Abstract
A short overview of currently available studies on the ecology of viruses in running waters is provided. Additionally, a survey was conducted on the dynamics of both viruses and bacteria in an isolated floodplain segment of the Danube River and in the main channel near Vienna (Austria) during the hydrologically most dynamic phase (spring – summer). The study evaluates the differences between the main channel and the floodplain segment for suspended particle abundance and quality in relation to bacterial and viral parameters; both free-living forms and those attached to particles are examined. The hydrological disconnection of these two contrasting sampling sites influenced particle abundance and quality as well as the distribution of free-living vs. attached bacteria and viruses. The per-cell activity of bacteria attached to particles was significantly higher than that of the free-living fraction, particularly in the isolated water body. The abundance of bacteria and viruses on particles depended on particle quality (size). In the main channel, bacteria were significantly more abundant on surfaces (per mm2) of suspended matter > 5 μm (aggregates with organic constituents) compared to particles < 5 μm (mostly mineral grains). In the isolated water body, both bacteria and viruses were more abundant on the larger particles/aggregates. Data from both locations revealed a positive correlation between abundance of particles > 5μm and attached viruses; free-living viruses were less abundant at high > 5μm particle loads. Only in the isolated floodplain section was viral abundance positively influenced by elevated per-cell productivity of potential host bacteria. The results demonstrate that system variability on a relatively small topographical scale (within a river-floodplain system) has consequences for microbial life, including viruses.
Keywords: River-floodplain, seston, bacterial activity, virus ecology
Inroduction
Following the discovery of high numbers of viruses in aquatic environments (now considered the most abundant biological entities in natural waters; Bergh et al., 1989), it soon was observed that this virioplankton is significantly linked to bacterio- and phytoplankton (Proctor and Fuhrman, 1990; Suttle et al., 1990). Since viruses infect all members of the microbial food web, they must be viewed as significant biological agents in microbial processes (for review see Wommack and Colwell, 2000; Weinbauer, 2004). Moreover, viral abundance is generally one to two orders of magnitude higher than that of bacterioplankton. Interestingly, there are indications that freshwater systems might differ from marine habitats regarding viral abundance and the ratio of viral to bacterial numbers (Maranger and Bird, 1995; Farnell-Jackson and Ward, 2003). Further surprising is that, although viruses play a significant role in marine and lake waters, many related aspects are largely ignored in research on the functioning of river systems.
For many years the presence of viruses in river water has been a significant public health issue in the context of the microbiological quality of this water resource. Drinking water is often produced from river water, which usually contains microorganisms pathogenic for humans. This led to investigations of the virological contamination in different riverine systems. Walter et al. (1989), for example, found a close connection between a sewage index and the determined virus levels. From the lower reach of the Danube River, Walter et al. (1990) reported on a pronounced virological risk in connection with producing drinking water. Skraber et al. (2002) concluded that bacteriophages are good indicators for pathogenic microorganisms present in water of the Moselle River. Their results showed that somatic coliphages are less sensitive to environmental factors (flow rate, temperature) than those bacteria traditionally used as indicators of faecal contamination. Therefore, somatic coliphages in river water apparently provide a more constant and reliable indicator of faecal contamination.
On the other hand, ecological studies on viruses in running water ecosystems are scarce. Mei and Danovaro (2004) detected some 18% bacterial mortality due to viruses in sediments of the Esino River. Baker and Leff (2004) investigated seasonal patterns of virus abundance in a northeast Ohio (USA) stream. A survey of nine streams in different regions of the USA revealed that virus abundance was not significantly different among streams or among sites within a stream (Olapade et al., 2006). Similarly, in a study of two rivers in the Czech Republic, no difference in abundance and distribution of viruses was found among sites within rivers and between rivers. Temporal variations, however, were large (Slováckova and Marsálek, in press). In a study of a lotic-lentic inland water network in tropical Sri Lanka, we have previously shown that most virus parameters, such as abundance or frequency of visibly infected cells, were positively linked to bacterial abundance and production as well as to organic nitrogen or certain phosphorus species. We calculated that between 13–46% of the prokaryotic standing stock suffered virusmediated mortality (Peduzzi and Schiemer, 2004).
Few relevant studies are available for a Danube backwater system near Vienna (Austria). One described seasonal variations of virus abundance and its potential impact on bacterial production in an isolated backwater pool (Mathias et al., 1995). Another study, conducted in an eutrophic, stagnant oxbow lake, suggested high prokaryotic mortality (up to 46% of the bacterial secondary production) due to viruses (Fischer and Velimirov, 2002). These studies did not consider the potential importance of suspended matter in the water column. The third investigation reported on virus-induced mortality in the sediment layer of another oxbow lake: viruses controlled up to 25% (average, 7%) of benthic bacterial secondary production (Fischer et al., 2003). Fischer et al. (2006) estimated simultaneously the significance of virus-induced lysis and protozoan grazing on benthic bacterial mortality. Their microcosm results indicated that viruses, heterotrophic nanoflagellates and ciliates together were an insufficient factor in regulating bacterial secondary production and bacterial standing stock in the investigated freshwater sediment (although they did contribute on average 12%).
Interactions between natural virus assemblages and suspended matter are scarcely documented. Particularly in river systems where suspended matter is a ubiquitous and important factor, very little information is available on the interaction of viruses with particles. In riverine floodplains, highly variable concentrations and the dynamics of suspended inorganic and organic particles are mainly determined by river morphology and hydrology (Meybeck, 1982; Richter et al., 1997). Particulate matter is transported from the river to side-arms as well as vice versa. A major driving force of such exchange processes is hydrological connectivity via surface waters (Junk et al., 1989; Galat et al., 1997). Depending on the hydrological connectivity of the floodplain with the main channel, the conditions within the floodplains can change from lotic to lentic. A study dealing with suspended particulate matter demonstrated the influence of particle size and quality on attached viruses. In a riverine wetland, surface type and potential host bacterial cells influenced the proliferation and distribution of viruses (Farnell-Jackson and Ward, 2003). The abundance of viruses attached to surfaces ranged from 1.3×106/cm2 on macrophytes to 1.1×107/cm2 on wood. The relevance of substratum type was also shown by Lemke et al. (1997): viral abundance associated with sediment exceeded that on leaves in a lotic system. A more recent study by our working group (Luef et al., 2007) was conducted in a dynamically connected floodplain segment and in the main channel of the Danube River downstream of Vienna. The study evaluated the level of connection between the main channel and the connected floodplain segment for particle abundance and quality as well as for bacterial and viral parameters. Variable water age (i.e. the time water was contained in a particular water body of the floodplain system, Luef et al., 2007) influenced bacterial and viral abundance and the bacterioplankton productivity in the surrounding water. The abundance of attached bacteria and viruses was not influenced by water age, but was influenced by particle quality. Particle-attached bacteria accounted on average 30.3% (± 3.1) of the total bacterial abundance. A variable and occasionally significant proportion of viruses, between 0.4% and 35.1%, was associated with suspended particulate material.
Following this short overview on the scarce information currently available on virus ecology in rivers, with our study we add new data by addressing the relevance of separated water bodies and suspended matter load in a river-floodplain system. In a preliminary study of the Danube River’s main channel and an adjacent isolated floodplain section representative for lentic floodplain conditions, we hypothesized that the limnological differences in these two adjoining environments influence viral distribution and parameters of their bacterial hosts in the water column and on suspended particles. Potentially significant driving forces are discussed. In a brief outlook we propose important questions that should be dealt with in future studies of riverine water bodies.
Material and methods
Study sites
The Danube near Vienna is a 9th-order river with an average annual discharge of 1950 m3/s and a slope of 0.045%. At this location, the Danube drains a 104,000 km2 area. Two contrasting sampling sites were investigated between April and July 1999 at weekly intervals. One sampling station was located in the main river channel at river kilometre 1890. For comparison, we also sampled an isolated side arm of the Danube floodplain area called “Lobau” on the north bank of the river. The investigated backwater system is located within the city area of Vienna; this former river channel is largely decoupled from the main river and prevailingly lentic. Surface connection is established only during very strong spate events (on average 17 days per year) when water enters this side arm in upstream direction via a narrow channel on its downstream end with low flow velocity. The sampling station was located in the central part of the stretch of this side arm approximately 5 km from the above-mentioned point of connection with the main river. A more detailed description of the investigated area is given in Hein et al. (1999).
Sampling
Water samples were taken with 10 L-carboys rinsed with 0.1 N HCl, tap and sample water. To assess bacterial and viral abundance and the number of suspended particles, 90 to 100 ml of the sample were fixed with formaldehyde (2% final concentration) immediately after sampling. The samples were kept refrigerated (+4°C) until analysis. Sampling depth was approximately 30–50 cm at all stations. The Danube station was sampled on 12 sampling events; on 18 May and 1 June 1999 no sampling could be conducted due to limited access to the site caused by a large flood. Lobau was sampled on 13 dates; no sampling occurred on 4 May at this station.
Particle abundance and quality
The fixed samples were filtered onto Whatman AnoDisc filters (pore size 0.2μm, 25 mm diameter). On each sampling date, two filters of each water sample were prepared. After gentle filtration (< 200 mm Hg), the filters were embedded in a mixture of glycerol and phosphate buffered saline onto microscope slides, stored in a refrigerator and analysed within less than a week. Particles were counted and sized (see below) under a light- and epifluorescence-microscope (Nikon E 800) at 500-fold magnification. Two size classes of particles, representing different microenvironments, were distinguished by considering the equivalent spherical diameter (ESD; i.e. the diameter of a sphere with equivalent volume to nonspherical-shaped particles; Riebesell, 1991; Peduzzi and Weinbauer, 1993): < 5 μm (mostly inorganic mineral particles) and > 5 μm (particles and aggregates with organic constituents; as described in Luef et al. 2007). On each filter, 20 fields were counted to determine the total number of particles; at least 20 particles of each size class were counted per field.
Bacterial and viral abundance, bacterial secondary production (BSP)
Samples were treated as described in detail in Luef et al. (2007). Briefly, 1 to 3 ml of the fixed samples were filtered onto Whatman AnoDisc filters (pore size 0.02μm, 25 mm diameter). Free-living and particle-attached bacteria as well as virus-like particles (VLPs) were stained with SYBR Green I (Noble and Fuhrman, 1998). Microorganisms were enumerated under an epifluorescence microscope (Nikon E 800) at 1250-fold magnification. On the filter approximately 20 particles of each size class were chosen randomly and inspected for the number of associated bacteria and virus-like particles (VLPs) as described in Luef et al. (2007). This method reveals abundance at least of surface-exposed microorganisms. Nucleic acid fluorescence from inside the aggregates might have been underestimated (see discussion), therefore our estimates may be conservative. The free-living, particle-associated and total fractions were assessed according to Luef et al. (2007). A virus-to-bacterium ratio (VBR) was calculated.
BSP of the free-living fraction was determined by pre-filtering samples through a 3.0 mm filter (Millipore TSTP, 47 mm diameter). For the total fraction, unfiltered samples were used. BSP was estimated using the [3H]-thymidine incorporation technique and applying a conversion factor proposed by Bell (1993). Triplicate samples (5 ml) were incubated for 0.5 to 1 h at in situ temperature in the laboratory. Two subsamples were pre-treated with formalin and served as a blank. The difference between the total BSP and the BSP of the free-living fraction was presumed to be the BSP of the attached fraction. The specific BSP (spBSP) was calculated by dividing the BSP by the respective bacterial abundance. No BSP measurements are available for 13 and 21 April and 22 June at the Lobau-site, and for 22 June at the Danube station.
Data Analysis
For statistical analysis, the software program SPSS 12.0 was used. A Spearman-Rho correlation test was performed to analyse the relations between measured variables (two-tailed, significance assumed at p < 0.05). Linear regression equations were calculated for selected variables. To test between sample differences a non-parametric Test (Mann-Whitney U-Test) was applied.
Results
Hydrology
An alpine regime governs the flow pattern of the Danube in Austria, with increased frequency of high discharge in spring/early summer due to snow-melt, precipitation or a combination of both. According to this, the investigation period from the mid-April to late July 1999 was dominated by relatively high discharge rates (Fig. 1). The water level of the Danube River remained almost permanently above mean water level (145.9 m above sea level). An annual spate occurred from mid-May to early June and a smaller one in early July. Only during the strong spate in May was the isolated Lobau connected to a certain degree via the narrow channel at its downstream end (see above).
Figure 1.
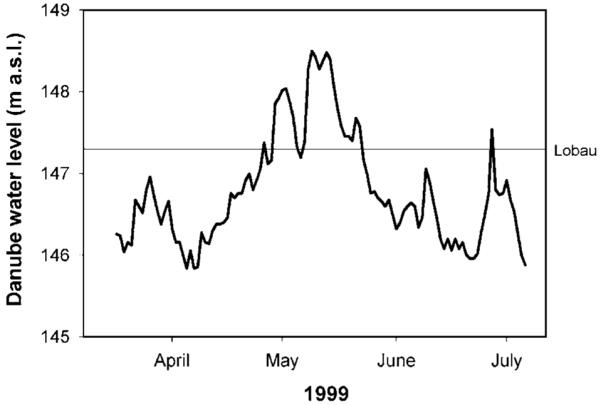
Danube River water level (m a.s.l. = meters above sea level) throughout the investigation period (April to July 1999). The horizontal reference line indicates the water level of connection between the Danube and the floodplain site Lobau.
Bacterial and viral dynamics
In the river channel of the Danube, the abundance of both bacterial fractions (free-living and attached) was quite variable during the investigation period. Free bacteria ranged between 0.2 and 2.1×106/ml, attached bacteria were even more variable fluctuating between 0.09 and 2.3×106/ml (Table 1). At the isolated Lobau section, free bacterial numbers were also variable (between 1.1 and 3.6×106/ml), attached bacteria ranged between 0.08 and 0.46×106/ml. On average, bacterial secondary production (BSP) of bacteria attached to suspended matter in the main channel was twice as high as that of free bacteria. At Lobau, the free-living bacteria exhibited higher BSP than the attached fraction (Table 1). At both sites, the per-cell productivity (spBSP) was about one order of magnitude higher for attached vs. free-living bacteria (Table 1).
Table 1.
Averages and range of values for bacterial (bacterial abundance, BSP = bacterial secondary production, spBSP = specific bacterial secondary production) and viral parameters (viral abundance, VBR = virus to bacterium ratio) for the free-living and attached fraction in the Danube River and in the floodplain site Lobau
| Danube channel |
Lobau |
|||||
|---|---|---|---|---|---|---|
| N | Range | Average | N | Range | Average | |
| Bacteriafree-living (106 ml−1) | 12 | 0.20–2.10 | 1.20 | 13 | 1.10–3.60 | 2.44 |
| Bacteriaattached (106 ml−1) | 12 | 0.09–2.30 | 0.87 | 13 | 0.08–0.46 | 0.26 |
| BSPfree-living (μg C L−1 h−1) | 11 | 0.16–1.19 | 0.66 | 10 | 0.14–3.85 | 1.21 |
| BSPattached (μg C L−1 h−1) | 11 | 0.36–2.14 | 1.18 | 10 | 0.14–1.64 | 0.79 |
| spBSPfree-living (fg C cell−1 h−1) | 11 | 0.08–3.80 | 0.90 | 10 | 0.05–1.40 | 0.47 |
| spBSPattached (fg C cell−1 h−1) | 11 | 0.19–12.00 | 3.24 | 10 | 0.87–10.00 | 4.79 |
| Virusesfree-living (107 ml−1) | 12 | 0.32–3.50 | 2.09 | 13 | 2.40–10.60 | 6.38 |
| Virusesattached(107 ml−1) | 12 | 0.02–0.69 | 0.19 | 13 | 0.01–0.26 | 0.09 |
| VBRfree-living | 12 | 4.11–34.96 | 19.79 | 13 | 14.01–48.19 | 27.23 |
| VBRattached | 12 | 0.53–13.51 | 3.18 | 13 | 0.08–6.78 | 4.05 |
In general, virus particles free in the water column were more abundant than attached forms. Free viruses in the main channel ranged from 0.3 to 3.5×107/ml; 0.02 to 0.69×107/ml virus particles were attached to suspended matter. In the Lobau water body, free viruses were much more abundant fluctuating between 2.4 and 10.6×107/ml; attached viruses varied between 0.01 and 0.26×107/ml. The virus-to-bacterium ratio (VBR) at both stations was always significantly lower (Mann-Whitney, p<0.005, n=25) on suspended matter than for the free fraction (see Table 1).
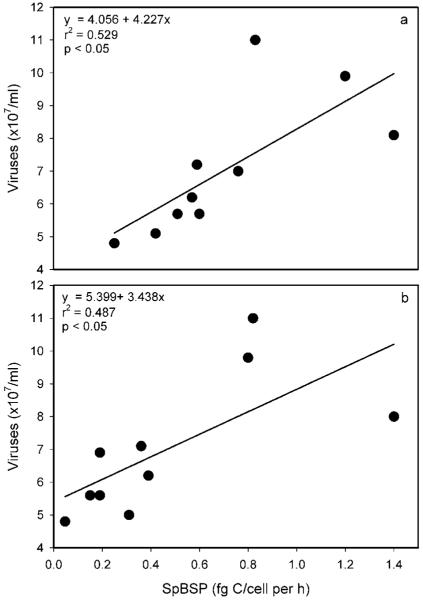
The per-cell activity of bacteria (spBSP) obviously influenced virus abundance, but only in the isolated Lobau waters. Figure 2a shows significant linear regressions between these two parameters for the total fractions (i.e. sum of free-living and attached microorganisms), and figure 2b for the free-living fraction alone.
Figure 2.
Relationship between the total specific bacterial secondary production (spBSP) and the total viral abundance (a), relationship between the spBSP of the free-living bacteria and the free-living viruses (b) in the isolated floodplain segment Lobau during the investigation period.
Bacteria and viruses on particles
To investigate whether suspended particle size is of importance for microorganism colonization, we separately inspected particles < 5μm (this fraction consists mainly of inorganic mineral material) and particles and aggregates > 5mm (with high content of organic material). At both sampling sites (pooled data from the entire investigation period), the larger particles were more densely (Mann-Whitney, always p < 0.05, n=36) colonized by bacteria than the small ”mineral” particles (based on particle surface area; see Fig. 3). However, colonization with viruses followed this pattern only at the lentic environment Lobau. In the Danube main channel, no significant difference in viral abundance on particle surfaces was observed (Mann-Whitney, p > 0.05, n = 36). When comparing the two sampling sites, only on particle surfaces of larger (“organic”) particles were significantly more bacteria (Mann-Whitney, p < 0.05, n = 50) present at the isolated section Lobau than in the Danube main channel. Viruses were significantly more abundant at Lobau on both suspended solid fractions (Mann-Whitney, small particles: p < 0.05, n = 36; large particles: p < 0.001, n = 50).
Figure 3.
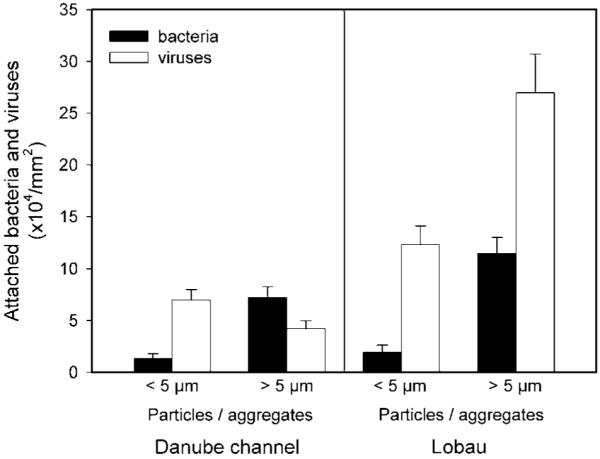
Abundance (mean ± S. E.) of bacteria and viruses attached to particles / aggregates in two size classes in the Danube and Lobau floodplain segment. At both stations, n for particles < 5 μm = 36 and for particles > 5 μm = 50.
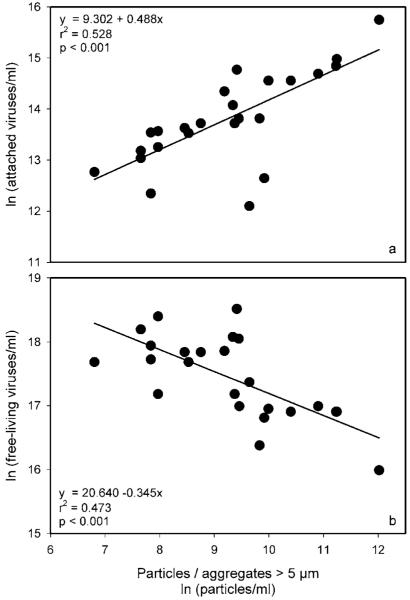
To evaluate whether the overall abundance of particles > 5μm (with organic content) had any effect on the fraction of attached microorganisms, we performed correlation analyses between these variables. In the Danube channel, both attached bacteria (Spearman, r = 0.66, p < 0.05, n = 12) and viruses (r = 0.99, p < 0.01, n = 12) were dependent on the abundance of suspended material. At the Lobau site, only viruses exhibited this type of dependency (r = 0.69, p < 0.01, n = 13). No such relationship was observed with small (“inorganic”) particles either in the main channel or in the Lobau water body. Linear regression models of data pooled from both environments (main channel and isolated section) are presented in figure 4a b, highlighting that the abundance of attached viruses is positively linked to the availability of suspended particles with higher organic content. The presence of free viruses exhibited a negative dependency on abundance of such > 5μm particulate material.
Figure 4.
Relationship between abundances of organic particles / aggregates > 5 μm and of attached viruses (a) and of free-living viruses (b) in the Danube and Lobau floodplain segment during the investigation period. All data were transformed to the natural logarithm prior to regression analysis. Based on a Grubb’s test for outliers (p < 0.01) in both regressions (panel a & b) one data point was not included in the analysis.
Discussion
Our study was conducted during the hydrologically dynamic period of the year (see Aspetsberger et al., 2002). Despite relatively high river discharge, the isolated floodplain section Lobau was largely lentic and clearly differed from the conditions in the main channel.
The overall bacterial abundance and BSP in the main channel and at Lobau were within the range reported in other studies (Hoch et al., 1995; Hein et al., 1999; Aspetsberger et al., 2002; Schiemer et al., 2006). We quantified two bacterial fractions – free-living and attached to suspended solids. In the Danube channel the abundance of both fractions (based on volume of water) remained on average within the same range (see Table 1), attached bacteria were less abundant at the Lobau site. A similar observation, although less pronounced, was made with BSP when comparing these two fractions. This was most likely due to the high suspended solid load in the main channel vs. the clearer water column of the isolated floodplain pool. High bacterial abundance bound to particles was also found in a study conducted in the main channel and another floodplain section that is strongly integrated into the river’s flow regime (Luef et al., 2007). Attached bacterial abundance can vary from a few percent to 98% of total bacterial abundance in various aquatic regions (Iriberri et al., 1987), mainly depending on particle abundance. In a head-water river-reservoir, Kondratieff and Simmons (1985) showed that attached bacteria were more important upstream (mean 42–45%) than in the reservoir and downstream (19–31%).
In contrast to bacteria, viruses were always more abundant in the free-living fraction in our study, particularly at the lentic site Lobau (ca. 3 times higher than in the main channel, see Table 1). This indicates favourable conditions for virus proliferation here. The lower VBR on suspended matter (compared to freely suspended viruses) in our study is comparable to other material as substratum for viruses. Lemke et al. (1997) reported that the VBR in different riverine sediments ranged from 0.7 to 1.2, while on leaves it ranged from 0.2 to 0.3. Fischer et al. (2003) found VBRs in Danube backwater sediments ranging from 0.9 to 3.2. The values in sediments of another riverine wetland ranged from 0.03 to 0.74, on aquatic macrophytes from 0.06 to 0.85 and on submerged wood from 0.01 to 0.86 (Farnell-Jackson and Ward, 2003). In lake snow, VBRs varied between 0.3 and 8.5 with an average of 4.7 (Simon et al., 2002).
This is one of the first attempts to quantify both bacteria and viruses together on suspended particles using epifluorescence microscopy, and our results probably merely reflect trends. Due to technical constraints (background fluorescence, only two-dimensional visualisation) the true abundance may be underestimated (Kirchman and Mitchell, 1982; Luef et al., 2007). However, our data indicate that viruses (and bacteria) can be abundant on particles and this apparently is linked to particle quality.
The hydrological regime of running waters is a major factor determining particulate organic matter, nutrients, phytoplankton and zooplankton as well as bacterio- and virioplankton dynamics in floodplains (Schagerl and Riedler, 2000; Baranyi et al., 2002; Hein et al., 2003, Luef et al., 2007). Different water age influences particle quality. Our results indicate that the quality of the particulate matter (size, organic constituents) affected the abundance of attached bacteria at both sampling sites, and of viruses only at the isolated location. Considering the surface area, those particles weakly colonised by bacteria (and at Lobau also by viruses) consisted mainly of inorganic components and were smaller than the heavily colonised particles. The strongly colonised particles were predominantly flocculent, and contained an organic matrix. A similar observation (for bacteria only) was reported by Berger et al. (1996) for the main channel of the Danube River. In a more pristine floodplain part of this Danube area, which is dynamically connected with the main river, the abundance of Alcian- and Commassie-stained suspended particles was positively related to water age, indicating an increasing importance of organic constituents in seston material under more lentic conditions (Luef et al., 2007). In the same study, moreover, Alcian-stained aggregates correlated with bacteria and viruses attached to the particles. In another investigation using stable isotope analysis, Aspetsberger et al. (2002) showed that water age significantly influenced POM quantity and composition. Our data (Fig. 3) indicate that the lentic conditions of the isolated section typically provide a more favourable particle substratum for virus colonization.
In general, the presence/abundance of suspended particles > 5μm in the water column of a riverine environment (either lotic or more lentic) appears to be significant for the distribution of viruses. Figure 4 clearly demonstrates that, as particles with organic constituents increase in the water body, more viruses are attached and fewer are freely suspended. Therefore, such particles apparently remove VLPs from the water column. We are currently still unable to predict whether these attached viruses are “inactivated” or play an active role in the particle microenvironment. Nevertheless, our results have considerable consequence for several environmental aspects of river ecosystems.
Isolated, mainly biologically controlled river-floodplain sections (particularly at low water levels) can be distinguished from more open and strongly hydrologically controlled parts (e.g. at higher water levels or the main channel; Tockner et al. 1999). Regarding viruses, our study must be considered preliminary to support a clear conclusion about controlling factors. However, since there is evidence that maintenance of abundant bacteriophage populations is dependent on an active bacterial host community (Wommack and Colwell, 2000; Peduzzi and Schiemer, 2004), the significant interrelationships between the per-cell productivity of bacteria (spBSP) and virus abundance in the isolated Lobau waters (see Fig. 2a b) suggest biological interaction between these two microbial compartments. No such significant correlation could be observed in the Danube River itself. In the main river, physical parameters like gauge and temperature generally prevail (Schiemer et al., 2006) and may have influenced virus abundance there.
The variability of the various subsystems in a river-floodplain is manifest in a set of limnological parameters, including sediment, suspended solids, nutrients and biota (Schiemer et al., 2006). Our research, conducted in two end-member subsystems of a river-floodplain (lotic vs. lentic), demonstrates that such variability also influences microbial life including viruses. Different water bodies apparently influence the particle quality along with their associated bacteria and viruses as well as microorganisms in the free water column. Particle-related bacteria and viruses are dependent on particle abundance, size and quality.
Our preliminary results hopefully stimulate further investigations on the microbial ecology of riverine and river-floodplain systems. Since particle origin (and quality) apparently is different in headwater floodplains compared to large lowland systems (Malard et al., 2006), there is still need for, e.g., quantification, sizing and characterization of particulate matter and the associated microorganisms in various subsystems of river-floodplains in relation to hydrologic events. Investigating whether disturbance by flooding influences virally induced mortality of prokaryotes in the various floodplain sections may be of particular importance. Work on marine systems indicates that viral lysis can profoundly affect the carbon cycle by converting cells into dissolved organic carbon (Fuhrman and Suttle, 1993), in a process described as the ”viral shunt” (Wilhelm and Suttle, 1999). In their model, these authors estimated that some 6–26% of photosynthetically fixed organic carbon is recycled back to dissolved organic material by viral lysis. Although viral lysis may release only a small fraction of the total pool of DOC and POC in a given time span, it could constitute a significant portion of the rapidly cycling carbon in the system. This is particularly relevant in river systems with a usually high proportion of aged and recalcitrant carbon (Raymond and Bauer, 2001). Nothing, however, is known about the effects of viral lysis on the carbon cycle in riverine systems. Therefore it may be asked what will be the consequences of viral lysis of prokaryotes on the microbial carbon cycle in the different subsystems and hydrological situations of a river-floodplain system. Future research should also consider the documentation of differences in the community structure of attached vs. ambient water viruses and hosts. The assessment of a potential viral impact on particle dissolution and aggregate formation (compare Peduzzi and Weinbauer, 1993) may also be of considerable relevance. Focusing on such topics will provide further important insights into the structure and function of aquatic microbial food webs, and into carbon cycling in a sequence of systems with varying particle load. It will also contribute to our understanding of microbially mediated processes in large river systems.
Acknowledgments
We thank the National Park Authority and the Austrian River Authority for enabling our research in the Danube Alluvial Zone National Park. Our research was funded by the Austrian Science Fund (P11720, P14721 and P17798). Thanks are due to T. Hein for logistic and intellectual support and to two anonymous reviewers for their valuable comments.
References
- Aspetsberger F, Huber F, Kargl S, Scharinger B, Peduzzi P, Hein T. Particulate organic matter dynamics in a river floodplain system: impact of hydrological connectivity. Archiv für Hydrobiologie. 2002;156:23–42. [Google Scholar]
- Baker PW, Leff LG. Seasonal patterns of abundance of viruses and bacteria in a Northeast Ohio (USA) stream. Archiv für Hydrobiologie. 2004;161:225–233. [Google Scholar]
- Baranyi C, Hein T, Holarek C, Keckeis S, Schiemer F. Zooplankton biomass and community structure in a Danube River floodplain system: effects of hydrology. Freshwater Biology. 2002;47:473–482. [Google Scholar]
- Bell RT. Estimating production of heterotrophic bacterioplankton via incorporation of tritiated thymidine. In: Kemp PF, Sherr BF, Sherr EB, Cole JJ, editors. Handbook of Methods in Aquatic Microbial Ecology. Lewis Publishers; Boca Raton, Fl.: 1993. pp. 495–503. [Google Scholar]
- Berger B, Hoch B, Kavka G, Herndl GJ. Bacterial colonization of suspended solids in the River Danube. Aquatic Microbial Ecology. 1996;10:37–44. [Google Scholar]
- Bergh Ø, Børsheim KY, Bratbak G, Heldal M. High abundance of viruses found in aquatic environments. Nature. 1989;340:467–468. doi: 10.1038/340467a0. [DOI] [PubMed] [Google Scholar]
- Farnell-Jackson EA, Ward AK. Seasonal patterns of viruses, bacteria and dissolved organic carbon in a riverine wetland. Freshwater Biology. 2003;48:841–851. [Google Scholar]
- Fischer UR, Velimirov B. High control of bacterial production by viruses in a eutrophic oxbow lake. Aquatic Microbial Ecology. 2002;27:1–12. [Google Scholar]
- Fischer UR, Wieltschnig C, Kirschner AKT, Velimirov B. Does virus-induced lysis contribute significantly to bacterial mortality in the oxygenated sediment layer of shallow oxbow lakes? Applied and Environmental Microbiology. 2003;69:5281–5289. doi: 10.1128/AEM.69.9.5281-5289.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer UR, Wieltschnig C, Kirschner AKT, Velimirov B. Contribution of virus-induced lysis and protozoan grazing to benthic bacterial mortality estimated simultaneously in microcosms. Environmental Microbiology. 2006;8:1394–1407. doi: 10.1111/j.1462-2920.2006.01032.x. [DOI] [PubMed] [Google Scholar]
- Fuhrman JA, Suttle CA. Viruses in marine planktonic systems. Oceanography. 1993;6:51–63. [Google Scholar]
- Galat DL, Kubisiak JF, Hooker JB, Sowa LM. Geomorphology, distribution and connectivity of lower Missouri River floodplain water bodies scoured by the flood of 1993. International Association of Theoretical and Applied Limnology. 1997;26:869–878. [Google Scholar]
- Hein T, Baranyi C, Heiler G, Holarek C, Riedler P, Schiemer F. Hydrology as a major factor determining plankton development in two floodplain segments and the River Danube, Austria. Archiv für Hydrobiologie, Supplement Large Rivers. 1999;11:439–452. [Google Scholar]
- Hein T, Baranyi C, Herndl GJ, Wanek W, Schiemer F. Allochthonous and autochthonous particulate organic matter in floodplains of the River Danube: the importance of hydrological connectivity. Freshwater Biology. 2003;48:220–232. [Google Scholar]
- Hoch B, Berger B, Kavka G, Herndl GJ. Remineralization of organic matter and degradation of the organic fraction of suspended solids in the River Danube. Aquatic Microbial Ecology. 1995;9:279–288. [Google Scholar]
- Iriberri J, Unanue M, Barcina I, Egia L. Seasonal variation in population density and heterotrophic activity of attached and free living bacteria in coastal waters. Applied and Environmental Microbiology. 1987;53:2308–2314. doi: 10.1128/aem.53.10.2308-2314.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junk WJ, Bayley PB, Sparks RE. The flood pulse concept in river-floodplain systems. Canadian Special Publication of Fisheries and Aquatic Sciences. 1989;106:110–127. [Google Scholar]
- Kirchman D, Mitchell R. Contribution of particle-bound bacteria to total microheterotrophic activity in five ponds and two marshes. Applied and Environmental Microbiology. 1982;43:200–209. doi: 10.1128/aem.43.1.200-209.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratieff PF, Simmons GM. Microbial colonization of seston and free bacteria in an impounded river. Hydrobiologia. 1985;128:127–133. [Google Scholar]
- Lemke M, Wickstrom C, Leff L. Preliminary study on the distribution of viruses and bacteria in lotic environments. Archiv für Hydrobiologie. 1997;141:67–74. [Google Scholar]
- Luef B, Aspetsberger F, Hein T, Huber F, Peduzzi P. Impact of hydrology on free-living and particle-associated microorganisms in a river-floodplain system (Danube, Austria) Freshwater Biology. 2007;52:1043–1057. [Google Scholar]
- Malard F, Uelinger U, Zah R, Tockner K. Flood-pulse and riverscape dynamics in a braided glacial river. Ecology. 2006;87:704–716. doi: 10.1890/04-0889. [DOI] [PubMed] [Google Scholar]
- Maranger R, Bird DF. Viral abundance in aquatic systems: a comparison between marine and fresh waters. Marine Ecology Progress Series. 1995;121:217–226. [Google Scholar]
- Mathias CB, Kirschner AKT, Velimirov B. Seasonal variations of virus abundance and viral control of bacterial production in a backwater system of the Danube River. Applied and Environmental Microbiology. 1995;61:3734–3740. doi: 10.1128/aem.61.10.3734-3740.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei ML, Danovaro R. Virus production and life strategies in aquatic sediments. Limnology and Oceanography. 2004;49:459–470. [Google Scholar]
- Meybeck M. Carbon, nitrogen, and phosphorus transport by world rivers. American Journal of Science. 1982;282:401–450. [Google Scholar]
- Noble RT, Fuhrman JA. Use of SYBR Green I for rapid epifluorescence counts of marine viruses and bacteria. Aquatic Microbial Ecology. 1998;14:113–118. [Google Scholar]
- Olapade OA, Brothers A, Crissman M, Gao X, Leff LG. Comparison of planktonic microbial communities among nine North American streams. Archiv für Hydrobiologie. 2006;165:221–239. [Google Scholar]
- Peduzzi P, Schiemer F. Bacteria and viruses in the water column of tropical freshwater reservoirs. Environmental Microbiology. 2004;6:707–715. doi: 10.1111/j.1462-2920.2004.00602.x. [DOI] [PubMed] [Google Scholar]
- Peduzzi P, Weinbauer MG. Effect of concentrating the virus-rich 2 – 200 nm size fraction of seawater on the formation of algal flocs (marine snow) Limnology and Oceanography. 1993;38:1562–1565. [Google Scholar]
- Proctor LM, Fuhrman JA. Viral mortality of marine bacteria and cyanobacteria. Nature. 1990;343:60–62. [Google Scholar]
- Raymond PA, Bauer JE. Riverine export of aged terrestrial organic matter to the North Atlantic Ocean. Nature. 2001;409:497–499. doi: 10.1038/35054034. [DOI] [PubMed] [Google Scholar]
- Richter BD, Baumgartner JV, Wigington R, Braun DP. How much water does a river need? Freshwater Biology. 1997;37:231–249. [Google Scholar]
- Riebesell U. Particle aggregation during a diatom bloom. I. Physical aspects. Marine Ecology Progress Series. 1991;69:273–280. [Google Scholar]
- Schagerl M, Riedler P. Phytoplankton composition in the River Danube floodplain system Regelsbrunner Au. Abhandlungen der Zoologisch-Botanischen Gesellschaft in Österreich. 2000;31:43–62. [Google Scholar]
- Schiemer F, Hein T, Peduzzi P. Hydrological control of system characteristics of floodplain lakes. Ecohydrology & Hydrobiology. 2006;6:7–18. [Google Scholar]
- Simon M, Grossart H-P, Schweitzer B, Ploug H. Microbial ecology of organic aggregates in aquatic ecosystems. Aquatic Microbial Ecology. 2002;28:175–211. [Google Scholar]
- Suttle CA, Chan AM, Cottrell MT. Infection of phytoplankton by viruses and reduction of primary productivity. Nature. 1990;347:467–469. [Google Scholar]
- Skraber S, Gantzer C, Maul A, Schwartzbrod L. Fates of bacteriophages and bacterial indicators in the Moselle river (France) Water Research. 2002;36:3629–3637. doi: 10.1016/s0043-1354(02)00063-5. [DOI] [PubMed] [Google Scholar]
- Slováčková H, Maršálek B. Virioplankton and microbial communities in two Czech rivers (Svratka and Morava River) Aquatic Sciences. in press. DOI 10.1007/s00027-008-8040-2. [Google Scholar]
- Tockner K, Pennetzdorfer D, Reiner N, Schiemer F, Ward JV. Hydrological connectivity, and the exchange of organic matter and nutrients in a dynamic river-floodplain system (Danube, Austria) Freshwater Biology. 1999;41:521–535. [Google Scholar]
- Walter R, Dumke R, Dürkop J, Guyer S, Krämer U. Virological investigations of the River Danube. Acta Hydrochimica et Hydrobiologica. 1990;18:333–343. [Google Scholar]
- Walter R, Macht W, Dürkop J, Hecht R, Hornig U, Schulze P. Virus levels in river waters. Water Research. 1989;23:133–138. [Google Scholar]
- Weinbauer MG. Ecology of prokaryotic viruses. FEMS Microbiology Reviews. 2004;28:127–181. doi: 10.1016/j.femsre.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Wilhelm SW, Suttle CA. Viruses and nutrient cycles in the sea. BioScience. 1999;49:781–788. [Google Scholar]
- Wommack KE, Colwell RR. Virioplankton: viruses in aquatic ecosystems. Microbiology and Molecular Biology Reviews. 2000;64:69–114. doi: 10.1128/mmbr.64.1.69-114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]