Abstract
Objective
This study examined the effects of the attention-deficit/hyperactivity disorder treatments, methylphenidate (MPH) and atomoxetine (ATM), on prefrontal cortex (PFC) function in monkeys and explored the receptor mechanisms underlying enhancement of PFC function at the behavioral and cellular levels.
Method
Monkeys performed a working memory task after administration of a wide range of MPH or ATM doses. The optimal doses were challenged with the α2-adrenoceptor antagonist, idazoxan, or the D1 dopamine receptor antagonist, SCH23390 (SCH). In a parallel physiology study, neurons were recorded from the dorsolateral PFC of a monkey performing a working memory task. ATM, SCH, or the α2 antagonist, yohimbine, were applied to the neurons by iontophoresis.
Results
MPH and ATM generally produced inverted-U dose-response curves, with improvement occurring at moderate doses, but not at higher doses. The beneficial effects of these drugs were blocked by idazoxan or SCH. At the cellular level, ATM produced an inverted-U dose-response effect on memory-related firing, enhancing firing for preferred directions (increasing “signals”) and decreasing firing for the nonpreferred directions (decreasing “noise”). The increase in persistent firing for the preferred direction was blocked by yohimbine, whereas the suppression of firing for the nonpreferred directions was blocked by SCH.
Conclusions
Optimal doses of MPH or ATM improved PFC cognitive function in monkeys. These enhancing effects appeared to involve indirect stimulation of α2 adrenoceptors and D1 dopamine receptors in the PFC. These receptor actions likely contribute to their therapeutic effects in the treatment of attention-deficit/hyperactivity disorder.
Keywords: methylphenidate, atomoxetine, attention-deficit/hyperactivity disorder, norepinephrine, dopamine
The symptoms of attention-deficit/hyperactivity disorder (ADHD) are consistent with dysfunction of the prefrontal cortex (PFC), a high-order cortical region that guides behavior, thought, and affect. The PFC accomplishes these “executive functions” by using representational knowledge of rules and goals to produce thoughtful, purposeful actions. In particular, it uses working memory (WM), a form of short-term memory in which information is held temporarily in the mind to provide top-down guidance.1 The PFC has extensive projections to inhibit inappropriate responses and regulate attentional processing in posterior cortical and subcortical regions, including projections through basal ganglia and cerebellar loops.2–5 The PFC also has projections to the arousal systems and thus can regulate its own monoamine and cholinergic inputs.6,7
The PFC in primates is regionally specialized for distinct functions. For example, the lateral surface, which is interconnected with motor and sensory association cortices, subserves WM, high-order decision-making, regulation of attention, and behavioral inhibition, whereas the ventromedial surface, which has connections with subcortical regions such as the amygdala, regulates emotional responses.2–4 Anatomic and physiologic studies in monkeys have revealed that PFC functions arise from networks of neurons that excite each other to keep information—such as goals and rules—“in mind.” PFC neuronal networks are comprised of pyramidal cells that excite each other through synapses on dendritic spines.1 The γ-aminobutyric acidergic interneurons also contribute to coordinate and sculpt network firing.1 This is an intricate and precise process that is highly vulnerable to error.
Top-down regulation by the PFC is altered in many patients with ADHD. Structural and functional imaging studies have shown evidence of smaller PFC volume, decreased PFC activity, weaker PFC white matter connections, and weaker resting connectivity in patients with ADHD.8–13 Similarly, many neuropsychologic studies have shown evidence of impaired PFC function, especially on tasks that require inhibition of inappropriate actions or sustained regulation of attention.14 Thus, different approaches have established that PFC function is weaker in patients with ADHD.
PFC function is powerfully modulated by the catecholamine neurotransmitters, dopamine (DA) and norepinephrine (NE). The PFC requires a narrow range of catecholamines to function optimally, such that too little or too much DA or NE impairs PFC function.15 These mechanisms have been studied in detail using spatial WM as a model of PFC function. Spatial WM is subserved by recurrent excitation among networks of PFC pyramidal cells in the PFC that are spatially “tuned” for a preferred spatial direction. 1,4 Under optimal conditions, PFC function is modulated by stimulation of postsynaptic α2A adrenoceptors on PFC pyramidal cells16 and by moderate stimulation of D1 DA receptors.17 Thus, α2A adrenoceptors enhance “signal” by strengthening network connections between neurons with similar tuning characteristics. This mechanism is accomplished by α2A inhibition of cyclic adenosine monophosphate (cAMP) production in spines, where cAMP signaling can open potassium channels (e.g., hyperpolarization-activated cyclic nucleotide gated cation [HCN] channels) that weaken network connectivity.16 Thus, blockade of α2A receptors in the monkey PFC leads to a collapse in PFC network firing,16 impaired WM,18 and weaker impulse control.19 Conversely, optimal stimulation of D1 receptors improves WM by decreasing “noise.” Moderate stimulation of D1 receptors increases cAMP signaling in a subset of nonpreferred network connections, weakening inappropriate responses.17 Loss of these NE and DA actions markedly impairs PFC function.20,21 However, too much catecholamine release—as can occur during exposure to uncontrollable stress or with very high doses of stimulant medication—impairs PFC function. 6 This impairment involves excessive cAMP-HCN channel signaling, which disconnects all PFC networks.17
ADHD is associated with abnormal catecholamine transmission. For example, polymorphisms in the gene that encodes for DA β-hydroxylase, the synthetic enzyme for NE, are associated with impaired executive function.22–24 Conversely, all approved treatments for ADHD enhance or mimic catecholamines in the PFC.25 The stimulants, methylphenidate (MPH; e.g., Ritalin [Novartis, Basel, Switzerland], Concerta [Ortho-McNeil-Janssen Pharmaceuticals, Inc., Beerse, Belgium]) and amphetamine (Adderall [Teva Pharmaceutical Industries Ltd., Petach Tikva, Israel], Vyvanse [Shire Pharmaceuticals, Wayne, PA]), and the nonstimulant, atomoxetine (ATM; Strattera [Eli Lilly and Company, Indianapolis, IN]), increase NE and DA transmission in the PFC,26,27 whereas guanfacine (Intuniv [Shire Pharmaceuticals]) and clonidine mimic NE actions at α2A receptors.15 Although previous works have often emphasized the role of DA in stimulant medications, recent biochemical studies have indicated that therapeutic doses of these agents actually have a greater effect on NE than DA levels in the PFC, while having more subtle effects on catecholamine release in subcortical structures.26 These neurochemical findings suggest that MPH and ATM may share therapeutic actions by enhancing catecholamine transmission in the PFC. The present study explored this hypothesis by examining the effects of a wide range of MPH and ATM doses on WM function in monkeys at the behavioral and cellular levels and determining the receptor mechanisms underlying their enhancing effects on PFC function. Specifically, this study investigated whether optimal doses of MPH and ATM enhanced PFC function by increasing catecholamine stimulation of α2 adrenoceptors and D1 DA receptors in the PFC.
METHOD
All procedures were approved by the institutional animal care and use committee at Yale University.
Subjects
Rhesus macaques (Macaca mulatta) were housed in pairs or individually under standard laboratory conditions. Highly palatable rewards (e.g., raisins) were used to minimize the need for dietary restriction. Water was provided ad libitum, and monkeys were fed monkey chow (Purina Mills, St Louis, MO) and fresh fruit immediately after testing. Great care was taken to minimize stress to the animals, e.g., by acclimating animals to each step of the procedures and minimizing or avoiding high doses of medication with untoward side effects.
Eighteen monkeys (16 female, two male) performed a manual version of the spatial WM task to examine cognitive performance. These monkeys ranged in age from 6 years (young adult) to 29 years (aged). Four monkeys were used in the physiology studies; one animal received ATM and α2 and D1 receptor compounds.
Behavioral Methods
Delayed Response Task to Test Spatial WM in Monkeys
Spatial WM was assessed using a manual delayed response task in a Wisconsin General Testing Apparatus, as previously described.28 In this task, the animal watched the experimenter place a food reward into one of two to six food wells and then cover the wells with identical cardboard plaques. The delay period followed, during which the experimenter lowered an opaque screen between the animal and the food wells, and the animal had to remember where the reward was hidden. At the end the of the delay period, the experimenter raised the opaque screen, and the animal reached for the reward; this procedure was repeated for 30 trials per day. The delay lengths were adjusted for each animal such that the animal showed a stable baseline performance of 60% to 80% correct, thus allowing room for improvements or impairments in performance after drug treatments. Animals were tested twice a week by experimenters who were familiar with the normative behavior of the monkeys, but were blind to the drug treatments.
Effects of MPH and ATM on PFC Cognitive Performance and Their Receptor Mechanisms
MPH (RTI International, Research Triangle Park, NC) or ATM (purchased as tomoxetine from Tocris Bioscience, Ellisville, MO) was administered over a wide range of doses to observe dose-response curves for each animal. MPH (0.1–2.0 mg/kg) or vehicle (water) was administered orally 30 minutes before testing, whereas ATM (0.001–5.0 mg/kg) or vehicle (water) was administered orally 1 hour before testing. Only one treatment was given per day, with at least a 10-day washout period between treatments. Treatments started with a relatively low dose that was gradually increased until the monkey showed impairment. The optimal dose was defined as that producing the best score and was repeated to ensure replication.
Enhancing doses were challenged with the α2-adrenoceptor antagonist, idazoxan (IDA; Sigma-Aldrich, St Louis, MO), or the D1 DA receptor antagonist, SCH23390 (SCH; Tocris Bioscience). These drugs were chosen based on previous experience with systemic administration.29,30 IDA (0.01– 0.5 mg/kg), SCH (0.001– 0.06 mg/kg), or vehicle (saline) was injected intramuscularly 1 hour before testing, in combination with MPH, ATM. or their respective vehicles. Pilot studies determined doses of IDA and SCH for each monkey that did not significantly impair WM performance on their own. Thus, any effects of the drugs on WM would be due to an interaction between the treatments, rather than nonspecific additive effects.
Each of the following four treatments were administered to each animal in counterbalanced order:
Role of α2 adrenoceptors in optimal ATM response: vehicle + vehicle versus ATM + vehicle versus vehicle + IDA versus ATM + IDA
Role of D1 receptors in optimal ATM response: vehicle + vehicle versus ATM + vehicle versus vehicle + SCH versus ATM + SCH
Role of α2 adrenoceptors in optimal MPH response: vehicle + vehicle versus MPH + vehicle versus vehicle + IDA versus MPH + IDA
Role of D1 receptors in optimal MPH response: vehicle + vehicle versus MPH + vehicle versus vehicle + SCH versus MPH + SCH
Data Analysis for PFC Cognitive Performance
The effects of IDA and SCH on optimal doses of MPH or ATM treatments were analyzed using a two-way analysis of variance (ANOVA) with repeated measures, with within-subject factors of medication (MPH or ATM versus vehicle) and antagonist challenge (IDA or SCH versus vehicle). User-defined contrasts explicitly tested whether IDA or SCH blocked the MPH or ATM response. Analyses were performed on a personal computer with a Windows platform using Systat software (Systat Software Inc, Chicago, IL).
Electrophysiological Methods
Effects of ATM on PFC Neuronal Firing and Their Receptor Mechanisms
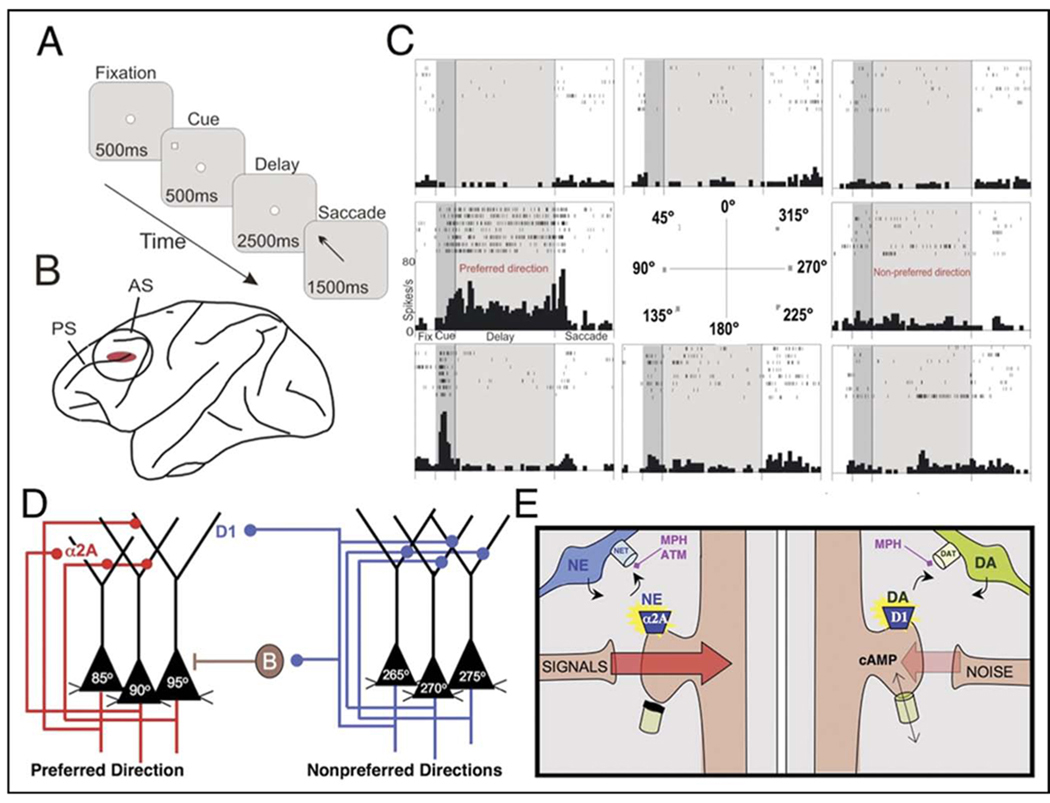
The effects of ATM on PFC neuronal firing patterns were examined in a monkey performing the oculomotor delayed response spatial WM task (Figure 1A), whereas single-unit recordings were performed in the dorsolateral PFC (Figure 1B).1,4,17,31 Under optimal conditions, pyramidal neurons in the dorsolateral PFC exhibit persistent firing during the delay periods for cues in a particular direction, called the preferred direction, but show no increase or even suppressed firing for other directions, called the nonpreferred directions1 (Figure 1C). Persistent firing during the delay period is thought to arise from networks of PFC pyramidal cells with shared spatial properties, engaged in recurrent excitation1,4 (Figure 1D). NE stimulation of α2A adrenoceptors appears to strengthen this network connectivity (Figure 1D,E). In contrast, the spatial tuning of the network arises from γ-aminobutyric acidergic lateral inhibition between these networks31 and from D1 DA receptor stimulation17 (Figure 1D,E).
FIGURE 1.
Physiological recordings from monkey prefrontal cortex. Note: (A) Monkeys performed the oculomotor delayed response. Each trial begins when the monkey fixates on a central point (fixation period). Next, a cue briefly appears in one of eight peripheral locations. A 2.5-second delay period follows, during which the monkey continues to maintain fixation. At the end of the delay period, the monkey makes a memory-guided saccade to the remembered cue location (response period) and is rewarded with juice if correct. Each test session consists of hundreds of trials, in which the cued location randomly changes for each trial, thus requiring the monkey to update working memory despite extensive, proactive interference. (B) Single-unit recording was performed in the dorsolateral prefrontal cortex, the region associated with spatial working memory in monkeys. (C) The firing patterns of a representative neuron in monkey dorsolateral prefrontal cortex. Under optimal conditions, neurons show delay-related firing for a preferred direction, but suppress firing for nonpreferred directions.1,4 This pattern is considered the cellular basis for spatial working memory. (D) The circuit basis for spatial working memory as proposed by Patricia Goldman-Rakic.1 Persistent firing during the delay period arises from recurrent excitation among pyramidal cells with similar spatial inputs from the parietal cortex. Spatial tuning is enhanced by γ-aminobutyric acidergic lateral inhibition,31 e.g., the basket cell (B) shown here, and by moderate levels of D1 dopamine (DA) receptor stimulation.17 (E) A working model of catecholamine influences on prefrontal cortical network connectivity. Under optimal conditions, appropriate inputs to a cell are strengthened by norepinephrine (NE) stimulation of α2A adrenoceptors, whereas inappropriate inputs are weakened by DA stimulation of D1 receptors. Changes in the strength of connections appear to be mediated by influence of cyclic adenosine monophosphate (cAMP) on the open state of ion channels near synaptic inputs. Although previous research emphasized the presynaptic actions of α2 adrenoceptors, it is now known that these receptors reside postsynaptically on prefrontal cortical neurons, and that the postsynaptic receptors are the site of prefrontal cortical cognitive enhancement with α2 agonists such as clonidine and guanfacine. Methylphenidate (MPH) blocks NE transporters and DA transporters, whereas atomoxetine (ATM) blocks NE transporters, thus enhancing catecholamine actions at these receptors. AS = arcuate sulcus; PS = principal sulcus.
Single-unit recordings were made from regularly spiking cells, which were presumed to be pyramidal cells, in the dorsolateral PFC; see Wang et al. for more detailed technical methods.16 A multibarrel iontophoretic electrode was filled with charged drug solutions, i.e., ATM, SCH, or the α2-adrenoceptor antagonist, yohimbine (YOH; Sigma-Aldrich). YOH was used instead of IDA because there were well-established parameters for iontophoresis of YOH from previous studies.16 Spike2 software (Cambridge Electronics Design, Cambridge, UK) revealed firing patterns for a particular cell. If the unit appeared to show spatially specific, delay-related firing online, drug treatments were applied by iontophoresis using a current of 5 to 50 nA with the appropriate polarity for each drug. After an initial control condition, dose-response curves for ATM were obtained by adjusting the ejection current in consecutive conditions. The optimal dose, which enhanced delay-related firing for the preferred direction or suppressed firing for the nonpreferred direction, was challenged by co-application of YOH or SCH. We were unable to examine the effects of MPH because this drug could not be reliably iontophoresed.
Data Analysis for PFC Neuronal Firing
We confirmed whether cells showed spatially specific, delay-related firing after the experiment. The firing rate was compared between the task epochs (fixation, cue, delay, response) and between the eight cue locations using a two-way ANOVA with repeated measures, with epoch and location as factors. User-defined contrasts tested whether the delay-related activity was enhanced for the preferred direction compared with the opposite, nonpreferred direction. The effects of various doses of ATM on neuronal activity were assessed using a one-way ANOVA with repeated measures, with condition (ATM versus control) as the factor. User-defined contrasts tested whether the optimal dose of ATM had a different effect from that with an excessive dose. The effects of YOH or SCH on optimal neuronal response with ATM were analyzed using a two-way ANOVA with repeated measures, with factors of medication (ATM versus vehicle) and antagonist challenge (YOH or SCH versus vehicle). User-defined contrasts revealed whether YOH or SCH blocked the ATM response. Statistical analyses were performed using Systat.
RESULTS
Behavioral Data
Effects of MPH and ATM on PFC Cognitive Performance
The effects of a range of doses of MPH (0.1–2.0 mg/kg) or ATM (0.001–5.0 mg/kg) were examined on spatial WM performance in the delayed response task. For each monkey, an optimal dose of MPH or ATM was found. In general, inverted-U-shaped dose-response curves were observed, whereby moderate doses improved and lower and higher doses impaired or had no effect on performance. However, there was much individual variability in the dose-response curves; thus, individual rather than average dose-response curves are shown (Figure 2A–F). Some of the variability in sensitivity to the drugs appeared to arise from the animal’s age. Drug potency was correlated with age for ATM (r = −0.63, p = .01), with older animals requiring lower doses of ATM than younger monkeys for improved performance, whereas there was a trend for MPH to be less potent in the older monkeys (r = 0.42, p = .14). WM capacity likely contributed to the individual variability, because monkeys with shorter delays and less food wells in the Wisconsin General Testing Apparatus tended to require higher MPH doses for optimal performance (r = −0.48, p = .070).
FIGURE 2.
Representative dose-response curves show generally inverted-U dose-response curves for methylphanidate (MPH) in monkeys B (A), M (B), and T (C) and for atomoxetine (ATM) in monkeys B (D), M (E), and T (F) while they performed the delayed response task. Note: Results represent number correct of a possible 30 trials. Monkey B also scored one trial correct with MPH 0.4 mg/kg, but this score was not included here, because it was uncharacteristic for her behavior; higher doses were not tested in monkey B, because she was switched to a different study.
Receptor Mechanisms Underlying Improvement of Cognitive Performance by MPH or ATM
We investigated whether MPH and ATM enhanced WM performance by stimulation of α2 adrenoceptors and D1 DA receptors by testing whether the α2-adrenoceptor antagonist, IDA, or the D1 receptor antagonist, SCH, blocked the enhancing effects of MPH or ATM on WM performance.
Methylphenidate
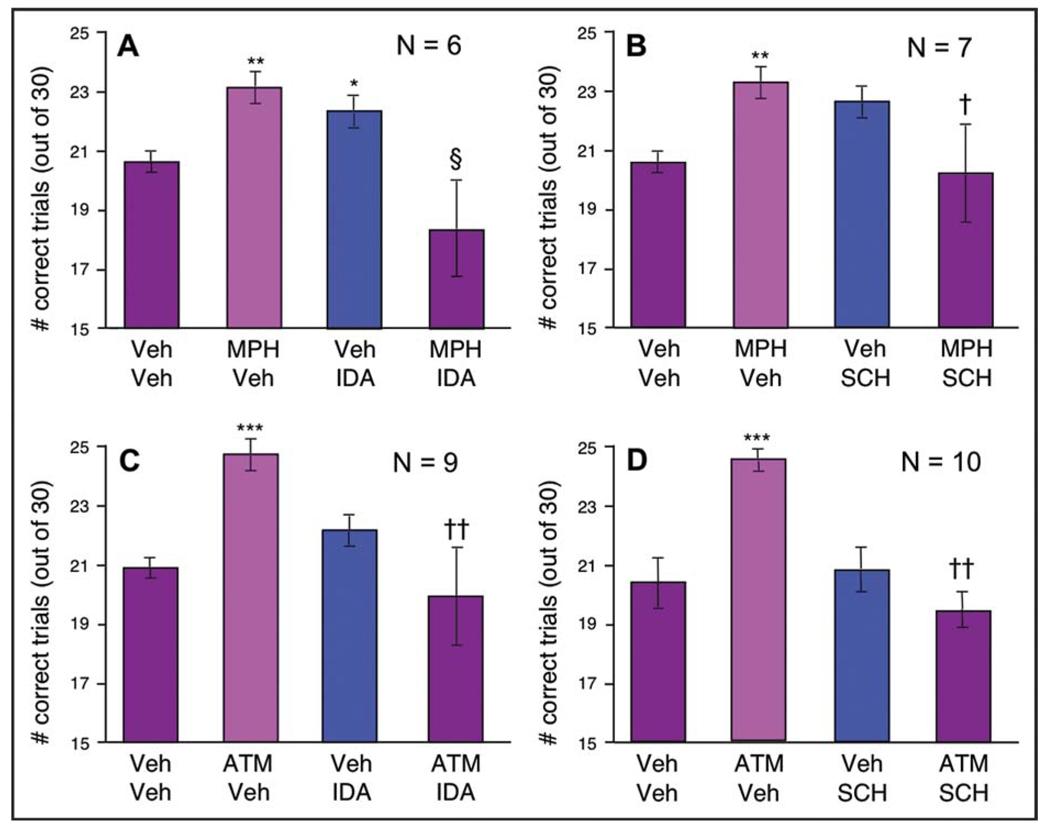
Idazoxan Reversed the Enhancing Effects of MPH (Figure 3A). A two-way ANOVA with repeated measures showed a significant interaction between MPH and IDA (F1,5 = 11.054, p = .021), whereas the main effects of MPH and IDA alone were not significant (MPH: F1,5 = 0.733, p > .05; IDA: F1,5 = 3.847, p > .05). User-defined contrasts revealed that MPH significantly improved performance compared with vehicle (MPH + vehicle versus vehicle + vehicle: F1,5 = 28.363, p = .003), whereas MPH + IDA treatment reversed this improvement (MPH + IDA versus MPH + vehicle: F1,5 = 7.915, p = .037). Because we chose doses of IDA that did not impair performance on their own, the analysis showed a small but significant improvement in performance after IDA treatment compared with baseline, which might arise from presynaptic drug actions (vehicle + IDA versus vehicle + vehicle: F1,5 = 12.091, p = .018). Thus, the ability of IDA to reverse the enhancing effects of MPH could not be due to additive effects of the two treatments.
FIGURE 3.
Optimal doses of methylphenidate (MPH) and atomoxetine (ATM) enhanced performance in the delayed response task, and this improvement was reversed by the α2-adrenoceptor antagonist, idazoxan (IDA) (A, C), or the D1 receptor antagonist, SCH23390 (SCH) (B, D). Note: Results represent the mean for number of trials correct, with error bars representing standard error of the mean; *p < .05 compared with vehicle; **p < .005 compared with vehicle; ***p < .001 compared with vehicle; §p < .05 compared with IDA + vehicle; †p < .01 compared with MPH + vehicle; ††p < .001 compared with ATM + vehicle. Veh + vehicle.
SCH also reversed the enhancing effects of MPH (Figure 3B). A two-way ANOVA with repeated measures showed a significant interaction between MPH and SCH (F1,6 = 25.583, p = .002), but no main effects of MPH or SCH (MPH: F1,6 = 0.05, p = .831; SCH: F1,6 = 0.666, p = .446). User-defined contrasts revealed that MPH significantly improved performance compared with vehicle (MPH + vehicle versus vehicle + vehicle: F1,6 = 44.954, p = .001), whereas MPH + SCH treatment reversed this improvement (MPH + SCH versus MPH + vehicle: F1,6 = 16.617, p = .007). SCH alone produced a nonsignificant improvement in performance compared with vehicle (vehicle + SCH versus vehicle + vehicle: F1,6 = 5.47, p = .058).
Atomoxetine
Idazoxan Blocked the Enhancing Effects of ATM (Figure 3C). A two-way ANOVA with repeated measures showed a significant interaction between ATM and IDA (F1,8 = 22.168, p = .002) and a significant main effect of IDA (F1,8 = 13.934, p = .006), but no main effect of ATM (F1,8 = 1.259, p = .294). User-defined contrasts revealed that ATM significantly improved performance compared with vehicle (ATM + vehicle versus vehicle + vehicle: F1,8 = 156.619, p < .0005), whereas ATM + IDA treatment reversed this improvement (ATM + IDA versus ATM + vehicle: F1,8 = 31.677, p < .0005). IDA alone had no significant effect on performance compared with vehicle in this experiment (vehicle + IDA versus vehicle + vehicle: F1,8 = 2.923, p = .126).
SCH also reversed the enhancing effects of ATM (Figure 3D). A two-way ANOVA with repeated measures showed a significant interaction between ATM and SCH (F1,9 = 40.146, p < .0005) and significant main effects of ATM (F1,9 = 8.947, p = .015) and SCH (F1,9 = 30.546, p < .0005). User-defined contrasts revealed that ATM significantly improved performance compared with vehicle (ATM + vehicle versus vehicle + vehicle: F1,9 = 104.425, p < .0005), whereas ATM + SCH treatment reversed this improvement (ATM + SCH versus ATM + vehicle: F1,9 = 122.575, p < .0005). SCH alone had no significant effect on performance compared with vehicle (F1,9 = 0.272, p = .614).
Electrophysiological Data
Effects of α2 NE or D1 DA Receptor Stimulation on PFC Neuronal Firing
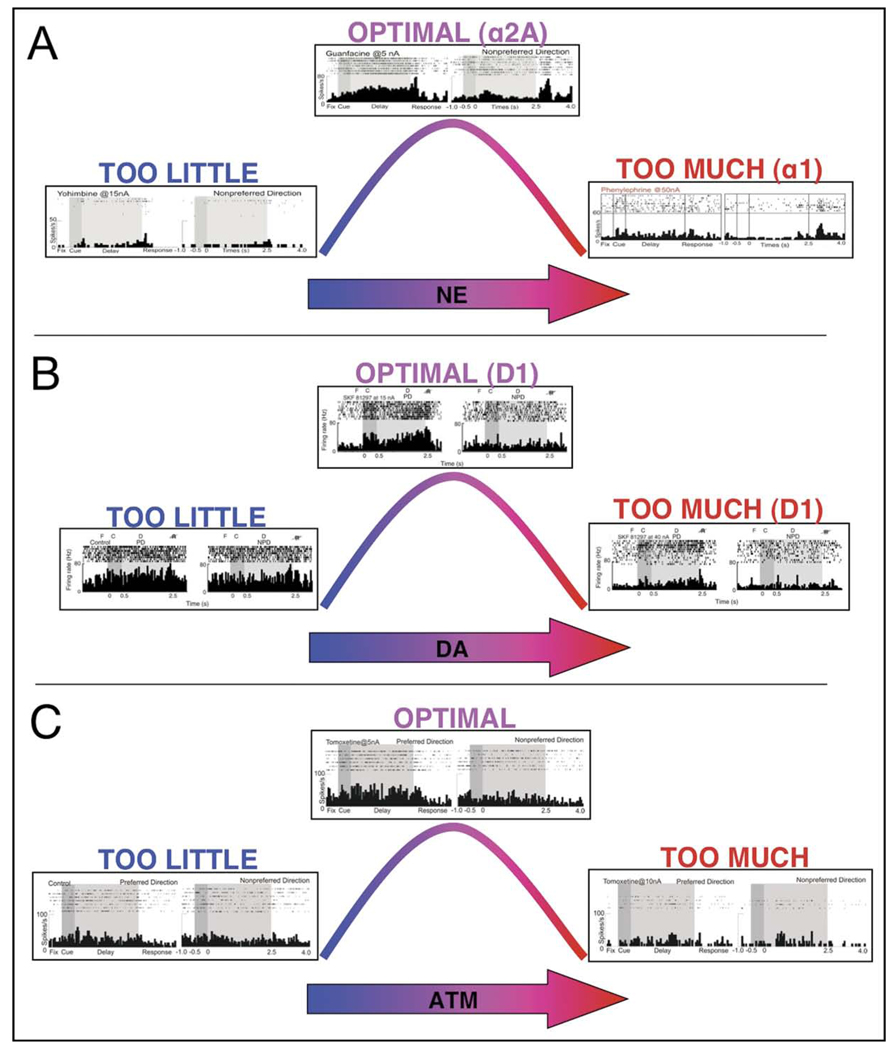
NE and DA had powerful effects on the delay-related firing of PFC neurons in monkeys performing a WM task. Stimulation of α2A NE receptors increased delay-related firing for the preferred direction of the neuron (Figure 4A), whereas moderate stimulation of D1 DA receptors decreased firing for the nonpreferred directions of the neuron (Figure 4B).16,17,32 These actions increased the signal-to-noise ratio of firing in a complementary manner. High levels of NE and DA suppressed PFC neuronal firing through α1 and excessive D1 receptor stimulation (Figure 4A,B). For simplicity, Figures 4 and 5 show data for the preferred direction and only one opposing nonpreferred direction for each neuron.
FIGURE 4.
Physiologic responses to catecholamines in monkey prefrontal cortex. Note: (A) Norepinephrine (NE), (B) dopamine (DA), and (C) atomoxetine (ATM) show inverted-U dose-response curves on delay-related activity in a monkey performing the oculomotor delayed response task. (A) Optimal levels of NE enhanced delay-related activity for the preferred direction via α2A adrenoceptors, whereas excessive levels suppressed firing via α1 and β1 adrenoceptors.16,32 (B) Optimal levels of DA enhanced spatial tuning by suppressing delay-related firing for the nonpreferred direction, whereas excessive levels suppressed firing for preferred and nonpreferred stimuli.17 (C) A low dose (5 nA) of ATM enhanced delay-related firing for the preferred direction, whereas a higher dose (10 nA) suppressed firing. Low and high doses were tested in four neurons; in the remaining cells, only a low or high dose was able to be tested within the testing session.
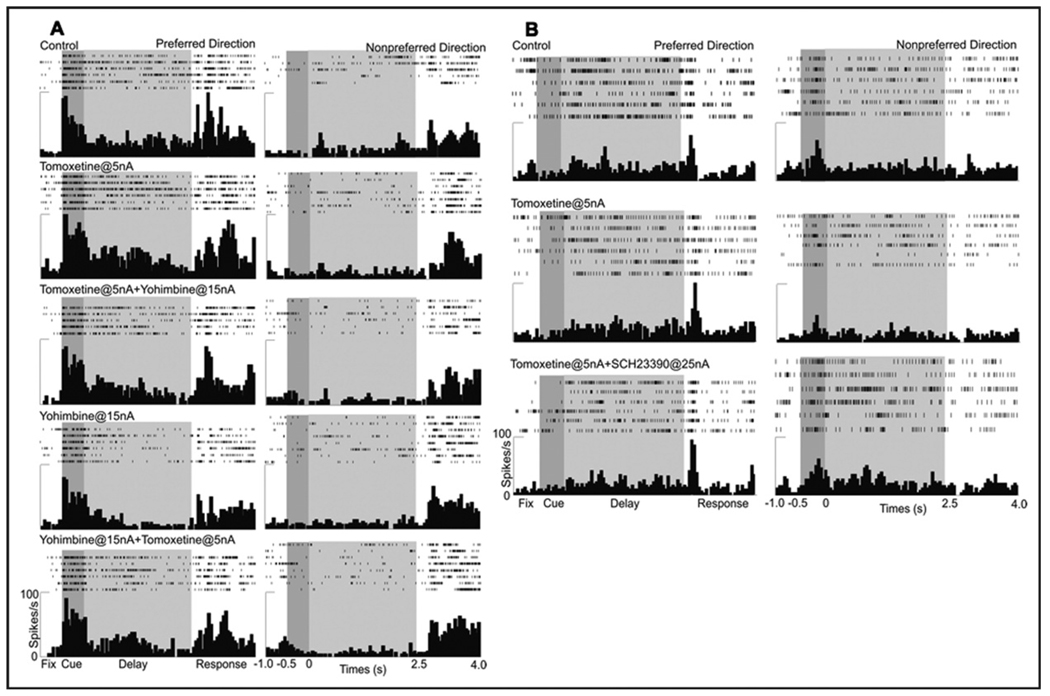
FIGURE 5.
Beneficial effects of atomoxetine were blocked by α2 or D1 receptor antagonists. Note: (A) A low dose of atomoxetine (5 nA) increased delay-related firing for the preferred direction (second row from top). This increase was blocked by co-iontophoresis of the α2 antagonist, yohimbine (third row from top). Subsequent application of yohimbine alone (15 nA) decreased delay-related firing (fourth row from top), and this decrease was reversed by addition of atomoxetine (5 nA; bottom row). (B) A low dose of atomoxetine (5 nA) enhanced spatial tuning by decreasing firing for the nonpreferred direction (middle row). This decrease was reversed by co-iontophoresis of SCH23390 (25 nA; bottom row).
Effects of ATM on PFC Neuronal Firing
The effects of ATM on neuronal firing patterns in the dorsolateral PFC were examined in a monkey performing the oculomotor delayed response task. A range of doses of ATM was applied to 17 neurons with spatially tuned, delay-related activity. As shown in Figure 4C, ATM treatment produced an inverted-U dose-response curve, whereby low doses enhanced delay-related firing or enhanced spatial tuning, whereas higher doses suppressed firing. Low doses of ATM (2–5 nA) increased delay-related firing for the cell’s preferred direction in nine of 12 neurons tested (p < .05 in four neurons, p < .001 in five neurons), i.e., increasing “signal.” In a small number of cells (n = 2), ATM (5 nA) decreased delay-related activity for the nonpreferred direction (p < .001), i.e., decreasing “noise.” Thus, low doses enhanced the signal-to-noise ratio of neuronal response during the delay period. In contrast, a higher dose of ATM (10 nA) nonspecifically decreased delay-related firing in five of five neurons tested (p < .001), similar to what has been seen with high doses of α1 NE or D1 DA agonists (Figure 4A,B). Low and high doses were tested in four of the 17 neurons; in these cells, low doses of ATM enhanced, whereas high doses of ATM suppressed, delay-related firing.
To investigate the receptor mechanisms by which ATM enhanced PFC neuronal activity, we challenged the effects of ATM with the α2-adrenoceptor antagonist, YOH, or the D1 antagonist, SCH. YOH blocked and/or reversed the enhancing effects of ATM (2–10 nA) on delay-related firing for the preferred direction in all seven cells (p < .001; Figure 5A). These results suggested that ATM’s beneficial effects on delay-related firing for the preferred direction involved enhanced NE stimulation of α2 receptors. Conversely, iontophoresis of SCH reversed the sculpting effects of ATM on firing for the nonpreferred direction (p < .001; Figure 5B). Although this response could be studied in only one neuron, the data support a D1 mechanism contributing to the ATM neuronal response.
DISCUSSION
This study found that moderate doses of MPH and ATM improved spatial WM in monkeys. The beneficial effects of these drugs were blocked by the α2-adrenoceptor antagonist, IDA, or the D1 DA receptor antagonist, SCH, consistent with previous findings in rats.33 Similar effects were observed at the cellular level within the PFC. Single-unit recording combined with iontophoresis showed that ATM produced an inverted-U dose-response effect on spatially tuned, persistent firing during an oculomotor spatial WM task. An optimal dose of ATM increased persistent firing for the preferred direction or decreased firing for the nonpreferred direction, thus enhancing the signal-to-noise ratio of the neuronal response. The increased persistent firing for the preferred direction was blocked by the α2 antagonist, YOH, whereas the suppression of firing for the nonpreferred direction was blocked by SCH. Taken together, these behavioral and physiological findings showed that the beneficial effects of MPH and ATM on WM involved enhanced endogenous stimulation of α2 adrenoceptors and D1 DA receptors in the PFC (Figure 1E). Other catecholamine receptors might have contributed; however, this study focused on α2 and D1 receptors, because they have been known to have prominent effects on dorsolateral PFC function. It was also likely that MPH and ATM had beneficial effects on behavior through actions outside the PFC, e.g., DA actions in the caudate (MPH) or catecholamine actions in the hippocampus (MPH or ATM). However, the dorsolateral PFC is the region most tightly connected to spatial WM tasks, and the iontophoresis of ATM onto PFC neurons produced results consistent with those of the behavioral studies.
Variability in Response to MPH and ATM
There was much individual variability in sensitivity to ATM and especially MPH. Such variability might result from different baseline catecholamine levels and receptor activity, which in turn might arise from differences in age, motivation, stress levels, sex hormones, or drug absorption or metabolism. We observed a trend of decreasing MPH efficacy with age. A similar profile has been observed in humans, whereby MPH was less effective in the elderly,34 which was also consistent with deficits in PFC function associated with aging (e.g., Moore et al.35). This pattern might relate to age-related declines in the DA system in humans36 and monkeys37 and to reductions in α2 adrenoceptor and possibly D1 receptor binding in the PFC of aged monkeys.35,38 Level of sex hormones was another factor that might contribute to variations in dose-response curves. Female primates—many of which are involved in this study—have cycling levels of estrogen that modulate the stress response. Shansky et al. showed that WM function of female rats with high estrogen levels is more sensitive to stress-induced impairment than female rats with low estrogen levels or male rats.39,40 Furthermore, polymorphisms in the DA transporter gene exist in many primate species, including rhesus macaques,41–44 which might differentiate extrasynaptic DA levels between individuals. However, polymorphisms in the NE transporter gene have not been investigated in the rhesus macaque. Because DA transporters are sparse in the PFC,45,46 the contribution of such polymorphisms to WM performance in this species is unknown. Although we strived to minimize day-to-day variations in baseline catecholamine levels (e.g., due to changes in arousal) by testing on a consistent schedule and by the same tester, there were likely daily factors that we could not control.
Receptor Mechanisms at Behavioral and Cellular Levels
The enhancing effects of MPH and ATM on behavioral performance were blocked by IDA or SCH, suggesting that stimulation of α2 and D1 receptors contributed to the beneficial effects of these medications. Similarly, the electrophysiological results were consistent with previous studies of the effects of selective α2A or D1 receptor agonists on PFC function. ATM, like the α2A agonist guanfacine, enhanced persistent firing for the preferred direction (Figures 4, 5A). This increase in firing is thought to arise from strengthened connectivity between PFC neurons with shared characteristics. Stimulation of postsynaptic α2A receptors on a subset of dendritic spines would inhibit cAMP, thus closing nearby ion channels and maintaining synaptic inputs onto the spines.16 Conversely, stimulation of D1 receptors on a separate set of spines may weaken connections for nonpreferred inputs by increasing the production of cAMP, thus opening nearby ion channels and decreasing the strength of synaptic inputs onto the spines.17 Similar effects of ATM and D1 agonists reducing “noise” can be seen in Figure 5B. In the present study, ATM more frequently increased firing for the preferred direction and less frequently decreased firing for the nonpreferred direction, suggesting that its effects on NE predominated over those on DA. That could be due to a number of factors, e.g., ATM could have greater effect on NE than DA in the primate PFC; endogenous DA levels in the PFC of young, healthy monkeys might be optimal, thus it would be difficult to see enhanced DA effects; and local application of ATM on PFC neurons might have less effect on DA than systemic administration of ATM, e.g., if ATM normally increases DA release in the PFC by actions in the ventral tegmental area. The efficacy of SCH in reversing the enhancing effects of systemic ATM on behavioral performance is consistent with this last interpretation.
There are some misconceptions about MPH in the literature, which should be addressed. One common misconception is that MPH is a selective blocker of the DA transporter, when it actually blocks NE and DA transporters. Indeed, rat studies have shown that MPH doses that produce blood levels similar to those in children taking MPH medication actually have a greater effect on NE than DA in the PFC.26 Another misconception is that it increases locomotor activity in normal subjects and decreases activity in subjects with ADHD. Many previous studies of MPH in rats have used excessive doses of MPH that increase locomotor activity and DA release in striatum.47 In contrast, lower doses that produce blood levels similar to those in patients with ADHD decrease locomotor activity in juvenile rats47,48 and improve WM and attentional function in adult rats.26,33 A similar pattern is observed in humans, where proper doses of MPH enhance PFC function in normal subjects49 and patients with ADHD.50,51 Furthermore, imaging studies of MPH effects in normal subjects reported enhanced efficiency of the PFC blood oxygen level-dependent (BOLD) response, in conjunction with improved WM performance.49 Thus, there is excellent correspondence between actions in patients with ADHD and in normal human and animal subjects. In the present study, we were unable to document MPH actions at the cellular level, because MPH could not be reliably iontophoresed. However, the behavioral results with systemic MPH administration were consistent with D1 and α2 receptor actions contributing to enhancing effects of MPH on WM.
Importance of Dose to PFC Function
The present results emphasize the importance of correct medication dosage for optimal PFC function. PFC function and neuronal firing are impaired by high levels of catecholamines.6 Consistent with these findings, the present study found that high doses of MPH or ATM impaired cognitive function or cell firing in many animals. These detrimental actions may also be seen in patients after excessive doses of medication, who complain of cognitive inflexibility.52 Even within a therapeutic dose range, there is likely variation in optimal dosing depending on current cognitive demands. For example, some tasks (e.g., arithmetic homework) may benefit from higher MPH doses that produce marked narrowing of attention (e.g., by enhancing catecholamine actions in sensory cortices and caudate), but these doses would be excessive for other tasks (e.g., multitasking) that require thoughtful, flexible PFC regulation of behavior. Even within the PFC, it is likely that different levels of catecholamines are required, depending on the cognitive operation engaged. Spatial WM in the present study benefited from D1 sculpting of inputs to maintain a narrow spatial location in WM, but other PFC functions that require broader inputs (e.g., attention- shifting or insight-learning) are worsened by these actions.53–55 Although it may be impossible to achieve an optimal dose under all circumstances, flexible dosing that respects environmental demands may maximize efficacy of ADHD medications. It is encouraging that the optimal doses of drugs in this study are comparable to typical doses used in patients with ADHD. We found that 0.1 to 1.2 mg/kg is optimal for MPH, whereas 0.1 to 1.0 mg/kg is often used in patients; and 0.01 to 5.0 mg/kg is optimal for ATM in this study, whereas 1.2 to 2.0 mg/kg is used for ATM in patients.
It is also noteworthy that in the present study, we observed beneficial effects of acute drug administration, although some patients with ADHD require chronic exposure (especially to ATM) for symptom relief. Acute administration of ATM has also been shown to improve behavioral inhibition in a stop-signal task in adults with ADHD56 and in normal adults,56–58 indicating that the present results are not specific to monkeys. MPH has also been shown to produce acute improvement in spatial WM and response inhibition in patients with ADHD.51,59 However, statistical improvement on a neuropsychologic task may require a lower threshold of drug efficacy than the amelioration of ADHD symptoms.
Summary: ADHD Medications and Catecholamine Actions in PFC
In this study, we have proposed mechanisms by which MPH and ATM improve PFC function in healthy monkeys. These mechanisms are likely to contribute to therapeutic effects in the PFC of patients with ADHD. Mehta et al. found that MPH improves WM performance and decreases regional cerebral blood flow in the dorsolateral PFC in normal adults.49 MPH is especially beneficial for subjects with lower baseline WM capacity than other subjects, suggesting that it may similarly enhance PFC function in patients with ADHD. Interestingly, MPH has also been associated with increased regional cerebral blood flow in the frontal cortex, in addition to improved behavioral symptoms in children with ADHD.60,61 The effect of ATM on PFC function has been investigated only in healthy adults. Chamberlain et al. showed that ATM improves inhibitory control in a stop-signal task and increases functional magnetic resonance imaging BOLD signals in the right inferior frontal gyrus in healthy adults, and that plasma levels of the drug correlates with the magnitude of BOLD responses during successful inhibition.58 Confirmation of these mechanisms in the PFC of patients with ADHD awaits further studies.
Our understanding of catecholamine actions in the PFC and their relevance to ADHD are expanding. In addition to WM functions of the dorsolateral PFC described in the present study, researchers are discovering the roles of catecholamines in other PFC operations such as various types of impulse control,62,63 attention regulation,64 cognitive flexibility, 65 and response to reward.66 Thus, it is hoped that we will have increasing ability to relate drug actions in the brain to therapeutic effects in patients.
Acknowledgments
This research was supported by a grant from Shire Pharmaceuticals to Dr. Arnsten as part of an overarching goal to further understanding of the neurobiological bases of ADHD and its treatment.
The authors thank Lisa Ciavarella, Tracy Sadlon, Sam Johnson, Michelle Benevento, and Jessica Thomas (employed by Yale University) for their invaluable technical expertise, and Benny Brunson and Daniel Torres (employed by Yale Animal Resources Center) for their superb care of our animals. This paper was written entirely by the authors, with no outside assistance from professional writers or statistical experts.
Footnotes
This article was reviewed under and accepted by Associate Editor James J. Hudziak, M.D.
Disclosure: Dr. Arnsten and Yale University have a license agreement and receive royalties from Shire Pharmaceuticals for the development of guanfacine (Intuniv™) for the treatment of ADHD and related disorders. Dr. Arnsten also performs consulting, teaching, and speaking engagements for Shire Pharmaceuticals. Dr. Arnsten has provided consultation to Eli Lilly and Co. in the past but not in regard to the present research. Dr. Gamo previously owned stock in Shire Pharmaceuticals but has since divested. Dr. Wang reports no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 2.Arnsten AFT, Castellanos FX. Neurobiology of attention regulation and its disorders. In: Martin A, Scahill L, Charney D, Leckman J, editors. Textbook of Child and Adolescent Pharmacology. New York: Oxford University Press; 2002. pp. 99–109. [Google Scholar]
- 3.Ghashghaei HT, Barbas H. Pathways for emotion: Interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- 4.Goldman-Rakic PS. The prefrontal landscape: Implications of functional architecture for understanding human mentation and the central executive. Phil Trans R Soc Lond B Biol Sci. 1996;351:1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- 5.Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- 6.Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnsten AF, Paspalas CD, Gamo NJ, Yang Y, Wang M. Dynamic network connectivity: a new form of neuroplasticity. Trends Cogn Sci. doi: 10.1016/j.tics.2010.05.003. [published online ahead of print June 14, 2010]. doi:10.1016/j.tics.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnsten AF. Fundamentals of attention-deficit/hyperactivity disorder: circuits and pathways. J Clin Psychiatry. 2006;67 suppl 8:7–12. [PubMed] [Google Scholar]
- 9.Seidman LJ, Valera EM, Makris N. Structural brain imaging of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1263–1272. doi: 10.1016/j.biopsych.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Kieling C, Goncalves RR, Tannock R, Castellanos FX. Neurobiology of attention deficit hyperactivity disorder. Child Adolesc Psychiatr Clin North Am. 2008;17:285–307. doi: 10.1016/j.chc.2007.11.012. viii. [DOI] [PubMed] [Google Scholar]
- 11.Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- 12.Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: An ALE meta-analysis. J Child Psychol Psychiatry. 2006;47:1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 13.Spencer TJ. Attention-deficit/hyperactivity disorder. Arch Neurol. 2002;59:314–316. doi: 10.1001/archneur.59.2.314. [DOI] [PubMed] [Google Scholar]
- 14.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 15.Arnsten AF. Catecholamine and second messenger influences on prefrontal cortical networks of “representational knowledge”: a rational bridge between genetics and the symptoms of mental illness. Cereb Cortex. 2007;17 suppl 1:i6–i15. doi: 10.1093/cercor/bhm033. [DOI] [PubMed] [Google Scholar]
- 16.Wang M, Ramos BP, Paspalas CD, et al. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 17.Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- 18.Li BM, Mei ZT. Delayed-response deficit induced by local injection of the alpha 2-adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav Neural Biol. 1994;62:134–139. doi: 10.1016/s0163-1047(05)80034-2. [DOI] [PubMed] [Google Scholar]
- 19.Ma CL, Arnsten AF, Li BM. Locomotor hyperactivity induced by blockade of prefrontal cortical alpha2-adrenoceptors in monkeys. Biol Psychiatry. 2005;57:192–195. doi: 10.1016/j.biopsych.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- 21.Li BM, Mei ZT. Delayed-response deficit induced by local injection of the alpha 2-adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav Neural Biol. 1994;62:134–139. doi: 10.1016/s0163-1047(05)80034-2. [DOI] [PubMed] [Google Scholar]
- 22.Greene CM, Bellgrove MA, Gill M, Robertson IH. Noradrenergic genotype predicts lapses in sustained attention. Neuropsychologia. 2009;47:591–594. doi: 10.1016/j.neuropsychologia.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Hess C, Reif A, Strobel A, et al. A functional dopamine-beta-hydroxylase gene promoter polymorphism is associated with impulsive personality styles, but not with affective disorders. J Neural Transm. 2009;116:121–130. doi: 10.1007/s00702-008-0138-0. [DOI] [PubMed] [Google Scholar]
- 24.Kieling C, Genro JP, Hutz MH, Rohde LA. The −1021 C/T DBH polymorphism is associated with neuropsychological performance among children and adolescents with ADHD. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:485–490. doi: 10.1002/ajmg.b.30636. [DOI] [PubMed] [Google Scholar]
- 25.Arnsten AF, Steere JC, Hunt RD. The contribution of alpha 2-noradrenergic mechanisms of prefrontal cortical cognitive function. Potential significance for attention-deficit hyperactivity disorder. Arch Gen Psychiatry. 1996;53:448–455. doi: 10.1001/archpsyc.1996.01830050084013. [DOI] [PubMed] [Google Scholar]
- 26.Berridge CW, Devilbiss DM, Andrzejewski ME, et al. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 27.Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- 28.Arnsten AF, Goldman-Rakic PS. Noise stress impairs prefrontal cortical cognitive function in monkeys: evidence for a hyperdopaminergic mechanism. Arch Gen Psychiatry. 1998;55:362–368. doi: 10.1001/archpsyc.55.4.362. [DOI] [PubMed] [Google Scholar]
- 29.Franowicz JS, Arnsten AF. The alpha-2a noradrenergic agonist, guanfacine, improves delayed response performance in young adult rhesus monkeys. Psychopharmacology (Berl) 1998;136:8–14. doi: 10.1007/s002130050533. [DOI] [PubMed] [Google Scholar]
- 30.Arnsten AF, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology (Berl) 1994;116:143–151. doi: 10.1007/BF02245056. [DOI] [PubMed] [Google Scholar]
- 31.Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABA(A) blockade of prefrontal cortical neurons engaged by working memory. J Neurosci. 2000;20:485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birnbaum SG, Yuan PX, Wang M, et al. Protein kinase C over activity impairs prefrontal cortical regulation of working memory. Science. 2004;306:882–884. doi: 10.1126/science.1100021. [DOI] [PubMed] [Google Scholar]
- 33.Arnsten AF, Dudley AG. Methylphenidate improves prefrontal cortical cognitive function through alpha2 adrenoceptor and dopamine D1 receptor actions: relevance to therapeutic effects in attention deficit hyperactivity disorder. Behav Brain Funct. 2005;1:2. doi: 10.1186/1744-9081-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turner DC, Robbins TW, Clark L, Aron AR, Dowson J, Sahakian BJ. Relative lack of cognitive effects of methylphenidate in elderly male volunteers. Psychopharmacology (Berl) 2003;168:455–464. doi: 10.1007/s00213-003-1457-3. [DOI] [PubMed] [Google Scholar]
- 35.Moore TL, Schettler SP, Killiany RJ, et al. Cognitive impairment in aged rhesus monkeys associated with monoamine receptors in the prefrontal cortex. Behav Brain Res. 2005;160:208–221. doi: 10.1016/j.bbr.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 36.van Dyck CH, Malison RT, Seibyl JP, et al. Age-related decline in central serotonin transporter availability with [(123)I]beta-CIT SPECT. Neurobiol Aging. 2000;21:497–501. doi: 10.1016/s0197-4580(00)00152-4. [DOI] [PubMed] [Google Scholar]
- 37.Goldman-Rakic PS, Brown RM. Regional changes of monoamines in cerebral cortex and subcortical structures of aging rhesus monkeys. Neuroscience. 1981;6:177–187. doi: 10.1016/0306-4522(81)90053-1. [DOI] [PubMed] [Google Scholar]
- 38.Bigham MH, Lidow MS. Adrenergic and serotonergic receptors in aged monkey neocortex. Neurobiol Aging. 1995;16:91–104. doi: 10.1016/0197-4580(95)80012-g. [DOI] [PubMed] [Google Scholar]
- 39.Shansky RM, Rubinow K, Brennan A, Arnsten AF. The effects of sex and hormonal status on restraint-stress-induced working memory impairment. Behav Brain Funct. 2006;2:8. doi: 10.1186/1744-9081-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shansky RM, Glavis-Bloom C, Lerman D, et al. Estrogen mediates sex differences in stress-induced prefrontal cortex dysfunction. Mol Psychiatry. 2004;9:531–538. doi: 10.1038/sj.mp.4001435. [DOI] [PubMed] [Google Scholar]
- 41.Inoue-Murayama M, Adachi S, Mishima N, et al. Variation of variable number of tandem repeat sequences in the 3′-untranslated region of primate dopamine transporter genes that affects reporter gene expression. Neurosci Lett. 2002;334:206–210. doi: 10.1016/s0304-3940(02)01125-4. [DOI] [PubMed] [Google Scholar]
- 42.Miller GM, Madras BK. Polymorphisms in the 3′-untranslated region of human and monkey dopamine transporter genes affect reporter gene expression. Mol Psychiatry. 2002;7:44–55. doi: 10.1038/sj.mp.4000921. [DOI] [PubMed] [Google Scholar]
- 43.Miller GM, Yatin SM, De La Garza R, II, Goulet M, Madras BK. Cloning of dopamine, norepinephrine and serotonin transporters from monkey brain: relevance to cocaine sensitivity. Brain Res Mol Brain Res. 2001;87:124–143. doi: 10.1016/s0169-328x(00)00288-6. [DOI] [PubMed] [Google Scholar]
- 44.Miller GM, De La Garza R, II, Novak MA, Madras BK. Single nucleotide polymorphisms distinguish multiple dopamine transporter alleles in primates: implications for association with attention deficit hyperactivity disorder and other neuropsychiatric disorders. Mol Psychiatry. 2001;6:50–58. doi: 10.1038/sj.mp.4000809. [DOI] [PubMed] [Google Scholar]
- 45.Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998;18:2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sesack SR, Hawrylak VA, Guido MA, Levey AI. Cellular and subcellular localization of the dopamine transporter in rat cortex. Adv Pharmacol. 1998;42:171–174. doi: 10.1016/s1054-3589(08)60720-6. [DOI] [PubMed] [Google Scholar]
- 47.Kuczenski R, Segal DS. Stimulant actions in rodents: implications for attention-deficit/hyperactivity disorder treatment and potential substance abuse. Biol Psychiatry. 2005;57:1391–1396. doi: 10.1016/j.biopsych.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 48.Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: Preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J Neurosci. 2002;22:7264–7271. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci. 2000;20:RC65. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mehta MA, Calloway P, Sahakian BJ. Amelioration of specific working memory deficits by methylphenidate in a case of adult attention deficit/hyperactivity disorder. J Psychopharmacol. 2000;14:299–302. doi: 10.1177/026988110001400314. [DOI] [PubMed] [Google Scholar]
- 51.Mehta MA, Goodyer IM, Sahakian BJ. Methylphenidate improves working memory and set-shifting in AD/HD: relationships to baseline memory capacity. J Child Psychol Psychiatry. 2004;45:293–305. doi: 10.1111/j.1469-7610.2004.00221.x. [DOI] [PubMed] [Google Scholar]
- 52.Dyme IZ, Sahakian BJ, Golinko BE, Rabe EF. Perseveration induced by methylphenidate in children: preliminary findings. Prog Neuropsychopharmacol Biol Psychiatry. 1982;6:269–273. doi: 10.1016/s0278-5846(82)80177-2. [DOI] [PubMed] [Google Scholar]
- 53.Crofts HS, Dalley JW, Collins P, et al. Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire an attentional set. Cereb Cortex. 2001;11:1015–1026. doi: 10.1093/cercor/11.11.1015. [DOI] [PubMed] [Google Scholar]
- 54.Tannock R, Schachar R. Methylphenidate and cognitive perseveration in hyperactive children. J Child Psychol Psychiatry. 1992;33:1217–1228. doi: 10.1111/j.1469-7610.1992.tb00940.x. [DOI] [PubMed] [Google Scholar]
- 55.Arnsten AFT, Vijayraghavan S, Wang M, Gamo NJ, Paspalas CD. Dopamine’s influence on prefrontal cortical cognition: actions and circuits in behaving primates. In: Iversen L, Iversen S, Dunnett S, Bjorklund A, editors. Dopamine Handbook. 1st ed. New York: Oxford University Press; 2009. pp. 230–248. [Google Scholar]
- 56.Chamberlain SR, Del Campo N, Dowson J, et al. Atomoxetine improved response inhibition in adults with attention deficit/hyperactivity disorder. Biol Psychiatry. 2007;62:977–984. doi: 10.1016/j.biopsych.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 57.Chamberlain SR, Muller U, Blackwell AD, Clark L, Robbins TW, Sahakian BJ. Neurochemical modulation of response inhibition and probabilistic learning in humans. Science. 2006;311:861–863. doi: 10.1126/science.1121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chamberlain SR, Hampshire A, Muller U, et al. Atomoxetine modulates right inferior frontal activation during inhibitory control: a pharmacological functional magnetic resonance imaging study. Biol Psychiatry. 2009;65:550–555. doi: 10.1016/j.biopsych.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 59.Aron AR, Dowson JH, Sahakian BJ, Robbins TW. Methylphenidate improves response inhibition in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2003;54:1465–1468. doi: 10.1016/s0006-3223(03)00609-7. [DOI] [PubMed] [Google Scholar]
- 60.Vaidya CJ, Austin G, Kirkorian G, et al. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci U S A. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim BN, Lee JS, Cho SC, Lee DS. Methylphenidate increased regional cerebral blood flow in subjects with attention deficit/hyperactivity disorder. Yonsei Med J. 2001;42:19–29. doi: 10.3349/ymj.2001.42.1.19. [DOI] [PubMed] [Google Scholar]
- 62.Bari A, Eagle DM, Mar AC, Robinson ES, Robbins TW. Dissociable effects of noradrenaline, dopamine, and serotonin uptake blockade on stop task performance in rats. Psychopharmacology (Berl) 2009;205:273–283. doi: 10.1007/s00213-009-1537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dalley JW, Mar AC, Economidou D, Robbins TW. Neurobehavioral mechanisms of impulsivity: Fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav. 2008;90:250–260. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 64.Robbins TW, Roberts AC. Differential regulation of fronto-executive function by the monoamines and acetylcholine. Cereb Cortex. 2007;17 suppl 1:i151–i160. doi: 10.1093/cercor/bhm066. [DOI] [PubMed] [Google Scholar]
- 65.Kehagia AA, Murray GK, Robbins TW. Learning and cognitive flexibility: frontostriatal function and monoaminergic modulation. Curr Opin Neurobiol. 2010;20:199–204. doi: 10.1016/j.conb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 66.Walker SC, Robbins TW, Roberts AC. Differential contributions of dopamine and serotonin to orbitofrontal cortex function in the marmoset. Cereb Cortex. 2009;19:889–898. doi: 10.1093/cercor/bhn136. [DOI] [PMC free article] [PubMed] [Google Scholar]