Abstract
The activation of heteromeric and homomeric nicotinic acetylcholine receptors was studied in Xenopus laevis oocytes to identify key structures of putative agonist molecules associated with the selective activation of homomeric α7 receptors. We observed that selectivity between α7 and α4β2 was more readily obtained than selectivity between α7 and α3β4. Based on structural comparisons of previously characterized selective and nonselective agonists, we hypothesize at least three chemical motifs exist that, when present in molecules containing an appropriate cationic center, could be associated with the selective activation of α7 receptors. We identify the three distinct structural motifs based on prototypical drugs as the choline motif, the tropane motif, and the benzylidene motif. The choline motif involves the location of an oxygen-containing polar group such as a hydroxyl or carbonyl separated by two carbons from the charged nitrogen. The tropane motif provides α7-selectivity based on the addition of multiple small hydrophobic groups positioned away from the cationic center in specific orientations. We show that this motif can convert the nonselective agonists quinuclidine and ethyltrimethyl-ammonium to the α7-selective analogs methyl-quinuclidine and diethyldimethyl-ammonium, respectively. We have shown previously that the benzylidene group of 3–2,4, dimethoxy-benzylidene anabaseine (GTS-21) converts anabaseine into an α7-selective agonist. The benzylidene motif was also applied to quinuclidine to generate another distinct family of α7-selective agonists. Our results provide insight for the further development of nicotinic therapeutics and will be useful to direct future experiments with protein structure-based modeling and site-directed mutagenesis.
The nicotinic acetylcholine receptors of the brain can be broadly divided into two classes: heteromeric β-subunit-containing receptors, and homomeric α7-type receptors. Homomeric α7-type receptors have emerged as an exciting potential therapeutic target for several indications, and this has encouraged the development of α7-selective agonists. This path of drug development relies on the consideration of both features that distinguish the heteromeric receptors from the homomeric receptors and features that distinguish selective agonists from nonselective agonists.
Both heteromeric, β-subunit-containing receptors and homomeric α7-type receptors are pentameric. The heteromeric neuronal nAChR contain at least one or more α subunits (α2–α6) and additional β subunits (β2–β4), with two agonist binding sites located at the interface between α and β subunits (Dani, 2001). Neuronal nicotinic receptor α subunits are classified as such based on sequence homology to the α subunits of muscle-type receptors (Heinemann et al., 1990), and essential conserved aspects of muscle and neuronal α subunits provide specialized subdomains that contribute to the primary face of an asymmetrical binding site for acetylcholine and other agonists. In contrast, there is structural homology between muscle-type γ, δ, and ε subunits and neuronal β2 and β4 subunits that provide in these subunits the specialized subdomains for the complimentary face of the agonist binding site (Le Novere et al., 2002b). Although the majority of heteromeric receptors in the mammalian brain are believed to contain just α4 and β2 subunits (Flores et al., 1992), minor populations may contain additional subunits in various configurations (Turner and Kellar, 2005). The specializations for forming an agonist binding site seem to be lacking in the muscle β1 and the neuronal α5 and β3 subunits, so these have been identified as “structural subunits” (Gotti et al., 2006).
At least two emergent properties are likely to have come from the specialization of the non-α subunits in the agonist binding sites. These two properties are the failure of heteromeric receptors to be activated efficiently by the ACh precursor choline (Papke et al., 1996), and the conversion of the receptors to desensitized states with high affinity for agonist, in parallel to or after activation (Higgins and Berg, 1988; Buisson and Bertrand, 2001). These features are common to all heteromeric nAChRs, including muscle-type receptors. In contrast to the heteromeric receptors, for the homomeric α7 receptors of the brain, choline is a fully efficacious agonist, and α7 receptors do not convert to high-affinity desensitized states.
The homomer-forming subunit α7 has been identified as phylogenetically ancestral to the more specialized subunits of the heteromeric receptors, and as such it contains subdomains able to contribute to either the primary or complimentary faces of up to five agonist binding sites per receptor (Le Novere et al., 2002a). Although some unique biophysical properties may emerge from the presence of so many potential agonist binding sites (Papke et al., 2000), it is presumably the lack of certain specializations in the binding site that has made it relatively easy to identify agonists that will activate α7 receptors but not heteromeric receptors like those containing α4 and β2 or α3 and β4.
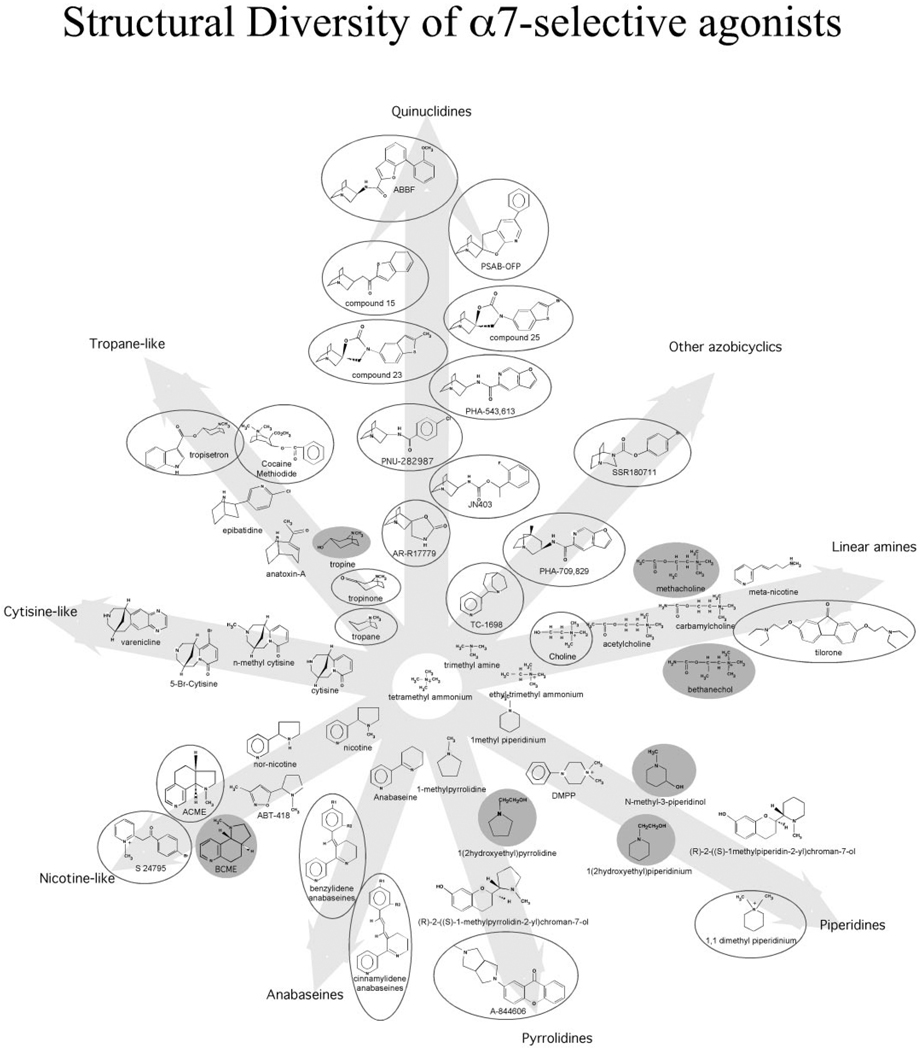
Our conceptual approach has been to classify “core agonist” structures because they represent different elaborations of the simplest cationic center of tetramethyl-ammonium (TMA). We have arranged into nine structurally related families of agonists, compounds that have been functionally characterized in the published literature by ourselves or others, or in unpublished studies conducted in our laboratory (Fig. 1). Agonists that selectively activate α7 nAChR have been identified in most of the structural classes. Table 1 provides summaries of the function studies that have been conducted on these various compounds.
Figure 1.
Structural diversity of selective activators of α7 nAChR. Multiple structural classes of nicotinic agonists are represented. Circled compounds are α7-selective. Compounds in filled gray circles had no significant agonist activity when tested at 300 µM on α7, α4β2, or α3β4 receptors (data not shown).
TABLE 1. Characterization of putative α7-selective agonists.
Compounds circled in Fig. 1 have been introduced into the literature as α7-selective agonists or partial agonists. However, the functional studies that provided the basis for this attribution have varied greatly in both the methods used and the number of receptor subtypes that were studied. Provided are brief synopses of the salient features of the studies cited and their conclusions. Note that in some cases, arguments for selectivity were based largely on binding data, which would not discriminate between agonists and antagonists, rather than on functional studies. Also, some functional assays relied on cell lines (e.g., PC12 cells), which vary in their nAChR expression from laboratory to laboratory or over time in a single laboratory. See Fig. 1 for structures.
| Compound | Expression System | nAChR Subtypes Studied | Effects on 5HT3 Receptors | Summary | Reference |
|---|---|---|---|---|---|
| A-844606 | X. laevis oocyte-transfected cells and IMR-32 cells | Human α7, α4β2, rat α7, putative α3β4 (IMR-32) | Not studied | Partial agonist of a7 with relatively little activation of other nAChR tested. Demonstrated to have antagonist activity for α4β2 and more potently for putative α3β4. Mechanism of antagonism not investigated. | Briggs et al., 2008 |
| ABBF | X. laevis oocytes | α7, α3β4, α4β2, Muscle (subunit composition not specified) | Antagonist | Putative full agonist for human α7 (EC50 ≈ 3 µM) and an antagonist for other nAChR tested (IC50 values of 1–8 µM). Mechanism of antagonism not investigated. Bound to 5HT3 receptors with high affinity (60 nM), comparable with rat brain membrane α7 sites (62 nM). | Boess et al., 2007 |
| ACME and ACME-B | X. laevis oocytes | α7, α3β4, α4β2 | Not studied | The rigid nicotine analogs ACME and ACME-B were partial agonists for rat α7 nAChR (Imax, 10 and 20% of that of ACh, respectively). ACME-B was 2-fold more potent (EC50 ≈ 26 µM) than the boron-free form ACME. Potential antagonist activity of non-α7 nAChR not investigated. | Papke et al., 2005b) |
| AR-R17779 | X. laevis oocytes | α7, α4β2, α3β4, α3β2, α3β3α5 | None | Initial characterization by Mullen et al. examined function at just α7 and no other nAChR. Claims for α7-selectivity was based solely on binding studies with rat brain membranes. Subsequent study by Papke et al. at other nAChR subtypes and the 5HT3 receptor. It was confirmed to be a full agonist for α7 with negligible agonist or antagonist activity for other receptors tested. | Mullen et al., 2000; Papke et al., 2004b |
| Benzylidene anabaseines | X. laevis ohocytes | α7, α2β2, α3β2, α3β4,α2β4,α3β4, α4β4 | Partial agonist and antagonist activity related to side groups on benzylidene ring | Numerous benzylidene anabaseines have been studied. They all seem to be α7-selective partial agonists but vary widely in both potency and efficacy. They are effective antagonists of other nAChR subtypes, and in some cases, the inhibition has been shown to be use-dependent. | Meyer et al., 1997, 1998a; Machu et al., 2001; Kem et al., 2004; Papke et al., 2004a |
| Choline | X. laevis oocytes | α1β1γδ, α3β4, α3β2, α4β2, α7, α9 | Not studied | Choline was initially identified as full agonist for α7 nAChR with little or no activity at heteromeric nAChR. The potency of choline for α7 is 10-fold lower than that of ACh. Antagonist activity was not studied in detail, although channel block is likely to occur at high (>1 mM) concentrations. Choline was later shown also to be an agonist for α9 nAChR (Verbitsky et al.). | Papke et al., 1996; Verbitsky et al., 2000 |
| Cinnamylidene anabaseines | X. laevis oocytes | α1β1γδ, α2β2, α3β2, α3β4, α4β4, α7 | Not studied | Numerous cinnamylidene anabaseines have been studied. They all seem to be α7-selective agonists or partial but vary widely in both potency and efficacy. They are effective antagonists of other nAChR subtypes, and in some cases, the inhibition has been shown to be use-dependent. | de Fiebre et al., 1995; Meyer et al., 1998b |
| Cocaine methiodide | X. laevis oocytes and transfected cells | α3β2, α3β4, α4β2, α1β1γδ, α7 | Not studied | Although cocaine was an antagonist at multiple nAChR subtypes, the methiodide derivative was a selective activator of rat, human, and chick α7 receptors expressed in oocytes and was also able to activate human α7 receptors transiently expressed in PC12 cells. Cocaine methiodide was a weak antagonist at other nAChR subtypes studied. | Francis et al., 2001 |
| Compound 15b | PC12 cells | α7 and possibly others | Not studied | No data provided to support selectivity. Relatively affinity for α7 supported by α-btx binding experiments but contrasting data for α4β2* receptors was not provided. Functional data were obtained from PC12 cells, which may express α7 and other nAChR subtypes. Selective antagonists were not used to confirm that evoked responses were α7-mediated. The (+) isomer of 15b had 5-fold greater agonist activity, but both isomers had similar affinity in binding assays. | Tatsumi et al., 2004 |
| Compound 23 | Cultured hippocampal neurons | α7 (putative α4β2* receptors in binding assays) | Affinity for 5HT3 receptors in binding experiments (Ki ≈ 10 nM), nearly as high as for putative α7 receptors (Ki ≈ 3 nM) | Only binding data are provided to support selectivity. There was high affinity for putative α7 and low affinity for α4β2* receptors. However, the data also indicated high affinity for 5HT3 receptors, but no functional tests of 5HT3 receptors were conducted. Functional data were obtained from cultured neurons, which predominantly express α7 nAChR, and selective antagonists were used to support the conclusion that α7 receptors were activated. Efficacy was only approximately 40%, and potency in the functional assay was 1000-fold lower than affinity obtained in binding assays. | Tatsumi et al., 2006 |
| Compound 25 | Cultured hippocampal neurons | α7 (putative α4β2* and muscle-type receptors in binding assays) | Not studied | Only binding data are provided to support selectivity. There was high affinity for putative α7 and low affinity for α4β2* and muscle type receptors. Functional data were obtained from cultured neurons that predominantly express α7 nAChR, and selective antagonists were used to support the conclusion that α7 receptors were activated. Efficacy was only approximately 40%, and potency in the functional assay was potency was 1000-fold lower than affinity obtained in binding assays. | Tatsumi et al., 2005 |
| Dimethyl piperidinium | X. laevis oocytes | α3β4, α4β2, α7 | Antagonist activity reported | A partial agonist (≥60% efficacy compared with ACh) selective for α7 among the limited number of receptor subtypes tested. Antagonist activity at α3β4 and 5HT3 receptors but not for α4β2. | Papke et al., 2005a |
| JN403 | Transfected cells and X. laevis oocytes | α1β1γδ, α3β4, α3β2, α4β2, α7 | Relatively low potency antagonist (IC50 ≈ 20 µM) | In the X. laevis oocyte experiments, JN403 was a relatively potent partial agonist, with an efficacy of approximately 50% of that of ACh. 5HT3 receptors and other nAChRs were evaluated with calcium flux measurements in transfected cells, and JN403 was antagonist in these experiments. | Feuerbach et al., 2007 |
| PHA-543,613 | Transfected cells and cell lines | Putative α3β4, α1β1γδ, putative α4β2* in rat brain membranes | Inhibitory activity at ≈ 600 nM | Functional data for α3β4 and α1β1γδ were obtained from calcium flux measurements, and the compound was a weak antagonist in these assays, although it was a much more potent antagonist at 5HT3 receptors. Interactions with putative α4β2 nAChR were only evaluated with very cursory binding experiments. Functional data were obtained from cultured neurons that predominately express α7 nAChR, and selective antagonists were used to support the conclusion that α7 receptors were activated. | Wishka et al., 2006 |
| PHA-709829 | Transfected cells and cultured hippocampal neurons | Putative α3β4, α1β1γδ, putative α4β2* in rat brain membranes | Inhibitory activity at ≈ 300 nM | Functional data for α3β4 and α1β1γδ were obtained from calcium flux measurements, and the compound was a weak antagonist in these assays, although it was a much more potent antagonist at 5HT3 receptors. Interactions with putative α4β2 nAChR were only evaluated with very cursory binding experiments. No functional data were presented for α7 nAChR. Affinity for α7 nAChR was supported only by binding data. | Acker et al., 2008 |
| PNU-282987 | Cultured hippocampal neurons and other unspecified | Putative α1β1γδ, α3β4, α7 | Functional antagonist of the IC50 ≈ 5 µM | There was negligible agonist or antagonist activity at either α1β1γδ or α3β4, but no data are shown, and methods for these determinations are not described. The claim for α7-selectivity is based on the limited data that 1 µM PNU-282987 displaced only 14% cytisine binding from rat brain membranes but displaced MLA binding from rat brain homogenates with a Ki of 27 nM. It was shown to evoke currents in rat hippocampal neurons in a concentration-dependent manner, but the data were insufficient to measure potency or efficacy. | Bodnar et al., 2005 |
| PSAB-OFP | Transfected cells and X. laevis oocytes | α2β4, α3β4, α4β4, α3,β2, α4β2, α7 | A 66% partial agonist, 10-fold more potent than 5HT | PSAB-OFP is a potent and efficacious agonist of α7 nAChR with little activity for other nAChRs. However, it is also a potent agonist for 5HT3 receptors. | Broad et al., 2002 |
| S 24795 | X. laevis oocytes and hippocampal interneurons in brain slices | α7, putative muscle type nAChR (Torpedo), ganglionic (IMR32 cells), and α4β2* and α7* in brain membranes | Not studied | Selectivity for α7 supported by binding studies not described in detail. Functional data from oocytes and hippocampal interneurons in brain slices support the conclusion that S 24795 is a partial agonist for α7 nAChR. | Lopez-Hernandez et al., 2007 |
| SSR-180711 | X. laevis oocytes cultured hippocampal neurons and transfected cells | α4β2, α7, α3β2, putative α3β4 (IMR32 cells) and α1β1γδ (TE671 cells) | Not studied | A relatively potent partial agonist with selectivity for α7 compared with other nAChR subtypes tested established by binding and voltage-clamp experiments. | Biton et al., 2007 |
| TC-1698 | X. laevis oocytes | α4β2* α3β2, α3β4 α1β1εδ α7 | Not studied | A potent full agonist for α7 nAChR with negligible activation of the heteromeric neuronal nAChR tested but with low-potency partial agonist activity for muscle nAChR. Effective competitive antagonist at α4β2 nAChR. | Marrero et al., 2004 |
| Tilorone | X. laevis oocyte-transfected cells and IMR-32 cells | Human α7, α4β2, rat α7, putative α3β4 (IMR-32) | Not studied | Partial agonist of α7 with relatively little activation of other nAChR tested. Demonstrated to have antagonist activity for α4β2 and more potently for putative α3β4. Mechanism of antagonism not investigated. | Briggs et al., 2008 |
| Tropane | X. laevis oocytes | α3β4, α4β2, α7 | Neither agonist nor antagonist activity detected | A partial agonist (25% efficacy compared with ACh) selective for α7 among the limited number of receptor subtypes tested. An antagonist for other nAChR tested but not for 5HT3 receptors. | Papke et al., 2005a |
| Tropinone | X. laevis oocytes | α3β4, α4β2, α7 | Neither agonist nor antagonist activity detected | A partial agonist (64% efficacy compared with ACh) selective for α7 among the limited number of receptor subtypes tested. Antagonist activity at α3β4 but not α4β2 or 5HT3 receptors | Papke et al., 2005a |
| Tropisetron | X. laevis oocytes | α3β4, α4β2, α7, and α1β1γδ for binding only | Competitive antagonist | A partial agonist (30% efficacy compared with ACh), selective for α7 among the limited number of receptor subtypes tested. An antagonist for other nAChRs tested and for 5HT3 receptors. | Macor et al., 2001; Papke et al., 2005a |
In addition to choline, a relatively simple structure associated with selective activation of α7 receptors is that of tropane (Papke et al., 2005a). We have published recently that unsubstituted quinuclidine will activate α7 receptors (Papke, 2006), and, as shown in Fig. 1, a quinuclidine provides the core agonist cationic center for many structurally complex α7-selective agonists. In the present study, we evaluate in greater detail the activity of choline and the simple amines TMA and ethyltetramethyl-ammonium (ETMA) and extend a preliminary report (Leonik et al., 2007) on quinuclidine and related compounds. Although quinuclidine itself is not α7-selective, we report that hydrophilic side groups, as well as both minimal and large hydrophobic substitutions, affect the activity profile of quinuclidines and can promote selectivity for the activation of α7.
Methods and Materials
Synthetic Chemistry
Solvents and reagents were purchased from Aldrich Chemical (Milwaukee, WI) and Acros Organics (Fairlawn, NJ). All solvents were dried overnight over CaH2 and were freshly distilled before use. 1H and 13C NMR spectra were obtained using VXR 300, Gemini 300 and 500, or Mercury 300 (300 MHz) spectrometers (Varian, Palo Alto, CA) in CDCl3, CD3OD, or D2O solvents. EI Mass spectra were obtained on a Finnigan MAT 95Q spectrometer (Thermo Fisher Scientific, Waltham, MA), or ESI spectra were obtained on a Bruker APEX II FTICR mass spectrometer (Bruker, Newark, DE). NMR spectral data for the new compounds synthesized are included in the supplemental material.
1-Methyl-1-azoniabicyclo[2.2.2]octane Iodide
To prepare 1-methyl-1-azoniabicyclo[2.2.2]octane Iodide (MQN) (Chen and Benoiton, 1976; Kaminski et al., 1978), a mixture of methyl iodide (0.54 ml), KHCO3 (0.34 g), and quinuclidine hydrochloride (50 mg) was stirred in methanol (6.8 ml) at room temperature for 12 h. The solvent was evaporated, and CHCl3 was added to the residue, and the mixture was stirred overnight. The mixture was filtered and the solvent evaporated under vacuum to afford methyl quinuclidine iodide as a white solid (85 mg) in 99% yield (m.p. 230°C, dec). 1H NMR (D2O): δ (in parts per million) 2.01 (m, 6H), 2.20 (m, 1H), 2.93 (s, 3H), 3.43 (t, 6H); 13C NMR (D2O): δ (in parts per million) 57.4, 52.3, 23.9, and 19.1; EI calculated for C8H16N (M+): 126.1277; found: 126.1286.
1-Ethyl-1-azoniabicyclo[2.2.2]octane Iodide
1-Ethyl-1-azoniabicyclo[2.2.2]octane Iodide (EQN) was synthesized by the same procedure described above using 0.55 ml of ethyl iodide. The reaction afforded N-ethyl quinuclidine iodide as a white solid (81 mg) in 90% yield (m.p. 210°C, dec). 1H NMR (D2O): δ (in parts per million) 1.31 (t, 3H), 2.01 (m, 6H), 2.22 (m, 1H), 3.22 (q, 2H), 3.40 (t, 6H); 13C NMR (D2O): δ (in parts per million) 60.0, 54.5, 23.8, 19.5, and 7.6. ESI-FT-ICR calculated for C9H18IN (2M+I+): 407.1918; found: 407.1921.
(E)- and (Z)-3-Benzylidene-1-azoniabicyclo[2.2.2]octane Chloride
The preparation of (E)- and (Z)-3-benzylidene-1-azoniabicyclo-[2.2.2]octane chloride (BQNE and BQNZ, respectively), was carried out as follows. The free base quinuclidinone was obtained by treating quinuclidinone hydrochloride with a 2 M aqueous solution of 2 M K2CO3 (20 ml). Then, this solution was extracted three times with ether (30 ml), and the solvent was evaporated under reduced pressure. To a suspension of NaH (60% in mineral oil, 0.23 g, 5.84 mmol) in 1,2-dimethoxy ethane (DME) (6.4 ml) was added drop-wise a solution of diethyl benzyl phosphonate (1.33 g, 5.84 mmol) in DME (2 ml) at room temperature under argon. After this addition, a solution of quinuclidinone (0.4 g, 2.54 mmol) in DME (1.78 ml) was added drop-wise. The reaction mixture was refluxed for 1.5 h. Then, the mixture was quenched carefully with water (30 ml), and the bulk of the DME was evaporated under reduced pressure. The aqueous phase was extracted with CH2Cl2 (3 × 30 ml), and the organic layer was dried over MgSO4 and evaporated under vacuum. The crude oil was purified by flash chromatography on silica gel using CH2Cl2/MeOH/Et3N at a ratio of 20:1:0.1 as eluent to give the Z-isomer (153 mg) in a 30% yield and the E-isomer in a (82 mg) 16% yield The hydrochloride salts of these isomers were obtained by adding ether-HCl to a solution of the free bases in a mixture of CH2Cl2/ether. For the Z-isomer: m.p. 200°C (dec); 1H NMR (CDCl3): δ (in parts per million) 2.07 (td, 4H), 2.78 (m, 1H), 3.26 (m, 4H), 4.15 (s, 2H), 6.45 (t, 1H), 7.15 to 7.39 (m, 5H); 13C NMR (CDCl3): δ (in parts per million) 144.5, 137.7, 128.9, 128.7, 126.7, 122.0, 56.0, 48.0, 34.1, and 28.1. ESI-FT-ICR calculated for C14H18N (M+): 200.1439; found: 200.1423. For the E-isomer: m.p. 200°C (dec); 1H NMR (CDCl3): δ (in parts per million) 2.01 (t, 4H), 3.4 (m, 5H), 4.06 (s, 2H), 6.49 (s, 1H), 7.17 to 7.39 (m, 5H); 13C NMR (CDCl3): δ (in parts per million) 135.1, 132.2, 128.6, 128.3, 127.5, 125.7, 54.2, 46.7, 24.4, and 23.7. ESI-FT-ICR calculated for C14H18N (M+): 200.1439; found: 200.1423.
(E)- and (Z)-3-(4-Methoxybenzylidene)-1-azoniabicyclo[2.2.2]-octane Chloride
(E)- and (Z)-3-(4-methoxybenzylidene)-1-azoniabicyclo-[2.2.2]octane chloride (MBQNE and MBQNZ, respectively) were synthesized using the same procedure described above. A suspension of NaH (60% in mineral oil, 0.868 g, 22.4 mmol) in DME (24 ml) was stirred at room temperature under argon. A solution of diethyl −4-methoxy benzylphosphonate (3.55 ml, 20.6 mmol) in DME (7 ml) was added drop-wise to that solution. After this, a solution of quinuclidinone as a free base (1.12 g, 8.96 mmol) in DME (6 ml) was added drop-wise. The reaction mixture was refluxed for 1.5 h and quenched with water. The DME was evaporated under vacuum, and the residue was dissolved in dichloromethane and washed with water. The organic layer was washed with brine and dried over MgSO4. The mixture was purified by flash chromatography (silica gel, CH2Cl2/MeOH 35:1). The two isomers, obtained as oils, were isolated as free bases giving the Z-isomer (400 mg) in 20% yield and the E-isomer (59 mg) in 3% yield. For the Z-isomer: 1HNMR (CDCl3): δ (in parts per million) 2.10 (td, 4H), 2.80 (t, 1H), 3.29 (dt, 2H), 3.39 (dt, 2H), 3.83 (s, 3H), 4.19 (s, 2H), 6.42 (t, 1H), 6.89 (d, 2H), 7.09 (d, 2H); 13C NMR (CDCl3): δ (in parts per million) 159.2, 131.1, 130.0, 128.2, 125.2, 114.6, 55.7, 54.4, 47.1, 32.2, and 25.3; EI calculated for C15H19NO (M+): 229.1467; found: 229.1475. For the E-isomer: 1H NMR (CDCl3): δ (in parts per million) 2.04 (m, 4H), 3.36 (m, 5H), 3.82 (s, 3H), 3.99 (s, 2H), 6.40 (s, 1H), 6.90 (d, 2H), 7.12 (d, 2H); 13C NMR (CDCl3): δ (in parts per million) 159.3, 131.1, 129.9, 127.9, 125.5, 114.4, 55.7,54.7, 47.0, 24.7, and 24.2. EI calculated for C15H19NO (M+): 229.1467; found: 229.1475.
Modeling
Molecular models of agonists were constructed with the Chem3D Ultra program (Cambridge Scientific, Cambridge, MA). The structures obtained were optimized with the molecular mechanics force field within Chem3D; in some cases, semiempirical calculations with AM1 parameters were used. Conformational searching was used to ensure that high-energy local minima were not used for the overlay calculations. Estimates of common molecular features between groups of agonists were made by using the overlay routine resident within the program suite. The positively charged nitrogen atom found in all agonists was always used as one of the pairs of points used to generate the overlaid structures.
Clustal Analysis
Clustal is an online utility to generate DNA or protein sequence alignments and comparisons. Binding loop protein sequences of α4β2, α3β4, and α7 were submitted to ClustalW2 online at The European Bioinformatics Institute web site (available at http://www.ebi.ac.uk/tools/clustalw2). Specifically, sequence of loops A, B, and C of the α was attached to the sequence of loops D, E, and F of the β or α7. Sequences selected for α4β2 and α3β4 were homologous to the loop sequence indicated for α7 (Stokes et al., 2004) and the acetylcholine binding protein (Brejc et al., 2001).
ACh Receptor Clones
The human nAChR receptor clones were obtained from Dr. Jon Lindstrom (University of Pennsylvania, Philadelphia, PA).
Preparation of RNA
After linearization and purification of cloned cDNAs, RNA transcripts were prepared in vitro using the appropriate mMessage mMachine kit from Ambion Inc. (Austin, TX).
Expression in Xenopus laevis Oocytes
Mature (>9 cm) female X. laevis African frogs (Nasco, Ft. Atkinson, WI) were used as a source of oocytes. Before surgery, the frogs were anesthetized by placing them in a 1.5 g/l solution of MS222 for 30 min. Oocytes were removed from an incision made in the abdomen.
Harvested oocytes were treated with 1.25 mg/ml collagenase (Worthington Biochemical Corporation, Freehold, NJ) for 2 h at room temperature in calcium-free Barth’s solution (88 mM NaCl, 1 mM KCl, 2.38 mM NaHCO3, 0.82 mM MgSO4, 15 mM HEPES, pH 7.6, and 12 mg/l tetracycline) to remove the follicular layer. Stage 5 oocytes were isolated and injected with 50 nl (5–20 ng) of each subunit cRNA. Recordings were normally conducted 2 to 5 days after injection.
Electrophysiology
Experiments were conducted using Opus-Xpress 6000A (Molecular Devices, Sunnyvale, CA). OpusXpress is an integrated system that provides automated impalement and voltage clamp of up to eight oocytes in parallel. Both the voltage and current electrodes were filled with 3 M KCl. The oocytes were clamped at a holding potential of −60 mV.
Data were collected at 50 Hz and filtered at 20 Hz. The oocytes were bath-perfused with Ringer’s solution. Agonist solutions were delivered from a 96-well plate using disposable tips. Flow rates were set at 2 ml/min for α7 and 4 ml/min for α4β2 and α3β4. Drug applications alternated between ACh controls and test solutions of ACh or other experimental agonists at varying concentrations.
Experimental Protocols and Data Analysis
Responses of α7 receptors are calculated as net charge (Papke and Papke, 2002), and responses of wild-type β subunit-containing receptors are reported as peak currents. Each oocyte received initial control applications of ACh, then experimental drug applications, and follow-up control applications of ACh. For α7 receptors, the control ACh concentration was 300 µM, a concentration sufficient to evoke a maximal net charge response (Papke and Papke, 2002). For α4β2 and α3β4 receptors, the ACh control was 100 µM. Responses to experimental drug applications were calculated relative to the preceding ACh control responses to normalize the data, compensating for the varying levels of channel expression among the oocytes. Mean values and standard errors (S.E.M.) were calculated from the normalized responses of at least four oocytes for each experimental concentration. For concentration-response relations, data were plotted using Kaleida-Graph 3.0.2 (Abelbeck Software, Reading, PA), and curves were generated from the Hill equation: response = (Imax[agonist]nH)/([agonist] nH) + (EC50)nH), where Imax denotes the maximal response for a particular agonist/subunit combination, and nH represents the Hill coefficient. Imax, nH, and the EC50 value were all unconstrained for the fitting procedures, except in the case of the ACh response curves. Because ACh is our reference full agonist, for the ACh concentration-response curves, the data were normalized to the observed ACh maximum, and the Imax of the curve fits were constrained to equal one.
Results
Choline and Quinuclidine Activation of α7, α4β2, and α3β4 nAChR
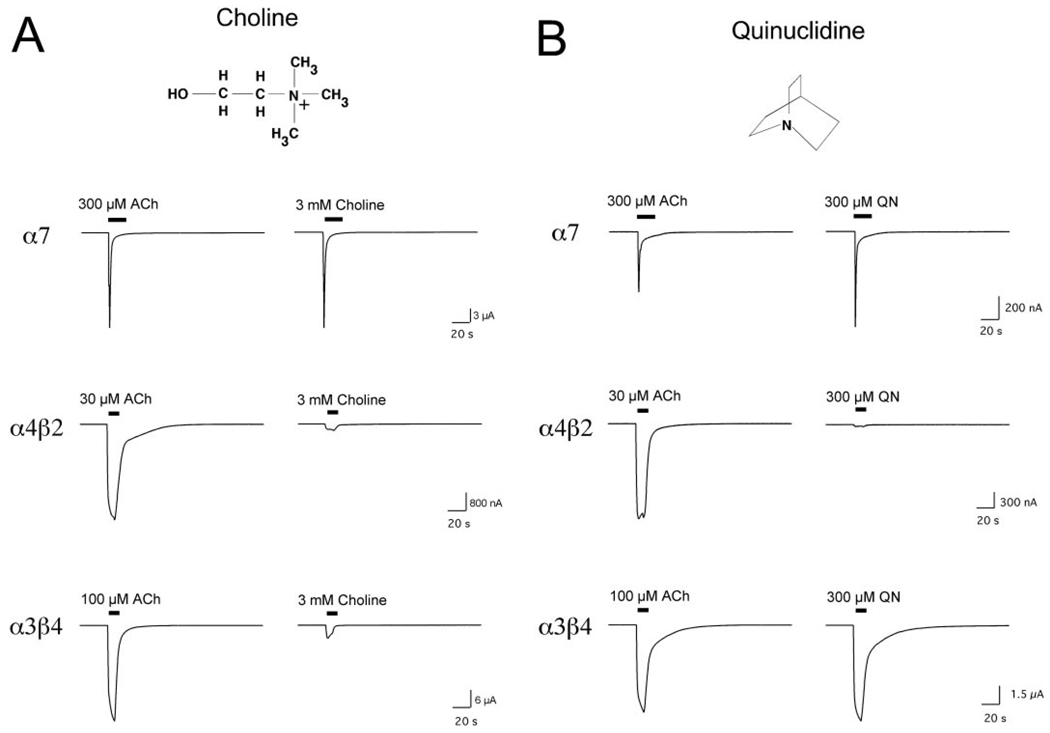
The only known α7-selective agonist naturally occurring in the vertebrate nervous system is choline, and, as published previously, choline is a full agonist with potency 10-fold lower than that of ACh. As shown in Fig. 2A, although choline is highly selective for α7, it does produce small but detectable responses from both α4β2 and α3β4 nAChR. When normalized to the maximal ACh-evoked responses in these cell types, the α3β4 were significantly more sensitive to choline than were the α4β2 receptors (p < 0.01), with responses to 3 mM choline equal to 4.5 ± 0.6% (n = 3) and 2.3 ± 0.3% (n = 5) of the ACh maximum for α3β4 and α4β2, respectively.
Figure 2.
A, Responses to ACh and choline obtained from single cells expressing either α7, α4β2, or α3β4 nAChR. Note that for α7, the 300 µM control is a fully efficacious ACh concentration for the net charge response. Choline is approximately 10-fold less potent than ACh, and therefore, 3mM choline evokes nearly identical responses with 300 µM ACh. For α4β2, the 30 µM ACh control is approximately the EC60 value for peak current, and 3 mM choline evokes responses that are only 2% of the ACh maximum. For α3β4, the 100 µM ACh control is approximately the EC45 value for peak current, and 3 mM choline evokes peak currents that are approximately 5% of the ACh maximum. B, responses to ACh and QN obtained from single cells expressing either α7, α4β2, or α3β4 nAChR. Note than for α7, the 300 µM control is a fully efficacious ACh concentration for the net charge response but not for peak current. QN is more potent than ACh and therefore evokes a larger peak current but the same net charge. For α4β2, the 30 µM ACh control is approximately the EC60 value for peak current, and for α3β4, the 100 µM ACh control is approximately the EC45 value for peak current.
Quinuclidine (QN) is a fully efficacious agonist of α7 receptors (Papke, 2006) and is a core element of many large α7-selective agonists (Fig. 1). We tested the agonist activity of QN on α3β4 and α4β2 nAChR, and, as shown in Fig. 2B, 300 µM QN was capable of producing maximal activation of α7. At this concentration, QN evoked currents from α4β2 receptors that were only 3.2 ± 0.7% of the ACh maximal responses (n = 5), whereas for α3β4 receptors, the QN-evoked responses were 46 ± 2% of the ACh maximum.
Our observations therefore suggest that to identify the key elements for an α7-selective agonist, comparisons between α7 and α3β4 will be of more use than comparisons between α7 and α4β2. A similar observation has been published previously (Efange et al., 2001) regarding piperidyl- and pyrrolidyl- chromans, which activated α7 and α3-containing nAChR much more efficaciously than α4-containing receptors. A Clustal analysis (Higgins, 1994) of α7, α3β4, and α4β2 sequences in the putative ACh binding loops (comparing α7 to α4 and α3 in loops A, B, and C and to β2 and β4 in loops D, E, and F; Brejc et al., 2001) indicated slightly greater sequence similarity between α7 and α3β4 than between α7 and α4β2. For these reasons, we focused our studies on α7 and α3β4 receptors. A good selectivity profile between these two subtypes will also have the therapeutic value of providing drugs with few potential side effects caused by the activation of ganglionic nAChR.
Activity Profiles for TMA, ETMA, Choline, and ACh on α7 and α3β4
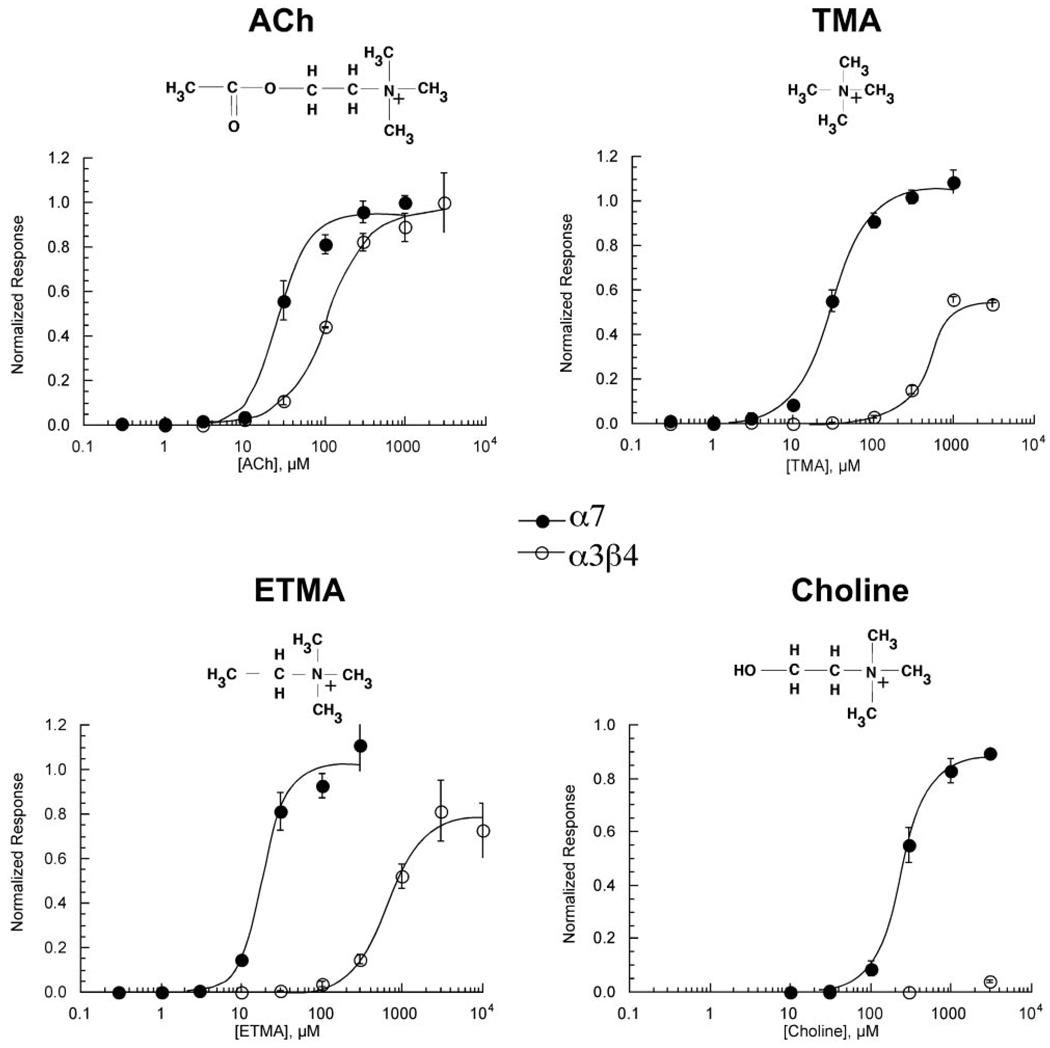
It has been reported previously that TMA and ETMA activate multiple subtypes of neuronal nAChR (Papke et al., 1996) when applied at high (1 mM) concentrations. Figure 3 shows full concentration-response studies for these compounds and for ACh and choline on α7 and α3β4 receptors (curve fit values provided in Table 2). All four of these quaternary amines were full agonists for α7, with only choline showing a significant decrease in potency compared with ACh. In contrast, both TMA and ETMA were only partial agonists of α3β4 receptors and also showed reduced potency compared with ACh (Table 2), whereas the activity of choline for α3β4 was too low to be characterized in regard to potency.
Figure 3.
The effect of ACh, TMA, ETMA, and choline on α7 and α3β4 nAChR subtypes. Concentration-response curves for α7-expressing oocytes were calculated based on net charge and those for α3β4 responses on peak current amplitudes. Responses were calculated relative to ACh control responses obtained from the same cells (see Materials and Methods) and subsequently normalized to the ACh maximum response determined in separate experiments (data not shown). Values represent the averages (± S.E.M.) of at least four oocytes.
TABLE 2.
Activation of α7 and α3β4 nAChR
| Agonist | α7 | α3β4 | ||
|---|---|---|---|---|
| Imax | EC50 | Imax | EC50 | |
| µM | µM | |||
| ACh | 1.0 ± | 27 ± 3 | 1.0 ± | 110 ± 9 |
| TMA | 1.1 ± 0.1 | 30 ± 3 | 0.55 ± 0.03 | 350 ± 190 |
| ETMA | 1.0 ± 0.1 | 19 ± 2 | 0.78 ± 0.04 | 660 ± 90 |
| Choline | 0.90 ± 0.1 | 243 ± 8 | 0.04 ± 0.01 | N.A. |
| dEdMA | 0.95 ± 0.06 | 197 ± 37 | 0.06 ± 0.01 | N.A. |
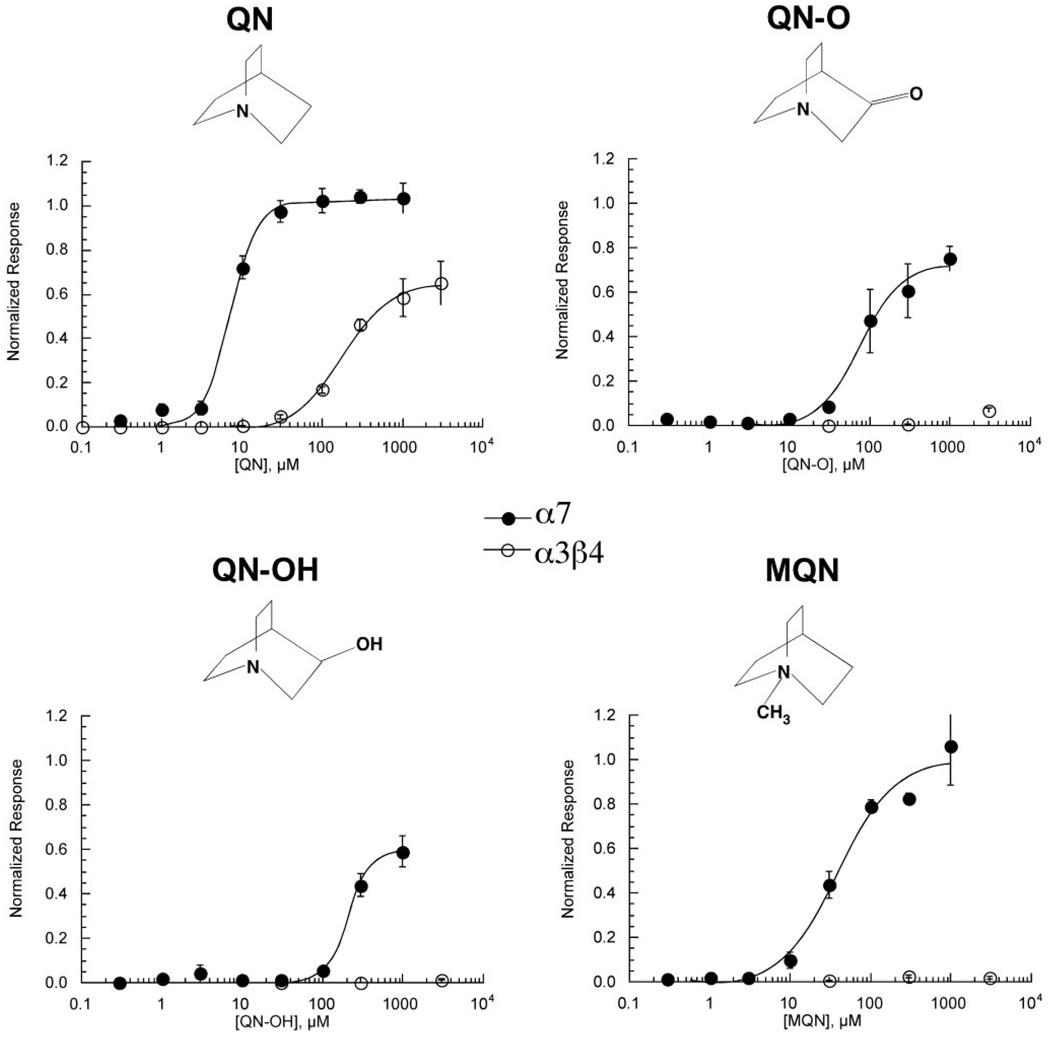
| QN | 1.03 ± 0.02 | 7.2 ± 0.5 | 0.64 ± 0.01 | 180 ± 10 |
| MQN | 1.0 ± 0.1 | 40 ± 8 | 0.025 ± 0.006 | N.A. |
| QN-O | 0.73 ± 0.04 | 78 ± 11 | ≥0.07± | >1000 |
| QN-OH | 0.60 ± 0.03 | 214 ± 15 | 0.01 ± | N.A. |
| BQNE | 0.28 ± 0.01 | 1.5 ± 0.2 | <0.01 | N.A. |
| BQNZ | 0.11 ± 0.01 | 7.3 ± 0.7 | 0.10 ± 0.01 | N.A. |
| MeOBQNE | 0.41 ± 0.03 | 1.3 ± 0.2 | <0.01 | N.A. |
| MeOBQNZ | 0.09 ± 0.01 | 1.1 ± 0.8 | <0.01 | N.A. |
N.A., not applicable.
Activity of Small Quinuclidine-Based Molecules on α7 and α3β4 nAChR
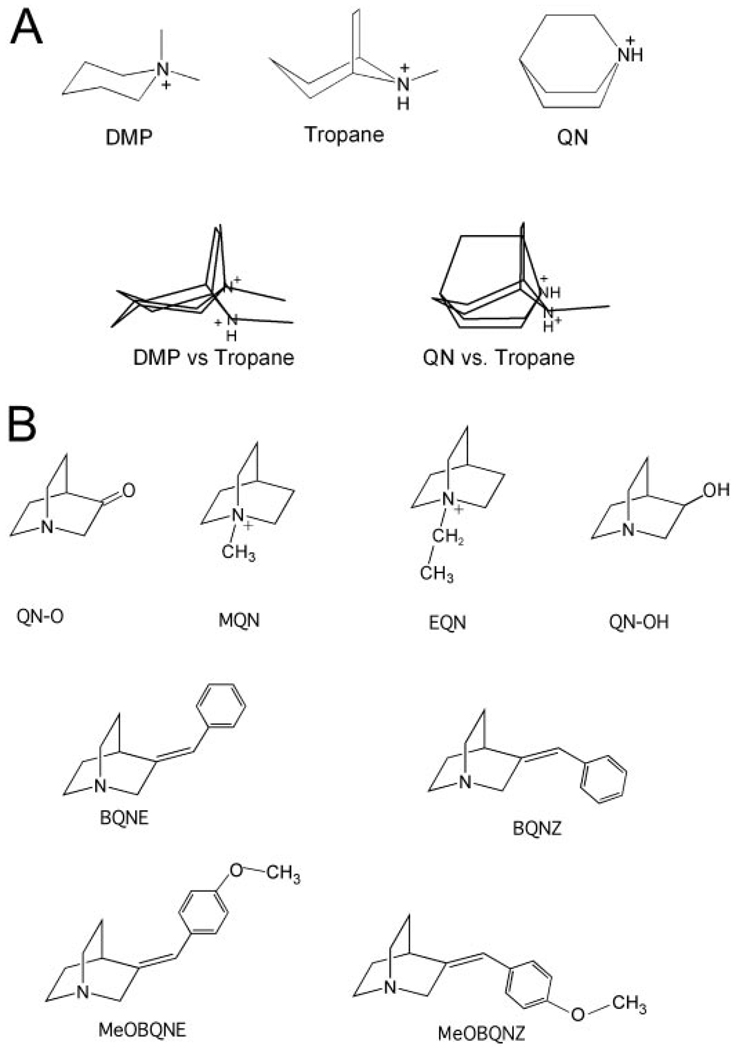
QN is an effective activator of α7 (Fig. 2B) and a partial agonist of α3β4 (Fig. 5). As shown in Fig. 4A, QN is comparable in size to the α7-selective agonists tropane and DMP (Papke et al., 2005a). However, although overlays of DMP and tropane show reasonably good alignment of the charged nitrogen and hydrophobic elements, the overlay of tropane and QN highlights the absence of a small hydrophobic element common to tropane and DMP, a single methyl group. We therefore sought to test the hypotheses that the addition of a methyl group to QN in a similar position (MQN, Fig. 4B) would produce a loss of α3β4 activity. Additional QN-related structures are also provided in Fig. 4B.
Figure 5.
The effect of quinuclidine compounds on α7 and α3β4 nAChR subtypes. Concentration-response curves for α7-expressing oocytes were calculated based on net charge and those for α3β4 responses on peak current amplitudes. Responses were calculated relative to ACh control responses obtained from the same cells (see Materials and Methods) and subsequently normalized to the ACh maximum response determined in separate experiments (data not shown). Values represent the averages (± S.E.M.) of at least four oocytes.
Figure 4.
Structural comparisons of nicotinic agonists. A, we have reported previously that the relatively simple tropane and DMPP molecules shown in the top row are selective activators of α7-receptors, and the overlay of these structures (second row, left) shows a similar disposition of the charge center and hydrophobic substituents. QN, which is not α7-selective, is compared with tropane (second row, right). This comparison suggests the addition of a methyl group to QN to produce MQN (B) would make a better match to the common features of tropane and DMP. B, structures of EQN, BQN compounds, and other quinuclidines as discussed in the text.
As shown in Fig. 5, QN was a potent and fully efficacious agonist for α7 and a partial agonist for α3β4, with an efficacy of 64% that of ACh. MQN was also fully effective for activating α7, although it was less potent than QN. With the addition of a methyl group to the QN core, essentially all activity was lost for α3β4 receptors, consistent with the pharmacophore hypothesis discussed above. It is interesting that the additional oxygen of quinuclidinone (QN-O) or the hydroxyl group of quinuclidinol (QN-OH) also caused a loss of α3β4 activation, and the spacing of these polar groups from the amine center of QN corresponds to the distance of the hydroxyl from the charged nitrogen in choline. QN-O and QN-OH were also reduced in efficacy for α7 compared with QN, and both showed decreases in potency compared with QN that that were equal to (in the case of QN-O) or greater than (in the case of QN-OH) the potency difference between ETMA and choline for α7.
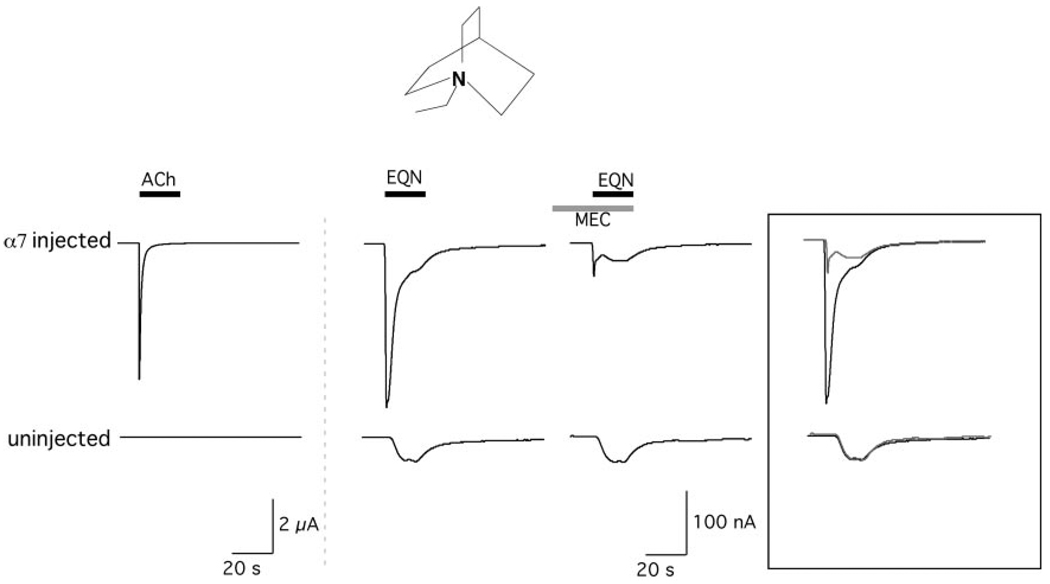
Ethyl-quinuclidine (EQN, Fig. 4) was also synthesized and tested (Fig. 6) to determine whether a somewhat larger hydrophobic extension in that domain would improve α7 agonist activity. EQN evoked receptor-specific current in α7-injected cells. However, it also evoked receptor-independent currents, which could be recorded in both injected and uninjected cells. The nAChR-mediated current could be identified because it was eliminated by the coapplication of 100 µM mecamylamine. In α7-injected cells, the mecamylamine-sensitive current was 58 ± 3% of the total current evoked by the application of 1 mM EQN (n = 4), and the net charge of the mecamylamine-sensitive current was only 23 ± 2% of that of the current evoked by the application of 300 µM ACh to the same cells. EQN also evoked currents in cells injected with α3β4, but these currents were comparable with the current evoked in uninjected oocytes and were not blocked by mecamylamine (data not shown). Therefore, although our data suggest that EQN is a weak partial agonist for α7 nAChR, the ability of EQN to activate receptor-independent currents limited our ability to study this agent in detail using the X. laevis oocyte expression system. Although it is clearly outside the scope of these experiments to investigate the nAChR-independent currents evoked by EQN in detail, we did wish to determine whether mediators of the response might be muscarinic AChRs, sometimes endogenously expressed by oocytes. Therefore, we compared the responses of uninjected oocytes with 1 mM EQN in Ringer’s solution with or without our normal 1 µM atropine and determined that the responses of uninjected oocytes to EQN were not significantly different in the presence or absence of the mAChR antagonist atropine (data not shown).
Figure 6.
Effects of EQN on cells expressing α7 and uninjected cells. Only α7-injected cells showed response to the application of 300 µM ACh. In contrast, inward currents were evoked by 1 mM EQN in both α7-injected and uninjected cells. However, in α7-injected cells, some portion of the current was mecamylamine-sensitive. The rightmost shows the EQN-evoked currents in the absence and presence of 100 µM mecamylamine.
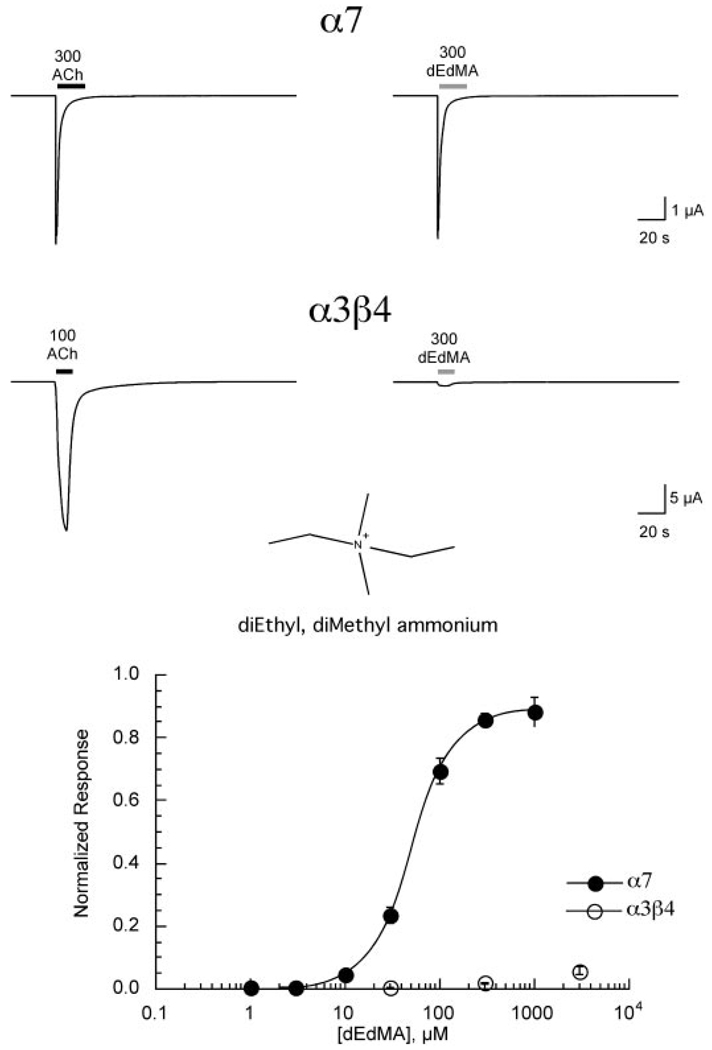
Selective Activation of α7 nAChR by Diethyl, Dimethyl Ammonium
Because the addition of a single methyl group was sufficient to convert QN into an α7-selective agonist, we hypothesized that a similar modification to the structure of ETMA would produce decreased activity on α3β4 receptors without a large decrease in α7 activation. As shown in Fig. 7, 300 µM diethyldimethylammonium (dEdMA) produced only approximately 2% activation of α3β4 receptors, and even at a concentration of 3 mM, α3β4 responses were only approximately 6% of the ACh maximum. In contrast to the greater than 10-fold difference in efficacy for α3β4, for α7, dEdMA was 90% as efficacious as ACh and only 2.5-fold less potent compared with ETMA.
Figure 7.
The effects of dEdMA on cells expressing α7 and α3β4. Representative traces are shown at the top compared with control ACh responses obtained from the same cells. For α7, the 300 µM control is a fully efficacious ACh concentration for the net charge response. For α3β4, the 100 µM ACh control is approximately the EC45 value for peak current. Concentration-response relationships are shown below. Values represent the averages (± S.E.M.) of at least four oocytes.
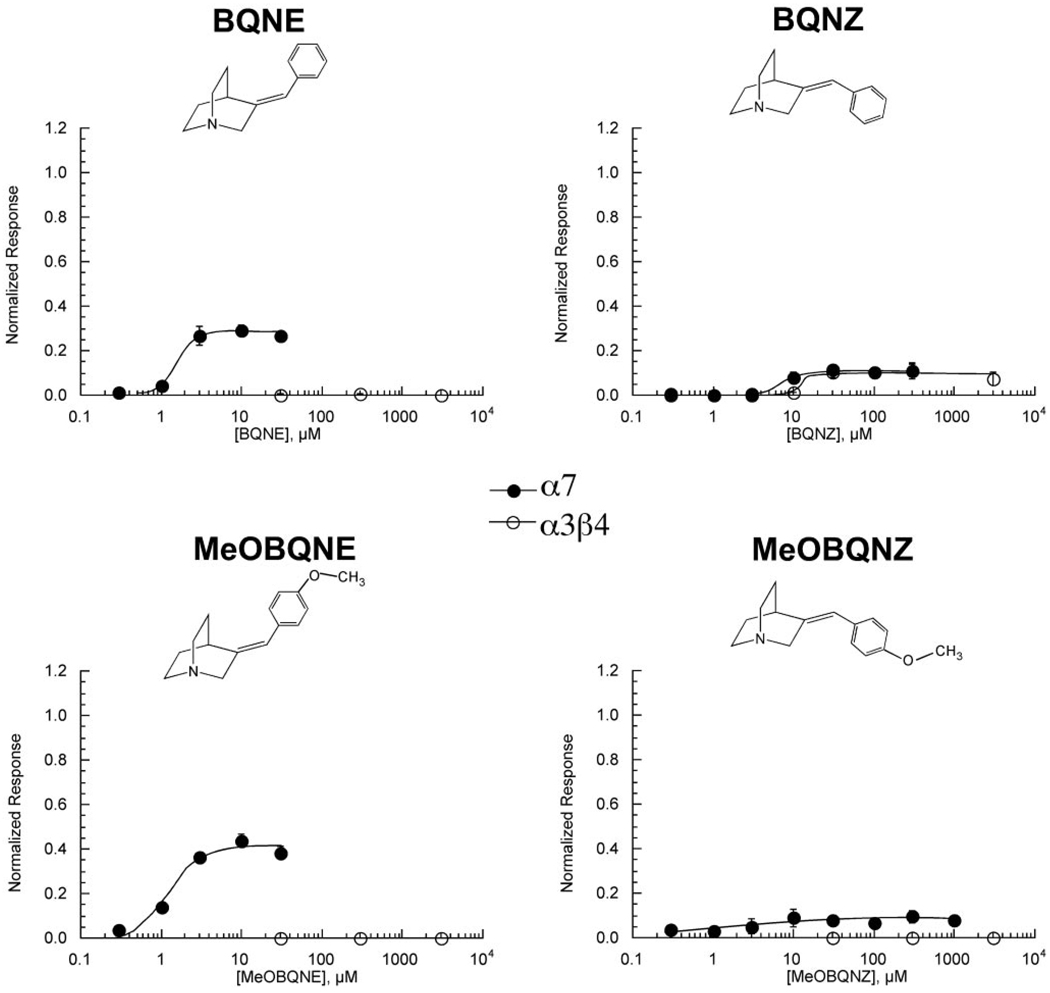
Activity of Benzylidene Quinuclidines on α7 and α3β4 nAChR
Although the addition of only a methyl group was sufficient to increase the α7-selectivity for a quinuclidine-based agonist, larger hydrophobic groups are also often tolerated in α7-selective agonists, often improving potency (Papke et al., 2005a), or, depending on additional side groups, affecting both potency and efficacy (Papke et al., 2004a). The conjugation of benzylidene groups to the nonselective agonist anabaseine account for the α7-selectivity of GTS-21 (also known as DMXBA) and related compounds (Papke et al., 2004a). Therefore we synthesized a series of benzylidene quinuclidines (Fig. 4B) to determine how effectively benzylidene groups would modulate the activity of quinuclidine-based agonists. Note that the relative orientation of the phenyl and quinuclidine groups around the double bond can lead to alternative E and Z structures (Fig. 4B). Both of these isomers were made with either an unsubstituted benzene group (BQNE and BQNZ) or with a methoxy group at the 4 position (MBQNE and MBQNZ). As shown in Fig. 8, the benzylidene quinuclidines in the E configuration were relatively potent α7 partial agonists, although less efficacious than QN or MQN (Table 1). The efficacy for α7 was further reduced for the benzylidene quinuclidines in the Z configuration. Whereas the activity of BQNE and MBQNE was α7-selective, BQNZ also produced significant activation of α3β4 receptors (Fig. 8) that was comparable with the activation of α7-expressing cells.
Figure 8.
The effect of benzylidene-quinuclidine compounds on α7 and α3β4 nAChR subtypes. Concentration-response curves for α7-expressing oocytes were calculated based on net charge and those for α3β4 responses on peak current amplitudes. Responses were calculated relative to ACh control responses obtained from the same cells (see Materials and Methods) and subsequently normalized to the ACh maximum response determined in separate experiments (data not shown). Values represent the averages (± S.E.M.) of at least four oocytes.
Activation and/or Inhibition of 5HT3 Receptors and Other nAChR
To more fully characterize the selectivity profile of the compounds used for this study, they were also tested at a concentration of 100 µM for their ability to either activate (when applied alone) or inhibit (when coapplied with agonist) 5HT3 receptors, as well as muscle type (α1β1εδ) and α4β2 nAChR expressed in oocytes (Table 3). Although most serotonin receptors are G-protein-coupled, 5HT3 receptors are members of the cysteine-loop super-family of ligand-gated ion channels, and, like α7 nAChR, 5HT3 receptor subunits form functional homomeric assemblies. Some α7-selective agonists characterized previously, such as tropisetron (Macor et al., 2001; Papke et al., 2005a), have been shown to be antagonists of 5HT3 receptors (Table 1). Likewise, benzylidene anabaseines have been shown to interact at these receptors, as either antagonists or partial agonists (Machu et al., 2001).
TABLE 3. Activation and/or inhibition of 5HT3 receptors and other nAChRs.
Agonist activity (Activity) was evaluated by applying the compounds at a concentration of 100 µM and comparing those responses with appropriate control responses obtained from the same oocytes. Controls were 10 µM serotonin, 3 µM ACh, and 30 µM ACh for the 5HT3, α1β1εδ, and α4β2 receptors, respectively. Those responses were then normalized to maximal agonist-evoked responses for each receptor subtype, as determined in other experiments. For 5HT3 receptors, the detection threshold was 5% of the maximal serotonin-evoked responses. ACh-evoked responses for muscle-type receptors are very large, so the detection threshold for responses evoked by the experimental compounds was 0.5% of the ACh maximum, and for α4β2 receptors, the detection threshold was 1% of the ACh maximum. Antagonist activity (Inhibition) was determined by comparing the activation produced by control applications of agonist with those produced by coapplication of agonists at the control concentrations plus the 100 µM concentration of the experimental compounds.
| Compound | 5HT3 | α1β1εδ | α4β2 | ||||
|---|---|---|---|---|---|---|---|
| Activity | Inhibition | Activity | Inhibition | Activity | Inhibition | ||
| % | % | ||||||
| QN | N | N.S. | 3 | N.S. | ≈2 | 74 ± 5 | |
| TMA | N | N.S. | <2 | N.S. | 19 ± 6 | N.S. | |
| ETMA | N | N.S. | N | N.S. | ≤2 | 40 ± 9 | |
| dEdMA | N | N.S. | N | N.S. | N | 52 ± 5 | |
| Choline | N | N.S. | N | N.S. | ≈1 | N.S. | |
| MQN | N | N.S. | N | N.S. | N | 72 ± 4 | |
| QN-O | N | N.S. | N | N.S. | N | N.S. | |
| QN-OH | N | N.S. | N | N.S. | N | N.S. | |
| BQNE | N | 94 ± 5 | N | 70 ± 7 | N | 77 ± 2 | |
| BQNZ | N | 95 ± 4 | ≈7 | N.S. | ≈1 | 74 ± 6 | |
| MeOBQNE | N | 94 ± 5 | N | 90 ± 2 | N | 81 ± 2 | |
| MeOBQNZ | N | 80 ± 16 | ≤1 | 76 ± 6 | N | 56 ± 4 | |
N, application of the drug at a concentration of 100 µM did not produce responses greater than our level of detection (i.e. distinct from application artifacts of 3–5 nA); N.S., there was no significant inhibition of the coapplication responses compared with control applications of agonist alone.
None of the quinuclidines or linear amines used for the α7 experiments produced detectable activation of 5HT3 receptors in oocytes. However, the benzylidene quinuclidines, like some benzylidene anabaseines, were effective antagonists, suggesting that the benzylidene group may allow these molecules to bind as competitive antagonists to the serotonin receptors.
The only compounds that produced detectable activation of muscle-type nAChRs were the non-α7-selective compounds, QN, TMA, and Z-forms of the benzylidene quinuclidines. These compounds, and to a lesser degree choline, also produced detectable responses in α4β2-expressing oocytes. These results support the classification of dEdMA, MQN, QN-O, QN-OH, and the E forms of the BQN compounds as α7-selective agonists. Several of the compounds that did not activate the muscle-type or α4β2 nAChR were inhibitory in coapplication experiments, suggesting that they may bind to but fail to activate these receptors or may have channel-blocking properties. Benzylidene anabaseines have been reported to both displace cytisine binding from α4β2-type receptors in brain membranes (Meyer et al., 1997) and produce use-dependent inhibition of α4β2 receptors expressed in X. laevis oocytes (Meyer et al., 1998a), so inhibition of these nAChR subtypes by the BQN compounds might be produced by either or both of these mechanisms.
Modeling of Multiple Pharmacophores for Selective Activation of α7 nAChR
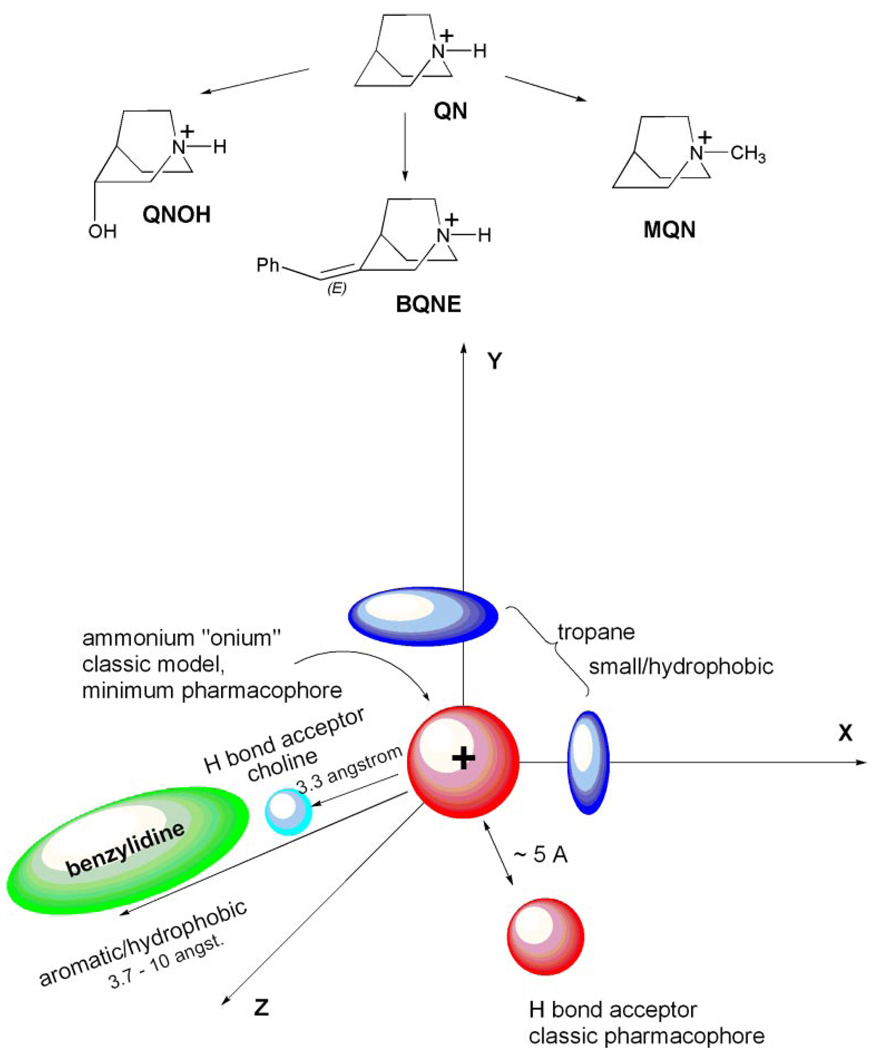
Figure 9, top, highlights how the nonselective agonist QN can lead to three unique α7-selective agonists. Figure 9, bottom, presents a model for three corresponding structural motifs to achieve selective activation of α7, and a typical (Beers and Reich, 1970) two-point pharmacophore model is represented to illustrate the geometric context of the selectivity model. This model was generated by overlaying a series of α7 agonists and identifying the common disposition of like atom types. Specifically, based on prototypical drugs using these motifs, we can identify them as the choline motif, the tropane motif, and the benzylidene motif.
Figure 9.
A. Three unique modifications of a nonselective agonist lead to selectivity. Quinuclidine is a nonselective α7 agonist, but its 3-hydroxy, 3-benzylidine, and N-methyl analogs are α7-selective. B, spatial model for α7-selectivity filters. Three key recognition motifs are superimposed on an arbitrary Cartesian frame of reference to illustrate the spatial relationships between the motifs. Red signifies the universal ammonium (cationic) recognition motif for all nAChRs and the H bond acceptor of the classic nAChR pharmacophore. The dark blue region represents the small tropane motif, which recognizes small aliphatic groups. The small light blue region represents the choline motif, a putative hydrogen bond acceptor. Green represents the benzylidene motif, an extended region that accommodates planar aromatic moieties. C, α-selective agonists superimposed on the spatial model. The top row (tropane motif) compares MQN, DMP, and tropane, illustrating that a common element is a small hydrophobic group resting above the x-y plane. The N-methyl group may serve to position the agonist to take advantage of this binding pocket. The bottom row (benzylidene motif) compares EBQN with BA. The common element for these two α7-selective agonists is an aromatic group in the third quadrant of the x-y plane.
The choline motif involves the location of an oxygen-containing polar group such as a hydroxyl or carbonyl separated by two carbons from the charged nitrogen. Note that the distance between the nitrogen and oxygen is ~3.4 Å, which, for example, in the case of 3-quinuclidinol, is probably too short to correspond to the hydrogen bond acceptor group described in existing nAChR pharmacophore models (Beers and Reich, 1970; Sheridan et al., 1986; Tonder et al., 2001). We therefore consider this selectivity motif to be distinct from the aforementioned pharmacophoric element.
The tropane motif relies on the recognition of small hydrophobic groups in a geometric relationship quite distinct from the benzylidine motif (discussed below). This motif is centered around the ammonium pharmacophore as illustrated in Fig. 9, bottom. The improvement in α7-selectivity obtained with the addition of a methyl group to quinuclidine and ethyltrimethyl-ammonium supports the hypothesis that tropane and other small selective agonists such as 1,1-dimethylpiperidine use a common (tropane) motif. This point is supported by the molecular overlay of QN and tropane shown in Fig. 4.
Like the tropane motif, the benzylidine motif is based on recognition of a hydrophobic group. However, it is spatially distinct from the tropane site. We consider that the α7 recognition site for this motif is extended in length, on the order of 7 to 10 Å from the charged nitrogen common to all agonists. This estimate is based on the inspection of models of benzylidene anabaseine, cinnamylidene anabaseine, and BQNE, in which the distance from the charged nitrogen to the end of the aromatic ring was measured. Furthermore, because substituents on this aromatic ring can modulate the activity of the agonist, the receptor pocket recognizing the benzylidene motif is likely to include residues that provide discrimination in addition to simple recognition of the aromatic ring system. It is interesting that the bridged nicotine analog ACME (Fig. 1) may provide an indication that the benzylidene selectivity filter could “start” as close as 3.7 Å from the charged nitrogen. In ACME, which is α7-selective, the ethyl chain, which constrains the pyridine ring from free rotation, is approximately in the same location as the benzylic carbon in compounds like benzylidene anabaseine and BQNE (Papke et al., 2005b).
Discussion
Although certain features have been proposed to be common to most α7-selective agonists, the diversity of ligands identified to be α7-selective is impressive (Mazurov et al., 2006). In general, drugs in commercial development have a cationic center, a hydrogen bond acceptor group, and one or more hydrophobic elements. The first two of these features were postulated in the earliest models for the pharmacophore for the muscle-type nAChR (Beers and Reich, 1970) and hence are not associated with α7-selectivity. However, the effective activation of both heteromeric and homomeric neuronal nAChRs by the simple quaternary amine TMA means that, although a hydrogen bond acceptor group may improve the activity for a specific type of ligand, it is not part of the minimal pharmacophore of a nonselective α7 agonist. The key element for α7 selectivity would then seem to be, in most cases, specific hydrophobic element(s). Most of the α7-selective agonists shown in Fig. 1 have very large hydrophobic groups, and many may be grouped into common classes based on the size and position of the groups. However, we have reported previously that the size of the hydrophobic element(s) need not be large (Papke et al., 2004b, 2005a,b). The data of the present study support the hypothesis that a hydrophobic element as small as a single methyl group can provide selective activation of α7 nAChR. Furthermore, this small hydrophobic recognition element is distinct from the one recognizing large hydrophobic residues. Our results therefore suggest that there are at least three independent and distinct structural motifs that can be associated with α7-selective agonists, which we have identified as the choline motif, the tropane motif, and the benzylidene motif.
Although agonists with the choline motif such as choline, QN-O, and QN-OH can selectively activate α7 receptors, the potency of these drugs is typically at least 10-fold lower than related agonists that lack the oxygen group (e.g., ETMA and QN). In the case of the QN compounds, there was also decreased efficacy in the α7-selective compounds using the choline motif. The choline motif is also present in four other inactive compounds closely related to α7 agonists (gray in Fig. 1), N-methyl-3-piperidinol, N,N-dimethyl-3-piperidinol, 1-(2-hydroxyethyl)piperidine, and 1-(2-hydroxyethyl)pyrrolidine (data not shown). In the case of N-methyl-3-piperidinol and N,N-dimethyl-3-piperidinol, the hydroxyl group resides in a piperidine ring with a chair-like conformation. Though structurally similar to QN-OH, this latter compound places the six-membered piperidine ring in a boat-like conformation because of the bridgehead. This alters and locks the orientation of the hydroxyl group, which could reasonably be critical for activity. In the case of the hydroxyethyl compounds, the choline motif is entirely exocyclic, and thus the attached five-or six-membered ring structure may clash with the receptor in seeking to bind favorably.
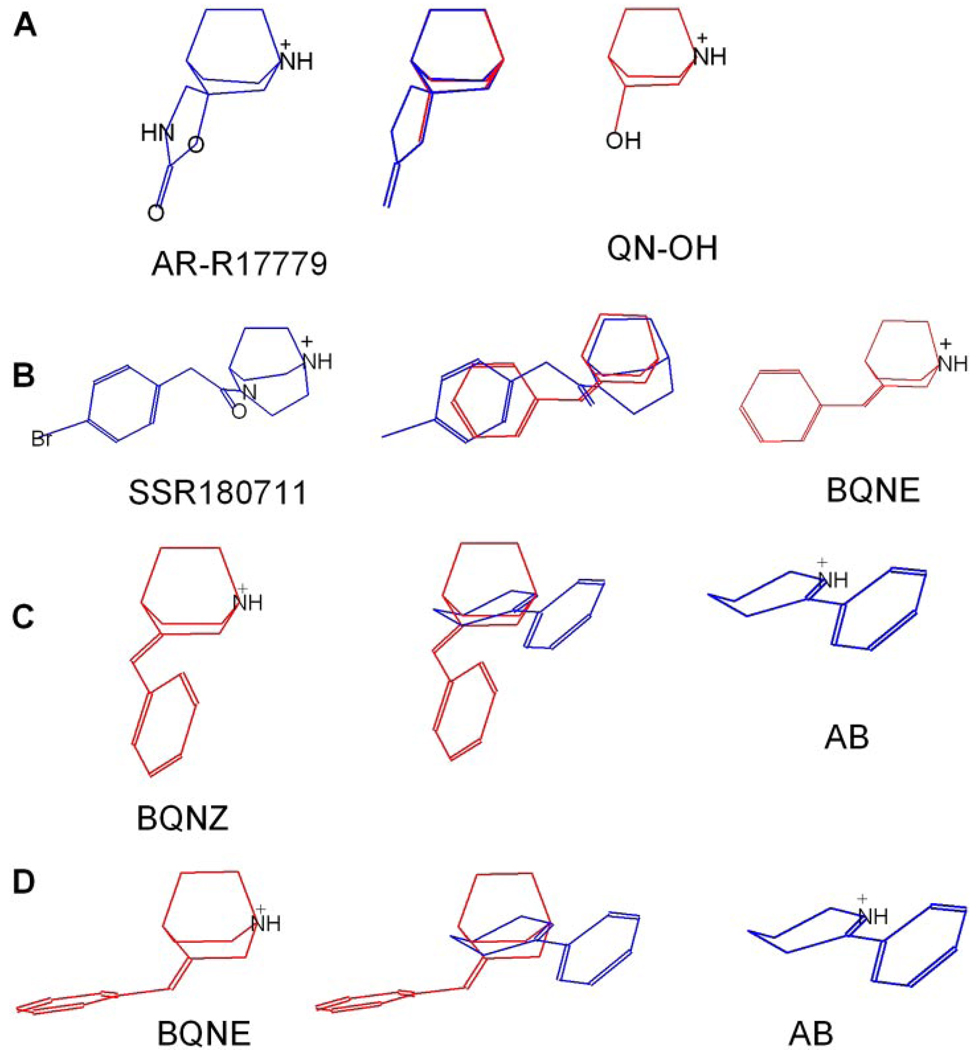
Because, the α7-selective compounds using the choline motif all seem to be less potent and/or efficacious for α7 than their most closely related nonselective analogs, the choline motif for α7-selectivity seems to apply some cost to functionality. One hypothesis is that perhaps the polar groups of choline and of more complex nonfunctional choline analogs such as tropine make a spurious hydrogen bond with the receptor that impedes the proper orientation of the ammonium analog in the binding site, reducing binding affinity and/or potency for activation. Comparison of AR-R17779 and 3-hydroxy quinuclidine by overlay shows a nearly perfect overlap (Fig. 10A). Because AR-R17779 cannot function as a hydrogen bond donor, it would avoid the spurious H-bond problem, and it is noteworthy that if AR-R17779 is modified so its oxygen atom becomes an H-bond donor (NH group) or a CH2 group, α7 binding affinity is abolished (Mullen et al., 2000). On the other hand, functional groups of the receptor-bound drugs may interfere with the conformational changes required for channel gating, and although this would not necessarily affect binding affinity for the resting state of the receptor, it might affect potency for activation. In summary, features of the choline motif that may strongly disfavor binding and/or activation of heteromeric receptors, may only do so mildly for α7s.
Figure 10.
Comparison of synthesized compounds with known α7-selective and nonselective agonists. The comparison illustrates how known quinuclidine-selective agonists can be classified in terms of whether they use a selectivity pharmacophore. The relative orientation of molecules coincides with the framework shown in Fig. 9. A, a comparison of AR-R17779 (blue) with QNOH (red), which share the choline motif. B, a comparison of SSR180711 (blue) with BQNE (red), both using the benzylidene motif. C, the Z-isomer of BQN places the phenyl ring close to the region occupied by the pyridine ring of nonselective anabaseine. D, overlay of BQNE and anabaseine. There was failure to match the benzylidene motif with the pyridine ring of anabaseine with any reasonable overlay scheme. In C and D, the red molecules are the BQN isomers, the blue molecule is anabaseine (AB).
The tropane motif provides α7-selectivity via recognition of small hydrophobic groups clustered about the ammonium nitrogen. Given that methylpiperidine is not α7-selective, but the addition of the second N-methyl group in the axial position above the piperidine ring plane renders it α7-selective, we suggest that the selectivity of MQN may not arise from the methyl group directly, but rather the methyl group may position the agonist so that the bridgehead CH2CH2 unit fits into the tropane motif of the receptor. In tropane itself, a similar effect may be operative. Furthermore, this tropane motif might be hypothesized to be the basis for other α7-selective agonists such as 3-acetoxy-1,1 dimethyl-piperidine. However, it is also present in DMPP, which is not α7-selective. Although DMPP has two nitrogens, the phenyl-substituted one is not basic, so an alternate binding mode with this position bearing the positive charge seems unlikely. It is possible that in binding to a non-α7 receptor, the phenyl group of DMPP provides a pathway for receptor activation that is unavailable to a simple piperidine. Whether or not this is the case for DMPP, it is quite clear that the position of the phenyl group with respect to the ammonium pharmacophore is quite critical because, as we discuss below, α7 selectivity can be conferred to a member of the quinuclidine family by the addition of a benzylidene group.
The third selectivity motif we identify is the benzylidene motif, in part because we have directly shown that the addition of a benzylidene group to quinuclidine in the BQN series can confer selectivity. Just as significant is the historic precedent of the first large family of synthetic α7-selective agonists, the benzylidene anabaseines such as GTS-21 (Papke et al., 2004a). In all probability, homologs of this motif account for the α7-selectivity of agonists such as PNU-282987, SSR180711, and others in Fig. 1 that have correspondingly large hydrophobic elements. Indeed, the molecular overlay of BQNE and SSR180711 shows an excellent coincidence of the aromatic moieties, using the charged nitrogen as an anchor point. (Fig. 10B).
The configuration of the double bond in the benzylidene motif is important for selective activation of α7 and significantly affects both the potency and efficacy of BQNs, because the E-isomers seem to be not only more selective but also more potent and efficacious (Table 1). However, this is only a modest difference, leading to the possibility that the recognition site in α7 for the benzylidene motif may be able to accommodate either geometric isomer. Another possibility is that the benzylidine motif does not apply to the Z-isomers. Instead, in the QNZ compounds, the phenyl ring may locate in the same region of the receptor occupied when agonists like nicotine or anabaseine are bound, using the “aryl-centroid” pharmacophoric site (Sheridan et al., 1986). BQNZ and the nonselective agonist AB were compared by overlaying the two structures, and we found that the aryl ring of BQNZ but not the aryl ring of BQNE, resided close to the pyridine ring of anabaseine.(Fig. 10, C and D). This and all of the preceding comparative analyses are predicated on the simplifying idea that a common single productive binding mode leading to activation exists. If this is not the case, comparative analysis would become exceptionally complex. The latter possibility is supported by mutational studies of the conserved Tyr188 residue in α7 and α4β2 (Horenstein et al., 2007). This residue has been hypothesized to play a key role in the sequence of intramolecular events coupling agonist binding to channel gating (Mukhtasimova et al., 2005). However, although the mutation of tyrosine 188 to phenylalanine had a large effect on ACh potency in α7, it had no effect on the potency of the benzylidene anabaseine 4OH-GTS-21 efficacy or potency in α7 (Horenstein et al., 2007). Further support for the hypothesis that gating can be initiated through multiple mechanisms came from the observation that the corresponding tyrosine to phenylalanine mutation in α4β2 had little apparent effect on activation by ACh but produced a 200-fold increase in the efficacy of 4OH-GTS-21.
The model presented in Fig. 9 incorporates the disposition of selectivity motifs in a relative sense, but ultimately, we seek to map this model into the receptor via mutagenesis studies, scanning cysteine accessibility mutagenesis analyses, homology modeling, and docking studies, in lieu of direct crystallographic data for α7 and α3β4 receptors. Preliminary inspection of homology models for α7 based on the crystal structure of snail ACh binding protein suggest that the large benzylidene motif will require extended interactions at the subunit interface. Ongoing work will address why heteromeric receptors cannot accommodate the aforementioned selectivity motifs in the activated state of the receptor and what the specific interactions are with α7. The results reported in this study demonstrate that α7-selective agonists may be obtained by swapping a subset of molecular features of one agonist class with another, for example, conferring selectivity to a quinuclidine by addition of a benzylidene group. This portability can be exploited for the design and synthesis of new α7 agonists.
Acknowledgments
We thank Clare Stokes, Lisa Jacobs, Chad Brodbeck, Sara Braley, and Dolan Abu-Aouf for technical assistance.
These studies were supported by National Institutes of Health grant GM57481.
ABBREVIATIONS
- nAChR
nicotinic acetylcholine receptor
- GTS-21
3–2,4,dimethoxy-benzylidene anabaseine
- TMA
tetramethyl-ammonium
- ETMA
ethyltetramethyl-ammonium
- dEdMA
diethyldimethylammonium
- QN
quinuclidine
- MQN
1-methyl-1-azoniabicyclo[2.2.2]octane iodide
- QN-O
quinuclidinone
- QN-OH
quinuclidinol
- EQN
1-ethyl-1-azoniabicyclo[2.2.2]octane iodide
- BQNE
E-3-benzylidene-1-azoniabicyclo[2.2.2]-octane chloride
- BQNZ
(Z)-3-benzylidene-1-azoniabicyclo[2.2.2]octane chloride
- MBQNE
(E)-3-(4-methoxybenzylidene)-1-azoniabicyclo[2.2.2]-octane chloride
- MBQNZ
(Z)-3-(4-methoxybenzylidene)-1-azoniabicyclo[2.2.2]octane chloride
- DME
1,2-dimethoxy ethane
- ACME
cis-1-methyl-2,3,3a,4,5,9b,-hexahydro-1H-pyrrolo[2,3-f]quinoline
- 5HT3
5-hydroxytryptamine-3
- ACh
acetylcholine
- MS222
tricaine methanesulfonate
References
- Acker BA, Jacobsen EJ, Rogers BN, Wishka DG, Reitz SC, Piotrowski DW, Myers JK, Wolfe ML, Groppi VE, Thornburgh BA, Tinholt PM, Walters RR, Olson BA, Fitzgerald L, Staton BA, Raub TJ, Krause M, Li KS, Hoffmann WE, Hajos M, Hurst RS, Walker DP. Discovery of N-[(3R,5R)-1-azabicyclo[3.2.1]oct-3-yl]furo[2,3-c]pyridine-5-carboxamide as an agonist of the alpha7 nicotinic acetylcholine receptor: in vitro and in vivo activity. Bioorg Med Chem Lett. 2008;18:3611–3615. doi: 10.1016/j.bmcl.2008.04.070. [DOI] [PubMed] [Google Scholar]
- Beers WH, Reich E. Structure and activity of acetylcholine. Nature. 1970;228:917–922. doi: 10.1038/228917a0. [DOI] [PubMed] [Google Scholar]
- Biton B, Bergis OE, Galli F, Nedelec A, Lochead AW, Jegham S, Godet D, Lanneau C, Santamaria R, Chesney F, et al. SSR180711, a novel selective alpha7 nicotinic receptor partial agonist: (1) binding and functional profile. Neuropsychopharmacology. 2007;32:1–16. doi: 10.1038/sj.npp.1301189. [DOI] [PubMed] [Google Scholar]
- Bodnar AL, Cortes-Burgos LA, Cook KK, Dinh DM, Groppi VE, Hajos M, Higdon NR, Hoffmann WE, Hurst RS, Myers JK, et al. Discovery and structure-activity relationship of quinuclidine benzamides as agonists of alpha7 nicotinic acetylcholine receptors. J Med Chem. 2005;48:905–908. doi: 10.1021/jm049363q. [DOI] [PubMed] [Google Scholar]
- Boess FG, De Vry J, Erb C, Flessner T, Hendrix M, Luithle J, Methfessel C, Riedl B, Schnizler K, van der Staay FJ, et al. The novel α7 nicotinic acetylcholine receptor agonist N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]-7-[2-(methoxy)phenyl]-1- benzofuran-2-carboxamide improves working and recognition memory in rodents. J Pharmacol Exp Ther. 2007;321:716–725. doi: 10.1124/jpet.106.118976. [DOI] [PubMed] [Google Scholar]
- Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- Briggs CA, Schrimpf MR, Anderson DJ, Gubbins EJ, Grønlien JH, Håkerud M, Ween H, Thorin-Hagene K, Malysz J, Li J, et al. Alpha7 nicotinic acetylcholine receptor agonist properties of tilorone and related tricyclic analogues. Br J Pharmacol. 2008;153:1054–1061. doi: 10.1038/sj.bjp.0707649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broad LM, Felthouse C, Zwart R, McPhie GI, Pearson KH, Craig PJ, Wallace L, Broadmore RJ, Boot JR, Keenan M, et al. PSAB-OFP, a selective alpha 7 nicotinic receptor agonist, is also a potent agonist of the 5-HT3 receptor. Eur J Pharmacol. 2002;452:137–144. doi: 10.1016/s0014-2999(02)02273-2. [DOI] [PubMed] [Google Scholar]
- Buisson B, Bertrand D. Chronic exposure to nicotine upregulates the human (alpha)4((beta)2 nicotinic acetylcholine receptor function. J Neurosci. 2001;21:1819–1829. doi: 10.1523/JNEUROSCI.21-06-01819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FCM, Benoiton NL. New method of quaternizing amines and its use in amino acid and peptide chemistry. Can J Chem. 1976;54:3310–3311. [Google Scholar]
- Dani JA. Overview of nicotinic receptors and their roles in the central nervous system. Biol Psychiatry. 2001;49:166–174. doi: 10.1016/s0006-3223(00)01011-8. [DOI] [PubMed] [Google Scholar]
- de Fiebre CM, Meyer EM, Henry JC, Muraskin SI, Kem WR, Papke RL. Characterization of a family of anabaseine-derived compounds reveals that the 3-(4)-dimethylaminocinnamylidine derivative (DMAC) is a selective agonist at neuronal nicotinic α7/[125I]α-bungarotoxin receptor subtypes. Mol Pharmacol. 1995;47:164–171. [PubMed] [Google Scholar]
- Efange SM, Tu Z, von Hohenberg K, Francesconi L, Howell RC, Rampersad MV, Todaro LJ, Papke RL, Kung MP. 2-(2-Piperidyl)- and 2-(2-pyrrolidyl-)chromans as nicotine agonists: synthesis and preliminary pharmacological characterization. J Med Chem. 2001;44:4704–4715. doi: 10.1021/jm010129z. [DOI] [PubMed] [Google Scholar]
- Feuerbach D, Nozulak J, Lingenhoehl K, McAllister K, Hoyer D. JN403, in vitro characterization of a novel nicotinic acetylcholine receptor alpha7 selective agonist. Neurosci Lett. 2007;416:61–65. doi: 10.1016/j.neulet.2007.01.045. [DOI] [PubMed] [Google Scholar]
- Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. A subtype of nicotinic cholinergic receptor in rat brain is composed of α4 and β2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol. 1992;41:31–37. [PubMed] [Google Scholar]
- Francis MM, Cheng EY, Weiland GA, Oswald RE. Specific activation of the alpha 7 nicotinic acetylcholine receptor by a quaternary analog of cocaine. Mol Pharmacol. 2001;60:71–79. doi: 10.1124/mol.60.1.71. [DOI] [PubMed] [Google Scholar]
- Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Heinemann S, Boulter J, Deneris E, Conolly J, Duvoisin R, Papke R, Patrick J. The brain nicotinic acetylcholine receptor gene family. Prog Brain Res. 1990;86:195–203. doi: 10.1016/s0079-6123(08)63177-5. [DOI] [PubMed] [Google Scholar]
- Higgins DG. CLUSTAL V: multiple alignment of DNA and protein sequences. Methods Mol Biol. 1994;25:307–318. doi: 10.1385/0-89603-276-0:307. [DOI] [PubMed] [Google Scholar]
- Higgins LS, Berg DK. A desensitized form of neuronal acetylcholine receptor detected by 3H-nicotine binding on bovine adrenal chromaffin cells. J Neurosci. 1988;8:1436–1446. doi: 10.1523/JNEUROSCI.08-04-01436.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horenstein NA, McCormack TJ, Stokes C, Ren K, Papke RL. Reversal of agonist selectivity by mutations of conserved amino acids in the binding site of nicotinic acetylcholine receptors. J Biol Chem. 2007;282:5899–5909. doi: 10.1074/jbc.M609202200. [DOI] [PubMed] [Google Scholar]
- Kaminski JJ, Knutson KW, Bodor N. Convenient method of anion-exchange in quaternary salts. Tetra. 1978;34:2857–2859. [Google Scholar]
- Kem WR, Mahnir VM, Prokai L, Papke RL, Cao X, LeFrancois S, Wildeboer K, Prokai-Tatrai K, Porter-Papke J, Soti F. Hydroxy metabolites of the Alzheimer's drug candidate DMXBA (GTS-21): their molecular properties, interactions with brain nicotinic receptors and brain penetration. Mol Pharmacol. 2004;65:56–67. doi: 10.1124/mol.65.1.56. [DOI] [PubMed] [Google Scholar]
- Le Novère N, Corringer PJ, Changeux JP. The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. J Neurobiol. 2002a;53:447–456. doi: 10.1002/neu.10153. [DOI] [PubMed] [Google Scholar]
- Le Novère N, Grutter T, Changeux JP. Models of the extracellular domain of the nicotinic receptors and of agonist- and Ca2+-binding sites. Proc Natl Acad Sci U S A. 2002b;99:3210–3215. doi: 10.1073/pnas.042699699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonik FM, Papke RL, Horenstein NA. Quinuclidines as selective agonists for alpha-7 nicotinic acetylcholine receptors. Bioorg Med Chem Lett. 2007;17:1520–1522. doi: 10.1016/j.bmcl.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Lopez-Hernandez G, Placzek AN, Thinschmidt JS, Lestage P, Trocme-Thibierge C, Morain P, Papke RL. Partial agonist and neuromodulatory activity of S 24795 for alpha7 nAChR responses of hippocampal interneurons. Neuropharmacology. 2007;53:134–144. doi: 10.1016/j.neuropharm.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Machu TK, Hamilton ME, Frye TF, Shanklin CL, Harris MC, Sun H, Tenner TE, Jr, Soti FS, Kem WR. Benzylidene analogs of anabaseine display partial agonist and antagonist properties at the mouse 5-hydroxytryptamine3A receptor. J Pharmacol Exp Ther. 2001;299:1112–1119. [PubMed] [Google Scholar]
- Macor JE, Gurley D, Lanthorn T, Loch J, Mack RA, Mullen G, Tran O, Wright N, Gordon JC. The 5-HT3 antagonist tropisetron (ICS 205–930) is a potent and selective alpha7 nicotinic receptor partial agonist. Bioorg Med Chem Lett. 2001;11:319–321. doi: 10.1016/s0960-894x(00)00670-3. [DOI] [PubMed] [Google Scholar]
- Marrero MB, Papke RL, Bhatti BS, Shaw S, Bencherif M. The neuroprotective effect of 2-(3-pyridyl)-1-azabicyclo[3.2.2]nonane (TC-1698), a novel α7 ligand, is prevented through angiotensin II activation of a tyrosine phosphatase. J Pharmacol Exp Ther. 2004;309:16–27. doi: 10.1124/jpet.103.061655. [DOI] [PubMed] [Google Scholar]
- Mazurov A, Hauser T, Miller CH. Selective alpha7 nicotinic acetylcholine receptor ligands. Curr Med Chem. 2006;13:1567–1584. doi: 10.2174/092986706777442011. [DOI] [PubMed] [Google Scholar]
- Meyer EM, Kuryatov A, Gerzanich V, Lindstrom J, Papke RL. Analysis of 40H-GTS-21 selectivity and activity at human and rat α7 nicotinic receptors. J Pharmacol Exp Ther. 1998a;287:918–925. [PubMed] [Google Scholar]
- Meyer EM, Tay ET, Papke RL, Meyers C, Huang GL, de Fiebre CM. Effects of 3-[2,4-dimethoxybenzylidene]anabaseine (DMXB) on rat nicotinic receptors and memory-related behaviors. Brain Res. 1997;768:49–56. doi: 10.1016/s0006-8993(97)00536-2. [DOI] [PubMed] [Google Scholar]
- Meyer EM, Tay ET, Zoltewicz JA, Meyers C, King MA, Papke RL, De Fiebre CM. Neuroprotective and memory-related actions of novel α7 nicotinic agents with different mixed agonist/antagonist properties. J Pharmacol Exp Ther. 1998b;284:1026–1032. [PubMed] [Google Scholar]
- Mukhtasimova N, Free C, Sine SM. Initial coupling of binding to gating mediated by conserved residues in the muscle nicotinic receptor. J Gen Physiol. 2005;126:23–39. doi: 10.1085/jgp.200509283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen G, Napier J, Balestra M, DeCory T, Hale G, Macor J, Mack R, Loch J, 3rd, Wu E, Kover A, et al. (−)-Spiro[1-azabicyclo[2.2.2]octane-3,5′-oxazolidin-2′-one], a conformationally restricted analogue of acetylcholine, is a highly selective full agonist at the alpha 7 nicotinic acetylcholine receptor. J Med Chem. 2000;43:4045–4050. doi: 10.1021/jm000249r. [DOI] [PubMed] [Google Scholar]
- Papke RL. Estimation of both the potency and efficacy of alpha7 nAChR agonists from single-concentration responses. Life Sci. 2006;78:2812–2819. doi: 10.1016/j.lfs.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Papke RL, Bencherif M, Lippiello P. An evaluation of neuronal nicotinic acetylcholine receptor activation by quaternary nitrogen compounds indicates that choline is selective for the α7 subtype. Neurosci Lett. 1996;213:201–204. doi: 10.1016/0304-3940(96)12889-5. [DOI] [PubMed] [Google Scholar]
- Papke RL, Meyer E, Nutter T, Uteshev VV. Alpha7-selective agonists and modes of alpha7 receptor activation. Eur J Pharmacol. 2000;393:179–195. doi: 10.1016/s0014-2999(00)00009-1. [DOI] [PubMed] [Google Scholar]
- Papke RL, Meyer EM, Lavieri S, Bollampally SR, Papke TA, Horenstein NA, Itoh Y, Porter Papke JK. Effects at a distance in alpha7 nAChR selective agonists: Benzylidene substitutions regulate potency and efficacy. Neuropharmacology. 2004a;46:1023–1038. doi: 10.1016/j.neuropharm.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Papke RL, Porter Papke JK. Comparative pharmacology of rat and human alpha7 nAChR conducted with net charge analysis. Br J Pharmacol. 2002;137:49–61. doi: 10.1038/sj.bjp.0704833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Porter Papke JK, Rose GM. Activity of alpha7-selective agonists at nicotinic and serotonin receptors expressed in Xenopus oocytes. Bioorg Med Chem Lett. 2004b;14:1849–1853. doi: 10.1016/j.bmcl.2003.09.104. [DOI] [PubMed] [Google Scholar]
- Papke RL, Schiff HC, Jack BA, Horenstein NA. Molecular dissection of tropisetron, an alpha7 nicotinic acetylcholine receptor-selective partial agonist. Neurosci Lett. 2005a;378:140–144. doi: 10.1016/j.neulet.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Papke RL, Zheng G, Horenstein NA, Dwoskin LP, Crooks PA. The characterization of a novel rigid nicotine analog with alpha7-selective nAChR agonist activity and modulation of agonist properties by boron inclusion. Bioorg Med Chem Lett. 2005b;15:3874–3880. doi: 10.1016/j.bmcl.2005.05.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan RP, Nilakantan R, Dixon JS, Venkataraghavan R. The ensemble approach to distance geometry: application to the nicotinic pharmacophore. J Med Chem. 1986;29:899–906. doi: 10.1021/jm00156a005. [DOI] [PubMed] [Google Scholar]
- Stokes C, Papke JK, Horenstein NA, Kem WR, McCormack TJ, Papke RL. The structural basis for drug selectivity between human and rat nicotinic α7 receptors. Mol Pharmacol. 2004;66:14–24. doi: 10.1124/mol.66.1.14. [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Fujio M, Satoh H, Katayama J, Takanashi S, Hashimoto K, Tanaka H. Discovery of the alpha7 nicotinic acetylcholine receptor agonists. (R)-3′-(5-Chlorothiophen-2-yl)spiro-1-azabicyclo[2.2.2]octane-3,5′-[1′,3′] oxazolidin-2′-one as a novel, potent, selective, and orally bioavailable ligand. J Med Chem. 2005;48:2678–2686. doi: 10.1021/jm049188d. [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Fujio M, Takanashi S, Numata A, Katayama J, Satoh H, Shiigi Y, Maeda J, Kuriyama M, Horikawa T, et al. (R)-3′-(3-Methylbenzo[b]thiophen-5-yl)spiro[1-azabicyclo[2,2,2]octane-3,5′ -oxazolidin]-2′-one, a novel and potent alpha7 nicotinic acetylcholine receptor partial agonist displays cognitive enhancing properties. J Med Chem. 2006;49:4374–4383. doi: 10.1021/jm060249c. [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Seio K, Fujio M, Katayama J, Horikawa T, Hashimoto K, Tanaka H. (+)-3-[2-(Benzo[b]thiophen-2-yl)-2-oxoethyl]-1-azabicyclo[2.2.2]octane as potent agonists for the alpha7 nicotinic acetylcholine receptor. Bioorg Med Chem Lett. 2004;14:3781–3784. doi: 10.1016/j.bmcl.2004.04.091. [DOI] [PubMed] [Google Scholar]
- Tønder JE, Olesen PH, Hansen JB, Begtrup M, Pettersson I. An improved nicotinic pharmacophore and a stereoselective CoMFA-model for nicotinic agonists acting at the central nicotinic acetylcholine receptors labelled by [3H]-N-methylcarbamylcholine. J Comput Aided Mol Des. 2001;15:247–258. doi: 10.1023/a:1008140021426. [DOI] [PubMed] [Google Scholar]
- Turner JR, Kellar KJ. Nicotinic cholinergic receptors in the rat cerebellum: multiple heteromeric subtypes. J Neurosci. 2005;25:9258–9265. doi: 10.1523/JNEUROSCI.2112-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbitsky M, Rothlin CV, Katz E, Elgoyhen AB. Mixed nicotinic-muscarinic properties of the alpha9 nicotinic cholinergic receptor. Neuropharmacology. 2000;39:2515–2524. doi: 10.1016/s0028-3908(00)00124-6. [DOI] [PubMed] [Google Scholar]
- Wishka DG, Walker DP, Yates KM, Reitz SC, Jia S, Myers JK, Olson KL, Jacobsen EJ, Wolfe ML, Groppi VE, et al. Discovery of N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]furo[2,3-c]pyridine-5-carboxamide, an agonist of the alpha7 nicotinic acetylcholine receptor, for the potential treatment of cognitive deficits in schizophrenia: synthesis and structure-activity relationship. J Med Chem. 2006;49:4425–4436. doi: 10.1021/jm0602413. [DOI] [PubMed] [Google Scholar]