Abstract
Our molecular understanding of somatosensation has been hampered by the difficulty in identifying the sensor in mammals. In a real breakthrough for the field, two proteins—Piezo 1 and 2—have recently been identified as the elusive mechano-sensors!
When Aristotle described the five senses in De Anima II in 350 BC, touch—which we now include in somatosensation—was considered to be the most primitive sense. Perhaps ironically, our understanding of the phylogenetically oldest sense—present in all five kingdoms of life—is still in its infancy. The primary mechanism for mechano-sensing has been linked to ion channels, which might be required to sense mechanical signals in many of the specialized nerve cells of our somatosensory system and various types of muscle cell, as well as in the epithelial and endothelial cells that line hollow organs such as the bladder and blood vessels. It is possible that all cells have the ability to sense mechanical forces, ranging from tiny touch signals—such as the brush of a feather—to relatively high changes in pressure—such as those that occur in blood vessels.
Mechano-sensitive channels in bacteria, which sense stretch and pressure, have been well characterized at the molecular level, but our understanding of stretch-sensing in mammalian cells remains limited. Calcium-permeable stretch-activated cation channels (SACs)—also referred to as MscCas—were discovered more than 20 years ago in chicken skeletal muscle and seem to be present in most, if not all, eukaryotic cells. These channels have a heterogeneous permeation profile and are gated by various mechanisms. These can be classified into two broad categories: direct or indirect gating. Direct gating involves changes in the plasma membrane tension, leading to alterations in the energetic equilibrium between membrane tension and channel closing, changes in the curvature of the plasma membrane or the rearrangement of cytoskeletal components tethered to the channel. In indirect gating, a channel receives signals from more distant sensors—such as G-proteins and phospholipases—which communicate with the channel by diffusible second messengers, the activation of kinases and the rearrangement of adhesion proteins such as integrins. Despite our efforts to understand mechano-sensation, the molecular identity of SACs remained unknown (Kung, 2005; Nilius, 2009; Pedersen & Nilius, 2007; Tsunozaki & Bautista, 2009)
In 1880, Jacques and Pierre Curie discovered an unusual characteristic of certain crystalline minerals: when they were subjected to a mechanical force, the crystals became electrically polarized. They termed this phenomenon “piezoelectric effect”, from the Greek word piezein (πιέζɛιν), meaning to press or squeeze. The Patapoutian lab in Scripps, La Jolla, California, has now identified a gene family that encodes at least two proteins that are involved in mechano-sensing and required for SAC activation (Coste et al, 2010). They have been named Piezo 1 and Piezo 2.
What is a SAC? We can provide a functional description, as the criteria that must be fulfilled by channels that are directly mechano-sensitive have been defined (Christensen & Corey, 2007): the latency of the current elicited by the stimulus should be faster than it is in second-messenger systems (about 5 ms); the kinetics of channel activation should depend on the amplitude of the stimulus; there should be a mechanical correlate of channel-gating and a sensory cell or organ that responds in the same range as the channel; and part of the channel and/or associated subunit has to move after a change in mechanical force. The putative SACs that have been characterized so far fulfil the first three, but not the last one of these criteria. SACs are non-selective cation channels that are permeable to Ca2+ and they have a single-channel conductance in a physiological environment of about 25 pS. They directly sense mechanical stimuli and respond within milliseconds to those signals, SAC activation is graded by the amount of stretch or pressure applied to the plasma membrane of a mechano-sensitive cell. SACs are normally desensitized during maintained mechano-stimulation.
Several candidate molecules have been proposed to be SACs, but none have been shown to fulfil all the criteria. It has been suggested that they belonged to the transient receptor potential (TRP) family of channels, but the most recent evidence indicates that TRPs are not the real SACs. There has now been a breakthrough: the expression of Piezo 1 and 2 in heterologous systems generates huge mechano-sensitive currents that match the properties of SACs! They induce depolarizing ionic currents, similar to the native SAC currents in various cell types, including sensory dorsal root ganglion neurons.
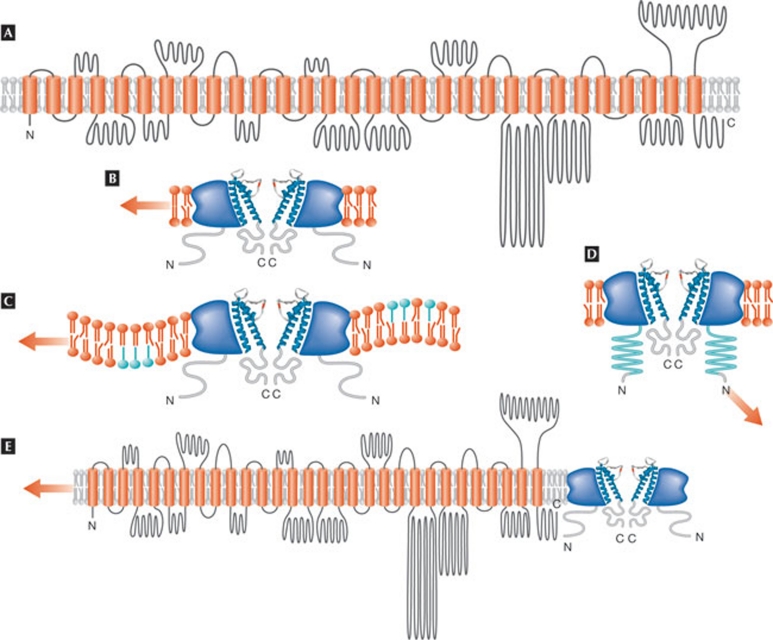
What are Piezos? The Patapoutian lab identified them using expression profiling and RNAi knockdown in mouse neuroblastoma cells, which have typical SACs. Fam38A (Piezo 1) and Fam38B (Piezo 2) are large transmembrane proteins with 24–36 predicted transmembrane domains (Fig 1A). These genes encode multipass transmembrane proteins that are conserved in protozoa, plants, invertebrates and vertebrates. Both are required for the activity of non-selective calcium-permeable stretch/pressure-activated SACs with different kinetic properties. Inactivation is delayed in Piezo 2.
Figure 1.
Piezo topology and gating models. (A) Topological model of Piezo 1 that predicts 30 transmembrane span domains (adapted from a structural model calculated by TMHMM prediction and kindly provided by Bertrand Coste and Ardem Patapoutian, redrawn by G. Owsianik, Leuven, Belgium). Other models, such as the one obtained with Phobius prediction, indicate the presence of 39 transmembrane span domains. (B) Mechano-sensitive channel gating by sensing plasma membrane tension, without interaction with any cellular structures. (C) Channel gating by changes in the membrane curvature. (D) Channel gating by force interaction with a structural substrate, such as the cytoskeleton, to which the channel is tethered. (E) Piezo might also be a mechano-sensing subunit of a mechano-sensitive channel complex, rather than pore-forming subunit (the red arrow indicates a force).
This is indeed an important finding in the hunt for SACs! These molecules can now be used to answer pertinent questions about SACs. The Patapoutian lab showed huge current amplitudes of SACs in cells that have the endogenous channel. The question remains—as a painful reminder of the ongoing search for volume-regulated anion channels—whether the expression of another protein, which might not be a channel, co-stimulates the expression of the endogenous actor, that is, the Piezo-SAC. It is most important to determine whether Piezos are actually channels; they are huge molecules, and topological models (Fig 1) do not clearly show where a pore region—which must be present for a protein to be called a channel—might be located. Measuring the response of the Piezos to the unavoidable toxin GsMTx-4 might help to resolve this (Bowman et al, 2007).
The gating mode of the Piezos is unknown. It is thought to be by direct activation, but there are several open questions. Is it sensing the lipid membrane tension? Is it sensing mechano-signals through tethering to another protein or the cytoskeleton? Is it due to changes in the curvature of the membrane (Fig 1B–D)? Could Piezo be interacting with an endogenous SAC and form a mandatory mechano-sensitive subunit rather than a channel (Fig 1E)? Are there more Piezos? SACs can differ in their threshold of activation, which can be low or high (Lumpkin & Caterina, 2007). They are also all transiently activated on mechano-stimulation. This activation can be classified as rapid, intermediate, slow or ultra-slow relaxation. What is the relaxation mechanism? Why is the relaxation voltage-dependent, that is, delayed at positive potentials (Hao & Delmas, 2010)? The relaxations of Piezo 1 and Piezo 2 are different and a comparison with more Piezos would help to solve the puzzle.
Although the Patapoutian paper shows the expression patterns of both Piezos, more sensitive data is required to reveal whether Piezos are present in mechano-sensory nerve endings or organs in the skin, as well as whether they exist in the inner ear.
Some outstanding questions in the field can now probably be solved. Is there a link to previously ‘identified' mechano-sensitive TRP channels, such as TRPC1 and TRPC6? None of these appear in the candidate gene list; TRPV2, TRPML2 and PKD1L2—which is linked to TRPP3—are all excluded. Can we finally forget about TRPs as mechanos? What is the situation with TRPA1? There is also an intriguing ‘side' result that is important for the community searching for volume-regulated anion channels; surprisingly, neither TMEM16A nor TMEM16F are mechano-sensitive—see the somewhat hasty prediction of TMEM16A as volume-regulated anion channel (Almaca et al, 2009).
Many years will, of course, be necessary in order to fully understand the function of Piezos as mechano-sensors, but this finding is undoubtedly a wonderful step forward!
References
- Almaca J et al. (2009) J Biol Chem 284: 28571–28578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman et al. (2007) Toxicon 49: 249–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen AP, Corey DP (2007) Nat Rev Neurosci 8: 510–521 [DOI] [PubMed] [Google Scholar]
- Coste B et al. (2010) Science 330: 55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Delmas P (2010) J Neurosci 30: 13384–13395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung C (2005) Nature 436: 647–654 [DOI] [PubMed] [Google Scholar]
- Lumpkin EA, Caterina MJ (2007) Nature 445: 858–865 [DOI] [PubMed] [Google Scholar]
- Nilius B (2009) Cell 139: 466–467 [DOI] [PubMed] [Google Scholar]
- Pedersen SF, Nilius B (2007) Meth Enzymol 428: 183–207 [DOI] [PubMed] [Google Scholar]
- Tsunozaki M, Bautista DM (2009) Curr Opin Neurobiol 19: 362–369 [DOI] [PMC free article] [PubMed] [Google Scholar]