Abstract
A multimarker approach may be useful for risk stratification in AMI (acute myocardial infarction) patients, particularly utilizing pathways that are pathophysiologically distinct. Our aim was to assess the prognostic value of PR3 (proteinase 3) in patients post-AMI. We compared the prognostic value of PR3, an inflammatory marker, with an established marker NT-proBNP (N-terminal pro-B-type natriuretic peptide) post-AMI. We recruited 900 consecutive post-AMI patients (700 men; age, 64.6±12.4 years) in a prospective study with follow-up over 347 (0–764) days. Plasma PR3 was significantly higher in patients who died [666.2 (226.8–4035.5) ng/ml; P<0.001] or were readmitted with heart failure [598 (231.6–1803.9) ng/ml, P<0.004] compared with event-free survivors [486.9 (29.3–3118.2) ng/ml]. Using Cox modelling, log10 PR3 [HR (hazard ratio), 3.80] and log10 NT-proBNP (HR, 2.51) were significant independent predictors of death or heart failure. When patients were stratified by plasma NT-proBNP (median, 1023 pmol/l), PR3 gave additional predictive value for death or heart failure, in both the patients in whom NT-proBNP level was above the median (log rank for trend, 12.54; P<0.0004) and those with NT-proBNP level below the median (log rank for trend, 3.83; P<0.05). Neither marker predicted recurrent AMI. In conclusion, this is the first report showing a potential role for the serine protease PR3 in determining mortality and incidence of heart failure following AMI, independent of established conventional risk factors. PR3 may represent a clinically useful marker of prognosis after an AMI as part of a multimarker strategy.
Keywords: myeloperoxidase, myocardial infarction, neutrophil, peptide, prognosis, proteinase 3
Abbreviations: AMI, acute myocardial infarction; AUC, area under the curve; BNP, B-type natriuretic peptide; CI, confidence interval; HR, hazard ratio; IL, interleukin; LVEF, left ventricular ejection fraction; LVWMI, left ventricular wall motion index; MAE, methyl-acridinium ester; MPO, myeloperoxidase; NT-proBNP, N-terminal pro-BNP; PR3, proteinase 3; ROC, receiver-operating characteristic; TNFα, tumour necrosis factor α
INTRODUCTION
Atherosclerosis is associated with inflammatory changes in the blood vessel wall [1], including macrophage and lymphocyte infiltration. There is an association between neutrophil count and the risk of future acute coronary syndromes [2], and recent evidence has documented elevated neutrophil activity in acute coronary syndromes [3,4]. Despite their role in plaque destabilization and acute coronary syndromes, it is not known whether neutrophil activation, with degranulation of their proteolytic enzymes might play a role in development of chronic heart failure and the risk of mortality following an AMI (acute myocardial infarction).
MPO (myeloperoxidase), an enzyme present within the azurophilic granules of neutrophils, may stimulate plaque destabilization by activation of metalloproteinases [5]. Plasma levels of MPO are raised in coronary artery disease [6] and predict outcome of patients presenting with chest pain [7] and acute coronary syndromes [8,9]. PR3 (proteinase 3) is another major component of neutrophil azurophilic granules, together with other serine proteases such as elastase and cathepsin G [10]. Azurophilic granules are released in response to powerful stimuli to neutrophils, whereas secretory granules (which also contain PR3 [11]) are released with weaker stimuli. PR3 may also be expressed on endothelial cells [12]. Neutrophil serine proteases may degrade extracellular matrix; in addition, PR3 has other deleterious effects which may be relevant to a role in pathogenesis of vasculitis in Wegener's granulomatosis and potentially in prognosis of patients post-AMI. These include induction of apoptosis through a caspase-like activity on endothelial cells [13], releasing activated TNFα (tumour necrosis factor α) from its nascent membrane-bound precursor form [14,15], activation of the pro-inflammatory mediators IL (interleukin)-1β [15] and IL-18 [16] and generation of angiotensin I and II from angiotensinogen [17]. Although PR3 and autoantibodies to it [ANCA (antineutrophil cytoplasmic antibodies)] are prime candidates for pathogenesis of vasculitis and tissue damage in the relatively uncommon autoimmune condition of Wegener's granulomatosis, the association of PR3 with morbidity and mortality in common medical vascular presentations such as AMI has never been examined.
PR3 is rapidly inactivated by irreversible binding to SERPIN A1 (α1-antitrypsin) [18,19], where the serine protease reacts with the reactive centre loop of the serpin, generating a covalently bound intermediate resulting in the distortion and inactivation of the protease [20]. Thus little free PR3 exists in plasma, and it is mainly present as a PR3–SERPIN A1 complex [18].
We hypothesized that measurement of such PR3–SERPIN A1 complexes would provide prognostic information on heart failure and mortality post-AMI, even when used in conjunction with recognized markers of prognosis such as BNP (B-type natriuretic peptide) or its precursor NT-proBNP (N-terminal pro-BNP) in order to establish a role for neutrophil activation in determining these adverse outcomes. Since PR3 is easily mobilized from secretory granules compared with azurophilic granules, it may also be a better marker of neutrophil activation than MPO.
MATERIALS AND METHODS
Study population
We recruited 900 consecutive patients with acute myocardial infarction admitted to the Coronary Care Unit of Leicester Royal Infirmary between 1 September 2003 and 31 July 2007. Written informed consent was obtained from patients, and the study complied with the Declaration of Helsinki and was approved by the local ethics committee. AMI was diagnosed if a patient had a plasma creatine kinase-MB elevation greater than twice normal or cardiac troponin I level >0.1 ng/ml with at least one of the following, chest pain lasting >20 min or diagnostic serial ECG changes consisting of new pathological Q waves or ST segment and T wave changes. Exclusion criteria included malignancy or recent surgery (within 1 month). Hypertension was defined as a blood pressure measurement of greater than 140/90 mmHg on two or more occasions or if the patient was on medication for hypertension. Hypercholesterolaemia was defined as a total cholesterol of greater than 5.0 mmol/l, or treatment with a statin. Diabetes was defined as a random plasma glucose of 11.1 mmol/l, fasting plasma glucose of 7 mmol/l or a history of diabetes on treatment.
Plasma samples
Blood samples were drawn at 2–5 days after the onset of chest pain for determination of plasma PR3–SERPIN A1 and NT-proBNP. After 15 min bed rest, 20 ml of blood was collected into tubes containing EDTA and aprotinin. All plasma was stored at −80 °C until assayed, blind to clinical details, in a single batch.
Echocardiography
Transthoracic echocardiography was performed in patients using a Sonos 5500 instrument (Philips Medical Systems), at 3–5 days post-admission. A 16-segment LVWMI (left ventricular wall motion index) based on the American Society of Echocardiography mode was derived by scoring each LV segment [1=normal, 2=hypokinesis, 3=akinesis and 4=dyskinesis (paradoxical motion)] and dividing the total by the number of segments scored. LVEF (left ventricular ejection fraction) was calculated using the biplane method of discs formula [21]. Impaired LV systolic function was defined as an LVEF <40% or a LVWMI >1.8.
NT-proBNP assay
Plasma NT-proBNP assay was measured using a non-competitive immunoluminometric assay as previously published [22]. Samples or NT-proBNP standards (10 μl) were incubated in ELISA plate wells coated with mouse monoclonal IgG directed to the C-terminal of NT-proBNP. Detection was with biotinylated rabbit N-terminal antibody followed by MAE (methyl-acridinium ester)-labelled streptavidin on an MLX plate luminometer (Dynex Technologies). The lower limit of detection was 0.3 pmol/l. This highly specific assay shows no cross-reactivity with ANP (atrial natriuretic peptide), BNP or CNP (C-type natriuretic peptide).
PR3–SERPIN A1 assay
Plasma PR3–SERPIN A1 was measured using a non-competitive immunoluminometric assay modified from a method published previously [18]. ELISA plate wells were coated with 200 ng of mouse monoclonal antibody to PR3 (clone 1B10, IgG2a; HyTest). Standards were produced by incubating PR3 with a 20-fold molar excess of SERPIN A1 (both from Sigma–Aldrich). Samples or standards (10 μl) were incubated with assay buffer (100 μl) in the coated wells for 24 h at 4 °C. Following washes, a specific SERPIN A1 rabbit antibody (Dako) was pipetted into the wells (20 ng/100 μl) and incubated for 3 h with shaking at 250 Hz at room temperature (22 °C). Following washing, a goat biotinylated anti-rabbit IgG (Rockland Immunochemicals), previously preadsorbed with human, rabbit and mouse serum proteins, at a dilution of 1:100000 was incubated within the wells for 1 h, followed by MAE-labelled streptavidin for another 1.5 h. Chemiluminescence was elicited with sequential injections of H2O2 in nitric acid, followed by NaOH containing cetyl trimethylammonium bromide, as described previously [22]. Intra- and inter-assay coefficients of variation were found to be less than 10%.
MPO assay
The MPO assay was based on a two-site non-competitive immunoluminometric assay which employed an ELISA plate immobilized monoclonal MPO antibody (100 ng/100 μl; Research Diagnostics) and a rabbit polyclonal antibody (50 ng/100 μl; Merck BioSciences), as described previously [23]. Standards and 10 μl of plasma were incubated in plates overnight, before washing and detection using goat biotinylated anti-rabbit IgG and streptavidin–MAE, as described for the PR3–SERPIN A1 assay. The MPO assays had inter- and intra-assay coefficients of variation <10%, with a lower limit of detection of 0.3 ng/ml.
End points
The primary end point was the combination of all-cause mortality and hospitalization for heart failure. Secondary end points included the occurrence of further myocardial infarction. Hospitalization for heart failure was defined as a hospital admission for which heart failure was the primary reason, requiring treatment with high-dose diuretics, inotropes or intravenous nitrate. Deaths were validated by reviewing the hospital record management systems and the Office of National Statistics Registry. Heart failure episodes were identified through the hospital record management systems and validated by contacting each patient. There was a minimum 60-day follow-up of all surviving patients.
Statistical analysis
Values are medians (range), unless otherwise stated. Statistical analyses were performed on SPSS Version 14.0. Continuous variables were compared using the Mann–Whitney U test. ANOVA with the Bonferroni correction for multiple comparisons was employed when more than two groups were compared. Correlation analysis employed Spearman's rho, and binary logistic regression and Cox proportional hazard analyses were used to develop models in order to test the independent predictive power for factors and variables. Plasma levels of NT-proBNP, MPO and PR3–SERPIN A1 were normalized by log-transformation. Thus ORs (odds ratios) and HRs (hazard ratios) refer to a 10-fold rise in the levels of these markers. Kaplan–Meier survival analysis was used to assess dichotomized values of NT-proBNP and PR3–SERPIN A1 and the log rank test was used for comparison of groups. ROC (receiver-operating characteristic) curves were generated and the AUC (area under the curve) was calculated to assess the accuracy of predictors [24]. A P value of less than 0.05 was deemed to be statistically significant.
RESULTS
Patient characteristics
The demographic features of the patient population are shown in Table 1. Median length of follow-up was 347 days with a range of 0–764 days. No patient was lost to follow-up. The index AMI was classed as STEMI (ST elevation MI) in 777 of the patients, 529 (68.1%) of whom received thrombolytic therapy. Echocardiographic data were available for 622 (69.1%) of the patients during the index admission. During follow-up, 92 (10.2%) of the 900 patients died, 66 (7.3%) experienced readmission with heart failure, 143 (15.9%) patients experienced either end point and 62 (6.9%) experienced hospitalization with recurrent AMI.
Table 1. Characteristics of patients in the study.
Values are means (S.D.) or numbers (%). ACE, angiotensin-converting enzyme; CK, creatine kinase.
| Charactertisitc | AMI patients |
|---|---|
| Number (n) (male) | 900 (700) |
| Age (years) | 64.6±12.4 |
| Previous medical history (n) | |
| Myocardial infarction | 165 (18.3%) |
| Angina pectoris | 195 (21.7%) |
| Hypertension | 391 (43.4%) |
| Diabetes mellitus | 192 (21.3%) |
| Hypercholesterolaemia | 221 (24.6%) |
| Current/ex-smokers | 560 (62.2%) |
| ST elevation AMI | 777 (86.3%) |
| Thrombolytic | 529 (58.8%) |
| Territory of infarct (n) | |
| Anterior | 369 (41.0%) |
| Inferior | 373 (41.4%) |
| Other/undetermined | 158 (17.6%) |
| Killip class on admission (n) | |
| I | 457 (50.7%) |
| II | 357 (39.7%) |
| III | 77 (8.6%) |
| IV | 9 (1.0%) |
| Peak CK (units/l) | 1157±1263 |
| Creatinine (μmol/l) | 103.9±35.4 |
| Medication (n) | |
| Aspirin | 794 (88.2%) |
| ACE inhibitors/angiotensin receptor blockers | 683 (75.9%) |
| β-Blockers | 721 (80.1%) |
| Statins | 701 (77.9%) |
Plasma PR3–SERPIN A1, NT-proBNP and MPO levels
Plasma levels of PR3–SERPIN A1 in patients with AMI ranged from 29.3 to 4035.5 ng/ml, with a median of 504.6 ng/ml, and were elevated compared with the established normal range [350 (110–580) ng/ml]. PR3–SERPIN A1 was significantly higher in patients who died [666.2 (226.8–4035.5) ng/ml; P<0.001 using Bonferroni's correction] or were readmitted with heart failure [598 (231.6–1803.9) ng/ml; P<0.004] compared with event-free survivors [486.9 (29.3–3118.2) ng/ml]. In contrast, levels of PR3–SERPIN A1 in those readmitted with recurrent AMI [482.5 (168.9–1753.9) ng/ml] were similar to those in event-free survivors. PR3–SERPIN A1 levels were similar in males and females, and in those with or without previous histories of hypertension, angina, AMI or heart failure, but were slightly elevated in those with diabetes [547.0 (76.9–4035.5) ng/ml] compared with non-diabetic patients [495.1 (29.3–3118.2) ng/ml; P<0.018].
Plasma PR3–SERPIN A1 levels were modestly correlated with plasma glucose (rs=0.08, P<0.03), log creatinine (rs=0.13, P<0.001), Killip class (rs=0.10, P<0.002), NT-proBNP (rs=0.25, P<0.001) and creatine kinase (rs=0.13, P<0.001) but had no relationship to age, site of infarction, presence or absence of ST elevation and whether thrombolysis was administered or not.
In confirmation of previous work, plasma NT-proBNP was higher in patients who died [5955.4 (9.02–14057.2) pmol/l] or were readmitted with heart failure [3106.6 (2.4–12282.9) pmol/l] compared with event-free survivors [806.8 (0.3–28886) pmol/l; P<0.001 for both]. NT-proBNP levels were higher in females [1951.7 (22.9–15732) pmol/l] compared with males [871.6 (0.3–28886) pmol/ml; P<0.001) and correlated with age (rs=0.46, P<0.001), plasma glucose (rs=0.15, P<0.001), log creatinine (rs=0.27, P<0.001), Killip class (rs=0.29, P<0.001) and creatine kinase (rs=0.13, P<0.001).
Plasma MPO was significantly higher in patients who died [36.7 (6.44–132.1) ng/ml; P<0.035 using Bonferroni's correction] compared with event-free survivors [24.6 (0.3–405.2) ng/ml]. In contrast those who were readmitted with heart failure [27.6 (6.5–210.8) ng/ml] or with recurrent AMI [23.9 (5.3–189.9) ng/ml] had similar values of MPO to event-free survivors. MPO levels were higher in females [30.3 (0.3–405.2) ng/ml] compared with males [23.9 (3.6–215.1) ng/ml; P<0.001] and in patients with diabetes [30.4 (5.1–238.7) ng/ml] compared with non-diabetic patients [24.0 (0.3–405.2) ng/ml; P<0.001] and were correlated with age (rs=0.12, P<0.001), plasma glucose (rs=0.10 P<0.009), log creatinine (rs=0.11, P<0.001) and Killip class (rs=0.11, P<0.002).
There was a modest correlation of plasma PR3–SERPIN A1 with impaired LV systolic function (rs=0.16, P=0.001) on the index admission (as defined by LVEF<40% or LVWMI>1.8 on echocardiography), with a stronger relationship demonstrated for NT-proBNP (rs=0.35, P=0.001). Plasma MPO was weakly correlated to PR3–SERPIN A1 and NT-proBNP (rs=0.11 and 0.12, respectively, P=0.002) but not to LV systolic function.
Primary end points: PR3–SERPIN A1 and NT-proBNP as predictors of death or heart failure
Binary logistic models were developed for prediction of death or heart failure, using the following clinical and demographic variables: age, gender, past history of AMI, hypertension or diabetes, Killip class >1, thrombolytic use, log creatinine, NT-proBNP, MPO and PR3–SERPIN A1. Independent predictors are reported in Table 2 and include NT-proBNP and PR3–SERPIN A1 as significant predictor variables. The Nagelkerke r2 was 0.35 suggesting a good fit of the model.
Table 2. Binary logistic regression model for the prediction of death and heart failure.
| Variable | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Age | 1.04 | 1.02–1.07 | 0.001 |
| Gender | 0.57 | 0.35–0.93 | 0.024 |
| Past history AMI | 2.28 | 1.43–3.74 | 0.001 |
| Killip class >1 | 1.66 | 1.05–2.63 | 0.032 |
| Log creatinine | 18.55 | 3.02–113.9 | 0.001 |
| Log NT-proBNP | 2.79 | 1.79–4.37 | 0.001 |
| Log PR3–SERPIN A1 | 6.42 | 2.25–18.3 | 0.001 |
The ROC curve for the predictive value of PR3–SERPIN A1 for the primary end point yielded an AUC of 0.65 [95% CI (confidence interval), 0.60–0.70; P<0.001); for NT-proBNP, the AUC was 0.78 (95% CI, 0.74–0.83; P<0.001). The logistic model combining the two markers yielded an AUC of 0.84 (95% CI, 0.80–0.88; P<0.001), which exceeded that of either peptide alone (P<0.003 by method of Hanley and McNeil [24]).
Cox proportional hazards modelling confirmed these findings, identifying the same independent predictors of death or heart failure apart from gender (Table 3). In both logistic and Cox models, plasma MPO was not an independent predictor of death or heart failure.
Table 3. Multivariate Cox proportional hazards regression model of the significant predictors of death or heart failure.
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| Age | 1.04 | 1.03–1.06 | 0.001 |
| Past history AMI | 1.52 | 1.06–2.17 | 0.023 |
| Killip class >1 | 1.60 | 1.08–2.37 | 0.02 |
| Log creatinine | 4.75 | 1.39–16.19 | 0.013 |
| Log NT-proBNP | 2.51 | 1.70–3.71 | 0.001 |
| Log PR3–SERPIN A1 | 3.80 | 1.78–8.14 | 0.001 |
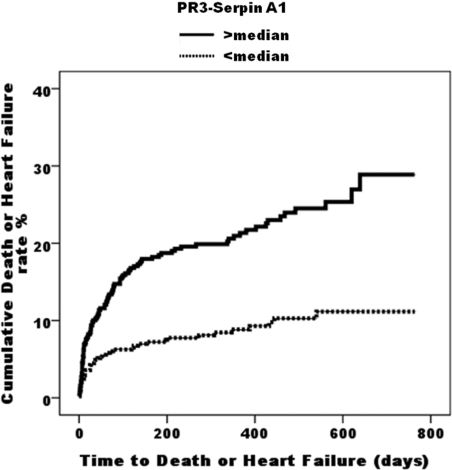
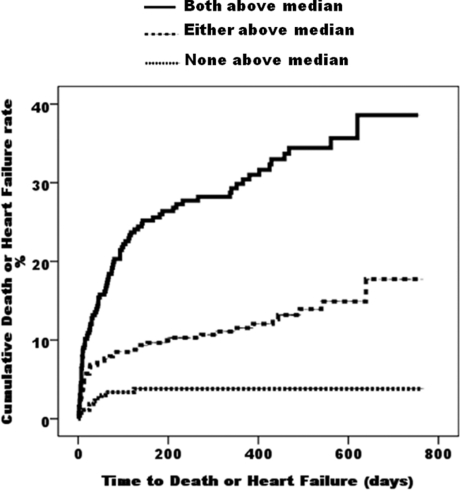
Kaplan–Meier survival analysis demonstrated a significantly better clinical outcome in patients with PR3–SERPIN A1 below the median (504.6 ng/ml) compared with those with PR3–SERPIN A1 above the median (log rank, 28.15; P<0.0001) (Figure 1). The event-free survival curves diverge early and continue to diverge even after 2 years. This was also true for NT-proBNP (log rank, 64.72; P<0.0001). When patients were stratified by plasma NT-proBNP (median 1023 pmol/l), PR3–SERPIN A1 gave additional predictive value for death or heart failure, in both the patients in whom NT-proBNP level was above the median (log rank for trend, 12.54; P<0.0004) and those with NT-proBNP level below the median (log rank for trend, 3.83; P<0.05). Those patients with both markers elevated above their respective medians had higher event rates than those with either marker elevated above the median, and these in turn had higher event rates than those with both markers below their respective medians (P<0.001 for all comparisons) (Figure 2).
Figure 1. Cumulative incidence plots showing time to adverse events (death or heart failure) in patients stratified by PR3–SERPIN A1.
Figure 2. Cumulative incidence plots showing time to adverse events (death or heart failure) in patients stratified according to whether both PR3–SERPIN A1 and NT-proBNP are below their respective median values, either marker being elevated or both markers elevated above median.
Troponin levels were available for 414 patients. Inclusion of troponin in the Cox model for death or heart failure led to the following HRs (and P values): age (1.07, P<0.001), Killip class >1 (1.83, P<0.04), log creatinine (6.05, P<0.06), past history of AMI (1.70, P<0.07), NT-proBNP (1.74, P<0.08), troponin (1.18, P=0.3), PR3–SERPIN A1 (2.83, P<0.06).
Echocardiography scans were analysable on 622 subjects, of which 203 showed evidence of LV systolic dysfunction (as defined by LVEF <40% or LVWMI >1.8). Inclusion of echocardiography evidence of systolic dysfunction into the Cox model for death or heart failure led to the following HRs (and P values): age (1.04, P<0.001), Killip class >1 (2.17, P<0.005), log creatinine (6.55, P<0.01), past history of AMI (1.40, P=0.13), NT-proBNP (1.92, P<0.007), LV systolic dysfunction (1.39, P=0.13) and PR3–SERPIN A1 (2.88, P<0.02).
Reclassification analyses for predictors of death or heart failure
The GRACE score [25] is a recognized risk predictor in AMI, enabling patients to be classified into low, intermediate and high risk groups for major adverse cardiac events after an acute coronary syndrome, and incorporates many of the above predictors for death or heart failure, e.g. age, history of AMI, elevated plasma creatinine, elevated biomarker levels, Killip class. In this present study, GRACE score was a significant predictor of death or heart failure [HR, 1.03 (95% CI, 1.02–1.04; P<0.0005]. In a model for predicting death or heart failure using GRACE score, NT-proBNP and PR3–SERPIN A1, all three remained significant predictors [HR (95% CI) were 1.02 (1.01–1.03); P<0.0005 for the GRACE score; 2.67 (1.79–3.99); P<0.0005 for NT-proBNP; and 4.08 (1.89–8.79); P<0.0005 for PR3–SERPIN A1].
Reclassification analyses were therefore performed using GRACE score, with the addition of NT-proBNP and PR3–SERPIN A1 as described by Pencina et al. [26]. NT-proBNP led to significant up-classification of patients with end points and down-classification of event-free survivors (Table 4), when added to the GRACE score (P<0.0002). PR3–SERPIN A1 in addition to the GRACE score also led to significant (P<0.02) up-classification of patients with end points, although when added to GRACE score with NT-proBNP, this up-classification was not significant (P=0.14).
Table 4. Reclassification analysis for death or heart failure using the GRACE score, with the addition of NTproBNP and/or PR3–SERPIN A1.
| (a) GRACE score compared with GRACE score with NT-proBNP | ||||
|---|---|---|---|---|
| Reclassification | ||||
| Parameter | Increased | Decreased | Correctly reclassified | P value |
| Patients without end points | 8.9% | 16.3% | 7.4% | 0.0002 |
| Patients with end points | 15.3% | 7.0% | 8.3% | |
| (b) GRACE score compared with GRACE score with PR3–SERPIN A1 | ||||
| Reclassification | ||||
| Parameter | Increased | Decreased | Correctly reclassified | P value |
| Patients without end points | 10.3% | 11.4% | 1.1% | 0.02 |
| Patients with end points | 9.1% | 2.3% | 6.8% | |
| (c) GRACE score with NT-proBNP compared with GRACE score with NT-proBNP and PR3–SERPIN A1 | ||||
| Reclassification | ||||
| Parameter | Increased | Decreased | Correctly reclassified | P value |
| Patients without end points | 8.6% | 7.9% | −0.7% | 0.14 |
| Patients with end points | 7.6% | 2.3% | 5.3% | |
Secondary end points: PR3–SERPIN A1 and NT-proBNP as predictors of myocardial infarction
Compared with survivors with no end points, patients who were readmitted with further AMI during follow-up had similar NT-proBNP [1480.7 (2.6–10645.9) compared with 806.8 (0.3–28886) pmol/l), PR3–SERPIN A1 levels [482.5 (168.9–1753.9) compared with 486.9 (29.3–3118.2) ng/ml) and MPO levels [23.9 (5.3–189.9) compared with 24.6 (0.3–405.2) ng/ml].
DISCUSSION
This study reports, for the first time, the prognostic potential of PR3–SERPIN A1 in a large cohort of patients with AMI, recruited prospectively from a single centre. This complex of a serine protease and its inhibitor provided prediction of death or heart failure, independently of established conventional risk factors such as renal function and clinical demographic information and the GRACE score. Moreover, this novel prognostic marker complements the information provided by an established marker of poor prognosis post-AMI, namely NT-proBNP. The use of PR3–SERPIN A1 for independent prediction of death or heart failure, together with troponin levels or echocardiographic evidence of systolic dysfunction, failed to achieve conventional levels of statistical significance due to the reduced number of subjects with data available for analysis. Reclassification analyses with the GRACE score suggested that PR3–SERPIN A1 had utility, mainly in up-classifying patients with end points, in contrast with NT-proBNP, which up-classified those with end points and down-classified those without end points. However, adding PR3–SERPIN A1 to models with GRACE score and NT-proBNP led to insignificant up-classification of patients with end points. The study may have been underpowered to detect significant reclassification in this analysis.
The measurement of PR3 levels in the post AMI setting is supported by strong evidence of its damaging role in cells, such as its proapoptotic activity [13] and its proinflammatory effect, by releasing the active forms of both TNFα, IL-1β, IL-18 [14–16] and the angiotensins [17]. These cytokines may be especially relevant to development of heart failure post-AMI, which still occurs despite therapy with inhibitors of the renin–angiotensin system. The involvement of such serine proteases in the development of heart failure and their association with increased risk of adverse outcome post-AMI may support the search for new agents that could attenuate the damaging effects of these enzymes. For example, there is preliminary evidence that elafin, a serine protease inhibitor could reduce the vascular and myocardial damage in experimental models of cardiac transplantation [27].
The source of circulating PR3 may be partly from degranulating, activated neutrophils, being present in both azurophilic and secretory granules. Neutrophils are known to infiltrate myocardium after infarction, although their direct contribution to the development of heart failure to mortality after AMI has not been clarified. Alternatively, or additionally, PR3 may be secreted from endothelial cells [12] or from lung parenchymal cells [28]. Pneumocytes in normal lung have been demonstrated to express PR3 [28], although expression is greatly up-regulated in Wegener's granulomatosis. Active PR3 is rapidly inactivated in the plasma by SERPIN A1 and alpha2-macroglobulin [10], the PR3-SERPIN A1 complex can therefore only be formed with an active protease and may therefore be a good measure of the amount of active enzyme that has been secreted. However, these plasma-borne inhibitors may not have rapid access to extravascular sites where the enzyme could be released post-AMI. The levels of the PR3–SERPIN A1 complex that we have demonstrated in patients who have a poor outcome post-AMI are very similar to those described in patients with active vasculitis in Wegener's granulomatosis [560 (110–3940) ng/ml] [29]. In normal subjects and those with inactive vasculitis, levels of approx. 350 (110–580) ng/ml have been reported [29], which are lower than our values for our event-free post-AMI survivors.
In this cohort of AMI patients, MPO did not provide independent prediction of death or heart failure, when models included NT-proBNP and PR3-SERPIN A1. MPO is localized within neutrophil azurophilic granules alone, which are released with strong stimuli [10], in contrast to the presence of an easily mobilizable pool of PR3 in secretory granules [11]. In addition, previous studies with MPO have included patients with acute coronary syndromes or chest pain [7,8], which may be a different spectrum from those with more myocardial damage. Indeed, Baldus et al. [8] reported prognostic potential of MPO in those with low or medium troponin T elevations, with poorer predictive potential in those with high troponin T levels.
Survival analysis revealed that those at highest risk were those with NT-proBNP elevated above the median of 1023 pmol/l and PR3–SERPIN A1 above the median of 504.6 ng/ml. It is uncertain whether intensification of therapy or provision of timely intervention procedures could have altered clinical outcome, but this remains to be investigated.
Limitations of the study
This was a single centre study, and the results need to be replicated in larger multicentre studies. There was a preponderance of ST elevation AMI, and the utility of PR3–SERPIN A1 in patients with other acute coronary syndromes should be investigated (e.g. in those with non-ST elevation AMI or chest pain). Larger studies may need to be performed to confirm the utility of PR3–SERPIN A1 in reclassification analyses.
Conclusion
This is the first report showing a potential role for the serine protease PR3 in determining mortality and incidence of heart failure following AMI, independent of established conventional risk factors and also the newly introduced biomarker NT-proBNP. The involvement of PR3 in pathophysiology of Wegener's granulomatosis may extend to more common presentations of vascular disease. A multimarker approach with clinical risk scores and either or both of PR3–SERPIN A1 and NT-proBNP may be useful for risk stratification in AMI patients, both markers providing complementary independent prediction, and the identification of such high risk patients may facilitate their subsequent management.
AUTHOR CONTRIBUTION
Leong Ng designed the study and constructed the assays; Sohail Khan recruited the patients; Sohail Khan and Joan Davies performed the echocardiography analysis; Leong Ng and Paulene Quinn performed the assays; Leong Ng, Sohail Khan, Iain Squire and Hafid Narayan analysed the data. The manuscript was prepared by all of the authors. All of the authors had full access to the data and take responsibility for its integrity and the accuracy of the analysis. All authors have read and agree to the manuscript as written.
FUNDING
This study is part of the research portfolio supported by the Leicester NIHR Biomedical Research Unit in Cardiovascular Disease and by the Van Geest Foundation. S.Q.K. was supported by a British Heart Foundation (BHF) Junior Research Fellowship [grant number FS/03/028/15486].
References
- 1.Libby P., Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111:3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 2.Friedman G. D., Klatsky A. L., Siegelaub A. B. The leukocyte count as a predictor of myocardial infarction. N. Engl. J. Med. 1974;290:1275–1278. doi: 10.1056/NEJM197406062902302. [DOI] [PubMed] [Google Scholar]
- 3.Naruko T., Ueda M., Haze K., van der Wal A. C., van der Loos C. M., Itoh A., Komatsu R., Ikura Y., Ogami M., Shimada Y., et al. Neutrophil infiltration of culprit lesions in acute coronary syndromes. Circulation. 2002;106:2894–2900. doi: 10.1161/01.cir.0000042674.89762.20. [DOI] [PubMed] [Google Scholar]
- 4.Buffon A., Biasucci L. M., Liuzzo G., D'Onofrio G., Crea F., Maseri A. Widespread coronary inflammation in unstable angina. N. Engl. J. Med. 2002;347:5–12. doi: 10.1056/NEJMoa012295. [DOI] [PubMed] [Google Scholar]
- 5.Fu X., Kassim S. Y., Parks W. C., Heinecke J. W. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J. Biol. Chem. 2001;276:41279–41287. doi: 10.1074/jbc.M106958200. [DOI] [PubMed] [Google Scholar]
- 6.Zhang R., Brennan M. L., Fu X., Aviles R. J., Pearce G. L., Penn M. S., Topol E. J., Sprecher D. L., Hazen S. L. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA, J. Am. Med. Assoc. 2001;286:2136–2142. doi: 10.1001/jama.286.17.2136. [DOI] [PubMed] [Google Scholar]
- 7.Brennan M. L., Penn M. S., Van Lente F., Nambi V., Shishehbor M. H., Aviles R. J., Goormastic M., Pepoy M. L., McErlean E. S., Topol E. J., et al. Prognostic value of myeloperoxidase in patients with chest pain. N. Engl. J. Med. 2003;349:1595–1604. doi: 10.1056/NEJMoa035003. [DOI] [PubMed] [Google Scholar]
- 8.Baldus S., Heeschen C., Meinertz T., Zeiher A. M., Eiserich J. P., Munzel T., Simoons M. L., Hamm C. W. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108:1440–1445. doi: 10.1161/01.CIR.0000090690.67322.51. [DOI] [PubMed] [Google Scholar]
- 9.Khan S. Q., Kelly D., Quinn P., Davies J. E., Ng L. L. Myeloperoxidase aids prognostication together with N-terminal pro-B-type natriuretic peptide in high-risk patients with acute ST elevation myocardial infarction. Heart. 2007;93:826–831. doi: 10.1136/hrt.2006.091041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pham C. T. Neutrophil serine proteases: specific regulators of inflammation. Nat. Rev. Immunol. 2006;6:541–550. doi: 10.1038/nri1841. [DOI] [PubMed] [Google Scholar]
- 11.Witko-Sarsat V., Cramer E. M., Hieblot C., Guichard J., Nusbaum P., Lopez S., Lesavre P., HalbwachsMecarelli L. Presence of proteinase 3 in secretory vesicles: evidence of a novel, highly mobilizable intracellular pool distinct from azurophil granules. Blood. 1999;94:2487–2496. [PubMed] [Google Scholar]
- 12.Mayet W. J., Csernok E., Szymkowiak C., Gross W. L., Meyer zum Buschenfelde K. H. Human endothelial cells express proteinase 3, the target antigen of anticytoplasmic antibodies in Wegener's granulomatosis. Blood. 1993;82:1221–1229. [PubMed] [Google Scholar]
- 13.Pendergraft W. F., Rudolph E. H., Falk R. J., Jahn J. E., Grimmler M., Hengst L., Jennette J. C., Preston G. A. Proteinase 3 sidesteps caspases and cleaves p21Waf1/Cip1/Sdi1 to induce endothelial cell apoptosis. Kidney. Int. 2004;65:75–84. doi: 10.1111/j.1523-1755.2004.00364.x. [DOI] [PubMed] [Google Scholar]
- 14.Robache-Gallea S., Morand V., Bruneau J. M., Schoot B., Tagat E., Realo E., Chouaib S., Roman-Roman S. In vitro processing of human tumor necrosis factor-α. J. Biol. Chem. 1995;270:23688–23692. doi: 10.1074/jbc.270.40.23688. [DOI] [PubMed] [Google Scholar]
- 15.Coeshott C., Ohnemus C., Pilyavskaya A., Ross S., Wieczorek M., Kroona H., Leimer A. H., Cheronis J. Converting enzyme-independent release of tumor necrosis factor alpha and IL-1β from a stimulated human monocytic cell line in the presence of activated neutrophils or purified proteinase 3. Proc. Natl. Acad. Sci. U.S.A. 1999;96:6261–6266. doi: 10.1073/pnas.96.11.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugawara S., Uehara A., Nochi T., Yamaguchi T., Ueda H., Sugiyama A., Hanzawa K., Kumagai K., Okamura H., Takada H. Neutrophil proteinase 3-mediated induction of bioactive IL-18 secretion by human oral epithelial cells. J. Immunol. 2001;167:6568–6575. doi: 10.4049/jimmunol.167.11.6568. [DOI] [PubMed] [Google Scholar]
- 17.Ramaha A., Patston P. A. Release and degradation of angiotensin I and angiotensin II from angiotensinogen by neutrophil serine proteinases. Arch. Biochem. Biophys. 2002;397:77–83. doi: 10.1006/abbi.2001.2687. [DOI] [PubMed] [Google Scholar]
- 18.Baslund B., Petersen J., Permin H., Wiik A., Wieslander J. Measurements of proteinase 3 and its complexes with alpha 1-proteinase inhibitor and anti-neutrophil cytoplasm antibodies (ANCA) in plasma. J. Immunol. Methods. 1994;175:215–225. doi: 10.1016/0022-1759(94)90364-6. [DOI] [PubMed] [Google Scholar]
- 19.Duranton J., Bieth J. G. Inhibition of proteinase 3 by α1-antitrypsin in vitro predicts very fast inhibition in vivo. Am. J. Respir. Cell. Mol. Biol. 2003;29:57–61. doi: 10.1165/rcmb.2002-0258OC. [DOI] [PubMed] [Google Scholar]
- 20.Stratikos E., Gettins P. G. Major proteinase movement upon stable serpin–proteinase complex formation. Proc. Natl. Acad. Sci. U.S.A. 1997;94:453–458. doi: 10.1073/pnas.94.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schiller N. B., Shah P. M., Crawford M., DeMaria A., Devereux R., Feigenbaum H., Gutgesell H., Reichek N., Sahn D., Schnittger I., et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. J. Am. Soc. Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 22.Omland T., Persson A., Ng L., O'Brien R., Karlsson T., Herlitz J., Hartford M., Caidahl K. N-terminal pro-B-type natriuretic peptide and long-term mortality in acute coronary syndromes. Circulation. 2002;106:2913–2918. doi: 10.1161/01.cir.0000041661.63285.ae. [DOI] [PubMed] [Google Scholar]
- 23.Ng L. L., Pathik B., Loke I. W., Squire I. B., Davies J. E. Myeloperoxidase and C-reactive protein augment the specificity of B-type natriuretic peptide in community screening for systolic heart failure. Am. Heart. J. 2006;152:94–101. doi: 10.1016/j.ahj.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 24.Hanley J. A., McNeil B. J. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 25.Eagle K. A., Lim M. J., Dabbous O. H., Pieper K. S., Goldberg R. J., Van de Werf F., Goodman S. G., Granger C. B., Steg P. G., Gore J. M., et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA, J. Am. Med. Assoc. 2004;291:2727–2733. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 26.Pencina M. J., D'Agostino R. B., Sr, D'Agostino R. B., Jr, Vasan R. S. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat. Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 27.Cowan B., Baron O., Crack J., Coulber C., Wilson G. J., Rabinovitch M. Elafin a serine elastase inhibitor, attenuates post-cardiac transplant coronary arteriopathy and reduces myocardial necrosis in rabbits afer heterotopic cardiac transplantation. J. Clin. Invest. 1996;97:2452–2468. doi: 10.1172/JCI118692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brockmann H., Schwarting A., Kriegsmann J., Petrow P., Gaumann A., Muller K. M., Galle P. R., Mayet W. Proteinase-3 as the major autoantigen of c-ANCA is strongly expressed in lung tissue of patients with Wegener's granulomatosis. Arthritis Res. 2002;4:220–225. doi: 10.1186/ar410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohlsson S., Wieslander J., Segelmark M. Increased circulating levels of proteinase 3 in patients with anti-neutrophilic cytoplasmic autoantibodies-associated systemic vasculitis in remission. Clin. Exp. Immunol. 2003;131:528–535. doi: 10.1046/j.1365-2249.2003.02083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]