Abstract
Purpose of review
The present review discusses recent reports showing that reciprocal changes in T helper interleukin-17-secreting CD4+ Th17 cells and CD4+CD25highFoxP3+ regulatory T cells (Tregs) may play a role in the progressive disease caused by the HIV and by simian immunodeficiency virus.
Recent findings
Studies in nonhuman primate models of lentiviral infection and in HIV-infected human individuals have shown that pathogenic infection is associated with loss of Th17 cells and an increase in the frequency of Tregs. Because interleukin-17 serves to maintain the integrity of the mucosal barrier, loss of Th17 cells may permit the increase in microbial translocation across the gastrointestinal mucosa that is observed in pathogenic lentiviral disease. It remains unclear, however, whether Th17 cells are preferentially infected or if, instead, their loss is induced by bystander effects of lentiviral infection, for example, the induction of indoleamine 2,3-dioxygenase.
Summary
Progressive lentiviral disease is associated with preferential depletion of Th17 cells and loss of Th17/Treg balance. Further analysis of such changes in the composition of subset CD4+ T helper and Tregs may shed new light on the immunopathology of HIV disease and suggest new strategies for therapeutic and preventive interventions.
Keywords: HIV; indoleamine 2,3-dioxygenase; regulatory T cells; Th17 cells
Introduction
The Th1/Th2 paradigm has shaped our understanding of adaptive immune responses by delineating the interactions that can occur between T helper subsets [1]. More recently, attention has focused on the reciprocal relationship betweenCD4+T cells secreting interleukin-17 (Th17 cells) and CD4+CD25highFoxP3+ regulatory T cells (Tregs). Cells of these two lineages are derived from a common progenitor [2–5,6••,7•,8] and their differentiation pathways are reciprocally modulated in a number of aberrant immune disorders related to host–pathogen interactions, inflammatory syndromes, autoimmune diseases, and primary immune deficiencies [9,10]. In the present article, we discuss the hypothesis that it is the relative balance of these two subsets, rather than the function of either alone, that may drive the immunopathology of progressive disease caused by simian immunodeficiency virus (SIV) and HIV.
The acute phase of SIV and HIV infection is a period of aberrant immune activation and rapid loss of CD4+T cells in lymphoid tissues such as the gastrointestinal tract. Early studies suggested that, during this time, resting memory CD4+ T cells are preferentially infected and activated effector memory T cells undergo apoptosis, and that early treatment with highly active antiretroviral therapy can reverse these effects [11,12]. In subsequent studies by Brenchley et al. [13], circulating levels of microbial products such as lipopolysaccharide (LPS) were found to be significantly increased in chronically infected HIV patients and in SIV-infected rhesus macaques, suggesting that breaches in the gut epithelial barrier facilitate microbial translocation and prompt the persistent immune activation that characterizes disease progression to the AIDS. By contrast, even though massive depletion of CD4+ T cells also occurs in the gastrointestinal tract of SIV-infected sooty mangabeys and African green monkeys (nonhuman primate species that do not progress to AIDS despite chronic infection with SIV) [14], microbial translocation is not observed [15,16]. Put together, these findings suggest that a critical determinant of disorder in lentiviral disease lies not so much in the quantity of CD4+ T cells lost but in qualitative changes that may instead relate to the relative composition of CD4+ T cell subsets.
The reciprocal development of Th17 and regulatory T cells in mucosal tissue
The microbial flora of the intestinal tract is composed primarily of bacteria that are commensal to and in some situations symbiotic with the host. In part, it is the role of the intestinal immune system to confer tolerance toward these organisms whereas also prompting immune responses against the occasional pathogen that enters [17,18•–20•]. At least two populations of CD3+CD4+ T cells have been found to be important in mediating this balance. Tregs expressing the transcription factor, forkhead box P3 (FoxP3), and Th17 cells secreting the cytokine, interleukin-17. Interestingly, Tregs and Th17 cells share common chemokine receptors (CCR6, CCR4) and homing properties (CCL20) [21] and are derived from a common progenitor cell [22–24], the differentiation of which is dependent upon the stimulation of mucosal dendritic cells and macrophages by microbial, parasitic, or fungal products [25–27] and cytokines including interleukin-23, interleukin-1β, and interleukin-6 [26,28].
Th17 cells are critical in the defense against bacteria and fungi, and also contribute to the homeostasis of enterocytes [2,29–31]. This T cell subset, however, has also recently been implicated in the pathology of human inflammatory bowel diseases (IBD) [32]. Th17 cells express the interleukin-23 receptor (IL23R) and, in genome-wide association studies, the IL23R as well as other genes involved in the differentiation of Th17 cells have been recognized as IBD susceptibility genes [33]. In small animal models of IBD and of autoimmune inflammation of the brain and nervous system, models that were typically thought to be Th1-dependent, mice deficient in the interleukin-23p19 subunit did not develop inflammation whereas those deficient in the interleukin-12p35 subunit did [34,35]. This seminal observation identified interleukin-23 as a critical driver of autoimmune inflammation and, in so doing, underscored the importance of the Th17 lineage. In subsequent studies, Annunziato et al. [36] described a population of human gut-associated lymphoreticular tissue (GALT) T cells that express both interleukin-17A and interferon-γ (IFNγ), raising the possibility that these cells may play a role in the pathophysiology of human IBD [36–38].
In contrast to the pro-inflammatory effects associated with Th17 cells, naturally arising CD4+CD25highFoxP3+ + Tregs induce tolerance against self antigens and prevent autoimmunity. As the initial description of such cells nearly 15 years ago by Sakaguchi et al. [39], their importance in various rodent models of experimental colitis has been highlighted by their ability to reverse both acute and chronic inflammation [40–46]. In studies of human IBD, however, such immunosuppressive effects have been less clearly demonstrated, prompting Maul et al. [47] to suggest that it may be more relevant to focus instead on the balance between Tregs and other proinflammatory T cell subsets (e.g., Th1 and Th17 cells). A number of studies have shown that FoxP3+ Tregs exert anti-inflammatory functions and control self-reactive T cells, including Th1, Th2, and Th17 cells [48,49]. In the context of acute and chronic infectious diseases, the net outcome of these effects remains unclear [50]. In the context of HIV and SIV disease, for instance, Tregs might decrease chronic immune activation, thereby slowing disease progression [51]; conversely, they might inhibit antiviral immune responses, thereby hastening disease progression [52,53]. Discriminating between these two extremes has been hampered by the difficulty of precisely identifying distinct subsets of FoxP3+ Tregs in vivo [54] and quantifying their mechanism(s) of suppression in vitro [55].
Studies in mice and in humans have defined a developmental link between Th17 cells and Tregs. Thus, transforming growth factor-β (TGF-β) is essential for the development of Th17 cells [2,3,56,57], primarily because it upregulates the retinoic acid receptor-related orphan receptor-γt (ROR-γt, encoded by RORc gene) [22,58], the master transcription factor of Th17 differentiation. Interestingly, TGF-β is also known to induce the Treg specific transcription factor, FoxP3, a key regulatory gene for the development of Tregs [59,60]. In a study first demonstrating the reciprocal nature of Treg versus Th17 differentiation, Bettelli et al. [4] found that the addition of interleukin-6 to TGF-β inhibits the generation of Tregs and induces the development of Th17 cells. Further support for the reciprocal development of Th17 and Treg cells was obtained in studies of retinoic acid [5,61] (which induces FoxP3, inhibits ROR-γt in Th17-inducing conditions and promotes the development of Tregs), of ligands of the aryl hydrocarbon receptor (AhR) [7•], and of Stat3 deficiency (a critical transcriptional factor related to Th17 development in humans [62•,63•] and directly involved in Treg contol over Th17 responses in mice [64••]). The above observations, coupled with the known effects of lentiviral infection of the gastrointestinal tract, beg the question: is it possible that the relative proportion of Th17 cells and Tregs might also influence the progression of lentiviral disease in vivo?
Sustained immune activation and the shift in Th17/regulatory T cells balance
Although CD4+ T cell depletion is the hallmark of HIV disease, the mechanisms leading to such depletion in vivo remain unclear. Possible mechanisms include viral lysis, apoptosis, and/or immune clearance of HIV-infected cells [65], bystander activation-induced cell death (AICD) and apoptosis of neighboring noninfected T cells [66,67], and impaired regenerative capacity, coupled with the destruction of essential hematopoietic progenitor cells and hastened by the chronic immune activation that attends HIV infection [13,68,69]. As early as 1984, Kotler et al. [70] observed histological abnormalities and lymphocyte depletion in GALT mucosa of infected individuals and first suggested a role for the mucosal immune system in disease progression. More recently, in HIV-infected patients, chronic immune activation has been found to be associated with a higher frequency of circulating T cells with an activated phenotype as well as with increased levels of pro-inflammatory cytokines and chemokines [71–73]. Such chronic CD4+ and CD8+ T cell activation ultimately leads to clonal exhaustion of memory T cell pools and also provides an increased frequency of target cells for viral infection, thus leading to increased viral loads and systemic dissemination [74,75].
To better define the role of Th17 and Tregs in lentiviral disease progression, we studied SIV infection in a pathogenic model (pigtailed macaques) and in a nonpathogenic model (African green monkeys), and found that disease progression was associated with the loss of Th17 cells and an increase in the frequency of CD4+FoxP3+ Tregs [76••]. The loss of Th17/Treg balance was associated with sustained systemic immune activation and a shift toward cellular stress pathways, nuclear factor-κB signaling, and Th1 profiles [76••,77••]. The loss of Th17 cells was also evident in blood and in rectosigmoid mucosal biopsies from chronically HIV-infected patients [78] and associated with sustained immune activation, microbial translocation, and disease progression (Favre et al., unpublished observation). Because Th17 cells can enhance host defenses against microbial agents [79–81], thus maintaining the integrity of the mucosal barrier [2,29–31,82,83], loss of Th17 cells in HIV disease might account for an increase in microbial translocation across the gastrointestinal mucosa [76••,78,84•]. A current working model proposes that maintaining robust Th17 function in mucosal tissues during HIV infection may prevent immune activation that would otherwise occur after microbial translocation and spread from the gut [75,85].
Possible mechanisms and consequences of loss of the Th17/regulatory T cells balance
The mechanisms responsible for selective depletion of Th17 cells during pathogenic SIV and HIV infection remain undefined. As IFNγ can directly impair subsequent Th17 development [86], the Th1/interleukin-12 prone inflammatory environment generated during pathogenic HIV and SIV infection may tilt the balance in the favor of Treg differentiation. However, as preferential Th17 depletion occurs quite quickly (e.g., in a matter of days after acute infection in nonhuman primates) [76••,87,88•], it seems more likely that destruction of existing Th17 cells must also occur. At this point, it is not clear whether these cells are preferentially infected by virus [88•] or, instead, indirectly destroyed as bystanders [78]. Cecchinato et al. [87] first showed that Th17 cells were infected by SIV and that depletion of Th17 cells in mucosal tissues strongly correlated with viral loads. Kader et al. [88•] extended this analysis to show that most Th17 cells in the peripheral blood were resting CD4+ T cells expressing high levels of integrin α4β7 heterodimer, and were preferentially infected and depleted during primary SIV infection. Ancuta et al. also found that peripheral blood memory CD4+ T cells expressing the chemokine receptor, CCR6 (a phenotype associated with Th17 cells and also Tregs [21,89]), displayed increased susceptibility to viral infection by CCR5 and CXCR4-tropic viruses in vitro and increased viral DNA content in HIV-infected individuals (Gosselin et al. Keystone 2009 and P. Ancuta, personal communication). Together, these results suggest that preferential HIV infection may account, at least in part, for the selective depletion of Th17 cells.
A number of considerations suggest, however, that viral and/or nonviral bystander mechanisms of selective Th17 depletion and Treg expansion are also likely at play. First, preferential infection of Th17 cells is not always found in viremic patients [78] or in SIV-infected macaques [87]. Second, the same virus inoculated in different hosts can result in massive Th17 depletion (in pathogenic infection in macaque) or not (in nonpathogenic infection in African green monkeys), despite similar viral loads [76••]. Finally, and perhaps most importantly, Tregs (and, to some degree, Th1 cells) are instead increased in fraction and/or numbers in lymphoid tissues during progressive HIV disease [53,76••, 90], even though they share common features with Th17 cells, including memory phenotype, chemokine receptors (CCR6, CCR4), homing properties (CCL20) [21], levels of CCR5 expression [89], and the propensity for lentiviral infection in vitro [91].
Apoptosis and AICD of noninfected CD4+ T cells have been correlated with chronic immune activation and inflammation during HIV disease progression [66,67]. One might speculate that selective loss of Th17 cells is due to the fact that they are more susceptible to cell death caused by such activation than are Tregs. For example, an intriguing developmental link exists between indoleamine 2,3-dioxygenase (IDO) metabolism and the differentiation of Th17 and Tregs from naive T cells. IDO is the rate-limiting enzyme involved in the catabolism of the amino acid tryptophan through the kynurenine pathway [92]. Tryptophan catabolites are able to induce the expression of FoxP3 and the generation of Tregs, and to suppress the expression of RORc and the generation of Th17 cells [93,94••]. Similarly, IDO-mediated tryptophan deprivation and the amino acid starvation response can induce Treg development and blunt Th17 conversion [95•,96]. Predominantly found in macrophages and dendritic cells, the expression of IDO is upregulated by interferons and by agonists of toll-like receptors [92]. When catalytically active, the enzyme suppresses T cell responses in a variety of settings, including autoimmune disorders [97], allograft rejection [98], viral infections [99], cancer [100], and pregnancy [101]. Such suppression is thought to occur either because IDO depletes the essential amino acid tryptophan and/or because it produces tryptophan catabolites that are toxic to T cells [102,103]. In either case, the ability of IDO to suppress immune responses has raised the possibility that it may contribute to the immunodeficiency found in individuals with progressive HIV disease [104]. As IDO metabolism is related both to the Treg to Th17 developmental switch and to HIV pathogenesis [104], we have explored the relationships between HIV disease, Th17 and Treg subsets, and IDO metabolism. In ongoing studies, we have now shown that enhanced IDO activity is associated with HIV disease progression, and that such activity results in an imbalance of Th17 cells and Tregs in the peripheral blood and in rectosigmoid tissue that is mediated by the tryptophan catabolite, 3-hydroxyanthranilic acid (Favre and Mold et al., submitted).
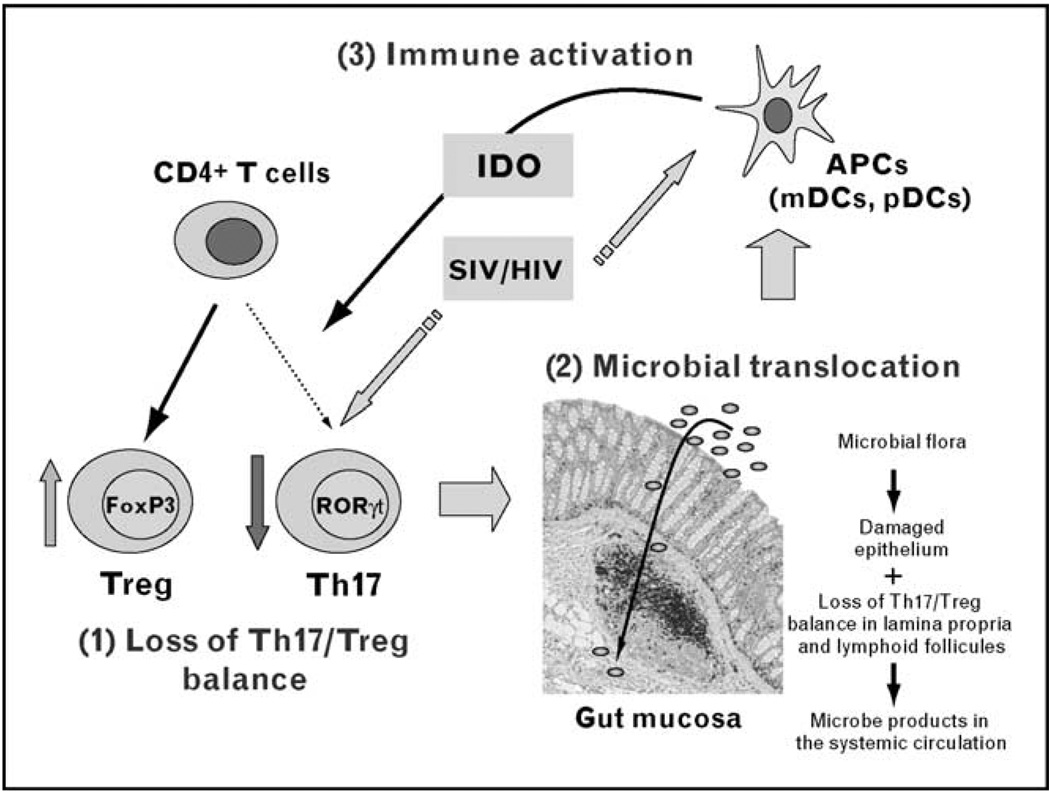
Although IDO-mediated depletion of Th17 cells could occur in concert with other mechanisms (e.g., bystander CD4+ T cell death through interactions with gp120/gp41 [105–107], Tat [108], Nef [109], or Vpr [110]), the induction of IDO may represent a critical initiating event that results in inversion of the Th17/Treg balance and in the maintenance of the chronic inflammatory state of progressive disease (see Fig. 1). This chronic activation of the IDO pathway diminishes the host’s capacity to generate Th17 cells and favors the generation of Tregs. The net outcome is a progressive loss of the mucosal immune barrier that is critically dependent upon Th17 cells coupled with a rise in Tregs, which may dampen effective T cell immune responses to HIV and other pathogenic organisms.
Figure 1. Loss of Th17/regulatory T cells balance and AIDS pathogenesis.
Direct simian immunodeficiency virus (SIV) or HIV infection and/or upregulation of indoleamine 2,3-dioxygenase (IDO) by plasmacytoid (pDCs) and myeloid (mDCs) dendritic cells leads to the preferential depletion of Th17 cells with a reciprocal increase of CD4+CD25highFoxP3+ regulatory T cells (Tregs) in peripheral blood and in the mucosa of the gastrointestinal tract (1). Loss of Th17/Treg balance results in progressive disruption of the mucosal barrier, permitting increased translocation of the gut microbial flora (2). Setting up a positive feedback loop, microbial products then activate antigen-presenting cells (APCs) in the mucosa and in lymphoid organs, enhancing local and systemic inflammation and immune activation, and resulting in sustained loss of the Th17/Treg balance (3). The picture of ‘gut mucosa’ represents the colonic mucosa of an SIV-infected monkey, with CD3+ T cells stained in black. Bacterial translocation from the lumen to the lamina propria is represented schematically. RORγt, receptor-related orphan receptor-γt.
Conclusion
Although additional work is required to clearly define the mechanisms of selective Th17 depletion as well as to definitively link changes in the Th17/Treg balance with the immunopathology of HIV infection, we believe that further attention to the balance of Th17 and Treg subsets will reveal much about the relationship between disease progression and inflammation. For example, several tryptophan derivatives are known to be natural ligands of the AhR, a receptor also involved in the balance of Th17 and Treg cells in vivo [7•,8]. An intriguing possibility for immune intervention may be to delineate the AhR signaling pathways associated with IDO metabolism so that strategies to blunt and to reverse Th17/Treg loss could be devised. For prophylactic vaccine strategies, on the contrary, it may be favorable to generate combined HIV-specific Th17 and Th1 responses in mucosal tissue that will sustain and/or generate a balanced representation of Th17/Treg and Th1 subsets therein. In this manner, the host may be able to establish an environment that is more likely to minimize inflammation and to limit the extent of viral replication and spread [111••].
Acknowledgement
The authors would like to thank Dr Jeff Mold for the photomicrograph shown in Fig. 1.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 196–197).
- 1.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 2.Mangan PR, Harrington LE, O’Quinn DB, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 3.Veldhoen M, Hocking RJ, Atkins CJ, et al. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 5.Mucida D, Park Y, Kim G, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 6. Zhou L, Lopes JE, Chong MM, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. The authors show that TGF-β-induced FoxP3 expression can restrain Th17 development, depending on the concentration of TGF-β and the presence of the inflammatory cytokines, interleukin-6, and interleukin-21
- 7. Quintana FJ, Basso AS, Iglesias AH, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. The authors show that activation of the AhR by environmental toxins and natural agonists derived from tryptophan differently regulates Treg and Th17 differentiation
- 8.Veldhoen M, Hirota K, Christensen J, et al. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J Exp Med. 2009;206:43–49. doi: 10.1084/jem.20081438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basso AS, Cheroutre H, Mucida D. More stories on Th17 cells. Cell Res. 2009;19:399–411. doi: 10.1038/cr.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ochs HD, Oukka M, Torgerson TR. TH17 cells and regulatory T cells in primary immunodeficiency diseases. J Allergy Clin Immunol. 2009;123:977–983. doi: 10.1016/j.jaci.2009.03.030. quiz 984–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guadalupe M, Reay E, Sankaran S, et al. Severe CD4+T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Q, Duan L, Estes JD, et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 13.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 14.Silvestri G, Paiardini M, Pandrea I, et al. Understanding the benign nature of SIV infection in natural hosts. J Clin Invest. 2007;117:3148–3154. doi: 10.1172/JCI33034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon SN, Klatt NR, Bosinger SE, et al. Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. J Immunol. 2007;179:3026–3034. doi: 10.4049/jimmunol.179.5.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandrea IV, Gautam R, Ribeiro RM, et al. Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. J Immunol. 2007;179:3035–3046. doi: 10.4049/jimmunol.179.5.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 18. Gaboriau-Routhiau V, Rakotobe S, Lecuyer E, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31:677–689. doi: 10.1016/j.immuni.2009.08.020. The present study highlight the interplay between commensal microbial flora, for example, segmented filamentous bacteria, and the generation of mucosal Th17 cells
- 19. Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. The present study highlight the interplay between commensal microbial flora, for example, segmented filamentous bacteria, and the generation of mucosal Th17 cells
- 20. Ivanov II, Frutos Rde L, Manel N, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. The present study highlight the interplay between commensal microbial flora, for example, segmented filamentous bacteria, and the generation of mucosal Th17 cells.
- 21.Acosta-Rodriguez EV, Rivino L, Geginat J, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 22.Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor ROR-gammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 23.Gavin MA, Rasmussen JP, Fontenot JD, et al. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 24.Yang XO, Pappu BP, Nurieva R, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denning TL, Wang YC, Patel SR, et al. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 26.van Beelen AJ, Zelinkova Z, Taanman-Kueter EW, et al. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27:660–669. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 27.LeibundGut-Landmann S, Gross O, Robinson MJ, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 28.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, et al. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 29.Ye P, Garvey PB, Zhang P, et al. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001;25:335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 30.Kleinschek MA, Muller U, Brodie SJ, et al. IL-23 enhances the inflammatory cell response in Cryptococcus neoformans infection and induces a cytokine pattern distinct from IL-12. J Immunol. 2006;176:1098–1106. doi: 10.4049/jimmunol.176.2.1098. [DOI] [PubMed] [Google Scholar]
- 31.Higgins SC, Jarnicki AG, Lavelle EC, et al. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells. J Immunol. 2006;177:7980–7989. doi: 10.4049/jimmunol.177.11.7980. [DOI] [PubMed] [Google Scholar]
- 32.Seiderer J, Elben I, Diegelmann J, et al. Role of the novel Th17 cytokine IL-17F in inflammatory bowel disease (IBD): upregulated colonic IL-17F expression in active Crohn’s disease and analysis of the IL17F p.His161Arg polymorphism in IBD. Inflamm Bowel Dis. 2008;14:437–445. doi: 10.1002/ibd.20339. [DOI] [PubMed] [Google Scholar]
- 33.Duerr RH, Taylor KD, Brant SR, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cua DJ, Sherlock J, Chen Y, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 35.Murphy CA, Langrish CL, Chen Y, et al. Divergent pro- and anti-inflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Annunziato F, Cosmi L, Santarlasci V, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi T, Okamoto S, Hisamatsu T, et al. IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn’s disease. Gut. 2008;57:1682–1689. doi: 10.1136/gut.2007.135053. [DOI] [PubMed] [Google Scholar]
- 38.Sakuraba A, Sato T, Kamada N, et al. Th1/Th17 immune response is induced by mesenteric lymph node dendritic cells in Crohn’s disease. Gastroenterology. 2009;137:1736–1745. doi: 10.1053/j.gastro.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 39.Sakaguchi S, Sakaguchi N, Asano M, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 40.Ishimaru N, Yamada A, Kohashi M, et al. Development of inflammatory bowel disease in Long-Evans Cinnamon rats based on CD4+CD25+Foxp3+ regulatory T cell dysfunction. J Immunol. 2008;180:6997–7008. doi: 10.4049/jimmunol.180.10.6997. [DOI] [PubMed] [Google Scholar]
- 41.Totsuka T, Kanai T, Nemoto Y, et al. RANK-RANKL signaling pathway is critically involved in the function of CD4+CD25+ regulatory T cells in chronic colitis. J Immunol. 2009;182:6079–6087. doi: 10.4049/jimmunol.0711823. [DOI] [PubMed] [Google Scholar]
- 42.Groux H, O’Garra A, Bigler M, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 43.Denning TL, Qi H, Konig R, et al. CD4+ Th cells resembling regulatory T cells that inhibit chronic colitis differentiate in the absence of interactions between CD4 and class II MHC. J Immunol. 2003;171:2279–2286. doi: 10.4049/jimmunol.171.5.2279. [DOI] [PubMed] [Google Scholar]
- 44.Kullberg MC, Jankovic D, Gorelick PL, et al. Bacteria-triggered CD4(+) T regulatory cells suppress Helicobacter hepaticus-induced colitis. J Exp Med. 2002;196:505–515. doi: 10.1084/jem.20020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oida T, Zhang X, Goto M, et al. CD4+CD25− T cells that express latency-associated peptide on the surface suppress CD4+CD45RBhigh-induced colitis by a TGF-beta-dependent mechanism. J Immunol. 2003;170:2516–2522. doi: 10.4049/jimmunol.170.5.2516. [DOI] [PubMed] [Google Scholar]
- 46.Uraushihara K, Kanai T, Ko K, et al. Regulation of murine inflammatory bowel disease by CD25+ and CD25− CD4+ glucocorticoid-induced TNF receptor family-related gene+ regulatory T cells. J Immunol. 2003;171:708–716. doi: 10.4049/jimmunol.171.2.708. [DOI] [PubMed] [Google Scholar]
- 47.Maul J, Loddenkemper C, Mundt P, et al. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–1878. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 48.Sakaguchi S, Powrie F. Emerging challenges in regulatory T cell function and biology. Science. 2007;317:627–629. doi: 10.1126/science.1142331. [DOI] [PubMed] [Google Scholar]
- 49.Chen X, Vodanovic-Jankovic S, Johnson B, et al. Absence of regulatory T cell control of TH1 and TH17 cells is responsible for the autoimmune-mediated pathology in chronic graft versus host disease. Blood. 2007;110:3804–3813. doi: 10.1182/blood-2007-05-091074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat Rev Immunol. 2007;7:875–888. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- 51.Kinter AL, Hennessey M, Bell A, et al. CD25(+)CD4(+) regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4(+) and CD8(+) HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J Exp Med. 2004;200:331–343. doi: 10.1084/jem.20032069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aandahl EM, Michaelsson J, Moretto WJ, et al. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J Virol. 2004;78:2454–2459. doi: 10.1128/JVI.78.5.2454-2459.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nilsson J, Boasso A, Velilla PA, et al. HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood. 2006;108:3808–3817. doi: 10.1182/blood-2006-05-021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 55.von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 56.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–591. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 57.Yang L, Anderson DE, Baecher-Allan C, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 61.Benson MJ, Pino-Lagos K, Rosemblatt M, et al. All-trans retinoic acid mediates enhanced Treg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Milner JD, Brenchley JM, Laurence A, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–776. doi: 10.1038/nature06764. The present study link genetic deficiency of Stat3 signaling with impaired Th17 development in human patients with Job’s syndrome, characterized by chronic mucosal bacterial and fungal infections.
- 63. de Beaucoudrey L, Puel A, Filipe-Santos O, et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med. 2008;205:1543–1550. doi: 10.1084/jem.20080321. The present study link genetic deficiency of Stat3 signaling with impaired Th17 development in human patients with Job’s syndrome, characterized by chronic mucosal bacterial and fungal infections.
- 64. Chaudhry A, Rudra D, Treuting P, et al. CD4+ Regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. This study shows that Tregs can adapt to their environment upon activation of STAT proteins and restrain pathogenic Th17 responses.
- 65.Lenardo MJ, Angleman SB, Bounkeua V, et al. Cytopathic killing of peripheral blood CD4(+) T lymphocytes by human immunodeficiency virus type 1 appears necrotic rather than apoptotic and does not require env. J Virol. 2002;76:5082–5093. doi: 10.1128/JVI.76.10.5082-5093.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meyaard L, Otto SA, Jonker RR, et al. Programmed death of T cells in HIV-1 infection. Science. 1992;257:217–219. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- 67.Gougeon ML, Montagnier L. Apoptosis in AIDS. Science. 1993;260:1269–1270. doi: 10.1126/science.8098552. [DOI] [PubMed] [Google Scholar]
- 68.Grossman Z, Meier-Schellersheim M, Sousa AE, et al. CD4+ T-cell depletion in HIV infection: are we closer to understanding the cause? Nat Med. 2002;8:319–323. doi: 10.1038/nm0402-319. [DOI] [PubMed] [Google Scholar]
- 69.Douek DC, Picker LJ, Koup RA. T cell dynamics in HIV-1 infection. Annu Rev Immunol. 2003;21:265–304. doi: 10.1146/annurev.immunol.21.120601.141053. [DOI] [PubMed] [Google Scholar]
- 70.Kotler DP, Gaetz HP, Lange M, et al. Enteropathy associated with the acquired immunodeficiency syndrome. Ann Intern Med. 1984;101:421–428. doi: 10.7326/0003-4819-101-4-421. [DOI] [PubMed] [Google Scholar]
- 71.Deeks SG, Kitchen CM, Liu L, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 72.Valdez H, Lederman MM. Cytokines and cytokine therapies in HIV infection. AIDS Clin Rev. 1997:187–228. [PubMed] [Google Scholar]
- 73.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kovacs JA, Lempicki RA, Sidorov IA, et al. Identification of dynamically distinct subpopulations of T lymphocytes that are differentially affected by HIV. J Exp Med. 2001;194:1731–1741. doi: 10.1084/jem.194.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brenchley JM, Price DA, Douek DC. HIV disease: fallout from a mucosal catastrophe? Nat Immunol. 2006;7:235–239. doi: 10.1038/ni1316. [DOI] [PubMed] [Google Scholar]
- 76. Favre D, Lederer S, Kanwar B, et al. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009;5:e1000295. doi: 10.1371/journal.ppat.1000295. The companion study [77••] shows for the first time that rapid Th17 depletion during acute infection is predictive of subsequent chronic immune activation and that the loss of Th17/Treg balance is directly related to pathogenic infection in nonhuman primates. Transcriptional profiling also revealed that an initial inflammatory response recedes in the case of nonpathogenic SIV infection but is sustained in the case of pathogenic infection.
- 77. Lederer S, Favre D, Walters KA, et al. Transcriptional profiling in pathogenic and nonpathogenic SIV infections reveals significant distinctions in kinetics and tissue compartmentalization. PLoS Pathog. 2009;5:e1000296. doi: 10.1371/journal.ppat.1000296. The companion study [76••] shows for the first time that rapid Th17 depletion during acute infection is predictive of subsequent chronic immune activation and that the loss of Th17/Treg balance is directly related to pathogenic infection in nonhuman primates. Transcriptional profiling also revealed that an initial inflammatory response recedes in the case of nonpathogenic SIV infection but is sustained in the case of pathogenic infection.
- 78.Brenchley JM, Paiardini M, Knox KS, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 80.McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006;27:17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 81.Kinugasa T, Sakaguchi T, Gu X, et al. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology. 2000;118:1001–1011. doi: 10.1016/s0016-5085(00)70351-9. [DOI] [PubMed] [Google Scholar]
- 82.Wu Y, Borde M, Heissmeyer V, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 83.Khader SA, Bell GK, Pearl JE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 84. Raffatellu M, Santos RL, Verhoeven DE, et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14:421–428. doi: 10.1038/nm1743. The authors show that mucosal Th17 depletion in chronically SIV-infected macaques is related to impaired mucosal barrier function after Salmonella typhimurium challenge.
- 85.Rice L, Orlow D, Ceonzo K, et al. CpG oligodeoxynucleotide protection in polymicrobial sepsis is dependent on interleukin-17. J Infect Dis. 2005;191:1368–1376. doi: 10.1086/428452. [DOI] [PubMed] [Google Scholar]
- 86.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 87.Cecchinato V, Trindade CJ, Laurence A, et al. Altered balance between Th17 and Th1 cells at mucosal sites predicts AIDS progression in simian immunodeficiency virus-infected macaques. Mucosal Immunol. 2008;1:279–288. doi: 10.1038/mi.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kader M, Wang X, Piatak M, et al. Alpha4(+)beta7(hi)CD4(+) memory T cells harbor most Th-17 cells and are preferentially infected during acute SIV infection. Mucosal Immunol. 2009;2:439–449. doi: 10.1038/mi.2009.90. This study showed that the colon homing receptor, integrin α4β7, is expressed at high levels on peripheral blood Th17 cells and that these cells are preferentially infected with SIV at early time points after infection. As the α4β7 receptor has been previously shown to bind to HIV gp120, it may also be involved in the tropism of SIV for Th17 cells.
- 89.Lim HW, Lee J, Hillsamer P, et al. Human Th17 cells share major trafficking receptors with both polarized effector T cells and FOXP3+ regulatory T cells. J Immunol. 2008;180:122–129. doi: 10.4049/jimmunol.180.1.122. [DOI] [PubMed] [Google Scholar]
- 90.Estes JD, Li Q, Reynolds MR, et al. Premature induction of an immunosuppressive regulatory T cell response during acute simian immunodeficiency virus infection. J Infect Dis. 2006;193:703–712. doi: 10.1086/500368. [DOI] [PubMed] [Google Scholar]
- 91.Moreno-Fernandez ME, Zapata W, Blackard JT, et al. Human regulatory T cells are targets for HIV infection and their susceptibility differs depending on the HIV-1 strain. J Virol. 2009;83:12925–12933. doi: 10.1128/JVI.01352-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 93.De Luca A, Montagnoli C, Zelante T, et al. Functional yet balanced reactivity to Candida albicans requires TRIF, MyD88, and IDO-dependent inhibition of RORc. J Immunol. 2007;179:5999–6008. doi: 10.4049/jimmunol.179.9.5999. [DOI] [PubMed] [Google Scholar]
- 94. Romani L, Fallarino F, De Luca A, et al. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451:211–215. doi: 10.1038/nature06471. This study shows the causal role of tryptophan catabolites and the indoleamine 2,3 dioxygenase (IDO) pathway in controlling the balance of interleukin-17 expressing T cells and Tregs in chronic inflammatory disease.
- 95. Sundrud MS, Koralov SB, Feuerer M, et al. Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response. Science. 2009;324:1334–1338. doi: 10.1126/science.1172638. This study showed that amino acid starvation responses, such as those induced by the IDO pathway can inhibit Th17 development in the mouse.
- 96.Sharma MD, Hou DY, Liu Y, et al. Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood. 2009;113:6102–6111. doi: 10.1182/blood-2008-12-195354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Platten M, Ho PP, Youssef S, et al. Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science. 2005;310:850–855. doi: 10.1126/science.1117634. [DOI] [PubMed] [Google Scholar]
- 98.Bauer TM, Jiga LP, Chuang JJ, et al. Studying the immunosuppressive role of indoleamine 2,3-dioxygenase: tryptophan metabolites suppress rat allogeneic T-cell responses in vitro and in vivo. Transpl Int. 2005;18:95–100. doi: 10.1111/j.1432-2277.2004.00031.x. [DOI] [PubMed] [Google Scholar]
- 99.Fuchs D, Moller AA, Reibnegger G, et al. Increased endogenous interferon-gamma and neopterin correlate with increased degradation of tryptophan in human immunodeficiency virus type 1 infection. Immunol Lett. 1991;28:207–211. doi: 10.1016/0165-2478(91)90005-u. [DOI] [PubMed] [Google Scholar]
- 100.Katz JB, Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev. 2008;222:206–221. doi: 10.1111/j.1600-065X.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 101.Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 102.Munn DH, Sharma MD, Baban B, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 103.Terness P, Bauer TM, Rose L, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196:447–457. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Murray MF. Tryptophan depletion and HIV infection: a metabolic link to pathogenesis. Lancet Infect Dis. 2003;3:644–652. doi: 10.1016/s1473-3099(03)00773-4. [DOI] [PubMed] [Google Scholar]
- 105.Banda NK, Bernier J, Kurahara DK, et al. Crosslinking CD4 by human immunodeficiency virus gp120 primes T cells for activation-induced apoptosis. J Exp Med. 1992;176:1099–1106. doi: 10.1084/jem.176.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vieillard V, Strominger JL, Debre P. NK cytotoxicity against CD4+ T cells during HIV-1 infection: a gp41 peptide induces the expression of an NKp44 ligand. Proc Natl Acad Sci U S A. 2005;102:10981–10986. doi: 10.1073/pnas.0504315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Boasso A, Herbeuval JP, Hardy AW, et al. HIV inhibits CD4+ T-cell proliferation by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood. 2007;109:3351–3359. doi: 10.1182/blood-2006-07-034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li CJ, Friedman DJ, Wang C, et al. Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science. 1995;268:429–431. doi: 10.1126/science.7716549. [DOI] [PubMed] [Google Scholar]
- 109.Swingler S, Mann A, Jacque J, et al. HIV-1 Nef mediates lymphocyte chemotaxis and activation by infected macrophages. Nat Med. 1999;5:997–1003. doi: 10.1038/12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jacotot E, Ravagnan L, Loeffler M, et al. The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J Exp Med. 2000;191:33–46. doi: 10.1084/jem.191.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Li Q, Estes JD, Schlievert PM, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–1038. doi: 10.1038/nature07831. This study shows that innate host responses can recruit target cells to the site of infection and facilitate the spread of infection. Conversely, the inhibition of such responses by antimicrobial and anti-inflammatory compounds can protect rhesus macaques from acute infection after repeated intravaginal exposure to high doses of SIV.