Abstract
Oxidized phospholipids (OxPLs) are increasingly recognized as pleiotropic lipid mediators demonstrating a variety of biological activities. In particular, OxPLs induce electrophilic stress response and stimulate expression of NF-E2-related factor 2 (NRF2)-dependent genes. The mechanisms of NRF2 upregulation in response to OxPLs, however, are incompletely understood. Here we show that upregulation of NRF2 by OxPLs depends on the activity of the CK2 protein kinase. Inactivation of CK2 by chemical inhibitors or gene silencing resulted in diminished accumulation of NRF2 and its target genes, GCLM, HMOX1, and NQO1, downstream in response to OxPLs. Furthermore, inhibition of CK2 suppressed NRF2-dependent induction of ATF4 and its downstream gene VEGF. Thus, inactivation of CK2 in OxPL-treated endothelial cells results in inhibition of the NRF2-ATF4-VEGF axis and is likely to produce antiangiogenic effects. This work characterizes novel cross-talk between CK2 and cellular stress pathways, which may provide additional insights into the mechanisms of beneficial action and side-effects of CK2 inhibitors.
Keywords: angiogenesis, vascular endothelial growth factor, NF-E2-related factor 2, oxidized lipids
Oxidation of polyunsaturated fatty acids leads to formation of a variety of lipid mediators, for example, prostaglandins, leukotrienes, isoprostanes, etc. Oxidized phospholipids (OxPLs) contain esterified oxidized residues and are increasingly recognized as pleiotropic lipid messengers demonstrating multiple biological activities including their ability to induce monocytic inflammation, procoagulant shift in endothelium, regulation of endothelial barrier function, and angiogenesis (1). More recently, OxPLs were shown to activate electrophilic and unfolded protein cellular stress responses, which promote adaptation of cells to nonlethal stress conditions (2, 3).
Transcription factor NF-E2-related factor 2 (NRF2) plays a key role in the induction of cytoprotective genes upon electrophilic stress. Under nonstress conditions, NRF2 is bound by KEAP1, which directs NRF2 to proteasomal degradation (4). Electrophilic compounds induce accumulation of NRF2 in the nucleus and activation of transcription of its target genes. In addition to this canonical mechanism of NRF2 activation, several protein kinases such as PERK (5), phosphatidylinositol-3-kinase (PI3K) (6, 7), and CK2 (8, 9) were demonstrated to regulate nuclear accumulation or transcriptional activity of NRF2.
OxPLs have been shown to induce expression of several cytoprotective genes containing NRF2 binding elements in their promoter regions [e.g., heme oxygenase 1 (HMOX1) (10); glutamate-cysteine ligase modifier subunit (GCLM) (11); glutamate-cysteine ligase catalytic subunit (GCLC) (11); and NAD(P)H dehydrogenase, quinone 1 (NQO1) (3)]. In addition, our previous data suggest that NRF2 plays a key role in the induction of vascular endothelial growth factor (VEGF) A and angiogenic reactions induced by OxPLs (12–14). According to our model, activation of NRF2 by OxPLs stimulates transcription of ATF4, which in turn directly transactivates the VEGF gene. Inhibition of any step in the NRF2-ATF4-VEGF axis by drugs or gene knockdown results in inhibition of angiogenic responses of endothelial cells (ECs) to OxPLs (12–14). In summary, NRF2-dependent transcription is an important mechanism mediating biological activity of OxPLs.
In this work, we identified the CK2 protein kinase as an additional molecule necessary for OxPL-induced upregulation of electrophilic genes. We showed that inhibition of CK2 by drugs or short interfering RNA (siRNA)-mediated knockdown results in diminished induction of the HO-1, GCLM, NQO1, as well as ATF4 and VEGF. Data suggest that active CK2 is a prerequisite for full induction of NRF2 and its target genes in OxPL-treated ECs. Thus, our data suggest CK2 as the potential target for regulation of electrophilic stress response and angiogenesis induced by OxPLs.
MATERIALS AND METHODS
Materials, lipids, and cell culture
4,5,6,7-Tetrabromobenzotriazole (TBB), [5-oxo-5,6-dihydroindolo-(1,2-a)quinazolin-7-yl]acetic acid (IQA), tetrabromocinnamic acid (TBCA), and LY294002 were purchased from Calbiochem. Wortmannin (Wort), tert-butylhydroquinone (tBHQ), emodin, and sulforaphane were from Sigma-Aldrich. NRF2 and ATF4 antibodies were from Santa Cruz Biotechnology. Antibodies to total AKT and its phosphorylated forms were purchased from Cell Signaling Technology.
Synthetic 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (PAPC; Avanti Polar Lipids) was oxidized by exposing a thin lipid film to air. Oxidation was monitored by thin-layer chromatography and electrospray ionization-mass spectrometry (15). Concentrations of OxPAPC were determined by phosphorus assay (16). Dried lipids were resuspended by vigorous vortexing in medium M199 supplemented with 2% fetal calf serum (FCS) prior to use in cell culture experiments.
Human umbilical vein ECs (HUVECs), human coronary artery ECs (HCAECs), and human artery ECs (HAECs) were purchased from Lonza and grown at 37°C in 5% Co2 in medium M199 containing 20% FCS, 1 U/ml heparin, 50 μg/ml bovine EC growth supplement (Technoclone), 2 mmol/l glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were used up to passage 5. Cells were stimulated with lipids and other agonists in medium M199 containing 2% FCS.
RNA isolation, cDNA synthesis, and quantitative PCR
Isolation of RNA from HUVECs was performed using Trizol reagent (Invitrogen). A GeneAmp RNA-PCR kit and oligod(T)16 primers (Applied Biosystems) were used for cDNA synthesis from 900 ng of total RNA. Quantitative real-time PCR (qPCR) was performed using a LightCycler (Roche Diagnostics) and FastStart SYBR Green Master Mix (Roche Diagnostics). Sequences of primers are available on request.
Western blotting
After cells were lysed in Laemmli buffer, protein samples were separated using SDS-polyacrylamide gels and transferred onto Immobilon-P membranes (Millipore) by electroblotting. Membranes were probed with rabbit antibodies against ATF4, NRF2, phospho-AKT, and total AKT. Anti-rabbit IgG conjugated with peroxidase and LumiGLO chemiluminescent substrate (Cell Signaling Technology) were used for detection of bound primary antibodies. A FluorChem HD2 imager (Alpha Innotech) was used for chemiluminescence detection.
NRF2 binding competition assay
NRF2 DNA interactions were additionally characterized using an ELISA-based TransAM NRF2 kit (Active Motif). The procedure is based on detection of NRF2 bound to consensus antioxidant response element (ARE) immobilized in a 96-well plate. Nuclear extracts from OxPAPC-stimulated and untreated HUVECs were prepared using NE-Per kit (Pierce) after 2 h of incubation. Nuclear extract protein (2 μg) was incubated in the plate containing immobilized consensus NRF2 binding site. Wells were washed three times, and bound NRF2 was detected by NRF2 antibody and secondary antibody conjugated with horseradish peroxidase. The signal was detected spectrophotometrically at 450 nm.
RNA interference
CK2α- and CK2β-specific and control siRNAs were purchased from Ambion. Cells were transfected with 100 nmol/l siRNA using polyethylenimin (PEI) reagent in serum- and antibiotic-free medium M199 for 4 h (17). Thereafter, medium was changed to medium M199 containing 20% FCS, and cells were grown for 48 h. Efficiency of silencing was checked at the mRNA level.
Statistical analysis
Data are expressed as means ± standard deviations obtained from two to four independent experiments and were analyzed by two-tailed Student's t-test. A P value less than 0.05 was considered significant.
RESULTS
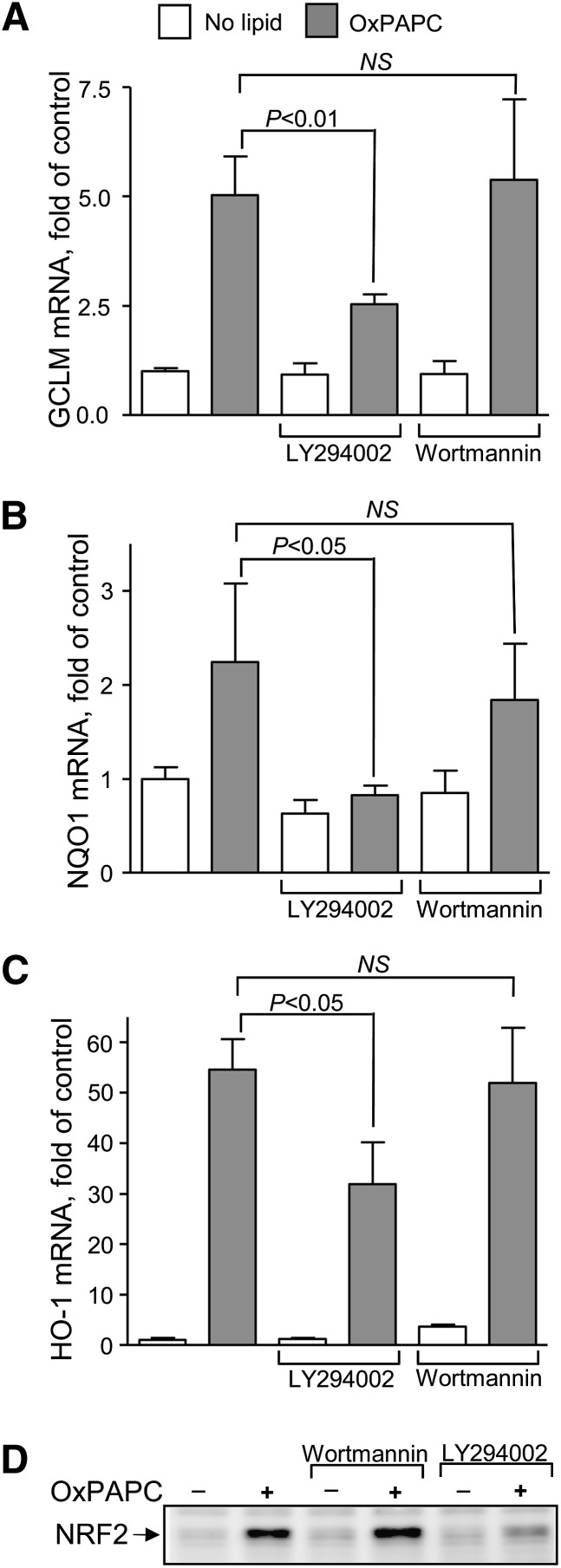
Previous studies have demonstrated that OxPLs induce NRF2 and electrophilic genes (3, 18, 19). However, the mechanisms of activation are only partially understood. A candidate signaling molecule is PI3K, which is well characterized as an important mediator of biological activity of OxPLs (20, 21) and, on the other hand, has been shown to play a critical role in the induction of NRF2 and NRF2-dependent genes in response to oxidative stressors and electrophilic compounds (7, 22–24). Based on these data, we asked whether PI3K plays a role in activation of electrophilic stress by OxPLs. Of the two PI3K inhibitors tested, LY294002 inhibited induction of the GCLM, NQO1, and HO-1 mRNAs and NRF2 protein, while Wort had no effect (Fig. 1). To ensure that Wort was active in our experiments, we analyzed OxPAPC-induced phosphorylation of AKT, which was inhibited both by LY294002 and by Wort (supplementary Fig. I). Based on these data, we hypothesized that PI3K is not involved in OxPL-induced upregulation of NRF2, and that LY294002 may inhibit another protein kinase which is important for induction of electrophilic response by OxPAPC.
Fig. 1.
Effect of PI3K inhibitors on induction of electrophilic genes and NRF2 by OxPAPC. A: GCLM mRNA was quantified in HUVECs stimulated with OxPAPC (130 µmol/l for 6 h) in the presence or absence of Wort (1 µmol/l or LY294002 (30 µmol/l). GCLM mRNA expression was quantified by real-time qPCR and normalized to β2-microglobulin mRNA levels. B, C: Effect of Wort and LY294002 on induction of NQO1 and HO-1 mRNAs levels by OxPAPC was studied as described above. D: Analysis of effects of Wort (1 µmol/l) and LY294002 (30 µmol/l) on the levels of NRF2 upon stimulation with OxPAPC (130 µmol/l, 2 h). Cell lysates were probed by Western blotting with antibodies against NRF2.
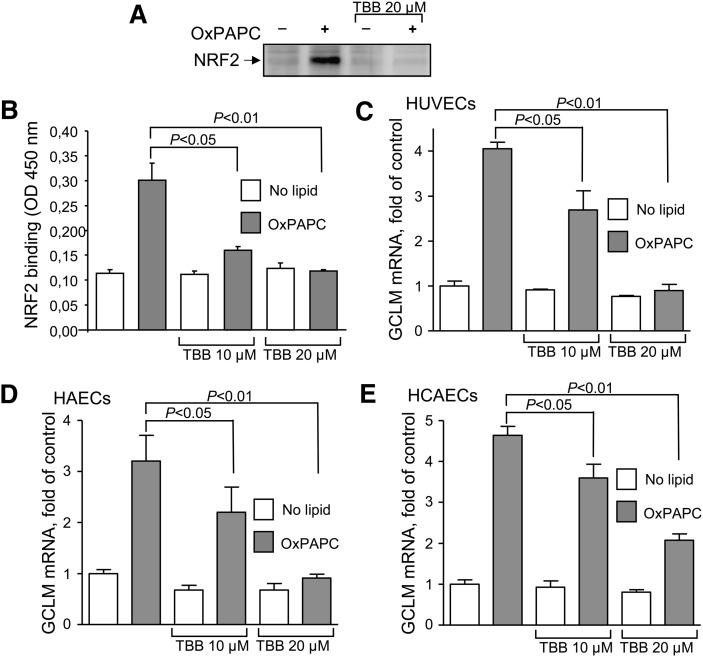
It is known that LY294002 inhibits the CK2 protein kinase with a potency similar to that of PI3K (26). In support of CK2 involvement, induction of the NRF2 protein and DNA binding activity (Fig. 2A, B), as well as upregulation of the electrophilic GCLM, NQO1, and HMOX1 genes by OxPLs (Fig. 2C and supplementary (Fig. II) were inhibited by the more specific CK2 inhibitor TBB. In addition to inhibiting HUVECs, the inhibitory action of TBB on the effects of OxPLs was observed in two types of aortic ECs (Fig. 2D, E). The action of TBB was independent of PI3K because TBB did not influence phosphorylation of AKT induced by OxPAPC (supplementary Fig. I). To ensure specificity of the effect, three additional CK2 inhibitors, TBCA, IQA, and emodin, were tested, and they also demonstrated inhibition of NRF2 accumulation and induction of GCLM in response to OxPAPC (supplementary Fig. III). The inhibitory effect did not result from nonspecific arrest of transcription in response to CK2 inhibitors, as TBB, TBCA, IQA, and emodin prevented induction of GCLM but did not inhibit upregulation of NRF2-independent genes (e.g., IL8 and PTGS2 encoding for COX-2) by OxPLs (supplementary Fig. III and IV).
Fig. 2.
CK2 inhibitor TBB attenuates effects of OxPAPC on induction of NRF2 and GCLM mRNA. A: The CK2 inhibitor TBB prevents induction of NRF2 by OxPAPC. At 30 min before stimulation with OxPAPC (130 µmol/l) HUVECs were pretreated with vehicle or TBB (20 µmol/l). After 2 h of stimulation, cell lysates were prepared and probed with NRF2 antibodies using Western blotting. B: NRF2/ARE DNA binding assay. Binding of NRF2 from control and OxPAPC-stimulated HUVEC nuclear extracts to immobilized oligonucleotide representing the consensus NRF2 binding site was quantified with an ELISA-based TransAM NRF2 kit and is expressed as an optical density of 450 nm. C, D, E: Effect of TBB on induction of GCLM mRNA levels by OxPAPC in different types of ECs (HAECs, HCAECs, and HUVECs, respectively). Before stimulation with OxPAPC (130 µmol/l, 6 h) cells were pretreated with TBB (20 µmol/l) for 30 min. Stimulation was stopped by addition of Trizol after 6 h. GCLM mRNA expression was quantified by real-time qPCR and normalized to β2-microglobulin mRNA levels.
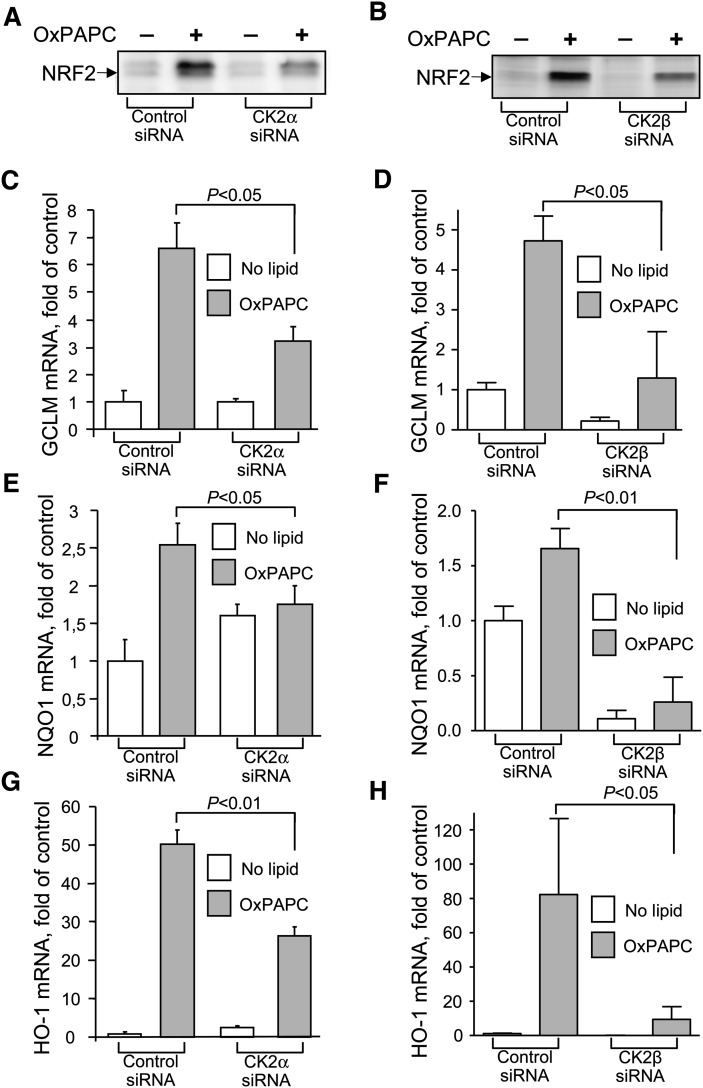
In order to obtain additional evidence for the importance of CK2 for OxPL-induced upregulation of NRF2 and its target genes and to avoid potential artifacts induced by inhibitory drugs, the levels of α and β subunits of CK2 were downregulated using siRNA. Silencing of CK2α or CK2β resulted in consistent reduction of NRF2 accumulation (Fig. 3A, B) and diminished levels of electrophilic gene transcripts in OxPAPC-treated cells (Fig. 3C–H). Thus, results of gene silencing are in good agreement with data for inhibition analysis.
Fig. 3.
siRNA-mediated knockdown of CK2 inhibits OxPAPC-induced upregulation of electrophilic genes and NRF2. A and B: Knockdown of CK2 inhibits OxPAPC-induced NRF2 increase. After 2 h of stimulation of control or CK2α siRNA (A) or CK2β siRNA (B) transfected HUVECs with OxPAPC (130 µmol/l) cell lysates were prepared and probed with antibodies against NRF2, using Western blotting. C–H: GCLM, NQO1, and HO-1 mRNA levels were quantified in control and CK2α (C, E, G) or CK2β (D, F, H) siRNA-transfected HUVECs treated with OxPAPC (130 µmol/l). Stimulation was stopped by addition of Trizol after 6 h. mRNA expression was quantified by real-time qPCR.
An important question raised by our findings was whether the involvement of CK2 is limited to the action of OxPLs or whether it plays a more general role in activation of electrophilic response. In support of the latter possibility, TBB inhibited upregulation of NRF2-dependent genes by compounds commonly used for induction of electrophilic stress, that is, tBHQ and sulforaphane (supplementary Fig. V). Thus, CK2 may be important for stress reactions induced not only by OxPLs but also by electrophilic compounds with completely different chemical structures.
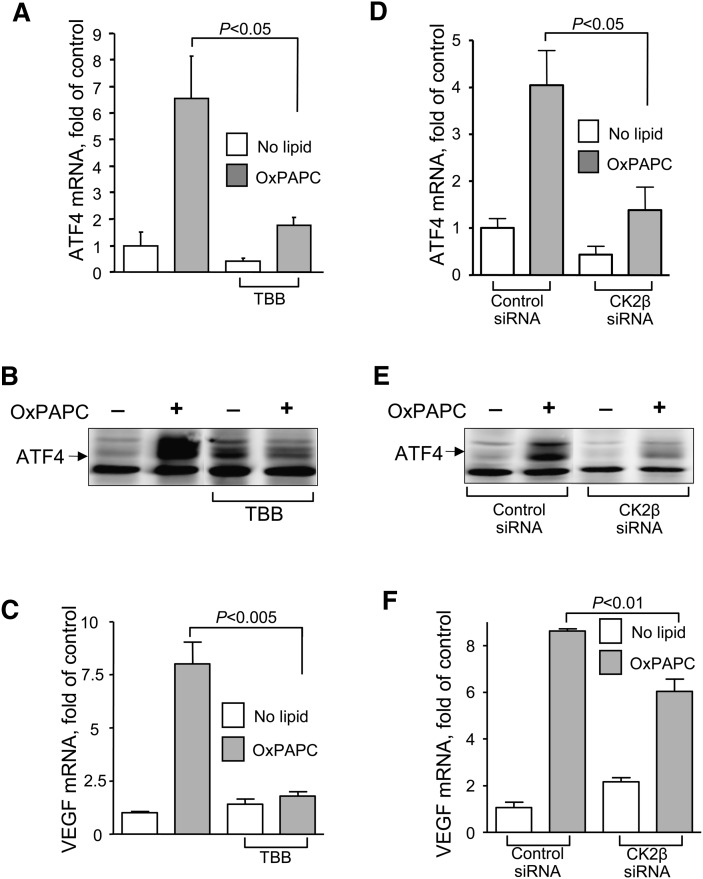
Finally, we tested whether inhibition of CK2 would suppress induction of ATF4 and VEGF by OxPAPC, which was described in our previous studies (13, 14). Treatment of ECs with TBB resulted in significant inhibition of OxPAPC-induced upregulation of ATF4 mRNA and protein (Fig. 4A, B). The same inhibitory effect was observed after transfection of cells with siRNA against CK2β (Fig. 4D, E). Furthermore, induction of VEGF in response to OxPAPC was also inhibited by TBB and siRNA against CK2β (Fig. 4C, F). Thus, inhibition of CK2 in OxPL-treated ECs results in suppression of the NRF2-ATF4-VEGF axis and is likely to produce antiangiogenic effects.
Fig. 4.
Induction of the ATF4-VEGF arm by OxPAPC depends on CK2. A, B, C: CK2 inhibitor TBB prevents induction of ATF4 mRNA, ATF4 protein, and VEGF mRNA by OxPAPC. At 30 min before addition of OxPAPC (130 μmol/l), HUVECs were pretreated either with vehicle or TBB (20 μmol/l). For ATF4 analysis, cell lysates were prepared after 4 h of incubation and probed with ATF4 antibodies. For ATF4 and VEGF mRNAs analysis, cells were harvested after 6 h of incubation, and mRNA level was measured by real-time qPCR. D, E, F: siRNA-mediated knockdown of CK2 decreases induction of ATF4 mRNA, ATF4 protein, and VEGF mRNA by OxPAPC. At 48 h after transfection with siRNA, HUVECs were stimulated with OxPAPC (130 μmol/l) for 4 h for ATF4 quantification by Western blotting and for 6 h for ATF4 and VEGF mRNA quantification by real-time qPCR.
DISCUSSION
The major finding of this study is that full induction by OxPLs of NRF2 and its target genes in venous and arterial ECs depends on the activity of CK2. Here we show that inhibition of CK2 by five chemically distinct drugs or siRNAs of the α or β subunits of the enzyme consistently resulted in diminished accumulation of NRF2 protein upon OxPL treatment, which in turn prevented induction of the NRF2-responsive GCLM, HO-1, NQO1, and ATF4 genes. Furthermore, inactivation of CK2 resulted in decreased induction of the ATF4-dependent gene VEGF, thus pointing to the importance of CK2 for the angiogenic activity of OxPLs.
Previously, it has been shown that CK2 directly phosphorylates NRF2, thus regulating its degradation rate and nuclear accumulation in response to chemical inducers of electrophilic stress (8, 9). Here we take a step further by showing that these molecular events are accompanied by important physiological effects such as induction of protective and angiogenic genes. Furthermore, our data suggest that this mechanism may be relevant not only to the action of toxic chemicals but also to naturally occurring molecules such as OxPLs that are known to accumulate in atherosclerotic vessels at concentrations similar to or higher than those used in our experiments (26). NRF2 is generally regarded as a protective factor in atherosclerosis (27), and therefore, whether long-term treatment with CK2 inhibitors may promote atherogenesis by downregulating electrophilic genes must be tested.
This study focused on ECs treated with atherogenic lipids; however, the ability of CK2 inhibitors to suppress activation of NRF2 suggests a straightforward link to other cell types and pathological processes, for example, tumor growth. Although induction of NRF2-dependent genes attenuates carcinogenesis, elevation of NRF2 expression in advanced tumors promotes their growth and resistance to chemotherapy (28). Conversely, CK2 is widely recognized as a prospective target in cancer treatment due its role in cell survival, angiogenesis, and other processes critical for tumor progression (29). We hypothesize that inhibition of electrophilic response leading to attenuated tumor cell survival and resistance to chemotherapy may represent an additional mechanism of antitumor activity of CK2 inhibitors.
In summary, this work characterizes the novel cross-talk between CK2 and cellular stress pathways activated by OxPLs and electrophilic compounds, thus providing additional insights into the mechanisms of beneficial action and potential side effects of CK2 inhibitors.
Supplementary Material
Footnotes
Abbreviations:
- EC
- endothelial cell
- GCLC
- glutamate-cysteine ligase catalytic subunit
- GCLM
- glutamate-cysteine ligase modifier subunit
- HAEC
- human artery endothelial cells
- HCAEC
- human coronary artery endothelial cells
- HUVEC
- human umbilical vein endothelial cells
- HO-1
- heme oxygenase 1
- NQO1
- NAD(P)H dehydrogenase, quinine 1
- NRF2
- NF-E2-related factor 2
- OxPAPC
- oxidized PAPC
- OxPLs
- oxidized phospholipids
- PAPC
- 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine
- PI3K
- phosphatidylinositol-3-kinase
- siRNA
- short interfering RNA
- VEGF
- vascular endothelial growth factor
This work was supported by Fonds zur Förderung wissenschaftlicher Forschung Grants P20801-B11 (V.N.B.) and P22267-B11 (O.V.O.), and by Österreichischer Forschungsförderungsgesellschaft (project 815445) (V.N.B.).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of five figures.
REFERENCES
- 1.Bochkov V. N., Oskolkova O. V., Birukov K. G., Levonen A. L., Binder C. J., Stockl J. 2010. Generation and biological activities of oxidized phospholipids. Antioxid. Redox Signal. 12: 1009–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gargalovic P. S., Gharavi N. M., Clark M. J., Pagnon J., Yang W. P., He A., Truong A., Baruch-Oren T., Berliner J. A., Kirchgessner T. G., et al. 2006. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 26: 2490–2496. [DOI] [PubMed] [Google Scholar]
- 3.Jyrkkanen H. K., Kansanen E., Inkala M., Kivela A. M., Hurttila H., Heinonen S. E., Goldsteins G., Jauhiainen S., Tiainen S., Makkonen H., et al. 2008. Nrf2 regulates antioxidant gene expression evoked by oxidized phospholipids in endothelial cells and murine arteries in vivo. Circ. Res. 103: e1–e9. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen T., Sherratt P. J., Nioi P., Yang C. S., Pickett C. B. 2005. Nrf2 controls constitutive and inducible expression of ARE-driven genes through a dynamic pathway involving nucleocytoplasmic shuttling by Keap1. J. Biol. Chem. 280: 32485–32492. [DOI] [PubMed] [Google Scholar]
- 5.Cullinan S. B., Zhang D., Hannink M., Arvisais E., Kaufman R. J., Diehl J. A. 2003. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol. Cell. Biol. 23: 7198–7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee J. M., Hanson J. M., Chu W. A., Johnson J. A. 2001. Phosphatidylinositol 3-kinase, not extracellular signal-regulated kinase, regulates activation of the antioxidant-responsive element in IMR-32 human neuroblastoma cells. J. Biol. Chem. 276: 20011–20016. [DOI] [PubMed] [Google Scholar]
- 7.Martin D., Rojo A. I., Salinas M., Diaz R., Gallardo G., Alam J., De Galarreta C. M., Cuadrado A. 2004. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J. Biol. Chem. 279: 8919–8929. [DOI] [PubMed] [Google Scholar]
- 8.Apopa P. L., He X., Ma Q. 2008. Phosphorylation of Nrf2 in the transcription activation domain by casein kinase 2 (CK2) is critical for the nuclear translocation and transcription activation function of Nrf2 in IMR-32 neuroblastoma cells. J. Biochem. Mol. Toxicol. 22: 63–76. [DOI] [PubMed] [Google Scholar]
- 9.Pi J., Bai Y., Reece J. M., Williams J., Liu D., Freeman M. L., Fahl W. E., Shugar D., Liu J., Qu W., et al. 2007. Molecular mechanism of human Nrf2 activation and degradation: role of sequential phosphorylation by protein kinase CK2. Free Radic. Biol. Med. 42: 1797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadl A., Huber J., Gruber F., Bochkov V. N., Binder B. R., Leitinger N. 2002. Analysis of inflammatory gene induction by oxidized phospholipids in vivo by quantitative real-time RT-PCR in comparison with effects of LPS. Vascul. Pharmacol. 38: 219–227. [DOI] [PubMed] [Google Scholar]
- 11.Gargalovic P. S., Imura M., Zhang B., Gharavi N. M., Clark M. J., Pagnon J., Yang W. P., He A., Truong A., Patel S., et al. 2006. Identification of inflammatory gene modules based on variations of human endothelial cell responses to oxidized lipids. Proc. Natl. Acad. Sci. U S A. 103: 12741–12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Afonyushkin T., Oskolkova O. V., Philippova M., Resink T. J., Erne P., Binder B. R., Bochkov V. N. 2010. Oxidized phospholipids regulate expression of ATF4 and VEGF in endothelial cells via NRF2-dependent mechanism: novel point of convergence between electrophilic and unfolded protein stress pathways. Arterioscler. Thromb. Vasc. Biol. 30: 1007–1013. [DOI] [PubMed] [Google Scholar]
- 13.Bochkov V. N., Philippova M., Oskolkova O., Kadl A., Furnkranz A., Karabeg E., Afonyushkin T., Gruber F., Breuss J., Minchenko A., et al. 2006. Oxidized phospholipids stimulate angiogenesis via autocrine mechanisms, implicating a novel role for lipid oxidation in the evolution of atherosclerotic lesions. Circ. Res. 99: 900–908. [DOI] [PubMed] [Google Scholar]
- 14.Oskolkova O. V., Afonyushkin T., Leitner A., von Schlieffen E., Gargalovic P. S., Lusis A. J., Binder B. R., Bochkov V. N. 2008. ATF4-dependent transcription is a key mechanism in VEGF up-regulation by oxidized phospholipids: critical role of oxidized sn-2 residues in activation of unfolded protein response. Blood. 112: 330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watson A. D., Leitinger N., Navab M., Faull K. F., Horkko S., Witztum J. L., Palinski W., Schwenke D., Salomon R. G., Sha W., et al. 1997. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J. Biol. Chem. 272: 13597–13607. [DOI] [PubMed] [Google Scholar]
- 16.Broekhuyse R. M. 1968. Phospholipids in tissues of the eye. I. Isolation, characterization and quantitative analysis by two-dimensional thin-layer chromatography of diacyl and vinyl-ether phospholipids. Biochim. Biophys. Acta. 152: 307–315. [DOI] [PubMed] [Google Scholar]
- 17.Baker A., Saltik M., Lehrmann H., Killisch I., Mautner V., Lamm G., Christofori G., Cotten M. 1997. Polyethylenimine (PEI) is a simple, inexpensive and effective reagent for condensing and linking plasmid DNA to adenovirus for gene delivery. Gene Ther. 4: 773–782. [DOI] [PubMed] [Google Scholar]
- 18.Garbin U., Fratta P. A., Stranieri C., Cominacini M., Pasini A., Manfro S., Lugoboni F., Mozzini C., Guidi G., Faccini G., et al. 2009. Cigarette smoking blocks the protective expression of Nrf2/ARE pathway in peripheral mononuclear cells of young heavy smokers favouring inflammation. PLoS ONE. 4: e8225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li R., Chen W., Yanes R., Lee S., Berliner J. A. 2007. OKL38 is an oxidative stress response gene stimulated by oxidized phospholipids. J. Lipid Res. 48: 709–715. [DOI] [PubMed] [Google Scholar]
- 20.Birukova A. A., Fu P., Chatchavalvanich S., Burdette D., Oskolkova O., Bochkov V. N., Birukov K. G. 2007. Polar head groups are important for barrier-protective effects of oxidized phospholipids on pulmonary endothelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 292: L924–L935. [DOI] [PubMed] [Google Scholar]
- 21.Cole A. L., Subbanagounder G., Mukhopadhyay S., Berliner J. A., Vora D. K. 2003. Oxidized phospholipid-induced endothelial cell/monocyte interaction is mediated by a cAMP-dependent R-Ras/PI3-kinase pathway. Arterioscler. Thromb. Vasc. Biol. 23: 1384–1390. [DOI] [PubMed] [Google Scholar]
- 22.Iwasaki K., Miwa Y., Haneda M., Uchida K., Nakao A., Kobayashi T. 2010. Significance of HLA class I antibody-induced antioxidant gene expression for endothelial cell protection against complement attack. Biochem. Biophys. Res. Commun. 391: 1210–1215. [DOI] [PubMed] [Google Scholar]
- 23.Nakaso K., Yano H., Fukuhara Y., Takeshima T., Wada-Isoe K., Nakashima K. 2003. PI3K is a key molecule in the Nrf2-mediated regulation of antioxidative proteins by hemin in human neuroblastoma cells. FEBS Lett. 546: 181–184. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y., Guan L., Wang X., Wen T., Xing J., Zhao J. 2008. Protection of chlorophyllin against oxidative damage by inducing HO-1 and NQO1 expression mediated by PI3K/Akt and Nrf2. Free Radic. Res. 42: 362–371. [DOI] [PubMed] [Google Scholar]
- 25.Davies S. P., Reddy H., Caivano M., Cohen P. 2000. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351: 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subbanagounder G., Leitinger N., Schwenke D. C., Wong J. W., Lee H., Rizza C., Watson A. D., Faull K. F., Fogelman A. M., Berliner J. A. 2000. Determinants of bioactivity of oxidized phospholipids. Specific oxidized fatty acyl groups at the sn-2 position. Arterioscler. Thromb. Vasc. Biol. 20: 2248–2254. [DOI] [PubMed] [Google Scholar]
- 27.Boon R. A., Horrevoets A. J. 2009. Key transcriptional regulators of the vasoprotective effects of shear stress. Hamostaseologie. 29: 39–43. [PubMed] [Google Scholar]
- 28.Lau A., Villeneuve N. F., Sun Z., Wong P. K., Zhang D. D. 2008. Dual roles of Nrf2 in cancer. Pharmacol. Res. 58: 262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruzzene M., Pinna L. A. 2010. Addiction to protein kinase CK2: a common denominator of diverse cancer cells? Biochim. Biophys. Acta. 1804: 499–504. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.