Abstract
Protein kinase C (PKC) isoenzymes are expressed and activated in a cell type-specific manner, and play an essential role in tissue-specific signal transduction. The presence of butyrate at millimolar concentrations in the colon raises the question of whether it affects the expression of PKC isoenzymes in the different cell types of the colonic epithelium. We investigated the protein expression levels of PKCγ, Thr514-phosphorylated PKCγ (pPKCγ-Thr514), and their subcellular distribution as affected by butyrate in a set of colon cancer cell lines. Thr514-phosphorylation of de novo synthesized PKCγ is the first step in priming of the inactive PKCγ before its release into the cytoplasm. For immunoblot analysis, we employed three antibodies, one against an unmodified sequence, mapping within 50 amino acids at its C-terminus, a second against pPKCγ-Thr514, and a third against pPKCγ-pan-Thr514. The antibody against an unmodified C-terminal peptide epitope did not recognize pPKCγ-Thr514, suggesting that phosphorylation at this site interferes with the binding of the antibody to the C-terminus. Marked butyrate-induced upregulation of PKCγ occurred in HT29 cells (colonocyte stem cells) and HT29-derived cell lines. However, in Caco2 and IEC-18 cells (differentiated intestinal epithelial cells), PKCγ was insensitive to upregulation, and present exclusively as pPKCγ-Thr514. Lovo and SW480 expressed higher levels of PKCγ. In HT29 cells, butyrate-induced upregulation of the non-phosphorylated PKCγ was observed in both the membrane and cytosolic fraction. In Caco2 cells, the Thr514-phosphorylated form was present at high levels in both fractions. The presence of unphosphorylated PKCγ in HT29 cells, and its complete absence in Caco2 cells demonstrates a cell type-dependent differential coupling of Thr514-phosphorylation with de novo synthesis of PKCγ in colon cancer cells.
Keywords: PKCγ, Thr514-phosphorylation, Butyrate, Caco2, HT29
1. Introduction
During their short life cycle of 3-4 days, colonic epithelial cells respond to a vast array of signals. On the apical side, the colonic epithelium is exposed to millimolar concentrations of the short-chain fatty acid butyrate. Numerous cell culture experiments have shown that butyrate, a histone deacetylase inhibitor, has manifold effects on normal as well as tumour cells, such as modulation of gene expression, induction of differentiation, arrest of the cell cycle and induction of cell death [reviewed in 1-4]. In the in vivo situation, a transformed clone has to expand, in spite of the presence of butyrate, so that the question arises as to how the signal transduction pathways of cells, and tumour cells arising from them, in the different layers of the colonic epithelium are adapted to the presence of butyrate.
Protein kinase C (PKC) isoenzymes are recognized as important regulators of homeostasis in the intestine [for reviews see 5-7]. PKC is a family of 12 serine/threonine kinases termed conventional or classical (α, β, and γ), novel (δ, ε, η and θ), atypical (ζ and ι/λ) and PKN and PKC-related (PKN1, PKN2 and PKN3) [8] that differ in cofactor requirements, tissue distribution, and substrate specificity, and are implicated in manifold cellular processes including proliferation, differentiation and cell death. Conventional PKCs are activated in response to increased concentrations of intracellular Ca2+ and diacylglycerol [for review see 9]. Novel PKCs are Ca2+-independent but diacylglycerol-dependent, and atypical PKCs are both, Ca2+- and diacylglycerol-independent. PKN kinases have a catalytic domain highly homologous to PKC family, and can be activated by Rho or arachidonic acid [10]. There are cellular systems in which PKCγ was activated by oxidative stress, without requiring elevated levels of diacylglycerol [11].
Although the exact mechanism and role of phosphorylation in PKC priming is not yet fully understood, it was suggested that a de novo synthesized conventional PKC first binds to a membrane, enabling a conformation that permits phosphoinositide-dependent protein kinase 1 (PDK-1) [12] to bind and phosphorylate a site in the activation-loop, which is Thr514 for PKCγ [13,14]. Phosphorylation at this site leads to a conformational change enabling phosphorylations at two carboxyl-terminal sites namely, the turn motif and the hydrophobic motif, as a result of which the fully phosphorylated conventional PKC is released from the membrane, and positioned in its primed, inactive form in the cytoplasm [15-17]. Binding of Ca2+ induces a low-affinity interaction with the membrane, where binding of the membrane-embedded cofactor diacyglycerol to the PKC results in high-affinity interaction of the PKC with the membrane. The energy of this interaction is used to release the pseudosubstrate from the substrate–binding cavity. In this open conformation, the mature PKC is present in its activated form and ready to bind substrates.
PKCs isoenzymes were suggested to play an important role at various stages of carcinogenesis [18-21]. Expression levels of PKC isoenzymes were found to be altered in colon cancer cell lines and carcinomas compared to normal intestinal epithelial tissue. In normal colonic epithelial tissue, PKC expression levels differ along the crypt. It can be expected that colon cancer cells at various stages of differentiation and malignancy differ considerably in their use and tuning of signal transduction pathways. The aim of our study was to examine the effect of butyrate on the expression levels of PKC isoenzymes in colon cancer cell lines representing different degrees of differentiation. For this reason, we selected for our study HT29 and HT29-derived cell lines (HT29-12, HT29-21, HT29cl.19a and HT29R) as a model related to colonic stem cells, Caco-2 and non-transformed rat intestinal epithelial IEC-18 cells as model for the differentiated phenotype, HCT116 cells for the colon cancer cell type with a low degree of differentiation, and Lovo, SW480 and DLD-1 cells representing dedifferentiated, highly advanced colon cancer cells. Since normal colonocytes as well as colon carcinoma cells have to grow in an environment exposed to millimolar concentrations of butyrate, and the butyrate concentration is determined by diet and bacterial flora of the colon, we were interested to examine whether butyrate modulates the expression levels of PKC isoenzymes, and thus influences an essential signal transduction system in these cells. The effects of the histone deacetylase inhibitor butyrate should also help understand the impact of histone deacetylase inhibitors generally on the expression of PKCs.
2. Materials and methods
2.1. Chemicals
Chemicals were purchased from Sigma-Aldrich (Darmstadt, Germany) unless indicated otherwise. Sodium-n-butyrate (BDH Chemicals Ltd, Poole, England) was dissolved in PBS, sterilized by filtration, and kept as a 2 M stock solution.
2.2. Cell culture
The HT29 (human colon adenocarcinoma)-derived cell lines, employed in the present study, represent stable, established cell lines. HT29-12 and HT29-21 were isolated based on their methotrexate-resistant phenotype [22], HT29cl.19a cells as resistant to 5 mM butyrate [23]. HT29R cells are resistant to butyrate-induced differentiation [24]. All the cell lines employed in this study namely, HT29, HT29-12, HT29-21, HT29R, Caco2, IEC-18, HCT116, Lovo, SW480 and DLD-1 cells were kindly provided as validated cell lines [25] by Dr. Albert Amberger, Tyrolean Cancer Research Institute, Innsbruck, Austria. The cells were cultured in Dulbecco's Modified Eagle's Medium (Sigma) supplemented with 10% FCS, 4 mM glutamine, 50 mg/l geneticin, at 37 °C, 5% CO2, and saturated humidity.
2.3. Western blot analysis
Butyrate-treated (10 mM sodium-n-butyrate, 24 h) cells and untreated controls (each from a 10 cm Petri dish) were washed twice with 10 ml pre-chilled PBS, and resuspended in (500 μl/10 cm tissue culture plate) ice-cold ZET buffer (50 mM Tris-HCl, pH 7.6, 10 mM EDTA, 1% Triton X-100), supplemented with 1x proteases inhibitor and phosphatases inhibitor cocktails (Roche Applied Science, UK), lysed by incubation on ice for 1 h, and centrifuged at 10 000 g, 30 min, 4°C. After transfer of the supernatants into fresh Eppendorf tubes, protein concentration was determined according to Bradford [26]. Equal amounts of proteins from the extracts were loaded onto 8-16% HEPES-SDS-polyacrylamide gels (Pierce, Rockford, IL, USA). Gels were blotted to Immobilon-P membranes (Millipore GmbH, Schwalbach, Germany) by a standard procedure [27]. For immunodetection, membranes were first blocked for 1 h with 5% (w/v) non-fat dry milk and 0.1% (v/v) Tween 20 in Tris-buffered saline (pH 7.5), at room temperature, and then treated with the antibodies and processed according to manufacturers' instructions. The following antibodies were used: rabbit polyclonal antibodies against PKCα, PKCγ, PKCδ, PKCε, PKCζ,, IGF1-R and tubulin; goat polyclonal antibody against pPKCε-Ser729 (all from Santa Cruz Biotechnology, Santa Cruz, CA, USA); rabbit polyclonal antibodies against pPKCα/β-Thr638/641, pPKC(pan)(γThr514), pPKCδ-Thr505 and pPKCζ-Thr410/403 (all from Cell Signaling Technology, Danvers, MA, USA); rabbit polyclonal antibody against pPKCγ-Thr514 and mouse monoclonal antibody against GAPDH (Millipore). PKCγ was detected with an antibody (C-19, Santa Cruz Biotechnology) mapping with an epitope of 15-25 amino acids within the last 50 amino acids at the C-terminus of PKCγ. As indicated above, two different antibodies were employed for the detection of Thr514-phospho-modified PKCγ (pPKCγ-Thr514). One of them (Milipore 07-878), generated against a peptide of human PKCγ with phosphorylated Thr514, is specific for pPKCγ-Thr514, while the other one pPKC(pan)(γThr514) detects not only Thr514-phosphorylated PKCγ but also PKCα, βI, βII, δ, ε, η and θ phosphorylated at a position homologous to Thr514 of PKCγ. Secondary antibodies conjugated with horseradish peroxidise were: goat anti-rabbit IgG, goat anti-mouse IgG (Jackson ImmunoResearch, Suffolk, UK) and donkey anti-goat IgG (Santa Cruz Biotechnology). Visualization of blots was performed with enhanced chemiluminescence by Immobilon Western (Millipore), and scanning with GelImager (INTAS, Göttingen, Germany).
To examine the kinetics of butyrate-induced upregulation of PKCγ, treatments with 10 mM sodium-n-butyrate for 12, 24 and 48 h were performed. Since in all investigated cell lines the 48 h butyrate treatment resulted in a rather high degree of apoptosis, a 24 h treatment with 10 mM butyrate was chosen for all further experiments.
2.4. Preparation of the cytosolic and membrane fractions
Cells cultured in 10 cm Petri dishes were pelleted by centrifugation (300 g), taken up in 200 μl lysis buffer A (20 mM Tris-HCl, pH 7.5, 2 mM MgCl2, 10 mM KCl, 10 mM β-glycerophosphate, 5 mM Na4P2O7, 2 mM EDTA, 2 mM EGTA, 2 mM DTT), supplemented with 1x protease inhibitor and phosphatase inhibitor cocktails (Roche), and kept on ice for 20 min. After homogenization (Dounce homogenizer, Pestle S, 30 strokes), the suspension was centrifuged at 500 g, 10 min, 4°C, to pellet nuclei and cell debris. The supernatant was centrifuged at 100 000 g for 60 min, 4°C, yielding the cytosolic fraction (supernatant 1) and a membrane pellet (pellet 1). The membrane pellet was washed twice with lysis buffer A, resuspended in lysis buffer B (homogenization buffer A with 2% Nonidet NP-40) and incubated for 1 h on ice under repeated vortexing. The resulting suspension was centrifuged at 100 000 g for 60 min, 4°C, to obtain the membrane fraction as supernatant (supernatant 2). Protein concentrations were determined according to Bradford (26), the fractions kept frozen at −80°C until analysis.
3. Results
3.1. Isoenzyme-selective, marked butyrate-induced upregulation of PKCγ in HT29 parental and HT29-derived cell lines
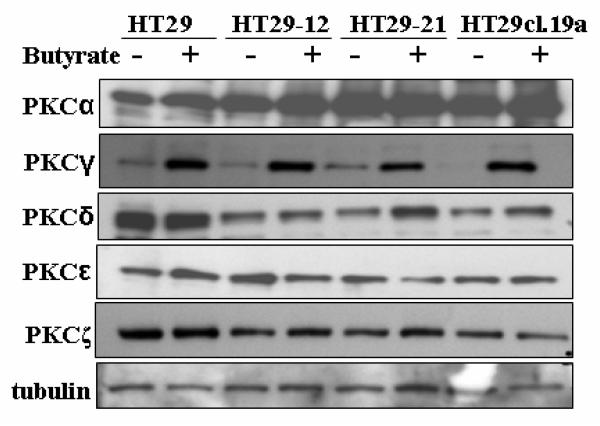
We examined the impact of butyrate on the expression levels of PKCα, γ, δ, ε and ζ, the isoenzymes which are expressed in parental HT29 and the HT29-derived cell lines HT29-12, HT29-21 and HT29cl.19a. HT29 is considered as the model for non-differentiated colonic stem cells. Fig. 1 shows that in these cell lines, butyrate treatment (10 mM, 24 h) resulted in marked upregulation of PKCγ, whereas the expression levels of the PKCs α, δ, ε and ζ did not change significantly.
Fig. 1.
Effect of butyrate on PKC isoenzyme protein expression levels in HT29 parental and HT29-derived cell lines. Cells were cultured under standard conditions as described in Materials and methods, and treated with 10 mM butyrate for 24 h. Lysates were subjected to Western blot analysis for the indicated PKC isoforms. Tubulin was probed as loading control. Representative blots are shown.
3.2. Butyrate-induced upregulation of Thr514-phosphorylated PKCγ occurs in HT29 and derived cell lines
The PKC priming process is a complex sequence of events, not yet fully understood. According to the current understanding [reviewed in 8,12], the newly-synthesized conventional PKCs associate with a membrane through interactions with the C1 and/or C2 domains. In this open conformation, PDK-1 is able to access its binding site at the hydrophobic motif in the kinase domain of the PKC and, once docked there, it phosphorylates PKCs on the activation loop. The release of PDK-1 from PKC is thought to be rate limiting [12]. Once phosphorylated on the activation loop (for PKCγ at Thr514), PKCs become auto-phosphorylated at two additional sites (the turn motif and the hydrophobic motif) leading to the release of an inactive conformation from the membrane to the cytoplasm. The primed, inactive form of the PKC, positioned in the cytoplasm, is now ready to respond to activation by cofactors. Conventional PKCs are activated by Ca2+ and diacyglycerol. Ca2+ binds to the C2 domain of cytosolic PKC and tethers it by a low-affinity interaction to the membrane. Subsequent binding of membrane-embedded diacylglycerol to the C1 domain results in high-affinity binding, release of the pseudosubstrate and thus activation of the now membrane-bound PKC.
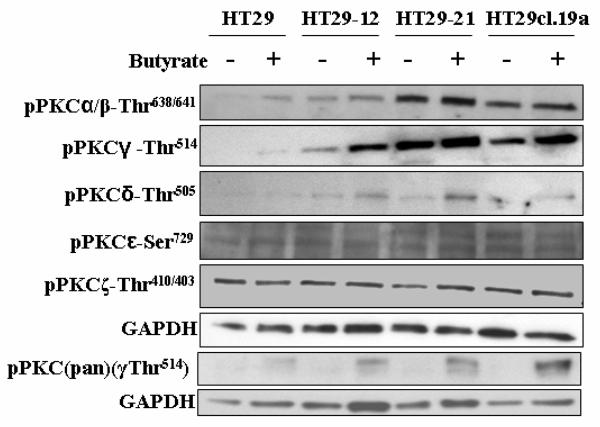
To examine whether and to what extent de novo synthesized PKCγ protein gets converted to the form phosphorylated at the activation-loop site, we performed also immunoblotting with a specific antibody against the Thr514-phosphorylated form of PKCγ (Fig. 2). Whereas in HT29 parental cells, pPKCγ-Thr514 was at the detection limit in the absence of butyrate, and butyrate treatment resulted in upregulation only to a level still near the detection limit, in the HT29-derived cell lines, especially in HT29-21, this modification was highly expressed. Butyrate treatment caused moderate upregulation of Thr514-phosphorylated PKCγ in HT29 parental as well as derived cell lines (Fig. 2), but clearly not to that extent as observed with the non-Thr514-phospho-modified form (Fig. 1). Thus, de novo synthesis of PKCγ and phosphorylation of its Thr514-position are not coupled. However, probing with pPKC(pan)Thr514-antibody showed considerable butyrate-induced upregulation, indicating in other PKC isoform(s) a closer coupling between butyrate-induced synthesis and phosphorylation (Fig.2). The pPKC(pan)Thr514-antibody recognizes not only pPKC-Thr514 but also other conventional pPKCs phosphorylated at a position homologous to Thr514.
Fig. 2.
Impact of butyrate on the level of pPKCγ-Thr514 and phosphorylated forms of other PKCs in parental HT29 and HT29-derived cell lines. Cells were treated with 10 mM butyrate for 24 h, and lysates were subjected to Western blot analysis with phospho-specific antibodies against the PKC isoforms. GAPDH was probed as a loading control. Representative blots are shown.
Furthermore, Fig. 2 shows also the butyrate-induced expression levels of phosphorylated forms (pPKCs) of the PKC isoenzymes α/β, δ, ε, ζ in the group of HT29 cell lines. While a tendency to butyrate-induced upregulation of pPKCα/β-Thr638/641 and to a more pronounced extent of pPKCδ-Thr505 could be detected in parental HT29 as also in the derived cell lines, the levels of pPKCε-Ser729 and pPKCζ-Thr410/403 did not change.
3.3. In Caco2 and IEC-18 cells PKCγ is present exclusively in the Thr514-phosphorylated form
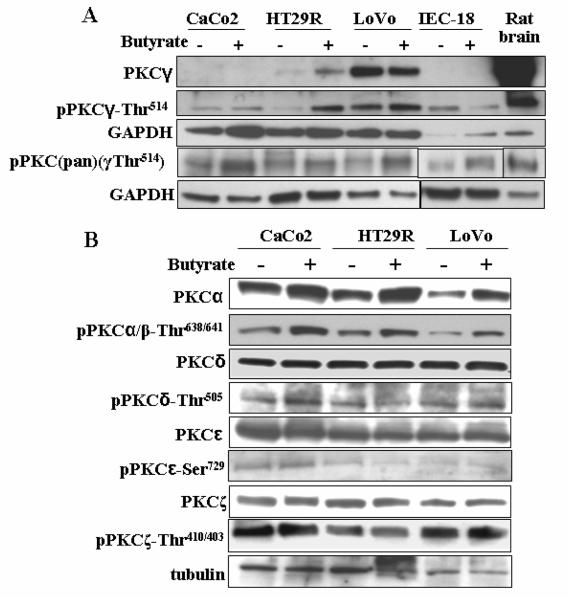
Next, we examined the impact of butyrate on the expression levels of PKCγ and its Thr514-modified form in Caco2 cells, which represent the model for differentiated colon cancer, and IEC-18 cells, which are normal, differentiated intestinal rat epithelial cells (Fig. 3A). Whereas PKCγ was detectable neither in untreated nor butyrate-treated Caco2 and IEC-18 cells, probing with antibodies against pPKCγ-Thr514 revealed a clearly detectable expression level Thr514-phosphorylated-PKCγ in these cells (Fig. 3A). One of the applied antibodies, raised against a Thr514-phosphorylated peptide of the human PKCγ sequence, recognizes specifically pPKCγ-Thr514; the other antibody pPKC(pan)γThr514 recognizes Thr514-phosphorylation not only in PKCγ but also in other PKC isoenzymes when this or a homologous position is phosphorylated. Thus, in Caco2 and IEC-18, PKCγ was detectable with antibodies against phospho-Thr514, but not with an antibody mapping with an epitope of 15-25 amino acids within the sequence of 50 amino acids at the C-terminus of PKCγ. This shows that the presence of a phosphate residue in the position of Thr514 (at the activation domain), made a domain within the 50 amino acids at the C-terminus inaccessible for an antibody. We also included authentic PKCγ from rat brain into the immunoblot analysis as a control for the electrophoretic mobility of PKCγ as well as the proper functioning of the antibodies. Fig. 3A also compares the differentiated cell types Caco2 and IEC-18 with HT29R (another HT29-derived cell line which is resistant to butyrate-induced differentiation) and Lovo cells (derived from a metastatic colon cancer tumour). The applied antibodies recognized PKCγ as well as pPKCγ-Thr514 in these cell lines. HT29R cells showed the characteristics of the HT29 group of cell lines, as in untreated HT29R cells, PKCγ was expressed at a marginal level, and became upregulated by butyrate. However, pPKCγ-Thr514 was more sensitive to upregulation by butyrate (Fig. 3A) than in HT29 and the other derived cell lines.
Fig. 3.
Effect of butyrate on PKC expression levels in Caco2, IEC-18, HT29R and Lovo cells. (A) The expression levels of PKCγ, pPKCγ-Thr514 and pPKC(pan)(γThr514) (the pan antibody detects phosphorylated sites in the activation domain of all conventional PKCs) in Caco2, IEC-18, HT29R, Lovo cells, and the impact of butyrate. Comparison with authentic PKCγ from rat brain. Cells were treated with 10 mM butyrate for 24 h. (B) The impact of butyrate on the non-modified and phospho-modified forms (pPKCs) of PKCα, PKCδ, PKCε, PKCζ. Representative blots are shown.
Interestingly, in Caco2 and IEC-18 cells, in contrast to the HT29 group of cell lines, pPKCγ-Thr514 was not upregulated by butyrate (Fig. 3A). Thus, in Caco2 and IEC-18 cells, the entire PKCγ was present in the Thr514-phospho-modified form, at high expression level, and insensitive to upregulation by butyrate.
Furthermore, Fig. 3B shows that in Caco2 as well as HT29R and Lovo cells, the isoenzymes PKCα, PKCδ, PKCε, PKCζ, pPKCζ, and their phosphorylated forms are detectable at high expression levels. Butyrate caused some upregulation of PKCα, pPKCα/β-Thr638/641 and pPKCδ-Thr505, whereas the levels of PKCδ, PKCε, PKCζ and pPKCζ-Thr410/403 remained unchanged.
3.4. PKCγ as well as its Thr514phospho-modified form are present at high expression levels in Lovo and SW480 cells
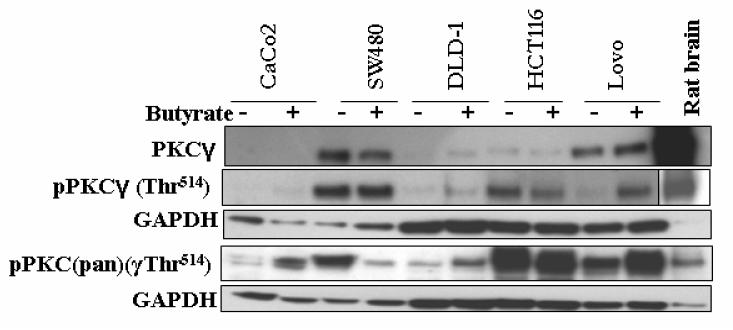
Next, we examined the expression profile of PKCγ in colon cancer cell lines that represent neither the undifferentiated colonic stem cell model nor differentiated intestinal epithelial cells. This group comprised HCT116, colon cancer cells with a low degree of differentiation, and Lovo, SW480 and DLD-1 cells which represent dedifferentiated highly advanced colon cancer. Fig. 4 shows that HCT116 cells (low degree of differentiation), closely resemble the differentiated intestinal cell types (Caco2 and IEC-18) with regard to PKCγ and pPKCγ expression pattern and its sensitivity to butyrate: the bulk of PKCγ was present as pPKCγ and insensitive to butyrate-induced upregulation.
Fig. 4.
The expression levels of PKCγ, pPKCγ-Thr514 and pPKC(pan)(γThr514) in Caco2, SW480, DLD-1, HCT116 and Lovo cells. Comparison with authentic PKCγ from rat brain. Cells were treated with 10 mM butyrate for 24 h. Lysates were subjected to Western blot analysis with antibodies as described in Materials and methods. GAPDH was probed as a loading control. Representative blots are shown.
PKCγ as well as its Thr514-phospho-modified form (pPKCγ) was highly expressed in SW480 and Lovo. In comparison to SW480 and Lovo, the expression of PKCγ was much lower in DLD-1, but it should be also mentioned that, as reported in a previous study, PKCγ is the major conventional PKC isoenzyme in DLD-1 cells [28]. Except for pPKCγ-Thr514 in Lovo cells, which was upregulated by butyrate (Figs. 3 and 4), in this group of cell lines PKCγ as well as its Thr514-phopho-modified form appeared to be insensitive to upregulation by butyrate. As DLD-1 cells are Dukes' grade C and SW480 cells Dukes' grade B, in this group of colon cancer cell lines there is no obvious correlation between PKCγ expression and the Dukes' grading of advanced cancer. Analysis of more cell lines, and of tumour samples will be necessary for exploring a possible correlation between PKCγ/pPKCγ expression and the malignant character of the tumour. But as far as PKCγ expression level and its sensitivity to butyrate is concerned, this group of cell lines differs from the undifferentiated (HT29) as well as the highly differentiated types (Caco2/IEC-18 cells) of colon cancer/colonic epithelial cells.
Interestingly, as shown in Fig. 4, probing with pPKC(pan)(γThr514) revealed a strong butyrate-induced upregulation of the phosphorylated PKC level in Caco2 and DLD-1, and marked downregulation in SW480. It should be pointed out that the pan-antibody detects not only phosphorylation of PKCγ at Thr514 but also other PKC isoenzymes phosphorylated at this or at a homologous position in the activation domain. Since these butyrate-induced effects are not seen with the antibody specific for pPKCγ-Thr14, it has to be considered that they are due to phosphorylation and dephosphorylation, respectively, of the activation domain in other PKC isoenzymes.
The above-mentioned observations were made after 24 h treatments of cells with 10 mM sodium n-butyrate, because after a 48 h treatment, some of the investigated cell lines exhibited considerable detachment and apoptosis. Especially, HT29R and Lovo cells appeared to be highly sensitive to butyrate. Butyrate-induced cell death in HT29R and the other HT29-derived cell lines was investigated in a previous study [24].
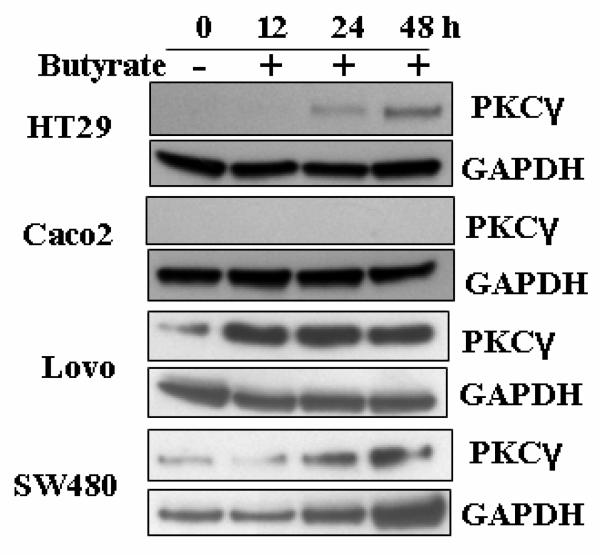
In order to examine the time-dependence of butyrate-induced changes in expression levels of PKCγ, cell lines representing the above-mentioned groups, namely HT29L, Caco2, Lovo and SW480 cells were compared after 12 h, 24 h and 48 h treatments with butyrate. For probing, the antibody (C-19, Santa Cruz Biotechnology) against the C-terminal domain of PKCγ was used, which specifically detects PKCγ which is not phosphorylated at Thr514. Fig. 5 shows that in HT29 cells, butyrate-induced upregulation of PKCγ becomes detectable after 12 h, and keeps increasing up to the 48 h time course measured. In Caco2 cells, PKCγ remained undetectable with this antibody over the whole treatment period, while in Lovo and SW480 cells, the high expression level of PKCγ remained unchanged (Fig.5). Also in DLD-1 and HCT116 cells the low expression level of PKCγ remained unchanged during the 48 h treatment period (blots of the latter two cell lines are not shown).
Fig. 5.
Kinetics of butyrate induced PKCγ expression levels. Cells were treated with 10 mM butyrate for 12, 24 and 48 h, and lysates were subjected to Western blot analysis with the antibody against non-Thr514-phosphorylated PKCγ. GAPDH was used as a loading control. Representative blots are shown.
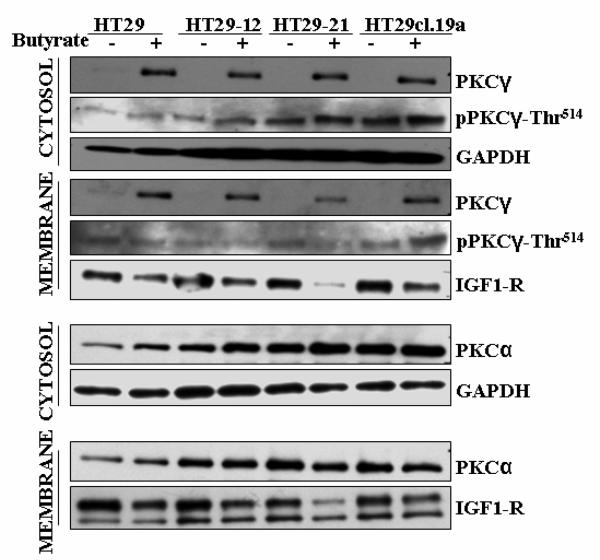
3.5. Marked butyrate-induced upregulation of PKCγ occurs in the cytosolic as well as membrane fraction in HT29-derived cell lines
We raised the question of how the expression levels of PKCα, PKCγ and pPKCγ-Thr514, and the butyrate-induced effects on them are manifested in their subcellular distributions. Fig. 6 shows the distribution of these isoforms between the cytosolic and membrane fraction, in the absence or presence of butyrate, in the group of HT29 cells. In all four cell lines, strong butyrate-induced upregulation of PKCγ and limited upregulation pPKCγ-Thr514 could be detected in the cytosolic as well as the membrane fraction. Thus, as also observed with the total cell extract (Figs. 1 and 2), the bulk of the upregulated PKCγ was detected non-phosphorylated in Thr514, suggesting that phosphorylation at this position is not tightly coupled to PKCγ synthesis. Moreover, as Fig. 6 shows, this upregulated non-phosphorylated form (sc-211, C-19, Santa Cruz Biotechnology) was located in both compartments, membrane-bound and free in the cytoplasm. Upregulation of the phospho-Thr514-modified form was very limited compared to non-phosphorylated PKCγ. Thus, the bulk of PKCγ upregulated under butyrate treatment was an inactive, non-phosphorylated, non-primed form of PKCγ, and there was a considerable fraction of newly-synthesized PKCγ molecules localized in the cytoplasm before their priming. Intracellular localization of PKCγ was also examined by immunofluorescence. No alterations in the distribution between the plasma membrane and cytoplasm, (i.e. between the plasma membrane and the interior of the cell), as a result of butyrate treatment were observed (data not shown).
Fig. 6.
Impact of butyrate on the subcellular distribution of PKCγ, pPKC-Thr514 and PKCα in HT29 parental and HT29-derived cell lines. After treatment of cells with 10 mM butyrate for 24 h, and fractionation, Western blot analysis was performed as described in Materials and methods. GAPDH and IGF1-R were used as a subcellular localization-specific marker proteins for the cytosolic and plasma membrane fractions, respectively. Representative blots are shown.
PKCα, examined for comparison, was detected in the cytosolic as well as membrane fractions (Fig. 6). The lack of major butyrate-induced changes in the cytosolic as well as membrane fractions, underline the selectivity of the butyrate-induced effect on expression of PKCγ.
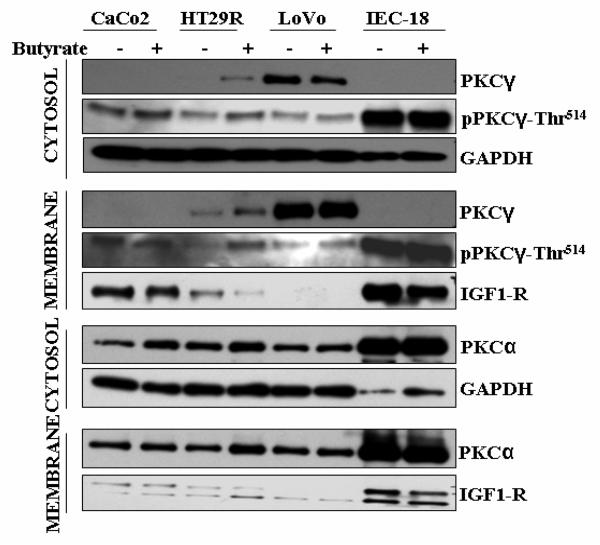
3.6. The impact of butyrate on the subcellular distribution of PKCγ in Caco2, IEC-18, HT29R and Lovo cells
Fig. 7 shows in Caco2, IEC-18, HT29R and Lovo cells, in the absence and presence of butyrate, the distribution of PKCα, PKCγ and pPKCγ-Thr514 between the cytosolic and the membrane fraction. In Caco2 and IEC-18 cells, with or without butyrate, PKCγ was not detectable with the antibody, mapping within a sequence of 50 amino acids at its C-terminus, either in the cytosolic or membrane fraction. However, in both cell lines, PKCγ could be detected at a high expression level as pPKCγ-Thr514, in the cytosolic as well as membrane fractions (Fig. 7). Thus, the analysis of the two fractions confirmed the results achieved with total cell extracts of Caco2 and IEC-18 cells (Fig. 3A) that in these cells the entire PKCγ is present in the pPKCγ-Thr514 form and its expression level is insensitive to butyrate. Moreover, since in both fractions under butyrate treatment the expression levels remained unchanged, butyrate treatment did not alter the distribution of pPKCγ-Thr514 between the cytosolic and membrane (particulate) fraction. In HT29R cells, as in the other cell lines of the HT29 group, butyrate caused marked upregulation of the non-phosphorylated form in both fractions, and as a special feature of HT29R also of the Thr514-phosphorylated form of PKCγ. In Lovo cells, a minor butyrate-induced decrease of the non-phosphorylated form was observed in the cytosolic fraction. A similar tendency to butyrate-induced decrease of the non-phopshorylated form was also observed with the total extract (Fig. 3). In these cell lines the intracellular localization of PKCγ was also examined by immunofluorescence. No changes in the distribution between the plasma membrane and cytoplasm (i.e. between plasma membrane and the interior of the cell), as a result of butyrate treatment, could be detected (data not shown).
Fig. 7.
Impact of butyrate on the subcellular distribution of PKCγ, pPKC-Thr514 and PKCα in Caco2, IEC-18, HT29R and Lovo cells. After treatment of cells with 10 mM butyrate for 24 h, and fractionation, Western blot analysis was performed as described in Materials and methods. GAPDH and IGF1-R were used as subcellular localization-specific marker proteins for the cytosolic and plasma membrane fractions, respectively. Representative blots are shown.
Furthermore, for comparison PKCα was also probed for in Caco2, IEC-18, HT29R and Lovo cells. As in the other investigated cell lines, PKCα was expressed at high levels in the cytosolic as well as membrane fractions, and this did not change under butyrate treatment (Fig. 7).
4. Discussion
According the generally accepted view, the de novo synthesized conventional PKC binds to a membrane creating an open conformation that permits phosphorylation by PDK-1 at a position in the activation loop domain, which, for PKCγ, is Thr514 [10,11]. This is followed by autophosphorylation of a position at the turn motif and another at the hydrophobic motif. With this, the maturation process is complete, the conventional PKC is primed and released as mature inactive PKC into the cytoplasm [14-17]. Upon activation, the primed PKC translocates to the plasma membrane. Several aspects of this maturation process are not yet fully clarified [8]. It is not known to which membrane the newly-synthesized conventional PKC binds and whether and to what extent the above-outlined scenario is generally applicable or whether one of several cell type-specific maturation mechanisms are in action. It should be pointed out that a study claimed that PKCγ was activated by oxidative stress [11]. The PKC maturation process is much less investigated than PKC activation, thus cell type-specific deviations from the canonical scenario can be expected. In this context, two unexpected observations of our study need to be mentioned. First, the large amount of non-Thr514-phosphorylated PKCγ detected in untreated Lovo and SW480 cells as well as butyrate-treated HT29 cells demonstrates that in these cell types activation-loop phosphorylation does not occur as an event coupled with de novo enzyme protein synthesis. Second, in Caco2 and IEC-18 cells, the entire PKCγ was present exclusively in the Thr514-phosphorylated form, at a high expression level. The fact that the non-phosphorylated form was not detectable suggests that in these differentiated intestinal epithelial cells, activation-loop phosphorylation is tightly coupled with de novo synthesis of PKCγ. The antibody which we employed (C-19, Santa Cruz Biotechnology) mapping with an epitope of 15-25 amino acids within the sequence of 50 amino acids at the C-terminus (i.e. within the sequence 647-697) of PKCγ, did not recognize the Thr514-phospho-modified form of PKCγ. Thus, either steric hindrance by the phosphate group or some sort of conformational change induced by the phosphate residue precludes the binding of the antibody to its C-terminal epitope. Investigations employing only this antibody would fail to detect highly expressed PKCγ in Caco2 and IEC-18 cells, and in every other cell line would also fail to detect the Thr514-phospho-modified portion of PKCγ.
The present study shows that colon cancer cell lines differ markedly not only in expression levels of PKCγ, but also in the proportions of the Thr514-phosphorylated form, and the sensitivity of both non-phosphorylated and Thr514-phosphorylated PKCγ to butyrate-induced upregulation. Based on the observations that in Caco2 and IEC-18 cells, two cell lines representative for the differentiated intestinal epithelial phenotype, PKCγ was detected exclusively in the Thr514-phospho-modified form, while in the undifferentiated and dedifferentiated cells large proportions of PKCγ persisted in the non-phosphorylated state, it is intriguing to speculate that non-primed PKCγ or activation-loop phopshorylation may be related to other functions besides priming. A recent study reported that binding domains of PKCα protein but not kinase activity was critical for glioma cell prolifetation and survival [28]. The marked differences in the proportions of the Thr514-phosphorylated form of PKCγ in the colon cancer cell lines may also indicate that priming, as a regulated process, may have its contribution to the cell type-specific expression and action of a PKC isoform.
The number of colon cancer cell lines investigated in the present study is not sufficient to claim specific correlations between PKCγ, pPKCγ-Thr514 expression level, its sensitivity to butyrate and the degree of differentiation. However, the results of the present study and available literature on PKCγ expression levels seem to indicate that the expression level of this isoenzyme is in some way associated with the differentiation status in colon cancer cell lines. The investigated cell lines can be tentatively classified into HT29 parental and HT29-derived cell lines, the model for colonic epithelial stem cells, in which PKCγ was expressed at a low level and became highly upregulated by butyrate. The pPKC-Thr514 form, present in HT29 cells as a substantial proportion of PKCγ, was less sensitive to upregulation by butyrate. In a second group, comprising Caco2 and IEC-18 cells, representing the differentiated intestinal epithelial phenotype, PKCγ was detected exclusively as pPKCγ-Thr514, and insensitive to butyrate-induced upregulation. pPKCγ-Thr514 was distributed equally between the cytosolic and membrane fraction. For further comparison we investigated several other cell lines, namely HCT116 adenocarcinoma cells with a low degree of differentiation; Lovo cells, which were originally isolated from a metastatic tumour (nodule in the left supraventricular region, Dukes' type C, grade IV); SW480 isolated from the primary adenocarcinoma tumour (Dukes' type B); DLD-1 isolated from a primary adenocarcinoma (Dukes' type C). The PKCγ expression profile of HCT116 cells with a low degree of differentiation resembled that of the differentiated Caco2 and IEC-18 cells, with the difference that non-phosphorylated PKCγ could be detected at a low expression level. Among the dedifferentiated cell lines representing highly advanced cancer, Lovo and SW480 cells were conspicuous for constitutively expressing high levels of non-phosphorylated as well as Thr514-phosphorylated PKCγ. Although, the expression level of PKCγ was lower in DLD-1 cells than in SW480 and Lovo, in a previous study it was stated that PKCγ was the major conventional PKC in DLD-1 cells [29].
There are only a few studies on the effects of butyrate on PKC isoenzyme profiles in colonic epithelial cells. In LIM1215 colon cancer cells, expressing the PKC isoforms α, ε, ζ, ι, butyrate specifically reduced the expression of PKCα and PKCε proteins [30]. In HCT116 cells, the protective effect of butyrate against deoxycholate-induced activation of PKCs was examined. Deoxycholate induced rapid translocation of PKCε but not PKCδ, and pretreatment with butyrate did not modify this response [31].
Until recently, PKCγ was primarily associated with nerve tissue [32-35], and implicated in tissue injury repair [14, 34]. Data on the expression of PKCγ in colon cancer cells or colon carcinomas are scarce. In reviews on PKC isoenzymes in colon carcinogenesis, PKCγ was not discussed either among the isoforms expressed in normal enterocytes and colonocytes, or among the PKC isoforms which play a role in cancer development [5, 36]. However, in a recent study with SW480 colon carcinoma cells, it was mentioned that in SW480, SW1222 and DLD-1 cells, PKCγ was expressed as the major conventional PKC [29]. Our study with a set of different colon cancer cell lines gives to some extent an explanation of this apparent discrepancy by demonstrating the colon cancer cell type-specific expression of PKCγ, which can be detected at higher expression levels in some dedifferentiated colon cancer cell lines (Lovo, SW480), or under butyrate treatment (HT29), or when probed with an antibody against its phospho-Thr514-modified-form (Caco2 and IEC-18). In a study employing COLO-205 cells, originally isolated as metastatic cells from the ascites fluid of a colon cancer patient (Dukes' type D), PKCγ and PKCε were detected as the only two isoforms [37]. Moreover, PKCγ was detected neither in normal colonocytes [38,39] nor in early adenomas [39].
The targets and function of PKCγ in intestinal epithelial cells and colon carcinomas are not known. We examined the phosphorylation of the potential PKCγ targets MARCKS and Elk-1 in HT29 cells as affected by butyrate, and could not detect any difference in the level of phosphorylations, in spite of the butyrate-mediated upregulation of PKCγ (data not shown). In previous studies [24] we investigated butyrate-induced differentiation, cell death and reactive oxygen species in HT29 and derived cell lines. Since the cell lines differed considerably in the above-mentioned butyrate-induced effects, the PKCγ expression and its sensitivity to butyrate, which is quite similar in these cell lines cannot be related to the above-mentioned phenomena.
There are indications, however, that PKCγ might be involved in cell migration. PKCγ and PKCδ were reported to be involved in insulin-like growth factor I-induced migration in colonic epithelial cells [40]. Furthermore, in protrusions and filipodia of migrating SW480 cells, PKCγ interacted with fascin, an actin-binding protein which is low or absent in normal colonic epithelia [29]. The results of the present study appear to be consistent with such a role of PKCγ, as it was detected at higher levels in cell lines derived from advanced or metastatic colon cancer cells. The lack of butyrate sensitivity of PKCγ in Caco2 and IEC-18 cells may be related to the stability of the differentiated surface of the colonic epithelium, which is in immediate contact with butyrate. Whether and in what way the different pools of non-primed and mature but inactive PKCγ are related to the amount of its activated form will be the subject of future studies.
Acknowledgements
This research was supported by grants of the Österreichische Forschungsgemeinschaft (Project MOEL 339) and Austrian Research Fund (P15039-B12, P17878-B12). We are indebted to Ms. Rajam Csordas-Iyer for critical reading and linguistic corrections.
Abbreviation
- PKC
protein kinase C
- pPKC
phospho-modified protein kinase C
References
- 1.Kruh J, Defer L, Tichonicky L. Effects of butyrate on cell proliferation and gene expression. In: Cummings JH, Rombeau JL, Sakata T, editors. Physiological and Clinical Aspects of Short-Chain Fatty Acids. Cambridge University Press; Cambridge: 1995. pp. 275–288. [Google Scholar]
- 2.Csordas A. Toxicology of butyrate and short-chain fatty acids. In: Hill MJ, editor. Role of Gut Bacteria in Human Toxicology and Pharmacology. Taylor and Francis; London: 1995. pp. 105–127. [Google Scholar]
- 3.Lindemann RK, Gabrielli B, Johnstone RW. Histone-deacetylase inhibitors for the treatment of cancer. Cell Cycle. 2004;3:1779–1788. [PubMed] [Google Scholar]
- 4.Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: molecular mechanism of action. Oncogene. 2007;26:5541–5552. doi: 10.1038/sj.onc.1210620. [DOI] [PubMed] [Google Scholar]
- 5.Black JD. Protein kinase C-mediated regulation of the cell cycle. Front. Biosci. 2000;5:D406–D423. doi: 10.2741/black. [DOI] [PubMed] [Google Scholar]
- 6.Di Mari JF, Mifflin RC, Powell DW. The role of protein kinase C in gastrointestinal function and disease. Gastroeneterology. 2005;128:2131–2146. doi: 10.1053/j.gastro.2004.09.078. [DOI] [PubMed] [Google Scholar]
- 7.Frey MR, Clark JA, Leontieva O, Uronis JM, Black AR, Black JD. Protein kinase C signalling mediates a program of cell cycle withdrawal in the intestinal epithelium. J. Cell Biol. 2000;151:763–778. doi: 10.1083/jcb.151.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat. Rev. Molec. Cell Biol. 2010;11:9–22. doi: 10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- 9.Griner EM, Kazanietz MG. Protein kinase C and other diacyglycerol effectors in cancer. Nat. Rev. Cancer. 2007;7:281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- 10.Mukai H. The structure and function of PKN, a protein kinase having a catalytic domain homologous to that of PKC. J. Biochem. 2003;133:17–27. doi: 10.1093/jb/mvg019. [DOI] [PubMed] [Google Scholar]
- 11.Lin D, Takemoto DJ. Oxidative activation of protein kinase Cγ through the C1 domain. J. Biol. Chem. 2005;280:13682–13693. doi: 10.1074/jbc.M407762200. [DOI] [PubMed] [Google Scholar]
- 12.Sonnenburg ED, Gao T, Newton AC. The phosphoinositide-dependent kinase, PDK-1, phosphorylates conventional protein kinase C isoenzymes by a mechanism that is independent of phosphoinositide 3-kinase. J. Biol. Chem. 2001;276:45289–45297. doi: 10.1074/jbc.M107416200. [DOI] [PubMed] [Google Scholar]
- 13.Dephoure N, Zhou C, Villén J, Beausoleil SA, Bakalarski CE, Elledge SJ, Gygi SP. A quantitative atlas of mitotic phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 2008;105:10762–10767. doi: 10.1073/pnas.0805139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnett ME, Madgwick DK, Takemoto DJ. Protein kinase C as a stress sensor. Cell. Signal. 2007;19:1820–1829. doi: 10.1016/j.cellsig.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newton AC. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem. J. 2003;370:361–371. doi: 10.1042/BJ20021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parekh DB, Ziegler W, Parker PJ. Multiple pathways control protein kinase C phosphorylation. EMBO J. 2000;19:496–503. doi: 10.1093/emboj/19.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards AS, Faux MC, Scott AC, Newton AC. Carboxy-terminal phosphorylation regulates the function of subcellular localization of protein kinase C βII. J. Biol. Chem. 1999;274:6461–6468. doi: 10.1074/jbc.274.10.6461. [DOI] [PubMed] [Google Scholar]
- 18.Murray NR, Weems J, Braun U, Leitges M, Fields AP. Protein kinase C βII and PKCι/λ: collaborating partners in colon cancer promotion and progression. Cancer Res. 2009;69:656–662. doi: 10.1158/0008-5472.CAN-08-3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fields AP, Calgano SR, Krishna M, Rak S, Leitges M, Murray NR. Protein kinase Cβ is an effective target for chemoprevention of colon cancer. Cancer Res. 2009;69:1643–1650. doi: 10.1158/0008-5472.CAN-08-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pysz MA, Leontieva OV, Bateman NW, Uronis JM, Curry KJ, Threadgill DW, Janssen K-P, Robine S, Velcich A, Augenlicht LH, Black AR, Black JD. PKCα tumor suppression in the intestine is associated with transcriptional and translational inhibition of cyclin D1. Exp. Cell Res. 2009;315:1415–1428. doi: 10.1016/j.yexcr.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gavrielides MV, Frijhoff AF, Conti CJ, Kazanietz MG. Protein kinase C and prostate carcinogenesis: targeting the cell cycle and apoptotic mechanisms. Curr. Drug Targets. 2004;5:431–443. doi: 10.2174/1389450043345380. [DOI] [PubMed] [Google Scholar]
- 22.Lesuffleur T, Violette S, Vasile-Pandrea I, Dussaulx E, Brabat A, Muleris M, Zweibaum A. Resistance to high concentrations of methotrexate and 5-fluorouracil of differentiated HT-29 colon-cancer cells is restricted to cells of enterocytic phenotype. Int. J. Cancer. 1998;76:383–392. doi: 10.1002/(sici)1097-0215(19980504)76:3<383::aid-ijc16>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 23.Augeron C, Laboisse CL. Emergence of permanently differentiated cell clones in a human colonic cancer cell line in culture after treatment with sodium butyrate. Cancer Res. 1984;44:3961–3969. [PubMed] [Google Scholar]
- 24.Domokos M, Jakus J, Szeker K, Csizinszky R, Csiko Gy., Neogrady Zs., Csordas A, Galfi P. Butyrate-induced cell death and differentiation are associated with distinct patterns of ROS in HT29-derived human colon cancer cells. Dig. Dis. Sci. 2009 doi: 10.1007/s10620-009-0820-6. doi:10.1007/s10620-009-0820-6. [DOI] [PubMed] [Google Scholar]
- 25.Parson W, Kirchebner R, Mühlmann R, Renner K, Kofler A, Schmidt S, Kofler R. Cancer cell line identification by short tandem repeat profiling: power and limitations. FASEB J. 2005;19:434–436. doi: 10.1096/fj.04-3062fje. [DOI] [PubMed] [Google Scholar]
- 26.Bradford MM. A rapid and sensitive for the quantitation of microgram quantitites of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 27.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. U.S.A. 1979;9:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cameron AJ, Procyk KJ, Leitges M, Parker PJ. PKC alpha protein but not kinase activity is critical for glioma cell proliferation and survival. Int. J. Cancer. 2008;123:769–779. doi: 10.1002/ijc.23560. [DOI] [PubMed] [Google Scholar]
- 29.Parsons M, Adams JC. Rac regulates the interaction of fascin with protein kinase C in cell migration. J. Cell Sci. 2008;121:2805–2813. doi: 10.1242/jcs.022509. [DOI] [PubMed] [Google Scholar]
- 30.Rickard KL, Gibson PR, Wilson NJ, Mariadason JM, Phillips WA. Short-chain fatty acids reduce expression of specific protein kinase C isoforms in human colonic epithelial cells. J. Cell Phys. 2000;182:222–231. doi: 10.1002/(SICI)1097-4652(200002)182:2<222::AID-JCP11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 31.Looby E, Long A, Keelleher D, Volkov Y. Bile acid dexycholate induces differential subcellular localisation of the PKC isoenzymes β1, ε and δ in colonic epithelial cells in a sodium butyrate insensitive manner. Int. J. Cancer. 2005;114:887–895. doi: 10.1002/ijc.20803. [DOI] [PubMed] [Google Scholar]
- 32.Furness JB, Hind AJ, Ngui K, Robbins HL, Clerc N, Merrot T, Tjandra JJ, Poole DP. The distribution of PKC isoforms in enteric neurons, muscle and intestinal cells of the human intestine. Histochem. Cell Biol. 2006;126:537–548. doi: 10.1007/s00418-006-0190-5. [DOI] [PubMed] [Google Scholar]
- 33.Saito N, Shirai Y. Protein kinase Cγ (PKCγ): Function of neuron specific isotype. J. Biochem. 2002;132:683–687. doi: 10.1093/oxfordjournals.jbchem.a003274. [DOI] [PubMed] [Google Scholar]
- 34.Chou W-H, Messing RO. Protein kinase C isoenzymes in stroke. Trends Cardiovasc. Med. 2005;15:47–51. doi: 10.1016/j.tcm.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Aronowski J, Grotta JC, Strong R, Waxham MN. Interplay between the gamma isoform of PKC and calcineurin in regulation of vulnerability to focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2000;20:343–349. doi: 10.1097/00004647-200002000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Black JD. Protein kinase C isoenzymes: guilt by omission. Gastroenterology. 2001;120:1868–1872. doi: 10.1053/gast.2001.25287. [DOI] [PubMed] [Google Scholar]
- 37.Lin S-Y, Liang Y-C, Ho Y-S, Tsai S-H, Pan S, Lee W-S. Involvement of both extracellular signal-regulated kinase and c-jun N-terminal kinase pathways in the 12-O-tetradecanoyphorbol-13-acetate-induced upregulation of p21Cip1 in colon cancer cells. Molec. Carcinogenesis. 2002;35:21–28. doi: 10.1002/mc.10070. [DOI] [PubMed] [Google Scholar]
- 38.Vestovsek G, Byrd A, Frey MR, Petrelli NJ, Black JD. Colonocyte differentiation is associated with increased expression and altered distribution of protein kinase C isozymes. Gastroenterology. 1998;115:75–85. doi: 10.1016/s0016-5085(98)70367-1. [DOI] [PubMed] [Google Scholar]
- 39.Assert R, Kötter R, Bisping G, Scheppach W, Stahlnecker E, Müller KM, Dusel G, Schatz H, Pfeiffer A. Anti-proliferative activity of protein kinase C in apical compartments of human colonic crypts: evidence for a less activated protein kinase C in small adenomas. Int. J. Cancer. 1999;80:47–53. doi: 10.1002/(sici)1097-0215(19990105)80:1<47::aid-ijc10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 40.André F, Rigot V, Remacle-Bonnet M, Luis J, Pommier G, Marvaldi J. Protein kinases C-γ and –δ are involved in insulin-like growth factor I-induced migration of colonic epithelial cells. Gastroenterology. 1999;116:64–77. doi: 10.1016/s0016-5085(99)70230-1. [DOI] [PubMed] [Google Scholar]