Abstract
BACKGROUND
Acute kidney injury (AKI) after coronary artery bypass graft (CABG) surgery is associated with increased postoperative morbidity and mortality. We hypothesized that increased plasma neutrophil gelatinase-associated lipocalin (NGAL) measured immediately after separating from cardiopulmonary bypass (CPB) would predict AKI after CABG surgery.
METHODS
In a retrospective observational study, we examined the value of plasma NGAL measured after CPB for predicting the risk of developing AKI (defined as a ≥50% increase in serum creatinine from preoperative levels) in 879 patients after CABG surgery using multivariable logistic regression. Area under the curve of receiver operating characteristic curves was analyzed to assess sensitivities, specificities, and cutoff points for postoperative plasma NGAL levels to predict AKI.
RESULTS
Seventy-five patients (8.6%) developed postoperative AKI. Plasma NGAL levels measured after CPB were higher in patients who subsequently developed AKI than in those who did not (AKI: 268.8 ng/mL [207.5–459.5 ng/mL], median [interquartile range], vs no AKI: 238.4 ng/mL [172.0–319.1 ng/mL]; P < 0.001) and remained higher through postoperative day 4. An optimal serum plasma NGAL cutoff of 353.5 ng/mL at the post-CPB time point had a sensitivity of 38.7%, specificity of 81.5%, and a positive predictive value of 16.3% for predicting AKI. In our multivariate regression model, post-CPB plasma NGAL levels >353.5 ng/mL were independently associated with postoperative AKI (odds ratio, 2.3; 95% confidence interval, 1.5–6.5; P = 0.002).
CONCLUSION
An early increase of post-CPB plasma NGAL is associated with AKI in adult patients undergoing CABG surgery, although the sensitivity is low. Therefore, assessing early plasma NGAL alone has limited utility for predicting AKI in this patient population.
Acute kidney injury (AKI) occurs in up to 30% of adult patients after coronary artery bypass graft (CABG) surgery with cardiopulmonary bypass (CPB), and it is associated with increased mortality, longer hospital length of stay, and greater resource utilization.1–4 Although clinical risk factors associated with AKI after cardiac surgery have been identified,5–7 current biomarkers of renal dysfunction lack diagnostic sensitivity in the early postoperative period.8 Most notably, serum creatinine may not peak until several days after tubular insult, potentially delaying diagnosis and possible treatment of renal injury.9 Thus, there is a strong need to identify early and reliable markers of postoperative kidney dysfunction.
Animal studies have demonstrated an increase in urinary neutrophil gelatinase-associated lipocalin (NGAL) expression after renal ischemic injury.10 Subsequent clinical studies have shown increased urinary NGAL levels to be highly sensitive and specific for predicting postoperative AKI as early as 2 hours after pediatric cardiac surgery.11 More recently, Wagener et al.12 demonstrated a significant association between increased urinary NGAL levels and AKI in adult cardiac surgical patients. Despite this correlation, further analysis revealed a low sensitivity of urinary NGAL for predicting postoperative AKI in this adult cardiac surgical cohort.
The value of early plasma NGAL levels as a predictive marker of postoperative AKI in adult cardiac surgical patients is not known. Therefore, our aim was to evaluate the utility of early postoperative plasma NGAL levels for predicting postoperative AKI in adult patients undergoing CABG surgery with CPB.
METHODS
Study Design and Patient Population
Consecutive patients scheduled for primary, CABG-only surgery at the Brigham and Women’s Hospital, Boston, MA, and the Texas Heart Institute, St. Luke’s Episcopal Hospital, Houston, TX, between August 2001 and June 2007 were eligible for enrollment into the CABG Genomics Program (http://clinicaltrials.gov/show/NCT00281164). CABG Genomics is an IRB-approved prospective observational study examining adverse outcomes after CABG surgery. All patients gave written informed consent before enrollment. Exclusion criteria for enrollment into the parent study were age <20 years, preoperative hematocrit <25%, and history of leukocyte-rich blood product transfusion ≤30 days before surgery. To address the specific aim of this study, patients with a history of preoperative renal replacement therapy, undergoing off-pump CABG surgery, receiving intraoperative aprotinin therapy, or missing preoperative serum creatinine or post-CPB plasma NGAL values were excluded from analysis.
Study Protocol
Demographic data, preoperative risk factors, and perioperative surgical and anesthetic management were recorded for all patients. Arterial whole blood samples were obtained preoperatively, immediately after separation from CPB, and on the 4 consecutive days after surgery (postoperative days [PODs] 1–4) and preserved in plastic tubes containing EDTA. Blood samples were immediately centrifuged at 2800 rpm for 15 minutes at 4°C, and plasma was aliquoted and stored in liquid-phase nitrogen (approximately 190°C). Batched samples were sent to Biosite (San Diego, CA) for analysis of plasma NGAL and serum creatinine. Plasma NGAL was measured using Triage® NGAL, a fluorescence- based immunoassay, in conjunction with the Triage Meter (Biosite, San Diego, CA).13 The Triage NGAL has a minimal detectable dose of 60 ng/mL and an upper range end of 2000 ng/mL. Serum creatinine was measured preoperatively and on PODs 1 to 4 using a buffered kinetic Jaffe reaction without deproteinization, with a measuring range for this assay of 0.17 to 24.9 mg/dL (Cobas C systems, Roche Diagnostics, Tucson, AZ).14,15 To maintain consistency with previously published literature, AKI was defined as a ≥50% increase from baseline in postoperative serum creatinine level during the first 4 PODs above the preoperative baseline measurement.16
Statistical Analysis
Areas under the curve of receiver operating characteristic (ROC) curves were analyzed to assess the optimal cutoff value for postoperative plasma NGAL levels for predicting AKI. The optimal NGAL cutoff level was defined as the value that provided the highest sensitivity and specificity for predicting AKI. Wilcoxon rank sum test and Fisher exact test were used to compare continuous and categorical variables, respectively. To adjust for clinical risk factors for postoperative AKI, variables with P ≤ 0.20 on univariate analysis were entered into the model using stepwise regression. The final AKI model included body mass index (BMI), history of hypertension or diabetes mellitus, duration of CPB, and perioperative administration of blood product. Demographic variables, including age, gender, race, and institution, were forced into the final models. Normally distributed data are expressed as mean ± SD, and nonnormally distributed data are expressed as median and interquartile range (IQR). Estimates of effect are expressed as odds ratios (ORs) with corresponding 95% confidence limits. P ≤ 0.05 was considered statistically significant. JMP 7.0.2 and SAS 9.1.3 (SAS Institute, Cary, NC) were used for statistical analysis.
RESULTS
Between August 2001 and June 2007, 1141 adult patients were enrolled in the CABG Genomics study. Patients with a history of preoperative renal replacement therapy (n = 3), undergoing off-pump CABG surgery (n = 36), and missing preoperative serum creatinine (n = 7) or plasma NGAL values after CPB (n = 224) were excluded from subsequent analysis. No patient received aprotinin. After exclusions, 879 patients were analyzed for the primary outcome of AKI (Table 1). The 75 patients (8.6%) who developed postoperative AKI were more likely to be female, have a higher BMI, have a lower preoperative hematocrit, have received perioperative blood product transfusion, spend a longer time on CPB, and to have received an intraoperative epinephrine infusion.
Table 1.
Baseline Demographic and Perioperative Characteristics of 879 Patients With and Without Acute Kidney Injury (AKI) After CABG Surgery With CPB
| AKI (n = 75) | No AKI (n = 804) | P | |
|---|---|---|---|
| Demographic data | |||
| Age at surgerya (y) | 65 ± 12 | 65 ± 10 | 1.00 |
| Female gender (%) | 29 | 19 | 0.02 |
| Caucasian (%) | 69 | 70 | 0.84 |
| Institution A (%) | 32 | 28 | 0.57 |
| Body mass indexa (kg/m2) | 31.3 ± 6.6 | 29.4 ± 5.7 | 0.01 |
| Preoperative data | |||
| Current smoker, (%) | 9 | 13 | 0.42 |
| Past smoker (%) | 63 | 69 | 0.24 |
| Diabetes mellitus (type I or II), (%) | 44 | 33 | 0.06 |
| Hypertension (%) | 84 | 76 | 0.15 |
| Hypercholesterolemia (%) | 64 | 75 | 0.05 |
| Stroke (%) | 8 | 5 | 0.52 |
| Left ventricular ejection fractionb (%) | 53 (40–60) | 55 (5–60) | 0.33 |
| Hematocrit (%)a | 38.4 ± 5.2 | 40.0 ± 4.9 | 0.01 |
| Serum creatinineb (mg/dL) | 1.1 (1.0–1.3) | 1.1 (0.9–1.3) | 0.34 |
| Plasma NGALb (ng/mL) | 75.8 (60–110.5) | 69.5 (54.8–94.0) | 0.08 |
| Preoperative medicationsc | |||
| Aspirin (%) | 69 | 75 | 0.26 |
| HMG CoA reductase inhibitors (%) | 69 | 76 | 0.22 |
| β-blockers (%) | 77 | 78 | 0.94 |
| ACE inhibitors (%) | 51 | 48 | 0.70 |
| Calcium channel blockers (%) | 20 | 14 | 0.13 |
| Nonaspirin platelet inhibitors (%) | 19 | 24 | 0.31 |
| Intraoperative data | |||
| Blood product transfusiond (%) | 70 | 59 | 0.08 |
| Cardiopulmonary bypass timeb (min) | 113 (82–138) | 97 (67–121) | <0.01 |
| Cross-clamp timeb (min) | 80 (55–104) | 71 (46–91) | 0.06 |
| Epinephrine infusion (%) | 41 | 26 | 0.02 |
CABG = coronary artery bypass graft; CPB = cardiopulmonary bypass; ACE = angiotensin-converting enzyme; ns = not significant; NGAL = neutrophil gelatinase-associated lipocalin.
Normally distributed continuous data are expressed as mean ± SD.
Nonnormally distributed continuous data are expressed as median (interquartile range).
History of any use at any time before surgery.
Packed red blood cells, fresh frozen plasma, and/or platelets given intraoperatively up until postoperative day 2.
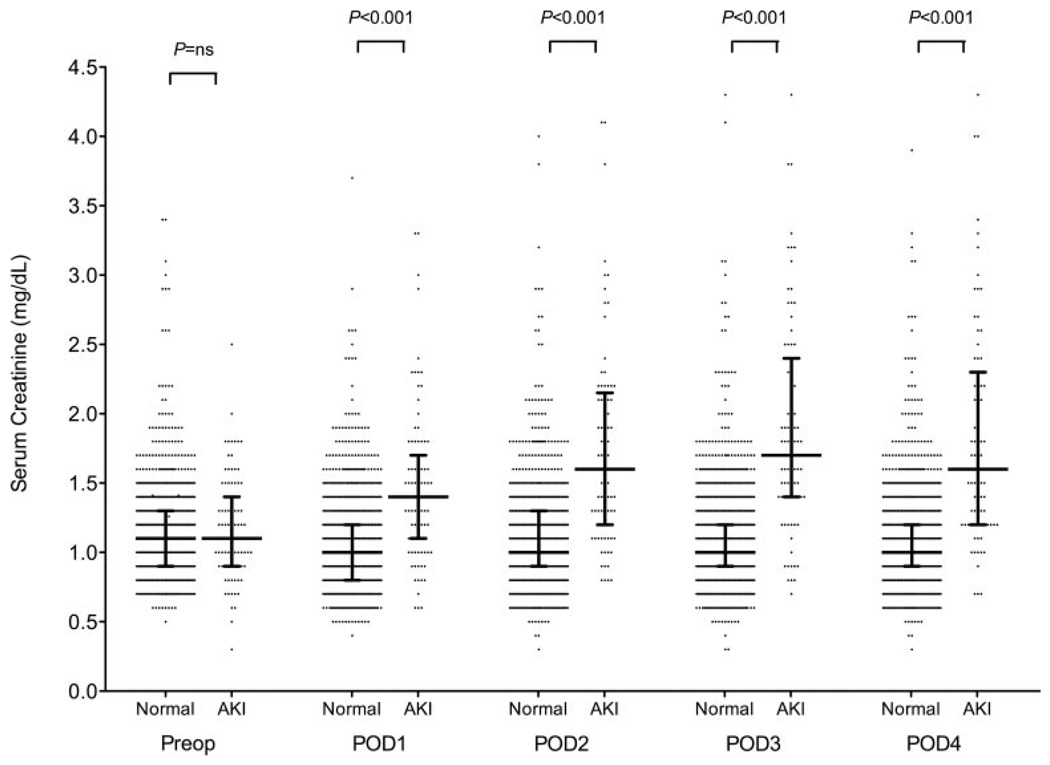
Preoperative serum creatinine levels did not differ between patients who subsequently developed AKI and those who did not (AKI: 1.1 mg/dL [1.0–1.3 mg/dL], median [IQR], vs no AKI: 1.1 mg/dL [0.9–1.3 mg/dL]; P = 0.34) (Fig. 1). Serum creatinine levels were elevated on POD 1 in patients who developed AKI compared with patients who did not develop AKI (AKI: 1.4 mg/dL [1.0–1.7 mg/dL] vs no AKI: 1.0 mg/dL [0.8–1.2 mg/dL]; P < 0.001), remained significantly elevated at all postoperative time points, and peaked on POD 3 in patients who developed AKI.
Figure 1.
Perioperative serum creatinine levels in patients who developed acute postoperative kidney injury and in those who did not after coronary artery bypass graft (CABG) surgery with cardiopulmonary bypass (CPB). Data represented as scatter plot with median and interquartile range and dichotomized by patients who did not develop acute kidney injury (AKI) (Normal) and those who did (AKI). Serum creatinine measured in increments of 0.1 mg/dL. P values represent differences between Normal and AKI columns at each corresponding time point. Preop = preoperative; POD = postoperative day.
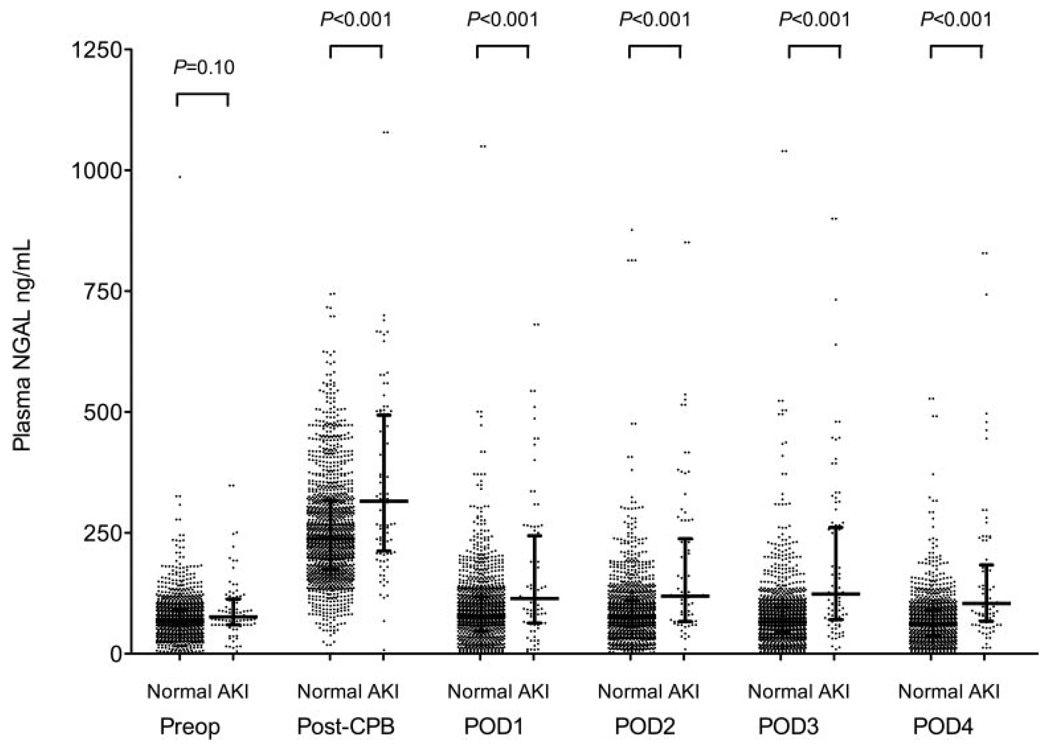
Preoperative plasma NGAL levels were not different in patients who developed AKI compared with patients who did not (AKI: 75.8 ng/mL [60.0–110.5 ng/mL], median [IQR], vs no AKI: 69.5 ng/mL [54.8–94.0 ng/mL]; P = 0.10) (Fig. 2). Plasma NGAL levels were highest immediately after CPB and were higher in patients who developed AKI compared with patients who did not (AKI: 268.8 ng/mL [207.5–459.5 ng/mL] vs no AKI: 238.4 ng/mL [172.0–319.1 ng/mL]; P < 0.001). Plasma NGAL levels decreased toward baseline on POD 1 both in patients who subsequently developed AKI and in those who did not (AKI: 115.0 ng/mL [62.8–232.6 ng/mL] vs no AKI: 83.9 ng/mL [41.9–122.4 ng/mL]; P < 0.001) but remained increased through POD 4 compared with baseline preoperative levels in patients who developed AKI.
Figure 2.
Perioperative plasma neutrophil gelatinase-associated lipocalin levels in patients who developed acute postoperative kidney injury and in those who did not after coronary artery bypass graft (CABG) surgery with cardiopulmonary bypass (CPB). Data represented as scatter plot with median and interquartile range and dichotomized by patients who did not develop acute kidney injury (AKI) (Normal) and those who did (AKI). P values represent differences between Normal and AKI columns at each corresponding time point. Preop = preoperative; post-CPB = immediately after separating from cardiopulmonary bypass; POD = postoperative day.
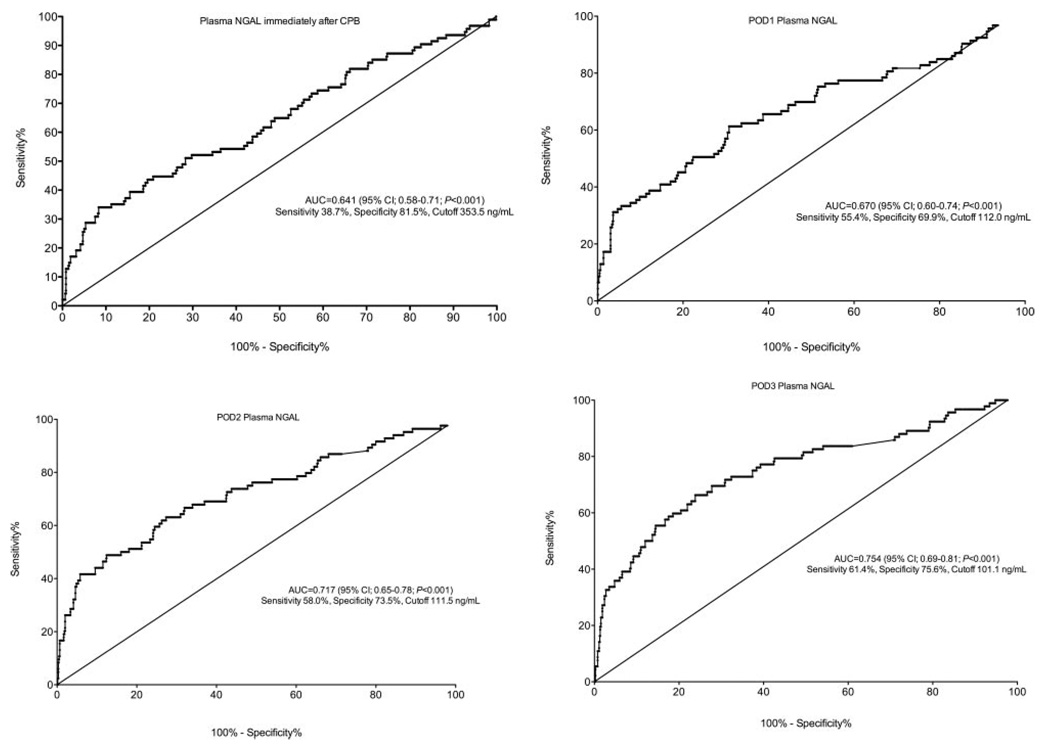
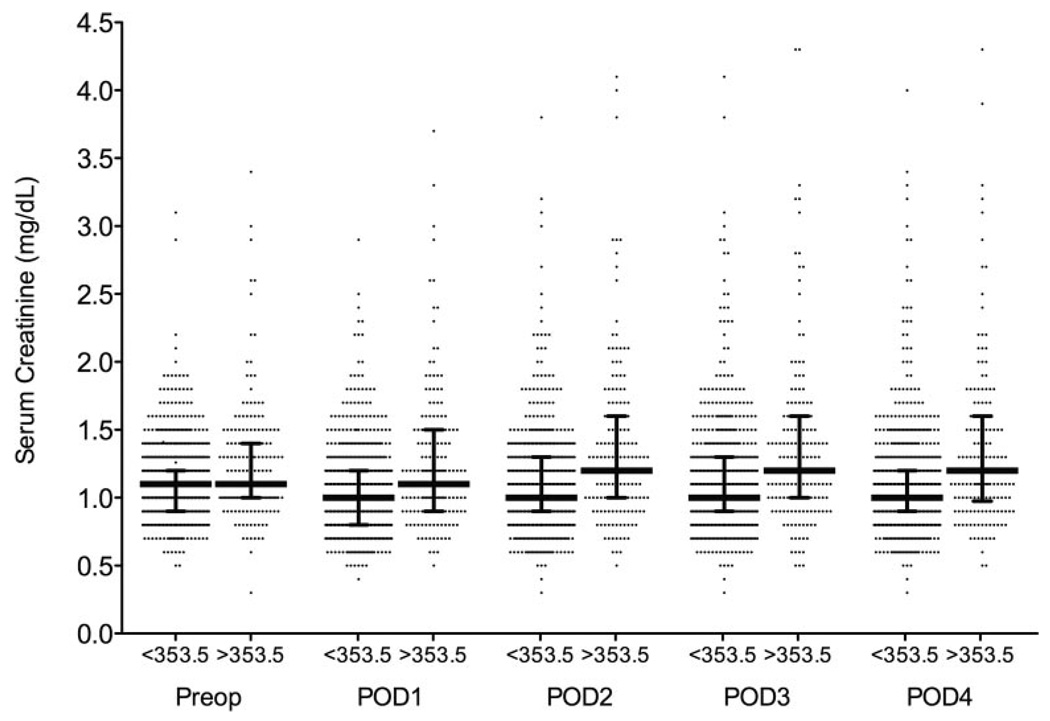
The ROC curves are shown in Figure 3. An optimal serum plasma NGAL cutoff of 353.5 ng/mL measured immediately after CPB was determined by the ROC curve to correspond with a sensitivity of 38.7%, specificity of 81.5%, a positive predictive value of 16.3% for predicting AKI, and a C-index of 0.641. We did not evaluate time points beyond POD 3 because serum creatinine peaks at this time point. The defined optimal cutoff of 353.5 ng/mL measured immediately after CPB was correlated with postoperative AKI (univariate model r2 = 0.026; OR, 2.6; 95% confidence interval [CI], 1.4–4.4; P = 0.0003). After adjusting for clinically relevant risk factors for postoperative AKI using multivariable logistic regression analysis, plasma NGAL levels >353.5 ng/mL measured immediately after CPB remained independently associated with postoperative AKI (multivariable model r2 = 0.067; OR, 2.3; 95% CI, 1.5–6.5; P = 0.002). In this multivariable model, BMI and duration of CPB were also independently associated with AKI (OR, 1.05; 95% CI, 1.00–1.09; P = 0.04 vs OR, 1.01; 95% CI, 1.00–1.01; P = 0.01, respectively). The median serum creatinine levels did not differ significantly at any perioperative time point between patients stratified by plasma NGAL levels (more than or less than 353.5 ng/mL measured immediately after CPB) (Fig. 4).
Figure 3.
Receiver operating characteristic curves for postoperative plasma neutrophil gelatinase-associated lipocalin (NGAL) levels to predict acute postoperative kidney injury after coronary artery bypass graft (CABG) surgery with cardiopulmonary bypass (CPB). POD = postoperative day; AUC = area under the curve; CI = confidence interval.
Figure 4.
Perioperative serum creatinine levels in patients with plasma neutrophil gelatinase-associated lipocalin (NGAL) levels more than or less than the determined optimal cutoff value of 353.5 ng/mL measured immediately after cardiopulmonary bypass (CBP). Data represented as scatter plot with median and interquartile range. POD = postoperative day.
DISCUSSION
A reliable biomarker for predicting AKI early after cardiac surgery might result in earlier therapeutic intervention to limit associated morbidity. In a cohort of relatively healthy surgical patients undergoing primary, nonemergent CABG-only surgery, we found an 8.6% incidence of AKI. After adjusting for clinically relevant perioperative risk factors, plasma NGAL levels measured immediately after CPB were independently associated with postoperative AKI. However, despite this association, the corresponding sensitivity and specificity of plasma NGAL for predicting AKI was 38.7% and 81.5%, respectively, with a positive predictive value of 16.3%. Thus, these data suggest that assessing early plasma NGAL alone has limited utility for predicting AKI after CABG surgery.
Recent findings suggest that urinary NGAL is a sensitive and specific marker of kidney injury in both adult and pediatric patients undergoing cardiac surgery with CPB.11,13,17 In adult cardiac surgical patients, Wagener et al.18 demonstrated that increased urinary NGAL measured 3 hours after surgery was associated with the highest risk for developing postoperative AKI. More recently, Wagener et al.12 confirmed these findings in a larger study of adult cardiac surgical patients by demonstrating that urinary NGAL levels measured immediately after surgery reflect the extent of intraoperative renal damage sooner and more effectively than postoperative serum creatinine levels. However, the authors report an area under the curve of 0.61 at the 18-hour postoperative time point with a corresponding sensitivity of only 39.2%. The authors explain this unanticipated finding as a result of the limitations of currently available definitions for acute postoperative renal injury, namely, postoperative serum creatinine levels.
In this study, we hypothesized that plasma NGAL measured immediately after separating from CPB could serve as an effective marker of postcardiac surgical renal injury. However, despite a significant association between post-CPB plasma NGAL levels and postoperative AKI, the corresponding sensitivity, specificity, and positive predictive values of plasma NGAL for predicting AKI was low. Alternatively, the model performance for the univariate association between early postoperative plasma NGAL level and AKI was low, meaning that plasma NGAL explains only a small fraction of the variables associated with postoperative AKI. As clinically relevant risk factors for postoperative AKI were added to the multivariable model, we observed an improvement in overall model performance to predict postoperative AKI. Therefore, although increased plasma NGAL levels are clearly associated with postoperative AKI, the remaining variability may explain the poor overall predictive value of plasma NGAL in this cohort.
Although the strengths of this study are its sample size and power to detect an association between plasma NGAL and postoperative AKI in a low-risk population of adult cardiac surgical patients with a relatively low incidence of AKI, there are limitations that warrant consideration. First, our primary outcome of AKI defined as a ≥50% increase in postoperative serum creatinine from preoperative baseline levels represents only one of a few recently defined classes of AKI. In recognizing the potential implications of relatively small changes in serum creatinine levels on clinical outcomes, the Acute Dialysis Quality Initiative group proposed a classification scheme for AKI termed RIFLE (Risk of renal dysfunction, Injury to the kidney, Failure of kidney function, Loss of kidney function, and End-stage kidney disease).16 More recently, the Acute Kidney Injury Network proposed a modification to the RIFLE classification, whereby only 3 classes of kidney injury are recognized.19 Despite these recommendations, definitions of AKI after cardiac surgery continue to vary.6,7,20,21 In the interest of consistency and reproducibility, we chose to define AKI as ≥50% increase in postoperative serum creatinine from preoperative baseline levels, similar to recently published reports describing the value of urinary NGAL as a predictor of AKI in both pediatric and adult cardiac surgical cohorts.11,13,17,18 Although some authors have stipulated that an increase in postoperative serum creatinine levels must occur within 48 hours of surgery to ensure that AKI is related to the inciting event,19 these consensus criteria were not specific for cardiac surgical patients, because increase in serum creatinine can occur as late as 72 hours after cardiac surgery.22 Thus, we accepted an increase in serum creatinine that occurred within 96 hours after surgery in defining our outcome. Second, although our data identify a distinct peak for plasma NGAL that occurs immediately after CPB, our study design precludes us from identifying the true peak, because we did not sample additional time points between the post-CPB time point and POD 1. In studies that examined the value of urinary NGAL as a marker of AKI, more frequent measurements revealed that urinary NGAL does not peak until 3 to 4 hours after surgery. Accordingly, we may be underestimating the peak, and as such, the predictive value of plasma NGAL as a marker of AKI. Third, emerging evidence suggests that NGAL is expressed by injured renal tubules after ischemic insult.11 Therefore, an immediate postoperative increase in urinary NGAL levels after cardiac surgery may be a more sensitive marker of ongoing renal injury, whereas the immediate increase in plasma NGAL levels may reflect ongoing injury in an array of tissue beds.10,23–25 A prospectively designed study to examine both perioperative serum and urine NGAL levels at more frequent time points (possibly every 2 hours for the first 18–20 hours) after cardiac surgery might not only address the predictive value of each biomarker but also better define an optimal time for measuring postoperative NGAL. Finally, our findings may be subject to selection bias of low-risk patients. Thus, there may be greater value for plasma NGAL as a marker of AKI in a subset of more critically ill patients undergoing cardiac surgery.
CONCLUSION
Increased plasma NGAL levels measured immediately after CPB are associated with AKI after CABG surgery. However, the low predictive value of early plasma NGAL level limits its utility for diagnosing postcardiac surgical renal damage. A prospective study design with more frequent sampling around the time point immediately after CPB may better define a plasma NGAL level with higher predictive value for AKI after adult cardiac surgery.
ACKNOWLEDGMENTS
The authors acknowledge the outstanding contributory efforts of James Gosnell, RN, Kujtim Bodinaku, MD, Jai Madan, MD, MPH, and Svetlana Gorbatov, MPH, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, and Juliette Dean, RN, James Chen, RN, Jacques Estephan, RN, and Isabella Candelaria, BS, Baylor College of Medicine Division of Cardiovascular Anesthesia, Texas Heart Institute, St. Luke’s Episcopal Hospital, Houston, TX.
STUDY FUNDING
Supported by the Scholars in Clinical Science Program, National Institutes of Health (NCRR) K30 grant RR022292-07 (to TEP and JDM); Faculty Scholar in Translational Anesthesia Research (STAR) Award, Department of Anesthesiology, Perioperative and Pain Medicine, BWH (to TEP); and National Institutes of Health K23 HL68774-01A1 (to SCB).
Footnotes
DISCLOSURE
Dr. Body received in-kind research support from Biosite Incorporated, San Diego, CA.
REFERENCES
- 1.Mangano CM, Diamondstone LS, Ramsay JG, Aggarwal A, Herskowitz A, Mangano DT. Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Ann Intern Med. 1998;128:194–203. doi: 10.7326/0003-4819-128-3-199802010-00005. [DOI] [PubMed] [Google Scholar]
- 2.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 3.Lassnigg A, Schmid ER, Hiesmayr M, Falk C, Druml W, Bauer P, Schmidlin D. Impact of minimal increases in serum creatinine on outcome in patients after cardiothoracic surgery: do we have to revise current definitions of acute renal failure? Crit Care Med. 2008;36:1129–1137. doi: 10.1097/CCM.0b013e318169181a. [DOI] [PubMed] [Google Scholar]
- 4.Bove T, Calabro MG, Landoni G, Aletti G, Marino G, Crescenzi G, Rosica C, Zangrillo A. The incidence and risk of acute renal failure after cardiac surgery. J Cardiothorac Vasc Anesth. 2004;18:442–445. doi: 10.1053/j.jvca.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 5.Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;104:343–348. doi: 10.1016/s0002-9343(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 6.Mehta RH, Grab JD, O’Brien SM, Bridges CR, Gammie JS, Haan CK, Ferguson TB, Peterson ED. Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation. 2006;114:2208–2216. doi: 10.1161/CIRCULATIONAHA.106.635573. [DOI] [PubMed] [Google Scholar]
- 7.Thakar CV, Worley S, Arrigain S, Yared JP, Paganini EP. Influence of renal dysfunction on mortality after cardiac surgery: modifying effect of preoperative renal function. Kidney Int. 2005;67:1112–1119. doi: 10.1111/j.1523-1755.2005.00177.x. [DOI] [PubMed] [Google Scholar]
- 8.Boldt J, Wolf M. Identification of renal injury in cardiac surgery: the role of kidney-specific proteins. J Cardiothorac Vasc Anesth. 2008;22:122–132. doi: 10.1053/j.jvca.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006;1:19–32. doi: 10.2215/CJN.00240605. [DOI] [PubMed] [Google Scholar]
- 10.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 11.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 12.Wagener G, Gubitosa G, Wang S, Borregaard N, Kim M, Lee HT. Urinary neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery. Am J Kidney Dis. 2008;52:425–433. doi: 10.1053/j.ajkd.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 13.Dent CL, Ma Q, Dastrala S, Bennett M, Mitsnefes MM, Barasch J, Devarajan P. Plasma neutrophil gelatinase-associated lipocalin predicts acute kidney injury, morbidity and mortality after pediatric cardiac surgery: a prospective uncontrolled cohort study. Crit Care. 2007;11:R127. doi: 10.1186/cc6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartels H, Böhmer M. Micro-determination of creatinine [in German] Clin Chim Acta. 1971;32:81–85. doi: 10.1016/0009-8981(71)90467-0. [DOI] [PubMed] [Google Scholar]
- 15.Fabiny DL, Ertingshausen G. Automated reaction-rate method for determination of serum creatinine with the CentrifiChem. Clin Chem. 1971;17:696–700. [PubMed] [Google Scholar]
- 16.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, Syed H, Ali S, Barasch J, Devarajan P. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008;3:665–673. doi: 10.2215/CJN.04010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagener G, Jan M, Kim M, Mori K, Barasch JM, Sladen RN, Lee HT. Association between increases in urinary neutrophil gelatinase-associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology. 2006;105:485–491. doi: 10.1097/00000542-200609000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown JR, Cochran RP, Dacey LJ, Ross CS, Kunzelman KS, Dunton RF, Braxton JH, Charlesworth DC, Clough RA, Helm RE, Leavitt BJ, Mackenzie TA, O’Connor GT. Perioperative increases in serum creatinine are predictive of increased 90-day mortality after coronary artery bypass graft surgery. Circulation. 2006;114:I409–I413. doi: 10.1161/CIRCULATIONAHA.105.000596. [DOI] [PubMed] [Google Scholar]
- 21.Loef BG, Epema AH, Smilde TD, Henning RH, Ebels T, Navis G, Stegeman CA. Immediate postoperative renal function deterioration in cardiac surgical patients predicts in-hospital mortality and long-term survival. J Am Soc Nephrol. 2005;16:195–200. doi: 10.1681/ASN.2003100875. [DOI] [PubMed] [Google Scholar]
- 22.Ryckwaert F, Boccara G, Frappier JM, Colson PH. Incidence, risk factors, and prognosis of a moderate increase in plasma creatinine early after cardiac surgery. Crit Care Med. 2002;30:1495–1498. doi: 10.1097/00003246-200207000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Cowland JB, Borregaard N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase-associated lipocalin from humans. Genomics. 1997;45:17–23. doi: 10.1006/geno.1997.4896. [DOI] [PubMed] [Google Scholar]
- 24.Kjeldsen L, Johnsen AH, Sengelov H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268:10425–10432. [PubMed] [Google Scholar]
- 25.Bundgaard JR, Sengelov H, Borregaard N, Kjeldsen L. Molecular cloning and expression of a cDNA encoding NGAL: a lipocalin expressed in human neutrophils. Biochem Biophys Res Commun. 1994;202:1468–1475. doi: 10.1006/bbrc.1994.2096. [DOI] [PubMed] [Google Scholar]