Abstract
We present a detailed comparative ontogenetic analysis of pseudanthia of representatives of all three subtribes of Euphorbieae (Euphorbiinae, Neoguillauminiinae, Anthosteminae) in order to clarify their homologies and interpretation. The cyathium of Euphorbia and its allies (subtribe Euphorbiinae) closely resembles a bisexual flower but is traditionally interpreted as an inflorescence bearing clusters of highly reduced male flowers surrounding a single terminal female flower. Previously unreported characters are (1) male flowers formed one above the other in the male inflorescences of some Euphorbiinae, (2) late-developing perianthlike structures in some male flowers of Neoguillauminia cleopatra, (3) evidence for a bracteate origin of the female perianth in Anthosteminae and Neoguillauminiinae, and (4) spatiotemporally independent formation of abscission zone and perianth. Indistinct boundaries between inflorescence, flower, and floral organs demonstrate that defining the cyathium neither as an inflorescence nor as a flower is entirely satisfactory and indicate a “hybrid” flower/inflorescence nature of the cyathium. Based on our current knowledge and the existing phylogenetic context, it is most parsimonious to suggest that the cyathium evolved from a determinate thyrse with a terminal female flower surrounded by dichasial male partial inflorescences. We speculate that the cyathium was formed because of strong condensation and possible overlap between expression zones of regulatory genes.
Keywords: cyathium, Euphorbia, Euphorbiaceae, Euphorbieae, flower, Malpighiales, ontogeny, pseudanthium
“What is the cyathium, this astounding performer?” —(Croizat, 1937b, p. 525)
A distinguishing feature of Euphorbia and its close allies in subtribe Euphorbiinae is the unique inflorescence, termed a cyathium (from the Greek kyathos = cup or dipper). This structure closely resembles a bisexual flower and is therefore apparently an extreme form of pseudanthium (Classen-Bockhoff, 1990, 1991). It contrasts strongly in organization with inflorescences of the vast majority of other angiosperms, including other Euphorbiaceae. Although Linnaeus (1753) initially described it as a flower—a view upheld by authorities such as Payer (1857) and Baillon (1858)—the cyathium has long been interpreted as an inflorescence by most authors since Lamarck (1786), Jussieu (1789, 1824), and Brown (1814).
The cyathium is a cuplike structure of fused bracts, often with distinct extrafloral nectar glands and showy petaloid appendages, enclosing a single terminal stalked gynoecium (interpreted as a perianthless female flower) surrounded by four or five groups of stamens (interpreted as partial inflorescences of perianthless male flowers) (Fig. 1a). Eichler (1878) summarized several features in support of a pseudanthial (i.e., inflorescence) interpretation of the Euphorbia cyathium (see also Weberling, 1981): (1) similarity with related genera (particularly Anthostema) in which both sexes possess a perianth; (2) a constriction within the “stamen” and in some species morphological differences between the upper (filament) and lower (pedicel) regions [this constriction is regarded as indicating the position of a suppressed perianth (e.g., Brown, 1814; Eichler, 1878; Weberling, 1981)]; (3) a perianth present in female structures in some species; (4) formation of male elements almost simultaneously with their subtending bracts; (5) the fact that flowers are generally unisexual among other Euphorbiaceae, so the occurrence of bisexual flowers would be unusual; and (6) movement of the female flower during anthesis.
Fig. 1.
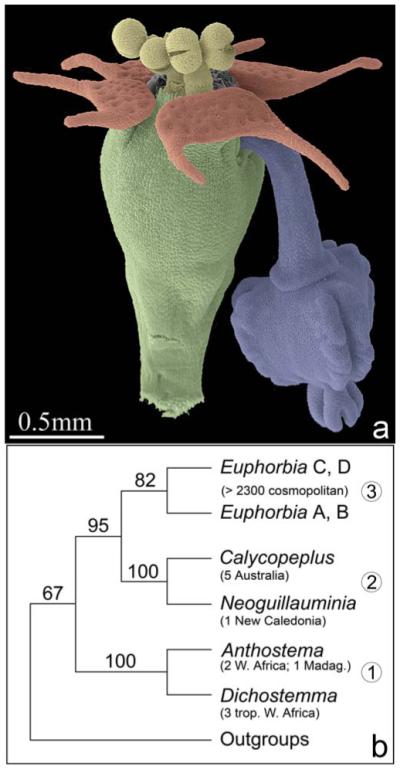
Cyathium morphology and systematic relationships in tribe Euphorbieae. (a) Colored scanning electron micrograph of anthetic cyathium of Euphorbia peplus showing involucre (green), nectaries (red) at rim of involucre, male flowers (yellow), and a single female flower (blue) hanging out of the involucre. (b) Summary tree of relationships in Euphorbieae based on Steinmann and Porter’s (2002) weighted maximum parsimony analysis of the ITS region, with bootstrap percentages greater than 50% above branches. Clade 1 = Anthosteminae, 2 = Neoguillauminiinae, 3 = Euphorbiinae split into two clades: clade (A), including species from Africa and Madagascar (A + B), and primarily Laurasia (B), and the cosmopolitan clade (C + D), in which all other genera recognized in Euphorbiinae are nested. Number and distribution of species are in parentheses below taxon name.
Within Euphorbiaceae, pseudanthial (i.e., strongly flower-like) inflorescences are not restricted to Euphorbieae but also occur in Dalechampia and Pera (Pax and Hoffmann, 1931; Webster, 1994). These two genera were both formerly placed in Acalyphoideae but are now considered only distantly related, either to each other or to Euphorbia. Indeed, Wurdack et al. (2005) placed Pera in its own subfamily Peroideae. Thus, there have apparently been at least three independent origins of pseudanthial inflorescences within Euphorbiaceae. Of these, Euphorbieae is by far the most species-rich, with over 2300 species (Radcliffe-Smith, 2001), and the cyathium of subtribe Euphorbiinae (representing the bulk of these species) can therefore be seen as a key innovation.
However, the current interpretation of the cyathium is mostly based on comparative studies dating from the 19th and early 20th centuries (e.g., Payer, 1857; Baillon, 1858; Michaelis, 1924; Haber, 1925). No study to date has included late floral ontogeny in Euphorbiinae. Furthermore, previous studies have mostly focused on subtribe Euphorbiinae (e.g., Hoppe and Uhlarz, 1982; Hoppe, 1985) rather than related groups; a broader comparative developmental study within subtribe Euphorbiinae is lacking. Ontogenetic studies of the related subtribes Neoguillauminiinae and Anthosteminae are entirely lacking, although they are extremely important for interpretation of the cyathium.
The uniqueness of the cyathium and its apparent morphological intermediacy make it a good subject for evaluating questions about flower and inflorescence evolution in angiosperms, in parallel with similar studies on distantly related angiosperms with obscure flower–inflorescence boundaries, such as the monocot family Triuridaceae (Rudall, 2003; Rudall and Bateman, 2006) and the early-divergent angiosperm Hydatellaceae (Rudall et al., 2007). Our goal in this paper is to utilize the modern phylogenetic context in Euphorbiaceae as a background for new comparative morphological and ontogenetic data. These new data will provide a basis for evaluation of floral and inflorescence homologies in Euphorbieae and allow us to establish hypotheses about the evolution of the cyathium that will ultimately be testable using developmental genetics.
Taxonomic context
Recent molecular analyses (reviewed by Wurdack et al., 2004) have demonstrated the polyphyly of Euphorbiaceae sensu lato and dispersed its constituent members throughout a novel order Malpighiales (e.g., Angiosperm Phylogeny Group, 1998, 2003; Tokuoka and Tobe, 2006; Soltis et al., 2007). Within subfamily Euphorbioideae, however, the monophyly of the tribe Euphorbieae has been broadly accepted since its description by Blume (1825) because of the distinctive pseudanthium. (A further potential synapomorphy for Euphorbieae is the presence of rod-shaped starch grains in the latex, though similar grains occur in a few species of the putative sister tribe Hippomaneae [Mahlberg, 1975; Rudall, 1987].) One exception to this consensus was Baillon (1858), who excluded Anthostema from Euphorbieae because he interpreted the pseudanthium of Euphorbieae as a flower and the reproductive structure in Anthostema as an inflorescence in which the female flower is in a lateral position.
Webster’s (1994) classification of Euphorbiaceae subdivided Euphorbieae into three subtribes, Anthosteminae, Neoguillauminiinae, and Euphorbiinae, all of which were upheld as monophyletic in the molecular analyses of Steinmann and Porter (2002) and Wurdack et al. (2005) (Fig. 1b). Taxonomic circumscription within subtribe Euphorbiinae has mostly focused on cyathial characters (Baillon, 1858; Boissier, 1866; Müller Argoviensis, 1874; Pax and Hoffmann, 1931; Croizat, 1936, 1937a, b). Bentham (1878, p. 522) noted that “the genus Euphorbia … is exceedingly varied in habit and primary inflorescence, but so uniform in the arrangement of the cymule and structure of the flower that its division into good sections is a matter of the greatest difficulty.” Recent cladistic analyses of molecular sequence data by Steinmann and Porter (2002), Haevermans et al. (2004), and Bruyns et al. (2006) have broadly supported this statement. Steinmann and Porter (2002) and Bruyns et al. (2006) found a paraphyletic Euphorbia with all other currently recognized genera of subtribe Euphorbiinae (i.e., Chamaesyce, Monadenium, Pedilanthus, and Synadenium) nested within it. Steinmann and Porter (2002) discussed the systematic consequences of either splitting Euphorbia or lumping the segregated genera into a large Euphorbia. Subsequently, Steinmann (2003) and Bruyns et al. (2006) made the first attempts to recombine the embedded taxa within Euphorbia. However, in the present study we maintain “traditional” taxon names to highlight the morphological differences, particularly within the cyathium. Steinmann and Porter (2002, p. 453) concluded that “within Euphorbia, the majority of the currently recognized subgenera are either paraphyletic or polyphyletic.” Phylogenetic studies based on morphological data (Park, 1996; Park and Elisens, 2000; Park and Backlund, 2002) contrast strongly with the molecular results. Thus, clarification of cyathial characters is important for the systematics of the subtribe.
MATERIALS AND METHODS
Ontogenetic data of reproductive structures of 41 species, representing all three subtribes of Euphorbieae and other members of Euphorbiaceae, were investigated using scanning electron microscopy (SEM, Appendix 1). Fresh material was fixed in formalin–acetic acid–alcohol (FAA: 70% alcohol, formaldehyde and glacial acetic acid, 85:10:5) and transferred to 70% ethanol before examination. For SEM, material was dissected in 70% ethanol, dehydrated through an ethanol series to absolute ethanol, and critical point dried using a Balzers CPD 020 (BAL-TEC AG, Liechtenstein). Dried material was further dissected and mounted onto specimen stubs using nail polish, coated with platinum using an Emitech K550 sputter coater (Emitech, Ashford, UK), and examined using a Hitachi cold field emission SEM S-4700-II (Hitachi High Technologies Corp., Tokyo, Japan). In total, ca. 3000 SEM micrographs were analyzed.
RESULTS
Cyathium ontogeny in Euphorbiinae
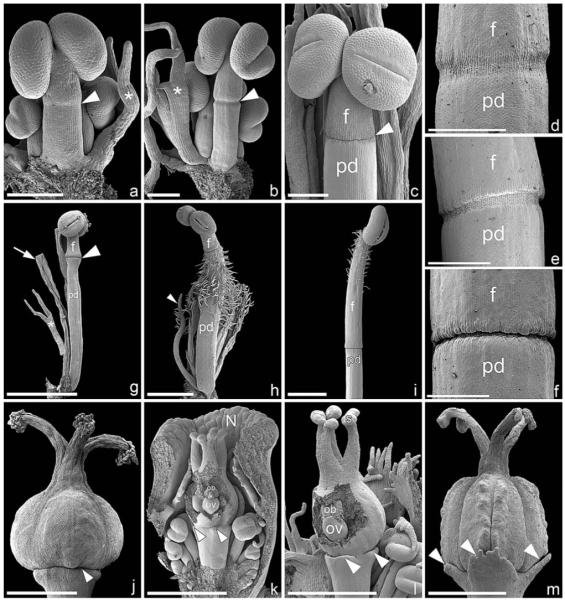
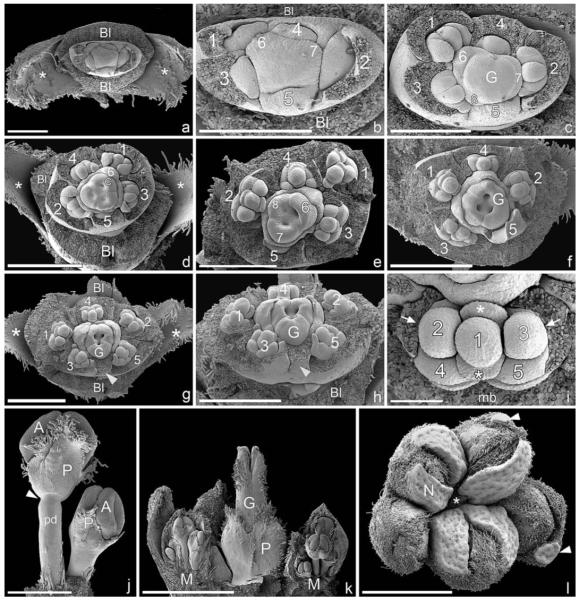
In Euphorbia myrsinites (shown here in detail as a typical example for Euphorbiinae), the cyathium is initiated as a more or less circular primordium preceded by two sequentially formed bracteoles (Fig. 2a). Five male elements are formed in either a clockwise or counterclockwise spiral, which commences on the adaxial side of the cyathium (Fig. 2b, c). Subtending bracts are initiated more or less simultaneously with each male element and soon fuse to form a uniform involucre (Fig. 2d). Depending on the direction of the original sequence of initiation, formation of male flowers (i.e., stamens) starts either on the left or the right side of the primary male primordia (Fig. 2d, e). At this early developmental stage, there are distinct gaps between male elements 1–3, 3–5, and 2–4 (Fig. 2d, e). Later, the involucre begins to close and four nectaries are formed on its rim (Fig. 2f). Within the involucre, male structures begin to differentiate and form anthers. At this developmental stage, the original direction of the spiral initiation of male elements remains visible (Fig. 2g, i). Within a single male partial inflorescence, male flowers are formed centrifugally, either in a distinct zigzag pattern (Fig. 2h, j, k) or one above the other (Fig. 2l). In neither pattern is there a clear distinction between male flower primordium and stamen primordium because anther formation starts immediately after the male primordium is formed (Fig. 2h, j–l). Associated male prophylls are normally completely absent (Fig. 2h, j), though in E. ammak, E. teke, and E. petraea we found unusual filamentous structures at the base of the male flowers that could be interpreted as prophylls (Fig. 2k, l). Other filamentous structures are formed later in ontogeny and are not easy to associate with particular male flowers (Fig. 3a, b, g, h). Their number is not correlated with the number of male flowers, and sometimes they become branched (Fig. 3b, g). In most species investigated, there is no anatomical difference between filament and pedicel, but they are apparently demarcated by a constriction formed late in ontogeny (Fig. 3a–f). The constriction acts as an abscission zone after anthesis—i.e., after the pollen has been shed (Fig. 3g). In E. obesa and E. globosa, we found hairs on the pedicel (Fig. 3h), while in Pedilanthus tithymaloides and P. cymbiferus, hairs were present on the filament only (Fig. 3i).
Fig. 2.
Early ontogeny of cyathium and differentiation of male elements in Euphorbiinae (SEM). (a–i) Euphorbia myrsinites. (a) Cyathium primordium preceded by two sequentially formed bracteoles. (b) Initiation of male primordia in a clockwise spiral sequence (1–5). The subtending bracts formed more or less simultaneously with the male elements (best seen in 4). (c) Somewhat older developmental stage than (b) showing counterclockwise formation of male elements (1–5) and their subtending bracts (arrowheads). (d) Bracts fused to form a cuplike involucre. Formation of male flower initials (1–5) in a counterclockwise spiral. The second order primordia (1′–5′) formed to the right of each first order primordium. Note the paired appearance of male elements 1/4 and 2/5 and isolated position of 3 associated with the formation of broad gaps (arrowheads). The gynoecium is initiated in the cyathium center, showing three ovule initials (asterisks) associated with three carpellary bulges. (e) Somewhat older developmental stage than (d), involucre removed. Clockwise formation of male elements with distinctive gaps between 1/3, 2/4, and 3/5 (arrowheads). (f) Formation of four nectar glands on ridges of involucre. The three carpellary bulges enlarged and partially enclosing the still visible ovules (asterisks). (g) Sequential maturation of male flowers in a clockwise direction, apparently following the order of initiation. The three gaps between the male elements are still clearly visible. (h) Abaxial lateral view of (g) showing centrifugal maturation of male flowers (1–3). (i) Frontal view of older cyathium in which the order of the counterclockwise formation of male primordia is still detectable (1–5) and in which carpel closure is in progress. (j) Euphorbia globosa, lateral view of male element, showing centrifugal differentiation of male flowers following an alternating left to right zigzag pattern. (k) E. ammak, lateral view of male element, similar to (j) but with distinct filamentous structures flanking male flowers (arrowheads along rim and asterisks along center). (l) E. petraea, male element showing centrifugal differentiation of male flowers (1–3) following a pattern in which each flower lies above the subsequent one. The male flowers are flanked by filamentous structures (arrowheads). Bar = 100 μm in a–c; 200 μm in d–l. A, cyathial apex; Bl, bracteole; G, gynoecium; In, involucre; N, nectary.
Fig. 3.
Male and female flower differentiation in Euphorbiinae (SEM). (a–c) Euphorbia pteroneura, differentiation of male flowers. (a) Late formation of constriction (arrowhead) when the anther is already formed. Note filamentous structure (asterisks), basally slightly fused with male flower. (b, c) Ongoing formation of constriction (arrowhead), which divides the male structure into a lower “pedicel” and upper “filament.” Note somewhat flattened filamentous structure (asterisk). (d–f) Pedilanthus tithymaloides, details of constriction formation. (g) E. pteroneura, male flower dissected out of mature cyathium. The constriction (arrowhead) divides the male structure into a lower “pedicel” and upper “filament.” The constriction acts as an abscission zone along which filaments break off after pollen is shed from the anther. The pedicel of the male flower remains on the cyathium (arrow). Note branched filamentous structure (asterisk). (h) E. obesa, male flower with hairy pedicel (pd) and glabrous filament (f). Note hairy filamentous structures (one marked with arrowhead). (i) P. cymbiferus, upper part of male flower with hairy filament and glabrous pedicel. (j) E. pteroneura, naked gynoecium sits on pedicel without obvious sign of female perianth (arrowhead). (k–m) Monadenium rhizophorum, late formation of perianthlike structures. (k) Onset of formation of perianthlike structure (arrowheads). Note female flower already distinctly stalked and ovule already formed within opened gynoecium. The nucellus protrudes out of the ovule (asterisk), and an obturator is present. Note distinct horseshoe-shaped nectary. (l) Somewhat older stage, showing slightly enlarged perianthlike structures at base of gynoecium (arrowheads). The ovule is enlarged and the obturator completely encloses the nucellar beak. The smooth surface of the stigmas (one labelled with S) contrasts with the papillate surface of the styles. (m) Mature female flower with three perianthlike structures (arrowheads) at base of gynoecium. The carpel shows a distinct longitudinal furrow. Bar = 200 μm in a–f; 1 mm in g–m. f, filament; pd, pedicel; N, nectary; ob, obturator; ov, ovule; s, stigma.
As soon as all five male elements are visible, the center of the cyathium becomes dome-shaped (Fig. 2c), and differentiation of the female structure (flower/gynoecium) commences in parallel with formation of male flowers (Fig. 2d). As with male structures, there is no clear distinction between the female flower primordium and gynoecium primordium (Fig. 2c, d). At this developmental stage, three ovule initials become visible, and the tricarpellary nature of the gynoecium becomes evident (Fig. 2d, e). The gynoecium closes late so that the ovule initials are visible for a long period (Fig. 2f, g, i). In the mature cyathium, the gynoecium is borne on the pedicel (which is by definition the floral stalk of the female flower). In most species the gynoecium lacks preceding organs (Fig. 3j), though in Monadenium rhizophorum, E. ammak, and E. teke a trimerous perianthlike structure is formed at its base late in ontogeny (Fig. 3k–m).
Pseudanthium ontogeny in Neoguillauminiinae
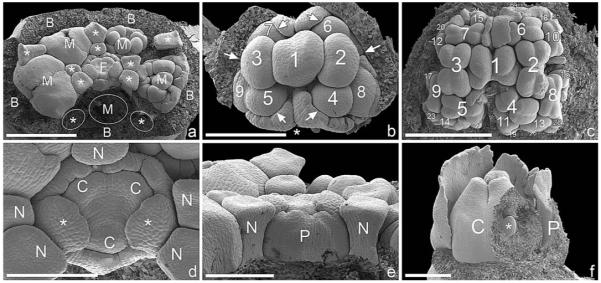
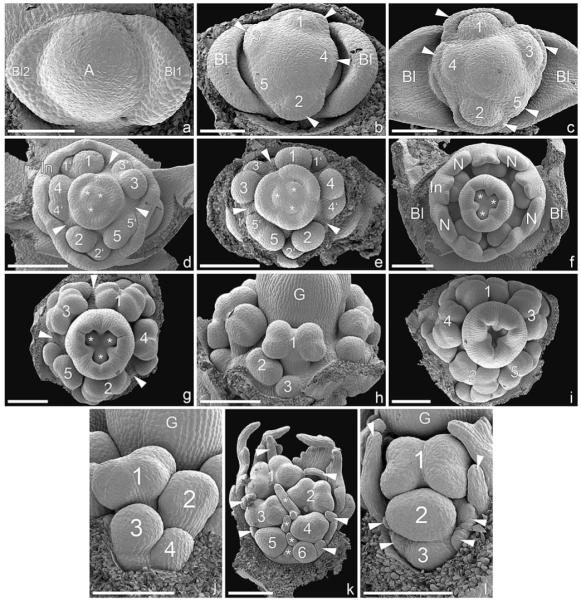
In Neoguillauminia cleopatra, a central trimerous female flower is surrounded by five male elements, each subtended by a petaloid white bract (removed in Fig. 4a). Each male element is flanked by pairs of nectaries, which arise from the pseudanthium base (Fig. 4a, d, e). Within each male element, a central male flower is formed first, which is associated with the petaloid bract of the entire male partial inflorescence. Subsequent male flowers are formed in pairs (Fig. 4b), and each male flower is subtended by a bract. Flowers 2 and 3 flank the first flower, flowers 4 and 5 are formed in an abaxial position, followed by flowers 6 and 7 in an adaxial position and flowers 8 and 9 again in an abaxial position. Subsequently, the pairwise formation is disrupted, and flowers (10–28 in Fig. 4c) are formed in a more or less irregular sequence with some spiral tendencies. Distinction between male flower primordium and stamen primordium is impossible because anther formation commences immediately after the male primordia become visible (Fig. 4b). Late in male flower development, when the anthers are already borne on a distinct stalk, a constriction is formed; this constriction acts as an abscission zone as soon as the pollen is shed (Fig. 5a–c). In some male flowers, we observed a lobed (possibly perianthlike) structure that is formed late in ontogeny, in parallel with the formation of the abscission zone (Fig. 5d–f).
Fig. 4.
Early developmental stages of pseudanthium ontogeny and formation and differentiation of male flowers in Neoguillauminia cleopatra (SEM). (a) Pseudanthium with five male elements (one in abaxial position removed), each subtended by a bract, which was removed during dissection. Each male element is flanked by two nectaries (asterisks). (b) Male element showing order of male flower initiation. Each flower is subtended by a bract, arrows pointing from the bract toward the subtended flower. The bract subtending the first initiated flower has been removed (asterisk). Central flower is formed first, and remaining flowers (2–9) are formed pairwise in rapid succession. (c) Older developmental stage with anthers of older flowers already formed. The orientation of the anther alternates; i.e., anthers of flowers 2 and 3 at a right angle to flower 1, anthers of flowers 4–7 at a right angle to flowers 2 and 3, and so on. (d) Female flower showing early formation of perianth with two larger lobes in transversal position (asterisks) and several smaller lobes in abaxial and adaxial positions. All parts of the perianth are already fused, as also seen in (e). Three carpels are formed. (e) Lateral view of (d) showing female perianth and two nectaries arising from the pseudanthium receptacle. (f) Tricarpellate female flower, perianth partly removed and ovary opened to reveal one ovule initial (asterisk). Bar = 1 mm in a, c; 400 μm in b, d–f. B, bract; C, carpel; F, female flower; M, male partial inflorescences; N, nectary; P, perianth.
Fig. 5.
Male flower differentiation and mature female flower of Neoguillauminia cleopatra (SEM). (a) Male flower, late formation of constriction below the already visible anther. (b) Somewhat older male flower with more prominent constriction. (c) Mature male flower with abscission zone dividing it into basal pedicel and apical filament. The two thecae are distinctly apart from each other. (d) Late formation of constriction, and perianthlike structure above it (arrowheads). (e) Somewhat older developmental stage than (d). (f) Male flower showing constriction and perianthlike structure (arrowhead) above it. (g) Mature female flower, ovary dissected and one style removed to show young ovule and obturator. Perianth partly removed. Note that flower sits on a short pedicel and constriction occurs below insertion of perianth. (h) Outer surface of perianth. (i) Detail of outer perianth surface showing densely arranged stomata. Bar = 500 μm in a, b, d–f; 1 mm in c, g, h; 300 μm in i. f, filament; ob, obturator; ov, ovule; P, perianth; pd, pedicel.
The female flower consists of three carpels surrounded by a perianth even at the earliest available stages (Fig. 4a, d, e). Despite the clear tricarpellary nature of the flower, the merism (merosity) of the perianth is difficult to determine. We found two larger lobes in transversal orientation and several smaller lobes in abaxial and adaxial positions. The perianth lobes appear to be fused from inception (Fig. 4e). Because of its dimensions during early developmental stages, a protective function for the gynoecium is possible (Fig. 4f); in the mature flower, the perianth encloses the base of the gynoecium. At this developmental stage, the perianth is a massive structure with the surface covered by stomata, so that it has a distinctly leaflike appearance (Fig. 5g–i).
Pseudanthium ontogeny in Anthosteminae
Dichostemma glaucescens
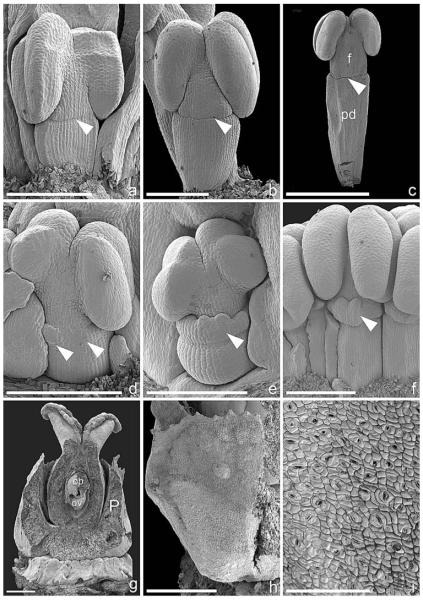
The pseudanthium is tetramerous and preceded by an unpaired prophyll in lateral position and two bracteoles in a median position (Fig. 6a–c). Two male elements are initiated first, in a transverse position, each subtended by a bract (Fig. 6a). Shortly after, two male elements are initiated in a median position, each subtended by a bract (Fig. 6b). Both bracts and interbracteate regions of the involucre, which later bears nectaries on the inside, enlarge (Fig. 6c) and soon enclose the male elements completely (Fig. 6d).
Fig. 6.
Pseudanthium formation in Dichostemma glaucescens (SEM). (a–f) Early stages. (a) Young pseudanthium subtended by bract and preceded by two bracteoles in the median plane. Arrowhead points to a pseudanthial primordium in the axil of the abaxial bracteole. Two male elements and their subtending bracts formed almost simultaneously in a transversal position. Pseudanthial apex is still undifferentiated. (b) Somewhat older developmental stage with four male elements and their subtending bracts formed. Female flower is initiated in pseudanthium center. Note two preceding bracteoles in a median position and a third unpaired prophyll to the left (asterisk). (c) Enlarged male bracts that partially enclose male elements; nectaries are formed between male bracts. Female perianth initiated as four separate primordia (asterisks). (d) Somewhat older developmental stage, two male bracts removed, nectar glands and female perianth are more prominent. (e) Involucre removed, female perianth has begun to bend inward (especially transversal regions), male elements have begun to enlarge. (f) Perianth removed from female flower in (e). Four horseshoe-shaped carpel initials are visible (borders of one highlighted). (g–l) Formation and dedifferentiation of male elements. (g) Young male element with two lateral prophylls (MBl) and central depression (asterisk). (h) Male perianth is in the form of a horseshoe-shaped primordium. (i) Stamen primordium surrounded by ring-shaped primordium. (j) Perianth almost encloses the young stamen. (k) Male inflorescence with a central male flower formed first (1), followed by pairs of male flowers (2+3, 4+5, 6+7), which have formed in rapid succession. (l) View of abaxial side of (k) showing difference in size of flowers 4–5 due to rapid succession of initiation. Bar = 200 μm in a–f; 100 μm in g–l. A, pseudanthial apex; C, carpel; B, bract; Bl, bracteole; F, female flower; M, male element; mb, male bract; MBl, male prophyll; N, nectary; P, perianth; S, stamen.
Within the male elements, two prophylls are initiated in a lateral–adaxial position, and a depression becomes visible in the center of the primordium (Fig. 6g). While the prophylls enlarge, a horseshoe-shaped primordium is initiated directly on the male inflorescence primordium, and no distinct male flower primordium is discernible. Soon after, the horseshoe-shaped primordium becomes circular and forms the male perianth (Fig. 6h–j). In the center of the perianth, a primordium is initiated; this could be designated a stamen primordium (Fig. 6i). Within the male inflorescence, this flower is flanked by pairs of male flowers, which are initiated in rapid succession (Fig. 6k, l). Following two transverse-adaxial flowers, flowers 4 and 5 are initiated in an abaxial position, followed by flowers 6 and 7 in an adaxial position.
At early developmental stages, the perianth encloses the anther almost completely (Figs. 6j–l, 7a). Later, when the anther is already clearly visible and only basally surrounded by the perianth, a constriction is formed (Fig. 7b), which acts as an abscission zone after the pollen is shed (Fig. 7c, d).
Fig. 7.
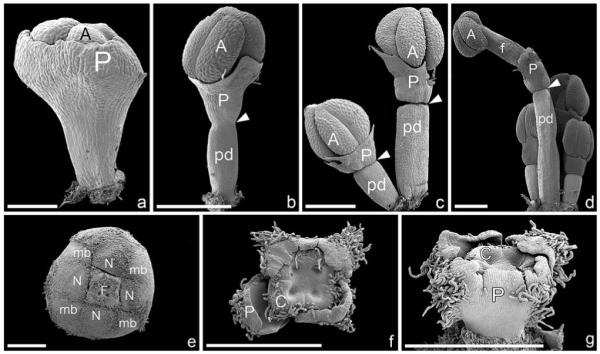
Male and female flower differentiation in Dichostemma glaucescens (SEM). (a–d) Male flower differentiation. (a) Perianth encloses most parts of male flower; only a small apical portion of the anther (A) is visible. (b) Formation of a constriction (arrowhead), which marks the end of the floral pedicel. Only the base of the enlarged anther is covered by the male perianth. (c) Complete constriction in male flowers (arrowheads) leaving a distinct mark between floral pedicel and perianth. (d) Group of male flowers, one mature, with a distinct pedicel, abscission zone (arrowhead), filament, and anther. (e) Pseudanthium partly enclosed by male bracts, and nectar glands, which are bent inwards; an apical opening leaves the female flower unprotected. (f) Female flower composed of four carpels (one labeled C) and four perianth parts (one labeled P). Both organs are externally hairy. (g) Lateral view of female flower, showing perianth fused and externally hairy. Bar = 100 μm in a; 500 μm in b–g. A, anther; C, carpel; f, filament; F, female flower; mb, male bract; N, nectar gland; P, perianth; pd, pedicel.
Simultaneous with the formation of the two median male elements, the female structure becomes evident in the center of the pseudanthium (Fig. 6b). Soon after, a tetramerous perianth is initiated (Fig. 6c, d), which starts to bend inward but never fully encloses the gynoecial apex (Figs. 6e, 7e–g). Gynoecial tetramery becomes evident early, when four horseshoe-shaped carpel initials are formed (Fig. 6f). Later in development, the gynoecium remains visible because closure of the involucre, and the female perianth is incomplete (Fig. 7e–g). The outside of the female perianth and the outer surface of the gynoecium are hairy (Fig. 7f, g).
Pseudanthium ontogeny in Anthosteminae
Anthostema madagascariense
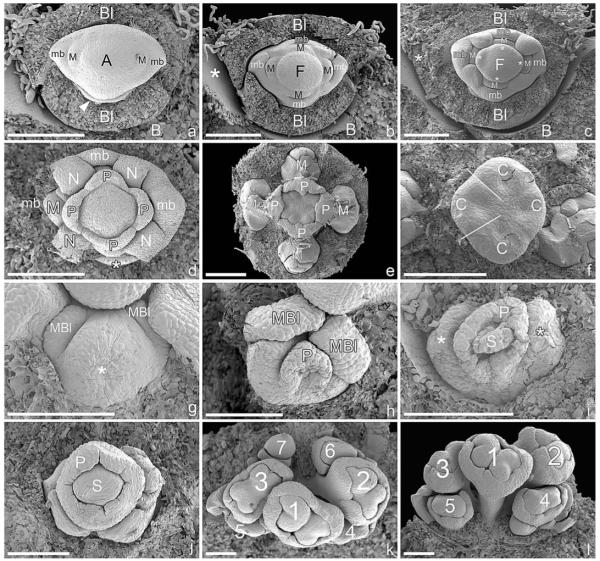
This species possesses a central trimerous gynoecium surrounded by four or five male elements. The pseudanthium is preceded by two prophylls in a transversal position and two median bracteoles (Fig. 8a). The male elements and their subtending bracts, respectively, are initiated in a spiral sequence starting in a lateral-adaxial position (Fig. 8b–f). The spiral continues toward the female flower, so that formation of the female perianth follows the original spiral of the male elements (Fig. 8b–g).
Fig. 8.
Ontogeny of pseudanthium in Anthostema madagascariense (SEM). (a) Young pseudanthium preceded by two median bracteoles, flanked by two further transversal prophylls (asterisks). (b) Helical formation of four male inflorescences in the axils of bracts (1–4). The fifth bract becomes part of the perianth of the central female flower. The remaining two perianth parts of the female flower are also formed in succession, following the spiral of the male inflorescences (5–7). (c) Somewhat older developmental stage, showing a similar condition as in (a), except that all three perianth parts of the female flower formed separately and in addition to the fifth bract (5) of the male inflorescence “whorl.” The gynoecium has begun to differentiate. (d) Pseudanthium preceded by two transversal bracteoles (Bl; adaxial/upper bracteole only partly visible to the left) and by two further lateral prophylls (asterisks). Five male inflorescences have formed in a spiral (1–5). The fifth inflorescence in an abaxial position remains small or rudimentary. The gynoecium/female flower is preceded by a single larger organ (6), which is in a position following the spiral of the male inflorescences. The rest of the perianth forms a more or less uniform ringlike structure. The three carpels of the syncarpous gynoecium are visible (one labeled C). (e) Five male elements formed in a spiral (1–5), the first one lying somewhat off the “male whorl” and the fifth one small or reduced. Similar to (d), the formation of the first female tepal follows the male spiral, and even the three carpels show a distinct developmental gradation (6–8) but in reverse direction of the male spiral. (f) Only four male inflorescences have formed. The position of the potential fifth male inflorescence is filled with a larger structure belonging to the perianth of the female flower. (g) Similar developmental stage to (f), but with five male inflorescences. The female tepal in an abaxial/lower position has been removed and seems to be part of the male inflorescence “whorl” (arrowhead). (h) Abaxial view of (g) showing insertion of tepal in a position where the other male inflorescences have formed (arrowhead). (i) Male inflorescence subtended by male bract. A central flower is formed first (1) and preceded by two lobes in median position, which later form the perianth (asterisks). Flowers 2 and 3 arise in transversal position, each subtended by a bract (arrow). Flowers 4 and 5 are initiated in abaxial position. (j) Male flowers with anthers partly enclosed by perianth. The constriction is present in the left flower (arrowhead), while the right flower has no sign of a constriction yet. Note the similarity with male flowers of Dichostemma glaucescens (Fig. 7a–d). (k) Longisection of pseudanthium with central female flower flanked by two male inflorescences. The female flower consists of a trimerous gynoecium basally enclosed by a perianth. (l) Frontal view of pseudanthium in which the central female flower is reduced (asterisk). Note the distinct nectaries (one marked with N) along the rims of the male bracts. Two nectaries are distinctly smaller (arrowheads). Bar = 300 μm in a–c; 500 μm in d–h, j–l; 100 μm in i. A, anther; C, carpel; Bl, bracteole; G, gynoecium; mb, male bract; N, nectar gland; P, perianth; pd, pedicel.
The fifth male element either develops fully (Fig. 8g) or is suppressed or even completely absent, in which case the first perianth part of the female flower is initiated in the position of the original fifth male element (Fig. 8d–f). In cases with five male elements, the phyllotaxis of the female perianth follows the male spiral (Fig. 8b, c), and in one instance we found the abaxial part of the female perianth located in the “whorl” of the male elements (even though the female flower is formed more or less in the pseudanthium center: Fig. 8a–c). In cases with only four male elements, we found an enlarged female perianth part in the position of the fifth male element (Fig. 8f).
Male flower initiation resembles that of Dichostemma glaucescens and Neoguillauminia cleopatra in that a central flower is formed first and is subsequently flanked by pairs of further developing male flowers (Fig. 8i). A male perianth is formed early as two median lobes flanking the male flower primordium (Fig. 8i).
With respect to the male perianth (Fig. 8j) and late formation of the constriction in male flowers, this species closely resembles Dichostemma glaucescens. In the mature pseudanthium, the female flower is basally enclosed by a perianth and flanked by male inflorescences (Fig. 8k). Massive nectaries are formed along the rim of the male bracts (Fig. 8l).
DISCUSSION
Boundary between stamen and male flower
All male organs in the tribe Euphorbieae possess a late-developing constriction that becomes a post-anthetic abscission zone. This constriction is generally considered to indicate the stamen–flower boundary; the region below it is interpreted as the male pedicel and the region above it as the filament (e.g., Brown, 1814; Eichler, 1878; Weberling, 1981). In some Euphorbieae the constriction is associated with adjacent lobes (normally interpreted as perianthlike sepaloid appendages) that occur immediately above it. However, our results show that the relative timing of development of constriction and lobes is not linked: the constriction invariably develops late in ontogeny, but the lobed appendages develop either early (in Anthosteminae) or late (in Neoguillauminiinae), and in Euphorbiinae appendages are entirely lost. In Neoguillauminia cleopatra the appendages are more reminiscent of lobing at a domain boundary than a perianth (Fig. 5d–f).
Thus, the male structures of subtribe Neoguillauminiinae are apparently intermediate in some respects between those of Anthosteminae and Euphorbiinae. The occurrence of perianth-like structures in some male flowers of Neoguillauminia, documented here for the first time, seems to support the hypothesis of a stepwise reduction of the male perianth within Euphorbieae (and also in different flowers within the pseudanthium of Neoguillauminia). The male flower of Anthosteminae is broadly accepted as the ancestral type in Euphorbieae (Brown, 1814; Müller Argoviensis, 1872; Eichler, 1878; Weberling, 1981). It possesses a small synsepalous perianthlike structure that is initiated early in ontogeny and has a late-developing constriction at its base. If this interpretation is correct, the male flower consists of a perianth whorl (probably a calyx, as it is not showy) surrounding a single terminal filament bearing an anther.
However, we note that alternative explanations are possible; specifically that at least some of the perianthlike structures could represent novel appendages, and the abscission zones may not always demarcate the base of the flower. The angiosperm stamen has a high degree of morphological flexibility. Different types of staminal outgrowths and/or appendages occur in species of a wide range of angiosperm families, such as the magnoliids Chloranthaceae (Kong et al., 2002) and Hernandiaceae (Endress and Lorence, 2004), the monocots Alliaceae (Rudall et al., 2002) and Pontederiaceae (Strange et al., 2004), and the eudicots Simaroubaceae (Franceschinelli and Thomas, 2000) and Loasaceae (Hufford, 2003).
Furthermore, abscission zones are common in flowers of Euphorbiaceae, but not always at the base of the flower. For example, in male flowers of Dalechampia and Acalypha (Euphorbiaceae), an abscission zone is formed within the pedicel so that the male flower abscises with part of the male pedicel and the rest of the flower (i.e., the perianth is located well above the abscission zone: G. Prenner, personal observations). Müller Argoviensis (1872) described abscission zones within the filament in Tetraplandra, within the stylar column in Algernonia, and beneath the base of the ovary in Stillingia. Among other angiosperms, abscission of organ parts within a (true) flower has been reported for Piper methysticum and Zippelia begoniaefolia (both Piperaceae) in which an abscission zone occurs below the anther (Prakash et al., 1994; Han-Xing and Tucker, 1995). Eichler (1878) mentioned a constriction in the stamen of Alchemilla, similar to that of euphorbs, but interpreted it as an entirely staminal structure. In the gynoecium of Eriope (Lamiaceae), a similar mid-stylar constriction, formerly interpreted as demarcating two morphologically distinct structures, was reinterpreted as an abscission zone (Rudall, 1981). Müller Argoviensis (1872, p. 66) noted (possibly correctly) that “on its own, the articulation verifies nothing.” However, he highlighted the presence of a perianth above the constriction in Anthostema (first mentioned by Brown, 1814 and illustrated by Jussieu, 1824) in support of a floral interpretation of the male structure in Euphorbiinae, in which the male perianth is reduced.
Our observations partially contradict those of Müller Argoviensis (1872) that in male flowers of some Brazilian euphorbias the pedicel is frequently more solid and brown than the filament, which is relatively glabrous, subtle and pale, and that in E. cotinoides only the pedicel is hairy while the filament remains glabrous (see also Weberling, 1981). We found hairy pedicels and glabrous filaments in Euphorbia obesa and E. globosa (Fig. 3h), but the reverse in Pedilanthus tithymaloides and P. cymbiferus, in which the “pedicel” is glabrous and the “filament” is hairy (Fig. 3i). Furthermore, in E. quadrilatera the “filament” is bright red, whereas the “pedicel” is white. Apart from these examples, most Euphorbiinae that we observed did not differ below and above the constriction. The similarity of the regions above and below the constriction was also mentioned by Müller Argoviensis (1872) as a character of most euphorbs.
Boundary between male flower and inflorescence
In Euphorbiinae the boundaries of the male partial inflorescence and male flower are also blurred: anther formation commences immediately after the male floral primordium becomes visible, without separate formation of a stamen primordium. Similarly, in Dichostemma glaucescens (Anthosteminae) the boundary between male inflorescence and male flower is unclear; the male perianth is formed directly on the male inflorescence primordium, and no distinct male floral primordium is discernible. Early developmental stages in Dichostemma resemble early stages of Centranthus ruber (Valerianaceae), except that in Centranthus the flower is formed on a clearly defined floral primordium, and the circular structure surrounding the stamen primordium is of petaloid origin (Roels and Smets, 1996; Endress, 1999). This indicates that in Dichostemma there is a distinct developmental shortening (paedomorphosis). Analogous to the female perianth (see later), this truncated developmental pathway raises the question of organ identity. What is the nature of the male “perianth,” and which genetic pathways are responsible for its formation?
Furthermore, within Euphorbiinae, prophylls associated with individual male flowers are completely absent from some species (e.g., Euphorbia donii, E. lathyris, E. myrsinites, Monadenium elegans, Pedilanthus tithymaloides), whereas in others (E. ammak, E. petraea, E. teke) we found filamentous structures associated with the male flowers, which could be interpreted as vestigial prophylls (Fig. 2k, l). Confirmation of possible evolutionary trends requires a more robust and well-resolved phylogeny that includes all the relevant species. Currently, it is not clear whether reduction of prophylls is a derived character within Euphorbiinae or how many times prophylls were lost.
In addition to putative prophylls, we frequently found other unusual filamentous structures within the cyathium (Fig. 3a, b, g, h). Warming (1870) also noted these and highlighted (1) their late formation (i.e., after male flower initiation); (2) their position, which does not allow a distinct association with particular male flowers; and (3) their number, which is frequently not correlated with the number of male flowers (sometimes only one is present, or sometimes they exceed the number of male flowers). He therefore interpreted these structures as trichomes, which are formed instead of true leaves, analogous to the pappus in Asteraceae, the perigone in Cyperaceae, or prickles in Cactaceae.
Assuming that each stamen represents a male flower, our results show that male partial inflorescences in both Anthosteminae and Neoguillauminiinae possess dichasial characteristics with a central male flower successively flanked by pairs of further male flowers (Figs. 4b, 6k, 8i, 9H). This contrasts with reports by Radcliffe-Smith (2001) and Steinmann and Porter (2002) of monochasial male subflorescences in Neoguillauminia. We found that in Neoguillauminia the dichasial pattern is disrupted during later development, so that the ultimate flowers are formed in an irregular sequence with some spiral tendencies. This observation is important for interpretation of the male partial inflorescences in Euphorbiinae. The distinct zigzag pattern of male flower formation in Euphorbiinae (first correctly interpreted as a cincinnus by Wydler, 1845) could be derived from a dichasium similar to that of Anthosteminae and Neoguillauminiinae, by alternating suppression of the right and left prophyll product, resulting in a scorpioid cyme or cincinnus (Fig. 9H, I). Single-flowered male partial “inflorescences,” as found in some species of Chamaesyce (e.g., C. prostrata and C. hirta; see Venkata Rao, 1971), could represent the endpoint of this reduction series (Fig. 9K). Our novel discovery of a second pattern of flower formation in Euphorbiinae, in which male flowers are formed one above the other (Fig. 2l), could be derived either by a reduction series from a dichasium via a scorpioid cyme (by suppression of every second flower) or directly from a dichasium (Fig. 9). Once again, a more robust and bettersampled phylogeny is required to resolve this.
Fig. 9.
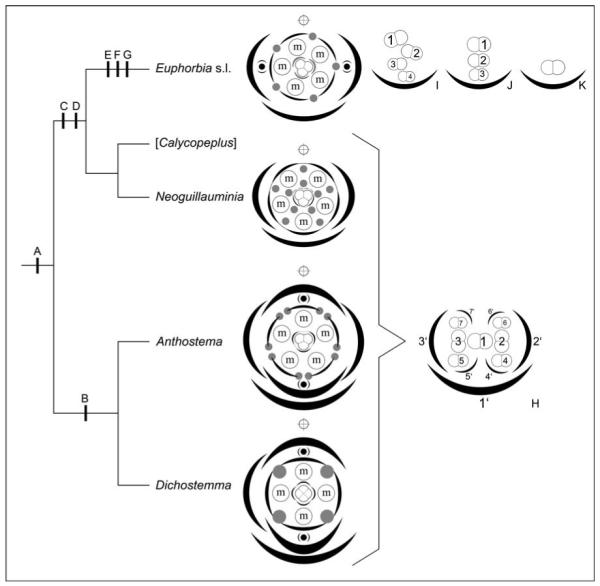
Optimization of evolutionary transitions on summary tree of relationships in tribe Euphorbieae (based on Steinmann and Porter’s [2002] weighted maximum parsimony analysis of the ITS region), with diagrams of pseudanthia and male partial inflorescences inserted next to the appropriate taxon (H–K). Calycopeplus (in square brackets) not included because of lack of material. A = Condensation of inflorescence. B = Reduction of outer cyathial prophylls in transversal position. C = Switch from centripetal to centrifugal formation of male organs. D = Switch of cyathial bracteoles (= inner prophylls) from median to transversal position. E = Reduction of male inflorescences from dichasial pattern (see H) to monochasial patterns (see I–K). F = Loss of male perianth. G = Switch from centripetal to centrifugal formation of female organs. Diagrams show stylized nectaries (gray dots) and stylized male partial inflorescences (encircled “m”). Occasional female perianthlike structures, as found in some Euphorbia and allies, are indicated as gray arcs. Pseudanthia are subtended by an abaxial bract and 2–4 preceding prophylls. All taxa except Neoguillauminia possess axial pseudanthia (black dots with preceding prophylls) either in median (Dichostemma and Anthostema) or transversal position (Euphorbia s.l.). Main axis indicated by a crossed circle above each diagram. The diagram of Anthostema madagascariense, in which the pseudanthium is variable in terms of number and structure of different elements, is drawn after Fig. 8g, without incorporating the “odd” position of the adaxial/lower female perianth. (H) Diagram of dichasial male partial inflorescence as found in Anthostema, Dichostemma and Neoguillauminia. Only the first seven flowers are shown and numbered in order of their initiation (1–7) as are their subtending bracts (1′–7′). Male perianth was omitted. (I) Diagram of monochasial male partial inflorescence (cincinnus) as found in most Euphorbiinae. Occasional prophyll-like structures and frequent other filamentous structures were omitted for clarity. (J) Diagram of monochasial male partial inflorescences with male flowers formed one above the other. Prophyll-like structures were omitted. (K) Highly reduced single-flowered male partial “inflorescence” as found in some species of Chamaesyce.
Another noteworthy feature is the almost simultaneous formation of male partial inflorescences and their associated bractlike structures (Fig. 2b, c). This was first noted by Warming (1870) as evidence for the inflorescence nature of the cyathium (see also Eichler, 1878); he considered that it can only be a bract and its axillary bud, rather than two distinct whorls of a flower. However, today we know that simultaneous formation of organs of adjacent whorls (i.e., common primordia) does occur in flowers of some groups (e.g., Primulaceae: Caris and Smets, 2004), especially in groups with fascicled androecia, such as Malpighiales. Thus, near-simultaneous formation of bract and male element is of limited value for interpretation of the cyathium as an inflorescence.
Boundary between gynoecium and female flower
In all species investigated here, the female flower is formed in a central (terminal) position. This location is of particular interest for Anthostema madagascariense because Baillon (1858), Venkata Rao (1971), and Radcliffe-Smith (2001) suggested that in Anthostema the female flower is lateral, which is important in interpretation of the cyathium (see later). In Euphorbiinae, carpel formation is initiated immediately after the central (female) primordium becomes visible, so there is no clear-cut distinction between the primordium of the female flower and the gynoecium.
Weberling (1981) reported that in Euphorbia the female flower initially hangs out of the cyathium and becomes erect later in anthesis. The opposite condition, i.e., initially erect and later hanging female flowers, occurs in cyathia of Cubanthus (P. Berry, University of Michigan, personal communication). In support of the floral identity of the female element in the cyathium, he considered that this type of movement is known from flowers but not from gynoecia (see also Eichler, 1878). However, this view is contradicted by similar movement of the gynophore in Arachis hypogaea (Leguminosae) and Atamisquea emarginata (Capparidaceae: Medan and Ponessa, 2003) and by movement of the style in Scrophularia (Scrophulariaceae) or Clerodendrum (Verbenaceae).
Within most Euphorbiinae, female flowers consist entirely of a syncarpous gynoecium, and a perianth is completely absent. Even in species that are normally regarded as possessing a female perianth, our results show that these lobed perianthlike structures are formed late in development when formation and differentiation of the gynoecium is almost complete (e.g., Monadenium rhizophorum, Fig. 3k–m). They could be interpreted as vestigial remnants of a perianth but (as in some male structures) could equally represent lobing at a domain boundary. Payer (1857), and Baillon (1858), arguing for the floral nature of the cyathium, interpreted protrusions at the base of the carpel as discs (“disque hypogyne”).
Somewhat similar late-developing structures are relatively common around the ovary base. They include the disc nectaries of Acanthaceae (Schönenberger and Endress, 1998), Lamiaceae (Petanidou et al., 2000), Leguminosae (Prenner, 2002), and other Euphorbiaceae (G. Prenner and P. J. Rudall, unpublished observations). Late-developing gynophores occur in both eudicots and monocots. For example, the “gynophore lobes” of the sedge Ficinia (Vrijdaghs et al., 2005) closely resemble the late-developing “perianthlike structures” that we have observed in Euphorbia ammak, E. teke, and Monadenium rhizophorum. The gynoecium in Sphaerosepalaceae (Malvales) is also frequently “sunken” into a gynophore (Horn, 2004). Within rosids, gynophores and androgynophores are concentrated in Brassicales, Malvales, Sapindales, and Fabales (Endress and Matthews, 2006), but there are scattered reports of gynophores in Malpighiales such as Podostemaceae (Ameka et al., 2002, 2003; Moline et al., 2006), Passifloraceae (Bernhard, 1999), and Ochnaceae (Dwyer, 1967), and androgynophores in Malesherbiaceae (Engler and Prantl, 1894; Gengler-Nowak, 2003) and Rafflesiaceae (Blarer et al., 2004).
With respect to their late developmental timing, and their location as pedicel outgrowths, the “perianthlike structures” that we observed around the ovary base resemble some epicalyces (e.g., in Dipsacales: Roels and Smets, 1996). Within Euphorbiaceae, an epicalyx also occurs in pistillate flowers of Croton sect. Decalobium (Webster, 1993). Epicalyces occur in a wide range of other angiosperms (e.g., Tofieldiaceae, Dirachmaceae, Rosaceae, Malvaceae), though not all are homologous (Ronse Decraene and Miller, 2004).
By contrast, female flowers of Neoguillauminia and Anthosteminae possess an early-formed perianth, which could be regarded as the primitive character state within Euphorbieae. In particular, formation of the female perianth in Anthostema madagascariense follows the phyllotaxis of initiation of male partial inflorescences. In cases of only four male elements (i.e., one element is reduced), the third female “sepal” is located in the male “whorl.” This could indicate that in A. madagascariense the female perianth is of bracteate origin and that in the ancestral inflorescence there were more than five male elements surrounding a central female flower. The position of the female perianth (in all species investigated in front of the carpels instead of alternating with them) could also be seen as an indication of bracteate origin, suggesting that male inflorescences were originally located in the axils of these bracts but were lost during evolution. Further evidence for a possible bracteate nature of the female perianth in Euphorbieae is the leaflike appearance of the female perianth in Neoguillauminia cleopatra (Fig. 5g–i). However, the earliest developmental stages, on which a more accurate interpretation could be based, were not available for this investigation.
Von Balthazar and Endress (2002) found a similar condition in Buxaceae, in which bractlike phyllomes precede the reproductive organs. They interpreted these structures as a “weakly differentiated perianth” and compared them with other extant early divergent and core eudicots. They regarded plasticity in perianth structure in this group as a primitive character indicating an “experimental phase” in floral evolution leading to a later canalization of a perianth with sepals and petals. The more derived phylogenetic placement of Euphorbiaceae, together with the presence of “true” sepals and petals in other Euphorbiaceae, suggest a different evolutionary pathway in this family. Phyllomes of bracteate origin in Anthostema (and possibly other early divergent Euphorbieae) could be seen as an additional innovation after complete loss of a true perianth. The occurrence of rather different perianthlike structures in some Euphorbiinae could be seen either as a further novel innovation or as vestigial remnants of the “perianth” in Anthosteminae and Neoguillauminiinae. Thus, analogous to von Balthazar and Endress’s (2002) interpretation in Buxaceae, we could define an “experimental phase” in floral evolution in Euphorbieae but at a rather different hierarchical level within angiosperms. It will be interesting to determine whether in this case “sleeping” developmental programs are switched on or new developmental pathways are initiated.
Regarding the timing of formation of perianthlike structures in Euphorbieae, in both male and female structures, we found a switch from centripetal perianth formation (i.e., the perianth is formed first, followed by stamen or gynoecium initiation) to centrifugal formation, with late-developing perianthlike structures (Fig. 9). This transition occurs in male flowers of Neoguillauminia and in female flowers of some taxa of Euphorbiinae. Analogous transitions from centripetal to centrifugal organ formation occur frequently throughout the flowering plants, including other Malpighiales (e.g., Flacourtiaceae s.l.: Bernhard and Endress, 1999) and more distantly related taxa (e.g., the monocot family Triuridaceae: Rudall and Bateman, 2006). Their broader significance will be the subject of further review.
Evolution of nectaries and their petaloid appendages
Hoppe and Uhlarz (1982) and Hoppe (1985) focused on interpretation of the unusual nectar glands of Euphorbiinae and their petaloid appendages, including an extensive literature review. Hoppe and Uhlarz (1982, p. 63) interpreted the glands on the rim of the involucre as interpetiolar stipules and the appendages as “dorsal effigurations of the leaf-ground.” However, our observations on early divergent Euphorbieae seem to indicate a somewhat different picture. In Anthostema, nectariferous tissue is formed along the margins of the bracts that subtend the male inflorescence (Fig. 8l), and in Neoguillauminia, the nectaries arise in pairs from the pseudanthium base, flanking the male inflorescences (Fig. 4a, d, e). These results do not necessarily support the stipulate nature of the nectaries in some species, especially because the homology of the different nectariferous structures in Euphorbieae is questionable. Similarly, the petaloid appendages in Euphorbieae may be nonhomologous; in Neoguillauminia, they are formed by the bracts of the male partial inflorescences, while in species of Euphorbiinae with petaloid cyathia, they are outgrowths of the nectary situated in between the male inflorescence bracts (see also Hoppe and Uhlarz, 1982; Hoppe, 1985). Species of Anthosteminae examined here do not have petaloid appendages associated with the pseudanthium. Further detailed ontogenetic studies are currently underway to clarify the nature of nectaries and petaloid structures in Euphorbieae.
In addition to these petaloid structures, which are integrated parts of the cyathium, other structures, such as cyathial bracteoles (e.g., in Euphorbia milii), cyathial bracts (e.g., in E. leucocephala), and even leaves (e.g., in E. pulcherrima) can become petaloid in Euphorbiinae.
Evolution of the cyathium
Venkata Rao and Ramalakshimi (1968) interpreted the single-staminate, naked male flowers of Euphorbiinae as derived by a reduction series from an ancestral androecium of 10 stamens (in two whorls of five), as in Jatropha and Manihot. However, this leaves many questions about the evolution of the rather complex cyathium unanswered (e.g., the origin of the male inflorescences and the evolution of the peculiar structure of the cyathium with a single central female flower surrounded by male partial inflorescences).
Gilbert (1994) speculated that the cyathium evolved from a synflorescence of axillary bisexual cymes (thyrse), which could be either dichasial or monochasial, or initially dichasial with monochasial ultimate braches (as frequently seen in species of Jatropha). Starting from this hypothetical synflorescence, the cyathium could have evolved by extreme condensation of the main inflorescence axis, reduction of the central cyme to a single female flower, reduction of female flower(s) in the surrounding whorl of cymes, and fusion of their associated subtending bracts and stipular glands to form the involucre. A related (but rather different) scenario, discussed by Gilbert (1994), is that the cyathium evolved from a single cyme with a terminal female flower and lateral male flowers (as also seen in different species of Jatropha). This hypothesis is based on Croizat’s (1937a, p. 406) idea that the tetramerous Dichostemma is “the key genus of the systematic complex at hand” and the assumption that four-lobed involucres represent the plesiomorphic condition for Euphorbieae.
Our results, combined with the results of molecular investigations, provide some support for both interpretations within different members of the tribe. Both strictly tetramerous cyathia (in Dichostemma) and cyathia with a strong tendency to pentamery (in Anthostema) occur in early divergent Euphorbieae (Fig. 9). The tetramerous pseudanthium of Dichostemma glaucescens would support Croizat’s (1937a) hypothesis. In Neoguillauminia cleopatra, the two enlarged lateral female perianth lobes could also be seen as evidence that this species evolved from a species ontogenetically similar to Dichostemma (with lateral elements formed first) and that the fifth male element is the result of a secondary gain. Coupled with the switch from a tetramerous gynoecium in Dichostemma to trimery in Neoguillauminia, this could also explain the somewhat irregular lobing of the female perianth in abaxial and adaxial positions (see Fig. 4d). However, in contrast to Gilbert’s (1994) interpretation of a cymose architecture in Dichostemma, our ontogenetic studies indicate that the pseudanthium in Dichostemma is also a determinate thyrse (as in Anthostema) with a terminal female flower and—in contrast to Anthostema—two pairs of male inflorescences in a decussate phyllotaxis (while Anthostema has four to five male partial inflorescences in a spiral phyllotaxis).
The starting point of a second possible scenario is Anthostema, in which the pseudanthium consists of four to five male elements initiated in a spiral and a central trimerous gynoecium (Fig. 9). The bauplan of a pentamerous pseudanthium of Anthostema is very similar to the cyathium of Euphorbiinae, except that the nectaries are an integral part of the involucre in Euphorbiinae, while they are found at the rim of male inflorescence prophylls in Anthostema (Figs. 2f, 8l, 9). Regarding the origin of the pseudanthium in Anthostema, we favor a modified version of Gilbert’s (1994) hypothesis: the most likely precursor is a thyrse terminated by a single female flower surrounded by cymose male partial inflorescences that are formed in a spiral around the main inflorescence axis.
It is noteworthy that the evolutionary pathways outlined are apparently accompanied by loss of lateral prophylls (which are present in Anthosteminae, in addition to bracteoles) and by a switch of the pseudanthial orientation (bracteoles transverse in Anthosteminae vs. lateral in Euphorbiinae) (Fig. 9). This indicates that the cyathium evolved not only by a “simple” reduction series, but also by major reorganizations of the entire bauplan of the pseudanthium.
To confirm that the cyathium is derived from an inflorescence, a comparative study of expression patterns of floral meristem identity genes such as LEAFY is currently underway (G. Prenner, unpublished observations). In Arabidopsis, LEAFY is expressed in floral primordia but not in the inflorescence meristem (Weigel et al., 1992; Sessions et al., 2000). Thus, if a similar pattern occurs in Euphorbiinae, we would expect LEAFY to be expressed in floral primordia but not in the cyathium primordium or the primordia of male elements.
A further (not necessarily exclusive) possibility is that partial loss of flower meristem identity occurred in ancestral Euphorbieae, resulting in structures combining features of a flower and an inflorescence, as in leafy mutants of Arabidopsis, in which lower inflorescence nodes form secondary short shoots rather than flowers and a bract primordium subtending an axillary apex is formed instead of a flower primordium (Ratcliffe et al., 1998). When the axillary apex is clear of the influence of the subtending bract, the inflorescence architecture gene TFL1, which maintains indeterminate growth, is suppressed, thereby terminating the shoot. Other floral identity genes (AP1 and CAL) become activated at upper nodes in the main inflorescence in these mutants, resulting in formation of carpellate flowers rather than shoots.
Baum and Donoghue (2002) speculated that in Arabidopsis LEAFY confers some floral properties (specifically, lack of internode elongation) upon the inflorescence meristem and tentatively suggested that this supports the notion that the identity of flowers and inflorescences can become mixed. Bomblies et al. (2003) also showed that in maize duplicate FLORICAULA/LEAFY homologs zfl1 and zfl2 control inflorescence architecture and flower patterning. In partial contrast, Buzgo et al. (2004), working on the early-divergent angiosperm Amborella, found gradual transitions between flower, inflorescence, and vegetative shoots, and concluded that “genes responsible for meristem identity may also regulate shoot development in a gradual mode.” Thus, there are strong indications for a “hybrid” flower/inflorescence nature of the cyathium (blurred boundaries), deducible not only from our ontogenetic studies, but also from the existing theoretical genetic background as found in other taxa.
Troll (1928) was convinced about the inflorescence nature of the cyathium but nevertheless emphasized that it is controlled by the same “Gestalt” as a radial flower. This could be viewed as an early hybrid flower/inflorescence interpretation. Rutishauser et al. (in press) discussed a broad range of different plant structures that appear not to follow the “classical model” or “discontinuum model” of discrete units on several hierarchical levels. These structures show fuzzy or blurred boundaries and intermediates that are difficult to categorize in a classical model, which apparently cause “identity crises” at different hierarchical levels. Different structures within the cyathium could represent further examples of such identity crises.
Terminal flowerlike structures, often with obscure organ identity, are widespread in angiosperms, especially in species with indeterminate inflorescences such as spikes, racemes, or spadices (Sokoloff et al., 2006). In some cases the entire terminal pseudanthium is very similar to a true flower. Furthermore, pseudanthia are frequently associated with morphological novelties of obscure identity, such as the unusual filamentous structures that we have observed at the bases of male flowers (Fig. 2k, l), though these could be interpreted as prophylls. Unusual filamentous structures are of interest because they sometimes occur in place of flowers, both in other angiosperms (e.g., alismatid monocots: Sokoloff et al., 2006) and in some artificially induced Arabidopsis mutants. For example, filamentous structures of obscure identity occur in place of flowers in ufo mutant phenotypes of Arabidopsis (Levin and Meyerowitz, 1995). Sawa et al. (1999) interpreted filamentous “type B structures” in Arabidopsis fil mutants as immature flowers that form a peduncle but lack a receptacle and floral organs. Analogous to this, we speculate that filamentous structures in the cyathium of Euphorbiinae could also be immature or highly reduced male flowers that represent remnants of the original dichasial male inflorescence. This would explain the variable number of filamentous structures and the fact that they are not associated with particular male flowers.
Finally, we should briefly discuss the somewhat unorthodox hypothesis that the cyathium is a quite extraordinary hermaphrodite flower. This interpretation had been occasionally revived in different forms. For example, Lodkina (1986) interpreted the cyathium as a flower. She noted strong similarities between stamen and ovule development and analogized the entire cyathium to an ovary. Each of Eichler’s (1878) arguments for the pseudanthial nature of the cyathium can be partially contradicted, and it is probably only the unique mixture of different characters that strongly favors a pseudanthial interpretation of the cyathium. Even the “fact” that flowers are largely unisexual in Euphorbiaceae was contradicted by Michaelis (1924), who observed that hermaphrodite flowers are not rare (“absolut nicht selten”) in Euphorbiaceae and who listed 15 genera for which hermaphrodite flowers have been reported (see also Penzig, 1921). For example, in Ricinus communis, hermaphrodite flowers frequently occur at the junction of male and female flowers (Michaelis, 1924; Shifriss, 1956; George and Shifriss, 1967; P. J. Rudall and G. Prenner, unpublished observations). In Euphorbieae, a genetic stabilization of these “transitional” flowers could have been the starting point for hermaphrodite flowers.
Conclusions
Our investigation emphasizes the indistinct boundaries between the inflorescence, flower, and floral organs in Euphorbieae, which could result from overlap between expression zones of regulatory genes. Thus, neither inflorescence nor flower is entirely satisfactory for defining the cyathium; both interpretations leave open issues such as the absence of distinct floral/organ primordia and the apparently hybrid nature of many structures. As with other strongly flowerlike inflorescences, notably those of Saururaceae and other perianthless Piperales (Remizowa et al., 2005), more than one possible scenario exists for the evolution of the Euphorbia cyathium (Fig. 9). It could be (1) derived from an Anthostema-like strongly condensed thyrse, terminated by a trimerous female flower, and spirally surrounded by dichasial male partial inflorescences; (2) derived from a similar but Dichostemma-like thyrse with a terminal tetramerous female flower and male partial inflorescences in decussate phyllotaxis; (3) a “hybrid” structure resulting from partial loss of flower meristem identity, and hence combining features of a flower and an inflorescence; or least plausibly (4) a true flower with a unique combination of features.
Improved understanding of the broader comparative morphological and phylogenetic context is necessary to evaluate these hypotheses. Our knowledge of the highly diverse flowers and inflorescences in Euphorbiaceae remains surprisingly rudimentary, and the only comprehensive surveys date back to the 19th and early 20th centuries (e.g., Baillon, 1858; Michaelis, 1924). We have yet to examine material of the remaining genus of Euphorbieae (subtribe Neoguillauminiinae), Calycopeplus, an Australian genus with a pseudanthium consisting of four clusters of male (partial?) inflorescences surrounding a central trimerous female flower enclosed by a four- to six-lobed perianth. Male flowers are naked and have a constriction as in other Euphorbieae. Nectar glands are small or absent (Klotzsch, 1844; Planchon, 1861; Boissier, 1866; Bentham, 1873; Pax and Hoffmann, 1931; Croizat, 1937a; Radcliffe-Smith, 2001). Additional ongoing work on developmental genetics of the flower and inflorescence formation in Euphorbieae will enable us to improve our understanding not only of the evolution of the cyathium, but also of the evolution of flowers and inflorescences within Euphorbiaceae.
Appendix 1
Voucher information for taxa used in this study. Unless otherwise stated, material investigated was either collected from a wild source; cultivated at the Royal Botanic Gardens, Kew, UK (indicated as HK plus accession number) or the Graz Botanic Garden, Austria (BGG); or was obtained from the Herbarium spirit collection at the Royal Botanic Gardens, Kew (K plus accession number) and Wageningen, Netherlands (WAG). (An asterisk indicates that species is illustrated with SEM micrographs.)
Family–Tribe–Subtribe
Species—Source; Voucher.
Euphorbiaceae–Euphorbieae–Anthosteminae
Anthostema madagascariense Baill.*—La Convalescence, Mayotte; Barthelat FB 1632. Dichostemma glaucescens Pierre*—WAG 117. Gabon, Ogooue-Maritime; WAG 328. WAG 3531. Cameroon, South Province; WAG 5482. WAG 6166.
Euphorbiaceae–Euphorbieae–Euphorbiinae
Chamaesyce maculata Small—Birge Hall, Botany Greenhouses, UW-Madison, USA; Prenner 756. Euphorbia ammak Schweinf.*—HK 1977–1245; Prenner 757. E. amygdaloides L.—Mixnitz, Austria; Prenner 698, 699. E. barrelieri Savi—HK 1996–1103; Prenner 758. E. delphinensis Ursch & Leandri—HK 1981–4757; Prenner 759. E. dendroides L.—BGG s.n.; Prenner 625. E. donii Oudejans—HK 1990–2788; Prenner 760. E. globosa Sims*—HK 1960–31437; Prenner 761. E. lathyris L.—BGG s.n.; Prenner 647. E. leucodendron Drake—HK 1998–193; Prenner 762. E. myrsinites L.*—HK 1940–16401; Prenner 763. E. obesa Hook.f.*—HK 1981–5643; Prenner 764. E. peplus L.*—BGG s.n.; Prenner 615. E. petraea S. Carter*—HK 1978–2262; Prenner 765. E. pteroneura A. Berger*—HK 1965–24820; Prenner 766. E. splendens Boj. ex Hook.—BGG s.n., Prenner 624. E. stapfii A. Berger—HK 1977–3565; Prenner 767. E. stygiana H. C. Watson—HK 1996–1105; Prenner 768. E. teke Schweinf. ex Pax—Nkose Island, Uganda; K 5226. E. tirucalli L.—BGG s.n., Prenner 640. E. tubiglans Marloth ex R. A. Dyer—HK 1997–6448; Prenner 769. Monadenium coccineum Pax—HK 1954–54107; Prenner 770. M. elegans S. Carter—HK 1990–1801; Prenner 771. M. laeve Stapf—HK 1970–3230; Prenner 772. M. rhizophorum P. R. O. Bally*—HK 1960–28203; Prenner 773. M. torrei L. C. Leach—HK 1977–200; Prenner 774. Pedilanthus cymbiferus Schltdl.*—HK 1964–40104; Prenner 775. P. tithymaloides Poit.*—HK 1984–2163; Prenner 776. Synadenium glaucescens Pax—HK 1979–6244; Prenner 777. S. grantii Hook f.—BGG s.n., HK 1978–2265; Prenner 616, 708, 778.
Euphorbiaceae–Euphorbieae–Neoguillauminiinae
Neoguillauminia cleopatra (Baill.) Croizat*—New Caledonia; Tokuoka NC010, NC028.
Other Euphorbiaceae
Acalypha siamensis Oliv. ex Gage—HK 1973–12219; Prenner 779. Dalechampia magnistipulata G. L. Webster & W. S. Armbruster—HK 2004–2623; Prenner 780. Jatropha lobata Müll. Arg.—Ethiopia, N. end of Dissei Island; K 7630. J. parvifolia Chiov.—Kenya, Voi district, Sabaki; K 31530. J. spicata Pax—Kenya, Voi district, Tsavo National Park; K 31526. Mabea cf. nitida Spruce ex Benth.—Bolivia, Pando; K 53788. Mercurialis annua L.—HK s.n.; Prenner 781. Ricinus communis L.—HK s.n.; Prenner 782.
Footnotes
The authors thank D. Baum, P. Berry, N. I. Cacho, P. Endress, M. Gilbert, M. Remizova, R. Rutishauser, D. Sokoloff, and A. Weber for useful discussion, and V. Steinmann for help with identification of plant material. T. Tokuoka and H. Tobe (Kyoto University, Japan), F. Barthelat (Direction de l’agriculture et de la forêt de Mayotte), and J. Wieringa (Nationaal Herbarium Nederland, Wageningen University branch) kindly provided material. M. Cheek and P. Hoffmann supplied helpful logistic support in Cameroon and the Kew Herbarium. G.P. acknowledges funding from the Austrian Science Fund (FWF) (project J2504) for the main project and from Synthesys (GB-TAF-685) for a short visit to RBG Kew during the initial phase.
LITERATURE CITED
- Ameka GK, Clerk GC, Pfeifer E, Rutishauser R. Developmental morphology of Ledermanniella bowlingii (Podostemaceae) from Ghana. Plant Systematics and Evolution. 2002;237:165–183. [Google Scholar]
- Ameka GK, Pfeifer E, Rutishauser R. Developmental morphology of Saxicolella amicorum and S. submersum (Podostemaceae: Podostemoideae) from Ghana. Botanical Journal of the Linnean Society. 2003;139:255–273. [Google Scholar]
- Angiosperm Phylogeny Group An ordinal classification for the families of flowering plants. Annals of the Missouri Botanical Garden. 1998;18:531–553. [Google Scholar]
- Angiosperm Phylogeny Group An update of the Angiosperm phylogeny group classification for the orders and families of flowering plants: APG II. Botanical Journal of the Linnean Society. 2003;141:399–436. [Google Scholar]
- Baillon H. Étude générale du groupe des Euphorbiacées. V. Masson; Paris, France: 1858. [Google Scholar]
- Baum DA, Donoghue MJ. Transference of function, heterotopy and the evolution of plant development. In: Cronk QCB, Bateman RM, Hawkins JA, editors. Developmental genetics and plant evolution. Taylor & Francis; London, UK: 2002. pp. 52–69. [Google Scholar]
- Bentham G. Flora australiensis: a description of the plants of the Australian territory. Vol. 6. Thymeleae to Dioscorideae. Reeve & Co.; London, UK: 1873. [Google Scholar]
- Bentham G. Notes on Euphorbiaceae. Journal of the Linnean Society. 1878;17:185–267. [Google Scholar]
- Bernhard A. Flower structure, development, and systematics in Passifloraceae and in Abatia (Flacourtiaceae) International Journal of Plant Sciences. 1999;160:135–150. [Google Scholar]
- Bernhard A, Endress PK. Androecial development and systematics in Flacourtiaceae s. l. Plant Systematics and Evolution. 1999;215:141–155. [Google Scholar]
- Blarer A, Nickrent DL, Endress PK. Comparative floral structure and systematics in Apodanthaceae (Rafflesiales) Plant Systematics and Evolution. 2004;245:119–142. [Google Scholar]
- Blume CL. Bijdragen tot de flora van Nederlandsch Indie, part 12. Lands Drukkerij; Batavia, Netherlands: 1825. [Google Scholar]
- Boissier E. Euphorbieae. In: de Candolle A, editor. Prodromus systematis naturalis regni vegetabilis, part. 2. Vol. 15. V. Masson; Paris, France: 1866. pp. 1–189. [Google Scholar]
- Bomblies K, Wang R-L, Ambrose BA, Schmidt RJ, Meeley RB, Doebley J. Duplicate FLORICAULA/LEAFY homologs zfl1 and zfl2 control inflorescence architecture and flower patterning in maize. Development. 2003;130:2385–2395. doi: 10.1242/dev.00457. [DOI] [PubMed] [Google Scholar]
- Brown R. General remarks, geographical and systematical, on the botany of Terra Australis. In: Flinders M, editor. A voyage to Terra Australis; undertaken for the purpose of completing the discovery of that vast country. II. G. and W. Nicol; London, UK: 1814. pp. 533–613. Appendix no. III. [Google Scholar]
- Bruyns PV, Mapaya RJ, Hedderson T. A new subgeneric classification for Euphorbia (Euphorbiaceae) in southern Africa based on ITS and psbA-trnH sequence data. Taxon. 2006;55:397–420. [Google Scholar]
- Buzgo M, Soltis SS, Soltis DE. Floral developmental morphology of Amborella trichopoda (Amborellaceae) International Journal of Plant Sciences. 2004;165:925–947. [Google Scholar]
- Caris PL, Smets EF. A floral ontogenetic study on the sister group relationship between the genus Samolus (Primulaceae) and the Theophrastaceae. American Journal of Botany. 2004;91:627–643. doi: 10.3732/ajb.91.5.627. [DOI] [PubMed] [Google Scholar]
- Classen-Bockhoff R. Pattern analysis in pseudanthia. Plant Systematics and Evolution. 1990;171:57–88. [Google Scholar]
- Classen-Bockhoff R. Anthodien, Pseudanthien und Infloeszenzblumen. Beiträge zur Biologie der Pflanzen. 1991;66:221–240. [Google Scholar]
- Croizat L. On the classification of Euphorbia. I. How important is the cyathium? Bulletin of the Torrey Botanical Club. 1936;63:525–531. [Google Scholar]
- Croizat L. Notes on Euphorbiaceae, with a new genus and a new subtribe of the Euphorbieae. Philippine Journal of Science. 1937a;64:397–412. [Google Scholar]
- Croizat L. On the classification of Euphorbia. II. How should the cyathium be interpreted? Bulletin of the Torrey Botanical Club. 1937b;64:523–536. [Google Scholar]
- Dwyer JD. Flora of Panama, part VI, family 119, Ochnaceae. Annals of the Missouri Botanical Garden. 1967;54:25–40. [Google Scholar]
- Eichler AW. Blüthendiagramme construiert und erläutert. W. Engelmann; Leipzig, Germany: 1878. [Google Scholar]
- Endress PK. Symmetry in flowers: diversity and evolution. International Journal of Plant Sciences. 1999;160(6 Supplement):S3–S23. doi: 10.1086/314211. [DOI] [PubMed] [Google Scholar]
- Endress PK, Lorence DH. Heterodichogamy of a novel type in Hernandia (Hernandiaceae) and its structural basis. International Journal of Plant Sciences. 2004;165:753–763. [Google Scholar]
- Endress PK, Matthews ML. First steps towards a floral structural characterization of the major rosid subclades. Plant Systematics and Evolution. 2006;260:223–251. [Google Scholar]
- Engler A, Prantl K. Die natürlichen Pflanzenfamilien. Abteilung. W. Engelmann; Leipzig, Germany: 1894. III. Teil, 6. [Google Scholar]
- Franceschinelli EV, Thomas WW. Simaba guianensis subsp. huberti, a new Venezuelan taxon of Simaroubaceae. Brittonia. 2000;52:311–314. [Google Scholar]
- Gengler-Nowak KM. Molecular phylogeny and taxonomy of Malesherbiaceae. Systematic Botany. 2003;28:333–344. [Google Scholar]
- George WL, Shifriss O. Interspersed sexuality in Ricinus. Genetics. 1967;57:347–356. doi: 10.1093/genetics/57.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert MG. The relationships of the Euphorbieae (Euphorbiaceae) Annals of the Missouri Botanical Garden. 1994;81:283–288. [Google Scholar]
- Haber JM. The anatomy and morphology of the flower of Euphorbia. Annals of Botany. 1925;39:657–705. [Google Scholar]
- Haevermans T, Hoffmann P, Lowry PP, II, Labat J-N, Randrianjohany E. Phylogenetic analysis of the Madagascan Euphorbia subgenus Lacanthis based on ITS sequence data. Annals of the Missouri Botanical Garden. 2004;91:247–259. [Google Scholar]
- Han-Xing L, Tucker SC. Floral ontogeny of Zippelia begoniaefolia and its familial affinity: Saururaceae or Piperaceae? American Journal of Botany. 1995;82:681–689. [Google Scholar]
- Hoppe J. Die Morphogenese der Cyathiendrüsen und ihrer Anhänge, ihre blattypologische Deutung und Bedeutung. Botanische Jahrbücher für Systematik. 1985;105:497–581. [Google Scholar]
- Hoppe J, Uhlarz H. Morphogenese und typologische Interpretation des Cyathiums von Euphorbia-Arten. Beiträge zur Biologie der Pflanzen. 1982;56:63–98. [Google Scholar]
- Horn JW. The morphology and relationships of the Sphaerosepalaceae (Malvales) Botanical Journal of the Linnean Society. 2004;144:1–40. [Google Scholar]
- Hufford L. Homology and developmental transformation: models for the origins of the staminodes of Loasaceae subfamily Loasoideae. International Journal of Plant Sciences. 2003;164(5 Supplement):409–439. [Google Scholar]
- Jussieu A. Genera plantarum. Herissant and Barrois; Paris, France: 1789. [Google Scholar]
- Jussieu A. De Euphorbiacearum generibus medicisque earumdem viribus. Didot Junioris; Paris, France: 1824. [Google Scholar]
- Klotzsch JF. Euphorbiaceae Juss. In: Lehmann C, editor. Plantae Preissianae. Vol. 1. Meissner; Hamburg, Germany: 1844. pp. 174–180. [Google Scholar]
- Kong H-Z, Lu A-M, Endress PK. Floral organogenesis of Chloranthus sessilifolius, with special emphasis on the morphological nature of the androecium of Chloranthus (Chloranthaceae) Plant Systematics and Evolution. 2002;232:181–188. [Google Scholar]
- Lamarck J-B. Encyclopédie méthodique. II. Botanique; Paris and Plomteux, Liege, France: 1786. Euphorbe ou Tithymale, Euphorbia, Caractère Générique. [Google Scholar]
- Levin JZ, Meyerowitz EM. UFO: an Arabidopsis gene involved in both floral meristem and floral organ development. Plant Cell. 1995;7:529–548. doi: 10.1105/tpc.7.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnaeus C. Species plantarum. L. Salvius; Stockholm, Sweden: 1753. [Google Scholar]
- Lodkina MM. Mode of development of the cyathium of Euphorbiaceae: evidence of its floral nature [translated from Russian] In: Tikhomirov VN, editor. Sources of information in phylogenetic plant systematics. Moscow University Press; Nauka, Moscow, Russia: 1986. pp. 43–44. [Google Scholar]
- Mahlberg PG. Evolution of the laticifer in Euphorbia as interpreted from starch grain morphology. American Journal of Botany. 1975;62:577–583. [Google Scholar]
- Medan D, Ponessa G. Movement-assisted dichogamy in Atamisquea emarginata (Capparaceae) Plant Systematics and Evolution. 2003;236:195–205. [Google Scholar]
- Michaelis P. Blütenmorphologische Untersuchungen an den Euphorbiaceen unter besonderer Berücksichtigung der Phylogenie der Angiospermenblüte. Botanische Abhandlungen. 1924;3:1–150. [Google Scholar]
- Moline PM, Les D, Philbrick CT, Novelo RA, Pfeifer E, Rutishauser R. Comparative morphology and molecular systematics of Podostemum (including Crenias)—American river weeds (Podostemaceae) Botanische Jahrbücher für Systematik. 2006;126:427–476. [Google Scholar]
- Müller Argoviensis J. Bestätigung der R. Brown’schen Ansicht über das Cyathium der Euphorbien. Flora. 1872;55:65–71. [Google Scholar]
- Müller Argoviensis J. Euphorbiaceae. In: de Martius CFP, Eichler AG, editors. Flora Brasiliensis, enumeratio plantarum in Brasilia. Vol. 11. F. Fleischer; Leipzig, Germany: 1874. pp. 293–751. part 2. [Google Scholar]
- Park K-R. Phylogeny of New World subtribe Euphorbiinae (Euphorbiaceae) Korean Journal of Plant Taxonomy. 1996;26:235–256. [Google Scholar]
- Park K-R, Backlund A. Origin of the cyathium-bearing Euphorbieae (Euphorbiaceae): phylogenetic study based on morphological characters. Botanical Bulletin of Academia Sinica. 2002;43:57–62. [Google Scholar]
- Park K-R, Elisens WJ. A phylogenetic study of tribe Euphorbieae (Euphorbiaceae) International Journal of Plant Sciences. 2000;161:425–434. doi: 10.1086/314262. [DOI] [PubMed] [Google Scholar]
- Pax F, Hoffmann K. Euphorbiaceae. In: Engler A, Prantl K, editors. Die natürlichen Pflanzenfamilien nebst ihren Gatttungen und wichtigeren Arten, insbesondere den Nutzpflanzen. W. Engelmann; Leipzig, Germany: 1931. pp. 11–251. [Google Scholar]
- Payer JB. Traité d’organogénie camparée de la fleur. V. Masson; Paris, France: 1857. [Google Scholar]
- Penzig AJO. Pflanzenteratologie systematisch geordnet. Gebrüder Bornträger; Berlin, Germany: 1921. [Google Scholar]
- Petanidou T, Goethals V, Smets E. Nectary structure of Labiatae in relation to their nectar secretion and characteristics in a Mediterranean shrub community—does flowering time matter? Plant Systematics and Evolution. 2000;225:103–118. [Google Scholar]
- Planchon MJ-E. La vraie nature de la fleur des euphorbes expliquée par un nouveau genre d’euphorbiacées. Bulletin de la Société Botanique de France. 1861;8:29–33. [Google Scholar]
- Prakash N, Brown JF, Yue-Hua W. An embryological study of kava, Piper methysticum. Australian Journal of Botany. 1994;42:231–237. [Google Scholar]
- Prenner G. Floral development in Fabaceae: new aspects in selected genera. Institute of Plant Sciences, University Graz, Austria; 2002. Ph.D. thesis. [Google Scholar]
- Radcliffe-Smith A. Genera Euphorbiacearum. Royal Botanic Gardens; Kew, UK: 2001. [Google Scholar]
- Ratcliffe OJ, Amaya I, Vincent CA, Rothstein S, Carpenter R, Coen ES, Bradley DJ. A common mechanism controls the life cycle and architecture of plants. Development. 1998;125:1609–1615. doi: 10.1242/dev.125.9.1609. [DOI] [PubMed] [Google Scholar]
- Remizowa M, Rudall PJ, Sokoloff D. Evolutionary transitions among flowers of perianthless Piperales: inferences from inflorescence and flower development in the anomalous species Peperomia fraseri (Piperaceae) International Journal of Plant Sciences. 2005;166:925–943. [Google Scholar]
- Roels P, Smets E. A floral ontogenetic study in the Dipsacales. International Journal of Plant Sciences. 1996;157:203–218. [Google Scholar]
- Ronse Decraene LP, Miller AG. Floral development and anatomy of Dirachma socotrana (Dirachmaceae): a controversial member of the Rosales. Plant Systematics and Evolution. 2004;249:111–127. [Google Scholar]
- Rudall PJ. Flower anatomy of the subtribe Hyptidinae (Labiatae) Botanical Journal of the Linnean Society. 1981;83:251–262. [Google Scholar]
- Rudall PJ. Laticifers in Euphorbiaceae—a conspectus. Botanical Journal of the Linnean Society. 1987;94:143–163. [Google Scholar]
- Rudall PJ. Monocot pseudanthia revisited: floral anatomy and systematics of the mycoheterotrophic family Triuridaceae. International Journal of Plant Sciences. 2003;164(Supplement 5):S307–S320. [Google Scholar]
- Rudall PJ, Bateman RM. Morphological phylogenetic analysis of Pandanales: testing contrasting hypotheses of floral evolution. Systematic Botany. 2006;31:223–238. [Google Scholar]
- Rudall PJ, Bateman RM, Fay MF, Eastman A. Floral anatomy and systematics of Alliaceae with particular reference to Gilliesia, a presumed insect mimic with strongly zygomorphic flowers. American Journal of Botany. 2002;89:1867–1883. doi: 10.3732/ajb.89.12.1867. [DOI] [PubMed] [Google Scholar]
- Rudall PJ, Sokoloff DD, Remizowa M, Conran JG, Davis JI, Macfarlane TD, Stevenson DW. Morphology of Hydatellaceae, an anomalous aquatic family recently recognized as an early-divergent angiosperm lineage. American Journal of Botany. 2007;94:1073–1093. doi: 10.3732/ajb.94.7.1073. [DOI] [PubMed] [Google Scholar]
- Rutishauser R, Grob V, Pfeifer E. Plants are used to having identity crises. In: Minelli A, Fusco G, editors. Evolutionary developmental biology. Cambridge University Press; Cambridge, UK: In press. [Google Scholar]
- Sawa S, Ito T, Shimura Y, Okada K. FILAMENTOUS FLOWER controls the formation and development of Arabidopsis inflorescences and floral meristems. Plant Cell. 1999;11:69–86. doi: 10.1105/tpc.11.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönenberger J, Endress PK. Structure and development of the flowers in Mendoncia, Pseudocalyx, and Thunbergia (Acanthaceae) and their systematic implications. International Journal of Plant Sciences. 1998;159:446–465. [Google Scholar]
- Sessions A, Yanofsky MF, Weigel D. Cell–cell signaling and movement by the floral transcription factors LEAFY and APETALA1. Science. 2000;289:779–781. doi: 10.1126/science.289.5480.779. [DOI] [PubMed] [Google Scholar]
- Shifriss O. Sex instability in Ricinus. Genetics. 1956;41:265–280. doi: 10.1093/genetics/41.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff DD, Rudall PJ, Remizowa M. Flower-like terminal structures in racemose inflorescences: a tool in morphogenetic and evolutionary research. Journal of Experimental Botany. 2006;57:3517–3530. doi: 10.1093/jxb/erl126. [DOI] [PubMed] [Google Scholar]
- Soltis DE, Gitzendanner MA, Soltis PS. A 567-taxon data set for angiosperms: the challenges posed by Bayesian analyses of large data sets. International Journal of Plant Sciences. 2007;168:137–157. [Google Scholar]
- Steinmann VW. The submersion of Pedilanthus into Euphorbia (Euphorbiaceae) Acta Botanica Mexicana. 2003;65:45–50. [Google Scholar]
- Steinmann VW, Porter JM. Phylogenetic relationships in Euphorbieae (Euphorbiaceae) based on ITS and ndhF sequence data. Annals of the Missouri Botanical Garden. 2002;89:453–490. [Google Scholar]
- Strange A, Rudall PJ, Prychid CJ. Comparative floral anatomy of Pontederiaceae. Botanical Journal of the Linnean Society. 2004;144:395–408. [Google Scholar]
- Tokuoka T, Tobe H. Phylogenetic analyses of Malpighiales using plastid and nuclear DNA sequences, with particular reference to the embryology of Euphorbiaceae sens. str. Journal of Plant Research. 2006;119:599–616. doi: 10.1007/s10265-006-0025-4. [DOI] [PubMed] [Google Scholar]
- Troll W. Organisation und Gestalt im Bereich der Blüte. J. Springer; Berlin, Germany: 1928. [Google Scholar]
- Venkata Rao C. Anatomy of the inflorescence of some Euphorbiaceae with a discussion on the phylogeny and evolution of the inflorescence including the cyathium. Botanical Notiser. 1971;124:39–64. [Google Scholar]
- Venkata Rao C, Ramalakshmi T. Floral anatomy of Euphorbiaceae. I. Some non-cyathium taxa. Journal of the Indian Botanical Society. 1968;47:278–300. [Google Scholar]
- Von Balthazar M, Endress PK. Development of inflorescences and flowers in Buxaceae and the problem of perianth interpretation. International Journal of Plant Sciences. 2002;163:847–876. [Google Scholar]
- Vrijdaghs A, Goetghebeur P, Muasya AM, Caris P, Smets E. Floral ontogeny in Ficinia and Isolepis (Cyperaceae), with focus on the nature and origin of the gynophore. Annals of Botany. 2005;96:1247–1264. doi: 10.1093/aob/mci276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warming E. Über die Entwicklung des Blütenstandes von Euphorbia. Flora. 1870;53:385–397. [Google Scholar]
- Weberling F. Morphologie der Blüten und der Blütenstände. Eugen Ulmer; Stuttgart, Germany: 1981. [Google Scholar]
- Webster GL. A provisional synopsis of the sections of the genus Croton (Euphorbiaceae) Taxon. 1993;42:793–823. [Google Scholar]
- Webster GL. Classification of the Euphorbiaceae. Annals of the Missouri Botanical Garden. 1994;81:3–32. [Google Scholar]
- Weigel D, Alvarez J, Smyth DR, Yanofsky MF, Meyerowitz EM. LEAFY controls floral meristem identity in Arabidopsis. Cell. 1992;69:843–859. doi: 10.1016/0092-8674(92)90295-n. [DOI] [PubMed] [Google Scholar]
- Wurdack KJ, Hoffmann P, Chase MW. Molecular phylogenetic analysis of uniovulate Euphorbiaceae (Euphorbiaceae sensu stricto) using plastid rbcL and trnL-F DNA sequences. American Journal of Botany. 2005;92:1397–1420. doi: 10.3732/ajb.92.8.1397. [DOI] [PubMed] [Google Scholar]
- Wurdack KJ, Hoffmann P, Samuel R, De Bruijn A, Van Der Bank M, Chase MW. Molecular phylogenetic analysis of Phyllanthaceae (Phyllanthoideae pro parte, Euphorbiaceae sensu lato) using plastid rbcL DNA sequences. American Journal of Botany. 2004;91:1882–1900. doi: 10.3732/ajb.91.11.1882. [DOI] [PubMed] [Google Scholar]
- Wydler H. Morphologische Beiträge. Flora. 1845;28:449–456. [Google Scholar]