Abstract
Theoretical models of addiction suggest that attentional bias for substance-related cues should be associated with self-reported craving. The authors evaluated the strength of the association by performing a meta-analysis on 68 independent data sets from which correlation coefficients between subjective craving and attentional bias indices were derived. Additional stratified analyses were conducted to identify any variables that might moderate the association between craving and attentional bias. The primary meta-analysis indicated a significant, albeit weak (r = .19), association between attentional bias and craving. Stratified analyses revealed that the association was larger for illicit drug and caffeine craving than for alcohol and tobacco craving, larger for direct measures of attention (eye movement measures and event-related potential measures) than for indirect behavioral measures of attentional bias, and larger when craving strength was high than when it was low (all ps < .05). The size of the correlation did not differ among patients in treatment and individuals who were not seeking treatment. These results suggest that attentional bias and craving are related phenomena, although the relationship is generally modest and appears to be moderated by various factors. Theoretical implications are discussed.
Keywords: attentional bias, craving, meta-analysis
In the context of substance abuse, “attentional bias” refers to the observation that substance-related cues tend to grab the attention of experienced substance users (see Field & Cox, 2008; Franken, 2003; Robbins & Ehrman, 2004; Rooke, Hine, & Thorsteinnsson, 2008). Theoretical models posit that such attentional biases are clinically meaningful, in that they either cause or index the underlying processes that cause substance-seeking behavior. However, one current source of controversy (see Wiers & Stacy, 2006) concerns the mechanisms by which attentional biases influence substance-seeking behavior. Broadly speaking, some models (e.g., Tiffany, 1990) suggest that substance-related cues are automatically detected and can influence substance-seeking behavior in the absence of conscious experience or awareness; according to these models, these processes are clearly separable from aspects of “explicit” or consciously reportable cognitive processes, such as subjective craving or intentions to use substances. Other models (e.g., Franken, 2003; Kavanagh, Andrade, & May, 2005) suggest that attentional biases are subjectively experienced by substance users and that they interact with other aspects of subjective experience (e.g., craving) in order to influence substance use. These different classes of models are not necessarily incompatible. It is possible that attentional bias leads to substance use through its interactions with conscious experience in some circumstances (e.g., among substance users who are attempting to quit) but that in other circumstances (e.g., among substance users who are not attempting to limit their substance use) any influence of attentional bias on behavior may be relatively automatic.
Our goal in this article is to investigate the nature of the association between subjective craving and attentional bias: If the association between the two variables is robust, this would have important implications for future theoretical developments, specifically for models of substance abuse and subjective craving but also in the broader context of research into emotional states (both appetitive and aversive) and their cognitive correlates. In this article we report the results of meta-analyses in which we examined the relationship between indexes of attentional bias and subjective craving and the factors that appear to influence the correlation between the two variables.
Emotional and Motivational States and Their Behavioral, Cognitive, and Physiological Correlates
Emotional states are associated with highly motivated behaviors (e.g., Frijda, 1986) that are important for survival and propagation (e.g., escape from predators, sexual behavior, the search for and consumption of food). To quote Lang (1995), “Emotions result when novel circumstances prevent completion of cued behavior. Thus, emotions quintessentially occur in a behavioural hiatus, as states ‘experienced’, then reported on and evaluated” (p. 373).
There is a broad consensus that, despite the diversity of emotional experience, emotions reflect the activation of one of two broadly defined motivational states, which can be termed appetitive and aversive (Lang, Bradley, & Cuthbert, 1998; cf. Dickinson & Dearing, 1979; Konorski, 1967). Research into emotional states in humans often begins with the study of (self-reported) subjective feelings, although it has long been recognized that emotional states have correlates in other response domains, such as physiology (e.g., heart rate, skin conductance), behavior (e.g., overt approach and avoidance), and cognition (see Lang, 1994; Lang et al., 1998). For example, Lang and colleagues have demonstrated that, relative to negative emotional states, positive emotional states are associated with increased heart rate, increased zygomatic facial activity (“smiling”), and reduced corrugator facial activity (“frowning”; see Lang et al., 1998). These investigators have also studied modulation of the startle reflex during passive viewing of pleasant photographs (e.g., a smiling baby) and unpleasant photographs (e.g., a mutilated corpse). When a loud and unexpected noise (a startle probe) is presented to human participants, the startle reflex—which can be measured by the magnitude of eye blinks—is engaged. This startle reaction is a primitive defensive reflex that serves to reduce the risk of injury and also acts to interrupt ongoing behavior, thereby increasing the resources available to deal with an imminent threat (Lang et al., 1998). A consistent observation is that presentation of an unpleasant photograph before onset of an auditory startle probe can increase the magnitude of the startle reflex, whereas presentation of a pleasant photograph can reduce the magnitude of the startle reflex (see Lang et al., 1998). Therefore, the experience of a potent emotional state (either positive or negative) is associated with modulation of the startle reflex, in addition to other physiological changes.
Regarding cognitive correlates of emotional states, Lang et al. (1998) noted that highly valenced photographs (i.e., those that elicit a potent positive or negative emotional response) also elicit increased arousal, as indicated by elevated skin conductance and subjective ratings of arousal. Such strongly valenced and highly arousing photographs also seem to influence attentional processes: Participants opt to view them for longer than neutral photographs (Lang et al., 1998). Further evidence of increased attentional processing of strongly valenced photographs comes from studies that use electroencephalographic recording. Such studies demonstrate that the cortical slow waves elicited by strongly valenced photographs are larger than those elicited by neutral photographs (see Lang, Bradley, & Cuthbert, 1997). In the context of passive picture viewing, enhanced slow waves are likely to reflect increased attentional processing of, or updating of working memory in response to, the photograph that is presented (Coull, 1998; Schupp, Flaisch, Stockburger, & Junghofer, 2006).
In summary, positive and negative emotional states are associated with different patterns of physiological reactivity that include changes in heart rate, facial expressions, and modulation of the startle reflex. Furthermore, the emotional response to highly valenced photographs also includes some changes in cognitive processing; photographs that elicit a potent emotional state (either positive or negative) receive more extensive attentional processing than photographs that do not. Other researchers have attempted to clarify the attentional correlates of aversive emotional states. Using various experimental psychology paradigms, many of which are described in detail in a later section of this paper, researchers have established that patients with anxiety disorders tend to preferentially direct their attention toward threat-related stimuli (e.g., MacLeod, Mathews, & Tata, 1986). Attentional bias is seen across the range of anxiety disorders. For example, patients with spider phobia direct their attention toward photographs of spiders (e.g., Lavy & van den Hout, 1993); patients with social phobia have an attentional bias for social threat cues (i.e., threatening facial expressions; e.g., Mogg, Philippot, & Bradley, 2004), whereas patients with generalized anxiety disorder exhibit an attentional bias for general threatening information (Hayes & Hirsch, 2007). However, attentional biases are not limited to patients with anxiety disorders. Within the general population (i.e., those who do not meet diagnostic criteria for any anxiety disorder), there is a clear link between naturally occurring variation in trait anxiety and attentional bias for threat-related cues (e.g., Fox, Russo, Bowles, & Dutton, 2001); for a recent meta-analysis, see Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, and van Ijzdendoorn (2007). Therefore, a large body of evidence indicates that aversive emotional states are associated with biases in cognitive processing and, specifically, with increased attentional processing of threat-related cues.
With regard to appetitive motivational states, individuals who are currently experiencing high levels of hunger exhibit an attentional bias for food-related stimuli (Mogg, Bradley, Hyare, & Lee, 1998), and food-related cues elicit enhanced cortical slow waves among hungry, compared to food-sated, participants (Stockburger, Weike, Hamm, & Schupp, 2008). We conclude this section by noting that two prototypical examples of appetitive and aversive motivational/emotional states—hunger and anxiety, respectively—are associated with distinct patterns of physiological reactivity; most important, both types of motivational state are associated with biases in selective attention for motivationally relevant stimuli.
Craving as Affect: Its Relationship to Behavior, Physiology, and Cognition
When substance users are presented with substance-related cues (e.g., when a lit cigarette is shown to a tobacco smoker), they typically respond with physiological changes reflective of increased arousal, such as elevated skin conductance and heart rate, coupled with increased subjective craving (see meta-analysis by Carter & Tiffany, 1999). Furthermore, a widely held belief among clinicians is that substance-related cues increase the likelihood of substance-seeking behavior or substance consumption, and there is anecdotal clinical evidence (e.g., Marlatt, 1996) as well as experimental evidence (e.g., Perkins, Epstein, Grobe, & Fonte, 1994) for this. Baker, Morse, and Sherman (1987) were among the first researchers to speculate on the nature of subjective substance craving and its physiological and behavioral correlates. As a development of the conceptualization of affect proposed by Lang and colleagues, Baker et al. (1987) suggested that substance craving is a type of affect that reflects a “drug acquisitive motivational state” (p. 258); like other affects, such as anxiety, it has phenomenological (subjectively experienced), behavioral, and physiological components.
In their theoretical model, Baker et al. (1987) proposed two distinct urge networks. The positive affect urge network is a motivational system that mediates pursuit of appetitive rewards; it is most likely to be activated in continuing drug users (i.e., individuals who are not attempting to limit their drug use and who are not currently experiencing withdrawal symptoms), and it can be activated by direct drug effects, positive mood states, the presence of drug-related cues, or information that the drug is available. According to the model, activation of this urge network is reflected in increased subjective craving coupled with positive affect and physiological changes that mirror those produced by the pharmacological effects of the drug itself. By contrast, the negative affect urge network is activated in response to drug withdrawal states, negative moods evoked by any other means (e.g., environmental stressors, interpersonal conflict), and information that the drug is not currently available. Activation of this network is also characterized by elevated craving, but in this instance subjective craving should be associated with negative affect and physiological responses that resemble those seen during drug withdrawal. An important prediction made by the model is that for both positive and negative affect networks, the subjective (e.g., self-reported craving) and physiological indices of network activation should show greater coherence (i.e., a larger positive correlation) as activation of the network increases.
Although some of the predictions made by the model have not been supported by subsequent evidence (e.g., cue-induced physiological changes and subjective craving are not highly correlated; Tiffany, 1990), some supportive evidence has been mustered in its favor. For example, Zinser, Baker, Sherman, and Cannon (1992) demonstrated that among smokers who were undergoing cigarette withdrawal, urge strength was positively correlated with negative affect but not positive affect. However, among smokers who were not undergoing withdrawal the reverse pattern was seen: Urge strength was positively correlated with positive affect but not negative affect. Many of the predictions made by the model have not been thoroughly investigated, but we discuss it here because it was one of the very first theoretical models to operationalize craving as a form of affect that, like other affects, has distinct subjective, physiological, and behavioral components. Although Baker et al. (1987) did not specifically discuss attentional biases as a correlate of subjective craving, they did acknowledge the possibility of such correlation by stating, for example, that activation of the positive affect urge network should be associated with “increased attention to dominant response options” (p. 304), whereas activation of the negative affect urge network should “increase the incentive value of the goal object” (p. 310). Placed in context, these predictions imply that the experience of subjective craving should be associated with preferential attentional processing of drug-related cues. In the next section we discuss recent theoretical models that make more explicit predictions about attentional biases in addiction and their hypothesized association with subjective craving.
Attentional Bias in Substance Users and Its Relationship to Subjective Craving
Robinson and Berridge (1993) proposed the incentive-sensitization theory, in which they suggested that substance-related cues acquire incentive-motivational properties. The central tenet of the theory is that repeated administration of a substance of abuse produces a dopaminergic response that becomes sensitized (i.e., progressively larger) with each new substance administration. This process causes the substance to be perceived as particularly salient and to acquire strong motivational properties, so that subjective cravings for the substance develop. Through classical conditioning, a substance-related cue acquires these incentive-motivational properties; as a consequence, the cue “grabs attention, becomes attractive and ‘wanted,’ and thus guides behavior to the incentive” (Robinson & Berridge, 1993, p. 261). Thus the model suggests that subjective craving and attentional bias reflect the same underlying process, and therefore one might expect the two to be correlated. However, Robinson and Berridge do also suggest that the incentive-motivational properties of substance-related cues can drive substance-seeking behavior in the absence of conscious awareness. This might imply that subjective craving and attentional bias can be decoupled in some circumstances.
A more recent extension of the model (Franken, 2003) is broadly consistent with the incentive-sensitization theory. Substance-related cues are flagged as salient, after extensive experience of substance use, and they grab the substance user’s attention, as a consequence of cue-induced dopamine release in the corticostriatal circuit. However, Franken’s model also suggests that subjective craving and attentional bias have mutual excitatory relationships. That is, when substance-related cues become the focus of attention, subjective craving increases; this, in turn, increases the salience of substance-related cues, and so on, until ultimately the substance is sought out and self-administered. Other models, which are more explicitly grounded in cognitive psychology, make similar predictions. For example, Ryan (2002) argued that “cue reactivity and the experience of craving are meaningfully related to perceptual and cognitive processes that occur before, during, and after cue exposure” (p. 68). The central tenet of Ryan’s model is that substance-related stimuli receive preferential attentional processing, and this is a major determinant of subjective craving in response to these stimuli. Like Franken’s (2003) model, Ryan’s model describes a reciprocal relationship between subjective craving and attentional bias for substance-related cues, such that an increase in craving increases the attention that is paid to substance-related cues and vice versa (see also Field & Cox, 2008).
Finally, the elaborated intrusion theory of desire (Kavanagh et al., 2005) is a general model of subjective motivational states (e.g., substance craving and hunger) that makes similar predictions. The theory suggests that subjective substance craving can initially be experienced as an “intrusion,” perhaps caused by internal states (e.g., withdrawal symptoms) or external cues (e.g., the sight of someone smoking). Once the substance user becomes aware of the craving, he or she “elaborates” on it, for example, by ruminating on the craving itself or by maintaining attentional focus on the external cues that triggered it (i.e., the sight of another person smoking). The elaboration, in turn, increases the strength of the subjective craving. This model, then, also implies that selective attentional processing of substance-related stimuli has a bidirectional causal relationship with subjective craving.
In summary, numerous theoretical models make the prediction that substance-related stimuli will capture the attention of people who use or abuse the addictive substance. These models converge on the prediction that attentional bias and subjective craving should be correlated with each other in the majority of circumstances.
Attentional Bias: Measurement Issues and Subcomponents of Attention
Tasks Used to Measure Attentional Bias
The most widely used test of attentional bias is a modified version of the classic Stroop task (Stroop, 1935), sometimes termed the addiction Stroop (Cox, Fadardi, & Pothos, 2006). During the task, words are presented in different colored fonts. Typically, two categories of words are presented—substance-related and emotionally neutral—and these word categories are matched on variables such as word length and number of syllables. Participants are instructed to quickly and accurately identify the color in which words are printed and to attempt to ignore the semantic content of the words. Attentional bias is indexed as the difference between participants’ mean color-naming reaction time on trials with substance-related words and on those with neutral words. It is assumed that slower color naming on trials with the substance-related words indicates automatic processing of the semantic content of the words, which impairs the color naming. A recent meta-analysis of addiction Stroop studies revealed robust color-naming interference produced by alcohol- and tobacco-related words in alcohol abusers and tobacco smokers, respectively (Cox et al., 2006).
In dual-task procedures, participants are exposed to substance-related stimuli while they perform a cognitively demanding task. For example, Sayette and Hufford (1994) exposed smokers to discrete smoking-related or neutral cues and instructed them to rapidly respond to auditory probe stimuli at the same time. Participants were slower to respond to the probes when smoking-related stimuli were presented than when neutral stimuli were presented. This outcome may indicate that the smoking-related cues were the focus of attention at the time.
In the visual probe task (e.g., Ehrman et al., 2002), a substance-related stimulus (most commonly, a picture) and a matched control stimulus are simultaneously presented on a computer screen. When the stimuli disappear, a visual probe appears in the location that one of the stimuli occupied. In one variant of the task, called the attentional cuing task, only one pictorial stimulus is presented on each trial, and it is either substance-related or neutral (e.g., Franken, Kroon, & Hendriks, 2000; Stormark, Field, Hugdahl, & Horowitz, 1997). In both variants of the task, participants are instructed to respond rapidly to the probe, and their reaction times to probes that replace substance-related stimuli are compared with those to probes that replace neutral stimuli. Because participants generally respond faster to probes that appear in a region of a visual display to which they are attending (Posner, Snyder, & Davidson, 1980), attentional bias for substance-related cues is inferred when participants are faster to respond to probes that replace substance-related stimuli than to probes that replace control stimuli. Therefore, the attentional bias index is derived by subtracting reaction times to probes that replace substance-related stimuli from reaction times to probes that replace neutral stimuli. As reviewed by Field and Cox (2008), studies employing visual probe and attentional cuing tasks have revealed attentional biases for substance-related cues in abusers of alcohol, cannabis, cocaine, heroin, and tobacco. Of note, three studies reported that alcohol-dependent patients who were currently receiving treatment exhibited significant attentional avoidance of alcohol-related cues when those cues were presented for 500 ms or longer (i.e., they were slower to respond to probes that replaced alcohol-related than to neutral stimuli; Noel et al., 2006; Stormark et al., 1997; Townshend & Duka, 2007). Therefore, although the majority of studies that used the visual probe task have revealed the predicted attentional biases, there is also evidence that alcohol-dependent patients in treatment respond in a completely different way (i.e., they show attentional avoidance).
Munafò, Johnstone, and Mackintosh (2005) and Waters, Heishman, Lerman, and Pickworth (2007) adapted the attentional blink task to measure attentional biases in tobacco smokers. The rationale for the task rests on the observation that, when two stimuli are briefly presented in sequence, participants are relatively unlikely to report perception of the second stimulus. In other words, the attentional system appears to “blink” for a short period after one stimulus has been detected and attended to. However, some stimuli, particularly those that are emotionally valenced (e.g., Keil & Ihssen, 2004), lead to a marked diminution of the attentional blink effect. This suggests that those stimuli are able to grab the attention. Both Munafò et al. (2005) and Waters et al. (2007) employed an attentional blink task in which the second stimulus presented was either a smoking-related word or a smoking-unrelated word. In the Waters et al. study, among tobacco smokers, the magnitude of the attentional blink effect (as inferred from recall of the second stimulus presented) was significantly diminished when that stimulus was a smoking-related word rather than a smoking-unrelated word. These results suggest that smokers are more likely to attend to smoking-related words when those words are presented under conditions in which awareness of stimuli is usually severely restricted. Such a pattern is indicative of attentional bias.
In the experimental tasks described thus far, the allocation of attention must be indirectly inferred on the basis of reaction time (in the case of the Stroop task, visual-probe task, or dual task) or stimulus recall (in the case of the attentional blink task). As discussed in this section, the rationale for these inferences is generally sound, although it is important to note that these tasks do not provide a direct measure of selective attention; one should not assume a perfect relationship between task performance and the allocation of selective attention. However, more direct measures of visuospatial attention are available. In particular, eye movements provide an excellent indicator of the current focus of selective attention: In most situations, participants are attending to the area of the visual field that is currently foveated (i.e., the focus of gaze; Kowler, 1995). Some investigators have monitored participants’ eye movements during the presentation of substance-related cues. For example, one research group (Rosse et al., 1997) assessed the relationship between the subjective craving and eye movements of cocaine users when they were presented with cocaine-related cues. More recently, researchers (e.g., Field, Eastwood, Bradley, & Mogg, 2006; Mogg, Bradley, Field, & De Houwer, 2003) have measured the eye movements of participants as they complete the visual probe task and have directly measured how participants allocate their attention when substance-related and matched control pictures compete for their attention. As recently reviewed by Field and Cox (2008), these studies have demonstrated that substance users, but not nonusers, maintain their gaze longer on substance-related cues than on control cues.
The final class of studies that we consider in this meta-analysis are those that measure electrophysiological signals, in particular, event-related potentials (ERPs), in response to substance-related and control cues. As discussed previously, “late” ERP components (i.e., ERPs that emerge around 300 ms after stimulus onset) provide a direct measure of enhanced attentional processing of the presented stimulus (Schupp et al., 2006, 2007). In research with substance users, late (or slow) positive waves, such as the P3 and the subsequent P3-related late positive potential (LPP), are compared in response to substance-related and neutral cues. Several ERP studies have demonstrated larger slow/late positive waves in response to substance-related stimuli compared to neutral stimuli, in substance abusers compared with controls (e.g., Franken, Stam, Hendriks, & van Den Brink, 2003; Littel & Franken, 2007; Namkoong, Lee, Lee, Lee, & An, 2004). Such results suggest enhanced attentional processing of substance-related cues in substance abusers.
Do All Tasks Measure the Same Thing? Initial Orienting and Delayed Disengagement
The distinction between the initial orienting of selective attention and the maintenance or disengagement of attention is recognized as an important one (Corbetta & Shulman, 2002). In this section, we discuss how performance on different tasks can be understood in terms of rapid orienting of attention versus delayed disengagement of attention. The addiction Stroop is generally conceptualized as a measure of involuntary semantic processing of substance-related words. Such processing occurs in very early stages of cognitive processing (see review and meta-analysis by Cox et al., 2006). However, some evidence suggests that Stroop interference might be attributable to slower attentional processes, such as delayed disengagement of attention. The addiction Stroop task can be understood as a variant of the emotional Stroop task, in which participants are required to name the color of emotionally valenced words. Phaf and Kan (2007) conducted a meta-analysis of 70 published emotional Stroop studies and concluded that the emotional Stroop effect seems to reflect a relatively slow process (the disengagement of attention) rather than a rapid, automatic processing bias.
Although these arguments relate to the emotional Stroop task, there is reason to believe that they might also apply to the addiction Stroop task. Evidence for “carryover” effects in tobacco smokers, heroin users, and cannabis users has been found in several studies (Cane, Sharma, & Albery, 2008; Waters, Sayette, Franken, & Schwartz, 2005; Waters, Sayette, & Wertz, 2003). In five independent data sets described in these reports, color-naming performance was slowed on trials in which a neutral word was presented and that had been preceded by a trial in which a substance-related word was presented, so the substance-related words produced a slowdown in color-naming performance that persisted into the following trial. Furthermore, Cane et al. (2008) demonstrated that carryover effects produced by cannabis-related words can persist over several subsequent trials. In their meta-analysis of addiction Stroop studies, Cox et al. (2006) concluded that color-naming interference was strongest when addiction-related words were presented in a “blocked” format, in which all substance-related words are presented in a discrete block and all neutral words are presented in a different block. Compared with use of an “unblocked” presentation format, in which substance-related and neutral words are intermixed, use of a blocked presentation format maximizes the cumulative influence of carryover effects; overall color-naming interference is more pronounced as a result. We interpret the presence of these carryover effects as indicative of a slow cognitive process that persists even after the substance-related cues have been removed from the stimulus display. Therefore, Stroop interference is likely to reflect the delayed disengagement of attention.
Attentional bias indices derived from dual-task procedures are also likely to reflect delayed disengagement of attention rather than rapid attentional orienting toward substance-related cues. This is because these procedures are conceptually similar to the modified Stroop task, in that participants are given a primary task (in the case of dual-task procedures, responding to an auditory probe) and are simultaneously presented with a substance-related cue. The substance-related cue is thought to impair performance on the primary task because the participant is relatively slow to disengage his or her attention from the substance-related cue. It is difficult to argue that the observed interference reflects a bias in the rapid orienting of attention toward the substance-related cue, because when the probe stimulus is presented, the participant is already attending to the substance-related cue.
With regard to the visual probe task, several investigators have suggested that by manipulating the amount of time that substance-related pictures are presented on the computer screen (the stimulus onset asynchrony, or SOA), one can investigate biases in the initial orienting versus disengagement of attention from those cues (see, e.g., Bradley, Field, Mogg, & De Houwer, 2004). The basic reasoning is that a short SOA permits participants to make only one shift of attention toward one of the stimuli, and therefore the reaction time index of attentional bias is likely to reflect a bias in the initial orienting of attention. With longer SOAs, participants are able to make multiple shifts of attention between the two different stimuli, and therefore the attentional index is likely to reflect a bias in the maintenance or disengagement of attention. But what constitutes a short versus a long SOA? In the anxiety and addiction literature, researchers have generally used an SOA of between 50 and 200 ms to detect biases in initial orienting. The rationale for assuming that this SOA will measure biases in initial orienting appears sound: Basic perceptual research with simple stimuli suggests that when a simple visual cue is presented, participants typically require around 50 ms to shift their attention to the cue (Duncan, Ward, & Shapiro, 1994). In addition, participants typically require at least 150 ms to disengage their attention from one simple visual cue and redirect it to another that is presented in a different spatial location (Theeuwes, 2005). It seems, therefore, that when two fairly complex stimuli are presented together (as happens on each trial of a visual probe task) for anywhere between 50 and 200 ms, any attentional bias that is observed (based on reaction times to probes that replace one of the stimuli) must reflect the stimulus toward which participants initially directed their attention. Within the time frame of 200 ms, a second shift of attention is not possible.
To infer biases in the maintenance of attention, researchers have generally used an SOA of 500 ms or longer. According to the logic outlined above, SOAs of 500 ms or longer are sufficient to allow multiple shifts of attention between the different stimuli, and therefore any attentional bias that is detectable at stimulus offset is likely to reflect a bias in the maintenance of attention on that stimulus. This logic is supported by findings from two recent studies (Field et al., 2006; Schoenmakers, Wiers, & Field, 2008), which demonstrated that, in cannabis users and heavy drinkers, the magnitude of attentional bias for substance-related cues (as inferred from reaction times to probes) was significantly positively correlated with the amount of time that participants maintained their gaze on the substance-related cue (compared to the control cue), when a 2,000-ms SOA was used.
With regard to the attentional blink task, it seems likely that the index of attentional bias derived from this task reflects a bias in the orienting of attention. Blink survival, the dependent variable in this task, refers to the percentage of substance-related words that “survive” the attentional blink. A higher percentage means that more substance-related words were consciously perceived when available attentional resources were limited due to ongoing processing of the first stimulus in the sequence (see Waters et al., 2007).
Eye movement monitoring provides a direct measure of initial orienting and delayed disengagement processes. Several studies (e.g., Field et al., 2006; Mogg et al., 2003; Schoenmakers et al., 2008) measured the eye movements of substance users as they completed a visual probe task, in which pairs of substance-related and matched control pictures were presented side by side on a computer screen. Eye movement monitoring permits the measurement of the duration of eye movement fixations directed toward substance-related versus control pictures, which is a direct measure of the maintenance or delayed disengagement of attention. Some of these studies (e.g., Mogg et al., 2003) also measured the direction of the initial shift in gaze during the task, and this index reflects the initial orienting of attention.
Finally, we consider event-related potentials to be a relatively direct measure of attentional processing, and the ERP components that we consider in this report (late positive waves, such as the P3 and LPP, and slow positive waves) are likely to reflect delayed disengagement of attention from substance-related cues, rather than initial orienting of attention toward those cues. Early ERP components, such as the P1 and N1, which occur within 200 ms of stimulus onset, are thought to index attentional orienting (see, e.g., Pourtois, Grandjean, Sander, & Vuilleumier, 2004). The ERP studies considered in this meta-analysis have not consistently demonstrated differentiation of these early ERP components in response to substance-related cues versus neutral cues among substance users. By contrast, late positive waves appear only after initial attentional orienting has occurred, and these ERP components represent sustained attention or delayed disengagement of attention (Schupp et al., 2006).
In summary, the following measures of attentional bias were considered in the meta-analysis reported here: (a) measures of the duration of eye movement fixations on substance-related cues (eye movement “dwell time”); (b) measures of the direction of initial shift in eye movements when substance-related and control cues were simultaneously presented during visual probe tasks (eye movement “initial orienting”); (c) reaction time measures obtained from the visual probe task; (d) reaction time measures obtained from the modified Stroop task; (e) reaction time measures obtained from dual task procedures; (f) blink survival from the attentional blink task; and (g) ERPs in response to drug-related cues. We focused on late ERP components (P3/LPP/slow wave), as none of the existing studies reported correlations between subjective craving and early ERP components.
Craving
A Definition of Craving
We define craving as a subjectively experienced motivational state that fluctuates over time. We also restrict our definition of craving to that of an appetitive motivational state (i.e., the desire to consume) rather than an avoidant motivational state, which might comprise the desire to limit use (cf. Davis, 1984); we note that no existing studies have examined relationships between attentional bias and self-report measures of this avoidant motivational state (e.g., the Avoidance subscale of the Approach and Avoidance of Alcohol Questionnaire; McEvoy, Stritzke, French, Lang, & Ketterman, 2004). This review is primarily concerned with subjective craving for psychoactive drugs, but this does not necessarily imply that substance (drug) craving is qualitatively or quantitatively different from other types of craving, such as food craving (e.g., Nijs, Franken, & Muris, 2007).
It may be helpful to explain what we believe craving is not. We do not equate craving with substance self-administration (cf. Markou et al., 1993), so for this reason any comparison with animal models of craving (in which craving is inferred from rates of self-administration of the substance) is not appropriate here. Although there is some evidence that subjective craving is correlated with substance self-administration in humans (see Drobes & Thomas, 1999), this association is not always apparent. For example, humans have the ability to experience strong cravings and choose not to take substances. In addition, one prominent theory of addiction has convincingly challenged the commonly held belief that subjective craving is the cause of substance self-administration (Tiffany, 1990). Indeed, human laboratory work investigating associations between self-reported craving and substance self-administration in the laboratory has sometimes (e.g., Willner, Hardman, & Eaton, 1995) but not always (e.g., Field, Mogg, & Bradley, 2005b) revealed associations between the two.
Measurement Issues
Self-reported craving is frequently assessed with questionnaire items that require individuals to indicate the strength of their current craving or their level of endorsement of items such as “I am craving alcohol right now,” “I have an urge to drink alcohol right now,” or “I have a desire to drink alcohol right now.” It is important to emphasize that we are primarily interested in this momentary subjective motivational state and that not all available questionnaires are suitable for assessing this fluctuating state. For example, the Obsessive Compulsive Drinking Scale (Anton, 2000) includes questions that assess the frequency and severity of alcohol-related cognitions, including craving, in general terms rather than questions that assess the current level of craving.
It is unlikely that any psychometric measure of craving, or any other subjective state, will provide a pure readout of the subjective state that it purports to measure. As outlined in a previous review of craving measurement (Sayette et al., 2000), there are a number of reasons for this. For example, researcher and research participant are unlikely to be in complete agreement regarding the meaning of terms such as craving, urge, and desire. Furthermore, it is likely that at least some research participants are unable to accurately assess their own internal states, and there may be instances in which participants are unwilling to give an accurate report of their feelings to a researcher (see Marissen, Franken, Blanken, van den Brink, & Hendriks, 2005). As such, self-report measures provide the best measure, and probably the only available measure, of subjective craving, but it is unrealistic to expect a perfect relationship between self-reported craving and the state that the participant is currently experiencing.
With regard to the specific instruments used to assess craving, a great deal of research has assessed momentary self-reported craving with single-item visual analogue scales (VAS) of the type discussed above. However, various researchers have highlighted limitations with this particular form of measurement (e.g., Sayette et al., 2000; Tiffany & Drobes, 1991; Tiffany, Singleton, Haertzen, & Henningfield, 1993). To highlight just two possible limitations: Single-item scales may have limited reliability compared with multiple-item scales, and the choice of terminology in the item (for example, asking about “craving” vs. asking about “urge” or “desire”) may mean different things to different individuals. On the basis of these and other potential limitations of single-item scales (for a detailed overview, see Sayette et al., 2000), researchers have devised and validated multi-item craving questionnaires. As a result of these research efforts, well-validated multi-item questionnaires now exist for the assessment of craving for substances such as alcohol (Bohn, Krahn, & Staehler, 1995; Love, James, & Willner, 1998; Singleton, Tiffany, & Henningfield, 1994; see Drobes & Thomas, 1999), nicotine (Cox, Tiffany, & Christen, 2001; Tiffany & Drobes, 1991), cannabis/marijuana (Heishman, Singleton, & Liguori, 2001), cocaine (Sussner et al., 2006; Tiffany et al., 1993), amphetamines (James, Davies, & Willner, 2004), and heroin (Franken, Hendriks, & van den Brink, 2002). Multi-item questionnaires such as these are arguably more reliable than single-item scales, although their use does raise additional issues (see Sayette et al., 2000). For example, (a) responding to multiple questions about craving might lead to rumination on craving, which could increase craving strength; (b) participants might use their response to the initial item to anchor their responses to remaining items; and (c) participants might deliberately respond in a similar way to all items in an attempt to appear consistent, which could contribute to the apparent high reliability of multi-item scales. Furthermore, in some circumstances it may be preferable to use a single-item scale rather than a multi-item questionnaire. Examples include studies in which multiple craving measurements are required or in which time for craving assessment is constrained.
With some exceptions (most notably the Alcohol Urge Questionnaire from Bohn et al., 1995; see Drummond & Phillips, 2002; MacKillop, 2006), factor analyses of multi-item questionnaires have yielded a multifactorial structure to self-reported craving. Although the individual items in these questionnaires were generated to assess craving for different substances, we suggest that striking similarities can be seen in terms of the factors of craving that are identified. The above-mentioned questionnaires identify factors representing desires or urges to use the substance (as would be expected), but they also identify other factors, including intentions to use, expectations of reinforcement from using (often subdivided into expectations of pleasure and expectations of relief from negative affect and withdrawal), and perceived control over substance use. Although these factors appear to represent distinct entities (based on factor analyses), the intercorrelations between factors are often large. For example, the correlation between the two factors of the brief form of the Questionnaire of Smoking Urges is .80 (Cox et al., 2001). In addition, combined scores on multifactorial questionnaires tend to correlate highly with VAS measures of craving (e.g., Rosenberg & Mazzola, 2007; Sussner et al., 2006). Given this, we argue that although multi-item, multifactorial questionnaires and single-item VAS might differ in terms of their reliability and validity, they measure the same construct.
Two other important issues warrant consideration. First, the underlying factor structure of multi-item questionnaires may be different for different populations. For example, Singleton et al.’s (1994) Alcohol Craving Questionnaire was factor analyzed by two different research groups, and their findings were inconsistent; Love et al. (1998) identified a three-factor structure in social drinkers, whereas Raabe, Grusser, Wessa, Podschus, and Flor (2005) identified a two-factor structure in a sample of alcohol abusers. A similar discrepancy is seen with the Desires for Alcohol Questionnaire. In this case, a three-factor structure emerged for social drinkers, but a four-factor structure emerged for alcoholics (Clark, 1994; Love et al., 1998). The second issue is that when an “average” craving score is taken from multi-item questionnaires, these average scores have high levels of internal reliability (e.g., Cox et al., 2001; Sussner et al., 2006), even though the questionnaires have an underlying multifactorial structure.
Our primary purpose in this review is to investigate the association between self-reported craving and attentional bias for substance-related cues. We did not examine correlations between attentional bias and the different subscales from multi-item questionnaires for two, largely pragmatic reasons. First, as detailed above, the internal reliability of multi-item craving questionnaires appears to be excellent, even when an average craving score is analyzed (as well as when different factors are analyzed separately); second, the theoretical models discussed previously do not make any clear predictions about which components of craving (e.g., urge, intentions, expectancy, control) should be most closely correlated with attentional bias, so we were unable to make a priori predictions that some components of craving should be more closely correlated with attentional bias than others. Even if we had taken this approach, the fact that researchers have been forced to use different questionnaires to assess craving for different substances means that this approach would confound questionnaire type with the type of substance for which craving is being assessed.
Method
Brief Overview of Rationale and Hypothesis
As discussed in the previous sections, contemporary theoretical models suggest that attentional bias for substance-related cues and subjective substance craving should be positively correlated. The most appropriate method for comprehensively addressing this issue is meta-analysis, which permits one to use multiple data sets to examine the size and consistency of a relationship between two given variables and any factors that may moderate this relationship (Rosenthal & DiMatteo, 2001).
Selection of Studies
Only peer-reviewed studies (published or accepted for publication) were included in the meta-analysis. Potential studies were initially identified on the basis of searches in PubMed, PsycINFO, and Scopus; several review articles (Cox et al., 2006; Field & Cox, 2008; Franken, 2003; Robbins & Ehrman, 2004) and relevant chapters in recent books (Munafò & Albery, 2006; Wiers & Stacy, 2006) were also consulted. Titles and abstracts of all potentially relevant articles were then inspected, and those that appeared to measure attentional bias or ERP reactivity in substance users were selected for further investigation. The reference sections of these published papers were consulted for additional publications.
We then consulted all articles to examine if the procedure involved the measurement of both subjective craving and attentional bias or ERP reactivity. Studies in which subjective craving or attentional bias/ERP reactivity were not measured were excluded. For the remaining articles, correlations were obtained directly from the article when possible; alternatively, corresponding authors were contacted and asked to provide raw data or to calculate correlation coefficients. Through this process we were able to obtain correlation data from the majority of published studies.
All studies included in the meta-analysis constituted independent samples. Data from Waters et al. (2007) were not included, as the sample (N = 55) was a subset of participants who had taken part in a different study (Leventhal et al., 2007; N = 199). The Leventhal et al. (2007) study was included. Similarly, data from Waters, Shiffman, Bradley, and Mogg (2003) were not included, as the sample (N = 141) was a subset of a larger sample of participants (N = 158) who had taken part in a different study, which was itself written up in two different reports (Waters, Shiffman, Sayette, et al., 2003, 2004). We took an average correlation from the latter, larger sample (i.e., the average of the correlations reported in Waters, Shiffman, Sayette, et al., 2003, 2004).
Selection of Variables: Primary Meta-Analysis
Correlation coefficients between indices of attentional bias and subjective craving were included in the meta-analysis. Attentional bias indices were generally derived by calculating the difference between reactivity to substance-related and control cues (e.g., reaction time, duration of eye movement dwell time, amplitude of late ERP components), although in the case of studies that used a dual task procedure, the dependent measure was simply reaction time when in the presence of a substance-related cue. With regard to ERP studies, ERP measurement yields many variables (in the studies that we considered, data from 16 to 32 electrodes were available). We focused on the frontal electrodes, because most studies reported the correlation between the amplitude of a late potential on a frontal electrode site and self-reported craving. We used the frontal midline electrode (Fz) if available; if Fz was not available, we used the most proximate frontal electrode that was reported.
In the majority of studies, only one measure of attentional bias and one measure of craving were obtained, so the correlations between these variables were included. Other studies included multiple measures of craving (e.g., a VAS and a multi-item questionnaire) and/or multiple different indices of attentional bias (e.g., a Stroop task and a visual probe task, both eye movement and reaction time measures derived from a visual probe task). In these cases, we obtained correlations between each measure of attentional bias and each measure of craving, and we included the average correlation coefficient for each sample in the primary meta-analysis.
Several of the studies that were included reported the results of an experimental manipulation on craving and attentional biases. Manipulations included pharmacological challenge (Duka & Townshend, 2004; Field, Mogg, & Bradley, 2005b; Franken, Hendriks, Stam, and van den Brink, 2004) and mood manipulation (e.g., Bradley, Garner, Hudson, & Mogg, 2007; Field & Powell, 2007). For those studies that employed a between-subjects design, the different experimental manipulations constituted independent groups, so we included correlations from these studies separately for each group. For those studies that used a within-subjects design, we included the average correlation obtained from the different experimental conditions.
Potential Moderating Variables Considered in Stratified Analyses
We conducted the following additional stratified analyses in order to consider the influences of a number of potential moderating variables on the relationship between subjective craving and attentional bias.
1. The type of substance-related cue presented: tobacco (k = 37), alcohol (k = 17), or “other” (cannabis/marijuana, heroin, cocaine, and caffeine). The latter substance-related cues were combined owing to the small number of available studies (k = 14 studies in total). We had no a priori hypothesis that the magnitude of the correlation would be larger for any particular type of substance cue.
2. Treatment-seeking status of participants: currently in treatment or seeking treatment (k = 15) or not in treatment (k = 53). On the basis of theoretical models that suggest that the correlates of subjective craving may differ among patients in treatment compared to patients who are not (e.g., Baker et al., 1987), coupled with evidence suggesting reduced levels of craving among patients in treatment (and therefore reduced variance in subjective craving; Wertz & Sayette, 2001b) and demonstrations of overt attentional avoidance of alcohol-related cues among alcohol-dependent patients in treatment (Noel et al., 2006; Stormark et al., 1997; Townshend & Duka, 2007), we hypothesized that the correlation between attentional bias and subjective craving would be significantly larger among individuals who were not in treatment than among patients who were in treatment.
We conducted supplementary stratified analyses in order to examine the potential moderating roles of a number of other factors, as detailed below. These analyses were considered separately from those described above, because some data sets contributed one correlation coefficient to each of the different arms of these stratified analyses, as detailed below.
3. The type of craving measure used: (a) single-item VAS (k = 35) or (b) multi-item questionnaire (k = 42). Given that multi-item scales may be more reliable than single-item VAS, we hypothesized a larger correlation when multi-item questionnaires were used.
4. The type of attentional bias task used: (a) visual probe task (k = 30; this included studies that used either eye movements or manual reaction times, or both, to infer attentional bias); (b) modified Stroop task (k = 26); (c) ERP P300/LPP or slow wave (k = 7); or (d) dual task procedures and “others” (k = 13). Ten samples that employed a form of dual-task procedure were included in the meta-analysis. For practical purposes, these samples were combined with three samples in which unique methods were used to assess attentional bias: Rosse et al. (1997) measured the duration of eye movement fixations on a crack cocaine pipe, whereas Munafò et al. (2005; two independent samples) used an attentional blink task in tobacco smokers. We had no a priori hypothesis that the magnitude of the correlation would be larger for any particular type of attentional bias task.
5. Measures of attentional bias that reflect (a) initial orienting toward (k = 12) versus (b) delayed disengagement of attention from (k = 68) substance-related cues. We collapsed attentional bias measures into those that measure the orienting of attention (eye movement orienting, visual probe task reaction times with SOAs of 200 ms or less, or blink survival from the attentional blink task) and those that measure the delayed disengagement of attention (eye movement gaze duration, ERP measures, visual probe task reaction times with SOAs of 500 ms or more, and reaction times obtained from modified Stroop and dual task procedures). We had no a priori hypothesis that the magnitude of the correlation would be larger for any particular attentional subcomponent.
6. Direct (k = 15) versus indirect (k = 59) measures of attention. As previously discussed, the majority of attentional bias measures can be classed as indirect measures of attention, as the allocation of attention is inferred from a secondary measure (most commonly, reaction time). The only exceptions are studies that employed eye movement monitoring or the study of ERP components, as these arguably constitute more direct measures of selective attention. We compared the magnitude of the correlation between attentional bias and subjective craving for samples that used an eye movement initial orienting measure, an eye movement gaze duration measure, or an ERP measure (k = 15) and for samples that used one or more indirect measures of attention (k = 59). We hypothesized that the correlation would be larger among studies that used direct versus indirect measures of attentional bias, as direct measures arguably provide a more valid measure of selective attention.
7. We considered whether the magnitude of the correlation between subjective craving and attentional bias would be greater among participants in whom craving was relatively high. Baker, Morse, and Sherman (1987) and Sayette and colleagues (Sayette, Martin, Hull, Wertz, & Perrott, 2003; Sayette, Martin, Wertz, Shiffman, & Perrott, 2001) have suggested that there is greater coherence between self-report measures of subjective craving and physiological and cognitive indices when individuals are experiencing a high level of craving. One plausible hypothesis (M. A. Sayette, personal communication, November 8, 2007) is that this is also true for the association between attentional bias for substance-related cues and subjective craving. In order to investigate this hypothesis, we considered the results from 12 samples in which subjective craving was experimentally manipulated with a pharmacological challenge, deprivation, cue exposure, or negative mood induction. In each of these studies, craving was significantly higher after the experimental manipulation (compared to a control condition or control group), and correlations between attentional bias and subjective craving were available after both the “high craving” and “low craving” (control) manipulations. We note here that we did not include some studies in this stratified meta-analysis, as in those studies the experimental manipulation did not produce a significant change in subjective craving (studies excluded on these grounds were Duka & Townshend, 2004; Franken, Hendriks, et al., 2004; Hitsman et al., 2008; Munafò, Mannie, Cowen, Harmer, & McTavish, 2007; Wertz & Sayette, 2001a).
Statistical Approach
Data were initially analyzed within a fixed-effects framework, and we pooled individual study effect sizes (r) using inverse variance methods to generate a summary r and 95% confidence interval (CI). A fixed-effects framework assumes that the underlying effect is constant across studies, and between-study variation is considered to be due to chance or random variation. The assumption was checked using a chi-square test of goodness of fit for homogeneity. The significance of the pooled r was determined using a Z test.
Where there was evidence of a significant correlation between attentional bias and self-reported craving in the presence of significant between-study heterogeneity, we employed a random-effects framework, with rs pooled using DerSimonian and Laird methods. A random-effects framework assumes that between-study variation is due both to chance or random variation and to an individual study effect. Random-effects models are more conservative than fixed-effects models and generate a wider confidence interval. The significance of the pooled d was determined with a Z test.
We created funnel plots that would assess potential ascertainment bias (as might be caused by publication bias) by plotting individual study effect size against the standard error of the effect size. Ascertainment bias was assessed with Egger’s test (Egger, Davey Smith, Schneider, & Minder, 1997). In the presence of bias, a corrected pooled effect size estimate was calculated with Duval and Tweedie’s trim-and-fill method (Duval & Tweedie, 2000). This method removes studies with outlying effect size values until symmetry is achieved and then replaces these along with imputed “mirror” values in order to retain symmetry.
We used meta-regression to determine the relationship between effect size estimate and the proportion of male versus female participants. Primary analyses were further stratified by type of substance cue presented (alcohol, tobacco, other) and treatment-seeking status of participants (treatment-seeking, not treatment seeking) in order to assess potential moderating effects of these variables. Additional stratified analyses assessed the effects of craving measure (multi-item questionnaire, VAS), attentional bias measure (modified Stroop, visual probe task, ERP, other), subcomponent of attention (orienting, disengagement), direct versus indirect measures of attention (eye movement or ERP measures vs. all other measures), and the current strength of subjective craving (high craving, low craving).
Results
Characteristics of Studies
Characteristics of the studies included in the meta-analyses are summarized in Table 1. A total of k = 56 studies published between 1993 and 2008, comprising k = 68 independent samples, contributed to the primary meta-analysis. Of the studies assessing attentional bias, 37 were for tobacco cues, 17 were for alcohol cues, 5 were for heroin cues, 5 were for cocaine cues, 3 were for cannabis cues, and 1 was for caffeine cues. Fifteen samples were obtained from participants who were currently in treatment or seeking treatment; the remaining 53 samples comprised participants who were not currently seeking treatment. Of the 68 samples, 33 used a multi-item measure of craving, 26 used a VAS measure, and 9 included both multi-item and VAS measures. A visual probe task was used to assess attentional bias in 25 samples, and a modified Stroop task was used in 18 samples. Both visual probe and modified Stroop tasks were used in an additional 5 samples. Seven studies monitored ERP reactivity, and 10 studies used a dual task procedure (one of these, the study reported in Waters et al., 2003, 2004, also used a modified Stroop task). Two studies, comprising three independent groups, used unique methods to assess attentional bias: Rosse et al. (1997) measured eye movement gaze duration on a crack cocaine pipe, whereas Munafò et al. (2005) used an attentional blink task in combination with a modified Stroop task (two independent samples).
Table 1.
Summary of the Studies Included in the Primary Meta-Analysis
| Study | Sample size (substance users only) |
Type of substance cue presented |
Treatment seeking? |
Type of craving measure |
Type of task (attentional bias measure) | Correlation |
|---|---|---|---|---|---|---|
| Attwood et al. (2008) | 54 | Tobacco | No | Multi-item and VAS | Visual probe task, 500-ms SOA (reaction time) | .26 |
| Baxter & Hinson (2001; Exp. 2) | 77 | Tobacco | No | Multi-item | Dual task procedure (reaction time) | .18 |
| Bradley et al. (2003; Exp. 1) | 20 | Tobacco | No | Multi-item | Visual probe task, 500-ms SOA (reaction time) | .25 |
| Bradley et al. (2003; Exp. 2) | 24 | Tobacco | No | Multi-item | Visual probe task, 500- and 2,000-ms SOAs (reaction time) | .29 |
| Bradley et al. (2004) | 20 | Tobacco | No | Multi-item and VAS | Visual probe task, 200- and 2,000-ms SOAs (reaction time) | .25 |
| Bradley et al. (2007) | 10 | Tobacco | No | Multi-item | Visual probe task, 2,00-ms SOA (reaction time, eye movement initial orientation, gaze duration) |
.28 |
| Bradley et al. (2008) | 22 | Tobacco | No | Multi-item | Visual probe task, 200- and 2,000-ms SOAs (reaction time) | −.02 |
| Copersino et al. (2004) | 20 | Cocaine | Yes | Multi-item | Addiction Stroop task (reaction time) | .35 |
| Duka & Townshend (2004; placebo group) | 14 | Alcohol | No | Multi-item | Addiction Stroop task (reaction time) and visual probe task, 500-ms SOA (reaction time) |
.l8 |
|
Duka & Townshend (2004; 0.3 g/kg alcohol group) |
14 | Alcohol | No | Multi-item | Addiction Stroop task (reaction time) and visual probe task, 500-ms SOA (reaction time) |
.14 |
|
Duka & Townshend (2004; 0.6 g/kg alcohol group) |
14 | Alcohol | No | Multi-item | Addiction Stroop task (reaction time) and visual probe task, 500-ms SOA (reaction time) |
.05 |
| Field (2005) | 28 | Cannabis | No | Multi-item | Addiction Stroop task (reaction time) | .54 |
| Field, Christiansen, et al. (2007) | 90 | Alcohol | No | Multi-item | Addiction Stroop task (reaction time) | .42 |
| Field, Duka, et al. (2007) | 60 | Alcohol | No | Multi-item and VAS | Addiction Stroop task (reaction time) and visual probe task, 500-ms SOA (reaction time) |
.11 |
| Field & Eastwood (2005) | 40 | Alcohol | No | Multi-item and VAS | Visual probe task, 500-ms SOA (reaction time) | .21 |
| Field et al. (2004) | 40 | Alcohol | No | VAS | Visual probe task, 2,000-ms SOA (reaction time) | .34 |
| Field et al. (2006) | 23 | Cannabis | No | Multi-item | Visual probe task, 200-ms SOA (reaction time); visual probe task, 2,000-ms SOA (reaction time and eye movement gaze duration) |
−.07 |
| Field, Mogg, & Bradley (2004a) | 17 | Cannabis | No | Multi-item | Visual probe task, 500-ms SOA (reaction time) | .56 |
| Field, Mogg, & Bradley (2004b) | 23 | Tobacco | No | Multi-item and VAS | Visual probe task, 2,000-ms SOA (reaction time, eye movement initial orienting, eye movement gaze duration) |
.22 |
| Field, Mogg, & Bradley (2005a) | 50 | Alcohol | No | Multi-item and VAS | Visual probe task, 500-ms & 2,000-ms SOAs (reaction time) | .24 |
| Field, Mogg, & Bradley (2005b) | 19 | Tobacco | No | Multi-item and VAS | Visual probe task, 2,000-ms SOA (reaction time, eye movement initial orienting, eye movement gaze duration) |
.36 |
| Field & Powell (2007; stress induction group) | 22 | Alcohol | No | Multi-item | Visual probe task, 500-ms SOA (reaction time) | .58 |
| Field & Powell (2007; control group) | 22 | Alcohol | No | Multi-item | Visual probe task, 500-ms SOA (reaction time) | −.07 |
| Field, Rush, et al. (2007) | 30 | Tobacco | No | Multi-item | Addiction Stroop task (reaction time) | .11 |
| Franken et al. (2003) | 19 | Heroin | Yes | Multi-item | ERP (P3/slow wave) | .35 |
| Franken et al. (2008) | 23 | Cocaine | Yes | Multi-item | ERP (P3/slow wave) | .45 |
| Franken, Hendriks, et al. (2004) | 17 | Heroin | Yes | Multi-item | Addiction Stroop task (reaction time) | .08 |
| Franken, Hulstijn, et al. (2004) | 21 | Cocaine | Yes | Multi-item | ERP (P3/slow wave) | .55 |
| Franken, Kroon, & Hendriks (2000) | 16 | Cocaine | Yes | VAS | Visual probe task, 100-ms and 500-ms SOAs (reaction time) | .42 |
| Franken, Kroon, et al. (2000) | 21 | Heroin | Yes | VAS | Addiction Stroop task (reaction time) | .23 |
| Hitsman et al. (2008) | 14 | Tobacco | No | Multi-item | Addiction Stroop task (reaction time) | .22 |
| Hogarth et al. (2003; Exp. 1) | 10 | Tobacco | No | Multi-item | Visual probe task, 500-ms SOA (reaction time) | .34 |
| Hogarth et al. (2003; Exp. 2) | 36 | Tobacco | No | Multi-item | Visual probe task, 500-ms SOA (reaction time) | .21 |
|
Juliano & Brandon (1998; smoking available group only) |
32 | Tobacco | No | VAS | Dual task procedure (reaction time) | .38 |
| Leventhal et al. (2007) | 199 | Tobacco | No | Multi-item | Addiction Stroop task (reaction time) | .04 |
| Littel & Franken (2007) | 39 | Tobacco | No | Multi-item | ERP (slow wave) | .32 |
| Lubman et al. (2008) | 16 | Heroin | Yes | VAS | ERP (P3) | .54 |
| Marissen et al. (2006) | 110 | Heroin | Yes | Multi-item | Addiction Stroop task (reaction time) | .23 |
| Mogg & Bradley (2002) | 27 | Tobacco | No | Multi-item and VAS | Addiction Stroop task (reaction time) and visual probe task, 500-ms SOA (reaction time) |
.35 |
| Mogg et al. (2003) | 20 | Tobacco | No | Multi-item and VAS | Visual probe task, 2,000-ms SOA (reaction time and eye movement gaze duration) |
.52 |
| Mogg et al. (2005) | 41 | Tobacco | No | Multi-item | Visual probe task, 2,000-ms SOA (reaction time, eye movement initial orienting, eye movement gaze duration) |
.30 |
| Munafò et al. (2005; abstinent group) | 14 | Tobacco | No | VAS | Addiction Stroop task (reaction time) and attentional blink task (blink survival) |
.19 |
| Munafò et al. (2005; satiated group) | 20 | Tobacco | No | VAS | Addiction Stroop task (reaction time) and attentional blink task (blink survival) |
.25 |
| Munafò et al. (2007; tyrosine-balanced group) | 10 | Tobacco | No | Multi-item | Addiction Stroop task (reaction time) | .43 |
| Munafò et al. (2007; tyrosine-depleted group) | 10 | Tobacco | No | Multi-item | Addiction Stroop task (reaction time) | −.26 |
| Namkoong et al. (2004) | 16 | Alcohol | Yes | VAS | ERP (P3/slow wave) | .51 |
| Noel et al. (2006) | 36 | Alcohol | Yes | VAS | Visual probe task, 50-, 500-, and 1,250-ms SOAs (reaction time) | −.11 |
| Rosse et al. (1997) | 19 | Cocaine | Yes | Multi-item | Eye movement gaze duration | .42 |
| Sayette et al. (1994) | 24 | Alcohol | Yes | VAS | Dual task procedure (reaction time) | .52 |
| Sayette et al. (2001; abstinent heavy smokers) | 32 | Tobacco | No | VAS | Dual task procedure (reaction time) | .40 |
| Sayette et al. (2001; satiated heavy smokers) | 28 | Tobacco | No | VAS | Dual task procedure (reaction time) | .14 |
|
Sayette et al. (2001; abstinent tobacco chippers) |
30 | Tobacco | No | VAS | Dual task procedure (reaction time) | .35 |
|
Sayette et al. (2001; satiated tobacco chippers) |
30 | Tobacco | No | VAS | Dual task procedure (reaction time) | .06 |
| Sayette & Hufford (1994; Exp. 1) | 40 | Tobacco | No | VAS | Dual task procedure (reaction time) | .19 |
| Sayette & Hufford (1994; Exp. 2) | 31 | Tobacco | No | VAS | Dual task procedure (reaction time) | .23 |
| Schoenmakers et al. (2007) | 106 | Alcohol | No | Multi-item | Visual probe task, 500-ms SOA (reaction time) | .06 |
| Schoenmakers et al. (2008) | 23 | Alcohol | No | Multi-item | Visual probe task, 2,000-ms SOA (reaction time, eye movement initial orienting, eye movement gaze duration) |
.06 |
| Townshend & Duka (2007) | 35 | Alcohol | Yes | Multi-item | Visual probe task, 500-ms SOA (reaction time) | −.31 |
| van den Wildenberg et al. (2006) | 48 | Alcohol | No | VAS | Addiction Stroop task (reaction time) | −.03 |
| Warren & McDonough (1999) | 20 | Tobacco | No | VAS | ERP (slow wave) | −.14 |
| Waters et al. (2003, 2004) | 158 | Tobacco | Yes | VAS | Addiction Stroop task (reaction time) and dual task procedure (reaction time) |
.07 |
| Waters & Feyerabend (2000) | 48 | Tobacco | No | VAS | Addiction Stroop task (reaction time) | −.12 |
| Waters & Li (2008) | 22 | Tobacco | No | VAS | Addiction Stroop task (reaction time) | −.13 |
|
Wertz & Sayette (2001b; smoking available group) |
30 | Tobacco | No | VAS | Addiction Stroop task (reaction time) | −.08 |
|
Wertz & Sayette (2001b; smoking unavailable group) |
29 | Tobacco | No | VAS | Addiction Stroop task (reaction time) | .08 |
|
Wertz & Sayette (2001b; 50% chance of smoking group) |
31 | Tobacco | No | VAS | Addiction Stroop task (reaction time) | .13 |
| Yeomans et al. (2005) | 32 | Caffeine | No | VAS | Visual probe task, 500-ms SOA (reaction time) | .43 |
| Zack et al. (2001) | 16 | Tobacco | No | Multi-item | Addiction Stroop task (reaction time) | .51 |
Note. VAS = visual analogue scales; SOA = stimulus onset asynchrony; ERP = event-related potential; P3 = late positive wave.
Primary Meta-Analysis
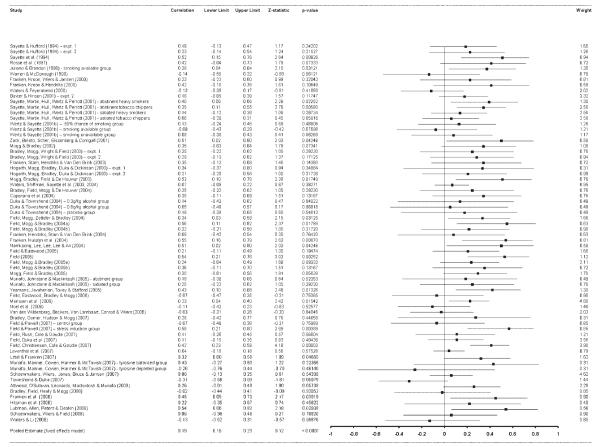
Summary data from the primary meta-analysis and all subsequent stratified analyses are shown in Table 2; a visual display of effect sizes can be seen in Figure 1. The primary meta-analysis (k = 68) indicated a significant positive correlation between attentional bias and self-reported craving (r = .19, 95% CI = 0.15–0.23, Z = 9.12, p < .001). There was evidence of significant between-study heterogeneity, χ2(67, N = 68) = 88.58, p = .040, I2 = 24.36, but when the data were analyzed within a random-effects framework, the correlation remained significant (p < .001).
Table 2.
Summary of Results
| Analysis | k | r | 95% CI | p |
|---|---|---|---|---|
| Primary meta-analysis | ||||
| All studies | 68 | .19 | 0.15, 0.23 | <.001 |
| Stratified by type of substance cue | ||||
| Alcohol | 17 | .17 | 0.09, 0.25 | <.001 |
| Tobacco | 37 | .16 | 0.11, 0.21 | <.001 |
| Other | 14 | .34 | 0.24, 0.43 | <.001 |
| Stratified by treatment-seeking status | ||||
| Treatment seeking | 15 | .20 | 0.11, 0.28 | <.001 |
| Non-treatment seeking | 53 | .19 | 0.14, 0.23 | <.001 |
| Stratified by type of craving measure | ||||
| Multi-item | 42 | .22 | 0.17, 0.27 | <.001 |
| VAS | 35 | .18 | 0.12, 0.24 | <.001 |
| Stratified by type of attentional bias task | ||||
| Modified Stroop | 26 | .15 | 0.09, 0.21 | <.001 |
| Visual probe | 30 | .19 | 0.11, 0.28 | <.001 |
| ERP (passive viewing) | 7 | .37 | 0.21, 0.51 | <.001 |
| Other | 13 | .20 | 0.11, 0.28 | <.001 |
| Stratified by subcomponent of attention | ||||
| Orienting | 12 | .08 | −0.05, 0.21 | .203 |
| Disengagement | 68 | .20 | 0.16, 0.24 | <.001 |
| Stratified by direct vs. indirect measures of attention | ||||
| Direct | 15 | .36 | 0.26, 0.46 | <.001 |
| Indirect | 59 | .18 | 0.13, 0.22 | <.001 |
| Stratified by current craving strength | ||||
| High craving | 12 | .23 | 0.15, 0.31 | <.001 |
| Low craving | 12 | .08 | 0.00, 0.17 | .061 |
Note. R values are correlations between subjective craving and attentional bias indices. CI = confidence interval; VAS = visual analogue scales; ERP = event-related potential.
Figure 1.
Effect sizes for individual studies included in the meta-analysis. The primary meta-analysis indicates a small but statistically significant positive correlation between attentional bias and subjective craving. Bars represent individual study effect sizes and 95% confidence intervals.
Publication bias
We observed asymmetry in the funnel plot of individual study effect size against the standard error of the effect size, and Egger’s test indicated formal evidence of potential publication bias, t(66) = 2.56, p = .013. When we corrected the pooled effect size estimate using Duval and Tweedie’s (2000) trim-and-fill method, based on the imputation of 17 hypothetical samples, the overall correlation was reduced (r = .13, 95% CI = 0.09–0.16) but remained nominally significant.
Effects of participant gender
Meta-regression of individual study effect size and the proportion of male versus female participants in the study did not indicate any evidence of association (p = .24).
Effect of substance cue type
Stratification by substance cue type (alcohol, tobacco, other) indicated a larger correlation among “other” substance cue types (r = .34, 95% CI = 0.24–0.43) than among alcohol and tobacco substance cue types (r = .17, 95% CI = 0.09–0.25 and r = .16, 95% CI = 0.11–0.21, respectively). This difference was statistically significant (p = .006).
Effect of treatment-seeking status of participants
Stratification by treatment-seeking status (seeking treatment, not seeking treatment) did not indicate any evidence of significant differences between participants who were seeking treatment and those who were not (p = .89).
Secondary Meta-Analyses
Stratified by craving measure
Stratification by type of craving measure (multi-item, VAS) did not indicate any evidence of significant differences between studies in which multi-item scales were used and those in which VAS were used (p = .29).
Stratified by type of attentional bias task
Stratification by type of attentional bias task (modified Stroop, visual probe, ERP, dual task, and other) revealed no significant differences between the different types of measure, although there was a marginally significant effect for a larger correlation when ERPs were used to measure attentional bias (p = .08).
Stratified by attentional subcomponent (initial orienting vs. delayed disengagement)
Stratification by the attentional subcomponent being measured (initial orienting vs. delayed disengagement) did not reveal a significant difference, although there was a marginally significant effect for a larger correlation for measures of delayed disengagement than for measures of initial orienting (p = .10).
Stratified by direct versus indirect measures of attention
Stratification by direct versus indirect measures of attention indicated a larger correlation among direct measures (r = .36, 95% CI = 0.26–0.46) than among indirect measures (r = .18, 95% CI = 0.13–0.22). This difference was statistically significant (p = .001).
Stratified by strength of craving at time of measurement
Stratification by relative craving strength (high, low) at the time of attentional bias measurement indicated a larger correlation when craving was relatively high (r = .23, 95% CI = 0.15–0.31) than when craving was relatively low (r = .08, 95% CI = 0.00–0.17); this difference was statistically significant (p = .015). Characteristics of the studies considered in this stratified analysis are shown in Table 3.
Table 3.
Summary of the Studies Included in the Stratified Meta-Analysis Exploring High vs. Low Craving Conditions
| Study | Type of craving manipulation |
Type of task (attentional bias measure) |
Correlation in high craving condition/group |
Correlation in low craving condition/group |
|---|---|---|---|---|
| Bradley et al. (2007) | Negative mood induction | Visual probe task, 2,000-ms SOA (reaction time, eye movement initial orienting, eye movement gaze duration) |
.41 | .15 |
| Field, Mogg, & Bradley (2004b) | Nicotine deprivation | Visual probe task, 2,000-ms SOA (reaction time, eye movement initial orienting, and eye movement gaze duration) |
.24 | .21 |
| Field, Mogg, & Bradley (2005b) | Alcohol priming | Visual probe task, 2,000-ms SOA (reaction time, eye movement initial orienting, eye movement gaze duration) |
.32 | .40 |
| Field & Powell (2007) | Stress | Visual probe task, 500-ms SOA (reaction time) |
.58 | −.07 |
| Field, Rush, et al. (2007) | Cue exposure | Addiction Stroop task (reaction time) | .21 | .00 |
| Leventhal et al. (2007) | Nicotine deprivation | Addiction Stroop task (reaction time) | .02 | .06 |
| Mogg & Bradley (2002) | Nicotine deprivation | Visual probe task, 500-ms SOA (reaction time) |
.32 | .38 |
| Munafò, Johnstone, & Mackintosh (2005) | Nicotine deprivation | Addiction Stroop task (reaction time) and attentional blink task (blink survival) |
.19 | .25 |
| Sayette et al. (2001) | Nicotine deprivation and smoking status (heavy smokers vs. tobacco chippers) |
Dual task procedure (reaction time) | .40 | .06 |
| Sayette & Hufford (1994) (Exper. 1) | Nicotine deprivation | Dual task procedure (reaction time) | .38 | .00 |
| Sayette & Hufford (1994) (Exper. 2) | Nicotine deprivation | Dual task procedure (reaction time) | .39 | .06 |
| Schoenmakers et al. (2008) | Alcohol priming | Visual probe task, 2,000-ms SOA (reaction time, eye movement initial orienting, eye movement gaze duration) |
.19 | −.08 |
Note. SOA = stimulus onset asynchrony.
Power Analysis
The overall effect size estimate (r = .19) indicated that the sample size needed for detection of this correlation with 80% power at α=.05 would be in excess of n = 212. This suggests that the studies conducted to date lack sufficient statistical power. Power calculations were performed with G*Power3 (Faul, Erdfelder, Lang, & Buchner, 2007).
Discussion
As hypothesized, the meta-analysis indicated a modest but statistically significant positive correlation between subjective craving and attentional bias. This result is consistent with predictions made by the diverse theoretical models that were discussed in the Introduction (Baker et al., 1987; Field & Cox, 2008; Franken, 2003; Kavanagh et al., 2005; Robinson & Berridge, 1993; Ryan, 2002). However, the overall correlation was small (r = .19), and this implies a low level of shared variance (less than 4%) between the two constructs. Furthermore, evidence of publication bias was detected, and when we corrected for this using a trim-and-fill procedure, the estimated overall correlation was reduced still further (to r = .13, which implies 2% shared variance between the two constructs). Although some of the theoretical models that were previously discussed do acknowledge the possibility that craving and attentional bias can be decoupled in certain circumstances (e.g., Robinson & Berridge, 1993), the size of the relationship between the two variables must be considered problematic for these models.
Our analysis revealed significant between-study heterogeneity, and the additional stratified analyses shed some light on the factors that appear to moderate the association between the two variables. These analyses also suggest a combination of conditions that might be expected to reveal a larger correlation between craving and attentional bias: studying users of illicit drugs or caffeine (rather than alcohol or tobacco), using direct rather than indirect measures to infer attentional processing and studying individuals in whom the current strength of craving is high rather than low. These factors are discussed in turn below.
First, the magnitude of the correlation was significantly larger when caffeine, cannabis, cocaine, and heroin cues were presented than when alcohol or tobacco cues were presented. It should be noted that only 14 data sets (21%) assessed the correlation involving substances other than tobacco or alcohol, and this category of other largely comprised studies investigating attentional bias for cues related to cannabis (k = 3), heroin (k = 5), and cocaine (k = 5). The small sample size did not permit us to statistically compare the correlations across these three illicit drugs, although inspection of the data in Table 1 suggests no consistent difference between these substances. The reason for the generally smaller correlation between craving and attentional bias for tobacco and alcohol cues is presently unclear, and future research should examine if this difference is robust.
Second, an additional stratified analysis revealed that the correlation between attentional bias and subjective craving was significantly larger in studies that directly measured selective attention than in studies that used other, indirect measures to infer the allocation of attention. Direct measures were eye movement monitoring or the measurement of ERPs; in contrast, the vast majority of indirect measures used manual reaction time to infer attentional processing. This difference was large; for direct measures, the average correlation was r = .36 (13% of shared variance), whereas for indirect measures, the average correlation was r = .18 (3% of shared variance). The most obvious explanation for this difference is that indirect measures, such as response time, provide only a crude indicator of attentional processes; one might expect these measures to yield an overall smaller correlation between attentional bias and other constructs (subjective craving, in this case). Researchers are encouraged to use eye movement monitoring or ERP recording in future studies on attentional biases in substance abuse, as these methods appear to be particularly sensitive.
Stratified analyses also revealed that among 12 data sets in which craving was experimentally manipulated, the correlation between craving and attentional bias was significantly larger when both were measured after an experimental manipulation that increased the strength of subjective craving (r = .23, 5% of shared variance) than after a control manipulation in which craving was lower (r = .08, less than 1% of shared variance). We know of at least one additional data set in which the observed correlation between craving and attentional bias (as measured with reaction time during a dual task procedure) was larger when craving was relatively high than when it was relatively low (Juliano & Brandon, 1998). Although this study was included in the primary meta-analysis, it could not be included in the subsequent stratified analysis comparing high and low craving conditions because the correlation from the low craving condition was not available. Therefore, the association between craving and attentional bias appears to be larger when the strength of subjective craving is relatively high at the time of assessment, and this finding is robust. This finding may be theoretically important, as it supports predictions made by some investigators (Baker et al., 1987; Sayette et al., 2001, 2003) that subjective craving should be most closely correlated with aspects of cognition and physiology when the current strength of craving is high. For example, in the context of Baker et al.’s (1987) model, activation of either positive or negative affect urge networks—which would be achieved by the experimental manipulations, such as deprivation, negative mood induction, and acute intoxication, that were used in the studies considered here—should be characterized by an increase in subjective craving and increased coherence between subjective craving and indices of urge network activation in other domains, which may include the cognitive domain. According to Baker et al., craving is a form of affect that shares important features with other affects (i.e., all appetitive and aversive emotional states). The present finding therefore has broader implications for our understanding of other emotional states and their correlates in other response domains.
With regard to the theoretical framework outlined by Lang et al. (1998), the present findings are consistent with the notions that the experience of an emotional state has correlates in terms of cognitive processing and that the correlations between indices derived from different response domains become more robust as activation of the emotional state increases. Our analysis provides some support for this claim in the context of an appetitive state—subjective craving—but future researchers are encouraged to examine whether similar processes are in operation in the context of aversive emotional states. For example, do subjective anxiety and attentional bias for threat cues become more closely correlated as the current strength of subjective anxiety increases?
A number of additional stratified analyses revealed marginally significant tendencies for other variables to moderate the strength of the association between subjective craving and attentional bias indices; these warrant a brief mention here. First, stratified analyses exploring different attentional bias tasks, or the subcomponents of attention that they measure, yielded some interesting findings. There was a marginally significant effect (p = .08) for a main effect of task type; however, inspection of the data in Table 2 reveals that this was due primarily to the overall larger correlation for ERP measures, which was previously discussed. The magnitude of the correlation did not differ when the other measures of attentional bias (modified Stroop, visual probe task, dual task procedures, and other) were compared with each other. Perhaps more interesting is the marginally significant effect (p = .10) for a larger association between craving and attentional bias when measures of the disengagement of attention from substance-related cues (r = .20, 4% of shared variance) were compared with measures of the initial orienting of attention (r = .08, less than 1% of shared variance). The small number of studies that employed a measure of initial orienting of attention (k = 12) may account for the failure of this factor to attain statistical significance, so we urge future researchers to follow up the findings reported here by including measures of both initial orienting and delayed disengagement of attention when they study attentional biases in addiction. If the demonstration of a larger correlation between craving and measures of the delayed disengagement of attention subsequently proves to be a robust one, this would be consistent with theoretical models that suggest that craving strength should be correlated with the extent of rumination on, or slow disengagement of attention from, substance-related cues (e.g., Kavanagh et al., 2005).
Finally, when the analysis was stratified by treatment-seeking status of participants, there was no apparent difference between participants who were currently receiving treatment for substance abuse and participants who were not seeking treatment. Thus we found no support for our hypothesis that the correlation would be reduced among patients in treatment compared to substance users who were not in treatment. This hypothesis was based on a number of observations: Alcohol-dependent patients in treatment appear to show overt attentional avoidance of alcohol-related cues, rather than attentional bias for them (Noel et al., 2006; Stormark et al., 1997; Townshend & Duka, 2007), and patients in treatment tend to report lower levels of craving than do individuals who are not currently seeking treatment (Wertz & Sayette, 2001a). It is also pertinent that Baker et al. (1987) specifically hypothesized that the physiological and cognitive correlates of subjective craving would be expected to differ among continuing substance users compared to patients in treatment. However, our analysis revealed that the association between craving and attentional bias was not moderated by the treatment-seeking status of participants. We conclude that the association between attentional bias and subjective craving appears to be moderated by a number of factors, but the treatment-seeking status of participants may not be one of them. Therefore, this aspect of Baker et al.’s urge network model may require modification.
We note that there was evidence of significant publication bias in the 68 data sets that were considered, both empirically (based on the numbers of papers that did not report the necessary correlational data) and formally (based on our funnel plot analyses). To better delineate the relationship between attentional bias and subjective craving, studies should consistently report these data (even if they are nonsignificant). However, we are confident that we managed to include almost every published data set in which attentional bias and subjective craving were measured in the same study, given our extensive contact with study authors and access to our own data. It is possible, of course, that some relevant studies remain unpublished, and we attempted to model the effect of this possibility using the trim-and-fill procedure. The results of this procedure suggest that our global estimate of the correlation between attentional bias and subjective craving might be an overestimate. It was also notable that all of the previously published studies examined biases in selective attention for visually presented substance cues, but substance-related cues can be detected in other sensory modalities, such as olfactory (e.g., the smell of whiskey), tactile (e.g., the handling of drug-related paraphernalia), and auditory (e.g., the click of a cigarette lighter). Therefore, the present meta-analysis is informative as to the association between subjective craving and biases in visual selective attention, but future researchers are encouraged to examine if craving is associated with bias in other sensory modalities as well. Finally, we were unable to examine the possible moderating influence of demographic variables such as race, ethnicity, or socioeconomic status on the association between subjective craving and attentional bias. This was because the majority of studies did not provide this type of information. We encourage future researchers to provide more information about their sample characteristics, so that these variables can be taken into consideration.
In summary, the present meta-analysis demonstrates that attentional bias for substance-related cues is positively correlated with the current level of substance craving, as predicted by numerous theoretical models. However, the overall correlation is very small (r = .19), and the relationship appears to be moderated by several factors. The magnitude of the correlation appears to be significantly larger for craving for illicit substances and caffeine than for craving for alcohol and tobacco, larger when selective attention is directly measured rather than indirectly inferred, and larger when subjective craving is relatively high at the time of measurement. These findings indicate important future directions for primary research and also indicate the likely sample sizes that will be necessary to confirm or refute these effects. Existing models of substance abuse that incorporate craving, attentional biases, and their interrelationships may require modification in the light of these findings, and these findings generate interesting questions for future research.
Acknowledgments
We are grateful to Brendan Bradley, Marc Copersino, Theodora Duka, Dan Lubman, Karin Mogg, Xavier Noel, Michael Sayette, Tim Schoenmakers, Andrew Waters, Esther van den Wildenberg, and Martin Zack for providing data in a format that enabled their inclusion in the meta-analyses we report. We thank Michael Sayette for his helpful suggestions regarding some aspects of the data analysis.
Contributor Information
Matt Field, School of Psychology, University of Liverpool, Liverpool, United Kingdom.
Marcus R. Munafò, Department of Experimental Psychology, University of Bristol, Bristol, United Kingdom
Ingmar H. A. Franken, Institute of Psychology, Erasmus University Rotterdam, Rotterdam, the Netherlands.
References
References marked with an asterisk indicate studies included in the meta-analysis.
- Anton RF. Obsessive-compulsive aspects of craving: Development of the Obsessive Compulsive Drinking Scale. Addiction. 2000;95:S211–S217. doi: 10.1080/09652140050111771. [DOI] [PubMed] [Google Scholar]
- *.Attwood AS, O’Sullivan H, Leonards U, Mackintosh B, Munafò MR. Attentional bias training and cue reactivity in cigarette smokers. Addiction. 2008;103:1875–1882. doi: 10.1111/j.1360-0443.2008.02335.x. [DOI] [PubMed] [Google Scholar]
- Baker TB, Morse E, Sherman JE. The motivation to use drugs: A psychobiological analysis of urges. In: Dienstbier RA, Rivers PC, editors. Nebraska Symposium on Motivation. Vol. 34. Alcohol and addictive behavior. University of Nebraska Press; Lincoln: 1987. pp. 257–323. [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- *.Baxter BW, Hinson RE. Is smoking automatic? Demands of smoking behavior on attentional resources. Journal of Abnormal Psychology. 2001;110:59–66. doi: 10.1037//0021-843x.110.1.59. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcoholism: Clinical and Experimental Research. 1995;19:600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- *.Bradley B, Field M, Mogg K, De Houwer J. Attentional and evaluative biases for smoking cues in nicotine dependence: Component processes of biases in visual orienting. Behavioural Pharmacology. 2004;15:29–36. doi: 10.1097/00008877-200402000-00004. [DOI] [PubMed] [Google Scholar]
- *.Bradley BP, Field M, Healy H, Mogg K. Do the affective properties of smoking-related cues influence attentional and approach biases in cigarette smokers. Journal of Psychopharmacology. 2008;22:737–745. doi: 10.1177/0269881107083844. [DOI] [PubMed] [Google Scholar]
- *.Bradley BP, Garner M, Hudson L, Mogg K. Influence of negative affect on selective attention to smoking-related cues and urge to smoke in cigarette smokers. Behavioural Pharmacology. 2007;18:255–263. doi: 10.1097/FBP.0b013e328173969b. [DOI] [PubMed] [Google Scholar]
- *.Bradley BP, Mogg K, Wright T, Field M. Attentional bias in drug dependence: Vigilance for cigarette-related cues in smokers. Psychology of Addictive Behaviors. 2003;17:66–72. doi: 10.1037/0893-164x.17.1.66. [DOI] [PubMed] [Google Scholar]
- Cane JE, Sharma D, Albery IP. The addiction Stroop task: Examining the fast and slow effects of smoking and marijuana-related cues. Journal of Psychopharmacology. 2008 doi: 10.1177/0269881108091253. 0, 0269881108091253v2. Advance online publication. doi: 10.1177/0269881108091253. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Clark D. Craving for alcohol. Journal of Psychopharmacology. 1994;9:73. [Google Scholar]
- *.Copersino ML, Serper MR, Vadhan N, Goldberg BR, Richarme D, Chou JCY, et al. Cocaine craving and attentional bias in cocaine-dependent schizophrenic patients. Psychiatry Research. 2004;128:209–218. doi: 10.1016/j.psychres.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Coull JT. Neural correlates of attention and arousal: Insights from electrophysiology, functional neuroimaging and psychopharmacology. Progress in Neurobiology. 1998;55:343–361. doi: 10.1016/s0301-0082(98)00011-2. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the Brief Questionnaire of Smoking Urges (QSU-brief) in laboratory and clinical settings. Nicotine and Tobacco Research. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Cox WM, Fadardi JS, Pothos EM. The addiction Stroop test: Theoretical considerations and procedural recommendations. Psychological Bulletin. 2006;132:443–476. doi: 10.1037/0033-2909.132.3.443. [DOI] [PubMed] [Google Scholar]
- Davis WA. The two senses of desire. Philosophical Studies. 1984;45:181–195. [Google Scholar]
- Dickinson A, Dearing MF. Appetitive–aversive interactions and inhibitory processes. In: Dickinson A, Boakes RA, editors. Mechanisms of learning and motivation. Erlbaum; Hillsdale, NJ: 1979. pp. 203–231. [Google Scholar]
- Drobes DJ, Thomas SE. Assessing craving for alcohol. Alcohol Research and Health. 1999;23:179–186. [PMC free article] [PubMed] [Google Scholar]
- Drummond DC, Phillips TS. Alcohol urges in alcohol-dependent drinkers: Further validation of the Alcohol Urge Questionnaire in an untreated community clinical population. Addiction. 2002;97:1465–1472. doi: 10.1046/j.1360-0443.2002.00252.x. [DOI] [PubMed] [Google Scholar]
- *.Duka T, Townshend JM. The priming effect of alcohol pre-load on attentional bias to alcohol-related stimuli. Psychopharmacology. 2004;176:353–362. doi: 10.1007/s00213-004-1906-7. [DOI] [PubMed] [Google Scholar]
- Duncan J, Ward R, Shapiro K. Direct measurement of attentional dwell time in human vision. Nature. 1994;369:313–315. doi: 10.1038/369313a0. [DOI] [PubMed] [Google Scholar]
- Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey-Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. British Medical Journal. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Bromwell MA, Lankford ME, Monterosso JR, O’Brien CP. Comparing attentional bias to smoking cues in current smokers, former smokers, and non-smokers using a dot-probe task. Drug and Alcohol Dependence. 2002;67:185–191. doi: 10.1016/s0376-8716(02)00065-0. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- *.Field M. Cannabis “dependence” and attentional bias for cannabis-related words. Behavioural Pharmacology. 2005;16:473–476. doi: 10.1097/00008877-200509000-00021. [DOI] [PubMed] [Google Scholar]
- *.Field M, Christiansen P, Cole J, Goudie A. Delay discounting and the alcohol Stroop in heavy drinking adolescents. Addiction. 2007;102:579–586. doi: 10.1111/j.1360-0443.2007.01743.x. [DOI] [PubMed] [Google Scholar]
- Field M, Cox WM. Attentional bias in addictive behaviors: A review of its development, causes, and consequences. Drug and Alcohol Dependence. 2008;97:1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- *.Field M, Duka T, Eastwood B, Child R, Santarcangelo M, Gayton M. Experimental manipulation of attentional biases in heavy drinkers: Do the effects generalise? Psychopharmacology. 2007;192:593–608. doi: 10.1007/s00213-007-0760-9. [DOI] [PubMed] [Google Scholar]
- *.Field M, Eastwood B. Experimental manipulation of attentional bias increases the motivation to drink alcohol. Psychopharmacology. 2005;183:350–357. doi: 10.1007/s00213-005-0202-5. [DOI] [PubMed] [Google Scholar]
- *.Field M, Eastwood B, Bradley BP, Mogg K. Selective processing of cannabis cues in regular cannabis users. Drug and Alcohol Dependence. 2006;85:75–82. doi: 10.1016/j.drugalcdep.2006.03.018. [DOI] [PubMed] [Google Scholar]
- *.Field M, Mogg K, Bradley BP. Cognitive bias and drug craving in recreational cannabis users. Drug and Alcohol Dependence. 2004a;74:105–111. doi: 10.1016/j.drugalcdep.2003.12.005. [DOI] [PubMed] [Google Scholar]
- *.Field M, Mogg K, Bradley BP. Eye movements to smoking-related cues: Effects of nicotine deprivation. Psychopharmacology. 2004b;173:116–123. doi: 10.1007/s00213-003-1689-2. [DOI] [PubMed] [Google Scholar]
- *.Field M, Mogg K, Bradley BP. Alcohol increases cognitive biases for smoking cues in smokers. Psychopharmacology. 2005a;180:63–72. doi: 10.1007/s00213-005-2251-1. [DOI] [PubMed] [Google Scholar]
- *.Field M, Mogg K, Bradley BP. Craving and cognitive biases for alcohol cues in social drinkers. Alcohol and Alcoholism. 2005b;40:504–510. doi: 10.1093/alcalc/agh213. [DOI] [PubMed] [Google Scholar]
- *.Field M, Mogg K, Zetteler J, Bradley BP. Attentional biases for alcohol cues in heavy and light social drinkers: The roles of initial orienting and maintained attention. Psychopharmacology. 2004;176:88–93. doi: 10.1007/s00213-004-1855-1. [DOI] [PubMed] [Google Scholar]
- *.Field M, Powell H. Stress increases attentional bias for alcohol cues in social drinkers who drink to cope. Alcohol and Alcoholism. 2007;42:560–566. doi: 10.1093/alcalc/agm064. [DOI] [PubMed] [Google Scholar]
- *.Field M, Rush M, Cole J, Goudie A. The smoking Stroop and delay discounting in smokers: Effects of environmental smoking cues. Journal of Psychopharmacology. 2007;21:603–610. doi: 10.1177/0269881106070995. [DOI] [PubMed] [Google Scholar]
- Fox E, Russo R, Bowles R, Dutton K. Do threatening stimuli draw or hold visual attention in subclinical anxiety? Journal of Experimental Psychology: General. 2001;130:681–700. [PMC free article] [PubMed] [Google Scholar]
- Franken IHA. Drug craving and addiction: Integrating psychological and neuropsychopharmacological approaches. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2003;27:563–579. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- *.Franken IHA, Dietvorst RC, Hesselmans M, Franzek EJ, van de Wetering BJ, van Strien JW. Cocaine craving is associated with electrophysiological brain responses to cocaine-related stimuli. Addiction Biology. 2008;13:386–392. doi: 10.1111/j.1369-1600.2008.00100.x. [DOI] [PubMed] [Google Scholar]
- *.Franken IHA, Hendriks VM, Stam CJ, van den Brink W. A role for dopamine in the processing of drug cues in heroin dependent patients. European Neuropsychopharmacology. 2004;14:503–508. doi: 10.1016/j.euroneuro.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Franken IHA, Hendriks VM, van den Brink W. Initial validation of two opiate craving questionnaires: The Obsessive Compulsive Drug Use Scale and the Desires for Drug Questionnaire. Addictive Behaviors. 2002;27:675–685. doi: 10.1016/s0306-4603(01)00201-5. [DOI] [PubMed] [Google Scholar]
- *.Franken IHA, Hulstijn KP, Stam CJ, Hendriks VM, van den Brink W. Two new neurophysiological indices of cocaine craving: Evoked brain potentials and cue modulated startle reflex. Journal of Psychopharmacology. 2004;18:544–552. doi: 10.1177/0269881104047282. [DOI] [PubMed] [Google Scholar]
- *.Franken IHA, Kroon LY, Hendriks VM. Influence of individual differences in craving and obsessive cocaine thoughts on attentional processes in cocaine abuse patients. Addictive Behaviors. 2000;25:99–102. doi: 10.1016/s0306-4603(98)00112-9. [DOI] [PubMed] [Google Scholar]
- *.Franken IHA, Kroon LY, Wiers RW, Jansen A. Selective cognitive processing of drug cues in heroin dependence. Journal of Psychopharmacology. 2000;14:395–400. doi: 10.1177/026988110001400408. [DOI] [PubMed] [Google Scholar]
- *.Franken IHA, Stam CJ, Hendriks VM, van den Brink W. Neurophysiological evidence for abnormal cognitive processing of drug cues in heroin dependence. Psychopharmacology. 2003;170:205–212. doi: 10.1007/s00213-003-1542-7. [DOI] [PubMed] [Google Scholar]
- Frijda NH. The emotions. Cambridge University Press; Cambridge, England: 1986. [Google Scholar]
- Hayes S, Hirsch CR. Information processing biases in generalized anxiety disorder. Psychiatry. 2007;6:176–182. [Google Scholar]
- Heishman SJ, Singleton EG, Liguori A. Marijuana Craving Questionnaire: Development and initial validation of a self-report instrument. Addiction. 2001;96:1023–1034. doi: 10.1046/j.1360-0443.2001.967102312.x. [DOI] [PubMed] [Google Scholar]
- *.Hitsman B, MacKillop J, Lingford-Hughes A, Williams TM, Ahmad F, Adams S, et al. Effects of acute tyrosine/phenylalanine depletion on the selective processing of smoking-related cues and the relative value of cigarettes in smokers. Psychopharmacology. 2008;196:611–621. doi: 10.1007/s00213-007-0995-5. [DOI] [PubMed] [Google Scholar]
- *.Hogarth LC, Mogg K, Bradley BP, Duka T, Dickinson A. Attentional orienting towards smoking-related stimuli. Behavioural Pharmacology. 2003;14:153–160. doi: 10.1097/00008877-200303000-00007. [DOI] [PubMed] [Google Scholar]
- James D, Davies G, Willner P. The development and initial validation of a questionnaire to measure craving for amphetamine. Addiction. 2004;99:1181–1188. doi: 10.1111/j.1360-0443.2004.00819.x. [DOI] [PubMed] [Google Scholar]
- *.Juliano LM, Brandon TH. Reactivity to instructed smoking availability and environmental cues: Evidence with urge and reaction time. Experimental and Clinical Psychopharmacology. 1998;6:45–53. doi: 10.1037//1064-1297.6.1.45. [DOI] [PubMed] [Google Scholar]
- Kavanagh DJ, Andrade J, May J. Imaginary relish and exquisite torture: The elaborated intrusion theory of desire. Psychological Review. 2005;112:446–467. doi: 10.1037/0033-295X.112.2.446. [DOI] [PubMed] [Google Scholar]
- Keil A, Ihssen N. Identification facilitation for emotionally arousing verbs during the attentional blink. Emotion. 2004;4:23–35. doi: 10.1037/1528-3542.4.1.23. [DOI] [PubMed] [Google Scholar]
- Konorski J. Integrative activity of the brain: An interdisciplinary approach. University of Chicago Press; Chicago: 1967. [Google Scholar]
- Kowler E. Eye movements. In: Kosslyn SM, Osheron DM, editors. Visual cognition. Harvard University Press; Cambridge, MA: 1995. pp. 215–265. [Google Scholar]
- Lang PJ. The varieties of emotional experience: A meditation on James–Lange theory. Psychological Review. 1994;101:211–221. doi: 10.1037/0033-295x.101.2.211. [DOI] [PubMed] [Google Scholar]
- Lang PJ. The emotion probe: Studies of motivation and attention. American Psychologist. 1995;50:372–385. doi: 10.1037//0003-066x.50.5.372. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Motivated attention: Affect, activation, and action. In: Lang PJ, Simons RF, Balaban MT, editors. Attention and orienting: Sensory and motivational processes. Erlbaum; Mahwah, NJ: 1997. pp. 97–135. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, motivation, and anxiety: Brain mechanisms and psychophysiology. Biological Psychiatry. 1998;44:1248–1263. doi: 10.1016/s0006-3223(98)00275-3. [DOI] [PubMed] [Google Scholar]
- Lavy E, van den Hout M. Selective attention evidenced by pictorial and linguistic Stroop tasks. Behavior Therapy. 1993;24:645–657. [Google Scholar]
- *.Leventhal AM, Waters AJ, Boyd S, Moolchan ET, Lerman C, Pickworth WB. Gender differences in acute tobacco withdrawal: Effects on subjective, cognitive, and physiological measures. Experimental and Clinical Psychopharmacology. 2007;15:21–36. doi: 10.1037/1064-1297.15.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Littel M, Franken IHA. The effects of prolonged abstinence on the processing of smoking cues: An ERP study among smokers, ex-smokers and never-smokers. Journal of Psychopharmacology. 2007;21:873–882. doi: 10.1177/0269881107078494. [DOI] [PubMed] [Google Scholar]
- Love A, James D, Willner P. A comparison of two alcohol craving questionnaires. Addiction. 1998;93:1091–1102. doi: 10.1046/j.1360-0443.1998.937109113.x. [DOI] [PubMed] [Google Scholar]
- *.Lubman DI, Allen NB, Peters LA, Deakin JFW. Electrophysiological evidence that drug cues have greater salience than other affective stimuli in opiate addiction. Journal of Psychopharmacology. 2008;22:836–842. doi: 10.1177/0269881107083846. [DOI] [PubMed] [Google Scholar]
- MacKillop J. Factor structure of the Alcohol Urge Questionnaire under neutral conditions and during a cue-elicited urge state. Alcoholism: Clinical and Experimental Research. 2006;30:1315–1321. doi: 10.1111/j.1530-0277.2006.00159.x. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. Journal of Abnormal Psychology. 1986;95:15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- Marissen MAE, Franken IHA, Blanken P, van den Brink W, Hendriks VM. The relation between social desirability and different measures of heroin craving. Journal of Addictive Diseases. 2005;24:91–103. doi: 10.1300/j069v24n04_07. [DOI] [PubMed] [Google Scholar]
- *.Marissen MAE, Franken IHA, Waters AJ, Blanken P, van den Brink W, Hendriks VM. Attentional bias predicts heroin relapse following treatment. Addiction. 2006;101:1306–1312. doi: 10.1111/j.1360-0443.2006.01498.x. [DOI] [PubMed] [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacology. 1993;112:163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- Marlatt GA. Taxonomy of high-risk situations for alcohol relapse: Evolution and development of a cognitive-behavioral model. Addiction. 1996;91(Suppl. 1):S37–S49. [PubMed] [Google Scholar]
- McEvoy PM, Stritzke WGK, French DJ, Lang AR, Ketterman RL. Comparison of three models of alcohol craving in young adults: A cross-validation. Addiction. 2004;99:482–497. doi: 10.1111/j.1360-0443.2004.00714.x. [DOI] [PubMed] [Google Scholar]
- *.Mogg K, Bradley BP. Selective processing of smoking-related cues in smokers: Manipulation of deprivation level and comparison of three measures of processing bias. Journal of Psychopharmacology. 2002;16:385–392. doi: 10.1177/026988110201600416. [DOI] [PubMed] [Google Scholar]
- *.Mogg K, Bradley BP, Field M, De Houwer J. Eye movements to smoking-related pictures in smokers: Relationship between attentional biases and implicit and explicit measures of stimulus valence. Addiction. 2003;98:825–836. doi: 10.1046/j.1360-0443.2003.00392.x. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Hyare H, Lee S. Selective attention to food-related stimuli in hunger: Are attentional biases specific to emotional and psychopathological states, or are they also found in normal drive states? Behaviour Research and Therapy. 1998;36:227–237. doi: 10.1016/s0005-7967(97)00062-4. [DOI] [PubMed] [Google Scholar]
- *.Mogg K, Field M, Bradley BP. Attentional and approach biases for smoking cues in smokers: An investigation of competing theoretical views of addiction. Psychopharmacology. 2005;180:333–341. doi: 10.1007/s00213-005-2158-x. [DOI] [PubMed] [Google Scholar]
- Mogg K, Philippot P, Bradley BP. Selective attention to angry faces in clinical social phobia. Journal of Abnormal Psychology. 2004;113:160–165. doi: 10.1037/0021-843X.113.1.160. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Albery IP. Cognition and addiction. Oxford University Press; Oxford, England: 2006. [Google Scholar]
- *.Munafò MR, Johnstone EC, Mackintosh B. Association of serotonin transporter genotype with selective processing of smoking-related stimuli in current smokers and ex-smokers. Nicotine and Tobacco Research. 2005;7:773–778. doi: 10.1080/14622200500259861. [DOI] [PubMed] [Google Scholar]
- *.Munafò MR, Mannie ZN, Cowen PJ, Harmer CJ, McTavish SB. Effects of acute tyrosine depletion on subjective craving and selective processing of smoking-related cues in abstinent cigarette smokers. Journal of Psychopharmacology. 2007;21:805–814. doi: 10.1177/0269881107077216. [DOI] [PubMed] [Google Scholar]
- *.Namkoong K, Lee E, Lee CH, Lee BO, An SK. Increased P3 amplitudes induced by alcohol-related pictures in patients with alcohol dependence. Alcoholism: Clinical and Experimental Research. 2004;28:1317–1323. doi: 10.1097/01.alc.0000139828.78099.69. [DOI] [PubMed] [Google Scholar]
- Nijs IMT, Franken IHA, Muris P. The modified Trait and State Food-Cravings Questionnaires: Development and validation of a general index of food craving. Appetite. 2007;49:38–46. doi: 10.1016/j.appet.2006.11.001. [DOI] [PubMed] [Google Scholar]
- *.Noel X, Colmant M, van der Linden M, Bechara A, Bullens Q, Hanak C, et al. Time course of attention for alcohol cues in abstinent alcoholic patients: The role of initial orienting. Alcoholism: Clinical and Experimental Research. 2006;30:1871–1877. doi: 10.1111/j.1530-0277.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Epstein LH, Grobe J, Fonte C. Tobacco abstinence, smoking cues, and the reinforcing value of smoking. Pharmacology Biochemistry and Behavior. 1994;47:107–112. doi: 10.1016/0091-3057(94)90118-x. [DOI] [PubMed] [Google Scholar]
- Phaf RH, Kan KJ. The automaticity of emotional Stroop: A meta-analysis. Journal of Behavior Therapy and Experimental Psychiatry. 2007;38:184–199. doi: 10.1016/j.jbtep.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Posner MI, Snyder CR, Davidson BJ. Attention and the detection of signals. Journal of Experimental Psychology. 1980;109:160–174. [PubMed] [Google Scholar]
- Pourtois G, Grandjean D, Sander D, Vuilleumier P. Electrophysiological correlates of rapid spatial orienting towards fearful faces. Cerebral Cortex. 2004;14:619–633. doi: 10.1093/cercor/bhh023. [DOI] [PubMed] [Google Scholar]
- Raabe A, Grusser SM, Wessa M, Podschus J, Flor H. The assessment of craving: Psychometric properties, factor structure and a revised version of the Alcohol Craving Questionnaire (ACQ) Addiction. 2005;100:227–234. doi: 10.1111/j.1360-0443.2005.00960.x. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN. The role of attentional bias in substance abuse. Behavioral and Cognitive Neuroscience Reviews. 2004;3:243–260. doi: 10.1177/1534582305275423. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rooke SE, Hine DW, Thorsteinnsson EB. Implicit cognition and substance use: A meta-analysis. Addictive Behaviors. 2008;33:1314–1328. doi: 10.1016/j.addbeh.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Rosenberg H, Mazzola J. Relationships among self-report assessments of craving in binge-drinking university students. Addictive Behaviors. 2007;32:2811–2818. doi: 10.1016/j.addbeh.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, DiMatteo MR. Meta-analysis: Recent developments in quantitative methods for literature reviews. Annual Review of Psychology. 2001;52:59–82. doi: 10.1146/annurev.psych.52.1.59. [DOI] [PubMed] [Google Scholar]
- *.Rosse RB, Johri S, Kendrick K, Hess AL, Alim TN, Miller M, et al. Preattentive and attentive eye movements during visual scanning of a cocaine cue: Correlation with intensity of cocaine cravings. Journal of Neuropsychiatry and Clinical Neurosciences. 1997;9:91–93. doi: 10.1176/jnp.9.1.91. [DOI] [PubMed] [Google Scholar]
- Ryan F. Detected, selected, and sometimes neglected: Cognitive processing of cues in addiction. Experimental and Clinical Psychopharmacology. 2002;10:67–76. doi: 10.1037//1064-1297.10.2.67. [DOI] [PubMed] [Google Scholar]
- *.Sayette MA, Hufford MR. Effects of cue exposure and deprivation on cognitive resources in smokers. Journal of Abnormal Psychology. 1994;103:812–818. doi: 10.1037//0021-843x.103.4.812. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Martin CS, Hull JG, Wertz JM, Perrott MA. Effects of nicotine deprivation on craving response covariation in smokers. Journal of Abnormal Psychology. 2003;112:110–118. [PMC free article] [PubMed] [Google Scholar]
- *.Sayette MA, Martin CS, Wertz JM, Shiffman S, Perrott MA. A multi-dimensional analysis of cue-elicited craving in heavy smokers and tobacco chippers. Addiction. 2001;96:1419–1432. doi: 10.1080/09652140120075152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Sayette MA, Monti PM, Rohsenow DJ, Gulliver SB, Colby SM, Sirota AD, et al. The effects of cue exposure on reaction time in male alcoholics. Journal of Studies on Alcohol. 1994;55:629–633. doi: 10.15288/jsa.1994.55.629. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Shiffman S, Tiffany ST, Niaura RS, Martin CS, Shadel WG. The measurement of drug craving. Addiction. 2000;95(Suppl. 2):S189–S210. doi: 10.1080/09652140050111762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Schoenmakers T, Wiers RW, Field M. Effects of a low dose of alcohol on cognitive biases and craving in heavy drinkers. Psychopharmacology. 2008;197:169–178. doi: 10.1007/s00213-007-1023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Schoenmakers T, Wiers RW, Jones BT, Bruce G, Jansen ATM. Attentional re-training decreases attentional bias in heavy drinkers without generalization. Addiction. 2007;102:399–405. doi: 10.1111/j.1360-0443.2006.01718.x. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Flaisch T, Stockburger J, Junghofer M. Emotion and attention: Event-related brain potential studies. Progress in Brain Research. 2006;156:31–51. doi: 10.1016/S0079-6123(06)56002-9. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Stockburger J, Codispoti M, Junghofer M, Weike AI, Hamm AO. Selective visual attention to emotion. Journal of Neuroscience. 2007;27:1082–1089. doi: 10.1523/JNEUROSCI.3223-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton EG, Tiffany ST, Henningfield JE. Development and validation of a new questionnaire to assess craving for alcohol. In: Harris LS, editor. Problems of drug dependence, 1994: Proceedings of the 56th annual scientific meeting, the College on Problems of Drug Dependence, Inc.; Rockville, MD: National Institute on Drug Abuse; 1995. p. 289. NIDA Research Monograph No. 153, vol. 2. [Google Scholar]
- Stockburger J, Weike AI, Hamm AO, Schupp HT. Deprivation selectively modulates brain potentials to food pictures. Behavioral Neuroscience. 2008;122:936–942. doi: 10.1037/a0012517. [DOI] [PubMed] [Google Scholar]
- Stormark KM, Field NP, Hugdahl K, Horowitz M. Selective processing of visual alcohol cues in abstinent alcoholics: An approach–avoidance conflict? Addictive Behaviors. 1997;22:509–519. doi: 10.1016/s0306-4603(96)00051-2. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reaction. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Sussner BD, Smelson DA, Rodrigues S, Kline A, Losonczy M, Ziedonis D. The validity and reliability of a brief measure of cocaine craving. Drug and Alcohol Dependence. 2006;83:233–237. doi: 10.1016/j.drugalcdep.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Theeuwes J. Irrelevant singletons capture attention. In: Itti L, Rees G, Tsotsos JK, editors. Neurobiology of attention. Elsevier Academic Press; London: 2005. pp. 418–424. [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: Role of automatic and nonautomatic processes. Psychological Review. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. British Journal of Addiction. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Singleton E, Haertzen CA, Henningfield JE. The development of a cocaine craving questionnaire. Drug and Alcohol Dependence. 1993;34:19–28. doi: 10.1016/0376-8716(93)90042-o. [DOI] [PubMed] [Google Scholar]
- *.Townshend JM, Duka T. Avoidance of alcohol-related stimuli in alcohol-dependent inpatients. Alcoholism: Clinical and Experimental Research. 2007;3:1349–1357. doi: 10.1111/j.1530-0277.2007.00429.x. [DOI] [PubMed] [Google Scholar]
- *.van den Wildenberg E, Beckers M, van Lambaart F, Conrod PJ, Wiers RW. Is the strength of implicit alcohol associations correlated with alcohol-induced heart-rate acceleration? Alcoholism: Clinical and Experimental Research. 2006;30:1336–1348. doi: 10.1111/j.1530-0277.2006.00161.x. [DOI] [PubMed] [Google Scholar]
- *.Warren CA, McDonough BE. Event-related brain potentials as indicators of smoking cue-reactivity. Clinical Neurophysiology. 1999;110:1570–1584. doi: 10.1016/s1388-2457(99)00089-9. [DOI] [PubMed] [Google Scholar]
- *.Waters AJ, Feyerabend C. Determinants and effects of attentional bias in smokers. Psychology of Addictive Behaviors. 2000;14:111–120. doi: 10.1037//0893-164x.14.2.111. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Heishman SJ, Lerman C, Pickworth W. Enhanced identification of smoking-related words during the attentional blink in smokers. Addictive Behaviors. 2007;32:3077–3082. doi: 10.1016/j.addbeh.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Waters AJ, Li Y. Evaluating the utility of administering a reaction time task in an ecological momentary assessment study. Psychopharmacology. 2008;197:25–35. doi: 10.1007/s00213-007-1006-6. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Sayette MA, Franken IHA, Schwartz JE. Generalizability of carry-over effects in the emotional Stroop task. Behaviour Research and Therapy. 2005;43:715–732. doi: 10.1016/j.brat.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Sayette MA, Wertz JM. Carry-over effects can modulate emotional Stroop effects. Cognition and Emotion. 2003;17:501–509. doi: 10.1080/02699930143000716. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Bradley BP, Mogg K. Attentional shifts to smoking cues in smokers. Addiction. 2003;98:1409–1417. doi: 10.1046/j.1360-0443.2003.00465.x. [DOI] [PubMed] [Google Scholar]
- *.Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Attentional bias predicts outcome in smoking cessation. Health Psychology. 2003;22:378–387. doi: 10.1037/0278-6133.22.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Cue-provoked craving and nicotine replacement therapy in smoking cessation. Journal of Consulting and Clinical Psychology. 2004;72:1136–1143. doi: 10.1037/0022-006X.72.6.1136. [DOI] [PubMed] [Google Scholar]
- Wertz JM, Sayette MA. Effects of smoking opportunity on attentional bias in smokers. Psychology of Addictive Behaviors. 2001a;15:268–271. [PMC free article] [PubMed] [Google Scholar]
- *.Wertz JM, Sayette MA. A review of the effects of perceived drug use opportunity on self-reported urge. Experimental and Clinical Psychopharmacology. 2001b;9:1–13. doi: 10.1037/1064-1297.9.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiers RW, Stacy AW, editors. Handbook on implicit cognition and addiction. Sage; Thousand Oaks, CA: 2006. [Google Scholar]
- Willner P, Hardman S, Eaton G. Subjective and behavioural evaluation of cigarette cravings. Psychopharmacology. 1995;118:171–177. doi: 10.1007/BF02245836. [DOI] [PubMed] [Google Scholar]
- *.Yeomans MR, Javaherian S, Tovey HM, Stafford LD. Attentional bias for caffeine-related stimuli in high but not moderate or non-caffeine consumers. Psychopharmacology. 2005;181:477–485. doi: 10.1007/s00213-005-0004-9. [DOI] [PubMed] [Google Scholar]
- *.Zack M, Belsito L, Scher R, Eissenberg T, Corrigall WA. Effects of abstinence and smoking on information processing in adolescent smokers. Psychopharmacology. 2001;153:249–257. doi: 10.1007/s002130000552. [DOI] [PubMed] [Google Scholar]
- Zinser MC, Baker TB, Sherman JE, Cannon DS. Relation between self-reported affect and drug urges and cravings in continuing and withdrawing smokers. Journal of Abnormal Psychology. 1992;101:617–629. doi: 10.1037//0021-843x.101.4.617. [DOI] [PubMed] [Google Scholar]