Mating activity | Increased sexual activity | No data | No data | No data | ||
| Sweetened solution preference test | Increased hedonic/pleasurable activity | Yes | Yes | Yes | ||
| Wheel running activity | ||||||
| FUST | ||||||
| CPP | ||||||
| ICSS | ||||||
| Forced swim or tail suspension tests | Increased escaping activity | Yes | Yes | Yes | ||
| Learned helplessness paradigm | ||||||
| Excessive involvement in pleasurable activities that have a high potential for painful consequences (e.g., engaging in unrestrained buying sprees, sexual indiscretions or foolish business investments) | Center activity in open field Elevated plus maze or zero maze Light/dark Reward/aversion test Novelty-induced hypophagia | More risk-taking (or less anxiety-like) activity | Yes | Yes | Yes | |
| Decreased need for sleep (e.g., feels rested after only 3 h of sleep) | Home cage activity monitoring | More activity and less rest in home cage | No data | Yes | Yes | |
| Home cage wheel running | ||||||
| Attention | Distractibility (i.e., attention too easily drawn to unimportant or irrelevant external stimuli) | There are potential tests, but these need to be further validated. | More distractible | No data | No data | No data |
| Exclusions | Manic symptoms are not due to neurological, endocrine or metabolic diseases | Brain morphology, biochemical tests and battery of motor-sensory tests | No obvious changes | Circadian dysfunction | Yes | Yes |
| Psychostimulant | Increased use | Psychostimulant-induced locomotion | No change or increased | Yes | Yes | Yes |
Abbreviations: CPP, conditioned place preference test; ERK1, extracellular signal-regulated kinase-1; FUST, female urine sniffing test; GluR6, glutamate receptor 6; ICSS, intracranial self-stimulation; NA, not applicable.
Table 3.
Affective vulnerability in humans and cross-species phenocopies of affective vulnerability in rodents
| Type | Affective vulnerability in humans | Suitable test/observation | Outcomes | GR transgenic | BAG1 transgenic | BAG1+/− |
|---|---|---|---|---|---|---|
| General | Absence of neurological diseases | Growth and appearance | No change | No change | No change | No change |
| General behavior | ||||||
| Euthymic mood | Batteries for depression and mania phenocopies | No change | No change | No change | No change | |
| Vulnerability to depression | Stress coping deficits | Immobility in forced swim and tail suspension tests | No change/more | Increased | No change | No change |
| Response to helplessness induction | Increased | No data | No change | No change | ||
| Recovery from helplessness | Less/lost | No data | Enhanced | Reduced | ||
| Response to chronic stress | Increased | No data | No data | No data | ||
| Recovery from chronic stress | Less/lost | No data | No data | No data | ||
| Response to monoamine depletion in tests for anhedonia-like behaviors | Increased | No data | No data | No data | ||
| Vulnerability to mania | Psychostimulants cause mania relapse | Psychostimulant challenge test | Increased | NA | Enhanced recovery | No change |
| Behavioral sensitization | Increased | Increased | Reduced | Increased | ||
| Circadian shift/jetlag triggers mania relapse | Circadian shift/sleep deprivation-induced behavioral changes | Increased | No data | No data | No data | |
| Treatments for depression and mania | Antidepressants | Behavioral response to antidepressants | Either (1) not effective; (2) prevent the induction of depression-like behaviors; or (3) worsen manialike behaviors | Increased | NA | No data |
| Mood stabilizers | Behavioral response to mood stabilizers | Not effective or preventive | No data | NA | No data |
Abbreviations: BAG1, Bcl-2-associated athanogene-1; GR, glucocorticoid receptor; NA, not applicable; Vmat2, vesicular monoamine transporter 2.
It should be emphasized that the translational study of BPD using the model animal approach rests upon making the same assumptions that are made in studies using animal models. First, the basic structure and biochemical function of the gene being studied is thought to be, to a certain degree, similar in both humans and rodents. It is important to recognize, however, that although the proteins encoded by the genes of human and rodents are highly homologous, the regulation of gene transcription by transcription factors or epigenetics, or the regulation of gene translation by microRNAs, can vary extensively between humans and rodents. Second, the gene–behavior relationships of some behaviors may be conserved only to a certain extent between the human and animal genomes. Clearly, human brains are far more complex than those of rodents, and rodents should not be viewed as miniature humans.20
We now turn our focus to research approaches that use model animals, as well as findings from these studies.
Mania
Mania can affect mood, thinking (processing and content) and behavior. Although many genes implicated in BPD are certainly being studied (see Craddock and Sklar21 and Kato22 for a review), some genes have been more comprehensively evaluated in behavioral experiments and seem to be most convincing in terms of their ability to encompass the multiple behavioral aspects of mania; for research purposes, these behavioral aspects are collectively referred to as excessive behavioral excitement.11 Any working definition for phenocopies of excessive behavioral excitement should include the following: the animal shows hyperactivity in multiple tests, increased goal-directed behavior, increased risk-taking activity, distractibility and reduced sleep or rest; furthermore, these deficits as a cluster can be experimentally distinguished from locomotor unrest, which is another behavioral abnormality, and cannot be alleviated by treatment with psychostimulants. Table 1 lists some of the tests proposed for monitoring these behaviors. The most promising genes believed to be associated with excessive behavioral excitement include: glycogen synthase kinase 3 (GSK-3), CLOCK, glutamate receptor 6 (GluR6 or GRIK2, one of the kainate receptors) and extracellular signal-regulated kinase-1 (ERK1, also known as MAP kinase-3).
Ample evidence implicates all of these genes in the pathophysiology of mood disorders. For instance, polymorphisms of GSK-3β have been associated with age of onset in BPD,23 as well as with therapeutic response to lithium treatment or sleep deprivation.24,25 Some studies suggest that polymorphisms of CLOCK and other components (ARNTL (BMAL1), BHLHB2, CSNK1E and PER3) of the molecular clock are associated with BPD risk,26,27 recurrence28 and age of onset.29 GluR6 (or GRIK2) resides on chromosome 6q21, which has been linked to BPD in several studies;30–34 however, whether GRIK2 polymorphisms per se contribute to the genetic risk of BPD in general or in some BPD pedigrees requires further investigation. It is interesting to note that mRNA levels of GluR6 were found to be significantly lower in the brain tissue of individuals with BPD.35,36 Finally, ERK1 and the ERK pathway are activated by treatment with mood stabilizers,37–40 and single gene and genome-wide association studies show significant and strong associations between BPD risk and polymorphisms of ERK pathway-modulating genes, such as BDNF (brain-derived neurotrophic factor),41 DISC1 (disrupted-in-schizophrenia 1),42,43 RASGRP1 (RAS guanyl releasing protein 1),7 EGFR (epidermal growth factor receptor)44 and GluR6.30–34 It is worth noting that genetic findings are not consistent across all studies, or consistent with findings from genome-wide association studies. Although reasons for such inconsistencies remain a matter of considerable debate, the interplay between risk genes and environmental factors are key issues of consideration.
As Table 1 highlights, behavioral studies used to assess cross-species phenocopies of different facets of excessive behavioral excitement have revealed that in mice, GSK-3β overexpression,45 mutant CLOCK (mCLOCK),46 GluR6 knockout (KO)11 and ERK1 KO12 all resulted in hyperactivity in the open field or in the locomotor activity test, and reduced immobility in the forced swim or tail suspension tests. mCLOCK, GluR6 KO and ERK1 KO mice also showed increased hedonic and risk-taking activity.11,46,12 In addition, GluR6 KO mice showed increased aggression during home cage observation, the social interaction test and the resident–intruder test, as well as changes in rest/activity pattern in their home cages.11
However, the interpretation of such behavioral data is complicated. For instance, although it is a featured abnormality of manic behavioral disturbances, hyperactivity per se is not specific to mania, and can also be observed in models of several other disorders, most notably attention deficit/hyperactivity disorder; it should also be noted that psychostimulants induce manic episodes in euthymic BPD patients47 and relieve symptoms in attention deficit/hyperactivity disorder patients.48 Consistent with the effects of psychostimulants in patients with BPD, psychostimulants aggravate, but do not relieve, hyperactivity in GluR6 KO, ERK1 KO and mCLOCK mice.
In addition, discussion and interpretation of these results have been aided by studies that clarify the ability of mood stabilizers to relieve these symptoms. For instance, in humans, lithium can reverse many of these manic symptoms, but there are no sufficient or convincing clinical data to support the notion that lithium therapy is effective in treating attention deficit/hyperactivity disorder. Furthermore, most behavioral abnormalities observed in mCLOCK and GluR6 KO mice respond to chronic treatment with lithium.11,46,12 The amphetamine supersensitivity noted in ERK1 KO mice also responded to chronic treatment with either lithium or valproate, as well as acute treatment with olanzapine; in addition, treatment with either chronic valproate or acute olanzapine reduced the baseline activity of ERK1 KO mice. Notably, lithium treatment did not significantly reduce the baseline activity of ERK1 KO mice, suggesting that ablation of ERK1—which is a target of mood stabilizers—compromised the behavioral effects of lithium. These treatment data also support the link between the phenocopies showed by these mice and a manic behavioral syndrome. The behavioral effects of mood stabilizers on behavioral disturbances in GSK-3β overexpressing mice have not yet been reported. It is important to note that even if behavioral phenocopies of model animals do not respond to treatment with existing mood stabilizers, the data can still support (though less conclusively) the role of implicated or suspected changes in affective regulation in BPD; this is related to the fact that existing mood stabilizers are not effective for all BPD patients. Indeed, such an outcome may yield clues as to the particular mechanisms of action of these physiological changes.
Another related issue is the extent to which hyperactivity showed in the open field test confounds results from tests for other behaviors, including risk-taking behaviors, aggression and escaping activity (in the forced swim or tail suspension tests). It is notable that behavioral clusters or activity patterns assessed through multiple tests seem relatively gene specific (Table 4). For instance, mCLOCK, GluR6 KO and ERK1 KO mice show similar patterns of behaviors—for example, hyperactivity in the open field test, less immobility in the forced swim test and more risk-taking or less anxious-like behaviors in anxiety-related tests. In contrast, deletion of the NMDAR2D (N-methyl-d-aspartate (NMDA) receptor type 2D) in rodents reduced total activity in the open field test, reduced immobility in the forced swim test and increased risk-taking behavior in the elevated plus maze.49 Deletion of δ-opioid receptors did not affect open field activity, but did increase activity in the open arms during the elevated plus test and reduced immobility in the forced swim test.50,51 Deletion of δ-opioid receptors increased open field activity in the open field test and increased anxious-like behaviors.51 Deletion of the γ-aminobutyric acid (GABA) B(1) and B(2) receptors did not change overall activity in the open field test, but did induce anxiety-like behaviors and reduced immobility.52,53 As Table 4 illustrates, the behavioral patterns showed in the open field, anxiety-related and immobility tests differ between strains of mice harboring different genetic alterations. Therefore, a cluster of behaviors highlighting a pattern of increased activity in the open field arena, increased activity in the open arms of the elevated plus maze and reduced immobility is likely to be specific for manipulating certain genes or pathways.
Table 4.
Genes and behavioral patterns
| Genes | Mutant stains | Open field test | Immobility tests | Anxiety-like/risk-taking tests |
|---|---|---|---|---|
| CLOCK | mCLOCK | Hyperactive | Less immobile | More risk-taking-like |
| GRIK2 (also known as GluR6) | GluR6−/− | Hyperactive | Less immobile | More risk-taking-like |
| MAP3 (also known as ERK1) | ERK1−/− | Hyperactive | Less immobile | More risk-taking-like |
| GRIN2D (also known as NMDAR2D or GluRe4) | GluRe4−/− | Hypoactive | Less immobile | More risk-taking-like |
| OPRM1 | mu-opioid receptor KO | No change | Less immobile | More risk-taking-like |
| OPRD1 | Oprd−/− | Hyperactive | More immobile | More anxious-like |
| GABBR1 | GABAB(1)−/− | Bell-shaped activity display | Less immobile | More anxious-like |
| GABBR2 | GABAB(2)−/− | No data | Less immobile | More anxious-like |
Abbreviations: GABBR1, γ-aminobutyric acid (GABA) B receptor, 1; GABBR2, GABA B receptor, 2; GRIK2, glutamate receptor, ionotropic, kainate 2; GRIN2D glutamate receptor, ionotropic, N-methyl D-aspartate 2D; MAP3, mitogen-activated protein kinase 3; OPRD1, opioid receptor, δ-1; OPRM1, opioid receptor, μ-1.
Bipolar depression
Depression affects mood, thinking and physiological and social–psychological functioning. The diagnosis of depression requires one of two core symptoms, that is, either depressed mood or anhedonia. Because mood cannot be directly assessed in rodents, anhedonia-like behaviors have become critical in evaluating behavioral clusters of cross-species phenocopies of depressive symptoms (see Table 2) to link the behavioral clusters being studied with clinical depression. Behavioral clusters without such a link to the core symptoms of depression provide evidence that is much more circumstantial.
Table 2.
Clinical features of depression and rodent phenocopies of depressive symptoms
| Domains | Clinical diagnostic features | Suitable animal tests | Expected outcomes | p11 −/− | Vmat2 −/+ |
|---|---|---|---|---|---|
| Depressed mood | Depressed mood most of the day, nearly every day, as indicated by either subjective report (e.g., feels sad or empty) or observations made by others (e.g., appears tearful) | Unknown | NA | NA | NA |
| In children and adolescents, can be irritable mood | Unknown | NA | NA | NA | |
| Anhedoni | Markedly diminished interest or pleasure in all, or almost all, activities most of the day, nearly every day (as indicated by either subjective account or observations made by others) | Home cage activity scan | Less | No data | No data |
| Sweetened solution preference | Less | Yes | Yes | ||
| FUST | Decreased | No data | No data | ||
| Wheel running | Decreased | No data | No data | ||
| Weight or appetite change | Significant weight loss when not dieting or weight gain (e.g., a change of > 5% of body weight in a month), or decrease or increase in appetite nearly every day. Note: In children, consider failure to make expected weight gains | Weight monitoring | Varied | No data | No data |
| Eating and drinking monitoring | Varied | No data | No data | ||
| Sleep disturbances | Insomnia or hypersomnia nearly every day | Home cage activity scan | Pattern change | No data | No data |
| Psychomotor activity change | Psychomotor agitation or retardation nearly every day (observable by others, not merely subjective feelings of restlessness or being slowed down) | Home cage activity scan | Slow/pattern change | No data | No data |
| Open field test | Slow/pattern change | Yes | Yes | ||
| Lost physical strength | Fatigue or loss of energy nearly every day | Home cage activity scan | Less | No data | No data |
| Treadmill | Less | No data | No data | ||
| Open field test | Less | Yes | Yes | ||
| Forced swim or tail suspension tests | More immobility | Yes | Yes | ||
| Low self esteem | Feelings of worthlessness or excessive or inappropriate guilt (which may be delusional) nearly every day (not merely self-reproach or guilt about being sick) | Unknown | NA | NA | NA |
| Cognitive disturbances | Diminished ability to think or concentrate, or indecisiveness, nearly every day (either by subjective account or as observed by others) | Unknown | NA | NA | NA |
| Hopelessness | Recurrent thoughts of death (not just fear of dying), recurrent suicidal ideation without a specific plan, or a suicide attempt or a specific plan for committing suicide | Unknown | NA | NA | NA |
| Exclusions | The symptoms are not due to the direct physiological effects of a substance (e.g., a drug of abuse, a medication) or a general medical condition (e.g., hypothyroidism) | Biological tests | No marked change | Yes | Yes |
| General health checklist | |||||
| Treatment | Lithium, lamotrigine | Treatment | Effective | No data | No data |
| Antidepressants | Treatment | Effective | Yes | Yes | |
| Ketamine | Treatment | Effective | No data | No data |
Abbreviations: FUST, female urine sniffing test; NA, not applicable; Vmat2, vesicular monoamine transporter 2.
In humans, anhedonia is loosely defined as an impaired capacity to experience or anticipate pleasure, and in rodents tests that assess anhedonia-like behaviors traditionally focus on a particular reward-seeking activity assessed in a given context; for example, sweet solution consumption or preference, wheel running, female urine sniffing or lever pressing or wheel running in the intracranial self-stimulation paradigm. Home cage and other activities (for example, novel object exploration or social interaction) can also provide useful information. Theoretically and empirically speaking, the outcomes of these tests or observations (Table 2) can be confounded by factors that by themselves do not constitute depression, such as locomotive dysfunction, lack of explorative curiosity or neophobia, specific sensory alterations, low sexual drive and loss of appetite. For all of these, one operational definition of anhedonia-like behaviors in rodents is that experimental animals show concurrent reductions in multiple activities known to be preferred by suitable control animals, and these reductions cannot be explained by any single cause alone. This definition means that anhedonia-like behaviors should be evaluated as a behavioral cluster or pattern assessed through multiple tests or tasks, but not as one type of activity in any given context. Anhedonia and other symptoms of depression can be observed in other neurological and psychiatric disorders, including schizophrenia, parkinsonism and dementia. Therefore, additional tests should be considered to examine whether genes associated with anhedonia-like behaviors/depression-like alterations are depression specific. Furthermore, the effects on such anhedonia-like behaviors of therapeutic agents used to treat various illnesses are also useful in evaluating this issue.
The diagnostic criteria for a depressive episode are the same for both BPD and major depressive disorder. Therefore, diagnosing bipolar depression requires both the occurrence of a current depressive episode as well as knowledge of an individual’s previous course of illness, for example, whether mania or hypomania had previously occurred. Bipolar depression can be treated with mood stabilizers such as lithium and valproate and, as suggested by a recent independent meta-analysis and meta-regression of individual patient data from five randomized trials, lamotrigine, although the overall effect of lamotrigine is modest.54 Treating bipolar depression with antidepressants alone can cause dysphoric symptoms, and even induce switch to a manic episode,55 and there is no convincing evidence suggesting that a combination of mood stabilizers and antidepressants is beneficial in treating bipolar depression. Very recent data suggest that the N-methyl-d-aspartate antagonist ketamine, which is added on to mood stabilizers, may rapidly relieve depressive symptoms in patients with bipolar depression.56 Although suitable tests exist for monitoring animal behaviors that cross-species phenocopy behavioral facets of depressive symptoms (Table 2), further refinement and validation of these tests are needed. In addition to the behavioral tests listed in Table 2, physiological and biological methods, such as electroencephalogram, can be used to monitor sleep disturbances.
It is important to note that some rodent behavioral displays, such as motivational deficits, ‘behavioral despair’, ‘helplessness’, submissiveness and anxiety-like abnormalities, are thought to be linked to clinical alterations frequently observed in depression, despite the fact that not all are diagnostic symptoms. Several animal models target these behaviors and are sensitive to chronic or repeated treatment with antidepressants, including the learned helplessness paradigm, the forced swim and tail suspension tests, the social defeat paradigm and the novelty-induced hypophagia test.13 In addition, some investigators have suggested that the behavioral effects of chronic treatment with antidepressants in the novelty-induced hypophagia test reflect the anxiolytic effects of chronic treatment with these agents observed in humans.57 Notably, much of the data used to assess these paradigms were generated from models designed to shed light on major depressive disorder or depressive disorders in general rather than bipolar depression in particular. However, conclusive data are lacking regarding whether or not the learned helplessness paradigm, the social defeat paradigm and the novelty-induced hypophagia test are sensitive to mood stabilizers and to lamotrigine administered in clinically relevant regimens.
Many candidate genes are being studied in antidepressant-sensitive behavioral paradigms or animal models. The influence of some genes (such as brain-derived neurotrophic factor and cAMP response element binding (CREB)) on behavior is brain region specific.58,59 For instance, blocking brain-derived neurotrophic factor and CREB in the hippocampus attenuated the effects of antidepressants, whereas in the ventral tegmental area, amygdala or nucleus accumbens, the blockage itself produced antidepressant-like effects or potentiated the effects of antidepressants. It remains unknown whether or not functional changes of these genes cause anhedonia-like behaviors.
The model animal approach is also used in translational studies analyzing the relationship between suspected genes and phenocopies of depressive symptoms. Functional manipulation of p11 was recently found to result in a phenotype conferring anhedonic-like behaviors and behavioral despair. Also known as S100A10, p11 is a member of the S100 protein family that mediates cell surface localization of the 5-HT1B receptor and several ion channels.60 p11 levels were lower in the brain tissues of depressed patients and of helpless animals, and higher in the brain tissues of animals treated with antidepressants or electroconvulsive therapy. p11 KO mice showed increased anxiety-like measures (thigmotaxis) in the open field test, and immobility in the tail suspension test. The mice also consumed less palatable 2% sucrose solution than their wild-type littermates. Thus, the mice showed a behavioral phenotype of depressive symptoms with two critical features: anhedonia-like behaviors and behavioral despair. It is presently unknown whether these features of p11 KO mice respond to treatment with various mood stabilizers, and it remains to be elucidated whether p11 alterations are involved in bipolar depression or, indeed, whether bipolar and unipolar depressive episodes share a common pathophysiological pathway.
Another promising finding was obtained from the study of vesicular monoamine transporter 2 (VMAT2) heterozygous (HET) KO mice. In brief, treatment with reserpine, which is an irreversible inhibitor of VMAT that transports cytoplasmic monoamine into secretory vesicles,61 depletes vesicular monoamine stores and precipitates depression in susceptible individuals. A specific haplotype of VMAT2 (also known as SLC18A2) was significantly associated with depressive symptoms in elderly men.62 VMAT2 HET KO mice were hypoactive in the open field test, showed less preference to sweetened but not quinine solutions and showed increased immobility in the forced swim and tail suspension tests.61 Treatment with antidepressants reduced the immobility of VMAT2 HET KO mice. However, the effects of antidepressants on motor retardation/lack of explorative behavior, anhedonia and increased immobility remain unknown, as do the behavioral effects of mood stabilizers and lamotrigine in VMAT2 HET KO mice. Direct evidence for the involvement of VMAT2 in the pathophysiology of bipolar depression is still lacking.
Vulnerability to BPD
Patients with BPD remitted from a manic or depressive episode are sometimes considered to be in a euthymic state in which they are vulnerable to relapse2 (Figure 1). Relapses can be triggered by physical stressors (such as lack of sleep or jetlag), social–psychological stressors,2 experimental use of psychostimulants47 and experimentally induced monoamine depletion63 (Figure 1). Interestingly, experimentally induced monoamine depletion causes some mood changes in healthy controls, but a full-blown depression in only individuals with a personal or family history of mood disorders.63 These unique clinical responses can be collectively considered as an affective vulnerability.
Several animal paradigms can be used to study the roles of candidate genes in affective vulnerability in animals13–16 (Table 3). These paradigms contain components of behavior induction and outcome observation. It should be noted that these paradigms encompass previously proposed animal models of depression or mania;64 however, their focus under the model animal approach is to analyze the interaction between implicated or suspected biological changes in BPD in tandem with behavioral inducers (such as foot shocks or psychostimulants) on outcome measures. Under such a design, the effects of these factors alone on outcome measures are not the focus of the investigation. Some of the most promising candidates include glucocorticoid receptors (GRs), and B-cell lymphoma 2 (Bcl-2)-associated athanogene (BAG1).
Considerable clinical data have shown that glucocorticoids are one of the few agents that are capable of triggering both depressive and manic episodes in individuals with BPD.2 Furthermore, alternative GR expression variants have been implicated in major depressive disorder by two independent groups of studies.65,66 Upbinding to glucocorticoids, GRs translocate from cytoplasm to nuclei and then regulate gene expression.67 This trafficking process is regulated by chaperones and co-chaperones including FKBP5 (FK506 binding protein 5, also known as FKBP51).67 Some, but not all, genetic studies show that variants of FKBP5 are associated with increased recurrence of depression,68 response to antidepressants,68–70 increased risk for BPD71 and symptoms of posttraumatic stress disorder in adults with a history of childhood abuse.72 These clinical data support the role of GRs in mood regulation.
GRov mice, a strain in which overexpression of GRs in neurons was achieved through use of a neuron-specific promoter,73 showed anxious-like behaviors in the elevated plus maze and light–dark box tests, and increased immobility in the forced swim test, suggesting that the mice were sensitive to stressful environmental conditions. Interestingly, the mice also showed enhanced cocaine-induced behavioral sensitization. Taken together, these results suggest that GRov mice seem to show an altered affective-like lability.
BAG1 is another co-chaperone involved in GR signaling.74 Through interaction with Hsp70/Hsc70, this protein regulates both glucocorticoid binding and gene transactivation of GRs.74 BAG1 is one of the common brain targets of chronic treatment with lithium and valproate,75 both of which upregulate BAG1 mRNA and protein levels and alter BAG1 function, including GR trafficking to nuclei. Mice with selective brain overexpression of BAG1 show less anxious-like behaviors in the elevated plus maze test, accelerated recovery from amphetamine-induced hyperlocomotion, blunted response to cocaine in the psychostimulant-induced behavioral sensitization paradigm and rapid spontaneous recovery from helplessness in the learned helplessness paradigm.76 BAG1 HET KO mice showed enhanced response to cocaine in the psychostimulant-induced behavioral sensitization paradigm and increased tendency to become helpless in the learned helplessness paradigm. 76 These data suggest that BAG1 regulates affective lability and resilience.
Additional considerations
Cognitive deficits
Clinically, to diagnose BPD, symptoms that can be explained by other medical conditions, such as organic brain diseases, must be excluded.2,77 Mild cognitive impairments in working memory and attention are known to be present in at least some patients with BPD, and these phenomena are often observed in schizophrenia. To address whether gene manipulation affects mood regulation as well as broadly influences multiple domains of brain function, predisposed animals can be examined using batteries of tests to assess general health, motor, sensory, learning and memory functioning and other neurological functions. These have been well-described elsewhere.20
Mood swings
Because the process of switching from depression to a state of mania or hypomania is a unique and core feature of BPD, another key issue is that of spontaneous mood swings. Although often triggered by particular stressors or medications, spontaneous mood relapses can occur in BPD without a clear external triggering event.2 If these mechanisms do exist, they must closely interact with, or even be integrated into, the mechanisms that underlie vulnerability. To study candidate genes for internal spontaneous swing mechanisms, long-term home cage activity monitoring of mice predisposed to known BPD risk factors can be helpful.
Accumulating data from both human genetic and post-mortem studies imply that mitochondrial dysfunction contributes to the etiology of BPD (for a review, see Kato22). Interestingly, a mutation (D181A) of mitochondrial DNA polymerase-γ(POLG) does not affect this enzyme’s polymerase activity; instead, it abolishes its 3′–5′ exonuclease activity and causes mitochondrial DNA deletion.78 Transgenic mice with brain-specific expression of mutant POLG accumulate brain mitochondrial DNA abnormalities similar to those found in the post-mortem brain of individuals with BPD.78 Although the mutant POLG mice seem normal on a variety of short-term tests, long-term home cage monitoring revealed that these mice have a unique activity pattern. Namely, they show altered activity immediately before and after the dark phase (which is the active phase for rodents) of the circadian cycle.78 This alteration can be further deteriorated by treatment with a tricyclic antidepressant known to induce mood switches in individuals with BPD. Female mutant POLG mice also show robust activity swings concurrent with the estrous cycle that can be stabilized by treatment with lithium. Taken together, the findings support the notion that mitochondrial dysfunction influences diurnal activity rhythms and the magnitude of rhythmic activity. Whether and how mitochondrial dysfunction drives these abnormal mood swings requires further investigation.
Closing remarks
This is an unprecedented time in BPD research. Owing to significant advances in human genetics, the identity of true susceptibility genes is starting to emerge. The influence of genetic risk variants on gene and gene network function continues to be extensively analyzed at the molecular, neural circuitry and behavioral levels. Although all animal approaches have well-known limitations in their ability to mirror human conditions, there is no doubt that animal behavioral research in general—and the model animal approach in particular—will have an irreplaceable role in analyzing the causal relationship between biological abnormalities resulting from genetic BPD risk variants, early-life environmental factors and behavioral manifestations of BPD. At the same time, a significant need exists to form a consensus regarding how to phenotype animals in mood disorders research, and how to improve the ability of behavioral research to monitor these alterations and the manner in which they relate to mood disorders. The reverse translation model animal approach outlined in this review can and should be viewed as one of many research avenues in overall BPD research. Future research advances in both biological information and behavioral methodology will be essential for the rapid development of true novel therapies for rapidly relieving mood symptoms, preventing mood relapses and halting the progression of this devastating disorder.
Acknowledgments
We gratefully acknowledge the support of the Intramural Research Program of the National Institute of Mental Health.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Belmaker RH. Bipolar disorder. N Engl J Med. 2004;351:476–486. doi: 10.1056/NEJMra035354. [DOI] [PubMed] [Google Scholar]
- 2.Goodwin FK, Jamison KR. Manic-Depressive Illness: Bipolar and Recurrent Unipolar Disorders. 2nd edn. New York: Oxford University Press; 2007. [Google Scholar]
- 3.Baum AE, Akula N, Cabanero M, Cardona I, Corona W, Klemens B, et al. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol Psychiatry. 2008;13:197, 207. doi: 10.1038/sj.mp.4002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato T. Molecular genetics of bipolar disorder and depression. Psychiatry Clin Neurosci. 2007;61:3–19. doi: 10.1111/j.1440-1819.2007.01604.x. [DOI] [PubMed] [Google Scholar]
- 5.Wellcome Trust Case Control Consortium. Genome-wide association study of 14 000 cases of seven common diseases and 3000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Post RM, Leverich GS. The role of psychosocial stress in the onset and progression of bipolar disorder and its comorbidities: the need for earlier and alternative modes of therapeutic intervention. Dev Psychopathol. 2006;18:1181–1211. doi: 10.1017/S0954579406060573. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira MA, O’Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L, et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008;40:1056–1058. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Insel TR. From animal models to model animals. Biol Psychiatry. 2007;62:1337–1339. doi: 10.1016/j.biopsych.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 10.Nestler EJ, Gould E, Manji H, Buncan M, Duman RS, Greshenfeld HK, et al. Preclinical models: status of basic research in depression. Biol Psychiatry. 2002;52:503–528. doi: 10.1016/s0006-3223(02)01405-1. [DOI] [PubMed] [Google Scholar]
- 11.Shaltiel G, Maeng S, Malkesman O, Pearson B, Schloesser RJ, Tragon T, et al. Evidence for the involvement of the kainate receptor subunit GluR6 (GRIK2) in mediating behavioral displays related to behavioral symptoms of mania. Mol Psychiatry. 2008;13:858–872. doi: 10.1038/mp.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engel SR, Creson TK, Hao Y, Shen Y, Maeng S, Nekrasova T, et al. The extracellular signal-regulated kinase pathway contributes to the control of behavioral excitement. Mol Psychiatry. 2009;14:448–461. doi: 10.1038/sj.mp.4002135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- 14.Dranovsky A, Hen R. Hippocampal neurogenesis: regulation by stress and antidepressants. Biol Psychiatry. 2006;59:1136–1143. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Donnell KC, Gould TD. The behavioral actions of lithium in rodent models: leads to develop novel therapeutics. Neurosci Biobehav Rev. 2007;31:932–962. doi: 10.1016/j.neubiorev.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Einat H. Different behaviors and different strains: potential new ways to model bipolar disorder. Neurosci Biobehav Rev. 2007;31:850–857. doi: 10.1016/j.neubiorev.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Bourin M, Masse F, Hascoet M. Evidence for the activity of lamotrigine at 5-HT(1A) receptors in the mouse forced swimming test. J Psychiatry Neurosci. 2005;30:275–282. [PMC free article] [PubMed] [Google Scholar]
- 18.Consoni FT, Vital MA, Andreatini R. Dual monoamine modulation for the antidepressant-like effect of lamotrigine in the modified forced swimming test. Eur Neuropsychopharmacol. 2006;16:451–458. doi: 10.1016/j.euroneuro.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Gould TD, O’Donnell KC, Dow ER, Du J, Chen G, Manji HK. Involvement of AMPA receptors in the antidepressant-like effects of lithium in the mouse tail suspension test and forced swim test. Neuropharmacology. 2008;54:577–587. doi: 10.1016/j.neuropharm.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crawley JN. Behavioral phenotyping strategies for mutant mice. Neuron. 2008;57:809–818. doi: 10.1016/j.neuron.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Craddock N, Sklar P. Genetics of bipolar disorder: successful start to a long journey. Trends Genet. 2009;25:99–105. doi: 10.1016/j.tig.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Kato T. Role of mitochondrial DNA in calcium signaling abnormality in bipolar disorder. Cell Calcium. 2008;44:92–102. doi: 10.1016/j.ceca.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Benedetti F, Bernasconi A, Lorenzi C, Pontiggia A, Serretti A, Colombo C, et al. A single nucleotide polymorphism in glycogen synthase kinase 3-beta promoter gene influences onset of illness in patients affected by bipolar disorder. Neurosci Lett. 2004;355:37–40. doi: 10.1016/j.neulet.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 24.Benedetti F, Serretti A, Colombo C, Lorenzi C, Tubazio V, Smeraldi E. A glycogen synthase kinase 3-beta promoter gene single nucleotide polymorphism is associated with age at onset and response to total sleep deprivation in bipolar depression. Neurosci Lett. 2004;368:123–126. doi: 10.1016/j.neulet.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 25.Benedetti F, Serretti A, Pontiggia A, Bernasconi A, Lorenzi C, Colombo C, et al. Long-term response to lithium salts in bipolar illness is influenced by the glycogen synthase kinase 3-beta −50 T/C SNP. Neurosci Lett. 2005;376:51–55. doi: 10.1016/j.neulet.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 26.Nievergelt CM, Kripke DF, Barrett TB, Burg E, Remick RA, Sadovnick AD, et al. Suggestive evidence for association of the circadian genes PERIOD3 and ARNTL with bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:234–241. doi: 10.1002/ajmg.b.30252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi J, Wittke-Thompson JK, Badner JA, Hattori E, Potash JB, Willour VL, et al. Clock genes may influence bipolar disorder susceptibility and dysfunctional circadian rhythm. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1047–1055. doi: 10.1002/ajmg.b.30714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benedetti F, Serretti A, Colombo C, Barbini B, Lorenzi C, Campori E, et al. Influence of CLOCK gene polymorphism on circadian mood fluctuation and illness recurrence in bipolar depression. Am J Med Genet B Neuropsychiatr Genet. 2003;123B:23–26. doi: 10.1002/ajmg.b.20038. [DOI] [PubMed] [Google Scholar]
- 29.Benedetti F, Dallaspezia S, Colombo C, Pirovano A, Marino E, Smeraldi E. A length polymorphism in the circadian clock gene Per3 influences age at onset of bipolar disorder. Neurosci Lett. 2008;445:184–187. doi: 10.1016/j.neulet.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Cichon S, Schumacher J, Muller DJ, Hurter M, Windemuth C, Strauch K, et al. A genome screen for genes predisposing to bipolar affective disorder detects a new susceptibility locus on 8q. Hum Mol Genet. 2001;10:2933–2944. doi: 10.1093/hmg/10.25.2933. [DOI] [PubMed] [Google Scholar]
- 31.Dick DM, Foroud T, Flury L, Bowman ES, Miller MJ, Rau NL, et al. Genomewide linkage analyses of bipolar disorder: a new sample of 250 pedigrees from the National Institute of Mental Health Genetics Initiative. Am J Hum Genet. 2003;73:107–114. doi: 10.1086/376562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McQueen MB, Devlin B, Faraone SV, Nimgaonkar VL, Sklar P, Smoller JW, et al. Combined analysis from eleven linkage studies of bipolar disorder provides strong evidence of susceptibility loci on chromosomes 6q and 8q. Am J Hum Genet. 2005;77:582–595. doi: 10.1086/491603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park N, Juo SH, Cheng R, Liu J, Loth JE, Lilliston B, et al. Linkage analysis of psychosis in bipolar pedigrees suggests novel putative loci for bipolar disorder and shared susceptibility with schizophrenia. Mol Psychiatry. 2004;9:1091–1099. doi: 10.1038/sj.mp.4001541. [DOI] [PubMed] [Google Scholar]
- 34.Schumacher J, Kaneva R, Jamra RA, Diaz GO, Ohlraun S, Milanova V, et al. Genomewide scan and fine-mapping linkage studies in four European samples with bipolar affective disorder suggest a new susceptibility locus on chromosome 1p35-p36 and provides further evidence of loci on chromosome 4q31 and 6q24. Am J Hum Genet. 2005;77:1102–1111. doi: 10.1086/498619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc Natl Acad Sci USA. 2007;104:10164–10169. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology. 2007;32:1888–1902. doi: 10.1038/sj.npp.1301312. [DOI] [PubMed] [Google Scholar]
- 37.Yuan PX, Huang LD, Jiang YM, Gutkind JS, Manji HK, Chen G. The mood stabilizer valproic acid activates mitogen-activated protein kinases and promotes neurite growth. J Biol Chem. 2001;276:31674–31683. doi: 10.1074/jbc.M104309200. [DOI] [PubMed] [Google Scholar]
- 38.Einat H, Yuan P, Gould TD, Li J, Du J, Zhang L, et al. The role of the extracellular signal-regulated kinase signaling pathway in mood modulation. J Neurosci. 2003;23:7311–7316. doi: 10.1523/JNEUROSCI.23-19-07311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hao Y, Creson T, Zhang L, Li P, Du F, Yuan P, et al. Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. J Neurosci. 2004;24:6590–6599. doi: 10.1523/JNEUROSCI.5747-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Creson TK, Yuan P, Manji HK, Chen G. Evidence for involvement of ERK, PI3K, and RSK in induction of Bcl-2 by valproate. J Mol Neurosci. 2009;37:123–134. doi: 10.1007/s12031-008-9122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan J, Sklar P. Genetics of bipolar disorder: focus on BDNF Val66Met polymorphism. Novartis Found Symp. 2008;289:60–72. doi: 10.1002/9780470751251.ch5. discussion 72–73, 87–93. [DOI] [PubMed] [Google Scholar]
- 42.Hashimoto R, Numakawa T, Ohnishi T, Kumamaru E, Yagasaki Y, Ishimoto T, et al. Impact of the DISC1 Ser704Cys polymorphism on risk for major depression, brain morphology and ERK signaling. Hum Mol Genet. 2006;15:3024–3033. doi: 10.1093/hmg/ddl244. [DOI] [PubMed] [Google Scholar]
- 43.Chubb JE, Bradshaw NJ, Soares DC, Porteous DJ, Millar JK. The DISC locus in psychiatric illness. Mol Psychiatry. 2008;13:36–64. doi: 10.1038/sj.mp.4002106. [DOI] [PubMed] [Google Scholar]
- 44.Sklar P, Smoller JW, Fan J, Ferreira MA, Perlis RH, Chambert K, et al. Whole-genome association study of bipolar disorder. Mol Psychiatry. 2008;13:558–569. doi: 10.1038/sj.mp.4002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prickaerts J, Moechars D, Cryns K, Lenaerts I, van Craenendonck H, Goris I, et al. Transgenic mice overexpressing glycogen synthase kinase 3beta: a putative model of hyperactivity and mania. J Neurosci. 2006;26:9022–9029. doi: 10.1523/JNEUROSCI.5216-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci USA. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anand A, Verhoeff P, Seneca N, Zoghbi SS, Seibyl JP, Charney DS, et al. Brain SPECT imaging of amphetamine-induced dopamine release in euthymic bipolar disorder patients. Am J Psychiatry. 2000;157:1108–1114. doi: 10.1176/appi.ajp.157.7.1108. [DOI] [PubMed] [Google Scholar]
- 48.Trinh JV, Nehrenberg DL, Jacobsen JP, Caron MG, Wetsel WC. Differential psychostimulant-induced activation of neural circuits in dopamine transporter knockout and wild type mice. Neuroscience. 2003;118:297–310. doi: 10.1016/s0306-4522(03)00165-9. [DOI] [PubMed] [Google Scholar]
- 49.Miyamoto Y, Yamada K, Noda Y, Mori H, Mishina M, Nabeshima T. Lower sensitivity to stress and altered monoaminergic neuronal function in mice lacking the NMDA receptor epsilon 4 subunit. J Neurosci. 2002;22:2335–2342. doi: 10.1523/JNEUROSCI.22-06-02335.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoo JH, Lee SY, Loh HH, Ho IK, Jang CG. Altered emotional behaviors and the expression of 5-HT1A and M1 muscarinic receptors in micro-opioid receptor knockout mice. Synapse. 2004;54:72–82. doi: 10.1002/syn.20067. [DOI] [PubMed] [Google Scholar]
- 51.Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, et al. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- 52.Mombereau C, Kaupmann K, Froestl W, Sansig G, van der Putten H, Cryan JF. Genetic and pharmacological evidence of a role for GABA(B) receptors in the modulation of anxiety- and antidepressant-like behavior. Neuropsychopharmacology. 2004;29:1050–1062. doi: 10.1038/sj.npp.1300413. [DOI] [PubMed] [Google Scholar]
- 53.Mombereau C, Kaupmann K, Gassmann M, Bettler B, van der Putten H, Cryan JF. Altered anxiety and depression-related behaviour in mice lacking GABAB(2) receptor subunits. Neuro-Report. 2005;16:307–310. doi: 10.1097/00001756-200502280-00021. [DOI] [PubMed] [Google Scholar]
- 54.Geddes JR, Calabrese JR, Goodwin GM. Lamotrigine for treatment of bipolar depression: independent meta-analysis and meta-regression of individual patient data from five randomised trials. Br J Psychiatry. 2009;194:4–9. doi: 10.1192/bjp.bp.107.048504. [DOI] [PubMed] [Google Scholar]
- 55.El-Mallakh RS, Ghaemi SN, Sagduyu K, Thase ME, Wisniewski SR, Nierenberg AA, et al. Antidepressant-associated chronic irritable dysphoria (ACID) in STEP-BD patients. J Affect Disord. 2008;111:372–377. doi: 10.1016/j.jad.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 56.Zarate CA. ACNP. Hollywood, FL: 2009. Dec 6–10, Ketamine in mood disorders: translational research from the clinic to the bench and back again. [Google Scholar]
- 57.Bodnoff SR, Suranyi-Cadotte B, Aitken DH, Quirion R, Meaney MJ. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology. 1988;95:298–302. doi: 10.1007/BF00181937. [DOI] [PubMed] [Google Scholar]
- 58.Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 59.Wang JW, Dranovsky A, Hen R. The when and where of BDNF and the antidepressant response. Biol Psychiatry. 2008;63:640–641. doi: 10.1016/j.biopsych.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 60.Svenningsson P, Chergui K, Rachleff I, Flajolet M, Zhang X, El Yacoubi M, et al. Alterations in 5-HT1B receptor function by p11 in depression-like states. Science. 2006;311:77–80. doi: 10.1126/science.1117571. [DOI] [PubMed] [Google Scholar]
- 61.Fukui M, Rodriguiz RM, Zhou J, Jiang SX, Phillips LE, Caron MG, et al. Vmat2 heterozygous mutant mice display a depressive-like phenotype. J Neurosci. 2007;27:10520–10529. doi: 10.1523/JNEUROSCI.4388-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Christiansen L, Tan Q, Iachina M, Bathum L, Kruse TA, McGue M, et al. Candidate gene polymorphisms in the serotonergic pathway: influence on depression symptomatology in an elderly population. Biol Psychiatry. 2007;61:223–230. doi: 10.1016/j.biopsych.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 63.Ruhe HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry. 2007;12:331–359. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- 64.Cryan JF, Slattery DA. Animal models of mood disorders: recent developments. Curr Opin Psychiatry. 2007;20:1–7. doi: 10.1097/YCO.0b013e3280117733. [DOI] [PubMed] [Google Scholar]
- 65.van Rossum EF, Binder EB, Majer M, Koper JW, Ising M, Modell S, et al. Polymorphisms of the glucocorticoid receptor gene and major depression. Biol Psychiatry. 2006;59:681–688. doi: 10.1016/j.biopsych.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 66.van West D, Van Den Eede F, Del-Favero J, Souery D, Norrback KF, Van Duijn C, et al. Glucocorticoid receptor gene-based SNP analysis in patients with recurrent major depression. Neuropsychopharmacology. 2006;31:620–627. doi: 10.1038/sj.npp.1300898. [DOI] [PubMed] [Google Scholar]
- 67.Grad I, Picard D. The glucocorticoid responses are shaped by molecular chaperones. Mol Cell Endocrinol. 2007;275:2–12. doi: 10.1016/j.mce.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 68.Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Putz B, et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- 69.Lekman M, Laje G, Charney D, Rush AJ, Wilson AF, Sorant AJ, et al. The FKBP5-gene in depression and treatment response–an association study in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) Cohort. Biol Psychiatry. 2008;63:1103–1110. doi: 10.1016/j.biopsych.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsai SJ, Hong CJ, Chen TJ, Yu YW. Lack of supporting evidence for a genetic association of the FKBP5 polymorphism and response to antidepressant treatment. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:1097–1098. doi: 10.1002/ajmg.b.30246. [DOI] [PubMed] [Google Scholar]
- 71.Willour VL, Chen H, Toolan J, Belmonte P, Cutler DJ, Goes FS, et al. Family-based association of FKBP5 in bipolar disorder. Mol Psychiatry. 2009;14:261–268. doi: 10.1038/sj.mp.4002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wei Q, Lu XY, Liu L, Schafer G, Shieh KR, Burke S, et al. Glucocorticoid receptor overexpression in forebrain: a mouse model of increased emotional lability. Proc Natl Acad Sci USA. 2004;101:11851–11856. doi: 10.1073/pnas.0402208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cato AC, Mink S. BAG-1 family of cochaperones in the modulation of nuclear receptor action. J Steroid Biochem Mol Biol. 2001;78:379–388. doi: 10.1016/s0960-0760(01)00114-5. [DOI] [PubMed] [Google Scholar]
- 75.Zhou R, Gray NA, Yuan P, Li X, Chen J, Chen G, et al. The anti-apoptotic, glucocorticoid receptor cochaperone protein BAG-1 is a long-term target for the actions of mood stabilizers. J Neurosci. 2005;25:4493–4502. doi: 10.1523/JNEUROSCI.4530-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maeng S, Hunsberger JG, Pearson B, Yuan P, Wang Y, Wei Y, et al. BAG1 plays a critical role in regulating recovery from both manic-like and depression-like behavioral impairments. Proc Natl Acad Sci USA. 2008;105:8766–8771. doi: 10.1073/pnas.0803736105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Diagnostic and Statistical Manual of Mental Disorders. 4th edn. Washington DC: American Psychiatric Publishing, Inc; 1997. American Psychiatric Association’s Committee on Psychiatric Diagnosis and Assessment. [Google Scholar]
- 78.Kasahara T, Kubota M, Miyauchi T, Noda Y, Mouri A, Nabeshima T, et al. Mice with neuron-specific accumulation of mitochondrial DNA mutations show mood disorder-like phenotypes. Mol Psychiatry. 2006;11:577–593. 523. doi: 10.1038/sj.mp.4001824. [DOI] [PubMed] [Google Scholar]