Abstract
It has been suggested that the interaction of antipsychotic medications with neuronal nicotinic receptors may increase the cognitive dysfunction associated with schizophrenia and may explain why current therapies only partially address this core feature of the illness. In the present studies we compared the effects of the atypical antipsychotics quetiapine, clozapine and N-desmethylclozapine to those of the typical antipsychotics haloperidol and chlorpromazine on the α4β2 and α7 nicotinic receptor subtypes. The binding of [3H]-nicotine to rat cortical α4β2 receptors and [3H]-methyllycaconitine to rat hippocampal α7 receptors was not affected by any of the compounds tested. However, Rb+ efflux evoked either by nicotine or the selective α4β2 agonist TC-1827 from α4β2 receptors expressed in SH-EP1 cells and nicotine-evoked [3H]-dopamine release from rat striatal synaptosomes were non-competitively inhibited by all of the antipsychotics. Similarly, α-bungarotoxin-sensitive epibatidine-evoked [3H]-norepinephrine release from rat hippocampal slices and acetylcholine-activated currents of α7 nicotinic receptors expressed in oocytes were inhibited by haloperidol, chlorpromazine, clozapine and N-desmethylclozapine. The inhibitory effects on nicotinic receptor function produced by the antipsychotics tested occurred at concentrations similar to plasma levels achieved in schizophrenia patients, suggesting that they may lead to clinically relevant effects on cognition.
Keywords: Antipsychotics, Nicotinic receptors, Cholinergic, Schizophrenia
1. Introduction
The well established fact that nicotine improves cognitive performance in animals and in humans suggests a potential role for neuronal nicotinic receptors (NNRs) in the cognitive deficits associated with various diseases and disorders, including schizophrenia (Levin et al., 2006; Levin and Rezvani, 2007; Martin and Freedman, 2007; Sacco et al., 2004). The two main NNR subtypes in the brain (α4β2 and α7) have both been implicated in cognitive processes (Bencherif and Schmitt, 2002), and a number of studies point to their involvement in schizophrenia. Abnormal expression of α4β2 and α7 NNRs was found in post-mortem hippocampus and cortex of schizophrenia patients (Breese et al., 2000; Marutle et al., 2001). In addition, the human α7 receptor gene located on chromosome-15 has been linked to sensory inhibition in familial schizophrenia (Freedman et al., 2003). It has long been known that blockade of the α7 receptor induces sensory gating deficits similar to those seen in schizophrenia (Luntz-Leybman et al., 1992) and that α7-selective agonists can improve sensory gating deficits in animal models such as the DBA/2 mouse (Martin and Freedman, 2007; Simosky et al., 2001). In clinical studies, nicotine ameliorates sensory gating deficits and smooth pursuit eye movement abnormalities in schizophrenics, providing a rationale for smoking as a form of self-medication (Kumari and Postma, 2005; Leonard et al., 2001).
The atypical antipsychotics exhibit advantages over the typical antipsychotics in treating schizophrenia due to their superior therapeutic outcome with respect to negative symptoms and to some extent cognition. For example, clozapine improves verbal fluency and attention, and risperidone has positive effects on working memory and attention (Meltzer and McGurk, 1999). There is some evidence that pro-cognitive effects of atypical anti-psychotics such as clozapine and its active metabolite N-des-methylclozapine may occur through M1 muscarinic cholinergic receptor-mediated mechanisms (Davies et al., 2005; Li et al., 2005; Weiner et al., 2004). However, few reports have addressed antipsychotic interactions with nicotinic cholinergic receptors. Clozapine and chlorpromazine are known to displace [3H]-nicotine in bovine chromaffin cells (Park et al., 2001) as does chlorpromazine in rat pheochromocytoma cells (Lee et al., 1999). The typical antipsychotic chlorpromazine is known to interact with functional NNRs as a non-competitive inhibitor (Heidmann et al., 1983; Heidmann and Changeux, 1986). More recently the atypical antipsychotic clozapine was shown to inhibit the function of α7 NNRs in oocytes and in hippocampal slice preparations (Singhal et al., 2007).
To date there has been no systematic assessment of the effects of atypical antipsychotics on NNR function. The present studies were undertaken to define the interactions of both typical (haloperidol and chlorpromazine) and atypical (quetiapine, clozapine and N-desmethylclozapine) antipsychotics with α4β2 and α7 NNRs. To do this we studied the effects of the compounds on receptor function in rat striatal synaptosomes (dopamine release), hippocampal slices (norepinephrine release), oocytes (changes in acetylcholine-evoked currents) and cell cultures (Rb+ efflux). To further characterize the interaction of antipsychotics with α4β2 NNRs, the selective α4β2 agonist TC-1827 (Bohme et al., 2004) was used to mimic administration of antipsychotics to smokers with high nicotine plasma levels. The results suggest that non-competitive inhibition of NNRs by atypical antipsychotics may have a clinically significant impact on cognitive function since inhibitory effects occur at concentrations within the range of those achieved in the plasma of human patients.
Portions of this work have been presented previously in abstract form: V.P. Grinevich, K.A. Sadieva, M. Bencherif. Interaction of typical and atypical antipsychotics with neuronal nicotinic receptors. Society for Neuroscience 35th Annual Meeting, 2005, November 12–16; Washington, DC. Program No. 951.11.2005 Abstract Viewer/Itinerary Planner. Washington, DC.
2. Materials and methods
2.1. Animals and materials
Female Sprague–Dawley rats weighing 150–200 g (Charles River Laboratories, Raleigh, NC) were housed individually (12/12-h light/dark cycle) with free access to food and water. All animal studies were carried out in accordance with the Declaration of Helsinki and/or with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health.
3,4-[7-3H(N)]-Dihydroxyphenylethylamine ([3H]-dopamine, 23.5 Ci/mmol), levo-[ring-2,5,6-3H]-norepinephrine ([3H]-norepinephrine, 53.0 Ci/mmol), L-(−)-[N-methyl-3H]-nicotine ([3H]-nicotine, 66.9 Ci/mmol) and 86RbCl (6.6–17.5 Ci/mmol) were purchased from PerkinElmer Life Science (Boston, MA). N-desmethylclozapine, methyllycaconitine and [3H]-methyllycaconitine, (25.4 Ci/mmol) were purchased from Tocris, Inc. (Ellisville, MO). Dulbecco’s phosphate-buffered saline (PBS) was purchased from Invitrogen Corporation, (Carlsbad, CA). Amphotericin B, α-bungarotoxin, chlorpromazine, clozapine, cytisine, haloperidol and S-(−)-nicotine were purchased from Sigma–Aldrich (St. Louis, MO). Quetiapine was purchased from Toronto Research Chemicals (North Work, Canada). Remaining materials were purchased from Sigma–Aldrich or Fisher Scientific (Pittsburgh, PA) unless specified otherwise in the text. TC-1827 was synthesized by Targacept, Inc.
2.2. Binding assays
The α4β2/SH-EP1 cells were harvested in ice-cold PBS (pH 7.4), centrifuged, and cell sediments were covered with 4 mL PBS and kept at −20 °C. After thawing, PBS was decanted and the cells were homogenized in 8 mL PBS with a Polytron (Brinkmann Instruments, Westbury, NY) at setting 6 for 15 s and centrifuged at 40,000 × g for 20 min (4 °C). The pellet was resuspended in 6 mL of ice-cold PBS and centrifuged again. The final pellet was resuspended in 6 mL of PBS for immediate use. Final preparations contained 0.16–0.20 mg/mL of total membrane protein.
[3H]-Nicotine and [3H]-methyllycaconitine were used to probe α4β2 and α7 nicotinic receptor binding sites, respectively, at final radioligand concentrations of 0.1–40 nM for saturation assays or at 5 nM for competition binding assays, as previously described (Grinevich et al., 2005). Binding was assayed in PBS buffer containing: 0.9 mM CaCl2; 2.67 mM KCl; 1.47 mM KH2PO4; 0.49 mM MgCl2; 137.93 mM NaCl and 4.29 mM Na2HPO4, pH 7.4 in 48-well plates. Each sample (assayed in duplicate or triplicate) contained 50 μL of test compound in solution at the desired concentration, 50 μL of buffer (total binding) or cytisine/methyllycaconitine solution (cytisine or methyllycaconitine were used at 10 μM final concentration to measure [3H]-nicotine or [3H]-methyllycaconitine nonspecific binding, respectively), 50 μL of 5× radioligand stock solution, and 100 μL of membrane suspension. Incubations were conducted for 2 h at 4 °C for [3H]-nicotine or at room temperature for [3H]-methyllycaconitine. Binding was terminated by dilution with cold PBS and immediate filtration onto GF/B filters (presoaked in 0.3% PEI) using a 48-sample, semi-auto harvester (Brandel, Gaithersburg, MD). After washing 3 times with ~1 mL of buffer, filters were transferred into scintillation vials filled with 3 mL of scintillation cocktail. Radioactivity was measured after 8–12 h using a liquid scintillation analyzer (model Tri-Carb 2200CA, PerkinElmer Life Sciences Inc., Boston, MA). Data expressed in dpm were transformed to fmol of bound radioligand per mg of total protein or as a percent of control [3H]-EPI binding (i.e., total – nonspecific). Competition assays using [3H]-nicotine binding to rat cortical membranes or [3H]-methyllycaconitine binding to rat hippocampal membranes were performed at 5 nM radioligand concentration. The procedure was identical to that described above for [3H]-EPI binding except that in these assays nonspecific binding was determined in the presence of 10 μM nicotine or 10 μM methyllycaconitine, respectively.
2.3. 86Rb+ efflux in SH-EP1 cells
Cells of the SH-EP1 human epithelial line stably transfected with α4β2 nicotinic receptors (α4β2/SH-EP1, kindly provided by Dr. R. Lukas, Barrow Neurological Institute, Phoenix, Arizona) were grown in Dulbecco’s modified Eagle’s medium (DMEM) with high glucose and L-glutamine supplemented with 10% horse serum, 5% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin (all from Invitrogen, Chicago, IL) and 0.25 μg/mL amphotericin B at 37 °C in 5% CO2/humidified air.
α4β2/SH-EP1 cells plated in 12-well clusters (Corning Inc., NY) and maintained to achieve confluency were loaded with 86Rb+ (3 h; 37 °C) in identical growth medium supplemented with 2 μCi/mL isotope, then rinsed twice with PBS, pH 7.4. After a 4 min exposure to cholinergic agonist or test compound, efflux medium samples were collected into scintillation vials, added to scintillation cocktail, and radioactivity (cpm) was measured using a liquid scintillation analyzer (model Tri-Carb 2200CA, PerkinElmer Life Sciences Inc., Boston, MA). Basal ion efflux was measured using samples containing no agonist and specific nicotinic receptor function was defined as evoked over basal level ion efflux in the presence of agonist (100% control, at maximal response). Responses elicited by test compounds at different concentrations were normalized (%) to control.
2.4. Neurotransmitter release assays
[3H]-dopamine release studies were performed using rat striatal synaptosomes as previously described (Bencherif et al., 1998). Briefly, rats (150–200 g) were killed by decapitation after anesthesia with 70% CO2. For synaptosomal preparations, striata from 2 animals were rapidly dissected and homogenized in 0.32 M sucrose containing 5 mM HEPES, pH 7.4 (7.5 mL per striatum). The tissue was then centrifuged at 1000 × g for 10 min and the pellet discarded. The supernatant was centrifuged at 12,000 × g for 20 min. The resultant pellet was resuspended in perfusion buffer (128 mM NaCl, 1.2 mM KH2PO4, 2.4 rnM KCl, 3.2 mM CaCl2, 1.2 mM MgSO4, 25 mM HEPES, l mM Ascorbic acid, 0.01 mM pargyline-HCl and 10 mM glucose, pH 7.4) and centrifuged at 25,000 × g for 15 min. The final pellet was resuspended in perfusion buffer (2 mL) oxygenated with 95% O2/5% CO2 (oxygenated buffer was used throughout all subsequent steps).
The synaptosomal preparation was incubated in perfusion buffer for 10 min at 37 °C in order to restore metabolic activity. The medium was then supplemented with 0.1 μM [3H]-dopamine for 10 min to promote neurotransmitter uptake into synaptosomes. Aliquots of [3H]-dopamine-loaded synaptosomes (100 μL) were transferred onto GF/B filters mounted into chambers of an 18-channel Brandel superfusion system (Brandel, Gaithersburg, MD). The tissues were washed for 8 min with the buffer (room temperature, flow rate of 1.2 mL/min), then perfused for another 8 min period with antipsychotic solutions (0.01–100 μM, each solution in separate flow channel) to equilibrate their interaction with nicotinic receptors. Control channels (nicotine-evoked full response) were perfused without antipsychotics. Then nicotine (10 μM) alone or in combination with antipsychotics was applied in the perfusion stream of all channels for 48 s. Fractions (24 s) were continuously collected from each channel throughout the experiment to capture basal outflow, agonist-induced [3H]-dopamine release, and to re-establish the baseline following the agonist application (up to 6 min). The perfusate was collected directly into scintillation vials, scintillation cocktail was added and radioactivity was measured by liquid scintillation counting (LS6000, Beckman Coulter, Inc., Fullerton, CA). Nicotine-evoked release of [3H]-dopamine was assessed by summing the radioactivity of fractional dopamine releases over the basal level of [3H] outflow, and was normalized to the basal level of [3H] outflow. Nicotine-evoked release of [3H]-dopamine in samples containing antipsychotics was expressed as a percentage of the effect of a maximally active concentration of 10 μM nicotine.
[3H]-Norepinephrine release assays were conducted using rat hippocampal slices and the same protocol as for [3H]-dopamine. Hippocampi from 2 rats were cross-chopped (McIlwain tissue chopper, Mickle Laboratory Engineering, Surrey, UK) at approximately 250 μm thickness, rinsed twice and immediately immersed in oxygenated perfusion buffer (see above, 2 mL). Then medium was supplemented with 50 nM [3H]-norepinephrine for 30 min at 37 °C for neurotransmitter uptake into slices. Epibatidine-evoked responses were used to measure the nicotinic receptor-mediated hippocampal norepinephrine release. Due to slow association kinetics, α-bungarotoxin (10 μM) was added during the probe-loading procedure for 60 min.
2.5. Electrophysiological studies
The antipsychotics (10 mM stock solutions in DMSO) were diluted in Ringer’s solution to the concentrations used in the experiments. The response to control applications of acetylcholine in the presence 1% DMSO (maximal final concentration) was not different from the response to acetylcholine alone (data not shown). Experiments were conducted using an OpusXpress 6000A (Axon Instruments, Union City, CA, USA). OpusXpress is an integrated system that provides automated impalement and voltage clamp of up to eight oocytes in parallel. Both the voltage and current electrodes were filled with 3 M KCl. Cells were voltage-clamped at a holding potential of −60 mV. Data were collected at 50 Hz and filtered at 20 Hz. Cells were bath-perfused with Ringer’s solution, and agonist solutions were delivered from a 96-well plate via disposable tips, which eliminated any possibility of cross-contamination. Flow rates were set at 2 mL/min. Drug applications alternated between acetylcholine controls and experimental agonists. Applications were 12 s in duration followed by 181-s washout periods. Responses were calculated as net charge (Papke and Porter Papke, 2002). For the determination of IC50, each oocyte received an initial control application of 60 μM acetylcholine, then co-application of 60 μM acetylcholine and antipsychotic at the desired concentration, and a follow-up control application of 60 μM acetylcholine. A control concentration of 60 μM was used since it was sufficient to evoke a large net charge response, yet exhibited responses slow enough to achieve even mixing of agonist and antagonist in a co-application protocol (Papke and Porter Papke, 2002). Responses to drug applications were normalized to the preceding acetylcholine control response, compensating for the varying levels of channel expression among the oocytes. Mean and S.E.M. were calculated from normalized responses of at least four oocytes for each experimental condition. The inhibition study was conducted by comparing acetylcholine net charge values across acetylcholine concentrations in the absence and in the presence of N-desmethylclozapine, at its IC50 level of 6 μM. Under both conditions (i.e. with and without N-desmethylclozapine) responses were normalized to the control response of 300 μM acetylcholine recorded from the same oocyte.
2.6. Calculations and statistics
Results calculated from observations performed in duplicate or triplicate were expressed as arithmetic means ± S.E.M., except for effective doses (EC50) and inhibition constants (IC50), which were expressed as geometric means (with 95% CI, confidence intervals). Maximal responses (Emax values) were obtained by averaging values generated from sigmoid nonlinear regression analyses of individual concentration–response curves. IC50 values and pseudo-Hill coefficients (nH) were determined from fixed and variable slope sigmoid fits, respectively. All data analyses were performed using GraphPAD Prism (GraphPAD, San Diego, CA).
3. Results
3.1. Lack of orthosteric interactions with α4β2 and α7 nicotinic receptors
The typical antipsychotics haloperidol and chlorpromazine, and the atypical antipsychotic clozapine and its major metabolite N-desmethylclozapine, were selected to examine whether antipsychotics interact directly with the orthosteric (i.e., agonist and competitive antagonist) binding sites of nicotinic receptors. [3H]-Nicotine was used to probe α4β2 NNR high affinity binding sites in isolated rat cortical tissue and in recombinant α4β2 NNRs expressed in SH-EP1 cells. [3H]-methyllycaconitine was used to probe α7 NNR binding sites in rat hippocampal preparations. None of the compounds tested inhibited binding of the high affinity orthosteric ligands at concentrations up to 100 μM. No displacement of 5 nM [3H]-nicotine or 5 nM [3H]-methyllycaconitine bound to nicotinic receptors was observed. Neither the dissociation constant (Kd) nor the maximal number of binding sites (Bmax) for saturation binding of [3H]-nicotine or [3H]-methyllycaconitine at 0.5–15 nM were affected (data not presented). Since nicotinic acetylcholine receptors have long been known to undergo allosteric modulation by non-competitive inhibitors (Heidmann et al., 1983) we conducted a series of studies (described below) to determine if the antipsychotics exhibited allosteric effects on α4β2 or α7 NNR function.
3.2. Inhibition of ion flux through α4β2 NNRs by antipsychotics
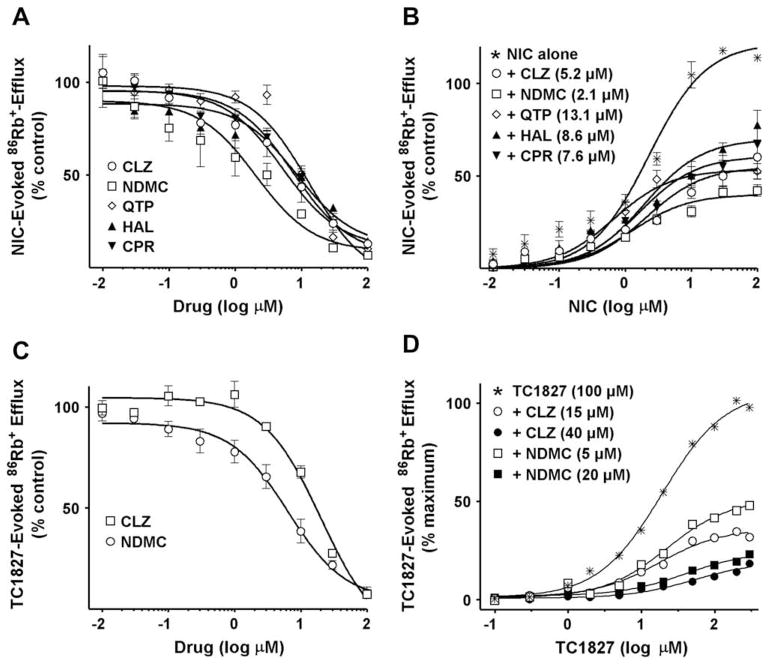
86Rb+ efflux was measured in SH-EP1 cells stably transfected with α4β2 nicotinic receptors (α4β2/SH-EP1 cells). The atypical antipsychotics quetiapine, clozapine and the clozapine metabolite N-desmethylclozapine were selected to examine whether members of this class of antipsychotics directly inhibit the channel permeability of α4β2 NNRs. The effects of the typical antipsychotics haloperidol and chlorpromazine were also assessed for comparison. Activation of 86Rb+ efflux by a sub-maximal concentration of nicotine (10 μM) was used to determine the relative effects of the antipsychotic drugs on agonist-induced ion flux. None of these compounds affected the basal 86Rb+ efflux by themselves (data not shown) but all of them completely inhibited nicotine-evoked 86Rb+ efflux. The inhibition potencies [IC50, mean] were as follows: clozapine, 5.2 μM, N-desmethylclozapine, 2.1 μM, quetiapine, 13.1 μM, haloperidol, 8.6 μM and chlorpromazine, 7.6 μM (Fig. 1A and Table 1). The mechanism of antipsychotic-induced inhibition of nicotinic receptor-channel permeability was further assessed by testing various concentrations of nicotine up to 100 μM, in the presence of antipsychotics at their respective IC50 concentrations (Fig. 1B). At concentrations above 1 μM nicotine activated 86Rb+ efflux in a dose-dependent manner, reaching a plateau in the concentration range 10–100 μM. All antipsychotics tested insurmountably inhibited nicotine-evoked 86Rb+ efflux, decreasing the maximal response (Emax) but not significantly affecting the potency (EC50 range 0.7–2.0 μM). These results support a non-competitive mechanism of inhibition of α4β2 NNRs by all compounds tested, in agreement with previous reports showing similar effects of chlorpromazine on Torpedo marmorata acetylcholine receptor preparations (Heidmann et al., 1983) and clozapine on α3β4 ganglionic nicotinic receptors (Heidmann et al., 1983; Jozwiak et al., 2004).
Fig. 1.
(A) Inhibition of nicotine (NIC)-evoked 86Rb+-efflux from α4β2/SH-EP1 cells by antipsychotics. Data are expressed as percent of control response evoked by 10 μM nicotine (Mean ± S.E.M. for 3–4 independent experiments). Curves were generated using nonlinear regression (individual data were best fitted to a one-site model) for clozapine (CLZ) (○), quetiapine (QTP) (◇), the clozapine metabolite N-desmethylclozapine(NDMC) (□), haloperidol (HAL) (▲) and chlorpromazine (CPR) (▼). (B) Insurmountable effect of antipsychotics at their IC50 concentrations on concentration–response relationship of nicotine for stimulation of 86Rb+-efflux from α4β2/SH-EP1 cells. Data are expressed as a percent of control response evoked by 10 μM nicotine (Mean ± S.E.M. of 3–4 independent experiments). Curves were generated using nonlinear regression (individual data were best fitted to a one-site model). (C) Inhibition of TC-1827-evoked 86Rb+-efflux from α4β2/SH-EP1 cells by clozapine (□) or N-desmethylclozapine (○). Data are expressed as percent of control response evoked by 100 μM TC-1827 (Means ± S.E.M. of 4 independent experiments). Curves were generated using nonlinear regression (individual data were best fitted to a one-site model). (D) Insurmountable effect of 15 μM (○) and 40 μM (●) clozapine, or 5 μM (□) and 20 μM (■) N-desmethylclozapine on the concentration–response relationship of TC-1827 for stimulation of 86Rb+-efflux from α4β2/SH-EP1 cells. Data expressed as a percent of control response evoked by 100 μM TC-1827 (Mean ± S.E.M. of 3 independent experiments). Curves were generated using nonlinear regression (individual data were best fitted to a one-site model).
Table 1.
Comparison of In Vitro Potencies of Antipsychotic Effects with Clinical Plasma Levels The IC50 values are shown for antipsychotic-mediated inhibition of nicotine-evoked Rb + efflux from α4β2/SH-EP1 cells, ACh-induced α7 current in oocytes, nicotine-evoked dopamine (DA) release from striatal synaptosomes and epibatidine-evoked norepinephrine (NE) release from hippocampal slices. Numbers in parentheses represent 95% confidence intervals. Maximum plasma concentrations (ng/mL) of antipsychotics represent values achieved in clinical studies, reported in the literature (supporting references at right). Maximum plasma levels (μM) were calculated from reported concentrations and known molecular weights of the individual compounds (left column).
| Anti-psychotic | Rb + Flux IC50 (μM) | α7 Current IC50 (μM) | DA Release IC50 (μM) | NE Release IC50 (μM) | Max. Plasma Levels (ng/mL) | Max. Plasma Levels (μM) | References |
|---|---|---|---|---|---|---|---|
| Clozapine | 5.2 (2.8–9.9) | 8.4 (3.8–18.8) | 5.3 (2.4–11.6) | 2.2 | 574 | 1.8 | Diaz et al., 2005 |
| MW = 326.8 | 880 | 2.7 | Freudenreich et al., 1996 | ||||
| 1088 | 3.3 | Perry et al., 1991 | |||||
| 1310 | 4.0 | Dumortier et al., 1998 | |||||
| 1920 | 5.9 | Wong et al., 2006 | |||||
| N-desmethyl-clozapine | 2.1 (0.9–4.7) | 3.2 (1.3–7.7) | 2.1 (1.1–3.9) | 2.2 | 420 | 1.3 | Flanagan et al., 2003 |
| 580 | 1.9 | Dumortier et al., 1998 | |||||
| MW = 312.8 | 720 | 2.3 | Wong et al., 2006 | ||||
| Quetiapine | 13.1 (9.1–18.9) | ND | 5.9 (1.1–20.9) | ND | 91 | 0.24 | Lin et al., 2004 |
| MW = 383.5 | 491 | 1.3 | Aichhorn et al., 2006 | ||||
| 1042 | 2.7 | Grimm et al., 2006 | |||||
| 1454 | 3.8 | Tauscher-Wisniewski et al., 2002 | |||||
| Chlorpromazine | 7.6 (4.5–12.7) | 9.1 (6.3–13.2) | 2.8 (1.8–19.6) | 1.7 | 124 | 0.4 | Della Corte et al., 1993 |
| MW = 355.3 | 422 | 1.2 | Chetty et al., 1996 | ||||
| 839 | 2.4 | Chetty and Miller, 2001 | |||||
| Haloperidol | 8.6 (4.2–17.6) | 10.5 (4.5–24.7) | 4.8 (1.2–6.7) | 0.8 | 86 | 0.23 | Roh et al., 2001 |
| MW = 375.9 |
ND = not done.
Since nicotine is a relatively non-selective NNR agonist, we evaluated the effects of the antipsychotics on α4β2/SH-EP1 cells activated by the pro-cognitive α4β2-selective compound TC-1827 (Bohme et al., 2004). We found that TC-1827 dose-dependently activates 86Rb+ efflux from α4β2/SH-EP1 cells, achieving maximal responses at concentrations of 100–300 μM with a potency [EC50, mean (95% CI) = 2.1 μM (1.5–3.5)] nearly an order of magnitude less than that of nicotine [18.9 μM (13.1–27.5)] (compare Fig. 1B and D). Activation of 86Rb+ efflux by 100 μM TC-1827 was fully inhibited by clozapine and N-desmethylclozapine with IC50 values of 20.3 μM (15.1–28.7) and 6.4 μM (3.2–7.7), respectively (Fig. 1C). This inhibition was also insurmountable by TC-1827 at concentrations as high as 300 μM, suggesting a non-competitive mechanism. This conclusion is further supported by the findings that EC50 values for TC-1827, when applied alone [18.9 μM (13.1–27.5)] or in the presence of 15 μM clozapine [17.6 μM (9.3–33.0)] or 5 μM N-desmethylclozapine [22.2 μM (12.0–41.0)], were not significantly different. However, these values were slightly higher in the presence of 40 μM clozapine or 20 μM N-desmethylclozapine, reflecting a typical rightward shift at high concentrations of the non-competitive inhibitor (Fig. 1D). In order to examine the dose dependency of the non-competitive mechanism and to exclude a possible ceiling effect, the actions of clozapine or N-desmethylclozapine on TC-1827- and nicotine-evoked responses were compared at moderate (15 and 1.5 μM, respectively) and at high (100 and 10 μM, respectively) concentrations of agonists. No significant differences in the potencies [IC50 values, mean (95% CI)] of clozapine [19.8 μM (10.2–38.6) and 20.8 μM (15.1–28.7)] or N-desmethylclozapine [8.2 μM (5.2–12.8) and 5.0 μM (3.2–7.7)] were found between these two conditions. Together these data confirm that clozapine and N-desmethylclozapine-induced inhibition is mediated entirely through a non-competitive mechanism.
3.3. Inhibition of nicotinic receptor electrophysiological responses by antipsychotics
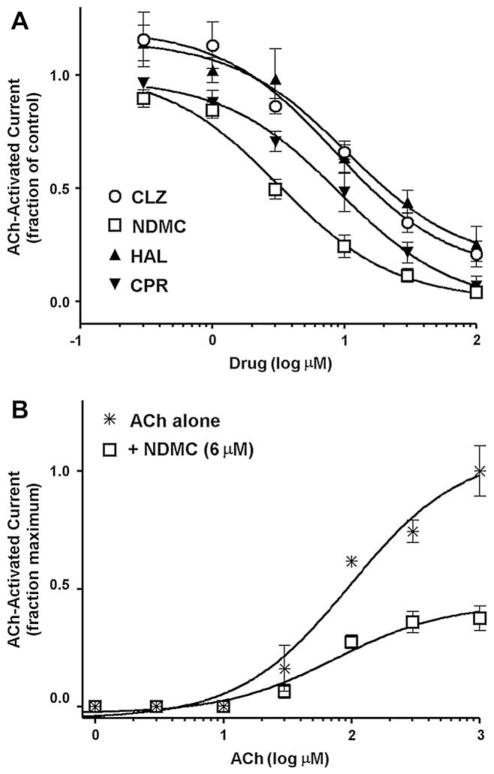
Acetylcholine-stimulated electrophysiological responses of Xenopus laevis oocytes expressing rat α7 NNRs were employed to study the effect of selected antipsychotics on receptor function. All antipsychotics tested (haloperidol, chlorpromazine, clozapine, N-desmethylclozapine), when co-applied with 60 μM acetylcholine produced reversible inhibition of ACh-evoked currents. Responses to consecutive applications of 30 and 100 μM ACh following drug applications recovered to 100 ± 6% and 87 ± 5% for clozapine, 90 ± 3% and 107 ± 6% for N-desmethylclozapine, 87 ± 6% and 97 ± 10% for haloperidol, and 113 ± 17% and 59 ± 6% for chlorpromazine. The potencies [IC50, mean] determined for inhibition of ACh-evoked currents were: clozapine, 8.4 μM, N-desmethylclozapine, 3.2 μM, haloperidol, 10.5 μM and chlorpromazine, 9.1 μM (Fig. 2A and Table 1). Variable slope sigmoid fits of the data revealed no visible cooperativity for interactions of the antipsychotics with α7 NNRs. The pseudo-Hill slopes (nH) were near unity for clozapine (−0.91), N-desmethylclozapine (−1.3), haloperidol (−1.1) and chlorpromazine (−0.84), consistent with a one-site model for their interactions. Co-application of 6 μM N-desmethylclozapine with increasing concentrations of ACh (1 μM–1 mM) resulted in insurmountable inhibition of net charge responses, reducing the maximal response up to 37.5 ± 5% (Fig. 2B). This effect of N-desmethylclozapine implies a non-competitive mechanism of action.
Fig. 2.
(A) Inhibition of acetylcholine (ACh, 60 μM)-stimulated electrophysiological (net charge) responses of Xenopus laevis oocytes expressing rat α7 nicotinic receptors by the antipsychotics clozapine (CLZ) (○), the clozapine metabolite N-desmethylclozapine (NDMC) (□), haloperidol (HAL) (▲) and chlorpromazine (CPR) (▼). Oocytes were voltage-clamped at −60 mV, and data were collected 50 Hz and filtered at 20 Hz. Acetylcholine and drugs were applied (12 s) in the perfusion stream of Ringer’s solution used to bathe (22 °C) the cells. Applications were with ACh alone, then with combination of ACh and antipsychotic, followed by ACh alone for a recovery control. Data are normalized to ACh-alone responses (Means ± S.E.M. for responses of at least 4 oocytes). Curves were generated using nonlinear regression (individual data were best fitted to a one-site model). (B) Insurmountable effect of N-desmethylclozapine (6 μM) on concentration–response relationship of ACh in evoking net charge responses in X. laevis oocytes expressing rat α7 nicotinic receptors. The experimental protocol and data analysis are the same as described for Fig. 2(A) above except that cells were pre-equilibrated with 6 μM N-desmethylclozapine alone for 10 s before the co-application of 6 μM N-desmethylclozapine and varying concentrations of ACh. Data are normalized to ACh-alone maximal response (Means + S.E.M. for responses of at least 4 oocytes).
3.4. Inhibitory effects of antipsychotics on neurotransmitter release
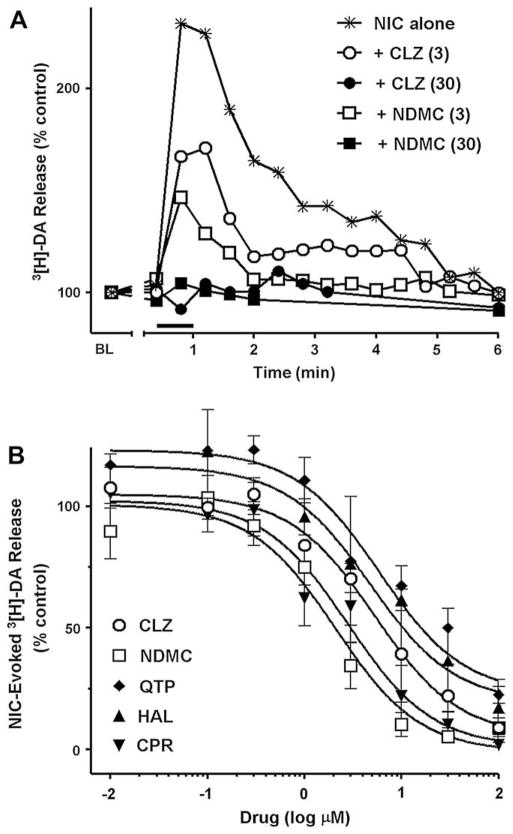
Nicotine-evoked [3H]-dopamine release from rat striatal synaptosomes was used to probe the function of presynaptic naturally-expressed α4β2-containing nicotinic receptors (Champtiaux et al., 2003). The present studies did not attempt to differentiate a6* NNR contributions to release. Nicotine application (10 μM for 48 s) rapidly produced a [3H]-dopamine release peak above that of basal release from perfused [3H]-dopamine-loaded synaptosomes (Fig. 3A). Nicotine-activated dopamine release returned to basal levels within 5 min after nicotine exposure. Pre-application of antipsychotics for 6 min followed by their co-application with 10 μM nicotine completely abolished the nicotine-evoked response. Representative data are shown for 30 μM clozapine and N-desmethylclozapine in Fig. 3A (filled symbols). The ratio of total agonist-evoked [3H]-dopamine release to the basal level of [3H]-dopamine release was used to quantify the effects of the antipsychotics. It was found that all drugs tested inhibited dopamine release in a concentration-dependent manner with potencies [IC50, mean]: clozapine, 5.3 μM, N-desmethylclozapine, 2.1 μM, quetiapine, 5.9 μM, haloperidol, 4.8 μM and chlorpromazine, 2.8 μM (Fig. 3B and Table 1).
Fig. 3.
(A) Representative fractional [3H]-dopamine release from rat striatal synaptosomes evoked by 10 μM nicotine alone (✳) or inhibited by 3 μM clozapine (CLZ) (○), 3 μM N-desmethylclozapine (NDMC) (□), 30 μM CLZ (●), and 30 μM NDMC (■). Data are expressed as % of basal release (BL = mean release of 3 preceding fractions). Each fraction was collected for 24 s. The bar above X axis shows the duration of nicotine application (48 s). Drugs were applied in perfusion stream for 6 min before nicotine and discontinued immediately after co-application with nicotine. (B) Inhibition of 10 μM nicotine-evoked total [3H]-dopamine release (sum of fractional releases) from rat striatal synaptosomes by CLZ (○), NDMC (□), quetiapine (QTP) (◇), haloperidol (HAL) (▲) and chlorpromazine (CPR) (▼). Experimental protocol was the same as described for Fig. 3(A). Data are expressed as % of control (nicotine alone) and are Means ± S.E.M. for 3–4 independent experiments. Curves were generated using nonlinear regression (individual data were best fitted to a one-site model).
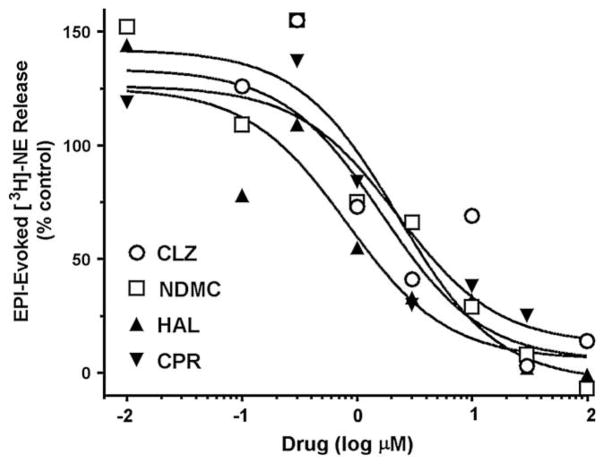
In additional studies, α-Bungarotoxin-sensitive epibatidine-evoked [3H]-NE release from rat hippocampal slices was used to evaluate the effects of the antipsychotics on α7 NNRs, a subtype known to be involved in memory function in this area of the brain. All of the antipsychotics tested, both atypical and typical, were found to completely inhibit responses to epibatidine with IC50 values in the range of 1–2 μM (Fig. 4). Quetiapine was not tested in the [3H]-NE release studies.
Fig. 4.
Inhibition of 100 nM epibatidine (EPI)-evoked total [3H]-Norepinephrine (NE) release (sum of fractional releases) from rat hippocampal slices by clozapine (CLZ) (○), N-desmethylclozapine (NDMC) (□), haloperidol (HAL) (▲) and chlorpromazine (CPR) (▼). Experimental protocol was the same as described for Fig. 3(A). Data are expressed as % of control (NE alone) and represent Means from representative experiments. Curves were generated using nonlinear regression (individual data were best fitted to a one-site model).
4. Discussion
The present results demonstrate that the atypical antipsychotics quetiapine, clozapine, and the clozapine active metabolite N-desmethylclozapine, as well as the typical antipsychotics haloperidol and chlorpromazine non-competitively inhibit the function of mammalian neuronal α4β2 and α7 NNRs with potencies comparable to that of the classical nicotinic receptor antagonist mecamylamine. This was observed for NNRs expressed both in recombinant systems (human SH-EP1 cells and frog oocytes) and in mammalian brain preparations (rat brain synaptosomes and hippocampal slices). With respect to functional inhibition, differences were noted in the potencies of individual antipsychotics but there did not appear to be substantial differences between the typical and atypical antipsychotic classes. Because the antipsychotics tested did not displace [3H]-nicotine or [3H]-methyllycaconitine binding to α4β2 and α7 NNRs, our data do not support a direct interaction with orthosteric binding sites. These results contrast with previous reports that the atypical antipsychotic clozapine inhibited [3H]-nicotine binding in bovine chromaffin cells (Park et al., 2001) and that the typical antipsychotic chlorpromazine inhibited [3H]-nicotine binding both in bovine chromaffin cells (Park et al., 2001) and in rat pheochromocytoma (PC-12) cells (Lee et al., 1999). The presence in those cell types of other non-α4β2 and non-α7 NNR subtypes with different binding characteristics may account in part for the discrepant findings. A number of studies have suggested that functional interactions of antipsychotics with cholinergic receptors are mediated through M1 muscarinic mechanisms (Davies et al., 2005; Friedman, 2004; Weiner et al., 2004). However, the typical antipsychotic chlorpromazine has long been known to interact with non-neuronal nicotinic cholinergic receptors as a non-competitive inhibitor (Heidmann et al., 1983; Heidmann and Changeux, 1986; Lee et al., 1999). A few reports have described functional inhibition of nicotinic receptors by atypical antipsychotics as well, including non-neuronal subtypes in bovine adrenal chromaffin cells (Park et al., 2001) and the vertebrate neuromuscular junction (Nguyen et al., 2002) and more recently the neuronal α7 subtype (Singhal et al., 2007).
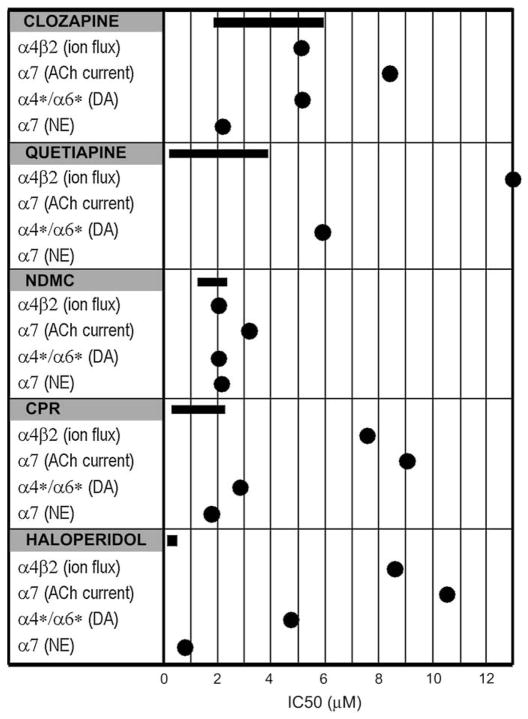
Nicotinic receptor-mediated cognitive effects have been demonstrated in numerous studies, both in animals and in humans (Levin et al., 2006) and the interaction of nicotine with antipsychotic medications is known to impact cognitive processes (Addy and Levin, 2002; Levin and Rezvani, 2007). For example, clinical studies have demonstrated that nicotine causes a dose-related reversal of haloperidol-induced impairments of memory performance in schizophrenics (Levin et al.,1996). By comparison, working memory deficits caused by the nicotinic antagonist mecamylamine are potentiated by haloperidol (Levin and Rose,1995). Our data indicate that both typical and atypical antipsychotics inhibit NNR function with potencies in the low micromolar range, as judged by agonist-stimulated ion flux and neurotransmitter release. Importantly, the IC50 values for all of the antipsychotics tested, with the exception of haloperidol, fall within or near the maximum plasma concentration ranges of antipsychotics observed in human patients (see Table 1 and Fig. 5). The net impact on cognition in schizophrenia, whether it is enhancement or impairment, cannot be predicted based on the present evidence but the potency of the inhibitory effects on α4β2 and α7 NNRs suggests that clinically relevant effects are possible.
Fig. 5.
Comparison of Antipsychotic Inhibitory Potencies to Clinical Plasma Levels The IC50 values for inhibition of receptor function by each of the antipsychotics were plotted for each of the NNR subtypes (●) and compared to the maximum plasma ranges achieved in clinical studies (–). Plasma ranges were derived from literature values shown in Table 1.
In schizophrenia patients, cognitive impairment may be the result of disease evolution but an iatrogenic component has been documented with haloperidol and other antipsychotics (Stip, 2006). These observations, together with the present data, suggest that a component of this cognitive impairment could be associated with their non-competitive inhibition of NNRs. For example, it is well established that chemical blockade with compounds such as mecamylamine (Newhouse et al., 1994) or MK-801 (Wang et al., 2007) or biological interference using animal knock-out models of either α4β2 (Hogg et al., 2003) or α7 NNRs (Young et al., 2007) can produce cognitive dysfunction. However the fact that the atypical antipsychotics are generally less likely to induce cognitive impairment suggests a more complicated relationship. Differential effects noted among some of the typical and atypical antipsychotic drugs are evidenced by studies showing that α7 NNRs contribute to the action of clozapine, but not haloperidol, in reversing auditory gating deficits in animal models (Simosky et al., 2003) and that the atypical neuroleptic risperidone is associated with time dependent and persistent negative effects on α7 NNRs (Terry et al., 2005). In addition, the effects of antipsychotics on cognition can differ, depending on which NNR subtypes are involved and where they are located. For example, blockade of α7 NNRs in the hippocampus with methyllycaconitine has been shown to enhance clozapine-induced memory impairment in rats while blockade of α4β2 NNRs with dihydro-β-erythroidine leads to clozapine-induced improvement in memory (Pocivavsek et al., 2006). The latter observation may suggest the possibility that for some NNR subtypes inhibition of receptor function is a feature, rather than a side effect, of the clinical efficacy of atypical antipsychotics and that inhibition is required for efficacy. However, underactivity of medial frontal cortical β2-containing NNRs increases clozapine-induced working memory impairment in rats (Levin et al., 2009). The dissociation of iatrogenic and disease-induced cognitive impairment, the differential effects of antipsychotic drugs on nicotinic receptors, and the observation that ligand-activated nicotinic receptor-mediated neurotransmitter release is influenced by antipsychotic drugs converge to suggest multiple mechanisms of action for antipsychotics. If NNR blockade is operative in vivo, the true potential of antipsychotics to alleviate cognitive impairment may be diminished to some extent by inhibition of key NNR subtypes. Adjunctive therapies based on nicotinic pharmacologies may either counteract this blockade or may be futile if the inhibition is insurmountable, although it is at least theoretically possible that non-competitive inhibition by the antipsychotics could be competitively antagonized by a ligand that affects their allosteric binding sites. The identification of atypical antipsychotics that enhance cognition but do not block NNR function would help to further clarify this issue. Clearly the final relative concentrations of these drugs in the synapse will define the overall outcome on cholinergic tone. In this regard, future studies involving a more in depth characterization of structure–activity relationships and pharmacokinetic/pharmacodynamic properties of the typical and atypical antipsychotics vis-à-vis NNR interactions would be instructive.
The heavy smoking behavior of schizophrenia patients will also certainly influence receptor interactions and neurotransmitter tone. This is consistent with previous proposals that their smoking behavior is at least partly motivated by the need to overcome undesirable side effects associated with antipsychotic agents (Barnes et al., 2006). However, there is also literature suggesting that both neuroleptic-naïve schizophrenics as well as in-patient schizophrenics display heavy smoking behavior and that this feature may be independent of side effects (McEvoy and Brown, 1999). The present results with the pro-cognitive α4β2-selective NNR agonist TC-1827 suggest that this NNR subtype may mediate complex interactions of nicotinic agonists with antipsychotics that affect cognitive tone, possibly providing additional motivation to smoke. Thus in the development of cognition-enhancing drugs for schizophrenia that target this NNR subtype, it should be considered that the presence of nicotine may prevent optimal activation of the receptors. The picture is further complicated by our emerging understanding of the structural and functional diversity of NNR subtypes. For example, the stoichiometry of α4β2 NNRs [(α4)2(β2)3 or (α4)3(β2)2] can impact sensitivity to agonist activation (Moroni et al., 2006). Similarly, α7 NNRs can exist both as homomeric [(α7)5] and heteromeric (α7β2*) subtypes (Liu et al., 2009). The present approaches have not probed these differences. Therefore additional studies are needed to fully understand the influence of NNR subforms. Hopefully a more complete understanding of drug–drug interactions between antipsychotics and NNR-targeted drugs will unlock the potential of NNR-based therapies in the management of positive and negative symptoms as well as cognitive deficits in neuropsychiatric disorders.
Acknowledgments
The authors are grateful to Dr. Kristen G. Jordan at Targacept, Inc. for helpful suggestions regarding the manuscript and to Khalima Sadieva at Targacept, Inc. and Clare Stokes at University of Florida for technical assistance. The financial support for these studies was provided by Targacept, Inc. and for the RL Papke studies, NIH grant GM57481.
References
- Addy N, Levin ED. Nicotine interactions with haloperidol, clozapine and risperidone and working memory function in rats. Neuropsychopharmacology. 2002;27:534–541. doi: 10.1016/S0893-133X(02)00327-5. [DOI] [PubMed] [Google Scholar]
- Aichhorn W, Marksteiner J, Walch T, Zernig G, Saria A, Kemmler G. Influence of age, gender, body weight and valproate comedication on quetiapine plasma concentrations. Int Clin Psychopharmacol. 2006;21:81–85. doi: 10.1097/01.yic.0000188213.46667.f1. [DOI] [PubMed] [Google Scholar]
- Barnes M, Lawford BR, Burton SC, Heslop KR, Noble EP, Hausdorf K, Young RM. Smoking and schizophrenia: is symptom profile related to smoking and which antipsychotic medication is of benefit in reducing cigarette use? Aust NZ J Psychiatry. 2006;40:575–580. doi: 10.1080/j.1440-1614.2006.01841.x. [DOI] [PubMed] [Google Scholar]
- Bencherif M, Schmitt JD. Targeting neuronal nicotinic receptors: a path to new therapies. Curr Drug Targets CNS Neurol Disord. 2002;1:349–357. doi: 10.2174/1568007023339094. [DOI] [PubMed] [Google Scholar]
- Bencherif M, Schmitt JD, Bhatti BS, Crooks P, Caldwell WS, Lovette ME, Fowler K, Reeves L, Lippiello PM. The heterocyclic substituted pyridine derivative (+/−)-2-(-3-pyridinyl)-1-azabicyclo[2.2.2]octane (RJR-2429): a selective ligand at nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 1998;284:886–894. [PubMed] [Google Scholar]
- Bohme GA, Letchworth SR, Piot-Grosjean O, Gatto GJ, Obinu MC, Caldwell WS, Laville M, Brunel P, Pellerin R, Leconte JP, Genevois-Borella A, Dubedat P, Mazadier M, Pradier L, Bencherif M, Benavides J. In vitro and in vivo characterization of TC-1827, a novel brain alpha4beta2 nicotinic receptor agonist with pro-cognitive activity. Drug Dev Res. 2004;62:26–40. [Google Scholar]
- Breese CR, Lee MJ, Adams CE, Sullivan B, Logel J, Gillen KM, Marks MJ, Collins AC, Leonard S. Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology. 2000;23:351–364. doi: 10.1016/S0893-133X(00)00121-4. [DOI] [PubMed] [Google Scholar]
- Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Lena C, Clementi F, Moretti M, Rossi FM, Le NN, McIntosh JM, Gardier AM, Changeux JP. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci. 2003;23:7820–7829. doi: 10.1523/JNEUROSCI.23-21-07820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetty M, Pillay VL, Moodley SV, Miller R. Response in chronic schizophrenia correlated with chlorpromazine, 7-OH-chlorpromazine and chlorpromazine sulfoxide levels. Eur Neuropsychopharmacol. 1996;6:85–91. doi: 10.1016/0924-977x(95)00047-s. [DOI] [PubMed] [Google Scholar]
- Chetty M, Miller R. Oral contraceptives increase the plasma concentrations of chlorpromazine. Ther Drug Monit. 2001;23:556–558. doi: 10.1097/00007691-200110000-00011. [DOI] [PubMed] [Google Scholar]
- Davies MA, Compton-Toth BA, Hufeisen SJ, Meltzer HY, Roth BL. The highly efficacious actions of N-desmethylclozapine at muscarinic receptors are unique and not a common property of either typical or atypical antipsychotic drugs: is M1 agonism a pre-requisite for mimicking clozapine’s actions? Psychopharmacology (Berl) 2005;178:451–460. doi: 10.1007/s00213-004-2017-1. [DOI] [PubMed] [Google Scholar]
- Della Corte L, Valoti M, Palmi M, Giovannini MG, Sgaragli GP. Pharmacokinetics of chlorimipramine, chlorpromazine and their N-dealkylated metabolites in plasma of healthy volunteers after a single oral dose of the parent compounds. J Pharm Pharmacol. 1993;45:825–829. doi: 10.1111/j.2042-7158.1993.tb05694.x. [DOI] [PubMed] [Google Scholar]
- Diaz FJ, de LJ, Josiassen RC, Cooper TB, Simpson GM. Plasma clozapine concentration coefficients of variation in a long-term study. Schizophr Res. 2005;72:131–135. doi: 10.1016/j.schres.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Dumortier G, Lochu A, Zerrouk A, Van NV, Colen de MP, Roche RD, Degrassat K. Whole saliva and plasma levels of clozapine and desmethylclozapine. J Clin Pharm Ther. 1998;23:35–40. doi: 10.1046/j.1365-2710.1998.00132.x. [DOI] [PubMed] [Google Scholar]
- Flanagan RJ, Yusufi B, Barnes TR. Comparability of whole-blood and plasma clozapine and norclozapine concentrations. Br J Clin Pharmacol. 2003;56:135–138. doi: 10.1046/j.1365-2125.2003.01821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Olincy A, Ross RG, Waldo MC, Stevens KE, Adler LE, Leonard S. The genetics of sensory gating deficits in schizophrenia. Curr Psychiatry Rep. 2003;5:155–161. doi: 10.1007/s11920-003-0032-2. [DOI] [PubMed] [Google Scholar]
- Freudenreich O, McEvoy JP, Cooper TB. Monitoring of clozapine blood levels in the real world. J Clin Psychopharmacol. 1996;16:90–91. doi: 10.1097/00004714-199602000-00023. [DOI] [PubMed] [Google Scholar]
- Friedman JI. Cholinergic targets for cognitive enhancement in schizophrenia: focus on cholinesterase inhibitors and muscarinic agonists. Psycho-pharmacology (Berl) 2004;174:45–53. doi: 10.1007/s00213-004-1794-x. [DOI] [PubMed] [Google Scholar]
- Grimm SW, Richtand NM, Winter HR, Stams KR, Reele SB. Effects of cytochrome P450 3A modulators ketoconazole and carbamazepine on quetiapine pharmacokinetics. Br J Clin Pharmacol. 2006;61:58–69. doi: 10.1111/j.1365-2125.2005.02507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinevich VP, Letchworth SR, Lindenberger KA, Menager J, Mary V, Sadieva KA, Buhlman LM, Bohme GA, Pradier L, Benavides J, Lukas RJ, Bencherif M. Heterologous expression of human {alpha}6{beta}-4{beta}3{alpha}5 nicotinic acetylcholine receptors: binding properties consistent with their natural expression require quaternary subunit assembly including the {alpha}5 subunit. J Pharmacol Exp Ther. 2005;312:619–626. doi: 10.1124/jpet.104.075069. [DOI] [PubMed] [Google Scholar]
- Heidmann T, Changeux JP. Characterization of the transient agonist-triggered state of the acetylcholine receptor rapidly labeled by the noncompetitive blocker [3H]chlorpromazine: additional evidence for the open channel conformation. Biochemistry. 1986;25:6109–6113. doi: 10.1021/bi00368a041. [DOI] [PubMed] [Google Scholar]
- Heidmann T, Oswald RE, Changeux JP. Multiple sites of action for noncompetitive blockers on acetylcholine receptor rich membrane fragments from torpedo marmorata. Biochemistry. 1983;22:3112–3127. doi: 10.1021/bi00282a014. [DOI] [PubMed] [Google Scholar]
- Hogg RC, Raggenbass M, Bertrand D. Nicotinic acetylcholine receptors: from structure to brain function. Rev Physiol Biochem Pharmacol. 2003;147:1–46. doi: 10.1007/s10254-003-0005-1. [DOI] [PubMed] [Google Scholar]
- Jozwiak K, Ravichandran S, Collins JR, Wainer IW. Interaction of noncompetitive inhibitors with an immobilized alpha3beta4 nicotinic acetylcholine receptor investigated by affinity chromatography, quantitative-structure activity relationship analysis, and molecular docking. J Med Chem. 2004;47:4008–4021. doi: 10.1021/jm0400707. [DOI] [PubMed] [Google Scholar]
- Kumari V, Postma P. Nicotine use in schizophrenia: the self medication hypotheses. Neurosci Biobehav Rev. 2005;29:1021–1034. doi: 10.1016/j.neubiorev.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Lee IS, Park TJ, Suh BC, Kim YS, Rhee IJ, Kim KT. Chlorpromazine-induced inhibition of catecholamine secretion by a differential blockade of nicotinic receptors and L-type Ca2+ channels in rat pheochromocytoma cells. Biochem Pharmacol. 1999;58:1017–1024. doi: 10.1016/s0006-2952(99)00181-1. [DOI] [PubMed] [Google Scholar]
- Leonard S, Adler LE, Benhammou K, Berger R, Breese CR, Drebing C, Gault J, Lee MJ, Logel J, Olincy A, Ross RG, Stevens K, Sullivan B, Vianzon R, Virnich DE, Waldo M, Walton K, Freedman R. Smoking and mental illness. Pharmacol Biochem Behav. 2001;70:561–570. doi: 10.1016/s0091-3057(01)00677-3. [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, Perkins A, Brotherton T, Qazi M, Berez C, Montalvo-Ortiz J, Davis K, Williams P, Christopher NC. Chronic underactivity of medial frontal cortical beta2-containing nicotinic receptors increases clozapine-induced working memory impairment in female rats. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:296–302. doi: 10.1016/j.pnpbp.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH. Nicotinic interactions with antipsychotic drugs, models of schizophrenia and impacts on cognitive function. Biochem Pharmacol. 2007;74:1182–1191. doi: 10.1016/j.bcp.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Rose JE. Acute and chronic nicotinic interactions with dopamine systems and working memory performance. Ann NY Acad Sci. 1995;757:245–252. doi: 10.1111/j.1749-6632.1995.tb17481.x. [DOI] [PubMed] [Google Scholar]
- Levin ED, Wilson W, Rose JE, McEvoy J. Nicotine-haloperidol interactions and cognitive performance in schizophrenics. Neuropsychopharmacology. 1996;15:429–436. doi: 10.1016/S0893-133X(96)00018-8. [DOI] [PubMed] [Google Scholar]
- Li Z, Huang M, Ichikawa J, Dai J, Meltzer HY. N-desmethylclozapine, a major metabolite of clozapine, increases cortical acetylcholine and dopamine release in vivo via stimulation of M1 muscarinic receptors. Neuro-psychopharmacology. 2005;30:1986–1995. doi: 10.1038/sj.npp.1300768. [DOI] [PubMed] [Google Scholar]
- Lin SN, Chang Y, Moody DE, Foltz RL. A liquid chromatographic-electrospray-tandem mass spectrometric method for quantitation of quetiapine in human plasma and liver microsomes: application to study in vitro metabolism. J Anal Toxicol. 2004;28:443–448. doi: 10.1093/jat/28.6.443. [DOI] [PubMed] [Google Scholar]
- Liu Q, Huang Y, Xue F, Simard A, DeChon J, Li G, Zhang J, Lucero L, Wang M, Sierks M, Hu G, Chang Y, Lukas RJ, Wu J. A novel nicotinic acetylcholine receptor subtype in basal forebrain cholinergic neurons with high sensitivity to amyloid peptides. J Neurosci. 2009;29:918–929. doi: 10.1523/JNEUROSCI.3952-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luntz-Leybman V, Bickford PC, Freedman R. Cholinergic gating of response to auditory stimuli in rat hippocampus. Brain Res. 1992;587:130–136. doi: 10.1016/0006-8993(92)91437-j. [DOI] [PubMed] [Google Scholar]
- Martin LF, Freedman R. Schizophrenia and the alpha7 nicotinic acetylcholine receptor. Int Rev Neurobiol. 2007;78:225–246. doi: 10.1016/S0074-7742(06)78008-4. [DOI] [PubMed] [Google Scholar]
- Marutle A, Zhang X, Court J, Piggott M, Johnson M, Perry R, Perry E, Nordberg A. Laminar distribution of nicotinic receptor subtypes in cortical regions in schizophrenia. J Chem Neuroanat. 2001;22:115–126. doi: 10.1016/s0891-0618(01)00117-x. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Brown S. Smoking in first-episode patients with schizophrenia. Am J Psychiatry. 1999;156:1120–1121. doi: 10.1176/ajp.156.7.1120a. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, McGurk SR. The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull. 1999;25:233–255. doi: 10.1093/oxfordjournals.schbul.a033376. [DOI] [PubMed] [Google Scholar]
- Moroni M, Zwart R, Sher E, Cassels BK, Bermudez I. alpha4beta2 nicotinic receptors with high and low acetylcholine sensitivity: pharmacology, stoichiometry, and sensitivity to long-term exposure to nicotine. Mol Pharmacol. 2006;70:755–768. doi: 10.1124/mol.106.023044. [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Corwin J, Lenox R. Age-related effects of the nicotinic antagonist mecamylamine on cognition and behavior. Neuro-psychopharmacology. 1994;10:93–107. doi: 10.1038/npp.1994.11. [DOI] [PubMed] [Google Scholar]
- Nguyen QT, Yang J, Miledi R. Effects of atypical antipsychotics on vertebrate neuromuscular transmission. Neuropharmacology. 2002;42:670–676. [PubMed] [Google Scholar]
- Papke RL, Porter Papke JK. Comparative pharmacology of rat and human alpha7 nAChR conducted with net charge analysis. Br J Pharmacol. 2002;137:49–61. doi: 10.1038/sj.bjp.0704833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park T, Bae S, Choi S, Kang B, Kim K. Inhibition of nicotinic acetylcholine receptors and calcium channels by clozapine in bovine adrenal chromaffin cells. Biochem Pharmacol. 2001;61:1011–1019. doi: 10.1016/s0006-2952(01)00577-9. [DOI] [PubMed] [Google Scholar]
- Perry PJ, Miller DD, Arndt SV, Cadoret RJ. Clozapine and norclozapine plasma concentrations and clinical response of treatment-refractory schizophrenic patients. Am J Psychiatry. 1991;148:231–235. doi: 10.1176/ajp.148.2.231. [DOI] [PubMed] [Google Scholar]
- Pocivavsek A, Icenogle L, Levin ED. Ventral hippocampal alpha7 and alpha4beta2 nicotinic receptor blockade and clozapine effects on memory in female rats. Psychopharmacology (Berl) 2006;188:597–604. doi: 10.1007/s00213-006-0416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh HK, Chung JY, Oh DY, Park CS, Svensson JO, Dahl ML, Bertilsson L. Plasma concentrations of haloperidol are related to CYP2D6 genotype at low, but not high doses of haloperidol in Korean schizophrenic patients. Br J Clin Pharmacol. 2001;52:265–271. doi: 10.1046/j.0306-5251.2001.01437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco KA, Bannon KL, George TP. Nicotinic receptor mechanisms and cognition in normal states and neuropsychiatric disorders. J Psychopharmacol. 2004;18:457–474. doi: 10.1177/0269881104047273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simosky JK, Stevens KE, Adler LE, Freedman R. Clozapine improves deficient inhibitory auditory processing in DBA/2 mice, via a nicotinic cholinergic mechanism. Psychopharmacology (Berl) 2003;165:386–396. doi: 10.1007/s00213-002-1285-x. [DOI] [PubMed] [Google Scholar]
- Simosky JK, Stevens KE, Kem WR, Freedman R. Intragastric DMXB-A, an alpha7 nicotinic agonist, improves deficient sensory inhibition in DBA/2 mice. Biol Psychiatry. 2001;50:493–500. doi: 10.1016/s0006-3223(01)01093-9. [DOI] [PubMed] [Google Scholar]
- Singhal SK, Zhang L, Morales M, Oz M. Antipsychotic clozapine inhibits the function of alpha7-nicotinic acetylcholine receptors. Neuropharmacology. 2007;52:387–394. doi: 10.1016/j.neuropharm.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Stip E. Cognition, schizophrenia and the effect of antipsychotics. Encephale. 2006;32:341–350. doi: 10.1016/s0013-7006(06)76162-0. [DOI] [PubMed] [Google Scholar]
- Tauscher-Wisniewski S, Kapur S, Tauscher J, Jones C, Daskalakis ZJ, Papatheodorou G, Epstein I, Christensen BK, Zipursky RB. Quetiapine: an effective antipsychotic in first-episode schizophrenia despite only transiently high dopamine-2 receptor blockade. J Clin Psychiatry. 2002;63:992–997. doi: 10.4088/jcp.v63n1106. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Gearhart DA, Mahadik SP, Warsi S, Davis LW, Waller JL. Chronic exposure to typical or atypical antipsychotics in rodents: temporal effects on central alpha7 nicotinic acetylcholine receptors. Neuroscience. 2005;136:519–529. doi: 10.1016/j.neuroscience.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Wang JH, Zhang B, Meng ZQ, Sun NL, Ma MX, Zhang HX, Tang X, Sanford LD, Wilson FA, Hu XT, Carlson S, Ma YY. Learning large-scale spatial relationships in a maze and effects of MK-801 on retrieval in the rhesus monkey. Dev Neurobiol. 2007;67:1731–1741. doi: 10.1002/dneu.20547. [DOI] [PubMed] [Google Scholar]
- Weiner DM, Meltzer HY, Veinbergs I, Donohue EM, Spalding TA, Smith TT, Mohell N, Harvey SC, Lameh J, Nash N, Vanover KE, Olsson R, Jayathilake K, Lee M, Levey AI, Hacksell U, Burstein ES, Davis RE, Brann MR. The role of M1 muscarinic receptor agonism of N-desme-thylclozapine in the unique clinical effects of clozapine. Psychopharmacology (Berl) 2004;177:207–216. doi: 10.1007/s00213-004-1940-5. [DOI] [PubMed] [Google Scholar]
- Wong JO, Leung SP, Mak T, Ng RM, Chan KT, Hon-Kee CH, Choi WK, Lai J, Wai-Kiu TA. Plasma clozapine levels and clinical response in treatment-refractory Chinese schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:251–264. doi: 10.1016/j.pnpbp.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Young JW, Crawford N, Kelly JS, Kerr LE, Marston HM, Spratt C, Finlayson K, Sharkey J. Impaired attention is central to the cognitive deficits observed in alpha 7 deficient mice. Eur Neuropsychopharmacol. 2007;17:145–155. doi: 10.1016/j.euroneuro.2006.03.008. [DOI] [PubMed] [Google Scholar]