The Ste20 kinase misshapen is essential for the invasive behaviour of ovarian epithelial cells in Drosophila
This study identifies the Ste-20-like kinase misshapen as a key, novel regulator of the levels and/or distribution of DE-Cadherin during collective cell migration.
Keywords: Ste20 kinases, cell migration, signalling, DE-cadherin
Abstract
Stationary-to-migratory transitions of epithelial cells have a key role in development and tumour progression. Border cell migration is a powerful system in which to investigate this transition in living organisms. Here, we identify the Ste20-like kinase misshapen (msn) as a novel regulator of border-cell migration in Drosophila. Expression of msn in border cells is independent of the transcription factor slow border cells and of inputs from all pathways that are known to control border-cell migration. The msn gene functions to modulate the levels and/or distribution of Drosophila E-cadherin to promote the invasive migratory behaviour of border cells.
Introduction
Cell migration has a key role in embryogenesis and in adult organisms. It is a complex process, the first step of which is often a stationary-to-migratory transition of epithelial cells. This transition involves changes in cell adhesion and polarity as well as the formation and extension of cellular protrusions in the direction of movement.
The border cells of the Drosophila ovary provide a useful system in which to study the mechanisms that control migration in vivo (Rorth, 2002; Montell, 2003). Drosophila oogenesis proceeds through 14 morphologically distinct stages (S1–S14). Each egg chamber contains one oocyte and 15 nurse cells surrounded by a monolayer of follicle cells, known as the follicular epithelium. Border cells are determined at the anterior pole of the follicular epithelium, and by S9 they delaminate and migrate posteriorly between the nurse cells until they contact the oocyte at S10 (Fig 1A–C). This migration is synchronized with the posterior movement of the remaining follicle cells. Finally, border cells migrate towards the dorsal side of the egg chamber. Border-cell migration is highly regulated; the migratory cluster is first specified as a group of two polar cells in a central position that, at S8, recruit 6–8 outer border cells. Once the cluster has formed, border cells acquire a motile polarity and become migratory through the reorganization of cell polarity molecules such as Fasciclin-II, Discs large (Dlg), Lethal (2) giant larvae, Par6 and Bazooka (Goode & Perrimon, 1997; Pinheiro & Montell, 2004; Szafranski & Goode, 2004). Border cells subsequently break contact with neighbouring cells, delaminate from the follicular epithelium and initiate migration as a cohesive group.
Figure 1.
Ovarian expression of misshapen. (A–C) Illustration of stage 8 (S8), S9 and S10 egg chambers. NCs, Oo, FCs, PCs (red) and BCs (green) are indicated. (D–F) Egg chambers from the msn06286 line stained for β-gal activity (green), the PC marker Fasciclin-III (FasIII; red) and the nuclear marker TO-PRO-3 (blue). Note that the border-cell cluster moves evenly with the main body follicle cells (empty arrowheads in E). BC, border cell; FC, follicle cell; β-gal, β-galactosidase; Fas III, Fasciclin-III; NC, nurse cell; Oo, oocyte; PC, polar cell.
Slow border cells (slbo), which codes for the Drosophila homologue of the CCAAT-enhancer-binding proteins (C/EBP) transcription factor, is a key regulator of border-cell migration. It is expressed in border cells before and during their migration, is essential for their motility and controls the expression of most genes required for their migration including shotgun (shg), the gene encoding Drosophila E-cadherin (DE-Cad) (Montell et al, 1992; Rorth et al, 2000). Regulation of DE-Cad is crucial for border cells, as a reduction or an increase in DE-Cad levels results in migration defects (Niewiadomska et al, 1999; Schober et al, 2005).
Four main signalling pathways are involved in border-cell determination and migration: Janus kinase/signal transducer and activator of transcription (Jak/Stat), ecdysone receptor, and the epidermal growth factor receptor (EGFR) and platelet-derived growth factor/vascular endothelial growth factor receptor (PVR) pathways. The Jak/Stat pathway is required for recruitment of outer border cells, acquisition of a motile status and migration itself (Silver & Montell, 2001; Silver et al, 2005). It acts both upstream and in parallel to Slbo to regulate the activity of genes essential for border-cell migration (Silver & Montell, 2001; Beccari et al, 2002). The timing of migration seems to be regulated by the ecdysone pathway (Bai et al, 2000). Finally, the EGFR and the PVR pathways act redundantly to guide border cells towards the oocyte (Duchek & Rorth, 2001; Duchek et al, 2001).
We aimed to isolate new genes required for border-cell migration, and identified the Ste20-like kinase misshapen (msn) as an essential gene in their migration. msn− border cells accumulate ectopic DE-Cad, and a reduction in DE-Cad function ameliorates the migration defects of msn-mutant border cells, we therefore propose that msn regulates DE-Cad turnover to facilitate their migration.
Results
msn is required for border-cell migration and recruitment
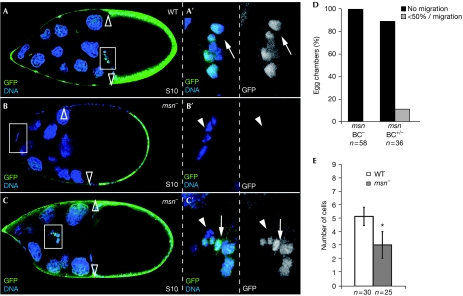
To examine the requirements for msn in border-cell migration, we first examined msn expression during oogenesis using lacZ enhancer trap lines msn06286, msnS138716 and msnj1E2 (Fig 1 and data not shown; Treisman et al, 1997). In all cases, from S2 to S8, msn-lacZ is detected at low levels in all follicle cells and at high levels in anterior and posterior polar cells. At S9, msn-lacZ is upregulated in the border-cell cluster during migration. The low levels present in the follicular epithelium gradually disappear at S9/10 (Fig 1D,F). Next, we analysed mosaic egg chambers containing clones of msn-mutant cells. We refer to ‘mutant' clusters when all border cells are homozygous for a given mutant allele and to ‘mixed' clusters when only a proportion of them are homozygous. Although wild-type border cells reach the oocyte at S10 (Fig 2A; supplementary Movie S1 online), we found that migration was completely blocked in all of the mosaic S10 egg chambers containing msn− border-cell clusters and these cells remained at the anterior pole of the egg chamber (n=58; Fig 2B,D). In addition, migration was blocked in 89% of the S10 egg chambers with mixed border-cell clusters (n=36; Fig 2D; supplementary Movie S2 online). The remaining 11% of mixed clusters showed a delayed migration, with cells advancing only half the distance to the oocyte (Fig 2C,D). In the latter cases, wild-type border cells were found at the front of the migrating cluster and the mutant cells were behind (Fig 2C). This phenomenon has also been observed in other mutants involved in border-cell migration, such as shg and tai (Niewiadomska et al, 1999; Bai et al, 2000).
Figure 2.
msn is required for border-cell migration. (A) WT and (B,C) msn mosaic stage 10 (S10) egg chambers. In all panels, homozygous mutant cells are labelled by the absence of GFP (green); primed letters (′) are magnifications of the white boxes. (A) At S10, posterior migration of border cells is complete. (B) An S10 msn102-mutant border-cell cluster stuck at the anterior pole of the egg chamber. The number of border cells in this msn− cluster is reduced compared with the wild type. (C) A delayed, mixed border-cell cluster containing wild-type cells at the front and msn− cells behind. (D) Quantification of the S10 migration phenotype of mutant (BC−) and mixed (BC+/−) clusters. (E) Quantification of the recruitment phenotype. Only mutant clusters were scored. An asterisk indicates a statistically significant difference (Student's test: P<0.01). Empty arrowheads, rear limit of main body follicle cells; arrowheads, mutant cells; arrow, wild-type cells. BC, border cell; GFP, green fluorescent protein; msn, misshapen; WT, wild type.
In addition to the migration defects, msn− clusters on average contained significantly fewer outer border cells compared with wild type (5±0.6 (n=30), msn− clusters: 3±0.9 (n=25); Fig 2B,E). Together, the above results show that msn is required for proper border cell migration and cluster recruitment. Here, we focus on the role of msn during this migration.
msn defines a new, slbo-independent pathway
As slbo is expressed and is required in border cells during their migration, we tested whether slbo expression was affected in msn− mixed clusters and observed that Slbo was expressed at similar levels in both wild-type and mutant border cells (supplementary Fig S1A online). In addition, expression of the polar-cell-specific marker Fasciclin-III was unaffected (supplementary Fig S1B online), indicating that msn is not required to specify border cells or to discriminate between polar cells and outer border cells. Furthermore, as msn-lacZ expression was not affected in slbo-mutant cells (supplementary Fig S1C online), we concluded that msn functions independently of slbo in the control of border-cell migration.
We then tested whether msn is a component of the other pathways known to regulate border-cell migration, such as the ecdysone, Jak/Stat, and EGFR and PVR pathways, and found that msn acts independently of them all (supplementary Fig S2 online).
msn is required for proper distribution of DE-Cad
Delamination and migration of the border-cell cluster requires changes in the distribution of the actin cytoskeleton and various cell adhesion molecules, including DE-Cad and Dlg. The behaviour of F-actin and Dlg in wild-type and msn-mutant border cells is indistinguishable. Thus, F-actin accumulates at the front facing the direction of movement (Verkhusha et al, 1999; supplementary Fig S3 online), and Dlg—which initially localizes to the anterior margin of polar cells (Fig 3A,B)—re-localizes to contacts between border cells in both genotypes during migration (Goode & Perrimon, 1997; Fig 3C,D). msn− border cells thereby retain cell polarity cues that allow the redistribution of polarity markers during their migration.
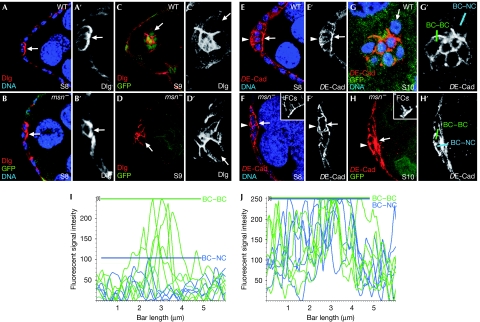
Figure 3.
msn regulates DE-Cad levels in border cells. At stage 8 (S8), (A) WT and (B) msn− border cells localize Dlg (red) at the front, facing the germ line. (C,D) At S9, Dlg is found at high levels at PC–BC and BC–BC interfaces and low levels at the BC–NC interface in both wild-type and msn− border cells. (E,F) DE-Cad distribution in S8 egg chambers is uniform around the surface of both (E) wild-type and (F) msn− border cells. (G,H) At S10, while in wild-type S10 clusters DE-Cad is found at high levels at the PC–BC and BC–BC boundaries and at low levels at BC–NC boundaries, msn− border cells also accumulate high DE-Cad levels at BC–NC interfaces. Insets in F and H are internal controls for DE-Cad staining in follicle cells. (I,J) Histogram of relative fluorescent intensities of DE-Cad along BC–BC (green) and BC–NC (blue) boundaries, as indicated in G′ and H′. Coloured bars indicate the maximum DE-Cad fluorescence intensity found at BC–BC and BC–NC boundaries. Arrows, BC–NC contacts; arrowheads, basal side of border cells. Primed letters are single channels of corresponding merged panels. BC, border cell; DE-Cad, DE-cadherin; Dlg, Discs large; FC, follicle cell; GFP, green fluorescent protein; msn, misshapen; NC, nurse cell; PC, polar cell; WT, wild type.
S8 wild-type follicle cells adjacent to polar cells upregulate DE-Cad levels, which mainly concentrate at the zonula adherens (Niewiadomska et al, 1999). At early S9, DE-Cad becomes uniformly distributed in a depolarized manner over the surface of border cells (Fig 3E; supplementary Movie S3 online). On border-cell migration, the highest levels of DE-Cad are seen at border-cell, polar-cell and mixed contacts, whereas contacts between border cells and nurse cells show lower levels of DE-Cad (Fig 3G,I; supplementary Movie S4 online). By contrast, although the initial distribution of DE-Cad in msn− cells is normal (Fig 3F; supplementary Movie S5 online), DE-Cad levels remain distributed in a non-polarized manner, with high levels in contacts between border cells and nurse cells (Fig 3H,J; supplementary Movie S6 online). Armadillo (Arm, β-catenin) distribution in msn− cells is indistinguishable from that of DE-Cad (supplementary Fig S3C online). Together with the normal accumulation of actin and Dlg in mutant cells, the aberrant localization of DE-Cad and Arm in msn− border cells demonstrates that msn− cells do not exhibit a general defect in cell polarity, but instead have a specific defect in the distribution of adherent junction components. This abnormal accumulation of DE-Cad complexes in msn− border cells is not a consequence of failed cell migration, as no defects in DE-Cad distribution are observed in other genes with similar phenotypes, such as jing or Hsp70 (Bai et al, 2000; Cobreros et al, 2008). The overexpression of UAS-msn using two different drivers—slbo-Gal4 and c306-Gal4—did not produce any visible phenotypes (data not shown).
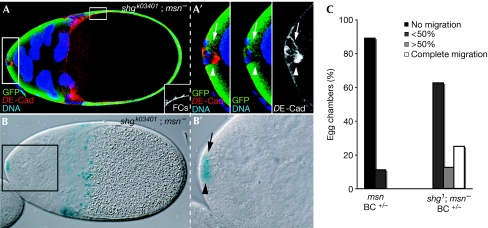
To test whether msn regulates DE-Cad at the transcriptional level, we examined both DE-Cad levels and β-galactosidase activity from the enhancer trap line shgk03401 in mixed clusters, with wild-type cells of the cluster as an internal control. msn− border cells with elevated levels of DE-Cad did not exhibit increased β-galactosidase activity compared with wild-type siblings (Fig 4A,B), indicating that msn does not affect shg transcription. Finally, we found that the abnormal levels of DE-Cad observed in msn− border cells were linked to inhibition of migration, as removing one copy of shg diminished the migration defects of msn mixed clusters (Fig 4C).
Figure 4.
msn does not regulate DE-cadherin transcription. S11 egg chamber carrying a mixed border-cell cluster stained with anti-GFP (green) and anti-DE-Cad (red), and assayed for β-galactosidase activity. (A) DE-Cad staining in msn− cells (white arrowheads) is abnormally elevated compared with sibling wild-type controls (white arrows). The inset shows an internal control for DE-Cad staining in follicle cells. (B) msn− cells (black arrowhead) show normal β-galactosidase activity (black arrow: sibling controls). (C) Reducing DE-Cad alleviates the border-cell migration defects found in msn mixed clusters. Migration is blocked in 89% of the mixed S10 border-cell clusters and delayed in 11% (n=36). In an shg−/+ background, migration is completed in 25% of the cases and delayed in the remaining 75% (n=15). Primed letters are magnifications of the corresponding white boxes. BC, border cell; DE-Cad, Drosophila E-cadherin; FC, follicle cell; GFP, green fluorescent protein; msn, misshapen; S11, stage 11; shg, shotgun.
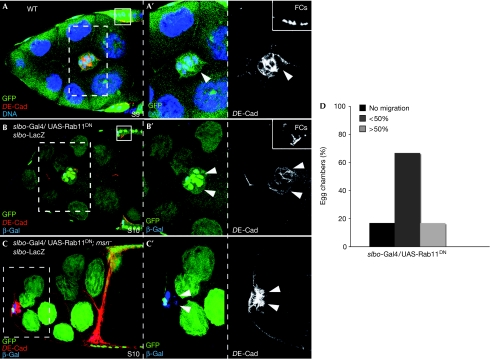
Endocytosis has been identified as a key step in regulating E-Cad turnover. In fact, DE-Cad is a known cargo of the Rab11 recycling endosomes in several contexts (Lock & Stow, 2005), and Rab11-mediated transport regulates cadherin-based adhesion in Drosophila wing and trachea development (Classen et al, 2005; Shaye et al, 2008). We found that Rab11-dependent transport was required for border-cell migration, as expression of Rab11DN (Marois et al, 2006) reduced DE-Cad levels substantially and delayed migration (Fig 5B,D). However, expression of Rab11DN was not able to rescue the abnormal levels of DE-Cad or the migration phenotype of msn− border cells (Fig 5C), suggesting that msn does not regulate DE-Cad through Rab11-mediated transport.
Figure 5.
Interfering with Rab11 function affects DE-Cad levels and border-cell migration. (A) S9 controls the border-cell cluster. (B,C) S9 border cells labelled with slbo-lacZ. (B) Overexpression of UAS-Rab11DN reduces DE-Cad levels and impairs migration. (C) Overexpression of UAS-Rab11DN does not rescue the migration defects or the elevated DE-Cad levels of msn− border cells. (D) Quantification of the border-cell migration phenotype of S10 egg chambers overexpressing UAS-Rab11DN. Small insets in A and B are internal controls of DE-Cad staining. Arrowheads, BCs. Primed letters are magnifications of white boxes. BC, border cell; DE-Cad, Drosophila E-cadherin; msn, misshapen; S9, stage 9; slbo, slow border cells; WT, wild type.
msn acts independently of JNK
The msn gene activates mitogen-activated protein kinase cascades in various organisms (Dan et al, 2001). In Drosophila, msn acts upstream from basket (Drosophila Jun amino-terminal kinase (JNK)) in dorsal closure (Su et al, 1998), but not during photoreceptor axon targeting (Ruan et al, 1999). In border cells, mutations in basket or overexpression of the JNK phosphatase puckered result in cluster dissociation and slightly delayed migration (Llense & Martin-Blanco, 2008). Similarly, we found that removal of either the Drosophila JNK kinase hep or the transcription factor Djun caused a delay in migration (supplementary Fig S4A,D online and data not shown). This suggests either that additional pathways cooperate in this process or that JNK is not downstream from msn in border-cell migration. We used a phospho-Jun antibody as a readout of JNK activation and found that this pathway was not altered in msn− border cells (supplementary Fig S4B online). To test for redundancy between the p38 kinase and JNK downstream from msn—as is the case in planar polarity generation (Paricio et al, 1999)—we analysed border cells lacking both hep and Dp38K/lic (n=36) and found that they behaved similarly to hep− border cells (supplementary Fig S4C online). Our observations indicate that msn acts independently of the JNK pathway to regulate border cells. Finally, a known target of the Ste20-like Nck-interacting kinase are the Ezrin–Radixin–Moesin proteins (Baumgatner et al, 2006). However, removal of the sole member of the Ezrin–Radixin–Moesin family in Drosophila, moesin, in border cells did not affect migration (supplementary Fig S4E online).
Discussion
A defining feature in stationary-to-migratory transitions of epithelial cells is the regulation of E-Cad distribution. Modulation of E-cad levels is also crucial during tumour progression (Peinado et al, 2007) and therefore understanding the molecular mechanisms behind this regulation is important to cancer biology.
The transition from stationary epithelial follicle cells to migratory border cells also requires tight regulation of DE-Cad levels and distribution (Niewiadomska et al, 1999; Bai et al, 2000). What are the signals that regulate DE-Cad levels in border cells? Several genes have been identified that, when mutated, result in increased or reduced DE-Cad levels and severe defects in border-cell migration, including slbo, tai, hnt, yan and Stat92E (Niewiadomska et al, 1999; Bai et al, 2000; Silver & Montell, 2001; Schober et al, 2005; Melani et al, 2008). However, a causal link between alterations in DE-Cad surface levels and migration defects has not been demonstrated. Here, we show that msn− border cells display abnormal accumulation of DE-Cad and Arm, and fail to delaminate from the epithelium. Importantly, a reduction in DE-Cad function ameliorates the msn− migration defects. Although msn might also control other aspects of the initiation of their migration, we propose that Msn acts to regulate proper redistribution of DE-Cad before migration by controlling the reorganization of DE-Cad-containing adhesion complexes, either by affecting its turnover in a Rab11-independent manner or by modifying DE-Cad directly or indirectly so that it can be efficiently recycled.
E-Cad activity during organogenesis and tumorigenesis is often regulated at the level of transcription (Peinado et al, 2007). For processes in which a fast and transient regulation of E-Cad is required—such as during Drosophila tracheal intercalation—transport of E-Cad has been identified as an alternative mechanism (Shaye et al, 2008). Our results and those of others suggest that endocytosis might also have a role in modulating E-Cad turnover during border-cell migration. Expression of dominant-negative forms of Rab11—as we have demonstrated here—or Rab5 in border cells (Schober et al, 2005) leads to a reduction or an increase, respectively, in DE-Cad levels at the cell cortex and in defective migration. As interference with Rab5 function causes a phenotype similar to that of blocking msn function, and as the activity of mitogen-activated protein kinase p38, downstream from msn, accelerates endocytosis by stimulating formation of the GDI–Rab5 complex (Cavalli et al, 2001), msn could activate Rab5-mediated endocytosis. However, the overexpression of an activated form of Rab5 in wild-type border cells was not sufficient to cause a consistent reduction of DE-Cad levels and we were therefore unable to test this hypothesis (supplementary Fig S5 online).
The role of Ste20 group kinases in cell migration seems to be pleiotropic and, in many instances, remains poorly understood. They modulate a variety of intracellular pathways through the extensive structural diversity of their non-catalytic domains. Caenorhabditis elegans NIK MIG-15 regulates guidance rather than migration itself (Su et al, 2000; Poinat et al, 2002), and mammalian Nik has been proposed to regulate the production of factors required for proper cell migration and not migration itself (Xue et al, 2001). Similarly to our results here, expression of dominant-negative forms of human Misshapen/NIKs-related kinase-β (hMINK-β) in MCF7 epithelial cells results in an upregulation of membrane-associated β-catenin, promoting cell–cell adhesion and inhibiting cell motility (Hu et al, 2004). The fact that msn is essential for the invasive behaviour of border cells supports a role for these kinases during invasion and/or tumorigenesis. In fact, human homologues of this family of kinases are broadly expressed in tumour cells and modulate cellular transformation, invasion and adhesion (Wright et al, 2003). The identification of downstream targets of Msn during border-cell migration and its mechanisms of action might result in the discovery of new proteins that contribute to tumour invasion.
Methods
Fly stocks. The following stocks were used: msn06286, msnj1E2, msn138716; msn102 FRT80B and msn172 FRT80B (Treisman et al, 1997; the null alleles, msn102 and msn172, gave rise to indistinguishable phenotypes so we refer only to the results obtained for msn102); ubi-GFP FRT80B; yw; P[lacW]shgK03401/CyO hs-flp; e22c-Gal4 UAS-flp; c306-Gal4 and slbo-Gal4 slbo-lacZ (BDSC); UAS-Rab11DN (Coumailleau et al, 2009).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank our colleagues and the Bloomington Drosophila Stock Center (BDSC) for the reagents and D. Montell for help with the in vivo analyses. Funding from the Spanish Ministerio de Ciencia e Innovación (MICINN), the EMBO Young Investigator Programme and the Junta de Andalucía is acknowledged.
Footnotes
The authors declare that they have no conflict of interest.
References
- Bai J, Uehara Y, Montell DJ (2000) Regulation of invasive cell behaviours by Taiman, a Drosophila protein related to AIB1, a steroid receptor coactivator amplified in breast cancer. Cell 103: 1047–1058 [DOI] [PubMed] [Google Scholar]
- Baumgatner M, Sillman AL, Blackwood EM, Srivastava J, Madson N, Schilling JW, Wright JH, Barber DL (2006) Tha Nck-interacting kinase phosphorylates ERM proteins for formation of lamellipodium by growth factors. Proc Natl Acad Sci USA 103: 13391–13396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beccari S, Teixeira L, Rorth P (2002) The JAK/STAT pathway is required for border cell migration during Drosophila oogenesis. Mech Dev 111: 115–123 [DOI] [PubMed] [Google Scholar]
- Cavalli V, Vilbois F, Corti M, Marcote MJ, Tamura K, Karin M, Arkinstall S, Gruenberg J (2001) The stress-induced MAP kinase p38 regulates endocytic trafficking via the GDI:Rab5 complex. Mol Cell 7: 421–432 [DOI] [PubMed] [Google Scholar]
- Classen AK, Anderson KI, Marois E, Eaton S (2005) Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Dev Cell 9: 805–817 [DOI] [PubMed] [Google Scholar]
- Cobreros L, Fernandez-Minan A, Luque CM, Gonzalez-Reyes A, Martin-Bermudo MD (2008) A role for the chaperone Hsp70 in the regulation of border cell migration in the Drosophila ovary. Mech Dev 125: 1048–1058 [DOI] [PubMed] [Google Scholar]
- Coumailleau F, Furthauer M, Knoblich JA, Gonzalez-Gaitan M (2009) Directional Delta and Notch trafficking in Sara endosomes during asymmetric cell division. Nature 458: 1051–1055 [DOI] [PubMed] [Google Scholar]
- Dan I, Watanabe NM, Kusumi A (2001) The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol 11: 220–230 [DOI] [PubMed] [Google Scholar]
- Duchek P, Rorth P (2001) Guidance of cell migration by EGF receptor signaling during Drosophila oogenesis. Science 291: 131–133 [DOI] [PubMed] [Google Scholar]
- Duchek P, Somogyi K, Jékely G, Beccari S, Rorth P (2001) Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell 107: 17–26 [DOI] [PubMed] [Google Scholar]
- Goode S, Perrimon N (1997) Inhibition of patterned cell shape change and cell invasion by Discs large during Drosophila oogenesis. Genes Dev 11: 2532–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Leo C, Yu S, Huang BC, Wang H, Shen M, Luo Y, Daniel-Issakani S, Payan DG, Xu X (2004) Identification and functional characterization of a novel human misshapen/Nck interacting kinase-related kinase, hMINK-β. J Biol Chem 279: 54387–54397 [DOI] [PubMed] [Google Scholar]
- Llense F, Martin-Blanco E (2008) JNK signaling controls border cell cluster integrity and collective cell migration. Curr Biol 18: 538–544 [DOI] [PubMed] [Google Scholar]
- Lock JG, Stow JL (2005) Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol Biol Cell 16: 1744–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marois E, Mahmoud A, Eaton S (2006) The endocytic pathway and formation of the Wingless morphogen gradient. Development 133: 307–317 [DOI] [PubMed] [Google Scholar]
- Melani M, Simpson KJ, Brugge JS, Montell D (2008) Regulation of cell adhesion and collective cell migration by hindsight and its human homolog RREB1. Curr Biol 18: 532–537 [DOI] [PubMed] [Google Scholar]
- Montell DJ (2003) Border-cell migration: the race is on. Nat Rev Mol Cell Biol 4: 13–24 [DOI] [PubMed] [Google Scholar]
- Montell DJ, Rorth P, Spradling AC (1992) Slow border cells, a locus required for a developmentally regulated cell migration during oogenesis, encodes Drosophila C/EBP. Cell 71: 51–62 [DOI] [PubMed] [Google Scholar]
- Niewiadomska P, Godt D, Tepass U (1999) DE-cadherin is required for intercellular motility during Drosophila oogenesis. J Cell Biol 144: 533–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paricio N, Feiguin F, Boutros M, Eaton S, Mlodzik M (1999) The Drosophila STE20-like kinase Misshapen is required downstream of the Frizzled receptor in planar polarity signaling. EMBO J 18: 4669–4678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H, Olmeda D, Cano A (2007) Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 7: 415–428 [DOI] [PubMed] [Google Scholar]
- Pinheiro EM, Montell DJ (2004) Requirement of Par-6 and Bazooka in Drosophila border cell migration. Development 131: 5243–5251 [DOI] [PubMed] [Google Scholar]
- Poinat PA, De Arcangelis S, Sookharefa S, Zhu X, Hedgecock EM, Labouesse M, Georges-Labouesse E (2002) A conserved interaction between β1 integrin/PAT-3 and Nck-Interacting Kinase/MIG-15 that mediates commissural axon navigation in C. elegans. Curr Biol 12: 622–631 [DOI] [PubMed] [Google Scholar]
- Rorth P (2002) Initiating and guiding migration: lessons from border cells. Trends Cell Biol 12: 325–331 [DOI] [PubMed] [Google Scholar]
- Rorth P, Szabo K, Texido G (2000) The level of C/EBP protein is critical for cell migration during Drosophila oogenesis and is tightly controlled by regulated degradation. Mol Cell 6: 23–30 [DOI] [PubMed] [Google Scholar]
- Ruan W, Pang P, Rao Y (1999) The SH2/SH3 adaptor protein Dock interacts with the Ste20-like kinase Misshapen in controlling growth cone motility. Neuron 24: 595–605 [DOI] [PubMed] [Google Scholar]
- Schober M, Rebay I, Perrimon N (2005) Function of the ETS transcription factor Yan in border cell migration. Development 132: 3493–3504 [DOI] [PubMed] [Google Scholar]
- Shaye DD, Casanova J, Llimargas M (2008) Modulation of intracellular trafficking regulates cell intercalation in the Drosophila trachea. Nat Cell Biol 10: 964–970 [DOI] [PubMed] [Google Scholar]
- Silver DL, Geisbrecht ER, Montell DJ (2005) Requirement for JAK/STAT signaling throughout border cell migration in Drosophila. Development 132: 3483–3492 [DOI] [PubMed] [Google Scholar]
- Silver DL, Montell DJ (2001) Paracrine signaling through the JAK/STAT pathway activates invasive behaviour of ovarian epithelial cells in Drosophila. Cell 107: 831–841 [DOI] [PubMed] [Google Scholar]
- Su Y-C, Maurel-Zaffran M, Treisman JE, Skolnik EY (2000) The Ste20 kinase Misshapen regulates both photoreceptor axon targeting and dorsal closure, acting downstream of distinct signals. Mol Cell Biol 20: 4736–4744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szafranski P, Goode S (2004) A Fasciclin 2 morphogenetic switch organizes epithelial cell cluster polarity and motility. Development 131: 2023–2036 [DOI] [PubMed] [Google Scholar]
- Treisman JE, Ito N, Rubin GM (1997) misshapen encodes a protein kinase involved in cell shape control in Drosophila. Gene 186: 119–125 [DOI] [PubMed] [Google Scholar]
- Verkhusha V, Tsukita S, Oda H (1999) Actin dynamics in lamellipodia of migrating border cells in the Drosophila ovary revealed by a GFP–actin fusion protein. FEBS Lett 445: 395–401 [DOI] [PubMed] [Google Scholar]
- Wright JH et al. (2003) The STE20 kinase HGK is broadly expressed in human tumor cells and can modulate cellular transformation, invasion, and adhesion. Mol Cell Biol 23: 2068–2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Wang X, Li Z, Gotoh N, Chapman D, Skolnik EY (2001) Mesodermal patterning defect in mice lacking the Ste20 NCK interacting kinase (NIK). Development 128: 1559–1572 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.