Abstract
Significant controversy still swirls around the regulation of extension by tip-growing cells, particularly during stable, oscillatory growth of pollen tubes. One explanation proposes that turgor pressure is both the controlling and driving force. We refute this hypothesis on theoretical and evidentiary grounds. Direct measurement of intracellular pressure reveals constant turgor even as growth rates change. Measured ion fluxes, notably potassium, are insufficient to account for the requisite osmotic changes. Water movement, and hence pressure gradients, occur throughout the cell, unrestricted to local domains. Increases in hydrostatic pressure alone would force water out of the cell rather than cause increased growth. We have recently demonstrated concomitant changes in the apical cell wall that account fully for observed changes in growth rate.
What controls pollen tube growth rate?
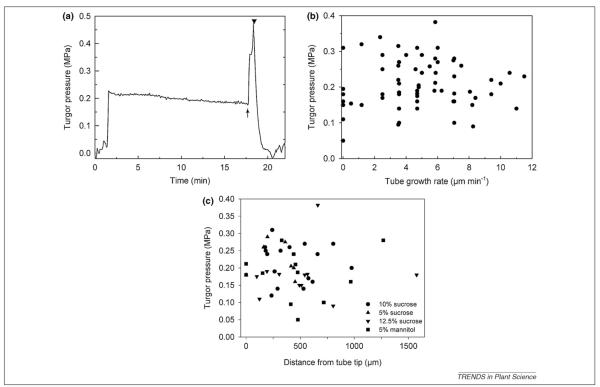
Pollen tube growth is a critical process in the life cycle of higher plants, connecting male and female gametophytes with a suitable path for sperm nucleus migration from pollen grain to micropyle (Box 1). Widely studied as a model for tip growth by plant cells [1-4], pollen tubes in vitro typically display regular oscillations in growth rate, varying by as much as 6-fold over periods of 20–50 s [5,6]. Some authors have suggested that oscillations in turgor pressure cause these changes in growth rate [7-12], an idea revisited in a recent review [12]: ‘There is substantial evidence that pollen tube cell wall extension growth is primarily dependent on changes in turgor pressure in the apical region’. Another review goes farther, proposing that ‘spatial non-equilibrium osmotic pressure [in a pollen tube] is predicted to be highest near the apex and diminish toward the distal tube’ (Figure 1 in [13]). Turgor pressure is indeed high in pollen tubes [0.1–0.4 MPa in lily (Lilium longiflorum) Thunb., mean of 0.21 MPa] [14] and is necessary for elongation. However, pressure probe measurements clearly show that turgor pressure does not change during growth (Figure 1a). Furthermore, growth rate per se is independent of turgor pressure (Figure 1b). To date, no published evidence exists demonstrating a significant correlation between oscillatory growth rates and either spatial or temporal patterns of internal pressure. On the contrary, two direct methods of measuring cellular hydrostatic pressure, the internal pressure probe [14] and microindentation [15], support the idea that turgor pressure does not change, even as growth rate does change.
Box 1. The race to the micropyle.
Pollen tubes elongate through diverse microenvironments on the way to the micropyle. In some flowers, the tube grows through a solid style, digesting polysaccharides and pushing between cells. In others, such as lily flowers, tube growth occurs along the sides of a transmitting tract, bounded by an internal gas space on one side and adhered to stylar tissue on the other [42]. Regardless of the circumstances, the evolutionary imperative operating upon the pollen tube selects for growth that is as fast as possible, regardless of changes in external osmotic concentration, physical resistance or gas phase oxygen tension [43]. Only one out of dozens of pollen tubes will successfully deliver a sperm cell to the egg, creating selection pressure for rapid growth [44].
Not only must the pollen tube grow quickly, it must also maintain polarity of growth even while constantly modulating the direction of growth. Successful pollen tubes are more likely to be the ones that have taken the most direct path to the micropyle, stimulated and guided by diffusible substances secreted by stylar tissues and synergid cells [45-48]. In vitro we often see growth patterns that reflect rapid elongation combined with changes in direction. In these examples, pollen tubes often seem to seek new directions, where they smoothly and rhythmically reorient their growth axis by tens of degrees alternately to either the left or the right. When tube growth is halted by external factors, such as non-plasmolytic shifts in osmotic potential, the tip usually swells and then reorganizes with a resumption of normal elongation [15,16]. The localized application of small aliquots of compounds in the growth medium, such as calcium chelators, causes pollen tubes to turn away from or to follow the artificial chemical gradients [49]. To be considered successful, any model proposed to explain growth regulation in pollen tubes must account for both the intensely directional and the spatially specific properties of the growth process.
Figure 1.
Turgor pressure of growing Lilium longiflorum (Lily) pollen tubes measured by pressure probe as described in [14]. (a) Time course of a representative experiment, showing very little change in turgor pressure during cell growth, until pressure was artificially increased and the cell burst, returning the probe to atmospheric pressure. Note that the tube wall withstood pressure over twice as large as that needed for growth before bursting. (b) In dozens of replicate experiments, growth rate was not correlated with turgor pressure. Even in the absence of growth cells maintained high turgor pressure. (c) Turgor pressure of lily pollen tubes in relation to impalement site of the pressure probe. Note the pressure values that were directly measured at the tube tip. Pollen grains were incubated in germination medium with various osmotica as indicated (unpublished data from [14]).
In this review, we critically examine the assumptions and data cited in support of the pressure control hypothesis and build a case for an alternative hypothesis: oscillatory pollen tube growth is not regulated by changes in pressure but rather by oscillations in the extensibility of the apical cell wall. Recent evidence of direct correlations between wall properties and growth rate during oscillatory growth [15,16] supports just such a mechanism. Indeed, given biological and biophysical realities detailed in Boxes 1 and 2, it is highly unlikely that changes in turgor pressure could ever be the source of the observed oscillations in growth rate.
Box 2. The rules of the race: thermodynamics, biophysics and cell growth.
Internal pressure in plant cells is generated by water movement across the plasma membrane in response to a gradient in water potential due mainly to concentration differences of osmotically active solutes. The water volume flow density jV for a plant cell membrane, which separates an interior, i, from an outer medium, o, is given by:
| (1) |
with V as the cell volume; A, cell surface area through which flux occurs; LP, hydraulic conductivity; P, turgor pressure; C, concentration of non-permeable solutes; CS, concentration of permeable solutes and σS, reflection coefficient for permeable solutes. The volume flow has a hydraulic driving force, the hydrostatic pressure gradient (P) and an osmotic driving force, the osmotic pressure gradient, Δπ=RT(ΔC+σSΔCS). In the presence of only non-permeable solutes, Equation 1 is simplified to
| (2) |
Thus, a water influx can occur only if Δπ>P, that is by a turgor pressure decrease, by increasing the osmolyte concentration, Ci, of the cytosol or by a decrease in external osmotically active solutes. The cell quickly reaches a balance point where the restraining force of the cell wall counter balances the outwardly directed turgor pressure and water uptake ceases. Because the cell contents behave as a viscous fluid, turgor pressure is exerted equally in all directions within the volume bounded by the cell wall.
In theory [17], it should be possible to deviate from the osmotic/turgor balance point to allow irreversible increase in volume, that is, growth, by two different mechanisms: an increase in turgor pressure might overcome the restraining capacity of the cell wall and cause it to yield or a decrease in the restraining force, through stress relaxation in the cell wall, would allow the existing turgor pressure to extend the cell wall. Because water movement in plant cells is passive, an increase in the turgor pressure requires a corresponding decrease in the internal water potential followed by an influx of water. Repeatedly, studies on plant cell growth reveal that cell wall loosening, resulting in stress relaxation, rather than turgor increase, emerges as the prime event underlying cell extension [50]. Because the cell, before stress relaxation, is at the balance point between outward-directed turgor pressure and the inward-directed restraint of the wall, when the cell wall polymer structure relaxes the existing turgor pressure will cause the wall to extend. An immediate consequence will be a momentary but slight reduction in the turgor pressure and a decrease in the water potential. Water will immediately flow into the cell re-establishing the original turgor pressure. In numerous plant cells, it is clear that water flow into the cell is a consequence not a cause of the growth event and that growth is dependent on stress relaxation of the cell wall [18,51-56].
The principles of cell growth developed in other plant cells apply to pollen tubes
Because pollen tubes differ from diffuse-growing plant cells by extending exclusively at their tips, conceivably they deviate from the biophysical rules (Box 2) derived from the study of other plant cells. We assert that this is unlikely because pollen tubes are not alone in exhibiting tip growth; this is a behavior shared with other tip-growing cells such as root hairs, fern and moss protonemata (e.g. Adiantum capillusveneris L. or Physcomitrela patens Hedwig.), algal rhizoids (e.g. Nitella spp.) and fungal hyphae (e.g. Saprolegnia ferax). The pollen tube cell wall, although enriched in pectins especially at the apical growing point, never-theless consists of typical wall polymers. Lastly, pollen tubes are clearly osmotically active, reacting to quick changes in external osmotic conditions by either plasmolysis or bursting, and adapting rapidly to smaller, slower changes in tonicity.
Large and rapid changes in turgor pressure are not feasible
Given the large and rapid changes in pollen tube growth rates (from 100 nm s−1 to 600 nm s−1 in lily over 10–25 s [5] and from 10 nm s−1 to up to 50 nm s−1 for tobacco (Nicotiana tabacum L.) [6], in the absence of changes to the pollen tube wall, according to the Lockhart Equation [17], the change in growth rate would have to be directly proportional to a change in turgor pressure. Because evidence to date from pressure probe and osmotic shift experiments suggests that pollen tube growth rates do not change until the cell bursts or begins to plasmolyze we must go to experiments with diffuse-growing cells for an estimate of the probable slope of a putative turgor pressure–growth rate relationship. In Chara internode cells and sunflower (Helianthus anuus L.) leaf cells, a change in the growth rate by 2-fold requires approximately a 0.05–0.1 MPa increase in turgor pressure [18]. Assuming a similar relationship in the lily pollen tube, to cause a 6-fold change in growth rate would require an increase in turgor pressure of 0.15–0.45 MPa. In addition to the fact that such increases in turgor pressure would cause bursting of the tube tip (Figure 4 in [14]), these changes would need to occur repeatedly every 20–50 s to account for the observed oscillations in growth rate.
How likely are large-scale osmotically driven turgor pressure changes in pollen tubes? Rapid, osmotically driven changes in cell shape and size do occur in other plants cells, such as stomatal guard cells and Mimosa leaflets. Assuming a typical pollen tube with a diameter of 10 μm, a length of 1000 μm, a volume of 78 500 μm3 and a surface area of 31 500 μm2, a minimal increase in turgor of 0.1 MPa corresponds to an increase in osmolytes of 42 mosmol kg−1 (approximately 40 mM K+). Peak K+ influx of 300 pmol cm−2 s−1 as measured in lily pollen tubes [19] would account for only 35 mM of the required 40 mM of osmolytes even with a total K+ uptake of 2.8 pmol over 30 s. A 6-fold change in growth rates caused by a turgor pressure increase of 0.3 MPa would require the uptake of three times more K+, leading to an increase of 120 mM K+ in the cytosol that cannot be explained by the measured K+ influx rates. Furthermore, transient increases in the cytosolic K+ concentration would result in transient membrane potential changes in growing pollen tubes, which have not been recorded so far in over 200 measurements of lily and tobacco pollen tubes (G. Obermeyer, unpublished data).
The accumulation and export of large amounts of solute, as occurs in stomatal guard cells, would require a huge expenditure of energy, as well as time. We must keep in mind that stomatal pores open over time periods that range from 0.5 to 1.5 h and not the tens of seconds typical for the oscillating pollen tube. In brief, this scenario is unrealistic for pollen tube growth, particularly for tubes growing between cells or in a hollow style, without a nearby source of ions as provided by the subsidiary cells adjacent to the guard cells. Moreover, a bulk osmotic change alone does not provide a mechanism for sustaining polarity or for changes in growth direction.
Direct measurements fail to indicate rapid or large-scale turgor changes during growth
Direct measurements of turgor pressure in growing lily pollen tubes [14], using the pressure probe, clearly showed that there was no change in the turgor pressure during growth, particularly in longer pollen tubes. Based on previous research [20], these cells would have been expected to oscillate in growth rate. Growth rate and turgor pressure were not correlated (Figure 1b): faster tubes did not have higher turgor pressure than slowly growing tubes. The imposition of a 2-fold increase in turgor pressure with the pressure probe routinely led to tube bursting at the tip, rather than an elevation in growth rate. Even small turgor pressure increases forced by the pressure probe to monitor pressure relaxation for Lp calculations did not cause an increase in growth rates. Rather, the tube tip became swollen and growth ceased. When pollen tubes were incubated in a medium of 3-fold higher osmolyte concentration the internal turgor pressure dropped only from 0.27 MPa to 0.18 MPa, providing evidence that the pollen tube retains a remarkable ability to adjust and maintain a normal or optimal turgor pressure for growth.
Despite very thorough measurements of turgor by pressure probe in growing lily pollen tubes [14],and clear definitive results, there have been targeted efforts to discount and dismiss the findings in this paper. For example, it has been suggested [8,9] that short lily pollen tubes such as those primarily studied in [14] do not oscillate and thus would not be expected to show varying turgor. Yet, other results [20] demonstrate that although short lily pollen tubes might not oscillate, they do exhibit irregular fluctuations with over 2-fold changes in the growth rate (see Figure 9c in [20]). For the pressure regulation model to work, turgor changes associated with those variations in growth rate should be observed and they were not. Another objection raised in Ref. [9] is that the time resolution of 1–2 s is poor and not adequate to show changes in turgor pressure. However, the growth rate oscillations occur during a 20- to 50-s period during which time there is sufficient resolution to observe any accompanying change in turgor pressure. Practically speaking, such relatively slow changes over time allow the experimenter to follow the turgor pressure change by adjusting the meniscus of the pressure probe. Any turgor pressure transients would have been readily noticed and monitored. Additionally, one can expect that a sudden and immediate increase in turgor pressure, achieved with a marked reduction in the external sucrose concentration (from 10% to 1%), should lead to a marked increase in thegrowth rate. With lily pollen tubes, such changes caused a shift in oscillatory profile [9] but not the predicted changes in growth rate. Although pollen tubes can adjust turgor pressure as necessary, within a resolution limit of 0.005 MPa (5 kPa [14]), they do not do so during in vitro growth of the lily pollen tube.
Localized pressure gradients probably do not occur
Pressure probe measurements have also been challenged on the grounds that the probe inserted in a region distal from the tip does not accurately record events that occur in the tip itself owing to pressure waves in localized compartments [21]. Perhaps the location of the cytoskeleton array and associated cytomechanical activities creates regions with different hydraulic properties [12] similar to a mechanism elaborated to explain surface blebbing in metastatic tumor cells [22]. With the cytoplasm modeled as a rigid microporous entity containing only 20% water [22] hypothetical pressure waves could be predicted with time constants of 10 s, over distances of 10 μm, seductively close to the dimensions needed to cause localized rhythmic expansion at the pollen tube tip. Yet even if it is poroelastic, mammalian cell cytoplasm only pushes against a cell membrane causing tensions of at the most hundreds of Pascals. Given the strength of the cell wall it will take pressure transients on the order of 100 000 Pa to locally increase the wall expansion rate, requiring the action of an actin network thousands of times more dense than that found in animal cells [23]. Such a structure is clearly absent from pollen tubes. Although there is a distinct F-actin fringe or collar in the apical domain of lily and tobacco pollen tubes [24-28], it is localized slightly back from the extreme apex, being positioned along the shank of the tube, and does not reside in that portion of the cytoplasm adjacent to where cell extension occurs. Like a stone dropped into a pond, any such transient pressure wave would cause ripples of pressure in all directions, including away from the tube tip, with a force well above that generated by the actin–myosin motors propelling starch grains and organelles. Should transient pressure waves occur, we should have observed ripples in organelle motion or at least rhythmic variations in the pressure probe measurements studied in [14], or the cantilever external probes used in [15], but no such phenomena have been reported.
Even if sufficient local pressure could be generated, is there evidence for the required cytoplasmic compartmentation in the pollen tube? Although large organelles, including plastids and the vacuoles, are prevented from entering the apical clear zone, there is no evidence that the smaller organelles, or water itself, are restricted in their motion. As can be readily observed through microscopic inspection of the growing pollen tube, cytoplasmic streaming is rapid (0.2–0.9 μm s−1) [29] and continuous along the entire length of the tube [30,31]. Organelles such as mitochondria, Golgi dictyosomes, elements of the endoplasmic reticulum and vesicles move rapidly and freely into the extreme apex of the tube. Injected fluorescent markers (fura-2-dextran, BCECF–dextran or GFP) inserted into the shank of the pollen tube more than 100 μm back from the apex rapidly and completely permeate all regions of the cytoplasm. Water and any associated pressure gradient or wave is thus able to move freely throughout the length of the pollen tube, and including notably the extreme apex of the cell.
Direct evidence for regions of high and low turgor pressure is also absent. The turgor pressure values in growing lily pollen tubes [14] were independent from the impalement site (Figure 1c). Even pressure values measured in the extreme tube tip (0 μm) were in the same range as values obtained from impalements of the pollen tube base. Furthermore, pressure pulses applied by the pressure probe impaling the pollen tube base lead to an immediate bursting of the tube tip (Figure 1a) thus supporting the view of the pollen tube cytosol being essentially one compartment. Because water is a non-compressible fluid, any pressures generated within the cytosol will be expressed uniformly throughout. A similar conclusion derives from studies of pollen tubes of tobacco that occasionally bifurcate and exhibit two growing tips. Although both growing points from the same tube oscillated, their frequencies were different [32], which would be impossible with oscillating turgor pressure.
Finally, without adequate compartmentalization or strong enough pressure waves at the tube tip, it seems unlikely that localized pressure gradients could be steep enough to sustain polarity and tip shape. During regular polarized growth, expansion rates must be maximal some-where near the tip of the cell and decrease to zero at the junction with the cylindrical shank of the tube, as elegantly conceptualized many years ago [33] and demonstrated in root hairs [34]. If pressure gradients were the cause of this anisotropy, they would have to be sustained and large over very short distances.
Water flux is bidirectional, responding to the water potential
Beginning with a growing pollen tube in steady-state with balanced water influx and turgor pressure, a localized increase in turgor pressure as postulated in [11,12,21] would drive water out of the pollen tube and thus not lead to a growth pulse. As illustrated by Equations 1 and 2 (Box 2) water can flow across the tube plasma membrane in either direction [35-37]. Furthermore, the hydrodynamics model [12] proposes water influx into the subapex and a simultaneous water efflux at the apex of the pollen tube that would require different turgor pressures or osmolyte concentration in both regions only 20–30 μm apart. As pointed out earlier, the turgor pressure is very likely to be constant throughout the pollen tube (Papex=Psub) and the osmolyte concentration (Co) in the surrounding medium (germination medium or style tissue) is also constant. Therefore, the only parameter that can be modulated is the cytosolic osmolyte concentration (Ci) with Cisubapex being higher than Ciapex to allow the postulated water influx at the subapex and the water efflux at the apex. This condition, where Cisubapex>Ciapex, will force a cytosolic water flux from the tube tip to the subapex rather than in the opposite direction thus contradicting the postulated water flow circuit.
Cell wall properties regulate growth and cell shape
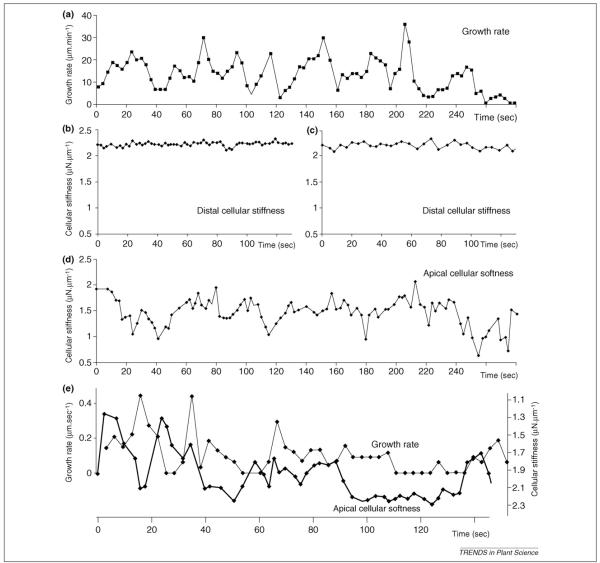
If turgor pressure does not oscillate, what then controls oscillations in pollen tube growth rate? In a recent study [16], the amount of wall material in the pollen tube apex clearly changed dramatically during oscillatory growth. Importantly, cross-correlation analysis indicates that this increase in wall material in lily pollen tubes anticipates the increase in growth rate by approximately 100° (full cycle=360°). A further statistical analysis reveals that the amount of wall material predicts, up to 90%, the magnitude and extent of the growth that follows. In addition, microindentation studies show that the pollen tube tip is indeed less rigid and that stiffness varies with the same frequency as oscillations in growth rate, with minimum stiffness, for example maximal extensibility, shifted in phase to 10–90 degrees before maximal growth (Figure 2) [15]. Although the exact biophysical and biochemical bases for changes in cell wall stiffness are not well understood, and are active areas of research, the magnitude of the softening does predict the magnitude of the growth pulse. Thus, pollen tube growth polarity and cell shape could readily be maintained by a gradient in wall viscoelasticity across the growing tip, as described in root hairs [34] and proposed for pollen tubes [38,39].
Figure 2.
Simultaneous measurements of growth rate and cell wall stiffness in oscillating Lilium pollen tubes (redrawn from [13]). (a) Typical oscillatory growth pattern. (b) Trace of cellular stiffness from the same cell and time period as (a), on the shank region of the tube. (c) Another trace of cellular stiffness in the shank, with the cantilever probe moved away and back to the same spot between measurements to mimic displacement. Note the absence of any oscillatory pattern in distal stiffness. (d) Time course of cell wall stiffness at the very tip of the cell for the same cell as (a–c). The probe was moved between each measurement to keep up with the growing tube. (e) Comparison of growth rate and wall softness (inverse of stiffness) of an oscillating growing cell, measured at the growing tip. Because of the similar shapes of the stiffness and growth rate curves it was possible to determine that the softening of the wall occurred approximately 10 s before a concomitant increase in growth rate.
Turgor pressure, at least at levels sufficient to deform the cell wall, does not change during oscillatory pollen tube growth, consonant with direct experimental observations [14,15]. Constant water influx down a gradient in water potential accounts for the volume enlargement during tube elongation [40]. Turgor pressure changes do not modulate wall expansion during oscillatory growth, rather wall loosening and stress relaxation in the apical cell wall oscillate and this activity underlies the control of oscillatory pollen tube growth. As noted by Harold [41]: ‘morphogenesis in walled cells results from localized compliance with the global force of turgor pressure’. The energy for growth derives from turgor; the control of rate and direction from the wall.
Acknowledgements
We thank our colleagues, notably Tobias Baskin and Eric Kramer, and members of our respective laboratory groups, for many helpful discussions during the course of this study. This project was supported in part by National Science Foundation Grants MCB-0516852 and MCB-0847876 to P.K.H., grants from the Fond zur Förderung der wissenschaftlichen Forschung (FWF, P17227 and P21298) to G.O. and grants from the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Fonds Québécois de la Recherche sur la Nature et les Technologies (FQRNT) to A.G.
Footnotes
* Dedicated to the memory of Jack Dainty (1919–2009), a pioneer in the field of plant biophysics who formulated essential physical concepts of ion and water transport across (plant) cell membranes.
References
- 1.Hepler PK, et al. Polarized cell growth in higher plants. Annu. Rev. Cell Dev. Biol. 2001;17:159–187. doi: 10.1146/annurev.cellbio.17.1.159. [DOI] [PubMed] [Google Scholar]
- 2.Cheung AY, Wu HM. Structural and signaling networks for the polar cell growth machinery in pollen tubes. Annu. Rev. Plant Biol. 2008;59:547–572. doi: 10.1146/annurev.arplant.59.032607.092921. [DOI] [PubMed] [Google Scholar]
- 3.Michard E, et al. The role of ion fluxes in polarized cell growth and morphogenesis: the pollen tube as an experimental paradigm. Int. J. Dev. Biol. 2009;53:1609–1622. doi: 10.1387/ijdb.072296em. [DOI] [PubMed] [Google Scholar]
- 4.Chebli Y, Geitmann A. Mechanical principles governing pollen tube growth. Funct. Plant Sci. Biotechnol. 2007;1:232–245. [Google Scholar]
- 5.Cárdenas L, et al. Pollen tube growth oscillations and intracellular calcium levels are reversibly modulated by actin polymerization. Plant Physiol. 2008;146:1611–1621. doi: 10.1104/pp.107.113035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michard E, et al. Tobacco pollen tubes as cellular models for ion dynamics: improved spatial and temporal resolution of extracellular flux and free cytosolic concentration of calcium and protons using PHluorin and YC3.1CaMelon. Sex. Plant Reprod. 2008;21:169–181. [Google Scholar]
- 7.Derksen J. Pollen tubes: a model system for plant cell growth. Botanica Acta. 1996;109:341–345. [Google Scholar]
- 8.Messerli MA, et al. Periodic increases in elongation rate precede increases in cytosolic Ca2+ during pollen tube growth. Dev. Biol. 2000;222:84–98. doi: 10.1006/dbio.2000.9709. [DOI] [PubMed] [Google Scholar]
- 9.Messerli MA, Robinson KR. Ionic and osmotic disruption of the lily pollen tube oscillator: testing proposed models. Planta. 2003;217:147–157. doi: 10.1007/s00425-003-0972-0. [DOI] [PubMed] [Google Scholar]
- 10.Plyushch TA, et al. Structural aspects of in vitro pollen tube growth and micropylar penetration in Gasteria verrucosa (Mill.) H. Duval and Lilium longiflorum Thunb. Protoplasma. 1995;187:13–21. [Google Scholar]
- 11.Zonia L, Munnik T. Life under pressure: hydrostatic pressure in cell growth and function. Trends Plant Sci. 2007;12:90–97. doi: 10.1016/j.tplants.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Zonia L, Munnik T. Uncovering hidden treasures in pollen tube growth mechanics. Trends Plant Sci. 2009;14:318–327. doi: 10.1016/j.tplants.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Zonia L. Spatial and temporal integration of signalling networks regulating pollen tube growth. J. Exp. Bot. 2010;61:1939–1957. doi: 10.1093/jxb/erq073. [DOI] [PubMed] [Google Scholar]
- 14.Benkert R, et al. The turgor pressure of growing lily pollen tubes. Protoplasma. 1997;198:1–8. [Google Scholar]
- 15.Zerzour R, et al. Polar growth in pollen tubes is associated with spatially confined dynamic changes in cell mechanical properties. Dev. Biol. 2009;334:437–446. doi: 10.1016/j.ydbio.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 16.McKenna ST, et al. Exocytosis precedes and predicts the increase in growth in oscillating pollen tubes. Plant Cell. 2009;21:3026–3040. doi: 10.1105/tpc.109.069260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lockhart JA. An analysis of irreversible plant cell elongation. J. Theor. Biol. 1965;8:264–275. doi: 10.1016/0022-5193(65)90077-9. [DOI] [PubMed] [Google Scholar]
- 18.Boyer JS. Cell wall biosynthesis and the molecular mechanism of plant enlargement. Funct. Plant Biol. 2009;36:383–394. doi: 10.1071/FP09048. [DOI] [PubMed] [Google Scholar]
- 19.Messerli MA, et al. Pulsatile influxes of H+, K+ and Ca2+ lag growth pulses of Lilium longiflorum pollen tubes. J. Cell Sci. 1999;112:1497–1509. doi: 10.1242/jcs.112.10.1497. [DOI] [PubMed] [Google Scholar]
- 20.Pierson ES, et al. Tip-localized calcium entry fluctuates during pollen tube growth. Dev. Biol. 1996;174:160–173. doi: 10.1006/dbio.1996.0060. [DOI] [PubMed] [Google Scholar]
- 21.Zonia LE, et al. Hydrodynamics and cell volume oscillations in the pollen tube apical region are integral components of the biomechanics of Nicotiana tabacum pollen tube growth. Cell Biochem. Biophys. 2006;46:209–232. doi: 10.1385/CBB:46:3:209. [DOI] [PubMed] [Google Scholar]
- 22.Charras GT, et al. Non-equilibration of hydrostatic pressure in blebbing cells. Nature. 2005;435:365–369. doi: 10.1038/nature03550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Money NP. Wishful thinking of turgor revisited: the mechanics of fungal growth. Fungal Genet. Biol. 1997;21:173–187. [Google Scholar]
- 24.Kroeger JH, et al. Microfilament orientation constrains vesicle flow and spatial distribution in growing pollen tubes. Biophys. J. 2009;97:1822–1831. doi: 10.1016/j.bpj.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lovy-Wheeler A, et al. Enhanced fixation reveals the apical cortical fringe of actin filaments as a consistent feature of the pollen tube. Planta. 2005;221:95–104. doi: 10.1007/s00425-004-1423-2. [DOI] [PubMed] [Google Scholar]
- 26.Vidali L, et al. Lifeact-mEGFP reveals a dynamic apical F-actin network in tip growing plant cells. PLoS One. 2009;4:e5744. doi: 10.1371/journal.pone.0005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kost B, et al. Cytoskeleton in plant development. Curr. Opin. Plant Biol. 1999;2:462–470. doi: 10.1016/s1369-5266(99)00024-2. [DOI] [PubMed] [Google Scholar]
- 28.Gossot O, Geitmann A. Pollen tube growth – coping with mechanical obstacles involves the cytoskeleton. Planta. 2007;226:405–416. doi: 10.1007/s00425-007-0491-5. [DOI] [PubMed] [Google Scholar]
- 29.Vidali L, et al. Actin polymerization is essential for pollen tube growth. Mol. Biol. Cell. 2001;12:2534–2545. doi: 10.1091/mbc.12.8.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bove J, et al. Magnitude and direction of vesicle dynamics in growing pollen tubes using spatiotemporal image correlation spectroscopy (STICS) Plant Physiol. 2008;147:1646–1658. doi: 10.1104/pp.108.120212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Win A, et al. Classification of organelle trajectories using region-based curve analysis. Cytometry. 1997;29:136–146. doi: 10.1002/(sici)1097-0320(19971001)29:2<136::aid-cyto6>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- 32.Geitmann A. Growth and Formation of the Cell Wall in Pollen Tubes of Nicotiana tabacum and Petunia hybrida. Verlag Hänsel-Hohenhausen; Egelsbach Frankfurt Washington: 1997. PhD thesis. [Google Scholar]
- 33.Green PB. Morphogenesis of the cell and organ axis: biophysical models. Brookhaven Symp. Biol. 1973;25:166–190. [Google Scholar]
- 34.Dumais J, et al. An anisotropic-viscoplastic model of plant cell morphogenesis by tip growth. Int. J. Dev. Biol. 2006;50:209–222. doi: 10.1387/ijdb.052066jd. [DOI] [PubMed] [Google Scholar]
- 35.Dainty J. Water relations of plant cells. Adv. Bot. Res. 1963;1:279–326. [Google Scholar]
- 36.Zimmermann U. Water relations of plant cells: pressure probe technique. Methods Enzymol. 1989;174:338–366. [Google Scholar]
- 37.Steudle E. Water flow in plants and its coupling to other processes: an overview. Methods Enzymol. 1989;174:183–225. [Google Scholar]
- 38.Geitmann A, Steer MW. The architecture and properties of the pollen tube cell wall. In: Malhó R, editor. The Pollen Tube: A Cellular and Molecular Perspective, Plant Cell Monographs. Springer Verlag; 2006. pp. 177–200. [Google Scholar]
- 39.Geitmann A, Parre E. The local cytomechanical properties of growing pollen tubes correspond to the axial distribution of structural cellular elements. Sex Plant Reprod. 2004;17:9–16. [Google Scholar]
- 40.Sommer A, et al. Ectopic expression of Arabidopsis thaliana plasma membrane intrinsic protein 2 aquaporins in lily pollen increases the plasma membrane water permeability of grain but not of tube protoplasts. New Phytol. 2008;180:787–797. doi: 10.1111/j.1469-8137.2008.02607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harold FM. To shape a cell: an inquiry into the causes of morphogenesis of microorganisms. Microbiol. Mol. Biol. Rev. 1990;54:381–431. doi: 10.1128/mr.54.4.381-431.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steer MW, Steer JM. Pollen tube tip growth. New Phytol. 1989;111:323–358. doi: 10.1111/j.1469-8137.1989.tb00697.x. [DOI] [PubMed] [Google Scholar]
- 43.Wilsen KL, Hepler PK. Sperm delivery in flowering plants. Bioscience. 2007;57:835–844. [Google Scholar]
- 44.Winsor JA, et al. Pollen competition in a natural population of Cucurbita foetidissima (Cucurbitaceae) Am. J. Bot. 2000;87:527–532. [PubMed] [Google Scholar]
- 45.Lord E. Adhesion and cell movement during pollination: cherchez la femme. Trends Plant Sci. 2000;5:368–373. doi: 10.1016/s1360-1385(00)01744-1. [DOI] [PubMed] [Google Scholar]
- 46.Okuda S, et al. Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature. 2009;458:357–361. doi: 10.1038/nature07882. [DOI] [PubMed] [Google Scholar]
- 47.Higashiyama T. Pollen tube attraction by the synergid cell. Science. 2001;293:1480–1483. doi: 10.1126/science.1062429. [DOI] [PubMed] [Google Scholar]
- 48.Higashiyama T, Hamamura Y. Gametophytic pollen tube guidance. Sex. Plant Reprod. 2008;21:17–26. [Google Scholar]
- 49.Malhó R, Trewavas AJ. Localized apical increases of cytosolic free calcium control pollen tube orientation. Plant Cell. 1996;8:1935–1949. doi: 10.1105/tpc.8.11.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cosgrove DJ. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005;6:850–861. doi: 10.1038/nrm1746. [DOI] [PubMed] [Google Scholar]
- 51.Geitmann A, Ortega JKE. Mechanics and modeling of plant cell growth. Trends Plant Sci. 2009;14:467–478. doi: 10.1016/j.tplants.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 52.Schopfer P. Biomechanics of plant growth. Am. J. Bot. 2006;93:1415–1425. doi: 10.3732/ajb.93.10.1415. [DOI] [PubMed] [Google Scholar]
- 53.Ray PM. Principles of plant cell expansion. In: Cosgrove DJ, Knievel DP, editors. Physiology of Cell Expansion During Plant Growth. American Society of Plant Physiologists; 1987. pp. 1–17. [Google Scholar]
- 54.Green PB. Mechanism for plant cellular morphogenesis. Science. 1962;138:1404–1405. doi: 10.1126/science.138.3548.1404. [DOI] [PubMed] [Google Scholar]
- 55.Green PB, Chen JC. Concerning the role of wall stresses in the elongation of the Nitella cell. Z. Wiss. Mikrosk. 1960;64:482–488. [PubMed] [Google Scholar]
- 56.Taiz L. Plant cell expansion: regulation of cell wall mechanical properties. Annu. Rev. Plant Physiol. 1984;35:585–657. [Google Scholar]