Abstract
Dissolved and particulate organic matter (DOM and POM) distribution, lignin phenol signatures, bulk elemental compositions, fluorescence indices and microbial plankton (algae, bacteria, viruses) in a temperate river floodplain system were monitored from January to November 2003. We aimed to elucidate the sources and compositions of allochthonous and autochthonous organic matter (OM) in the main channel and a representative backwater in relation to the hydrological regime. Additionally, bacterial secondary production was measured to evaluate the impact of organic carbon source on heterotrophic prokaryotic productivity. OM properties in the backwater tended to diverge from those in the main channel during phases without surface water connectivity; this was likely enhanced due to the exceptionally low river discharge in 2003. The terrestrial OM in this river floodplain system was largely derived from angiosperm leaves and grasses, as indicated by the lignin phenol composition. The lignin signatures exhibited significant seasonal changes, comparable to the seasonality of plankton-derived material. Microbially-derived material contributed significantly to POM and DOM, especially during periods of low discharge. High rates of bacterial secondary production (up to 135 μg C L−1 d−1) followed algal blooms and suggested that autochthonous OM significantly supported heterotrophic microbial productivity.
1. Introduction
Riverine dissolved and particulate organic matter (DOM and POM) are important components of the global carbon cycle and are the primary drivers of ecosystem functions in freshwater environments (Hedges et al., 2000; Battin et al., 2008). Production and transformation of OM in streams and rivers render the broad range of molecular forms typical for organic material in freshwater systems (Kaplan and Bott, 1989; Kim et al., 2006). Sources and properties of OM are key in controlling microbial processing and carbon cycling in aquatic ecosystems (Kaplan and Bott, 1989). Allochthonous OM from the catchment is often thought to prevail over autochthonous material derived from aquatic primary producers (Ertel et al., 1986; Battin, 1998), whereas carbon released from algae is typically more available for heterotrophic microorganisms (Azam and Cho, 1987; Kaplan and Bott, 1989).
Various environmental factors maintain complex temporal and spatial patterns among autochthonous and allochthonous OM fractions in aquatic systems. Autochthonous, microbially-produced carbon typically shows a pronounced seasonality due to phytoplankton growth driven by light, temperature and nutrient supply (Hein et al., 1999; Kirschner and Velimirov, 1999). Inputs and properties of terrestrially derived OM are determined by land cover of the catchment, riparian vegetation, upland soil profiles (Ertel et al., 1986; Hedges et al., 1986; Eckard et al., 2007) and precipitation and runoff (Buffam et al., 2001; Dalzell et al., 2007). Until recently, it was agreed that most of the terrestrially-derived organic carbon that enters running waters is already largely degraded in upland soils and is transported conservatively along streams and rivers (Ertel et al., 1986; Hedges et al., 1986). Recent studies indicate, however, that river OM is less recalcitrant and has a greater bioavailability than previously thought (Bianchi et al., 2004; Mayorga et al., 2005; Duan and Bianchi, 2006; Hernes et al., 2007; Battin et al., 2008).
In natural river floodplain systems and wet lands, lentic and lotic aquatic habitats cover a broad range of DOM and POM concentration and composition (Aspetsberger et al., 2002; Hein et al., 2003; Schiemer et al., 2006), that are strongly dependent on a dynamic hydrological regime. Hydrology affects OM properties via the connectivity between the main channel, backwaters (e.g. old tributaries and distributaries) and the surrounding watershed, which ultimately control the transfer of terrestrial and aquatic-derived OM in this ecosystem (Hedges et al., 1986; Tockner et al., 1999). Several studies focused on the flux of OM through river floodplain systems (Aspetsberger et al., 2002; Hein et al., 2003) and the importance of hydrology for phytoplankton development and transport (Hein et al., 1999). While characterization of dissolved organic carbon (DOC) and data concerning the terrestrial component exist for the catchments of large rivers (Ertel et al., 1986; Hedges et al., 2000; Onstad et al., 2000; Bianchi et al., 2004; Duan et al., 2007a,b) and marine environments (Moran and Hodson, 1994; Opsahl and Benner, 1997), few studies have examined the sub-habitats in river floodplain systems.
Here we report on a study concerning sources and composition of DOM and POM in the main channel and the backwaters of one of the largest semi-natural floodplains in Europe, the National Park of the river Danube, Austria. The general working hypothesis is that algal productivity is the primary driver of microbial production, while terrestrially-derived OM provides a stable background source of OM for bacterial growth. Our main objectives were to investigate the dynamic changes in OM concentration, sources and properties with respect to hydrological connectivity. The terrigenous component of OM was characterized via its lignin phenol signature. Lignin oxidation products provide a tracer for the source of vascular plant derived OM and for the extent of alteration that has occurred by way of microbial or photochemical degradation (Ertel et al., 1986; Hedges et al., 1986; Opsahl and Benner, 1995) or by leaching/sorption processes (Hernes et al., 2007). C:N ratio, δ13C values of POM and fluorescence index of DOM (McKnight et al., 2001) were applied to characterize chemical properties. We monitored the abundance of algae, bacteria and viruses to estimate the importance of microbially derived material to aquatic OM. Furthermore, bacterial secondary production was measured to detect possible correlations between organic carbon sources and heterotrophic prokaryotic activity. Finally, the hydrodynamics of the main channel of the river Danube were compared with a dynamically connected and semi-natural backwater.
2. Sampling and analytical methods
2.1. Site description and hydrological conditions
The National Park of the river Danube (Austria) includes the free flowing section of the river downstream of Vienna and its backwaters. At this location, the Danube drains a 104,000 km2 area. The hydrological conditions are governed by an alpine regime, resulting in highly variable flow and highest water level in early summer (Schiemer et al., 1999). The local vegetation of the Donauauen National Park is dominated by deciduous forest (65%), with grassland comprising 15%. The catchment is largely characterized by grassland and arable land (47%). About 37% of the catchment area consists of forest (Zessner et al., 2005), coniferous forest comprising a significant part of the Upper Danube catchment area (Vogt et al., 2007). Samples from the main channel of the Danube were taken near the town of Haslau. A backwater near the town of Regelsbrunn was chosen as an example of a floodplain pool dynamically interlinked with the main channel. This backwater is dominated by a former river channel, which at mean water level constitutes 82% of the total aquatic surface area within the floodplain section (Tockner et al., 1999). Inflow of river water is possible at a water level of 0.5 m below mean water, while full surface connectivity and lotic conditions are established at mean water (Schiemer et al., 1999). Discharge recordings of the river Danube were provided by the Austrian River Authority.
2.2. Sampling and water analysis
Surface water samples for OM properties, plankton and bacterial production were taken with acid- and water-rinsed polyethylene bottles (10 L) from Jan. to Nov. 2003 (Fig. 1). Samples for lignin phenol analysis were taken on 11 dates, with a focus on spring and early summer months. Temperature and conductivity were measured in the field. Phosphorus and nitrogen fractions (soluble reactive phosphorus, nitrate, ammonium) were determined following standard methods (Golterman et al., 1978) and dissolved organic nitrogen was measured and calculated by subtracting ammonium from Kjeldahl-nitrogen (Mühlhauser et al., 1987).
Fig. 1.
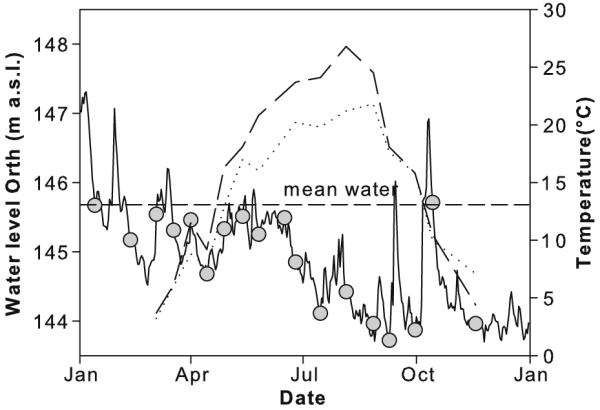
Water level in meters above sea level (m a.s.l., solid line), water temperature in main channel (dotted line) and in backwater (dashed line) during investigated period. Surface water connection of backwater to main channel was established 0.5 m below mean water (indicated as horizontal line). Grey circles indicate sampling dates.
2.3. OM properties
Pre-filtered water (Millipore, APF/F, pore size 0.7 μm) was used to estimate the concentration and properties of DOM. DOC concentration was measured using high temperature catalytic oxidation (HTCO) with a Shimadzu TOC 5000 C analyzer according to Benner and Strom (1993). Fluorescence was determined at an excitation wavelength of 370 nm using a Shimadzu RF-1501 spectrofluorometer; the ratio of fluorescence intensity at 450 nm to that at 500 nm was measured. A fluorescence index (FI) of about 1.2 is indicative of terrestrially derived OM, while a value of about 1.9 indicates microbially derived OM (McKnight et al., 2001).
For analysis of particulate organic carbon (POC) and particulate organic nitrogen (PON), 500–1000 mL water samples were concentrated on pre-weighed glass fibre filters (Millipore, APF/F). Filters were dried at 60 °C for 24 h, weighed and cut into sections. Elemental analysis was performed according to Cifuentes et al. (1996). Defined proportions of the filters were fumed over concentrated HCl (37.5%) to eliminate inorganic constituents and then ground in a ball mill. POC and PON were analyzed using continuous flow gas isotope ratio mass spectrometry. The elemental analyzer (EA 1200, CE Instruments, Italy) was interfaced via a ConFlo II device (Finnigan MAT, Germany) to the gas isotope ratio mass spectrometer (DeltaPLUS, Finnigan MAT). The standard for deviation for repeated measurements of δ13C values of a laboratory standard was 0.10%. The δ13C value was calculated as follows:
where R is the ratio of mass 45/mass 44 (carbon). Abundance is expressed in relation to the international standard Vienna Pee Dee Belemnite (V-PDB). Carbon isotopic signatures of OM can distinguish vascular plants employing the C3 pathway of CO2 uptake, plants using the C4 pathway, and freshwater phytoplankton (Smith and Epstein, 1971; Rau, 1978). Organic carbon and organic nitrogen measurements were used to calculate the C:N values for POM and DOM. Reproducibility of POC and PON measurements was below ±2.5%.
Duplicate samples for lignin phenol concentration and composition were analyzed using CuO oxidation and gas chromatography (Hedges and Ertel, 1982). Water samples (10 L) were filtered through glass fibre filters (Millipore, APF/F) to concentrate particulate matter. Filtered water samples (2–4 L) were acidified (pH 2) and DOM was isolated using solid phase extraction (C18 extraction disks, 3M Empore™, MN, USA; Louchouarn et al., 2000). Samples containing on average 8.2 mg OC were oxidized at 155 °C (3 h) with CuO under basic (8% NaOH) conditions. Oxidation products were acidified, extracted with ethyl acetate and dried under a stream of N2 (Gordon and Goni, 2003). Lignin phenols were then converted to trimethylsilyl derivatives and analyzed using a Varian 3800 gas chromatograph equipped with a fused silica capillary column (J&W Scientific DB-1) and a flame ionization detector. Major lignin oxidation products were identified by calibration with commercially available standards (Sigma; Opsahl and Benner, 1998; Aufdenkampe et al., 2007) and quantified with an average precision of ±15% using ethylvanillin as internal standard (Opsahl and Benner, 1997). Lignin phenol analysis of POM samples from GF/F filters generated problems during the extraction with ethyl acetate. In several samples a precipitate formed, which interfered with the separation of the aqueous and organic phases. Only unimpaired samples were included for further analysis.
The yields of eight lignin derived phenols, comprising the vanillyl (vanillin, acetovanillon, vanillic acid), syringyl (syringaldehyde, acetosyringone, syringic acid) and cinnamyl (p-coumaric acid, ferulic acid) families (V, S and C), were summed to determine the lignin concentration (in μg lignin phenols per L water) and the carbon normalized lignin yield Λ (mg lignin produced per 100 mg OC; Hedges and Ertel, 1982). The relative distribution of these phenol families differs among plant types. Weight ratios of S:V and C:V can be used as plant type indicators. Syringyl phenols are virtually absent from gymnosperm tissue (S:V = 0); among angiosperms, S:V can range from 0.5 to 8 (Ertel et al., 1986; Hedges et al., 1986; Opsahl and Benner, 1995). Cinnamyl phenols are produced in appreciable amounts from nonwoody tissues such as leaves, macrophytes and grass, but not from wood (Hedges et al., 1986). The vanillic acid:vanillin (Ad:Al)v ratio increases during humification and photochemical degradation (Opsahl and Benner, 1995; Hernes and Benner, 2003) and has been used to provide information about the diagenetic state of OM. However, this index has been shown to be especially sensitive to alteration during leaching-sorption effects (Hernes et al., 2007) and has to be interpreted with caution when comparing different size fractions. We therefore use the term “alteration state” to include all possible processes that lead to a change in the (Ad:Al)v ratio.
2.4. Plankton dynamics and bacterial secondary production
Ca. 1 L aliquots of water were filtered onto APF/C filters (Millipore, pore size 1.2 μm) and chlorophyll a (Chl a) was extracted with p.a. grade acetone (16 h, 4 °C) in the dark. Chl a concentration was measured spectrophotometrically, using a Hitachi U 2000 spectrophotometer (Lorenzen, 1967).
Formaldehyde-fixed water samples (1 to 3 mL) were stained with DAPI (Sigma–Aldrich) for bacterial abundance (BA), or SYBR Green I (Invitrogen) for viral abundance and filtered either onto a 0.2 μm black filter (Millipore, GTBP; for bacteria) or on 0.02 μm AnoDisc filters (Whatman; for viruses). Bacteria and virus-like particles (VLP) were enumerated in 30 randomly selected fields to account for 300 to 500 cells/VLP using epifluorescence microscopy (Nikon E800) (Porter and Feig, 1980; Noble and Fuhrman, 1998).
Bacterial secondary production (BSP) of the free-living bacterial fraction was determined by pre-filtering samples through a 3.0 μm filter (Millipore, TSTP). For the total bacterial community, unfiltered samples were used. BSP was assessed using the [3H]-thymidine incorporation technique and applying a conversion factor of 2 × 1018 cells produced per mol thymidine incorporated (Bell, 1993). Triplicate samples (5 mL) were incubated for 1 h at in situ temperature in the laboratory. Two sub-samples were treated with formalin and served as a blank. The difference between the total BSP and the BSP of the free-living fraction was presumed to be the BSP of the bacteria associated with particles.
2.5. Data analysis
Values from sampling sites and OM fractions were tested for significant differences using the Student’s t-test for paired samples. Correlation analysis was used to estimate the influence of hydrology on OM and plankton and to evaluate the agreement between the indicators of OM sources. Correlations were conducted with Pearson correlation analysis. Normal distribution of data was confirmed using Kruskal–Wallis-ANOVA. The software packages Microsoft Excel and SPSS 12.0 were applied for the statistical analyses.
3. Results
3.1. Characterization of hydrological and hydrochemical conditions
The year 2003 was characterized by exceptional low discharge and water levels below mean water, despite Jan. and Feb. and a short spate in Nov. Full surface water connectivity was hardly established that year (Fig. 1) and water exchange between the main channel and the backwater was largely limited to seepage. Discharge in the backwater ranged from 0.2 to 12.3 m3 s−1 (Table 1). The water temperature was between 3 °C and 22 °C in the main channel and between 4 °C and 27 °C in the backwater (Fig. 1). Mean conductivity averaged (±standard deviation) 469 ± 45 μS cm−2 in the main channel and 416 ± 49 μS cm−2 in the backwater; suspended solids concentrations were below 25 mg L−1 for most of the year (Table 1). Average nutrient concentrations were 668 ± 687 μg L−1 NO3-N, 25 ± 35 μg L−1 NH4-N and 4 ± 7 μg L−1 PO4-P in the backwater, compared to 1878 ± 539 μg L−1 NO3-N, 77 ± 50 μg L−1 NH4-N and 16 ± 12 μg L−1 PO4-P in the main channel.
Table 1.
Discharge, suspended solids and OM properties in main river channel and representative backwater of Danube (abbreviations as in text)
| Sampling date | Discharge (m3 s−1) | Suspended solids (mg L−1) |
DOM |
POM |
||||
|---|---|---|---|---|---|---|---|---|
| DOC (μg L−1) | DON (μg L−1) | FI (450/500 nm) | POC (μg L−1) | PON (μg L−1) | δ13C (‰) | |||
| Main river channel | ||||||||
| 13-Jan-03 | 2058.0 | n.d.a | 2796 | n.d.a | 1.18 | n.d.a | n.d.a | n.d.a |
| 11-Feb-03 | 1739.0 | n.d.a | 2473 | n.d.a | n.d.a | n.d.a | n.d.a | n.d.a |
| 4-Mar-03 | 1970.0 | 10.4 | n.d.a | 194 | n.d.a | 1702 | 265 | −32.6 |
| 18-Mar-03 | 1823.2 | 8.3 | 2411 | 234 | 1.53 | 495 | 116 | −28.9 |
| 1-Apr-03 | 1917.7 | 12.4 | n.d.a | 189 | 1.56 | 1339 | 247 | −30.3 |
| 14-Apr-03 | 1472.8 | 10.6 | 1954 | 198 | 1.50 | 721 | 155 | −31.8 |
| 28-Apr-03 | 1835.5 | 13.0 | 2004 | 224 | 1.47 | 1189 | 222 | −30.6 |
| 13-May-03 | 1950.2 | 16.9 | 1548 | 176 | 1.50 | 1105 | 256 | −29.0 |
| 26-May-03 | 1786.6 | 17.9 | 1974 | 218 | 1.44 | 1581 | 268 | −30.0 |
| 25-Jun-03 | 1560.3 | 21.9 | 1557 | 139 | 1.40 | 746 | 118 | −29.3 |
| 15-Jul-03 | 1212.6 | 12.7 | 1986 | n.d.a | 1.50 | 891 | 153 | −30.0 |
| 5-Aug-03 | 1348.0 | 17.0 | 1549 | 137 | 1.50 | 530 | 85 | −30.2 |
| 27-Aug-03 | 1151.9 | 14.9 | n.d.a | 162 | n.d.a | 537 | 84 | −28.1 |
| 9-Sep-03 | 1060.9 | 12.9 | 1391 | 113 | n.d.a | 345 | 53 | −29.0 |
| 30-Sep-03 | 1116.9 | 11.3 | 1541 | 156 | 1.48 | 336 | 50 | −29.2 |
| 14-Oct-03 | 2085.8 | 87.4 | 2815 | 321 | 1.42 | n.d.a | n.d.a | n.d.a |
| 18-Nov-03 | 1151.9 | 16.9 | 1677 | 100 | 1.50 | n.d.a | n.d.a | n.d.a |
| Backwater | ||||||||
| 13-Jan-03 | 11.3 | n.d.a | 2821 | n.d.a | 1.16 | n.d.a | n.d.a | n.d.a |
| 11-Feb-03 | 4.0 | n.d.a | 2036 | n.d.a | n.d.a | n.d.a | n.d.a | n.d.a |
| 4-Mar-03 | 8.6 | 13.1 | n.d.a | 192 | n.d.a | 716 | 146 | −27.4 |
| 18-Mar-03 | 5.3 | 7.3 | 2070 | 193 | 1.56 | 635 | 134 | −29.6 |
| 1-Apr-03 | 7.3 | 24.5 | 2177 | 196 | 1.52 | 2300 | 434 | −31.0 |
| 14-Apr-03 | 1.4 | 14.3 | 2267 | 236 | 1.50 | 2169 | 425 | −30.5 |
| 28-Apr-03 | 5.6 | 15.3 | 2615 | 268 | 1.43 | 2353 | 485 | −31.4 |
| 13-May-03 | 8.1 | 11.2 | 1832 | 194 | 1.49 | 1282 | 307 | −29.3 |
| 26-May-03 | 4.7 | 11.6 | 1847 | 215 | 1.48 | 1316 | 214 | −32.2 |
| 25-Jun-03 | 2.0 | 10.7 | 2526 | 234 | 1.39 | 1204 | 234 | −34.0 |
| 15-Jul-03 | 0.4 | 8.2 | n.d.a | n.d.a | 1.46 | 1232 | 208 | −35.9 |
| 5-Aug-03 | 0.8 | 12.5 | 2222 | 267 | 1.47 | 1029 | 179 | −34.6 |
| 27-Aug-03 | 0.3 | 13.4 | n.d.a | 237 | n.d.a | 825 | 145 | −32.0 |
| 9-Sep-03 | 0.2 | 8.1 | 2286 | 259 | n.d.a | 524 | 94 | −32.4 |
| 30-Sep-03 | 0.3 | 2.8 | 1737 | 166 | 1.46 | 265 | 49 | −28.9 |
| 14-Oct-03 | 12.3 | 27.6 | 2110 | 276 | 1.44 | 449 | 65 | −26.9 |
| 18-Nov-03 | 0.3 | 15.5 | 1283 | 88 | 1.54 | n.d.a | n.d.a | n.d.a |
Not determined.
3.2. OM properties
Correlation analysis revealed significant influence of hydrology on OM concentration and properties (Table 2). The water level of the Danube at the sampling dates was used as a measure of the daily flow conditions. Additionally, it was averaged over 20 days before sampling to include flow history. While some parameters (DOC and POC concentration, C:N values) were significantly correlated with both daily and averaged water level, some others [FI, lignin concentration, (Ad:Al)v of DOM] showed significant correlations only when water level history was included (Table 2).
Table 2.
Correlations of OM and plankton properties with daily water level and flow history, calculated as water level averaged over 20 days before sampling (Pearson correlation coefficients are given, significance at the 0.05 level is indicated by *, significance at the 0.01 level by **; abbreviations as in text)
| OC | Lignina | Λ | S:V | C:V | (Ad:Al)v | C:N | FI | δ13C | Chl a | BA | VLP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DOM | Plankton |
|||||||||||
| Daily water level | 0.558** | 0.405 | 0.076 | 0.134 | −0.277 | −0.269 | −0.510* | −0.327 | 0.549** | −0.195 | −0.438* | |
| Averaged water level | 0.574** | 0.600** | 0.315 | −0.052 | −0.442* | −0.630** | −0.457* | 0.674*** | 0.575** | −0.164 | −0.514** | |
| POM | ||||||||||||
| Daily water level | 0.473* | 0.513 | 0.458 | 0.504 | 0.259 | 0.427 | −0.392* | 0.208 | ||||
| Averaged water level | 0.407* | 0.607* | 0.324 | 0.163 | −0.041 | 0.232 | −0.475* | −0.067 | ||||
Lignin refers to lignin concentration in μg L−1 river water.
DOC concentration in both water bodies ranged between 1283 and 2821 μg L−1 and showed a slightly decreasing trend from Jan. to Nov. POC values were generally lower than DOC values, in spite of a clear peak in the backwater in April and May (265 to 2353 μg L−1). From June to Nov., POC concentration decreased in both water bodies (Fig. 2a, Table 1). No significant differences between sampling sites were detected.
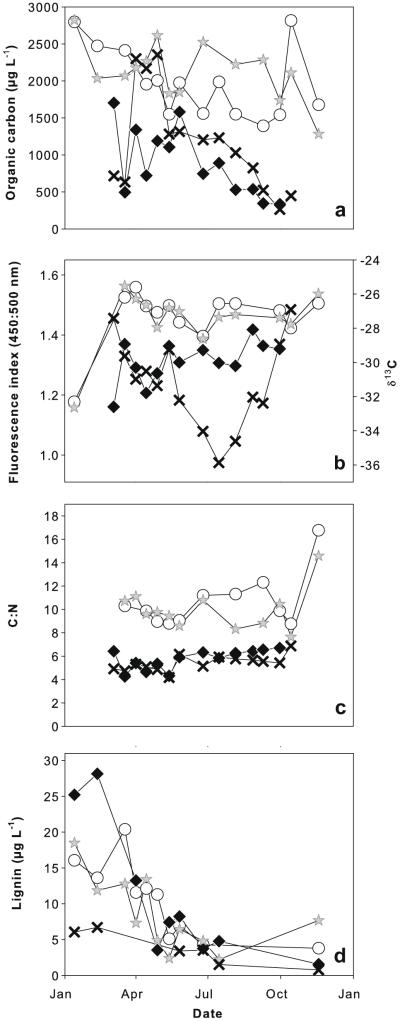
Fig. 2.
POM and DOM properties in main channel and backwater. (a) Organic carbon concentration, (b) FI of DOM and δ13C values of POM, (c) C:N ratio and (d) lignin concentration in river water. Symbols refer to: main channel DOM (white circles), backwater DOM (grey stars), main channel POM (black diamonds), backwater POM (black crosses).
At both locations, the FI of DOM showed a clear terrestrial signature in Jan., but averaged out around 1.5 for the rest of the year, which indicates a significant contribution from microbially derived OM (Fig. 2b, Table 1). No significant correlation between FI and Λ was detected, though both indicators reflect the importance of terrestrial vs. microbially derived OM. The δ13C signals of POM varied around a mean value of −30% over the whole sampling period in the main channel. Similar δ13C values were observed in the backwater in spring and autumn, while a clear depletion (−32% to −36%) occurred during summer (Fig. 2b, Table 1). The δ13C value was significantly correlated with Λ for POM (r = 0.69, p < 0.05, n = 9).
The C:N values of DOM ranged between 8 and 12 for most of the sampling period, but were conspicuously higher in late autumn (Fig. 2c). In late summer the backwater DOM exhibited lower C:N values than the main channel. No significant correlation with either lignin concentration, Λ or Chl a was detected. C:N values of POM were significantly lower (with an average of 5.6) than for DOM at both sites (p < 0.001, n = 20) and negatively correlated with Chl a (r = −0.57, p < 0.01, n = 27). C:N value for main channel POM was on average slightly, but significantly (p < 0.05, n = 13) higher than that of backwater POM (Fig. 2c).
Lignin concentration and Λ values decreased from late winter to summer and were mostly higher for the main channel than the respective backwater samples (Fig. 2d, Table 3), but differences were not significant. Lignin concentration of DOM was significantly correlated with DOC concentration (r = 0.57, p < 0.05, n = 19); the relationship between lignin and POC was not significant. The number of samples for lignin analysis of POM was limited due to the problems during processing described in Section 2.3. Furthermore, some samples for POC concentration were not available at several sampling dates, so some lignin yields could not be normalized to OC.
Table 3.
Lignin phenol yield from DOM and POM collected in main river channel and representative backwater of Danube (abbreviations as in text)
| Sampling date | DOM |
POM |
||||||
|---|---|---|---|---|---|---|---|---|
| V (μg L−1) | S (μg L−1) | C (μg L−1) | Λ (mg (100 mg OC)−1) | V (μg L−1) | S (μg L−1) | C (μg L−1) | Λ (mg (100 mg OC)−1) | |
| Main river channel | ||||||||
| 13-Jan-03 | 11.37 | 3.67 | 1.02 | 0.57 | 14.04 | 9.34 | 1.83 | n.d.a |
| 11-Feb-03 | 8.20 | 3.85 | 1.57 | 0.55 | 12.36 | 10.58 | 5.20 | n.d.a |
| 18-Mar-03 | 12.10 | 6.15 | 2.13 | 0.85 | disc.b | disc.b | disc.b | disc.b |
| 1-Apr-03 | 8.14 | 2.75 | 0.68 | n.d.a | 5.70 | 4.96 | 2.59 | 0.99 |
| 14-Apr-03 | 8.50 | 2.57 | 1.03 | 0.62 | disc.b | disc.b | disc.b | disc.b |
| 28-Apr-03 | 7.68 | 2.61 | 1.00 | 0.56 | 1.27 | 1.28 | 1.00 | 0.30 |
| 13-May-03 | 2.90 | 1.47 | 0.72 | 0.33 | 1.80 | 1.94 | 3.68 | 0.67 |
| 26-May-03 | 4.35 | 2.05 | 0.51 | 0.35 | 2.69 | 2.55 | 2.98 | 0.52 |
| 25-Jun-03 | 2.88 | 0.87 | 0.51 | 0.27 | 2.13 | 1.16 | 0.46 | 0.50 |
| 15-Jul-03 | n.d.a | n.d.a | n.d.a | n.d.a | 2.48 | 1.60 | 0.69 | 0.54 |
| 18-Nov-03 | 2.53 | 0.67 | 0.59 | 0.23 | 0.84 | 0.55 | 0.18 | n.d.a |
| Backwater | ||||||||
| 13-Jan-03 | 12.1 | 4.90 | 1.49 | 0.66 | 3.85 | 1.74 | 0.46 | n.d.a |
| 11-Feb-03 | 6.64 | 3.68 | 1.54 | 0.58 | 3.59 | 2.33 | 0.77 | n.d.a |
| 18-Mar-03 | 8.31 | 3.91 | 0.58 | 0.62 | disc.b | disc.b | disc.b | disc.b |
| 1-Apr-03 | 4.83 | 1.89 | 0.61 | 0.34 | disc.b | disc.b | disc.b | disc.b |
| 14-Apr-03 | 7.47 | 4.98 | 0.97 | 0.59 | disc.b | disc.b | disc.b | disc.b |
| 28-Apr-03 | 2.28 | 1.73 | 0.89 | 0.19 | disc.b | disc.b | disc.b | disc.b |
| 13-May-03 | 1.28 | 0.78 | 0.32 | 0.13 | disc.b | disc.b | disc.b | disc.b |
| 26-May-03 | 4.85 | 1.26 | 0.35 | 0.35 | 0.87 | 0.93 | 1.62 | 0.26 |
| 25-Jun-03 | 2.93 | 1.46 | 0.44 | 0.19 | 1.03 | 1.13 | 1.36 | 0.29 |
| 15-Jul-03 | 1.54 | 0.49 | 0.19 | n.d.a | 0.76 | 0.27 | 0.49 | 0.12 |
| 18-Nov-03 | 4.17 | 2.45 | 1.05 | 0.60 | 0.55 | 0.12 | 0.10 | n.d.a |
Not determined.
Discarded because of issues mentioned in Section 2.3.
To obtain an estimate of the relative importance of terrestrial sources for river OM, we compared lignin yields with values from the literature. Hernes et al. (2007) recently suggested that the percentage of vascular plant derived DOC should be calculated considering only the yield of vanillyl phenols, due to differences in sources and reactivity of syringyl and cinnamyl phenols. We used a value of 1.5 mg V per 100 mg DOC to represent a 100% vascular plant DOM end member, as reported by Hernes et al. (2007) for plant leachates submitted to sorption/desorption processes. The terrestrial proportion of DOM (XDOM) was thus calculated according to
This results in an average of 19 ± 9% terrestrially derived DOC, with values ranging from 5% in the backwater in May to 33% in the main channel in March.
We used the average of the Λ values reported by Cotrim da Cunha et al. (2001) for different plant tissues from a French river catchment to represent 100% vascular plant POM end member (4.9 mg lignin (100 mg POC)−1. The vascular plant derived percentage (XPOM) was calculated according to
The observed Λ values of river POM would thus refer to (±standard deviation) 10 ± 5% terrestrially derived POC, with a minimum of 3% in the backwater in July and a maximum of 20% in the main channel in April.
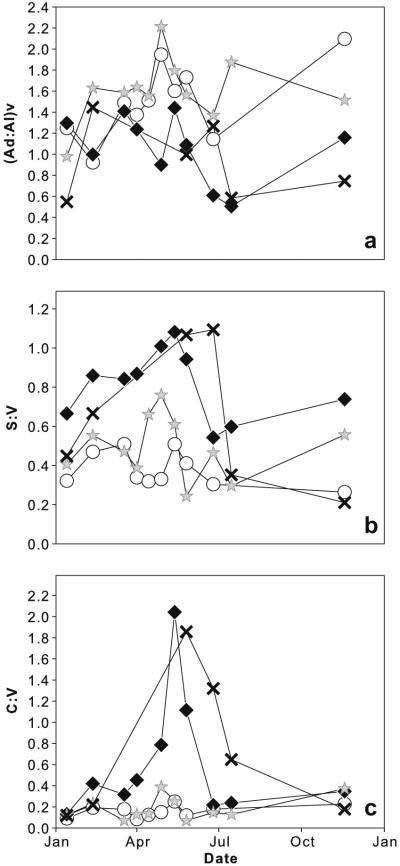
The ratio values of lignin oxidation products were generally similar between sampling sites. The (Ad:Al)v values showed no clear seasonal pattern (Fig. 3a). S:V and C:V ratios of DOM and POM peaked in late spring and early summer at both sampling sites (Fig. 3b and c). S:V and C:V were significantly correlated (r = 0.64, p < 0.01, n = 21 for DOM and r = 0.74, p < 0.01, n = 15 for POM). S:V of DOM was significantly (p < 0.05, n = 10) lower in the main channel than in the backwater; S:V of POM, C:V and (Ad:Al)v ratios did not differ significantly between the sampling sites. All indices differed significantly between the two OM fractions (p < 0.001, n = 15), S:V and C:V being higher and (Ad:Al)v values lower for particulate matter.
Fig. 3.
Ratios of lignin phenol groups in DOM and POM of main channel and backwater. (a) (Ad:Al)v ratio as indicator of alteration state; (b) S:V and (c) C:V ratios as indicators for plant sources. Symbols as in Fig. 2.
3.3. Plankton dynamics
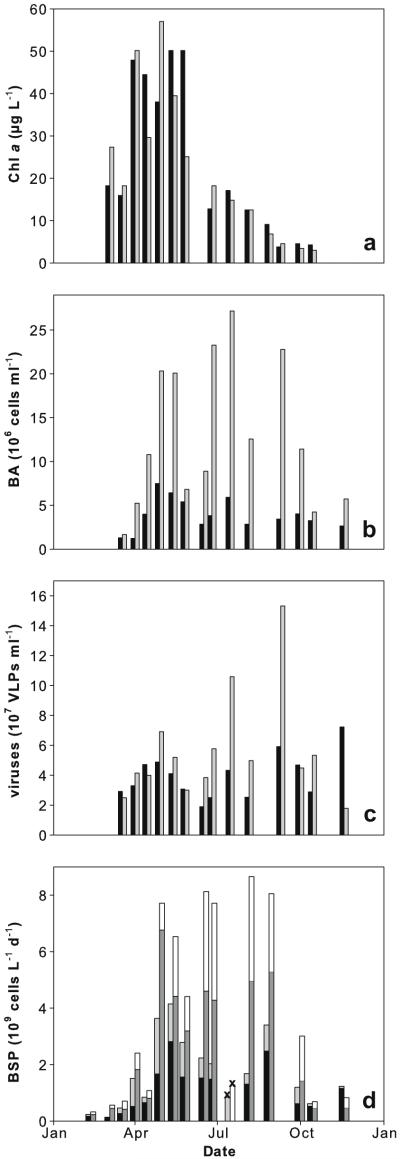
Chl a exhibited a pronounced seasonality, with significant algal blooms in March and May (up to 57 μg L−1) and decreasing values towards autumn (Fig. 4a, Table 4). Phytoplankton biomass was significantly correlated with POC (r = 0.72, p < 0.001, n = 27). BA ranged between 1.2 × 106 and 2.7 × 107 cells mL−1 and was significantly (p < 0.001, n = 14) higher in the backwater than in the main channel. Distinct peaks were observed in May, July and Sept. (Fig. 4b, Table 4). BA was significantly correlated with temperature (r = 0.56, p < 0.01, n = 26), while no significant correlation was observed between BA and Chl a or OC concentrations. VLP were most abundant in autumn, with up to 7.2 × 107 VLP mL−1 in the main channel and up to 1.5 × 108 VLP mL−1 in the backwater (Fig. 4c, Table 4). The ratio of viruses to bacterial cells ranged between 5.7 and 27.5 in the main channel and between 2.5 and 15 in the backwater (Table 4). VLP were significantly related to BA (r = 0.70, p < 0.001, n = 28). Chl a was positively and VLP negatively correlated with daily and 20 d-averaged water level, BA showing no significant correlation (Table 2).
Fig. 4.
Plankton dynamics in main channel (black bars) and backwater (grey bars): (a) chlorophyll a, (b) bacterial abundance and (c) virus-like particles. (d) BSP of free-living and the particle-associated bacterial community. Bars are stacked to yield total BSP in main channel and backwater. Main channel particle-associated bacteria (black bars), main channel free-living bacteria (light grey bars), backwater particle-associated bacteria (dark grey bars), backwater free-living bacteria (white bars). Crosses indicate dates when particle-associated BSP was not measured.
Table 4.
Plankton abundance parameters and BSP in main river channel and representative backwater of Danube (abbreviations as in text)
| Sampling date | Chl a (μg L−1) | BA (cells mL−1) | Viruses (VLP mL−1) | Virus:bacteria ratio | Part.-assoc.a BSP (cells L−1 d−1) | Free-living BSP (cells L−1 d−1) |
|---|---|---|---|---|---|---|
| Main river channel | ||||||
| 11-Feb-03 | n.d.b | n.d.b | n.d.b | n.d.b | 1.73 × 108 | 6.26 × 107 |
| 4-Mar-03 | 18.24 | n.d.b | n.d.b | n.d.b | 1.18 × 108 | 1.59 × 107 |
| 18-Mar-03 | 15.96 | 1.29 × 106 | 2.92 × 107 | 22.6 | 2.67 × 108 | 1.96 × 108 |
| 1-Apr-03 | 47.88 | 1.22 × 106 | 3.30 × 107 | 27.1 | 5.19 × 108 | 9.91 × 108 |
| 14-Apr-03 | 44.46 | 3.99 × 106 | 4.71 × 107 | 11.8 | 6.52 × 108 | 1.96 × 108 |
| 28-Apr-03 | 38.00 | 7.48 × 106 | 4.88 × 107 | 6.5 | 1.66 × 109 | 1.97 × 109 |
| 13-May-03 | 50.16 | 6.41 × 106 | 4.11 × 107 | 6.4 | 2.81 × 109 | 1.34 × 109 |
| 26-May-03 | 50.16 | 5.40 × 106 | 3.07 × 107 | 5.7 | 1.56 × 109 | 1.23 × 109 |
| 16-Jun-03 | n.d.b | 2.83 × 106 | 1.90 × 107 | 6.7 | 1.52 × 109 | 7.07 × 108 |
| 25-Jun-03 | 12.77 | 3.81 × 106 | 2.50 × 107 | 6.6 | 1.48 × 109 | 5.52 × 108 |
| 15-Jul-03 | 17.10 | 5.89 × 106 | 4.32 × 107 | 7.3 | n.d.b | 8.46 × 108 |
| 5-Aug-03 | 12.54 | 2.83 × 106 | 2.53 × 107 | 8.9 | 1.32 × 109 | 3.58 × 108 |
| 27-Aug-03 | 9.12 | n.d.b | n.d.b | n.d.b | 2.49 × 109 | 9.12 × 108 |
| 9-Sep-03 | 3.80 | 3.42 × 106 | 5.91 × 107 | 17.3 | n.d.b | n.d.b |
| 30-Sep-03 | 4.56 | 4.01 × 106 | 4.69 × 107 | 11.7 | 6.33 × 108 | 5.66 × 108 |
| 14-Oct-03 | 4.28 | 3.25 × 106 | 2.88 × 107 | 8.9 | 5.43 × 108 | 7.16 × 107 |
| 18-Nov-03 | n.d.b | 2.62 × 106 | 7.23 × 107 | 27.5 | 1.17 × 109 | 5.95 × 107 |
| Backwater | ||||||
| 11-Feb-03 | n.d.b | n.d.b | n.d.b | n.d.b | 2.29 × 108 | 9.69 × 107 |
| 4-Mar-03 | 27.36 | n.d.b | n.d.b | n.d.b | 4.46 × 108 | 1.19 × 108 |
| 18-Mar-03 | 18.24 | 1.65 × 106 | 2.49 × 107 | 15.0 | 4.16 × 108 | 2.88 × 108 |
| 1-Apr-03 | 50.16 | 5.24 × 106 | 4.14 × 107 | 7.9 | 1.82 × 109 | 5.84 × 108 |
| 14-Apr-03 | 29.64 | 1.08 × 107 | 4.00 × 107 | 3.7 | 8.07 × 108 | 2.73 × 108 |
| 28-Apr-03 | 57.00 | 2.03 × 107 | 6.91 × 107 | 3.4 | 6.76 × 109 | 9.58 × 108 |
| 13-May-03 | 39.52 | 2.01 × 107 | 5.19 × 107 | 2.6 | 4.42 × 109 | 2.11 × 109 |
| 26-Mby-03 | 25.08 | 6.81 × 106 | 3.00 × 107 | 4.4 | 3.19 × 109 | 1.22 × 109 |
| 16-Jun-03 | n.d.b | 8.87 × 106 | 3.84 × 107 | 4.3 | 4.60 × 109 | 3.52 × 109 |
| 25-Jun-03 | 18.24 | 2.33 × 107 | 5.77 × 107 | 2.5 | 4.28 × 109 | 3.43 × 109 |
| 15-Jul-03 | 14.82 | 2.72 × 107 | 1.06 × 108 | 3.9 | n.d.b | 1.28 × 109 |
| 5-Bug-03 | 12.54 | 1.25 × 107 | 4.98 × 107 | 4.0 | 4.96 × 109 | 3.70 × 109 |
| 27-Bug-03 | 6.84 | n.d.b | n.d.b | n.d.b | 5.29 × 109 | 2.76 × 109 |
| 9-Sep-03 | 4.56 | 2.28 × 107 | 1.53 × 108 | 6.7 | n.d.b | n.d.b |
| 30-Sep-03 | 3.42 | 1.14 × 107 | 4.49 × 107 | 3.9 | 1.43 × 109 | 1.58 × 109 |
| 14-Oct-03 | 3.04 | 4.24 × 106 | 5.33 × 107 | 12.6 | 4.61 × 108 | 2.27 × 108 |
| 18-Nov-03 | n.d.b | 5.72 × 106 | 1.78 × 107 | 3.1 | 4.69 × 108 | 3.59 × 108 |
Particle associated.
Not determined.
To obtain a crude estimate of the contribution of plankton to OM, we transformed the measured microbial parameters into organic carbon using conversion factors from the literature. Using a conversion factor for algal biomass of C:Chl a = 19 (Hein et al., 2003), the observed Chl a levels translated into 58 to 1083 μg carbon L−1. Assuming 20 fg carbon per bacterial cell (Lee and Fuhrman, 1987) and 0.2 fg carbon per VLP (Suttle, 2005), bacteria accounted for 24 to 543 μg carbon L−1 and viruses for 4 to 31 μg carbon L−1.
BSP (both particle-associated and free) ranged from 1.6 × 107 to 6.8 × 109 cell L−1 d−1; assuming 20 fg C per cell, this translates into 0.3 to 135.2 μg C L−1 d−1. It exhibited a clear seasonality (Fig. 4d, Table 4) and was correlated with water temperature (r = 0.761, p < 0.001). BSP of the free-living bacterial fraction was negatively correlated with Λ of DOM (r = −0.76, p < 0.001, n = 17). No significant correlation was found between BSP of the particle-associated bacterial fraction and Λ, nor between either fraction of BSP and Chl a. BSP was significantly higher for the attached than for the free-living bacterial community at both sites (p < 0.001, n = 30) and significantly higher in the backwater than in the main channel for both communities (free-living p < 0.05, n = 16, particle-associated p < 0.01, n = 15).
4. Discussion
The extremely hot and dry year of 2003 was likely the cause of the restricted surface water connection between the backwater and the main channel. This is in contrast to the usual high water level found in spring and early summer in the Danube (Schiemer et al., 1999; Tockner et al., 1999). The lack of significant connectivity resulted in apparently unrelated variation in POM properties (POC concentration and δ13C values) in the two water bodies (Fig. 2a and b). At very low water levels the separation was obviously extensive enough to lead to gradients even in the DOM quality, as indicated by different C:N values in summer (Fig. 2c). This is in agreement with an earlier study of the Danube, which found differences between floodplain pools at low water level regarding quality, rather than the quantity, of DOM (Peduzzi et al., 2008).
Hydrology exhibited a fast and dominating influence on DOC and POC concentration, either through input of terrestrial OM via runoff or through changes in autochthonous production due to nutrient and light availability. The impact of hydrology on lignin concentration was only significant when the water level history was included, suggesting a time lag between the change in water level and the corresponding change in OM quality. Significance of terrestrial OM, at least on DOC concentration, was indicated by the correlation between lignin concentration and DOC. However, decreasing percentages of terrestrial OM (Λ) in summer and the absence of a significant correlation between Λ and water level suggested additional factors determining the OC composition.
The lignin yields of river OM were conspicuously lower than values found in the Amazon river (Ertel et al., 1986; Hedges et al., 1986, 2000), but well within values reported from studies conducted in the USA (Onstad et al., 2000; Bianchi et al., 2004, 2007; Duan et al., 2007a; Eckard et al., 2007), France (Cotrim da Cunha et al., 2001) and Russia (Lobbes et al., 2000). While terrestrial input dominates in the Amazon river (Wissmar et al., 1981; Ertel et al., 1986; Hedges et al., 1986), several of the above studies suggest an important proportion of autochthonous production to river OM (Cotrim da Cunha et al., 2001; Bianchi et al., 2004, 2007; Duan et al., 2007a,b). Significant contribution of autochthonous OM also seems likely in the Danube river floodplain system.
We estimated the vascular plant derived percentages of POM in our riverine system from Λ values of fresh plants (Cotrim da Cunha et al., 2001). Lignin contents of plant debris vary among plant species and tissues, but also during diagenetic alteration (Opsahl and Benner, 1995). Even in the Amazon, where dilution of vascular plant OM by algal OM is small (Wissmar et al., 1981), the lignin content of POM has been shown to be equal to or less than in the respective plant tissues (Hedges et al., 1986). The 3 to 20% terrestrial POC in our study might therefore be regarded as a minimum estimate of vascular plant derived POM. The calculation of the terrestrial percentage of DOM proposed by Hernes et al. (2007) already takes into account the impact of leaching and sorption processes. However, microbial and photochemical processes might have altered the Λ values of DOM (Opsahl and Benner, 1998; Benner and Opsahl, 2001; Hernes and Benner, 2003), so the estimated 5 to 33% terrestrial DOC in our study contains uncertainty. However, the rather low values were consistent with the other indicators of a significant algal contribution to OM in this exceptional dry year (Tables 1 and 4).
The (Ad:Al)v values of DOM varied synchronously in the main channel and the backwater for most of the sampling period, suggesting some exchange of dissolved matter via seepage. In contrast, the variation in (Ad:Al)v values of POM was apparently unrelated in the two water bodies (Fig. 3a). Both POM and DOM (Ad:Al)v values were similar to those for several aquatic environments, where they have been interpreted as diagenetically altered OM (Hedges et al., 2000; Engelhaupt and Bianchi, 2001; Hernes and Benner, 2003). The significantly higher (Ad:Al)v values for DOM were also in accord with earlier studies (Hedges et al., 2000; Bernardes et al., 2004), leading to the conclusion that DOM is generally diagenetically more altered than POM. However, Hernes et al. (2007) recently found that leaching and sorption processes can account for a threefold increase in (Ad:Al)v values for the DOM fraction, thereby obscuring patterns caused by microbial alteration and photochemical alteration. Therefore, the DOM in the investigated river floodplain system might be as fresh, or even fresher than the POM.
Grasses and angiosperm leaves, as the main sources of terrestrial OM in the Danube river floodplain system, were consistent with the composition of the local vegetation, which is dominated by floodplain forest and agricultural land. Less pronounced seasonal changes in main channel DOM S:V and C:V ratios, and slightly lower S:V (Fig. 3b and c), might reflect some import of upstream, partly gymnosperm derived DOM from the Upper Danube catchment. However, S:V and C:V ratios are also subject to alteration during sorption/desorption processes, which can account for significant differences between DOM and POM (Hernes et al., 2007). During winter, spring and autumn, S:V values for river OM in our system were similar to those from catchments dominated by angiosperm forests (Ertel et al., 1986; Hedges et al., 1986, 2000). Peaks in S:V values in summer resembled values reported for watersheds with prevalent grassland or crop land (Bianchi et al., 2007; Dalzell et al., 2007; Eckard et al., 2007). C:V values of DOM ranged between values for angiosperm forests (Ertel et al., 1986) and values for crop land (Dalzell et al., 2007; Eckard et al., 2007). C:V ratios of POM were similar to values for grassland or crop land in early spring and autumn (Lobbes et al., 2000; Onstad et al., 2000; Bianchi et al., 2007) and exhibited even higher values in summer.
The observed strong seasonal variability in S:V and C:V ratios could be due to changes in source input, or the extent of photochemical and microbial alteration. Photooxidation has been shown to result in decreased S:V and unchanged or elevated C:V values, while microbial degradation afforded decreased C:V and unchanged S:V ratios (Opsahl and Benner, 1995, 1998; Benner and Opsahl, 2001; Hernes and Benner, 2003). Photooxidation in particular can affect lignin signatures within short periods of time, and the comparatively low suspended solids load in the investigated year (Aspetsberger et al., 2002; Preiner et al., 2008) enhanced the potential for photochemical processes. However, given the contrasting impacts on S:V and C:V ratios and the significant correlation of these two indices in our study, shifts in OM sources likely contributed to the observed patterns.
The conspicuous peak in S:V and C:V ratios in summer might represent input from crop land and grassland, reflecting agricultural activity. For instance, C:V values as high as 4.2 have been found for corn tissue by Dalzell et al. (2007). Other sources of syringyl and cinnamyl rich OM could include submersed macrophytes (Hedges et al., 1986; Engelhaupt and Bianchi, 2001), which are typical for disconnected floodplain pools in this river floodplain system (Schiemer et al., 2006). A significant input from pollen would also corroborate the observed seasonal pattern (Keil et al., 1998). This presumes, however, a direct and fast input of fresh plant material into the river and adds new complexity to the long held theory that most terrestrial OM in rivers originates from a pool of altered OM in soil (Ertel et al., 1986; Hedges et al., 1986). Source and quality of terrestrial carbon in this river floodplain system exhibited a distinct seasonality, similar to that observed for plankton.
The FI and C:N ratios of DOM indicated a mixture of terrestrial and microbial sources for most of the investigated period. The lack of a significant correlation between the FI and Λ of DOM may be due to photochemical alteration or sorption/desorption processes (McKnight et al., 2001). For instance, a significant decrease in FI was found during an irradiation experiment by Brooks et al. (2007). However, the FI responded to 20 d-averaged water level fluctuations, indicating significant hydrological control, as observed for other source indicators. An accumulation of N-rich DOM in the backwater in summer might be due to enhanced microbial degradation. The higher C:N values in autumn suggested a change in DOM sources, likely as result of the flow pulses in Sept./Oct.
The δ13C values of −27% to −36% in our study (Fig. 2b) were at the lower end of the range reported for C3 plants and within those assumed for microbial OM (Smith and Epstein, 1971; Rau, 1978). A δ13C signature of C4 plants in river POM might have been masked by a high proportion of microbial POM. The positive correlation with Λ further indicated that δ13C values were determined by shifts in terrestrial and microbial POM percentages, rather than shifts within the terrestrial POM fraction. The distinct drop in the δ13C values in the backwater suggested microbially-produced matter as an important fraction of POM in summer. The C:N values also indicated largely microbial POM. Low C:N values could also result from selective adsorption of N-rich molecules to particles (Aufdenkampe et al., 2001); however, the significant correlation of C:N values with Chl a indicate that a large part of PON was fixed in algal biomass.
Chl a exhibited distinct peaks (Fig. 4a) which exceeded levels typical for rivers (Wissmar et al., 1981; Castillo, 2000; Cotrim da Cunha et al., 2001). Chl a reached its maximum when the water level was close to mean water in spring and decreased during the low water period. Earlier studies of the Danube river floodplain system observed phytoplankton peaks around mean water level during falling discharge, likely due to a preceding input of inorganic nutrients and decreasing load of suspended solids (Hein et al., 1999). Assuming the conversion factor proposed by Hein et al. (2003) for the Danube floodplain, algae accounted for (average ± standard deviation) 43 ± 25% of POC. The significant correlation between Chl a and POC concentration also suggested algae as an important component of the POM.
Bacteria peaked in summer and autumn, when the estimated bacterial biomass accounted for up to 87% of the POC in the backwater. The highest BA and BSP levels occurred after the breakdown of the algal bloom. It is likely that bacterial productivity in summer was partly fuelled by the remnants of dead planktonic algae, especially in the backwater. High levels of benthic primary production in the backwater were found in a concomitant study (Preiner et al., 2008), which probably complemented the amount of fresh OM during the summer months. Consistent with this, an earlier study reported a shift from algal to bacterial dominance after flood pulses in this floodplain system (Hein et al., 1999). The rates of BSP were clearly higher than in rivers with low algal production (Benner et al., 1995), which further supports the assumption that algal production played a significant role for BSP.
Viruses can also be a significant source of nutrients for heterotrophic nanoflagellates if present at a virus:bacteria ratio > 50 (Gonzalez and Suttle, 1993). Assuming that the majority of viruses are planktonic and typically < 100 nm in diameter in the investigated system (Weinbauer, 2004; Luef et al., 2007), we regarded VLPs as part of the DOM (Weinbauer and Peduzzi, 1995). The carbon contribution of VLP constituted only a small percentage (<2%) of DOC. Together with low virus:bacteria values (Table 4), this indicated a rather limited importance of viruses as a source of organic carbon for the heterotrophic plankton during the investigated period.
Calculating algal, bacterial and viral OC using a single conversion factor certainly yields a rough estimation, and in fact the calculated algal biomass exceeded the measured POC concentration at one date. The Chl a content of algae depends on species, age and metabolic stage (Geider et al., 1997). Bacteria and viruses can vary more than one order of magnitude in size, but probably less in carbon content (Lee and Fuhrman, 1987; Weinbauer, 2004; Suttle, 2005). Nevertheless, though the respective contributions to OC might be overestimated at some dates, the data underline the importance of autochthonously produced OM in the studied system.
The data indicated a significant contribution of microbially derived material to OM in this temperate river floodplain system in the year 2003. Primarily algal derived POC has also been reported for several big river systems in the USA (Kendall et al., 2001). Earlier studies of the floodplains of the river Danube demonstrated the importance of autochthonous OC in floodplain pools (Aspetsberger et al., 2002; Hein et al., 2003), but suggested primarily terrestrial derived OC in the main channel. The exceptionally low discharge and low sediment loads in 2003 likely also resulted in increased phytoplankton density and reduced terrestrial OM concentration in the main channel, as compared to hydrologically average years (Aspetsberger et al., 2002; Preiner et al., 2008). Benthic algal productivity was probably an additional important source of fresh OM, especially in the backwater (Preiner et al., 2008). This rendered a large pool of presumably utilizable OM for bacterial growth (Azam and Cho, 1987; Kaplan and Bott, 1989). Terrestrial OM might be an important source for heterotrophic growth during phases with low algal production (i.e. at high water level and in winter), and the strong seasonality of terrestrial OM sources likely triggered changes in specialized bacterial communities.
Acknowledgements
We thank the National Park Authority and the Austrian River Authority for permission to conduct our research in the Danube Alluvial Zone National Park. We thank W. Wanek and the Department of Chemical Ecology and Ecosystem Research for stable isotope and elemental analyses and colleagues from Department of Freshwater Ecology for help in the field and in the lab. We are grateful for valuable suggestions from two anonymous reviewers. The work was supported by grants from the Austrian Science Fund (P14721 and P17798) to P.P.
References
- Aspetsberger F, Huber F, Kargl S, Scharinger B, Peduzzi P, Hein T. Particulate organic matter dynamics in a river floodplain system: impact of hydrological connectivity. Archiv für Hydrobiologie. 2002;156:23–42. [Google Scholar]
- Aufdenkampe AK, Hedges JI, Richey JE, Krusche AV, Llerena CA. Sorptive fractionation of dissolved organic nitrogen and amino acids onto fine sediments within the Amazon Basin. Limnology and Oceanography. 2001;46:1921–1935. [Google Scholar]
- Aufdenkampe AK, Mayorga E, Hedges JI, Llerena C, Quay PD, Gudeman J, Krusche AV, Richey JE. Organic matter in the Peruvian headwaters of the Amazon: compositional evolution from the Andes to the lowland Amazon mainstem. Organic Geochemistry. 2007;38:337–364. [Google Scholar]
- Azam F, Cho B. Bacterial utilization of organic matter in the sea. In: Fletcher M, Gray TRG, Jones JG, editors. Ecology of Microbial Communities. Cambridge University Press; Cambridge, UK: 1987. pp. 261–281. [Google Scholar]
- Battin TJ. Dissolved organic matter and its optical properties in a blackwater tributary of the upper Orinco river, Venezuela. Organic Geochemistry. 1998;28:561–569. [Google Scholar]
- Battin TJ, Kaplan LA, Findlay SEG, Hopkinson CS, Marti E, Packman AI, Newbold JD, Sabater F. Biophysical controls on organic carbon fluxes in fluvial networks. Nature Geoscience. 2008;1:95–100. [Google Scholar]
- Bell RT. Estimating production of heterotrophic bacterioplankton via incorporation of tritiated thymidine. In: Kemp PF, Sherr BF, Sherr EB, Cole JJ, editors. Handbook of Methods in Aquatic Microbial Ecology. CRC Press; Boca Raton, FL: 1993. pp. 495–503. [Google Scholar]
- Benner R, Opsahl S. Molecular indicators of the sources and transformations of dissolved organic matter in the Mississippi river plume. Organic Geochemistry. 2001;32:597–611. [Google Scholar]
- Benner R, Strom M. A critical evaluation of the analytical blank associated with DOC measurements by high-temperature catalytic oxidation. Marine Chemistry. 1993;41:153–160. [Google Scholar]
- Benner R, Opsahl S, Chin-Leo G, Richey JE, Forsberg BR. Bacterial carbon metabolism in the Amazon River system. Limnology and Oceanography. 1995;40:1262–1270. [Google Scholar]
- Bernardes MC, Martinelli LA, Krusche AV, Gudeman J, Moreira M, Victoria RL, Ometto JPHB, Ballester MVR, Aufdenkampe AK, Richey JE, Hedges JI. Riverine organic matter composition as a function of land use changes, southwest Amazon. Ecological Applications. 2004;14:S263–S279. [Google Scholar]
- Bianchi TS, Filley TR, Dria K, Hatcher PG. Temporal variability in sources of dissolved organic carbon in the lower Mississippi River. Geochimica et Cosmochimica Acta. 2004;68:959–967. [Google Scholar]
- Bianchi TS, Wysocki LA, Stewart M, Filley TR, McKee BA. Temporal variability in terrestrially-derived sources of particulate organic carbon in the lower Mississippi River and its upper tributaries. Geochimica et Cosmochimica Acta. 2007;71:4425–4437. [Google Scholar]
- Brooks ML, Meyer JS, McKnight DM. Photooxidation of wetland and riverine dissolved organic matter: altered copper complexation and organic composition. Hydrobiologia. 2007;579:95–113. [Google Scholar]
- Buffam I, Galloway JN, Blum LK, McGlathery KJ. A stormflow/baseflow comparison of dissolved organic matter concentrations and bioavailability in an Appalachian stream. Biogeochemistry. 2001;53:269–306. [Google Scholar]
- Castillo MM. Influence of hydrological seasonality on bacterioplankton in two neotropical floodplain lakes. Hydrobiologia. 2000;437:57–69. [Google Scholar]
- Cifuentes LA, Coffin RB, Solorzano L, Cardenas W, Espinoza J, Twilley RR. Isotopic and elemental variations of carbon and nitrogen in a mangrove estuary. Estuarine, Coastal and Shelf Science. 1996;43:781–800. [Google Scholar]
- Cotrim da Cunha L, Serve L, Gadel F, Blazi J-L. Lignin-derived phenolic compounds in the particulate organic matter of a French Mediterranean river: seasonal and spatial variations. Organic Geochemistry. 2001;32:305–320. [Google Scholar]
- Dalzell BJ, Filley TR, Harbor JM. The role of hydrology in annual organic carbon loads and terrestrial organic matter export from a Midwestern agricultural watershed. Geochimica et Cosmochimica Acta. 2007;71:1448–1462. [Google Scholar]
- Duan S, Bianchi TS. Seasonal changes in the abundance and composition of plant pigments in particulate organic carbon in the lower Mississippi and Pearl rivers. Estuaries and Coasts. 2006;29:427–442. [Google Scholar]
- Duan S, Bianchi TS, Sampere TP. Temporal variability in the composition and abundance of terrestrially-derived dissolved organic matter in the lower Mississippi and Pearl Rivers. Marine Chemistry. 2007a;103:172–184. [Google Scholar]
- Duan S, Bianchi TS, Shiller AM, Dria K, Hatcher PG, Carman KR. Variability in the bulk composition and abundance of dissolved organic matter in the lower Mississippi and Pearl rivers. Journal of Geophysical Research. 2007b;112:G02024. doi:10.1029/2006JG000206. [Google Scholar]
- Eckard RS, Hernes PJ, Bergamaschi BA, Stepanauskas R, Kendall C. Landscape scale controls on the vascular plant component of dissolved organic carbon across a freshwater delta. Geochimica et Cosmochimica Acta. 2007;71:5968–5984. [Google Scholar]
- Engelhaupt E, Bianchi TS. Sources and composition of high-molecular-weight dissolved organic carbon in a southern Louisiana tidal stream (Bayou Trepagnier) Limnology and Oceanography. 2001;46:917. [Google Scholar]
- Ertel JR, Hedges JI, Devol AH, Richey JE, De Nazare Goes Ribeiro M. Dissolved humic substances of the Amazon River system. Limnology and Oceanography. 1986;31:739–754. [Google Scholar]
- Geider RJ, MacIntyre HL, Kana TM. Dynamic model of phytoplankton growth and acclimation: responses of the balanced growth rate and the chlorophyll a:carbon ratio to light, nutrient limitation and temperature. Marine Ecology Progress Series. 1997;148:187–200. [Google Scholar]
- Golterman HL, Clymo RS, Ohnstad MAM. Methods for Physical and Chemical Analysis of Freshwater. Blackwell Scientific; Oxford, UK: 1978. [Google Scholar]
- Gonzalez JM, Suttle CA. Grazing by marine nanoflagellates on viruses and virus-sized particles: ingestion and digestion. Marine Ecology Progress Series. 1993;94:1–10. [Google Scholar]
- Gordon ES, Goni MA. Sources and distribution of terrigenous organic matter delivered by the Atchafalaya River to sediments in the northern Gulf of Mexico. Geochimica et Cosmochimica Acta. 2003;67:2359–2375. [Google Scholar]
- Hedges JI, Ertel JR. Characterization of lignin by gas capillary chromatography of cupric oxide oxidation products. Analytical Chemistry. 1982;54:174–178. [Google Scholar]
- Hedges JI, Clark WA, Quay PD, Richey JE, Devol AH, Santos UDM. Compositions and fluxes of particulate organic material in the Amazon River. Limnology and Oceanography. 1986;31:717–738. [Google Scholar]
- Hedges JI, Mayorga E, Tsamakis E, McClain ME, Aufdenkampe AK, Quay PD, Richey JE, Benner R, Opsahl S, Black B, Pimentel T, Quintanilla J, Maurice L. Organic matter in Bolivian tributaries of the Amazon River: a comparison to the lower mainstream. Limnology and Oceanography. 2000;45:1449–1466. [Google Scholar]
- Hein T, Heiler G, Pennetzdorfer D, Riedler P, Schagerl M, Schiemer F. The Danube restoration project: functional aspects and planktonic productivity in the floodplain system. Regulated Rivers: Research & Management. 1999;15:259–270. [Google Scholar]
- Hein T, Baranyi C, Herndl GJ, Wanek W, Schiemer F. Allochthonous and autochthonous particulate organic matter in floodplains of the River Danube: the importance of hydrological connectivity. Freshwater Biology. 2003;48:220–232. [Google Scholar]
- Hernes PJ, Benner R. Photochemical and microbial degradation of dissolved lignin phenols: implications for the fate of terrigenous dissolved organic matter in marine environments. Journal of Geophysical Research. 2003;108:3291. doi:10.1029/2002JC001421. [Google Scholar]
- Hernes PJ, Robinson AC, Aufdenkampe AK. Fractionation of lignin during leaching and sorption and implications for organic matter “freshness”. Geophysical Research Letters. 2007;34:L17401. doi:10.1029/2007GL031017. [Google Scholar]
- Kaplan LA, Bott TL. Diel fluctuations in bacterial activity on streambed substrata during vernal algal blooms: effects of temperature, water chemistry, and habitat. Limnology and Oceanography. 1989;34:718–733. [Google Scholar]
- Keil RG, Tsamakis E, Giddings C, Hedges JI. Biochemical distributions (amino acids, neutral sugars, and lignin phenols) among size-classes of modern marine sediments from the Washington coast. Geochimica et Cosmochimica Acta. 1998;62:1347–1364. [Google Scholar]
- Kendall C, Silva SR, Kelly VJ. Carbon and nitrogen isotopic compositions of particulate organic matter in four large river systems across the United States. Hydrological Processes. 2001;15:1301–1346. [Google Scholar]
- Kim S, Kaplan LA, Hatcher PG. Biodegradable dissolved organic matter in a temperate and a tropical stream determined from ultra-high resolution mass spectrometry. Limnology and Oceanography. 2006;51:1054–1063. [Google Scholar]
- Kirschner AKT, Velimirov B. Benthic bacterial secondary production measured via simultaneous 3H-thymidine and 14C-leucine incorporation, and its implication for the carbon cycle of a shallow macrophyte-dominated backwater system. Limnology and Oceanography. 1999;44:1871–1881. [Google Scholar]
- Lee S, Fuhrman JA. Relationships between biovolume and biomass of naturally derived marine bacterioplankton. Applied and Environmental Microbiology. 1987;53:1298–1303. doi: 10.1128/aem.53.6.1298-1303.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobbes JM, Fitznar HP, Kattner G. Biogeochemical characteristics of dissolved and particulate organic matter in Russian rivers entering the Arctic Ocean. Geochimica et Cosmochimica Acta. 2000;64:2973–2983. [Google Scholar]
- Lorenzen CJ. Determination of chlorophyll and phaeopigments: spectrometric equations. Limnology and Oceanography. 1967;12:343–346. [Google Scholar]
- Louchouarn P, Opsahl S, Benner R. Isolation and quantification of dissolved lignin from natural waters using solid-phase extraction and GC/MS. Analytical Chemistry. 2000;72:2780–2787. doi: 10.1021/ac9912552. [DOI] [PubMed] [Google Scholar]
- Luef B, Aspetsberger F, Hein T, Huber F, Peduzzi P. Impact of hydrology on free-living and particle-associated microorganisms in a river floodplain system (Danube, Austria) Freshwater Biology. 2007;52:1043–1057. [Google Scholar]
- Mayorga E, Aufdenkampe AK, Masiello CA, Krusche AV, Hedges JI, Quay PD, Richey JE, Brown TA. Young organic matter as a source of carbon dioxide outgassing from Amazonian rivers. Nature. 2005;436:538–541. doi: 10.1038/nature03880. [DOI] [PubMed] [Google Scholar]
- McKnight DM, Boyer EW, Westerhoff PK, Doran PT, Kulbe T, Andersen DT. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnology and Oceanography. 2001;46:38–48. [Google Scholar]
- Moran MA, Hodson RE. Dissolved humic substances of vascular plant origin in a coastal marine environment. Limnology and Oceanography. 1994;39:762–771. [Google Scholar]
- Mühlhauser HA, Soto L, Zahradnik P. Improvement of the Kjeldahl method for total nitrogen including acid-hydrolyzable phosphorus determinations in freshwater ecosystems. International Journal of Environmental Analytical Chemistry. 1987;28:215–226. [Google Scholar]
- Noble RT, Fuhrman JA. Use of SYBR Green I for rapid epifluorescence counts of marine viruses and bacteria. Aquatic Microbial Ecology. 1998;14:113–118. [Google Scholar]
- Onstad GD, Canfield DE, Quay PD, Hedges JI. Sources of particulate organic matter in rivers from the continental USA: lignin phenol and stable carbon isotope compositions. Geochimica et Cosmochimica Acta. 2000;64:3539–3546. [Google Scholar]
- Opsahl S, Benner R. Early diagenesis of vascular plant tissues: lignin and cutin decomposition and biogeochemical implications. Geochimica et Cosmochimica Acta. 1995;59:4889–4904. [Google Scholar]
- Opsahl S, Benner R. Distribution and cycling of terrigenous dissolved organic matter in the ocean. Nature. 1997;386:480–482. [Google Scholar]
- Opsahl S, Benner R. Photochemical reactivity of dissolved lignin in river and ocean waters. Limnology and Oceanography. 1998;43:1297–1304. [Google Scholar]
- Peduzzi P, Aspetsberger F, Hein T, Huber F, Kargl Wagner S, Luef B, Tachkova Y. Dissolved organic matter (DOM) and bacterial growth in floodplains of the Danube River under varying hydrological connectivity. Fundamental and Applied Limnology. 2008;171:49–61. [Google Scholar]
- Porter KG, Feig YS. The use of DAPI for identifying and counting aquatic microflora. Limnology and Oceanography. 1980;25:943–948. [Google Scholar]
- Preiner S, Drozdowski I, Schagerl M, Schiemer F, Hein T. The significance of side-arm connectivity for carbon cycling of large rivers. Freshwater Biology. 2008;53:238–252. [Google Scholar]
- Rau G. 13C depletion in a subalpine lake: carbon flow implications. Science. 1978;201:901–902. doi: 10.1126/science.201.4359.901. [DOI] [PubMed] [Google Scholar]
- Schiemer F, Baumgartner C, Tockner K. Restoration of floodplain rivers: the Danube restoration project. Regulated Rivers: Research & Management. 1999;15:231–244. [Google Scholar]
- Schiemer F, Hein T, Peduzzi P. Hydrological control of system characteristics of floodplain lakes. International Journal of Ecohydrology & Hydrobiology. 2006;6:1–18. [Google Scholar]
- Smith BN, Epstein S. Two categories of 13C/12C ratios for higher plants. Plant Physiology. 1971;47:380–384. doi: 10.1104/pp.47.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle CA. Viruses in the sea. Nature. 2005;437:356–361. doi: 10.1038/nature04160. [DOI] [PubMed] [Google Scholar]
- Tockner K, Schiemer F, Baumgartner C, Kum G, Weigand E, Zweimüller I, Ward JV. The Danube restoration project: species diversity patterns across connectivity gradients in the floodplain system. Regulated Rivers: Research & Management. 1999;15:245. [Google Scholar]
- Vogt J, Soille P, De Jager A, Rimaviciute E, Mehl W, Foisneau S, Bodis K, Dusart J, Paracchini ML, Haastrup P, Bamps C. A pan-European river and catchment database. EC-JRC; 2007. Report EUR 22920 EN. [Google Scholar]
- Weinbauer MG. Ecology of prokaryotic viruses. FEMS Microbiology Reviews. 2004;28:127–181. doi: 10.1016/j.femsre.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Weinbauer MG, Peduzzi P. Effect of virus-rich high molecular weight concentrates of seawater on the dynamics of dissolved amino acids and carbohydrates. Marine Ecology Progress Series. 1995;127:245–253. [Google Scholar]
- Wissmar RC, Richey JE, Stallard RF, Edmond JM. Plankton metabolism and carbon processes in the Amazon River, its tributaries, and floodplain waters, Peru, Brazil, May–June 1977. Ecology. 1981;62:1622–1633. [Google Scholar]
- Zessner M, Postolache C, Clement A, Kovacs A, Strauss P. Considerations on the influence of extreme events on the phosphorus transport from river catchments to the Sea. Water Science and Technology. 2005;51:193–204. [PubMed] [Google Scholar]