Abstract
Skin surface lipids (SSLs) arising from both sebaceous glands and skin removal form a complex lipid mixture composed of free fatty acids and neutral lipids. High-temperature gas chromatography coupled with electron impact or chemical ionization mass spectrometry was used to achieve a simple analytical protocol, without prior separation in classes and without prior cleavage of lipid molecules, in order to obtain simultaneously i) a qualitative characterization of the individual SSLs and ii) a quantitative evaluation of lipid classes. The method was first optimized with SSLs collected from the forehead of a volunteer. More than 200 compounds were identified in the same run. These compounds have been classified in five lipid classes: free fatty acids, hydrocarbons, waxes, sterols, and glycerides. The advantage to this method was it provided structural information on intact compounds, which is new for cholesteryl esters and glycerides, and to obtain detailed fingerprints of the major SSLs. These fingerprints were used to compare the SSL compositions from different body areas. The squalene/cholesterol ratio was used to determine the balance between sebaceous secretion and skin removal. This method could be of general interest in fields where complex lipid mixtures are involved.
Keywords: lipidomics, structural characterization, squalene, free fatty acids, sterols, waxes, glycerides, neutral lipids
Even if the barrier property of skin is localized in the stratum corneum (SC), skin surface lipids (SSLs) present in hydrolipidic film have a close relationship with SC lipids. Thus, SSLs participate in this barrier function (1–5).
SSLs are formed from a complex mixture of free fatty acids (FFAs) and neutral lipids arising from both sebaceous secretion and skin removal (6). Freshly liberated sebum contains predominantly triglycerides, wax esters, and squalene (7–9). Under the influence of lipase-producing microorganisms of human skin flora, triglycerides are partially hydrolyzed, producing FFAs, diglycerides, monoglycerides, and glycerol (10–12). In addition, continual removal of the uppermost layer of the skin leads to liberation, at the skin surface, of free and esterified cholesterols along with FFAs. Several lipid classes are then present in SSLs, and the complexity of the mixture is emphasized by the structural microheterogeneity within each class.
Hydrolipidic film represents an important modulator of cutaneous barrier functions (1–5), particularly in SC hydration. Moreover, sebum transports antioxidants to the skin surface (e.g., vitamin E), preventing aging (3, 13, 14). Free fatty acids contribute to the pH of skin surface (15–17). A particular acid (sapienic acid, C16:1Δ6) exhibits strong innate antimicrobial activity (18). Sebaceous lipids are involved in some inflammatory diseases such as acne (14, 19), seborrhea, or dermatitis (13). Maintaining the stability of the amounts and composition of SSL is of major importance to preserving skin barrier properties. Moreover, information provided by SSL analyses, such as fine profiling, squalene/cholesterol ratio, and intact glyceride patterns, contribute to the wide knowledge of physiological and pathological evolution of hydrolipidic film.
Our study falls within the framework of lipidomics, in which global SSLs profiles are determined in the entire sample, keeping the structural integrity of the compounds. Our purpose was to develop a simple analytical protocol using a noninvasive sampling method without time-consuming sample preparation steps that would provide a qualitative characterization of individual SSL compounds and a quantitative evaluation of different lipid classes.
Previously, SSLs were studied mostly after being separated into lipid classes, using thin-layer chromatography. The classes were then submitted to a quantitative analysis using photodensitometry (6, 20–22) and gas chromatography-flame ionization detection (GC-FID) (23). In other methods, each class was analyzed separately: FFAs (24–26), wax esters with or without cleavage (26–30), and triglycerides after cleavage (26, 31, 32). Individual compounds were identified using additional techniques such as HPLC/atmospheric pressure chemical ionization-mass spectrometry (APCI-MS) (30), GC-FID (24, 29, 32), GC-MS (24, 26), and electrospray ionization mass spectrometry (ESI-MS) (27).
GC-MS is the method most widely used for analyzing complex mixtures. The columns available today that are stable at high temperature are suitable for successful separations of compounds with high molecular weights (33–39).
Thus, high-temperature gas chromatography-mass spectrometry (HTGC-MS) was chosen to achieve the separation of SSLs. Both electron impact (EI) and chemical ionization (CI) procedures were necessary to obtain structural information and molecular masses. The protocol was optimized to preserve the integrity of thermolabile compounds and to provide good separation between the lipid classes and between individual compounds. Each compound was identified by its retention time, molecular mass, and fragmentation pattern and then assigned to the proper class. Thus, informative fingerprints of SSLs were obtained.
To illustrate this method, the differences among SSL compositions from different body areas have been studied. The peak area normalization with the response factor approach was applied (40). Relative amounts of lipid classes were determined, and the squalene/cholesterol ratio was assessed to describe the balance between sebum secretion and skin removal (41).
To sum up, this method gives structural information and detailed lipid profiles by using only one analytical protocol. It could be of a great interest for establishing the proof for medical treatment efficacy in diseases such as acne, atopic dermatitis, seborrhea, or psoriasis. More generally, this method can be extended to numerous other applications in medical, pharmaceutical, cosmetic, and alimentary fields where complex lipid mixtures are involved.
MATERIALS AND METHODS
Chemicals
Diethyl ether, isooctane, palmitic acid, monostearin (also glyceryl monostearate), squalene, cholesterol, palmityl palmitate, dipalmitin, cholesteryl palmitate, tripalmitin, pyridine, and N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) were obtained from Sigma-Aldrich (St. Louis, MO). Lipid-free absorbent papers were purchased from Rizla UK Ltd. (Pontypridd, South Wales, UK). Medical tapes, i.e., elastic tubular nettings for the application of gauzes and medications (Surgifix), were purchased from Fra Production Spa (Cisterna d'Asti, Italy).
Subject
After cleaning the body sampling areas with water and soap, 12 h before sampling, the volunteer did not apply any cosmetic or pharmaceutical product to these areas until the end of sampling. Skin surface lipids were collected from six areas (forehead, back, thorax, forearm, thigh, and calf) of a female volunteer, 26 years old, with healthy skin, living in France for more than 6 months before the date of sampling. Human samples were obtained following review and approval from an institutional review board, and informed consent was obtained from the volunteer.
Lipids collection
Two lipid-free absorbent papers were maintained on the defined area for 30 min, using medical tape, and then removed with tweezers and introduced into a closed vial. This step was repeated four times. The collected lipids were extracted from papers twice with 40 ml of diethyl ether. The solution was concentrated in a rotary evaporator at 30°C, then transferred into a 2-ml vial, and dried under a gentle stream of nitrogen. The dry extract was stored at −20°C until further use.
Derivatization of SSLs
The trimethylsilylation reagent consisted of BSTFA/pyridine, 50:50 (v/v). The dry extract was trimethylsilylated at room temperature for 30 min with 100 µl of reagent. The excess reagent was then removed using rotary evaporation at 30°C, and the dried residue was dissolved in 500 µl of isooctane. For forearm, thigh, and calf samples, the solution was directly injected (1 μl, on-column). For forehead, back, and thorax samples, the solution was diluted in isooctane (1:5; v/v) before injection.
Gas chromatography-mass spectrometry
A Thermo Scientific (Austin, TX) gas chromatography unit (Trace GC Ultra) equipped with an on-column injector was coupled to a quadrupole DSQII mass spectrometer via a high-temperature interface. The separation was achieved using a 30 m × 0.32 mm ZB-5HT capillary column (Phenomenex, Torrance, CA) coated with 0.1 µm of 5% diphenyl/95% dimethylpolysiloxane, connected to a 5-m 0.32-mm HT-deactivated tubing guard column. Helium was used as a carrier gas at a constant flow of 2 ml/min. The injector and transfer line temperatures were set to 80°C and 350°C, respectively. The oven temperature was programmed from 80°C to 240°C at 5°C/min; 240°C to 320°C at 2.5°C/min; and 320°C to 350°C at 1°C/min. EI mass spectra were recorded in the total ion current (TIC) monitoring mode. The operating conditions for EI-MS were source temperature at 250°C, ionizing energy at 70 eV, and scan range from m/z 45 to 1,000 with a period of 1 s. For CI-MS, ionizing energy was 120 eV, and ammonia was used as the reagent gas at a constant flow of 1ml/min.
RESULTS AND DISCUSSION
Optimization of HTGC-MS conditions
The SSL derivatization step was used to improve the detection of compounds having acid and/or alcohol functions. We checked to make sure that with the BSTFA/pyridine (50:50; v/v) mixture, a 30-min reaction at room temperature led to results similar to those obtained at 80°C. Thus, we chose cold silylation in order to prevent any thermodegradation of the samples (33).
On-column injection was used to avoid discrimination between analytes of very different volatility and to keep the structural integrity of thermolabile compounds like steryl esters.
A high-temperature stable capillary column with apolar stationary phase was chosen because it is chemically inert toward silylated compounds (42).
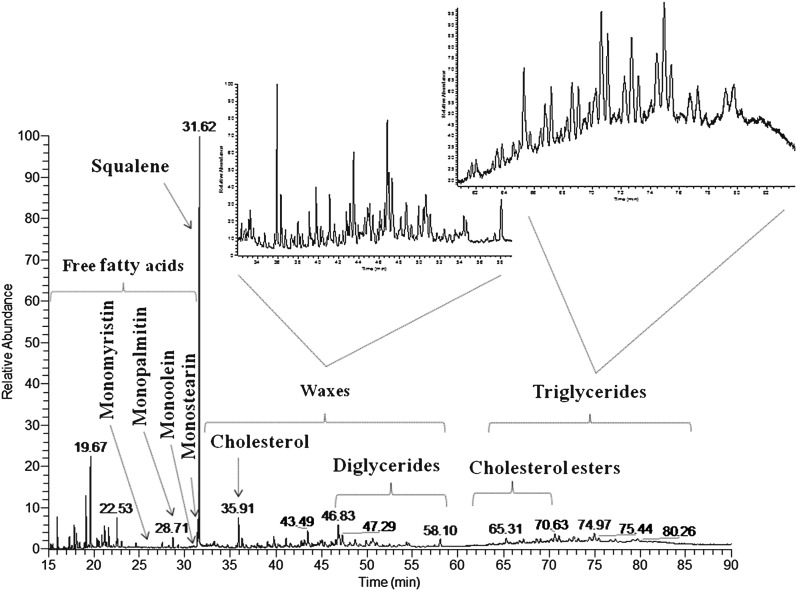
At the beginning of the study, rapid oven temperature programming was tested, from 80°C to 390°C at 30°C/min and an 8-min isothermal step at 390°C. That program led to a lot of coelutions, very high background noise at the end of the chromatogram, and a partial degradation of steryl esters. To remedy these problems, an optimized oven temperature program was achieved. A method with three temperature gradients was adopted: from 80°C to 240°C at 5°C/min to elute FFAs; then to 320°C at 2.5°C/min to elute wax esters and diglycerides; and finally to 350°C at 1°C/min to elute steryl esters and triglycerides (Fig. 1). Thus, selectivity was improved and coelutions and background noise were decreased, which provided mass spectra of better quality.
Fig. 1.
HTGC-MS profile of a trimethylsilylated SSL sample (analyzed as described in Materials and Methods).
Qualitative analysis of skin surface lipids
Samples were collected from the forehead of a female volunteer by using absorbent papers. This noninvasive method allows collecting exclusively SSLs.
In the SSL sample studied, a compound corresponding to a given chromatographic peak was identified through its retention time and MS data (molecular mass and fragmentation pattern) and assigned to the proper class regardless of isomers and unsaturation positions in order to establish the profiles of all SSLs.
Free fatty acids.
In addition to the molecular [M]+ ion, the [M-CH3]+ ion, due to the loss of a methyl from the trimethylsilyl (TMS) group, was used for identification. Under our chromatographic conditions, major FFA (trimethylsilyl esters) were contained between decanoic acid (C10:0), and tetracosenoic acid (C24:1) (Table 1). These acids have been partly described in a study by Green et al. (28) from C13 to C18. Due to the branching of chains or unsaturation positions, several FFA with the same total number of carbon atoms, the so-called carbon number, were eluted at different retention times. Hexadecenoic acid (C16:1) was the most abundant acid detected in the sample. According to different authors (31, 43), we could identify it as sapienic acid (C16:1Δ6).
TABLE 1.
Lipid classes identified in trimethylsilylated SSL samples analyzed by HTGC-MS
| Free Fatty Acids | Free Fatty Acids (continued) | RT | Hydrocarbons | Monoglycerides | ||||
|---|---|---|---|---|---|---|---|---|
| RT | RCO | RT | RCO | 29.29 | Squalene | RT | RCO | |
| 8.05 | C10:0 | 18.89 | C16:0 | 31.62 | Squalene | 25.81 | C14:0 | |
| 11.32 | C12:0 | 19 | C16:0 | 33.14 | Epoxysqualene | 28.26 | C16:0 | |
| 12.06 | C12:0 | 19.13 | C16:1 | 28.71 | C16:0 | |||
| 12.14 | C13:0 | 19.28 | C16:1 | RT |
Free sterols |
31 | C18:1 | |
| 12.9 | C13:0 | 19.4 | C16:0 | 35.91 | Cholesterol | 31.45 | C18:0 | |
| 13.09 | C13:0 | 19.53 | C16:0 | 38.22 | Lanosterol | |||
| 13.32 | C13:0 | 19.67 | C16:0 | 38.94 | 3 hydroxy lanosta-8,24-diene | Diglycerides |
||
| 13.46 | C13:0 | 19.57 | C16:0 | RT | R'CO | R’’CO | ||
| 14.05 | C13:0 | 19.85 | C16:0 | 46.83 | C15:0 | C16:0 | ||
| 14.34 | C13:0 | 20.21 | C17:1 | Cholesteryl esters |
47.67 | C14:0 | C18:0 | |
| 14.84 | C14:0 | 20.34 | C17:0 | RT | R1CO | 48.17 | C15:0 | C16:1 |
| 15.27 | C14:0 | 20.53 | C17:0 | 61.48 | C14:1 | 48.69 | C15:0 | C16:1 |
| 15.49 | C14:1 | 20.87 | C17:1 | 61.72 | C14:1 | 49.20 | C15:0 | C16:0 |
| 15.99 | C14:0 | 21.17 | C18:1 | 62.01 | C14:0 | 49.92 | C16:0 | C16:1 |
| 16.65 | C15:1 | 21.39 | C17:0 | 63.17 | C15:1 | 49.92 | C16:0 | C16:0 |
| 16.74 | C15:0 | 21.91 | C18:1 | 63.48 | C15:1 | 50.05 | C16:0 | C16:1 |
| 16.93 | C15:0 | 22.14 | C18:2 | 63.82 | C15:0 | 50.37 | C16:0 | C16:0 |
| 17.17 | C15:0 | 22.43 | C18:2 | 64.57 | C16:1 | 50.61 | C16:0 | C16:1 |
| 17.32 | C15:0 | 22.53 | C18:1 | 64.99 | C16:0 | 51.05 | C16:0 | C16:0 |
| 17.17 | C15:0 | 22.8 | C18:0 | 65.31 | C16:1 | 53.51 | C18:1 | C16:0 |
| 17.32 | C15:0 | 23.06 | C18:0 | 65.71 | C16:0 | 54.61 | C18:0 | C16:0 |
| 17.86 | C15:0 | 29.05 | C20:1 | 66.46 | C17:1 | 57.45 | C18:0 | C18:0 |
| 18.11 | C15:0 | 30.45 | C21:1 | 66.81 | C17:1 | 58.10 | C18:0 | C18:0 |
| 18.46 | C16:1 | 31.8 | C22:1 | 66.84 | C17:0 | |||
| 18.58 | C16:0 | 32.85 | C23:1 | 67.20 | C17:0 | |||
| 18.69 | C16:0 | 34.79 | C24:1 | 68.59 | C18:1 | |||
Several trimethylsilylated FFA with the same total carbon number were eluted at different retention times (RT, min); this could due to the branching of chains or unsaturation positions. RCO, R1CO, R′CO and R″CO, acid moieties.
Other fatty acids were detected in trace amounts: C7:0 (3.44 min), C8:0 (4.78 min), C9:0 (6.45 min), C11:0 (10.38 min), and C12:1 (12.26 min). Their amounts were too low to significantly affect the total sum of fatty acids. This result was consistent with the findings of Vantrou et al. (7) and James et al. (25).
Hydrocarbons.
Three main hydrocarbons were identified using the [M]+ and [M-CH3]+ ions (Table 1). The squalene, which is the marker of the sebaceous function (44, 45), was clearly the most abundant compound in the sample studied. In addition, epoxy-squalene was also detected. It could be an intermediate in the cholesterol synthesis from squalene (46) and/or the photo-oxidation squalene product (47).
Waxes.
For saturated waxes, RCOOR’, alcohol moieties and acid moieties were identified using [R’-H]+ and [RC(OH)2]+ fragments, respectively. When the fatty acid moiety is unsaturated, the characteristic fragment corresponds to [RCO-H]+. Identified waxes (Table 2) were contained between myristyl myristate (C28H56O2) and palmitoleyl lignocerate (C40H76O2). We were able to distinguish saturated waxes from unsaturated waxes, in which the acid moiety and/or the alcohol moiety could be unsaturated. Waxes having the same total carbon number and unsaturation number but with differences in the acid and alcohol chain lengths were coeluted. The fatty acid moieties identified were mostly hexadecenoic acid (C16:1) and hexadecanoic acid (C16:0), which suggests the importance of palmitic acid and its unsaturated counterpart in the physiological film of the skin. In the alcohol moieties, the lengths of saturated and monounsaturated hydrocarbon chains were much more varied, between C14 and C24. Waxes are not hydrolyzed on the skin surface (20). No free fatty alcohols were detected.
TABLE 2.
Wax compositions in trimethylsilylated SSL sample
| Waxes | Waxes (continued) | Waxes (continued) | ||||||
|---|---|---|---|---|---|---|---|---|
| RT | RCO | OR’ | RT | RCO | OR’ | RT | RCO | OR’ |
| 33.59 | C14:0 | C14:0 | 40.24 | C16:0 | C16:0 | 45.85 | C16:0 | C20:1 |
| 34.13 | C13:0 | C16:1 | C14:0 | C18:0 | C15:0 | C21:1 | ||
| C15:0 | C14:1 | C18:0 | C14:0 | C14:0 | C22:1 | |||
| 34.68 | C15:0 | C14:0 | 40.43 | C16:1 | C17:0 | C16:0 | C20:1 | |
| 34.75 | C13:0 | C16:1 | 40.68 | C16:0 | C17:1 | C16:1 | C20:0 | |
| 35.10 | C14:0 | C15:0 | C15:0 | C18:1 | 46.09 | C16:1 | C20:1 | |
| C15:0 | C14:0 | 41.13 | C16:1 | C17:0 | 46.26 | C18:0 | C18:1 | |
| 35.68 | C16:1 | C14:0 | C16:0 | C17:1 | C19:0 | C17:1 | ||
| 36.10 | C14:0 | C16:0 | 41.58 | C15:0 | C18:1 | 46.59 | C16:1 | C20:0 |
| C16:0 | C14:0 | C14:0 | C19:1 | 46.83 | C19:0 | C17:2 | ||
| 36.30 | C16:1 | C14:0 | C17:0 | C16:1 | 46.97 | C16:0 | C20:2 | |
| 36.40 | C14:1 | C16:0 | 42.06 | C15:0 | C18:0 | C16:1 | C20:1 | |
| 36.73 | C16:0 | C14:0 | C14:0 | C19:0 | 47.29 | C19:0 | C17:1 | |
| C14:0 | C16:0 | C16:0 | C17:0 | 47.69 | C16:0 | C20:0 | ||
| 37.33 | C15:0 | C16:1 | 42.44 | C16:0 | C18:1 | C18:0 | C18:0 | |
| C14:0 | C17:1 | C18:0 | C16:1 | 48.17 | C19:0 | C18:1 | ||
| 37.47 | C13:0 | C18:1 | 42.73 | C16:1 | C18:0 | C20:0 | C17:1 | |
| 37.65 | C14:1 | C17:0 | 42.94 | C16:0 | C18:1 | 48.69 | C20:0 | C17:1 |
| 37.99 | C16:1 | C15:0 | 43.15 | C18:0 | C16:2 | 49.20 | C16:0 | C21:0 |
| C15:0 | C16:1 | 43.49 | C16:1 | C18:0 | C16:1 | C21:0 | ||
| C14:0 | C17:1 | C16:0 | C18:0 | 49.92 | C16:1 | C22:1 | ||
| 38.43 | C15:0 | C16:0 | C18:0 | C16:0 | 50.61 | C16:1 | C22:1 | |
| C16:0 | C15:0 | 44.87 | C16:1 | C19:0 | 50.75 | C16:0 | C22:0 | |
| 39.11 | C16:1 | C16:0 | 45.07 | C16:0 | C19:0 | 51.05 | C16:1 | C22:0 |
| 39.25 | C15:0 | C17:1 | C15:0 | C20:0 | 52.44 | C16:1 | C23:1 | |
| 39.55 | C16:0 | C16:0 | C16:0 | C19:0 | 54.38 | C20:1 | C20:1 | |
| C14:0 | C18:0 | 45.35 | C15:0 | C20:0 | C16:1 | C24:1 | ||
| 39.78 | C16:1 | C16:0 | C14:0 | C21:0 | 54.75 | C16:1 | C24:0 | |
| 39.93 | C17:0 | C15:1 | C16:1 | C19:0 | ||||
For some retention times (RT, min), different waxes have been identified. They are grouped together in the same cells. RCO, acid moiety; OR′, alcohol moiety.
Sterol class.
This class is composed of free sterols and sterols esterified with fatty acids released by the sebum or the epidermis.
Free sterols.
Identification of free sterols (Table 1) was established by observation of specific ions: [M]+, [M-CH3]+, [M-TMSiOH]+, [M-CH3-TMSiOH]+, [M-TMSiO=CHCH= CH2]+. Cholesterol, which is the skin removal marker (17, 48, 49), was the main free sterol identified. Our method allowed us to detect the presence of lanosterol. It is considered a cholesterol synthesis intermediate from squalene, following the Kandutsch-Russel route (45, 46).
Steryl esters.
Using an adapted temperature program allowed us to characterize steryl esters without any thermal degradation. EI mass spectra showed the same fragment at m/z 368 for all steryl esters R1COOR2. It corresponds to the sterol moiety [R2-H]+ formed by R1COOH elimination and formation of a double bond in position 3. Therefore, CI-MS was necessary to observe the adduct ion [M+NH4]+ and identify the ester acyl group. All sterol esters identified were cholesteryl esters. The chain lengths of fatty acid varied from C14 to C18 (Table 1). The most abundant compound was cholesteryl hexadecenoate, eluted at 65.31 min.
Glyceride class.
As mentioned above, microorganisms living in the pilosebaceous canal and on the skin surface hydrolyze triglycerides (11, 12). Hence, the SSL glyceride class is composed of three subclasses, monoglycerides, diglycerides, and triglycerides.
One advantage of our method is the simultaneous detection of intact triglycerides and all their hydrolysis products (e.g., glycerol). TMS-glycerol was detected at 5.19 min (m/z 293 [M-CH3]+, m/z 218 [M-TMSiOH]+, and m/z 205 [M-TMSiOCH2]+).
Monoglycerides.
Five monoglycerides were identified (Table 1) using the ions [M-CH3]+, [RCO]+, and [RCO+74]+. It was also possible to distinguish sn-2-isomers, characterized by the [M-RCOOH]+ ion at m/z 218, from sn-1(3)-isomers, characterized by the [M-TMSiOCH2]+ ions and [TMSiO]2C2H3+ ion at m/z 205. Monopalmitin was the predominant monoglyceride identified in the sample.
Diglycerides.
Diglycerides were identified (Table 1) using the same ions, [M-CH3]+, [RCO]+, and [RCO+74]+. The last ion is the most abundant in sn-1,2-isomers spectra. In contrast, the [M-RCOOCH2]+ ion is the most abundant in sn-1,3-isomers spectra. The total carbon number ranged between C34 and C39.
Triglycerides.
EI mass spectra provided interesting information concerning the triglyceride structures, using ions [RCO]+, [RCO+74]+, and [M-RCOO]+, but the observation of molecular ions is difficult, and it was not possible to evaluate the total carbon number. Thus, it was necessary to perform CI-MS where the [M+NH4]+ ion intensity was high enough to obtain this information. Triglycerides were contained between C45H70O6 and C54H104O6. The chain lengths of the constitutive fatty acids varied between C12 and C18, which is consistent with previous results (31).
Only limited data have been reported for triglyceride structures because of their extremely high complexity. In this study, we found that the majority of triglycerides consisted of mixed triglycerides with different aliphatic hydrocarbon chain lengths in the same compound. The triglycerides were eluted in several groups according to their total carbon numbers. Each group shows several chromatographic peaks (between three and five peaks), with a decrease of retention times depending on the unsaturation number (Fig. 1, Table 3).
TABLE 3.
Triglyceride compositions in trimethylsilylated SSL samples
| Triglycerides | Triglycerides (continued) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| RT | R'CO | R’’CO | R’’’CO | TG | RT | R'CO | R’’CO | R’’’CO | TG |
| 63.16 | C13:0 | C12:1 | C17:1 | C45H82O6 | 66.77 | C15:1 | C15:0 | C14:0 | C47H88O6 |
| C16:0 | C14:1 | C12:1 | C15:0 | C15:0 | C14:1 | ||||
| C16:1 | C14:0 | C12:1 | C16:0 | C14:1 | C14:0 | ||||
| C16:1 | C14:1 | C12:0 | C16:1 | C14:0 | C14:0 | ||||
| C15:1 | C14:1 | C13:0 | 67.19 | C15:0 | C15:0 | C14:0 | C47H90O6 | ||
| 63.48 | C13:0 | C12:0 | C17:1 | C45H84O6 | C16:0 | C14:0 | C14:0 | ||
| C16:0 | C14:1 | C12:0 | 67.58 | C16:1 | C15:0 | C14:2 | C48H86O6 | ||
| C15:0 | C14:1 | C13:0 | C16:2 | C15:0 | C14:1 | ||||
| 63.84 | C13:0 | C12:0 | C17:0 | C45H86O6 | C16:2 | C15:1 | C14:0 | ||
| C16:0 | C14:0 | C12:0 | C16:1 | C15:2 | C14:0 | ||||
| C15:0 | C14:0 | C13:0 | C16:0 | C15:1 | C14:2 | ||||
| 64.77 | C14:1 | C14:1 | C15:0 | C46H84O6 | C16:0 | C15:2 | C14:1 | ||
| C16:1 | C13:0 | C14:1 | 67.85 | C16:1 | C15:1 | C14:1 | C48H86O6 | ||
| C16:1 | C14:1 | C13:0 | 68.28 | C16:0 | C15:1 | C14:1 | C48H88O6 | ||
| 64.99 | C14:1 | C14:0 | C15:0 | C46H86O6 | C16:1 | C15:0 | C14:1 | ||
| C16:1 | C13:0 | C14:0 | C16:1 | C15:1 | C14:0 | ||||
| C16:1 | C15:0 | C12:0 | 68.62 | C16:0 | C15:1 | C14:0 | C48H90O6 | ||
| C16:0 | C14:1 | C13:0 | C16:0 | C14:1 | C15:0 | ||||
| C16:1 | C14:0 | C13:0 | C16:1 | C14:0 | C15:0 | ||||
| C17:0 | C14:1 | C12:0 | 69.04 | C16:0 | C15:0 | C14:0 | C48H92O6 | ||
| C18:1 | C13:0 | C12:0 | 69.49 | C16:2 | C15:1 | C15:0 | C49H88O6 | ||
| 65.31 | C14:0 | C14:0 | C15:0 | C46H88O6 | C16:0 | C15:1 | C15:2 | ||
| C15:0 | C15:0 | C13:0 | C16:0 | C16:2 | C14:1 | ||||
| C16:0 | C15:0 | C12:0 | C16:1 | C16:2 | C14:0 | ||||
| C16:0 | C14:0 | C13:0 | 69.79 | C16:1 | C15:1 | C15:1 | C49H88O6 | ||
| C17:0 | C14:0 | C12:0 | C16:1 | C16:1 | C14:1 | ||||
| C17:0 | C13:0 | C13:0 | 70.23 | C16:1 | C15:1 | C15:0 | C49H90O6 | ||
| C18:0 | C13:0 | C12:0 | C16:0 | C15:1 | C15:1 | ||||
| 66.07 | C15:1 | C15:1 | C14:1 | C47H84O6 | C16:1 | C16:1 | C14:0 | ||
| C16:1 | C14:1 | C14:1 | C16:0 | C16:1 | C14:1 | ||||
| 66.45 | C15:1 | C15:0 | C14:1 | C47H86O6 | 70.63 | C16:1 | C15:0 | C15:0 | C49H92O6 |
| C15:1 | C15:1 | C14:0 | C16:1 | C16:0 | C14:0 | ||||
| C16:1 | C14:0 | C14:1 | 71.08 | C16:0 | C15:0 | C15:0 | C49H94O6 | ||
| C16:0 | C14:1 | C14:1 | C16:0 | C16:0 | C14:0 | ||||
| 71.47 | C16:0 | C17:1 | C14:2 | C50H90O6 | 74.05 | C14:1 | C16:1 | C18:1 | C51H92O6 |
| C16:0 | C17:2 | C14:1 | C15:1 | C16:1 | C17:1 | ||||
| C16:1 | C17:2 | C14:0 | C16:1 | C16:1 | C16:1 | ||||
| C16:2 | C17:1 | C14:0 | 74.45 | C16:0 | C16:1 | C16:1 | C51H94O6 | ||
| C16:2 | C17:0 | C14:1 | C14:0 | C16:1 | C18:1 | ||||
| C16:1 | C17:0 | C14:2 | 74.97 | C16:0 | C16:0 | C16:1 | C51H96O6 | ||
| C16:0 | C15:1 | C16:2 | C14:0 | C16:0 | C18:1 | ||||
| C16:0 | C15:2 | C16:1 | 75.44 | C16:0 | C16:0 | C16:0 | C51H98O6 | ||
| C16:2 | C15:0 | C16:1 | C14:0 | C16:0 | C18:0 | ||||
| 71.86 | C16:1 | C17:1 | C14:1 | C50H90O6 | C15:0 | C16:0 | C17:0 | ||
| C16:1 | C15:1 | C16:1 | 76.15 | C16:1 | C16:1 | C17:1 | C52H94O6 | ||
| 72.23 | C16:1 | C15:1 | C16:0 | C50H92O6 | C15:1 | C16:1 | C18:1 | ||
| C16:1 | C15:0 | C16:1 | 76.77 | C16:0 | C16:1 | C17:1 | C52H96O6 | ||
| C16:0 | C17:1 | C14:1 | C16:1 | C16:1 | C17:0 | ||||
| C16:1 | C17:1 | C14:0 | C15:1 | C16:0 | C18:1 | ||||
| C16:1 | C17:0 | C14:1 | C15:0 | C16:1 | C18:1 | ||||
| 72.72 | C16:0 | C15:1 | C16:0 | C50H94O6 | C15:1 | C16:1 | C18:0 | ||
| C16:1 | C15:0 | C16:0 | 77.25 | C16:1 | C16:0 | C17:0 | C52H98O6 | ||
| C16:0 | C17:0 | C14:1 | C16:0 | C16:0 | C17:1 | ||||
| C16:0 | C17:1 | C14:0 | C15:1 | C16:0 | C18:0 | ||||
| C16:1 | C17:0 | C14:0 | C15:0 | C16:1 | C18:0 | ||||
| 73.18 | C16:0 | C15:0 | C16:0 | C50H96O6 | C15:0 | C16:0 | C18:1 | ||
| C16:0 | C17:0 | C14:0 | 77.82 | C16:0 | C16:0 | C17:0 | C52H100O6 | ||
| 73.54 | C14:2 | C16:1 | C18:0 | C51H92O6 | C15:0 | C16:0 | C18:0 | ||
| C15:2 | C16:1 | C17:0 | 78.63 | C15:1 | C17:1 | C18:1 | C53H96O6 | ||
| C14:1 | C16:2 | C18:0 | C16:1 | C16:1 | C18:1 | ||||
| C15:1 | C16:2 | C17:0 | 79.19 | C15:1 | C17:1 | C18:0 | C53H98O6 | ||
| C14:0 | C16:2 | C18:1 | C15:1 | C17:0 | C18:1 | ||||
| C15:0 | C16:2 | C17:1 | C15:0 | C17:1 | C18:1 | ||||
| C14:2 | C16:0 | C18:1 | C16:1 | C16:1 | C18:0 | ||||
| C15:2 | C16:0 | C17:1 | C16:1 | C16:0 | C18:1 | ||||
| C14:1 | C16:0 | C18:2 | 79.73 | C15:0 | C17:0 | C18:1 | C53H100O6 | ||
| C15:1 | C16:0 | C17:2 | C15:0 | C17:1 | C18:0 | ||||
| C14:0 | C16:1 | C18:2 | C15:1 | C17:0 | C18:0 | ||||
| C15:0 | C16:1 | C17:2 | C16:1 | C16:0 | C18:0 | ||||
| C16:0 | C16:1 | C16:2 | C16:0 | C16:0 | C18:1 | ||||
| 80.26 | C15:0 | C17:0 | C18:0 | C53H102O6 | |||||
| C16:0 | C16:0 | C18:0 | |||||||
For some retention times (RT), different triglycerides (TG) have been identified. They are grouped together in the same cells. R′CO, R″CO and R‴CO acid moieties.
Quantitative analysis of skin surface lipids at six body areas
In order to compare the SSL compositions from different human body areas, six SSL samples were collected at the same time from six body areas from the same volunteer. The relative amount of each lipid class in the total amount of SSLs was estimated using the area normalization with response factors (40).
When mass spectrometry is used, the sensitivities corresponding to analytes of different lipid classes are different (40). Hence the TIC response may change significantly from one lipid class to another. This limitation can be removed by running one representative standard per lipid class to obtain the response factor (K) for each class, using the equation C = K × A, where C is the injected concentration of representative standard of one lipid class, K is the response factor of the lipid class studied, and A is the peak area of the representative standard.
Thus, one authentic compound representative for each lipid class was chosen, analyzed by chromatography using the same experimental conditions as those for studied samples, and used to calculate the response factor (mean K value ± SD) of the corresponding class, as follows: palmitic acid, 0.223 ± 0.019; monostearin, 0.373 ± 0.014; squalene, 0.084 ± 0.008; cholesterol, 0.086 ± 0.002; palmityl palmitate, 0.485 ± 0.087; dipalmitin, 0.278 ± 0.007; cholesteryl palmitate, 0.344 ± 0.017; and tripalmitin, 0.613 ± 0.154, obtained for six analyses on 6 different days.
Within each lipid class, peak areas were measured and summed. The sum was multiplied by the corresponding response factor, and then the percentages of each lipid class in the collected SSLs were calculated using the equation
where Xi is the assessed lipid class, i is the lipid class index, Ki is the lipid class response factor, and Ai is the sum of area peaks assigned to the same lipid class i.
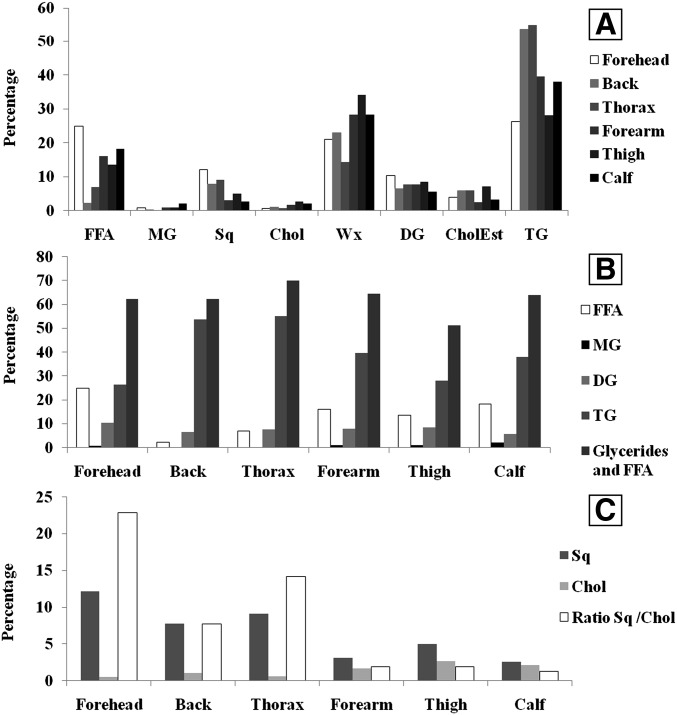
To sum up, six SSL samples were collected from six body areas, each SSL sample was run twice. The percentage of each lipid class in the total amount of SSLs was evaluated as shown in Fig. 2A.
Fig. 2.
Histograms of the mean (n = 2) percentages of lipid classes for SSLs collected from six body areas. (a) all classes, (b) FFAs and glycerides, (c) squalene, cholesterol, and squalene/cholesterol ratio. FFA, free fatty acids; MG, monoglycerides; Sq, squalene; Chol, cholesterol; Wx, waxes; DG, diglycerides; CholEst, cholesteryl esters; TG, triglycerides.
The total amount of SSLs collected was higher from body areas rich in sebaceous glands than from other areas. A factor 5 was observed between the total quantities collected from forehead, back, and thorax and total quantities collected from forearm, thigh, and calf. In contrast, the detection of the same lipid compounds from the six sampled areas indicated a homogeneous qualitative composition. Only the relative percentages of each lipid class varied according to the number of sebaceous glands present, the amount of skin removed, and cutaneous resident bacterial activity at the studied areas.
Figure 2A summarizes the overall centesimal lipid class composition of the SSLs from the six body areas studied. The sum of triglycerides and their hydrolysis compounds represented the major part of the SSLs, in agreement with findings by Greene et al. (6).
Figure 2B shows the inverse relationship between FFA and triglyceride percentages, in agreement with the findings by Downing et al. (20, 50). This finding highlights the advantage of our analytical protocol in the investigation of glyceride composition without prior cleavage. In fact, our protocol allowed us to observe the natural relationship between triglycerides and their hydrolysis products. In addition, the sum of total glycerides and FFA percentages was independent of the studied areas.
Sebaceous glands produce squalene and little or no cholesterol. Cholesterol is synthesized during cell differentiation from stratum basale to stratum corneum without accumulation of squalene (6, 13, 17, 44, 45). Thus, the squalene/cholesterol ratio in SSLs could reflect the relative activity of sebaceous gland and skin removal (41).
Figure 2C shows that the squalene percentage was higher in SSLs collected from areas rich in sebaceous glands than in SSLs from areas in which these glands were sparse. This finding is also in agreement with that of Greene et al. (6). This observation led us to choose the squalene as a sebaceous activity marker.
As expected, cholesterol percentage was higher in SSLs collected from areas sparse in sebaceous glands than in SSLs from areas rich in these glands (Fig. 2C). According to different authors, cholesterol could be used to follow skin removal (48, 49).
The squalene/cholesterol ratio represents the relative participation of both sebaceous and desquamation lipids in the SSLs. This ratio decreases significantly in areas in which sebaceous glands are sparse (Fig. 2).
However, it is difficult to interpret the variations of cholesteryl ester percentages between different body areas depending on the activity of both tissues studied. Furthermore, the density of the microbial population in the skin may influence the presence of cholesteryl esters in SSLs (51).
To sum up, this method, which uses only one analytical protocol, allowed a comparison of variations among lipid classes obtained from different body areas. The qualitative SSL profiles do not depend on the skin area studied and seem to be a personal feature. However, the quantitative inter-area variations were observed mainly for the squalene/cholesterol ratio and FFAs/glycerides pattern.
CONCLUSION
The aim of this study was to separate and identify SSLs in order to get SSL fingerprints. For this purpose, HTGC-MS was used with EI and CI ionization. SSL class identification assays were performed using specific mass fragments of each lipid class, molecular ions, and retention time of each compound. Despite the complexity of this lipid mixture, more than 200 compounds were individually identified and assigned to five lipid classes including FFA, hydrocarbons, waxes, glycerides, and free and esterified cholesterols. The dominant fatty acid in the set of lipid classes were hexadecenoic acid (C16: 1) and hexadecanoic acid (C16: 0). These findings suggest the important role of these two acids in the hydrolipidic film.
SSL compositions from six human body areas were studied. No qualitative change was detected, suggesting that SSL compositions are similar in the different body areas. The quantitative composition was assessed using peak area normalization with a response factor. We checked that the squalene percentage increases in areas rich in sebaceous glands, whereas the cholesterol percentage increases in areas poor in sebaceous glands. This balance between sebaceous activity and skin removal markers could be an indicator of the equilibrium between these two cutaneous activities. The study of glyceride class compounds without prior cleavage allowed us to observe the inverse relationship between the percentages of triglycerides and their hydrolysis products that could reflect the bacterial skin surface activity. Thus, the method developed during this work could be useful to evaluate the influence of treatments or environmental and nutrition factors on skin health. Moreover, it could detect skin lipid disorders related to skin diseases.
To sum up, this method gives structural information and detailed lipid profiles by using only one analytical protocol. It could be of a great interest for establishing the proof for medical treatment efficiency in diseases such as acne, atopic dermatitis, seborrhea or psoriasis.
More generally, other application areas involving complex lipid mixture investigation could benefit from this analytical approach.
Supplementary Material
Acknowledgments
We thank Caroline Baudoin and Stéphanie Brédif, Expanscience Laboratoires (Epernon, France), for valuable input and assistance.
Footnotes
Abbreviations:
- APCI
- atmospheric pressure chemical ionization
- CI
- chemical ionization
- EI
- electron impact
- FID
- flame ionization detector
- HTGC-MS
- high-temperature gas chromatography-mass spectrometry
- SC
- stratum corneum
- SSL
- skin surface lipid
- TIC
- total ion current
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two figures.
REFERENCES
- 1.Fluhr J. W., Mao-Qiang M., Brown B. E., Wertz P. W., Crumrine D., Sundberg J. P., Feingold K. R., Elias P. M. 2003. Glycerol regulates stratum corneum hydration in sebaceous gland deficient (Asebia) mice. J. Invest. Dermatol. 120: 728–737. [DOI] [PubMed] [Google Scholar]
- 2.Mavon A., Zahouani H., Redoules D., Agache P., Gall Y., Humbert P. 1997. Sebum and stratum corneum lipids increase human skin surface free energy as determined from contact angle measurements: a study on two anatomical sites. Colloids Surf. B Biointerfaces. 8: 147–155. [Google Scholar]
- 3.Thiele J. J., Weber S. U., Packer L. 1999. Sebaceous gland secretion is a major physiologic route of vitamin E delivery to skin. J. Invest. Dermatol. 113: 1006–1010. [DOI] [PubMed] [Google Scholar]
- 4.Pilgram G. S. K., van der Meulen J., Gooris G. S., Koerten H. K., Bouwstra J. A. 2001. The influence of two azones and sebaceous lipids on the lateral organization of lipids isolated from human stratum corneum. Biochim. Biophys. Acta. 1511: 244–254. [DOI] [PubMed] [Google Scholar]
- 5.Man M. Q., Xin S. J., Song S. P., Cho S. Y., Zhang X. J., Tu C. X., Feingold K. R., Elias P. M. 2009. Variation of skin surface pH, sebum content and stratum corneum hydration with age and gender in a large chinese population. Skin Pharmacol. Physiol. 22: 190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greene R. S., Downing D. T., Pochi P. E., Strauss J. S. 1970. Anatomical variation in the amount and composition of human skin surface lipid. J. Invest. Dermatol. 54: 240–247. [DOI] [PubMed] [Google Scholar]
- 7.Vantrou M., Venencie P. Y., Chaumeil J. C. 1987. Les lipides cutanés de surface chez l'homme: origine, synthése, régulation. Ann. Dermatol. Venereol. 114: 1115–1129. [PubMed] [Google Scholar]
- 8.Downing D. T., Wertz P. W., Stewart M. E. 1986. The role of sebum and epidermal lipids in the cosmetic properties of skin. Int. J. Cosmet. Sci. 8: 115–123. [DOI] [PubMed] [Google Scholar]
- 9.Saint-Léger D., Lévêque J-L. 1980. Les methodes d’évaluation quantitative des lipides de surface chez l'homme. Présentation d'une nouvelle procédure. Int. J. Cosmet. Sci. 2: 283–294.19467103 [Google Scholar]
- 10.Pablo G., Hammons A., Bradley S., Fulton J. E., Jr 1974. Characteristics of the extracellular lipases from corynebacterium acnes and staphylococcus epidermis. J. Invest. Dermatol. 63: 231–238. [DOI] [PubMed] [Google Scholar]
- 11.Shalita A. R. 1974. Genesis of free fatty acids. J. Invest. Dermatol. 62: 332–335. [DOI] [PubMed] [Google Scholar]
- 12.Nicolaides N. 1974. Skin lipids: their biochemical uniqueness. Science. 186: 19–26. [DOI] [PubMed] [Google Scholar]
- 13.Smith K. R., Thiboutot D. M. 2008. Thematic review series: Skin lipids. Sebaceous gland lipids: friend or foe? J. Lipid Res. 49: 271–281. [DOI] [PubMed] [Google Scholar]
- 14.Picardo M., Ottaviani M., Camera E., Mastrofrancesco A. 2009. Sebaceous gland lipids. Dermatoendocrinol. 1: 68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freinkel R. K., Shen Y. 1969. The Origin of Free Fatty Acids in Sebum II. J. Invest. Dermatol. 53: 422–427. [DOI] [PubMed] [Google Scholar]
- 16.Stefaniak A. B., Harvey C. J. 2006. Dissolution of materials in artificial skin surface film liquids. Toxicol. In Vitro. 20: 1265–1283. [DOI] [PubMed] [Google Scholar]
- 17.Feingold K. R. 2009. The outer frontier: the importance of lipid metabolism in the skin. J. Lipid Res. 50: S417–S422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wille J. J., Kydonieus A. 2003. Palmitoleic acid isomer (C16:1Δ6) in human skin sebum is effective against gram-positive bacteria. Skin Pharmacol. Physiol. 16: 176–187. [DOI] [PubMed] [Google Scholar]
- 19.Zouboulis C. C. 2004. Acne and sebaceous gland function. Clin. Dermatol. 22: 360–366. [DOI] [PubMed] [Google Scholar]
- 20.Downing D. T., Strauss J. S., Pochi P. E. 1969. Variability in the chemical composition of human skin surface lipids1. J. Invest. Dermatol. 53: 322–327. [DOI] [PubMed] [Google Scholar]
- 21.Marzouki Z. M., Taha A. M., Gomma K. S. 1987. Variability in the composition of human skin surface lipids in tropical climates. Trop. Geogr. Med. 39: 366–371. [PubMed] [Google Scholar]
- 22.Bonté F., Pinguet P., Chevalier J. M., Meybeck A. 1995. Analysis of all stratum corneum lipids by automated multiple development high-performance thin-layer chromatography. J. Chromatogr. B Biomed. Sci. Appl. 664: 311–316. [DOI] [PubMed] [Google Scholar]
- 23.Pappas A., Johnsen S., Liu J-C., Eisinger M. 2009. Sebum analysis of individuals with and without acne. Dermatoendocrinol. 1: 157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nazzaro-Porro M., Passi S., Boniforti L., Belsito F. 1979. Effects of aging on fatty acids in skin surface lipids. J. Invest. Dermatol. 73: 112–117. [DOI] [PubMed] [Google Scholar]
- 25.James A. T., Wheatley V. R. 1965. Studies of sebum. 6. The determination of the component fatty acids of human forearm sebum by gas–liquid chromatography. Biochem. J. 63: 269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nordstrom K. M., Labows J. N., McGinley K. J., Leyden J. J. 1986. Characterization of wax esters, triglycerides, and free fatty acids of follicular casts. J. Invest. Dermatol. 86: 700–705. [DOI] [PubMed] [Google Scholar]
- 27.Fitzgerald M., Murphy R. C. 2007. Electrospray mass spectrometry of human hair wax esters. J. Lipid Res. 48: 1231–1246. [DOI] [PubMed] [Google Scholar]
- 28.Green S. C., Stewart M. E., Downing D. T. 1984. Variation in sebum fatty acid composition among adult humans. J. Invest. Dermatol. 83: 114–117. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto A., Serizawa S., Ito M., Sato Y. 1987. Effect of aging on sebaceous gland activity and on the fatty acid composition of wax esters. J. Invest. Dermatol. 89: 507–512. [DOI] [PubMed] [Google Scholar]
- 30.Vrkoslav V., Urbanová K., Cvacka J. 2010. Analysis of wax ester molecular species by high performance liquid chromatography/atmospheric pressure chemical ionisation mass spectrometry. J. Chromatogr. A. 1217: 4184–4194. [DOI] [PubMed] [Google Scholar]
- 31.Marzouki Z. M. H., Taha A. M., Gomaa K. S. 1988. Fatty acid profiles of sebaceous triglycerides by capillary gas chromatography with mass-selective detection. J. Chromatogr. B Biomed. Sci. Appl. 425: 11–24. [DOI] [PubMed] [Google Scholar]
- 32.Robyn N. S., Anna B., George A. V., Neil J. M. 2008. The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglycerides. J. Dermatol. Sci. 50: 41–52. [DOI] [PubMed] [Google Scholar]
- 33.Myher J. J., Kuksis A. 1995. General strategies in chromatographic analysis of lipids. J. Chromatogr. B Biomed. Sci. Appl. 671: 3–33. [DOI] [PubMed] [Google Scholar]
- 34.Kuksis A., Myher J. J., Geher K. 1993. Quantitation of plasma lipids by gas-liquid chromatography on high temperature polarizable capillary columns. J. Lipid Res. 34: 1029–1038. [PubMed] [Google Scholar]
- 35.Moldovan Z., Jover E., Bayona J. M. 2002. Systematic characterisation of long-chain aliphatic esters of wool wax by gas chromatography-electron impact ionisation mass spectrometry. J. Chromatogr. A. 952: 193–204. [DOI] [PubMed] [Google Scholar]
- 36.Jover E., Moldovan Z., Bayona J. M. 2002. Complete characterisation of lanolin steryl esters by sub-ambient pressure gas chromatography-mass spectrometry in the electron impact and chemical ionisation modes. J. Chromatogr. A. 970: 249–258. [DOI] [PubMed] [Google Scholar]
- 37.Aichholz R., Lorbeer E. 1999. Investigation of combwax of honeybees with high-temperature gas chromatography and high-temperature gas chromatography-chemical ionization mass spectrometry: I. High-temperature gas chromatography. J. Chromatogr. A. 855: 601–615. [DOI] [PubMed] [Google Scholar]
- 38.Aichholz R., Lorbeer E. 2000. Investigation of combwax of honeybees with high-temperature gas chromatography and high-temperature gas chromatography-chemical ionization mass spectrometry: II: High-temperature gas chromatography-chemical ionization mass spectrometry. J. Chromatogr. A. 883: 75–88. [DOI] [PubMed] [Google Scholar]
- 39.Christie W. W. 2003. Determination of lipid profiles by gas chromatography. Lipids Analysis. 3rd edition Christie W. W., editor The Oily Press, Bridgwater (UK) 118–121. [Google Scholar]
- 40.Miller J. M. 2005. Chromatography: concepts and contrasts. 2nd edition John Wiley and Sons, Inc., Hoboken, NJ: 300–301. [Google Scholar]
- 41.Mélissopoulos A., Levacher C. 1998. La peau: structure et physiologie. 2nd edition Tec & Doc Lavoisier, Paris, France: 71–73. [Google Scholar]
- 42.Donike M. 1973. Die temperaturprogrammierte Analyse von Fettsäuretrimethylsilylestern: Ein kritischer Qualitätstest für gas-chromatographische Trennsäulen. Chromatographia. 6: 190–195. [Google Scholar]
- 43.Drake D. R., Brogden K. A., Dawson D. V., Wertz P. W. 2008. Thematic review series: skin lipids. Antimicrobial lipids at the skin surface. J. Lipid Res. 49: 4–11. [DOI] [PubMed] [Google Scholar]
- 44.Strauss J. S., Pochi P. E., Downing D. T. 1976. The sebaceous glands: twenty-five years of progress. J. Invest. Dermatol. 67: 90–97. [DOI] [PubMed] [Google Scholar]
- 45.Pappas A. 2009. Epidermal surface lipids. Dermatoendocrinol. 1: 72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nikkari T. 1974. Comparative chemistry of sebum. J. Invest. Dermatol. 62: 257–267. [DOI] [PubMed] [Google Scholar]
- 47.Ottaviani M., Alestas T., Flori E., Mastrofrancesco A., Zouboulis C. C., Picardo M. 2006. Peroxidated squalene induces the production of inflammatory mediators in HaCaT keratinocytes: a possible role in acne vulgaris. J. Invest. Dermatol. 126: 2430–2437. [DOI] [PubMed] [Google Scholar]
- 48.Epstein E. H., Jr, Williams M. L., Elias P. M. 1984. The epidermal cholesterol sulfate cycle. J. Am. Acad. Dermatol. 10: 869–870. [DOI] [PubMed] [Google Scholar]
- 49.Elias P. M., Crumrine D., Rassner U., Hachem J-P., Menon G. K., Man W., Choy M. H. W., Leypoldt L., Feingold K. R., Williams M. L. 2004. Basis for abnormal desquamation and permeability barrier dysfunction in RXLI. J. Invest. Dermatol. 122: 314–319. [DOI] [PubMed] [Google Scholar]
- 50.Downing D. T., Strauss J. S., Ramasastry P., Abel M., Lees C. W., Pochi P. E. 1975. Measurement of the time between synthesis and surface excretion of sebaceous lipids in sheep and man. J. Invest. Dermatol. 64: 215–219. [DOI] [PubMed] [Google Scholar]
- 51.Puhvel S. M. 1975. Esterification of [4–14C]cholesterol by cutaneous bacteria (Staphylococcus epidermidis, Propionibacterium acnes, and Propionibacterium granulosum). J. Invest. Dermatol. 64: 397–400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.