Abstract
Background
Shiga toxin (Stx)–producing Escherichia coli (STEC), especially O157:H7, cause bloody diarrhea, and in 3%–15% of individuals the infection leads to hemolytic uremic syndrome (HUS) or other complications. Use of antibiotics to treat STEC infections is controversial. Here, we describe the use of piglets to evaluate the efficacy and mechanism of action of antibiotics in these infections.
Methods
The effects of 2 antibiotics on STEC toxin production and their mechanisms of action were first determined by enzyme-linked immunosorbent assay and subsequently evaluated clinically in the gnotobiotic piglet infection model.
Results
In vitro treatment of clinical and isogenic strains with ciprofloxacin increased the production of Stx2 via phage induction but not the production of Stx1. Azithromycin caused no significant increase in toxin production. After treatment with ciprofloxacin, infected piglets had diarrhea and the severe fatal neurological symptoms associated with Stx2 intoxication. Characteristic petechial hemorrhages in the cerebellum were more severe in ciprofloxacin-treated animals than in control animals. In contrast, azithromycin-treated piglets survived the infection and had little or no brain hemorrhaging.
Conclusions
The increased in vitro toxin production caused by ciprofloxacin was strongly correlated with death and an increased rate of cerebellar hemorrhage, in contrast to the effect of azithromycin. The piglet is a suitable model for determining the effectiveness and safety of antibiotics available to treat patients.
In humans, especially children, infection with Shiga toxin (Stx)–producing Escherichia coli (STEC) is strongly associated with the development of hemolytic uremic syndrome (HUS), the leading cause of kidney failure in children [1–4] and acute encephalopathy [5–7]. In the United States, the most significant Stx-producing organisms are enterohemorrhagic E. coli (EHEC) of serotype O157 [8–11]. STEC strains produce 2 subclasses of Stx, Stx1 and Stx2 [12, 13]. Although similar in basic structure, binding specificity, and mode of action, the 2 toxins are immunologically distinct and are regulated in different ways [14]. There is now strong epidemiological evidence showing that HUS development is more closely associated with Stx2-producing strains than with Stx1-producing strains or strains that produce both toxins [15]. This association is further strengthened by studies in gnotobiotic piglets infected with isogenic strains producing Stx2, Stx1, or both: the development of systemic complications as the indicators of HUS was associated with the production of Stx2, and the greatest rate of development was associated with Stx2 production alone [16].
Currently there are no recommended treatment regimens to lessen the risk of HUS in patients [5, 17–19], and the available treatment regimens need to be reevaluated in an animal model. The normal first-line clinical treatment of an enteric infection, the administration of appropriate antibiotics, is highly controversial. Some investigators have concluded that the administration of antibiotics has no effect on the risk of HUS development [20–22], and some have even concluded that the risk is lessened after fosfomycin treatment [23]. Two epidemiological studies—a national surveillance study conducted by the Centers for Disease Control and Prevention and a case-control study involved in a network of participating centers in Washington, Idaho, Oregon, and Montana—found that antibiotic therapy for STEC enteritis significantly increased the risk of HUS [24, 25]. A recent review indicated that the rate of HUS development after O157:H7 infection may increase from 15% to 50% after antibiotic use [19]. The effects of antibiotics on Stx1 and Stx2 production and/or release may explain a possible antibiotic-related increase in HUS development. The genes for the toxins are usually carried on lysogenic phages. Adverse conditions often cause the induction of these temperate phages, and, as the phage replicates within the bacterium, large quantities of toxin are produced. It has been shown in vitro that treatment with certain antibiotics causes phage induction and subsequent increases in toxin levels [26]. To correlate in vitro results with in vivo treatment regimens of currently licensed antibiotics or to evaluate a novel class of antibiotics or medications before their clinical application requires a suitable animal model of infection in which detrimental and beneficial antibiotics can be reliably tested.
We reevaluated the effects of several antibiotics on toxin expression in vitro. For one antibiotic, ciprofloxacin, the mechanism of enhancing Stx2 production via phage induction has been validated. We report the development of an animal model system to allow for the testing of potentially beneficial and deleterious antibiotics. On the basis of our in vitro results and the treatment regimens for children, we evaluated the efficacy of antibiotics (ciprofloxacin and azithromycin) against E. coli O157:H7 infection in our animal model.
METHODS
E. coli O157:H7 strains
Strain 933 (Stx1 and Stx2 producer) was originally isolated from ground beef [27]. Strains 933-1C (Stx1 producer) and 933-2 (Stx2 producer) are its isogenic derivatives. All 3 isogenic strains have been well characterized genetically and have been used extensively by us in the piglet model of EHEC infection [28]. Strain 86-24 (Stx2 producer) was isolated from an outbreak in Walla Walla, Washington [29]. Strains 94–9004, 92–9140, 95–8061, 94–8052, 92–9204, 97–8075, and 97–8023 are all clinical isolates obtained from A. MacKenzie (Child and Youth Clinical Trial Network, Ottawa, Canada). For animal inoculation, a single colony from strain 86-24 was picked and cultured overnight in Luria-Bertani (LB) medium at 37°C, and the bacterial number was determined on the basis of optical density. The inocula were washed once with PBS and adjusted to the appropriate concentration.
ELISAs for Stx1 or Stx2
Two ELISAs using paired antibodies (4D3/rabbit anti-Stx1 for Stx1 and 3D1/5C12 for Stx2) were established on the basis of the previous protocol with modifications to detect both toxins in in vitro cultures, gut contents, and serum [30–33]. Purified Stx1 and Stx2, supplied by A. Kane (Tufts University Medical School, Boston, Massachusetts), were used to generate standard curves. The concentration of toxins in the samples was calculated with nonlinear regression using GraphPad Prism software (version 4) [34].
ELISA sample preparation in vitro or in vivo
To measure toxins released in the culture supernatant or associated with the cells, 1 mL of each antibiotic-treated culture was centrifuged to pellet the bacteria and collect the supernatant, which was sterile filtered and frozen at −20°C. The bacterial pellet was washed once in PBS by centrifugation at 5000 g for 10 min, resuspended with 0.5 mL of bacterial protein extraction reagent from Pierce Biotechnology, and homogenized by vortexing vigorously. The cell lysate was then centrifuged to collect the supernatant containing the cell-associated toxin. This supernatant was called “cell lysate” to differentiate it from the original culture supernatant. To measure Stx2 in serum or gut contents, serum and fecal samples diluted with PBS were used.
Basal level of Stx1 and Stx2
The basal level of Stx1 and Stx2 produced by isogenic strains in LB medium was determined by growing inocula for 24 h. Toxins released in culture supernatants and cell lysates were measured using ELISAs specific for either Stx1 or Stx2. The concentrations were expressed as nanograms per milliliter of toxin. The related data were analyzed with Student’s t test.
Antibiotics and MIC against E. coli O157:H7
The antibiotics selected, with the exception of gentamicin, have all been used to treat intestinal infections and are currently licensed in the United States. Cephalexin and fosfomycin were purchased from Sigma, ciprofloxacin was provided by Bayer Pharmaceuticals, azithromycin was purchased from Pfizer, and gentamicin was purchased from Gibco. Antibiotics were freshly prepared as stock solutions (10 mg/mL) in sterile distilled water and stored in aliquots at −20°C. Gentamicin was kept at room temperature, as recommended by the supplier.
The MIC for each antibiotic was determined using the broth dilution method, according to the recommendations of the National Committee for Clinical Laboratory Standards (1993) [35]. A single colony was initially grown in LB medium for 18 h at 37°C with aeration. Cells were adjusted to a final concentration of 1 × 105 cfu/mL in tubes containing antibiotics 2-fold serially diluted in LB medium in concentrations ranging from 32 to 0.125 µg/mL for cephalexin, fosfomycin, and azithromycin; 25 to 0.05 µg/mL for gentamicin; and 0.5 to 0.0005 µg/mL for ciprofloxacin. Cultures were then grown at 37°C with aeration for 18 h. The lowest dose of antibiotic that inhibited growth was defined as the MIC.
In vitro effects of antibiotics on toxin production
Samples were also obtained from cultures grown in subinhibitory concentrations of each antibiotic to determine cell concentration on the basis of optical density and to measure toxin levels by ELISA. Cultures not treated with antibiotics were grown as positive controls. The effects of antibiotics on Stx production in vitro were identified using 2-fold serial dilutions of the antibiotics in subinhibitory concentrations. The total toxin (cell-associated toxin plus toxin released in the supernatant) level was measured for each antibiotic dilution and compared with that in cultures with no antibiotic to determine the overall increase or decrease in toxin production. In addition, 2 antibiotics, ciprofloxacin and azithromycin, were tested for their effects on toxin production against various clinical isolates.
Construction of recA mutant strains and their response to ciprofloxacin
Isogenic recA mutants of strains 933-1C and 933-2 were derived by a protocol that has been described else-where [36], using plasmid pIM15 as a suicide vector. Mutants were confirmed by polymerase chain reaction (PCR) analysis and phenotypically by a lack of growth on LB agar plates containing 800 ng/mL mitomycin C, a concentration that did not significantly affect the growth of the parental strains. Strains 933-1C and 933-2 along with their isogenic recA mutants were treated with a subinhibitory concentration of ciprofloxacin, and their total toxin levels were measured by ELISA.
In vivo experiments
All piglets were delivered and housed at the Division of Infectious Disease, Tufts University Cummings School of Veterinary Medicine, in accordance with the approved procedures of the Institutional Animal Care and Use Committee at Tufts University. Forty-six gnotobiotic piglets from 10 litters were used. On day 1, the animals were delivered by cesarean section and maintained in plastic sterile microbiological isolators in a 26°C room [28]. On day 2, piglets were challenged by oral administration of 1 × 109 strain 86-24 bacteria. On day 3, piglets were either left untreated, treated with ciprofloxacin at the calculated dose per kilogram of body weight used in children (10 mg/kg, twice a day), or treated with azithromycin at the recommended pediatric dose (10 mg/kg on day 1 and 5 mg/kg for subsequent doses, once per day). Between days 3 and 14, piglets were observed for clinical signs, except for milk consumption, disposition, diarrhea, and hydration. Piglets were fed Similac (Abbott Laboratories) 3 times daily, for a total of 500–700 mL/day.
Piglets exhibiting neurological symptoms or >8% dehydration were euthanized, and tissues from intestines and brain were processed for histological evaluation. Gut contents were obtained for bacterial count and toxin assay. For bacterial counting, serially diluted fecal samples were plated on MacConkey agar and sorbitol MacConkey agar. The plates were cultured for 24 h at 37°C, and the bacterial number was averaged from both plates. All surviving piglets were euthanized.
Statistical analysis
Data were analyzed with the GraphPad Prism statistics and graphing program (GraphPad). Student’s t test was used for 2-group comparisons. Log-rank and χ2 tests were used to compare the survival curves for different treatments. Comparisons involving multiple groups were conducted using analysis of variance for significance followed by Dunnett’s multiple-comparison test to determine intergroup statistical significance. In all cases, differences were considered significant at P < .05.
RESULTS
Basal level of Stx1 and Stx2 of strain 933 and its derivatives, 933-1C and 933-2
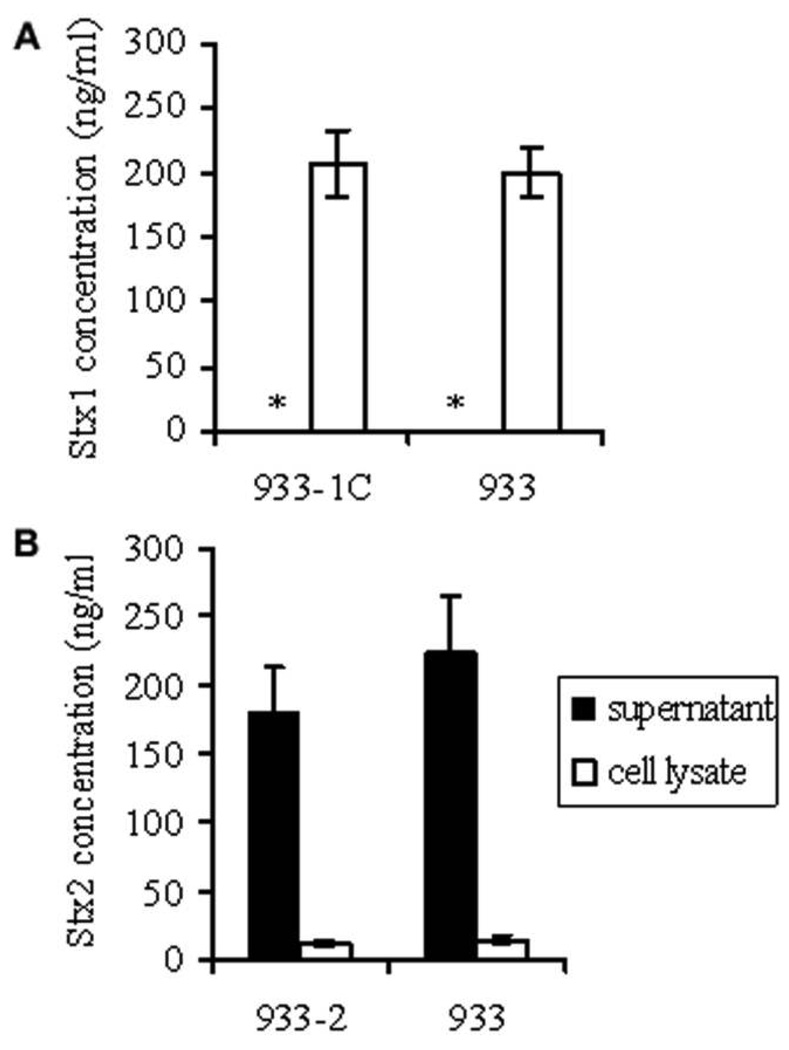
Stx1-producing strains (strain 933 and its isogenic derivative 933-1C) had the majority of the cell-associated toxin. The amount of Stx1 found in culture supernatants was very low (<1 ng/mL) and was near the limit of detection of the assay (figure 1A). Strains 933-1C and 933 had no significant difference in Stx1 production (P > .1). However, Stx2-producing strains (strain 933 and its isogenic derivative 933-2) showed >90% of the toxin released in culture supernatants, with no significant difference in Stx2 production between strains (P > .1). The total levels of Stx1 and Stx2 did not differ among all the isogenic strains (P > .1), with a mean of 200 ng/mL (figure 1).
Figure 1.
Toxin levels in culture supernatants and cell lysates. A, Shiga toxin (Stx) 1–producing strains 933-1C and 933. B, Stx2-producing strains 933-2 and 933. Stx in supernatants or cell lysates was detected by ELISA, and the concentration was determined on the basis of standard curves using purified Stx1 or Stx2. Results are expressed as mean ± SE toxin concentrations for 10 different inocula for each strain. Student’s t test was used for related 2-group comparisons. * Stx1 concentration <1 ng/mL.
MICs for antibiotics
MICs were determined for 5 antibiotics—cephalexin, fosfomycin, ciprofloxacin, azithromycin, and gentamicin—using the 3 isogenic strains 933, 933-1C, and 933-2. The MIC values were 8 µg/mL for cephalexin, 8 µg/mL for fosfomycin, 0.03 µg/mL for ciprofloxacin, 16 µg/ mL for azithromycin, and 1.5 µg/mL for gentamicin.
In vitro characterization of antibiotics against O157:H7
Supernatants and cell lysates from cultures grown in subinhibitory concentrations of each antibiotic were tested for Stx1 and Stx2 levels. Similar to findings in untreated control cultures, Stx1 was detected mostly in the cell lysates of 933 and 933-1C strains, whereas Stx2 was found mostly in the supernatants of 933 and 933-2 cultures (data not shown). The only exception was in cultures treated with 4 µg/mL fosfomycin; Stx1 was found only in the supernatant instead of being cell associated, probably because of cell lysis observed at this concentration. At concentrations <4 µg/mL fosfomycin, Stx1 was mainly cell associated. Stx2 release in cultures treated with fosfomycin had the same pattern as for cultures before and after exposure to the other antibiotics. With the exception of ciprofloxacin, which enhanced Stx1 production slightly, none of the tested antibiotics increased Stx1 production at subinhibitory concentrations. Ciprofloxacin increased Stx2 production from 10- to 250-fold and cephalexin and fosfomycin increased it slightly, but azithromycin and gentamicin had no effects on Stx2 production. Table 1 shows detailed results for all the antibiotics tested.
Table 1.
Effects of various antibiotics at subinhibitory concentrations on Shiga toxin (Stx) 1 and Stx2 production.
| Fold increase in Stx1a | Fold increase in Stx2a | |||
|---|---|---|---|---|
| Antibiotic | 933b | 933-1Cc | 933b | 933-2d |
| Cephalexin | 0.2–1.3 | 0.8–1.6 | 1.1–4.7 | 1.2–4.8 |
| Fosfomycin | 0.7–1.2 | 0.5–1.1 | 1.2–2.2 | 0.8–2.2 |
| Ciprofloxacin | 1.3–1.5 | 1–1.7 | 5–261 | 4–166 |
| Azithromycin | 0.2–0.7 | 0.2–0.7 | 0.1–1.2 | 0.1–1 |
| Gentamicin | 0.1–0.6 | 0.3–0.7 | 0.5–0.9 | 0.8–1.1 |
Toxin production values were calculated as fold increases (Stx concentration in the test tube with antibiotic/Stx concentration in control tube without antibiotic); the baseline production of Stx (in nonantibiotic-treated culture) is equivalent to 1. Results are given as the range of fold increases for the subinhibitory concentration of each antibiotic.
Wild-type Escherichia coli O157:H7 strain 933.
Isogenic Stx1-producing strain derived from strain 933.
Isogenic Stx2-producing strain derived from strain 933.
Another 8 clinical isolates were treated with ciprofloxacin and azithromycin at subinhibitory concentrations. Stx2 production was enhanced to various degrees by ciprofloxacin but not by azithromycin. In contrast, neither antibiotic had a significant effect on Stx1 production. Table 2 shows the effects of ciprofloxacin and azithromycin at subinhibitory concentrations on Stx1 or Stx2 production by the clinical isolates.
Table 2.
Effects of ciprofloxacin and azithromycin at subinhibitory concentrations on Shiga toxin (Stx) 1 and/or Stx2 production by various clinical isolates.
| Fold increase in Stxa | |||||
|---|---|---|---|---|---|
| Ciprofloxacin | Azithromycin | ||||
| Strain | Toxin | Stx1 | Stx2 | Stx1 | Stx2 |
| 86-24 | Stx2 | … | 67–188 | … | 0.2–0.8 |
| 94–9004 | Stx2 | … | 154–499 | … | 1–2 |
| 92–9140 | Stx2 | … | 111–418 | … | 0.025–0.5 |
| 95–8061 | Stx2c | … | 111–243 | … | 0.9–1.1 |
| 94–8052 | Stx1 | 1–2 | … | 1–2 | … |
| 92–9204 | Stx1 | 1–2 | … | 1–2 | … |
| 97–8075 | Stx1/Stx2 | 1–2 | ~500 | 1–2 | ~1 |
| 97–8023 | Stx1/Stx2 | 3–4 | 700 | 3–4 | 1 |
Toxin production values were calculated as fold increases (Stx concentration in the test tube with antibiotic/Stx concentration in control tube without antibiotic). Results are given as ranges for the subinhibitory concentration of each antibiotic.
In vitro treatment of recA mutants
The recA mutants, treated with the phage-inducing agent mitomycin C, failed to produce any phage plaques, indicating a defect in phage induction due to the absence of recA. Table 3 shows the comparison between recA+ and recA− strains in terms of toxin production. The recA status had a pronounced effect on the basal level of Stx2 but not Stx1. Treatment of cultures with subinhibitory concentrations of ciprofloxacin increased Stx2 levels only in recA+ cultures. No antibiotic-mediated toxin increases were seen in the recA− cultures.
Table 3.
Effect of the recA mutation on Shiga toxin (Stx) 1 and Stx2 production.
| Strain | Mean ± SE toxin productiona after treatment with ciprofloxacin at | |||
|---|---|---|---|---|
| 2.0 ng/mL | 1.0 ng/mL | 0.5 ng/mL | 0 ng/mL | |
| 933-1C Stx1 | 72 ± 3.4 | 62 ± 3.8 | 64 ± 3.2 | 70 ± 3.4 |
| 933-1C Stx1 recA− | 31 ± 4 | 53 ± 9 | 105 ± 3.8 | 71 ± 3.2 |
| 933-2 Stx2 | 3625 ± 510 | 2089 ± 479 | 1699 ± 425 | 627 ± 96 |
| 933-2 Stx2 recA− | 1.87 ± 1.52 | 1.07 ± 0.19 | 0.89 ± 0.12 | 1.33 ± 0.10 |
Toxin production values are given in ng/1 × 109 cells.
In vivo studies
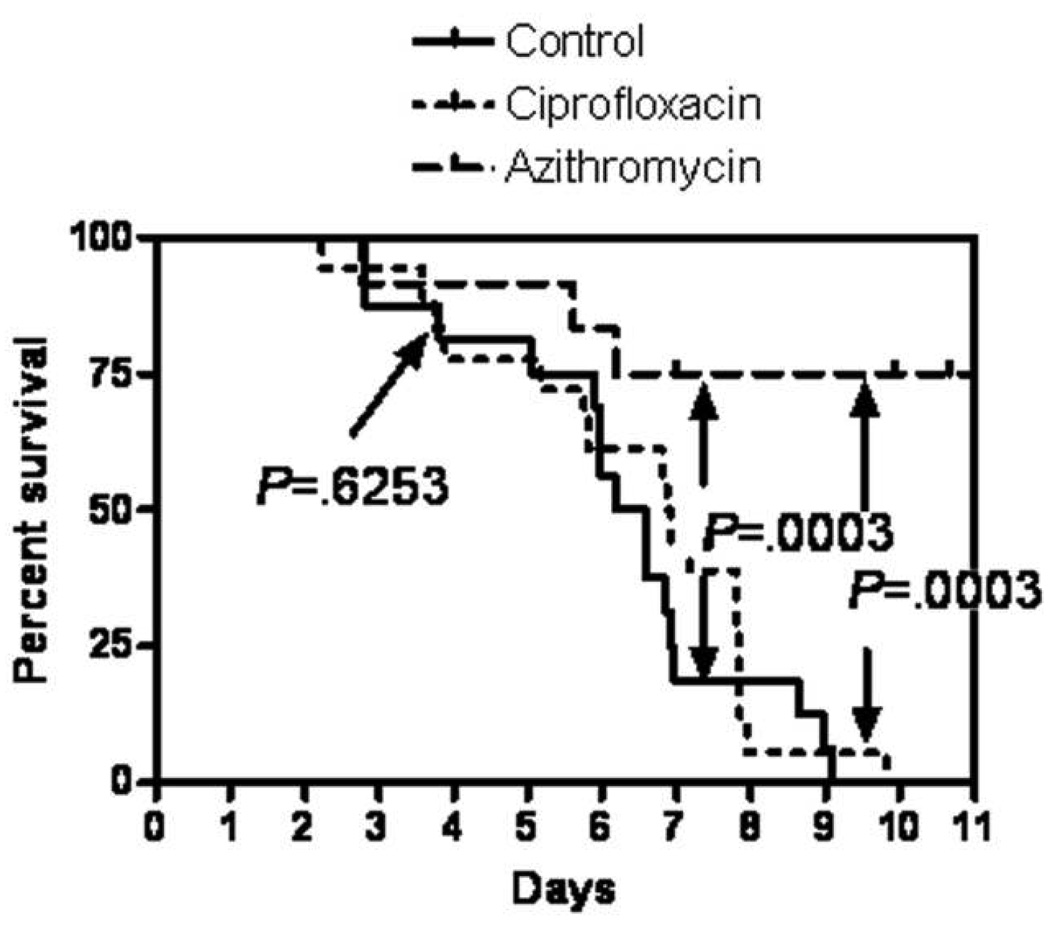
Azithromycin treatment significantly protected the infected piglets over both those left untreated and those treated with ciprofloxacin (P < .05, log-rank and χ2 tests). There were no significant differences in survival between ciprofloxacin-treated piglets and the untreated group (P ≥ .05). Piglet survival curves are shown in figure 2, and the clinical manifestations are summarized in table 4.
Figure 2.
Results of the survival experiment. Azithromycin but not ciprofloxacin had a statistically significant effect on the survival curve (for azithromycin vs. control and azithromycin vs. ciprofloxacin, P < .01; for ciprofloxacin vs. control, P ≥ .05 [log-rank and χ2 tests]).
Table 4.
Summary of in vivo findings.
| Piglet groupa | Diarrhea | Gut lesionsa | Central nervous system involvement | Bacterial countc | Toxin leveld | |
|---|---|---|---|---|---|---|
| Symptoms | Lesions | |||||
| 1 (n = 15) | 15 | 15 | 15 | 13 (87) | 118.0 ± 21.9 | 0.745 (0.390–1.024) |
| 2 (n = 16) | 16 | 1 | 16 | 14 (88) | 1.8 ± 1.4 | 0.066 (0.000–8.691) |
| 3 (n = 11) | 11 | 1 | 2 | 4 (36) | 22.8 ± 6.0 | 0.108 (0.034–0.193) |
NOTE. Data are no. (%) of piglets, unless otherwise indicated.
Group 1 piglets were infected with strain 86-24 only, group 2 piglets were infected with strain 86-24 and treated with ciprofloxacin, and group 3 piglets were infected with strain 86-24 and treated with azithromycin.
Gut lesions were caused by bacterial attachment-effacement to enterocytes and colonocytes.
Data are mean ± SE values given in 1 × 108 cfu/mL.
Data are median (interquartile range) values given in µg/mL.
Bacterial counts in the cecum of animals at necropsy revealed that both ciprofloxacin and azithromycin significantly lowered the number of organisms compared with those in untreated control animals (P < .05). Ciprofloxacin treatment had the most pronounced effect, yielding no viable organisms in the cecum or anywhere else in the intestines. Azithromycin treatment did not eliminate the organism from the intestinal tract but lowered the numbers of viable organisms compared with those in untreated control animals (table 4).
In a group of 4 piglets that were treated with ciprofloxacin, fecal shedding of strain 86–24 was monitored daily. Within 24–48 h after the beginning of antibiotic treatment, bacteria were no longer detected in fecal samples. Despite the absence of viable organisms, the same piglets went on to manifest severe neurological symptoms and were euthanized.
The toxin level in the cecum was also determined by ELISA. In the ciprofloxacin-treated group, animals that showed no viable organisms also had no measurable toxin. However, a few animals had measurable organisms, and the level of toxin in their gut content was many fold higher than that found in the untreated control animals. Azithromycin-treated animals had less cecal toxin than the untreated control animals (P < .05) (table 4).
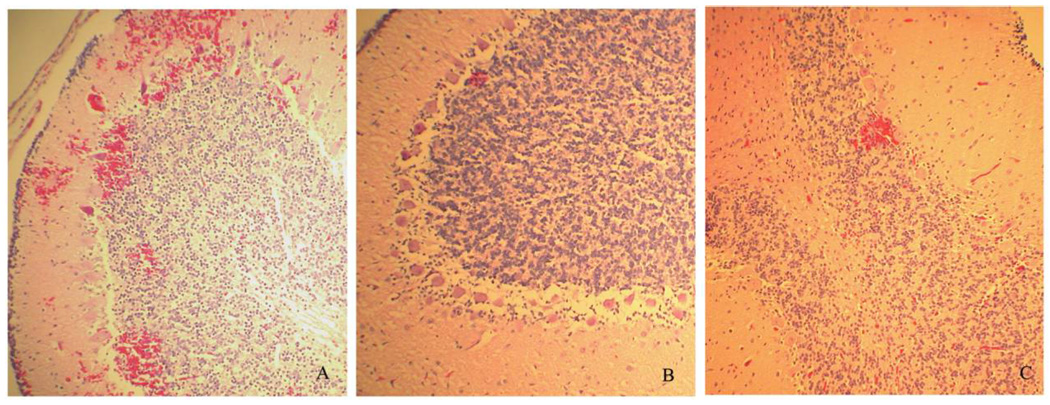
Histological evaluation of the cerebellum and ileocecum/spiral colon was performed on the basis of our previous observations. Of 15 piglets infected with strain 8624 only, 13 had focal hemorrhagic cerebellar lesions (severe in 3), and no cerebellar lesions were found in 2. Of 16 infected piglets treated with ciprofloxacin, 14 had hemorrhagic cerebellar lesions (severe in 8), and 2 had no observable lesions. However, of 11 infected piglets treated with azithromycin, only 4 had mild hemorrhagic cerebellar lesions, and 7 had no observable lesions. Representative cerebellar lesions are shown in figure 3. In infected piglets that did not receive antibiotic treatment, the surface mucosal epithelium was mildly eroded in some areas and was attached by large numbers of bacteria with small numbers of neutrophils in the subjacent lamina propria. Some piglets showed severe neutrophilic erosive colitis with crypt abscesses and large colonies of bacterial organisms along the enterocyte surface. A moderate quantity of neutrophilic cell debris was in the lumen. Attenuated crypt epithelium, submucosal edema, and small numbers of hemosiderin-laden macrophages in the mucosa were observed. Of 16 infected piglets treated with ciprofloxacin, 14 had hemorrhagic cerebellar lesions (severe in 8), and 2 had no observable lesions. Generally, no lesions were observed; there were mild lesions in only 1 ciprofloxacin-treated and 1 azithromycin-treated piglet. In addition, in 4 piglets given either ciprofloxacin or azithromycin only, no lesions were observed in either the cerebellum or the large intestines (data not shown).
Figure 3.
Characteristic histological changes in the cerebellum (hematoxylin-eosin staining; original magnification, ×100). A, Strain 86-24 plus ciprofloxacin. B, Strain 86-24 plus azithromycin. C, Strain 86-24 only.
DISCUSSION
Treatment with antibiotics is the first line of defense for many intestinal infections. However, antibiotic treatment for STEC infections is in disfavor because of epidemiological evidence indicating that the incidence of HUS may, in fact, increase with antibiotic use. Increased toxin production due to antibiotic exposure may be the cause of this increased risk of HUS. In the present study, the effects of several antibiotics were analyzed first in vitro and then in vivo. Stx1 and Stx2 production in strain 933 and its isogenic toxin deletion derivatives was studied using subinhibitory levels of several antibiotics, including the protein synthesis inhibitors azithromycin and gentamicin, the cell wall inhibitors fosfomycin and cephalexin, and a DNA gyrase inhibitor, ciprofloxacin. Although no significant increases in toxin production were seen for most of the antibiotics tested, dramatic increases in Stx2 levels were observed for ciprofloxacin treatment. Antibiotics that inhibit protein synthesis appear to cause no increases in toxin levels.
Because another study [37] noted a wide variation in the response to antibiotics among strains, the effects of ciprofloxacin and azithromycin on toxin production were tested using several additional clinical strains. Our results repeated the previous findings: ciprofloxacin did induce Stx2 production to varying degrees but did not induce Stx1 production, and azithromycin had no effect on either.
Because we have shown previously that phage induction yields high levels of toxin [36], we constructed isogenic strains that were deficient in phage induction to explore the mechanism by which ciprofloxacin treatment increases production of Stx2. Strain 933 derivatives were made recA− by suicide-vector mutagenesis. Mutants were confirmed genotypically by PCR and phenotypically by their sensitivity to the mutagen mitomycin C. When these strains were treated with ciprofloxacin, no increases in Stx2 levels were seen, indicating that phage induction was the mechanism by which ciprofloxacin increases toxin yields.
In vitro results are interesting and suggestive but do not necessarily reflect what might happen in vivo. In the in vitro studies, subinhibitory concentrations of antibiotics were used, and it is unclear how these levels reflect what happens in vivo with standard doses of antibiotics. We have shown previously that systemic complications manifested by neurological abnormalities can be observed in 30%–40% of newborn gnotobiotic piglets orally infected with strain 933, a producer of Stx1 and Stx2, and in 80%–90% of those infected with strain 86-24 or 933-2, both Stx2 producers [16]. In addition, Stx2-specific human monoclonal antibodies protect piglets from fatal systemic complications of Stx2 [33]. To test the effects of ciprofloxacin and azithromycin, gnotobiotic piglets were challenged orally 24 h after birth with strain 86-24 and then treated with antibiotics. As before, gnotobiotic piglets challenged orally with Stx2-producingEHEC strains showed systemic complications, manifested by watery diarrhea within 24–48 h and neurological abnormalities. In histological evaluation, these same animals also showed characteristic attaching and effacing lesions in the terminal ileum and colon. Neurological complications are caused by systemic delivery of toxin to the vasculature endothelium of the cerebellum, which results in occlusions.
To test the model, we chose one antibiotic, ciprofloxacin, that increased Stx2 yields dramatically in vitro and another, azithromycin, that had no significant effect on toxin production vitro. In the piglet experiments, ciprofloxacin treatment of piglets infected with strain 86-24 had no protective effect on the rate of systemic complications, although it was very effective in eliminating the organism from the gastrointestinal tract within 24 h after the start of treatment; correspondingly, it reduced the attaching and effacing lesions in the terminal ileum and spiral colon. However, despite the lack of shedding of the organisms, severe neurological symptoms developed 3–4 days after the start of antibiotic treatment, requiring the animals to be euthanized. In contrast, azithromycin treatment of infected piglets had a significant protective effect. Treatment reduced but did not eliminate the organism from the intestinal tract and consequently reduced the amount of toxin in the gut. Histological examination has further strengthened the clinical observations. Cerebellar tissue from ciprofloxacin-treated piglets exhibited a high number of petechial hemorrhagic lesions, in contrast to that from azithromycin-treated piglets.
The present study confirms the potential risk that some antibiotic treatments for EHEC infections may have on the rate of systemic complications. Ciprofloxacin caused a significant increase in toxin production in vitro. In our in vivo piglet model, however, despite eliminating the organism from the intestinal tract, ciprofloxacin proved fatal. It is likely that enough toxin was produced during the initial exposure to ciprofloxacin to overwhelm the animals several days later. This study is also significant because its results indicate that specific antibiotics may be effective in treating the infection. For example, although in vitro experiments revealed no significant increases in toxin production with azithromycin, it was effective in preventing systemic complications in vivo. Our findings underscore the importance of using an appropriate in vivo model to evaluate treatment regimens. The gnotobiotic piglet will provide a useful animal model for testing additional antibiotics to determine their efficacy in lowering the rate of systemic complications in EHEC infections.
Acknowledgments
The support and dedication of Donald J. Girouard, Jr., who performed the cesarean sections, and the technicians at the Division of Infectious Disease, Tufts University Cummings School of Veterinary Medicine, who cared for the piglets, are appreciated.
Financial support: Food and Waterborne Diseases Integrated Research Network, National Institutes of Health (grant N01-AI30050).
Footnotes
Potential conflicts of interest: none reported.
References
- 1.Karmali MA, Petric M, Lim C, Fleming PC, Arbus GS, Lior H. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J Infect Dis. 1985;151:775–782. doi: 10.1093/infdis/151.5.775. [DOI] [PubMed] [Google Scholar]
- 2.Gransden WR, Damm MA, Anderson JD, Carter JE, Lior H. Further evidence associating hemolytic uremic syndrome with infection by Verotoxin-producing Escherichia coli O157:H7. J Infect Dis. 1986;154:522–524. doi: 10.1093/infdis/154.3.522. [DOI] [PubMed] [Google Scholar]
- 3.Spika JS, Parsons JE, Nordenberg D, Wells JG, Gunn RA, Blake PA. Hemolytic uremic syndrome and diarrhea associated with Escherichia coli O157:H7 in a day care center. J Pediatr. 1986;109:287–291. doi: 10.1016/s0022-3476(86)80386-9. [DOI] [PubMed] [Google Scholar]
- 4.Thomas A, Chart H, Cheasty T, Smith HR, Frost JA, Rowe B. Vero cytotoxin-producing Escherichia coli, particularly serogroup O 157, associated with human infections in the United Kingdom: 1989–91. Epidemiol Infect. 1993;110:591–600. doi: 10.1017/s0950268800051013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iijima K, Kamioka I, Nozu K. Management of diarrhea-associated hemolytic uremic syndrome in children. Clin Exp Nephrol. 2008;12:16–19. doi: 10.1007/s10157-007-0007-4. [DOI] [PubMed] [Google Scholar]
- 6.Tzipori S, Sheoran A, Akiyoshi D, Donohue-Rolfe A, Trachtman H. Antibody therapy in the management of Shiga toxin-induced hemolytic uremic syndrome. Clin Microbiol Rev. 2004;17:926–941. doi: 10.1128/CMR.17.4.926-941.2004. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tzipori S, Chow CW, Powell HR. Cerebral infection with Escherichia coli O157:H7 in humans and gnotobiotic piglets. J Clin Pathol. 1988;41:1099–1103. doi: 10.1136/jcp.41.10.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voetsch AC, Kennedy MH, Keene WE, et al. Risk factors for sporadic Shiga toxin-producing Escherichia coli O157 infections in FoodNet sites, 1999–2000. Epidemiol Infect. 2007;135:993–1000. doi: 10.1017/S0950268806007564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varma JK, Greene KD, Reller ME, et al. An outbreak of Escherichia coli O157 infection following exposure to a contaminated building. JAMA. 2003;290:2709–2712. doi: 10.1001/jama.290.20.2709. [DOI] [PubMed] [Google Scholar]
- 10.Olsen SJ, Miller G, Breuer T, et al. A waterborne outbreak of Escherichia coli O157:H7 infections and hemolytic uremic syndrome: implications for rural water systems. Emerg Infect Dis. 2002;8:370–375. doi: 10.3201/eid0804.000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mead PS, Griffin PM. Escherichia coli O157:H7. Lancet. 1998;352:1207–1212. doi: 10.1016/S0140-6736(98)01267-7. [DOI] [PubMed] [Google Scholar]
- 12.Scotland SM, Willshaw GA, Smith HR, Rowe B. Properties of strains of Escherichia coli belonging to serogroup O157 with special reference to production of Vero cytotoxins VT1 and VT2. Epidemiol Infect. 1987;99:613–624. doi: 10.1017/s0950268800066462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acheson DW. Nomenclature of enterotoxins. Lancet. 1998;351:1003. doi: 10.1016/s0140-6736(05)78992-3. [DOI] [PubMed] [Google Scholar]
- 14.Gunzer F, Bohn U, Fuchs S, et al. Construction and characterization of an isogenic slt-ii deletion mutant of enterohemorrhagic Escherichia coli. Infect Immun. 1998;66:2337–2341. doi: 10.1128/iai.66.5.2337-2341.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ostroff SM, Tarr PI, Neill MA, Lewis JH, Hargrett-Bean N, Kobayashi JM. Toxin genotypes and plasmid profiles as determinants of systemic sequelae in Escherichia coli O157:H7 infections. J Infect Dis. 1989;160:994–998. doi: 10.1093/infdis/160.6.994. [DOI] [PubMed] [Google Scholar]
- 16.Donohue-Rolfe A, Kondova I, Oswald S, Hutto D, Tzipori S. Escherichia coli O157:H7 strains that express Shiga toxin (Stx) 2 alone are more neurotropic for gnotobiotic piglets than are isotypes producing only Stx1 or both Stx1 and Stx2. J Infect Dis. 2000;181:1825–1829. doi: 10.1086/315421. [DOI] [PubMed] [Google Scholar]
- 17.Orth D, Grif K, Zimmerhackl LB, Wurzner R. Prevention and treatment of enterohemorrhagic Escherichia coli infections in humans. Expert Rev Anti Infect Ther. 2008;6:101–108. doi: 10.1586/14787210.6.1.101. [DOI] [PubMed] [Google Scholar]
- 18.Goldwater PN. Treatment and prevention of enterohemorrhagic Escherichia coli infection and hemolytic uremic syndrome. Expert Rev Anti Infect Ther. 2007;5:653–663. doi: 10.1586/14787210.5.4.653. [DOI] [PubMed] [Google Scholar]
- 19.Serna At, Boedeker EC. Pathogenesis and treatment of Shiga toxin-producing Escherichia coli infections. Curr Opin Gastroenterol. 2008;24:38–47. doi: 10.1097/MOG.0b013e3282f2dfb8. [DOI] [PubMed] [Google Scholar]
- 20.Bell BP, Griffin PM, Lozano P, Christie DL, Kobayashi JM, Tarr PI. Predictors of hemolytic uremic syndrome in children during a large outbreak of Escherichia coli O157:H7 infections. Pediatrics. 1997;100:E12. doi: 10.1542/peds.100.1.e12. [DOI] [PubMed] [Google Scholar]
- 21.Pavia AT, Nichols CR, Green DP, et al. Hemolytic-uremic syndrome during an outbreak of Escherichia coli O157:H7 infections in institutions for mentally retarded persons: clinical and epidemiologic observations. J Pediatr. 1990;116:544–551. doi: 10.1016/s0022-3476(05)81600-2. [DOI] [PubMed] [Google Scholar]
- 22.Proulx F, Turgeon JP, Delage G, Lafleur L, Chicoine L. Randomized, controlled trial of antibiotic therapy for Escherichia coli O157:H7 enteritis. J Pediatr. 1992;121:299–303. doi: 10.1016/s0022-3476(05)81209-0. [DOI] [PubMed] [Google Scholar]
- 23.Ikeda K, Ida O, Kimoto K, Takatorige T, Nakanishi N, Tatara K. Effect of early fosfomycin treatment on prevention of hemolytic uremic syndrome accompanying Escherichia coli O157:H7 infection. Clin Nephrol. 1999;52:357–362. [PubMed] [Google Scholar]
- 24.Slutsker L, Ries AA, Maloney K, Wells JG, Greene KD, Griffin PM. A nationwide case-control study of Escherichia coli O157:H7 infection in the United States. J Infect Dis. 1998;177:962–966. doi: 10.1086/515258. [DOI] [PubMed] [Google Scholar]
- 25.Wong CS, Jelacic S, Habeeb RL, Watkins SL, Tarr PI. The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N Engl J Med. 2000;342:1930–1936. doi: 10.1056/NEJM200006293422601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimmitt PT, Harwood CR, Barer MR. Toxin gene expression by Shiga toxin-producing Escherichia coli: the role of antibiotics and the bacterial SOS response. Emerg Infect Dis. 2000;6:458–465. doi: 10.3201/eid0605.000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strockbine NA, Marques LR, Newland JW, Smith HW, Holmes RK, O’Brien AD. Two toxin-converting phages from Escherichia coli O157:H7 strain 933 encode antigenically distinct toxins with similar biologic activities. Infect Immun. 1986;53:135–140. doi: 10.1128/iai.53.1.135-140.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tzipori S, Gunzer F, Donnenberg MS, de Montigny L, Kaper JB, Donohue-Rolfe A. The role of the eaeA gene in diarrhea and neurological complications in a gnotobiotic piglet model of enterohemorrhagic Escherichia coli infection. Infect Immun. 1995;63:3621–3627. doi: 10.1128/iai.63.9.3621-3627.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffin PM, Ostroff SM, Tauxe RV, et al. Illnesses associated with Escherichia coli O157:H7 infections: a broad clinical spectrum. Ann Intern Med. 1988;109:705–712. doi: 10.7326/0003-4819-109-9-705. [DOI] [PubMed] [Google Scholar]
- 30.Acheson DW, Jacewicz M, Kane AV, Donohue-Rolfe A, Keusch GT. One step high yield affinity purification of Shiga-like toxin II variants and quantitation using enzyme linked immunosorbent assays. Microb Pathog. 1993;14:57–66. doi: 10.1006/mpat.1993.1006. [DOI] [PubMed] [Google Scholar]
- 31.Acheson DW, Keusch GT, Lightowlers M, Donohue-Rolfe A. Enzyme-linked immunosorbent assay for Shiga toxin and Shiga-like toxin II using P1 glycoprotein from hydatid cysts. J Infect Dis. 1990;161:134–137. doi: 10.1093/infdis/161.1.134. [DOI] [PubMed] [Google Scholar]
- 32.Donohue-Rolfe A, Kelley MA, Bennish M, Keusch GT. Enzyme-linked immunosorbent assay for shigella toxin. J Clin Microbiol. 1986;24:65–68. doi: 10.1128/jcm.24.1.65-68.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukherjee J, Chios K, Fishwild D, et al. Human Stx2-specific monoclonal antibodies prevent systemic complications of Escherichia coli O157:H7 infection. Infect Immun. 2002;70:612–619. doi: 10.1128/iai.70.2.612-619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Motulsky HJ, Ransnas LA. Fitting curves to data using nonlinear regression: a practical and nonmathematical review. FASEB J. 1987;1:365–374. [PubMed] [Google Scholar]
- 35.Smith MC, Sephel GC, Woodward SC. Spreadsheet templates for calculating precision according to NCCLS (National Committee for Clinical Laboratory Standards) guidelines. MLO Med Lab Obs. 1993;25:67–69. [PubMed] [Google Scholar]
- 36.Fuchs S, Muhldorfer I, Donohue-Rolfe A, et al. Influence of RecA on in vivo virulence and Shiga toxin 2 production in Escherichia coli pathogens. Microb Pathog. 1999;27:13–23. doi: 10.1006/mpat.1999.0279. [DOI] [PubMed] [Google Scholar]
- 37.Grif K, Dierich MP, Karch H, Allerberger F. Strain-specific differences in the amount of Shiga toxin released from enterohemorrhagic Escherichia coli O157 following exposure to subinhibitory concentrations of antimicrobial agents. Eur J Clin Microbiol Infect Dis. 1998;17:761–766. doi: 10.1007/s100960050181. [DOI] [PubMed] [Google Scholar]