Summary
Purpose
Calorie restriction can be anticonvulsant in animal models. The ketogenic diet was designed to mimic calorie restriction and has been assumed to work by the same mechanisms. We challenged this assumption by profiling the effects of these dietary regimens in mice subjected to a battery of acute seizure tests.
Methods
Juvenile male NIH Swiss mice received ketogenic diet or a normal diet fed in restricted quantities (continuously or intermittently) for ~ 12 days, starting at 3–4 weeks of age. Seizures were induced by the 6 Hz test, kainic acid, maximal electroshock, or pentylenetetrazol.
Results
The ketogenic and calorie-restricted diets often had opposite effects depending on the seizure test. The ketogenic diet protected from 6 Hz–induced seizures, whereas calorie restriction (daily and intermittent) increased seizure activity. Conversely, calorie restriction protected juvenile mice against seizures induced by kainic acid, whereas the ketogenic diet failed to protect. Intermittent caloric restriction worsened seizures induced by maximal electroshock but had no effect on those induced by pentylenetetrazol.
Discussion
In contrast to a longstanding hypothesis, calorie restriction and the ketogenic diet differ in their acute seizure test profiles, suggesting that they have different underlying anticonvulsant mechanisms. These findings highlight the importance of the 6 Hz test and its ability to reflect the benefits of ketosis and fat consumption.
Keywords: Intermittent fasting, Ketogenic diet, 6 Hz Test, Kainic acid
For the one-third of epilepsy patients who fail to achieve adequate seizure control with the two leading medications (Kwan & Brodie, 2000), treatment options include surgery for a resectable focus or dietary therapy (Guerrini, 2006; Freeman et al., 2007). The ketogenic diet, a high-fat diet that also restricts carbohydrate calories, was designed to mimic the beneficial effects of fasting on seizure control (Freeman et al., 2007).
Several successful rodent models have been developed to pursue the underlying mechanisms of seizure protection through metabolic interventions. Restricted daily calorie consumption is anticonvulsant in juvenile rats (Bough et al., 2003) and in epilepsy-like (EL/suz) mice, a model of partial-onset epilepsy with genetic and environmental antecedents (Greene et al., 2001). Calorie restriction (CR) achieved by intermittent fasting (fasting and feeding ad lib on alternate days) provides neuroprotection against intrahippocampal or systemic kainic acid injection in adult rats (Bruce-Keller et al., 1999; Contestabile et al., 2004; Youssef et al., 2008). CR and the ketogenic diet are generally assumed to share anticonvulsant mechanisms (Maalouf et al., 2009). However, juvenile mice on a ketogenic diet (6:1, ratio of fat-to-protein and carbohydrate) also weigh less, even without restricting intake (Samala et al., 2008), raising the possibility that CR is an important component of the ketogenic diet. In fact, a 4:1 ratio ketogenic diet was reported to prevent pentylenetetrazol (PTZ)–induced seizures in juvenile rats (Bough et al., 1999) and in the EL mouse, only when animals were restricted to 20–25% of their normal consumption (Mantis et al., 2004). Therefore, the relative contributions of high ketone levels versus CR are not clear (Bough & Rho, 2007).
If the ketogenic diet protects against seizures by the same underlying mechanism as CR, then these two metabolic interventions should have similar effects in multiple seizure tests. To confirm or dispel this hypothesis, we tested juvenile mice, corresponding to the patient age group for which dietary therapy is most frequently used for seizures. We found consistent differences, and even opposite outcomes with these dietary regimens, implying important differences in the underlying mechanisms of action between the ketogenic diet and CR by daily or intermittent fasting.
Methods
Animals and diet regimens
Male NIH Swiss mice (NCI, Frederick, MD, U.S.A.) aged 3–4 weeks were acclimatized to the animal care facility for at least 1 day, fasted overnight (14–18 h), housed three to four per cage, and then randomly divided into two to four different dietary schemes, including: (1) controls fed unrestricted normal rodent chow (Teklad Global 2018SX, Madison, WI, U.S.A.), (2) CR by intermittent fasting (CR-IF, 24 h fasting, 24 h normal chow unrestricted), (3) daily CR [CR-D, implemented by offering daily ~50% (by weight) of the normal rodent chow intake of mice in the intermittent fasting group, and further adjusted to match body weight of intermittent fasting mice (after a feeding day], and (4) unrestricted 6.5:1 ratio of fat-to-protein ketogenic diet (Research Diets D07091701, Brunswick, NJ, U.S.A.) that provides 93% of calories as fat, 7% of calories as protein, and only trace carbohydrate (Table S1). All animal protocols are approved by the Animal Care and Use Committee.
Seizure tests
Seizure tests similar to those used in the NINDS Anticonvulsant Screening Project were administered, including the 6 Hz test, intravenous PTZ, and maximal electroshock (MES) (Barton et al., 2001), as well as intraperitoneal and intravenous kainic acid. Intermittently fasted mice were tested after 24 h of feeding. Each mouse was seizure tested only once.
6 Hz Test
The 6 Hz test was administered as described previously (Brown et al., 1953; Hartman et al., 2008). Briefly, corneal stimulation (ECT Unit #57800, Ugo Basile North America, Collegeville, PA, U.S.A.) was performed at frequency 6 Hz, pulse width 0.2 ms, and shock duration 3 s. Seizures were defined as any typical activity (regardless of duration), including clonus, followed by immobility, facial muscle twitching, staring, automatisms including chewing and unilateral pawing, and a Straub tail.
Intraperitoneal kainic acid (ipKA)
Kainic acid (5.3 mg/ml PBS; Tocris Bioscience, Ellisville, MO, U.S.A.) was injected intraperitoneally at a dose of 22.5 mg kainic acid/kg mouse body weight. Seizure behaviors were scored for 2 h using a modified Racine scale (the highest score in a given 5-min block was used), as described previously (Morrison et al., 1996): 0, no seizure; 1, immobility; 2, forelimb and/or tail extension; 3, automatisms; 4, forelimb clonus, rearing, and/or falling; 5, repetition of stage 4; 6, tonic–clonic seizures; and 7, death.
Intravenous PTZ (ivPTZ) and kainic acid (ivKA)
Using a Mouse Tail Illuminator (Braintree Scientific, Braintree, MA, U.S.A.), a 30-gauge catheter with polyethylene tubing (Instech Solomon, Plymouth Meeting, PA, U.S.A.) was inserted into the tail vein and secured with tape. PTZ [10 mg/ml phosphate-buffered saline (PBS); Tocris Bioscience] was infused constantly at a rate of 0.05 ml/min using an infusion pump (KD Scientific, Holliston, MA, U.S.A.). Time to seizure behaviors was used to calculate the threshold dose for each endpoint: first twitch (generalized body jerk and/or sudden tail flick), clonus (sustained rhythmical body jerking and loss of the righting reflex, sometimes followed by periods of immobility), and tonus (tonic hindlimb extension). Kainic acid (5.3 mg/ml PBS; Sigma Aldrich, St. Louis, MO, U.S.A.) was infused constantly at a rate of 0.125 ml/min, and mice were observed for behavior arrest, clonus, or tonus.
Maximal electroshock (MES) threshold
Mice were pretreated with tetracaine 0.5% ophthalmic solution (Bausch & Lomb, Tampa, FL, U.S.A.) before current was delivered from a Rodent Shocker 221 (Harvard Apparatus, Holliston, MA, U.S.A.). Shock duration was 0.2 s; current settings were adjusted in a staircase-like manner based on responses noted in serial testing, similar to the 6 Hz test. Scoring was based on the presence or absence of tonic hindlimb extension (i.e., extension of the hindlimbs to 180 degrees in the rostral-caudal plane).
Biochemical studies
Glucose and ketone testing
Twenty-five percent of mice in each dietary regimen were chosen randomly 1 h before seizure testing, exposed for <10 s to isoflurane (Abbott Laboratories, North Chicago, IL, U.S.A.), and blood from tail clippings was used to measure glucose and β-hydroxybutyrate (Precision Xtra system; Abbott Laboratories). This testing appears to have no effect on seizure scores.
Other hematology and biochemical studies
Littermates that did not undergo seizure testing were sacrificed after undergoing isoflurane anesthesia followed by decapitation. Trunk blood obtained for a complete blood count and comprehensive metabolic panel was measured. These studies were performed at the same times and conditions as seizure tests.
Statistics
Comparisons between two groups were made using Student’s t-test, whereas comparisons among more than two groups were made using an analysis of variance (ANOVA) with post hoc Tukey multiple comparison tests for normally distributed data, or a Kruskal-Wallis and Dunn’s multiple comparison tests for data that were not normally distributed. Seizure thresholds in the 6 Hz and MES threshold tests were calculated with a probit analysis using Minitab 15 (Minitab Inc., State College, PA, U.S.A.). Data were expressed as mean ± standard error of the mean (SEM). Differences between groups (all two-tailed) were considered statistically significant when the probability of error was <0.05 (p < 0.05) in seizure tests. In the complete blood count and biochemical tests, a Bonferroni correction for multiple comparisons was applied, yielding a level of significance for the complete blood count of p < 0.008 and for blood chemistries of p < 0.002.
Results
Intermittent fasting is detrimental in the 6 Hz seizure test
Consistent with previous studies (Hartman et al., 2008; Samala et al., 2008), juvenile mice on the ketogenic diet for 11–13 days were significantly protected from 6 Hz–induced seizures compared to controls on normal rodent chow (Fig. 1A,B). To determine if the ketogenic diet mimics the effects of CR in 6 Hz–induced seizures, seizure thresholds were also determined for two different calorie-restricted regimes. Juvenile mice were fed normal rodent chow intermittently (alternate days) or daily in restricted amounts. In sharp contrast to the ketogenic diet, CR by either regimen significantly increased susceptibility to 6 Hz–induced seizures (Fig. 1A,B). These analyses were performed on the maximum number of animals with matching body weights during the time course (Fig. 1C) and on the day of testing (Fig. 1D) for the three experimental diets. Therefore, the disparate effects of the ketogenic and calorie-restricted diets cannot be explained by differences in body weight, although all animals on these diets weighed less than those on the normal (unrestricted) diet. Furthermore, when all animals tested were analyzed for seizure outcomes regardless of weight, the results remained unchanged (Fig. S1; for complete datasets, see Table S2). To further test for any influences from body weight, animals with lower weights (below the mean weight) for each diet group were compared to heavier mice (above mean weight) for the same diet regimen. Seizure phenotypes were strikingly similar for both the upper and lower weight groups within each diet type (Fig. S1). Therefore, CR alone cannot explain the beneficial effects of the ketogenic diet in juvenile mice.
Figure 1.
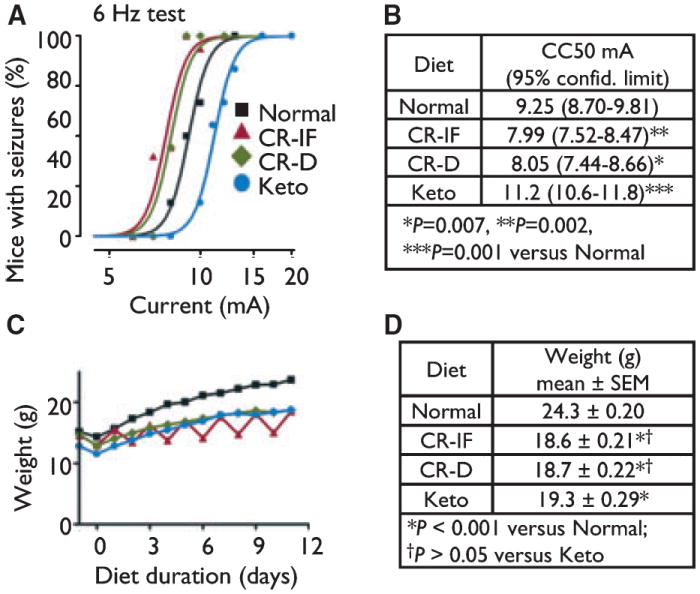
Calorie restriction (intermittent and daily) and the ketogenic diet have opposite effects on 6 Hz–induced seizures. (A) Probability of seizure events at the indicated currents was determined by a probit analysis. Results presented are for weight-matched mice fed normal rodent chow without restriction (Normal, n = 43 mice), mice fasted (24 h) and fed unrestricted normal rodent chow (24 h) on alternate days (CR-IF, n = 61), mice fed restricted amounts of normal rodent chow daily (CR-D, n = 42), and mice fed the ketogenic diet (Keto, n = 37), tested in three to four independent animal cohorts in three to four independent experiments. Because the curves shown represent a probability function, not all points lie on the curve. (B) The current where 50% of mice had convulsions (CC50) was derived from data in panel A (larger animal numbers were tested near the CC50 to increase sensitivity of the assay). (C) Weights of animals shown in panel A through day 11 (before animals were removed from each set for testing on days 11–13). (D) Body weights on the day of seizure testing for all mice in panel A, mean ± SEM; analyzed by one-way ANOVA with Tukey’s multiple comparison test.
Intermittent fasting for 2 weeks protects against kainic acid–induced seizures
In contrast to these findings with the 6 Hz test, others reported that intermittent fasting protects adult rats from kainic acid–induced neurodegeneration (Bruce-Keller et al., 1999; Youssef et al., 2008). Therefore, we compared intermittent fasting and the ketogenic diet by observing seizure behaviors following intraperitoneal injection of kainic acid (ipKA, Fig. 2A). In juvenile mice, intermittent fasting significantly protected against seizures induced by ipKA, compared to an unrestricted normal diet and the ketogenic diet (Fig. 2A). This protective effect of intermittent fasting was also reflected by reduced time spent at seizure scores of 2 (forelimb and/or tail extension) through 7 (death) (Fig. 2B), a longer latency to onset of seizure of score 2 compared to other diet groups (Fig. 2C), with minimal effect on the maximum seizure score (Fig. 2D). In contrast, the ketogenic diet was associated with an increased number of epochs spent in seizure stages 2 through 7, although other parameters were not significantly affected (Fig. 2B). Therefore, intermittent fasting, but not the ketogenic diet, protects against ipKA-induced seizures in juvenile mice. Only weight-matched animals were included in these analyses, eliminating any effects of body weight on seizure outcome (Fig. 2E,F), and the results were the same when all animals tested were analyzed (Fig. S2). To determine if failure by the ketogenic diet was specific to the intraperitoneal injection route, we also tested intravenously administered kainic acid. However, no differences were noted between mice fed the normal versus ketogenic diet in any of the seizure behaviors measured (behavior arrest, clonus, or tonus), when animals were weight matched or not (Figs S3 and S4). Therefore, the ketogenic diet failed to protect against kainic acid–induced seizures regardless of delivery route. Furthermore, the ketogenic diet and intermittent fasting have nearly opposite effects on seizure outcomes depending on whether seizure activity was induced by 6 Hz or kainic acid.
Figure 2.
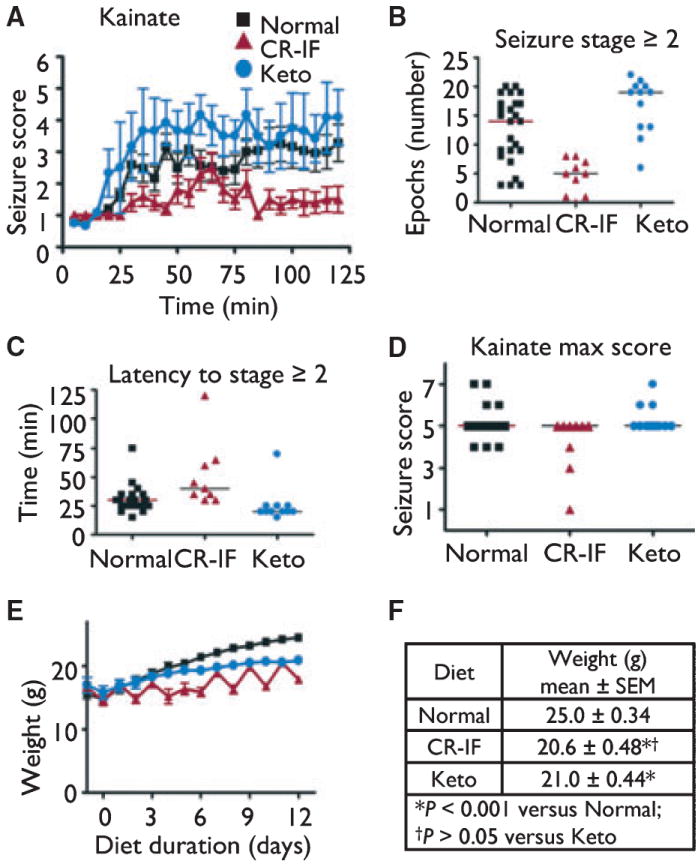
Intermittent fasting (CR-IF), but not the ketogenic diet, protects against seizures induced by kainic acid injected intraperitoneally (i.p.). (A) Mean seizure scores (±SEM) were assessed in 5-min blocks for two groups of weight-matched mice tested independently. Mice were fed as described for Fig. 1; (Normal, n = 25 mice; CR-IF, n = 9; Keto, n = 12). (B) Number of 5-min blocks spent in seizure stage ≥2 for animals in panel A (Normal vs. CR-IF & CR-IF vs. Keto, p < 0.001; Normal vs. Keto, p > 0.05; analyzed by one-way ANOVA with Tukey’s multiple comparison test). Bar represents the group median. (C) Latency to seizure score ≥2 (bar represents group median) for animals in panel A (Normal vs. CR-IF & Normal vs. Keto, p < 0.05; CR-IF vs. Keto, p < 0.001; analyzed by Kruskal-Wallis test with Dunn’s multiple comparison test). Bar represents the group median. (D) Maximum seizure scores for mice in panel A (CR-IF vs. Keto, p < 0.05; Normal vs. CR-IF & Normal vs. Keto, p > 0.05; analyzed by Kruskal–Wallis test with Dunn’s multiple comparison test). Bar represents the group median. (E) Weight of animals shown in panel A (until the first animals were removed for seizure testing). (F) Body weights on the day of seizure testing for mice in panel A, mean ± SEM; analyzed by one-way ANOVA with Tukey’s multiple comparison test.
Intermittent fasting is detrimental in the MES threshold test
In the maximal electroshock (MES) test, a sine wave pulse is delivered, rather than the square wave pulse used in the 6 Hz test. By testing a range of currents, we found that the MES threshold for intermittent fasting was lower than for controls (Fig. 3A,B). Therefore, intermittent fasting had similar detrimental effects in both the MES and the 6 Hz test. Variations of the MES test have shown inconsistent results with the ketogenic diet (Millichap et al., 1964; Uhlemann & Neims, 1972; Martillotti et al., 2006), but we detected no significant effects of the ketogenic diet in MES. These results cannot be explained by any differences in body weight (Fig. 3C,D), and similar results were observed even when animal were not matched for weights (Fig. S5; for complete datasets, see Table S3).
Figure 3.
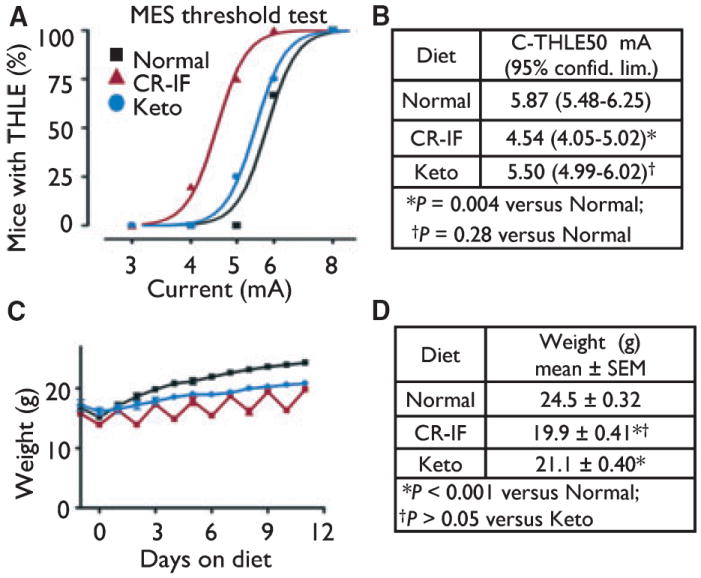
Intermittent fasting is detrimental in the MES test. (A) Probability of seizure events at the indicated currents was determined for weight-matched mice by a probit analysis. Results presented are for three independent animal cohorts in three independent experiments (Normal, n = 33 mice; CR-IF, n = 14; Keto, n = 20). Because the curves shown represent a probability function, not all points lie on the curve. (B) The current where 50% of mice had tonic hindlimb extension (C-THLE50) was derived from data in panel A. (C) Weights of animals shown in panel A (up until the first animals were removed for testing). (D) Body weights on the day of seizure testing for mice in panel A, mean ± SEM; analyzed by one-way ANOVA with Tukey’s multiple comparison test.
Intermittent fasting does not protect against ivPTZ-induced seizures
CR by intermittent fasting had no effect on any seizure behavior in the ivPTZ test (Fig. 4A–C). These results were determined for animals with weights similar to those tested with the other seizures tests (Fig. 4D). Our results have a power of 0.91 to detect a difference in latency to clonus between unrestricted normal diet and intermittently fasted mice, indicating that we are unlikely to have missed a phenotype. Two other groups have reported that the ketogenic diet also fails to protect against seizures induced by PTZ in juvenile mice (Uhlemann & Neims, 1972; Samala et al., 2008) and, therefore, was not tested here. Thus the PTZ test appears to be insensitive to both the ketogenic and calorie-restricted dietary therapies in mice.
Figure 4.
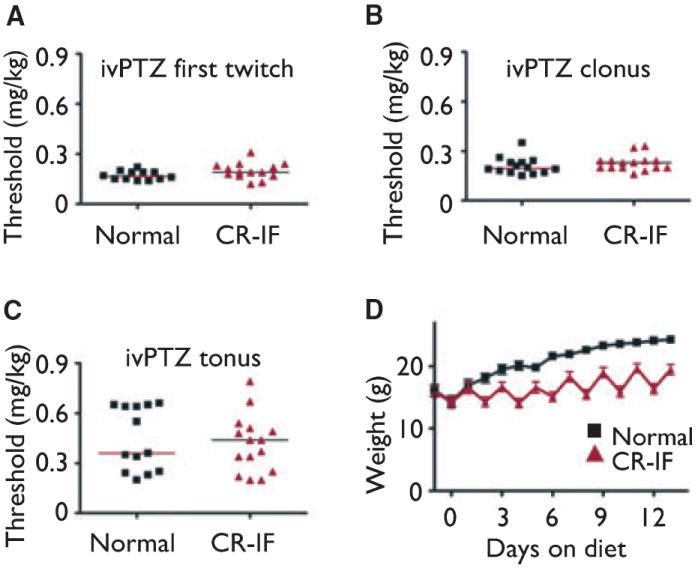
Dietary interventions lack a PTZ seizure test phenotype. (A) Threshold dose for first twitch (A), clonus (B), and tonus (C) (p > 0.05 for each). Bars show the median for each group. Results presented are for mice fed as described in Fig. 1 (Normal, n = 12 mice; CR-IF, n = 14), tested in three independent experiments. (D) Weights of animals shown in panel A (up until the first animals were removed for testing). Body weights on the day of seizure testing for mice in panel A–C: Normal, 23.9 ± 0.49; CR-IF, 19.5 ± 0.86; p = 0.001 (Student’s t-test).
Glucose and ketone levels are not related to seizure protection in the 6 Hz or kainic acid tests
Glucose levels were significantly lower (Fig. 5A) and levels of the ketone β-hydroxybutyrate were significantly higher (Fig. 5B) in mice fed the ketogenic diet compared to others, confirming the intended effects of this diet. Calorie-restricted mice also had lower glucose levels that were intermediate between controls and ketogenic animals, but there was no statistically significant difference between the two different CR regimens. However, because lower blood glucose levels were noted in all experimental dietary groups that had both elevated (e.g., ketogenic diet) and decreased (e.g., intermittent fasting and daily CR) seizure thresholds in the 6 Hz test and nearly opposite results in the kainic acid test, it seems unlikely that blood glucose levels are the major contributor to seizure protection. Levels of the ketone β-hydroxybutyrate in the two CR models were indistinguishable from those of controls (Fig. 5B). Given the seizure protection afforded by the ketogenic diet in the 6 Hz test, there may be an influence of ketones on 6 Hz seizure threshold. However, elevated ketones do not appear to play a protective role in kainic acid tests. Calorie intake by mice fed the ketogenic diet is greater than that for other dietary regimens (Fig. 5C). Therefore, the amount or ratio of fat to carbohydrate could be important in predicting seizure protection in these tests.
Figure 5.
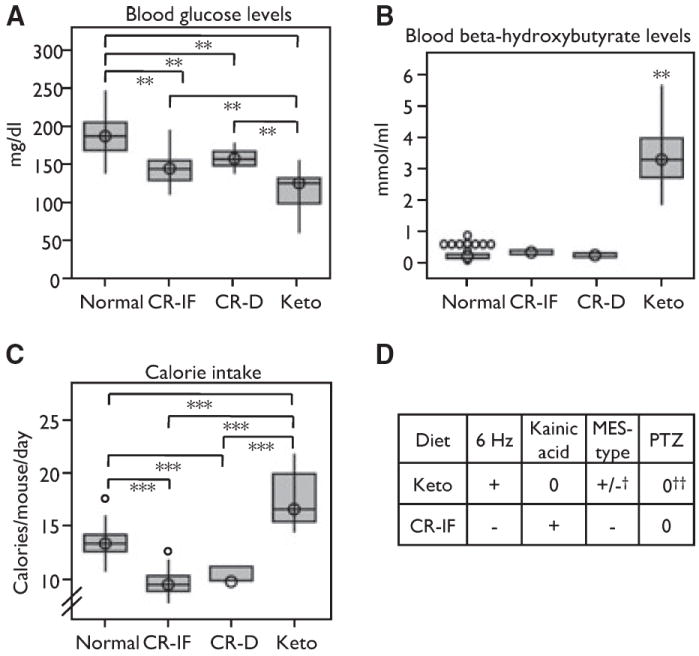
Low blood glucose does not correlate with seizure protection. (A) Boxplots showing blood glucose levels in all four diet groups (**p < 0.01). Results presented are for mice fed as described in Fig. 1 (Normal, n = 45 mice; CR-IF, n = 39; CR-D, n = 11; Keto, n = 25). (B) Boxplots showing blood β-hydrox-ybutyrate levels, ***p < 0.001 for all comparisons versus Keto (Normal, n = 44 mice; CR-IF, n = 39; CR-D, n = 11; Keto, n = 25). (C) Boxplot showing calories consumed per mouse based on food weight per each cage, uncorrected for weight (***p < 0.001) (Normal, n = 39 cages; CR-IF, n = 41 cages; CR-D, n = 11 cages; Keto, n = 28 cages). (D) Chart comparing seizure tests among different dietary regimens from this study, except as noted. [+, protective compared to Normal; −, more severe seizure phenotypes compared to Normal; 0, no effect; +/−, mixed results; †(Millichap et al., 1964; Uhlemann & Neims, 1972; Martillotti et al., 2006); ‡(Uhlemann & Neims, 1972; Samala et al., 2008)].
Intermittent fasting is not associated with major changes in complete blood count or biochemical tests
One potential explanation for decreased 6 Hz and MES seizure threshold in intermittently fasted mice is an adverse effect of the dietary intervention on nutritional status or electrolyte levels. Normal rodent chow and the ketogenic diets have different levels of specific vitamins and minerals (Table S1). However, compared to the recommended daily requirements (Reeves et al., 1993) and food intake per mouse for each feeding regimen, the diets tested in this study had no deficiencies, with one exception. The ketogenic diet contains one-third the amount of protein as normal rodent chow. Protein reduction is required to achieve the high fat levels required for ketosis. However, intermittently fasted mice were similar in size to those on the ketogenic diet and had normal serum total protein levels. In addition, no differences were detected in complete blood cell counts or in a comprehensive metabolic panel, except for a higher blood urea nitrogen and albumin levels in intermittently fasted mice (Tables S4 and S5). Therefore, major biochemical differences do not appear to contribute to differences in seizure tests. However, we cannot eliminate the possibility that relevant nutrition-related differences were not detected in this battery of assays.
Discussion
The ketogenic diet, which limits carbohydrate intake but provides calories via increased fat intake, was originally designed to mimic the beneficial effects of fasting on seizure control (Freeman et al., 2007). However, we show here that CR (intermittent or daily) and the ketogenic diet, administered to growing juvenile mice for 1.5–2 weeks, have opposite outcomes in the 6 Hz test, where the ketogenic diet protects and the calorie-restricted regimens are detrimental, despite similar body weights. Conversely, intermittent fasting protects in the kainic acid tests, whereas the ketogenic diet had no effect or modestly exacerbated seizure severity. Results presented herein and elsewhere indicate that dietary interventions of any type appear to have no effect on PTZ-induced seizures. Taken together, the anticonvulsant mechanisms that underlie CR and the ketogenic diet appear to be distinct in juvenile NIH Swiss mice (Fig. 5D). Therefore, these results challenge the assumption that the ketogenic diet protects in epilepsy and other illnesses by the same mechanisms as CR (Mantis et al., 2004; Yamada, 2008; Maalouf et al., 2009). Our findings are consistent with another study that found differences between CR and the ketogenic diet in the in vitro maximal dentate activation test, an electro-physiologic paradigm that measures seizure threshold (Bough et al., 2003).
Novel mechanisms predicted by the 6 Hz test
The two mouse seizure tests used to screen new compounds in the NIH Anticonvulsant Screening Program (Barton et al., 2001), PTZ and variations of the MES test, fail to reveal consistent protective benefit of the ketogenic diet. Yet these seizure tests are used to reject compounds from further consideration, passing over potentially therapeutic compounds that mimic the ketogenic diet. Therefore, the 6 Hz test may be useful for screening new compounds that mimic the ketogenic diet.
Compared to mice on a normal diet, mice on the 6.5:1 ketogenic diet consistently weighed less, even though food intake was not restricted. Therefore, ketosis, weight reduction, and the accompanying low glucose levels may all contribute to protection by the ketogenic diet. However, weight reduction and (moderately) reduced glucose levels (without ketosis) that occurred with CR were not sufficient to protect against 6 Hz–induced seizures. Consistent with these findings, alterations in blood glucose levels do not correlate with efficacy of the ketogenic diet in children consuming the ketogenic diet for seizure control (Bergqvist et al., 2007). However, it is important to note that ketone bodies typically are not used as fuel by the brain unless circulating levels of glucose are low (Veech, 2004). Nevertheless, abnormally low and high glucose levels can be proconvulsant (Kaul et al., 1980; Schwechter et al., 2003). Although weight reduction is not sufficient to explain the effects of the ketogenic diet in the 6 Hz test, weight reduction may be an important component. This is supported by epileptic EL mice, which show seizure protection with low body weight and blood glucose only if the 4:1 ketogenic diet was fed in restricted amounts (Mantis et al., 2004). The ketogenic diet was the only metabolic intervention that protected against 6 Hz–induced seizures, and the only diet that caused elevated blood ketones, suggesting a functional correlation.
The exact means by which the 6 Hz test induces seizures is unknown, but based on the seizure semiology and current delivery through corneal electrodes, it appears to activate forebrain structures. Consistent with this, immediate early gene expression is upregulated in the amygdala, pyriform cortex, and dentate gyrus after 6 Hz test induction (Barton et al., 2001). This is in contrast to kainic acid (which activates receptors in the CA3 region of the hippocampus), PTZ [which binds to γ-aminobutyric acid (GABA) receptors], and the maximal electroshock test (which activates hindbrain structures and identifies medicines that act at sodium channels) (Smith et al., 2007). Therefore, the 6 Hz test appears to induce seizures through unique pathways, consistent with our finding that the 6 Hz test distinguishes different anticonvulsant diets.
Controversies and commonalities with kainic acid–induced seizures
Our results showed that intermittent fasting protects in the kainic acid test are consistent with studies in older rodents. Adult rats after 8 weeks of intermittent fasting were protected against neurodegeneration due to intrahippocampal injection of kainic acid (Bruce-Keller et al., 1999). Intermittent fasting for 6 months in adult rats appears to preserve GABAergic interneurons in the anterior hippocampus after subcutaneous kainic acid administration (Contestabile et al., 2004). Similarly, long-term potentiation was preserved in kainic acid–treated rat hippocampal slices after intermittent fasting for 7–10 weeks (Youssef et al., 2008). Herein we show that intermittent fasting protects juvenile mice after only 2 weeks of dietary intervention, although a relatively short treatment period is required to complete the diet regimen before adulthood. Whether intermittent fasting alters the pharmacokinetics of ipKA or ivPTZ (and thus, affects sensitivity to these convulsants) is unknown, but this is not a concern for electroconvulsive tests.
Our data showing that the ketogenic diet fails to protect or worsens seizures in the kainic acid test are consistent with a recent report showing that C3Heb/FeJ mice are more susceptible to ipKA after consuming a ketogenic diet (Samala et al., 2008), but disagrees with a study of Institute of Cancer Research mice that shows a prolonged latency to seizure onset after ipKA (Noh et al., 2003). Conflicting results also exist for rats consuming a ketogenic diet that are undergoing kainic acid seizure tests. More consistent with our study, rats on a 4:1 ketogenic diet experienced more severe convulsions (Bough et al., 2002), whereas another study showed mild protection from spontaneous recurrent seizures when a ketogenic diet was started after kainic acid–induced status epilepticus (Muller-Schwarze et al., 1999). Mouse strain has been reported to affect kainic acid–induced seizures with normal diets (Schauwecker & Steward, 1997). Therefore, differences in animal strain, age, and species, as well as differences in diet components and duration may influence susceptibility to this convulsant.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge expert technical assistance provided by Shifa Zhou and information provided by Matthew Ricci, PhD (Research Diets). This study was supported by a Neurological Sciences Academic Development Award K12NS001696 (ALH), a generous gift from the Becker Family Foundation (ALH), and NIH grant NS037402 (JMH).
Footnotes
Disclosure
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Additional Supporting Information may be found in the online version of this article:
Table S1. Comparison of normal diet (2018SX), ketogenic diet (D07091701), and AIN-93G standards (Reeves et al., 1993).
Table S2. Data for 6 Hz test.
Table S3. Data for MES threshold test.
Table S4. Complete blood count results for unrestricted normal diet (Norm) and intermittent fasting (CR-IF) groups.
Table S5. Comprehensive metabolic panel results for unrestricted normal diet (Norm) and intermittent fasting (CR-IF) groups.
Figure S1. Calorie restriction (intermittent and daily) and the ketogenic diet have opposite effects on 6 Hz–induced seizures.
Figure S2. Intermittent fasting (CR-IF), but not the ketogenic diet, protects against seizures induced by kainic acid injected intraperitoneally (ip).
Figure S3. The ketogenic diet does not protect against intravenous kainic acid–induced seizures.
Figure S4. The ketogenic diet does not protect against intravenous kainic acid–induced seizures (without thresholds being corrected for weight).
Figure S5. Intermittent fasting is detrimental in the MES test.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Barton ME, Klein BD, Wolf HH, White HS. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res. 2001;47:217–227. doi: 10.1016/s0920-1211(01)00302-3. [DOI] [PubMed] [Google Scholar]
- Bergqvist AG, Schall JI, Richard EL, Gallagher PR, Stallings VA. Predictive power of first morning glucose and the ketogenic diet. Neuropediatrics. 2007;38:193–196. doi: 10.1055/s-2007-992816. [DOI] [PubMed] [Google Scholar]
- Bough KJ, Valiyil R, Han FT, Eagles DA. Seizure resistance is dependent upon age and calorie restriction in rats fed a ketogenic diet. Epilepsy Res. 1999;35:21–28. doi: 10.1016/s0920-1211(98)00125-9. [DOI] [PubMed] [Google Scholar]
- Bough KJ, Gudi K, Han FT, Rathod AH, Eagles DA. An anticonvulsant profile of the ketogenic diet in the rat. Epilepsy Res. 2002;50:313–325. doi: 10.1016/s0920-1211(02)00086-4. [DOI] [PubMed] [Google Scholar]
- Bough KJ, Schwartzkroin PA, Rho JM. Calorie restriction and ketogenic diet diminish neuronal excitability in rat dentate gyrus in vivo. Epilepsia. 2003;44:752–760. doi: 10.1046/j.1528-1157.2003.55502.x. [DOI] [PubMed] [Google Scholar]
- Bough KJ, Rho JM. Anticonvulsant mechanisms of the ketogenic diet. Epilepsia. 2007;48:43–58. doi: 10.1111/j.1528-1167.2007.00915.x. [DOI] [PubMed] [Google Scholar]
- Brown WC, Schiffman DO, Swinyard EA, Goodman LS. Comparative assay of an antiepileptic drugs by psychomotor seizure test and minimal electroshock threshold test. J Pharmacol Exp Ther. 1953;107:273–283. [PubMed] [Google Scholar]
- Bruce-Keller AJ, Umberger G, McFall R, Mattson MP. Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann Neurol. 1999;45:8–15. [PubMed] [Google Scholar]
- Contestabile A, Ciani E, Contestabile A. Dietary restriction differentially protects from neurodegeneration in animal models of excitotoxicity. Brain Res. 2004;1002:162–166. doi: 10.1016/j.brainres.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Freeman JM, Kossoff EH, Hartman AL. The ketogenic diet: one decade later. Pediatrics. 2007;119:535–543. doi: 10.1542/peds.2006-2447. [DOI] [PubMed] [Google Scholar]
- Greene AE, Todorova MT, McGowan R, Seyfried TN. Caloric restriction inhibits seizure susceptibility in epileptic EL mice by reducing blood glucose. Epilepsia. 2001;42:1371–1378. doi: 10.1046/j.1528-1157.2001.17601.x. [DOI] [PubMed] [Google Scholar]
- Guerrini R. Epilepsy in children. Lancet. 2006;367:499–524. doi: 10.1016/S0140-6736(06)68182-8. [DOI] [PubMed] [Google Scholar]
- Hartman AL, Lyle M, Rogawski MA, Gasior M. Efficacy of the ketogenic diet in the 6-Hz seizure test. Epilepsia. 2008;49:334–339. doi: 10.1111/j.1528-1167.2007.01430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul CL, David J, Grewal RS. The incidence of electroshock and pentylenetetrazole (Metrazol) induced convulsions in hypoglycaemic and acute and chronic hyperglycaemic states in mice. Pharmacol Res Commun. 1980;12:791–803. doi: 10.1016/s0031-6989(80)80083-x. [DOI] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- Maalouf M, Rho JM, Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev. 2009;59:293–315. doi: 10.1016/j.brainresrev.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantis JG, Centeno NA, Todorova MT, McGowan R, Seyfried TN. Management of multifactorial idiopathic epilepsy in EL mice with caloric restriction and the ketogenic diet: role of glucose and ketone bodies. Nutr Metab (Lond) 2004;1:11. doi: 10.1186/1743-7075-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martillotti J, Weinshenker D, Liles LC, Eagles DA. A ketogenic diet and knockout of the norepinephrine transporter both reduce seizure severity in mice. Epilepsy Res. 2006;68:207–211. doi: 10.1016/j.eplepsyres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Millichap JG, Jones JD, Rudis BP. Mechanism of anticonvulsant action of ketogenic diet. studies in animals with experimental seizures and in children with petit mal epilepsy. Am J Dis Child. 1964;107:593–604. [PubMed] [Google Scholar]
- Morrison RS, Wenzel HJ, Kinoshita Y, Robbins CA, Donehower LA, Schwartzkroin PA. Loss of the p53 tumor suppressor gene protects neurons from kainate-induced cell death. J Neurosci. 1996;16:1337–1345. doi: 10.1523/JNEUROSCI.16-04-01337.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Schwarze AB, Tandon P, Liu Z, Yang Y, Holmes GL, Stafstrom CE. Ketogenic diet reduces spontaneous seizures and mossy fiber sprouting in the kainic acid model. Neuroreport. 1999;10:1517–1522. doi: 10.1097/00001756-199905140-00023. [DOI] [PubMed] [Google Scholar]
- Noh HS, Kim YS, Lee HP, Chung KM, Kim DW, Kang SS, Cho GJ, Choi WS. The protective effect of a ketogenic diet on kainic acid-induced hippocampal cell death in the male ICR mice. Epilepsy Res. 2003;53:119–128. doi: 10.1016/s0920-1211(02)00262-0. [DOI] [PubMed] [Google Scholar]
- Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Samala R, Willis S, Borges K. Anticonvulsant profile of a balanced ketogenic diet in acute mouse seizure models. Epilepsy Res. 2008;81:119–127. doi: 10.1016/j.eplepsyres.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Schauwecker PE, Steward O. Genetic determinants of susceptibility to excitotoxic cell death: implications for gene targeting approaches. Proc Natl Acad Sci USA. 1997;94:4103–4108. doi: 10.1073/pnas.94.8.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwechter EM, Veliskova J, Velisek L. Correlation between extra-cellular glucose and seizure susceptibility in adult rats. Ann Neurol. 2003;53:91–101. doi: 10.1002/ana.10415. [DOI] [PubMed] [Google Scholar]
- Smith M, Wilcox KS, White HS. Discovery of antiepileptic drugs. Neurotherapeutics. 2007;4:12–17. doi: 10.1016/j.nurt.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlemann ER, Neims AH. Anticonvulsant properties of the ketogenic diet in mice. J Pharmacol Exp Ther. 1972;180:231–238. [PubMed] [Google Scholar]
- Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids. 2004;70:309–319. doi: 10.1016/j.plefa.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Yamada KA. Calorie restriction and glucose regulation. Epilepsia. 2008;49(suppl 8):94–96. doi: 10.1111/j.1528-1167.2008.01847.x. [DOI] [PubMed] [Google Scholar]
- Youssef FF, Ramchandani J, Manswell S, McRae A. Adult-onset calorie restriction attenuates kainic acid excitotoxicity in the rat hippo-campal slice. Neurosci Lett. 2008;431:118–122. doi: 10.1016/j.neulet.2007.11.064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


