Abstract
15-Oxygenated cholesterol species such as 5α-cholest-8(14)ene-3β,15α-diol (15HC) and 3β-hydroxy-5α-cholest-8(14)-en-15-one (15KC) are commercially available synthetic products unlikely to occur in biological systems. Surprisingly, Farez et al. recently reported that these two steroids occur in human circulation at levels considerably higher than those of any other endogenous oxysterol [Farez, M. et al. 2009. Toll-like receptor 2 and poly(ADP-ribose) polymerase 1 promote central nervous system neuroinflammation in progressive EAE. Nat. Immunol. 10: 958–964]. The levels were reported to be increased in patients with multiple sclerosis in a progressive phase and the authors suggested that this could be utilized diagnostically. Based on extensive in vitro experiments exposing cells to the same high levels of 15HC as found in vivo (1000 ng/ml) the authors concluded that 15HC may be an important pathogenetic factor in multiple sclerosis. Using combined gas chromatography-mass spectrometry we fail to detect significant plasma levels of 15HC either in healthy controls or in patients with multiple sclerosis (levels < 2 ng/ml). If 15KC is present in these plasma samples, the concentration of it must be <10 ng/ml. Our failure to detect significant levels of the above steroids could not be due to loss during hydrolysis and work-up because recovery of the added two oxysterols was close to 100%. Autoxidation of lipoprotein-bound cholesterol resulted in extensive conversion of cholesterol into 7-oxygenated but not 15-oxygenated sterols. We conclude that if present there are trace amounts only of the above 15-oxygenated steroids in human circulation and that the role of such oxysterols as pathogenetic factors and biomarkers must be reconsidered.
Keywords: oxysterols; 5α-cholest-8(14)ene-3β,15α-diol; 3β-hydroxy-5α-cholest-8(14)-en-15-one; autoxidation
Oxysterols are oxygenated metabolites of cholesterol. Mainly based on in vitro experiments, they have been ascribed a number of regulatory roles in connection with inflammation, neurodegeneration, and atherosclerosis (1). They are present in trace amounts only in biological systems and there is a controversy with respect to the role they play under in vivo conditions. Most in vitro experiments with oxysterols have been carried out at unphysiological concentrations and without taking into account that cholesterol, present at levels 103–105 in excess of oxysterols in most biological systems, may counteract the effect.
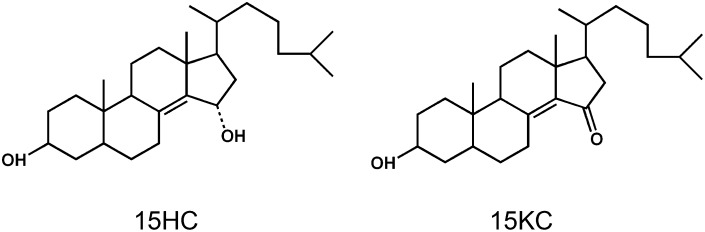
5α-Cholest-8(14)ene-3β,15α-diol (15HC) and 3β-hydroxy-5α-cholest-8(14)-en-15-one (15KC) are commercially available synthetic products of 7-dehydrocholesterol (Fig. 1). From a metabolic point of view, it appears unlikely that they are formed in biological systems. To our knowledge, there is only one report in the literature about the occurrence of 15-oxygenated C27-steroids in animal tissues; Emmons et al. (2) reported the presence of 15KC in rat skin and hair. The possibility was not excluded, however, that this steroid may have been formed by autoxidation of 5α-cholest-8(14)en-3β-ol or by the action of bacteria or other microorganisms in the skin and hair.
Fig. 1.
Structures of 5α-cholest-8(14)en-3β,15α-diol (15HC) and 3β-hydroxy-5α-cholest-8(14)-en-15-one (15KC).
Recently, Farez et al. (3) reported in a prestigious journal that these two steroids occur in human circulation at levels considerably higher than those of any other endogenous oxysterols like 24S- and 27-hydroxycholesterol and 7α-hydroxycholesterol. The levels were reported to be increased in patients with multiple sclerosis in a progressive phase and it was suggested that this could be used diagnostically. In addition, the authors made extensive in vitro experiments exposing cells to the same high levels of 15HC as they found in vivo (1,000 ng/ml). Based on the results, the authors suggested that 15HC is an important pathogenetic factor in the development of multiple sclerosis.
It was stated in the article that gas chromatography-mass spectrometry was used for the assay of the 15-oxygenated steroids in the circulation, but no details were given. With respect to methodology, a reference was given to a paper that only included assay of 7-oxocholesterol (4). Initial attempts to get more information from the authors failed.
The article by Farez et al. (3) has evoked great interest among clinical scientists interested in multiple sclerosis. We, as well as some other groups involved in steroid analyses, have been asked to set up a suitable method for assay of the above 15-oxygenated steroids.
In the present work, we describe our attempts to confirm the results by Farez et al. (3). In spite of considerable efforts, we failed to detect significant levels of the above two oxysterols in plasma from healthy controls as well as from patients with multiple sclerosis at different stages of the disease. Our failure to confirm the results by Farez et al. cannot be due to loss during extraction or work-up because the recovery of the two steroids added to human plasma was close to 100%.
MATERIALS AND METHODS
Materials
The unlabeled and deuterium labeled oxysterols were those used in a previous work (5). 15HC and 15KC were obtained from Avanti.
Patients and controls
Plasma samples were obtained from an in-house biobank containing samples collected during routine neurological diagnostic work. In total, 58 subjects were included in this study. Multiple sclerosis patients (n = 33; mean age 47.3, range 26–62 years, 21 females and 12 males) fulfilling McDonald's criteria (6) consisted of relapsing-remitting multiple sclerosis (RRMS) (n = 18) and secondary progressive multiple sclerosis (SPMS) (n = 15). The control cohort (n = 25; mean age 45.0, range 22–78 years, 15 females and 10 males) consisted of subjects with other neurological diseases (n = 12) and healthy donors (n = 13). Classification and scoring of multiple sclerosis patients was assessed by a trained neurologist. For RRMS, a relapse was defined as an increase with ≥1 point on the expanded disability status scale with a duration of at least 1 week before sampling, whereas remission was defined as a stable status >3 months prior to sampling. SPMS was defined as an initial relapsing-remitting disease course followed by more than 12 months of continuous worsening of neurological function, with or without occasional relapses. At the time of sampling, two RRMS patients were on immunomodulatory treatment including IFN-β1a and Natalizumab. The study was approved by the regional ethics committee and written informed consent was obtained from all patients.
Assay of oxysterols
The method used for assay of the oxysterols was essentially the one described by Dzeletovic et al. (5). In short, plasma is spiked with 2H6-7α-hydroxycholesterol, 2H6-7β-hydroxycholesterol, 2H6-7-oxocholesterol, 2H5-24-hydroxycholesterol, 2H3-25-hydroxycholesterol, 2H5-27-hydroxycholesterol. The EDTA plasma is then hydrolyzed by treatment for 2 h with 0.35M potassium hydroxide at room temperature in an argon atmosphere followed by separation of cholesterol from the oxysterols by silica column chromatography and conversion of the oxysterol fraction into trimethylsilyl ether. An Agilent 6890 gas chromatograph equipped with a HP-5-MS column (30m, 0.25 mm ID) is used combined with an Agilent 5973 mass selective detector. The temperature of the transfer line is 280°C and the initial temperature of the column is 180°C for 1 min followed by an increase with 20°C/min up to 250°C. The temperature is then increased to 300°C with an increase of 5°C/min followed by 8 min at this temperature. The mass spectrometer is operated in the selected ion monitoring mode, and two or four ions are detected at the same time. For details with respect to the ions used for assay of each individual oxysterol, see ref. 5. The quantitations were made with use of standard mixtures of the above deuterium labeled internal standards with the authentic compounds.
In the initial attempts to detect 15HC and 15KC, the same procedure as above was used with the exception that no deuterium labeled internal standard was added. The ions at m/z 456, m/z 434, and m/z 343 were used for assay of 15HC and the ion m/z 472 was used for assay of 15KC (see Figs. 3, 4). In the quantitation of endogenous and added 15HC, 2H6-7β-hydroxycholesterol was used as internal standard and the quantitations were performed with use of standard mixtures of 15HC and 2H6-7β-hydroxycholesterol. In the quantitation of endogenous and added 15KC, 2H6-7-oxocholesterol was used as internal standard and standard mixtures of 15KC and 2H6-7-oxocholesterol were used in the quantitations.
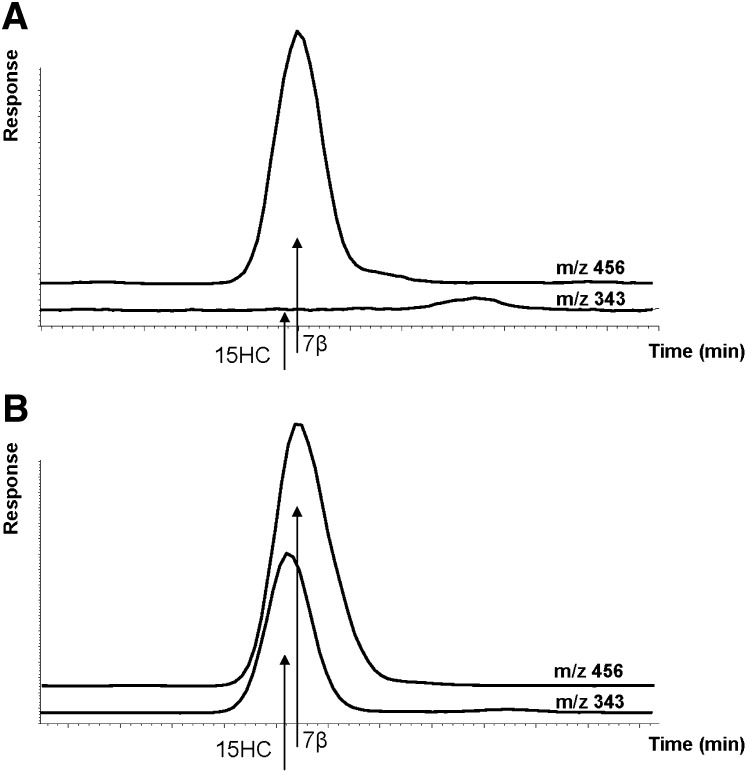
Fig. 3.
Ion chromatogram of trimethylsilyl ether of a purified extract of a plasma sample (A) and of the same plasma sample to which 90 ng/ml of 5α-cholest-8(14)ene-3β,15α-diol (15HC) had been added (B). 7β: 7β-hydroxycholesterol.
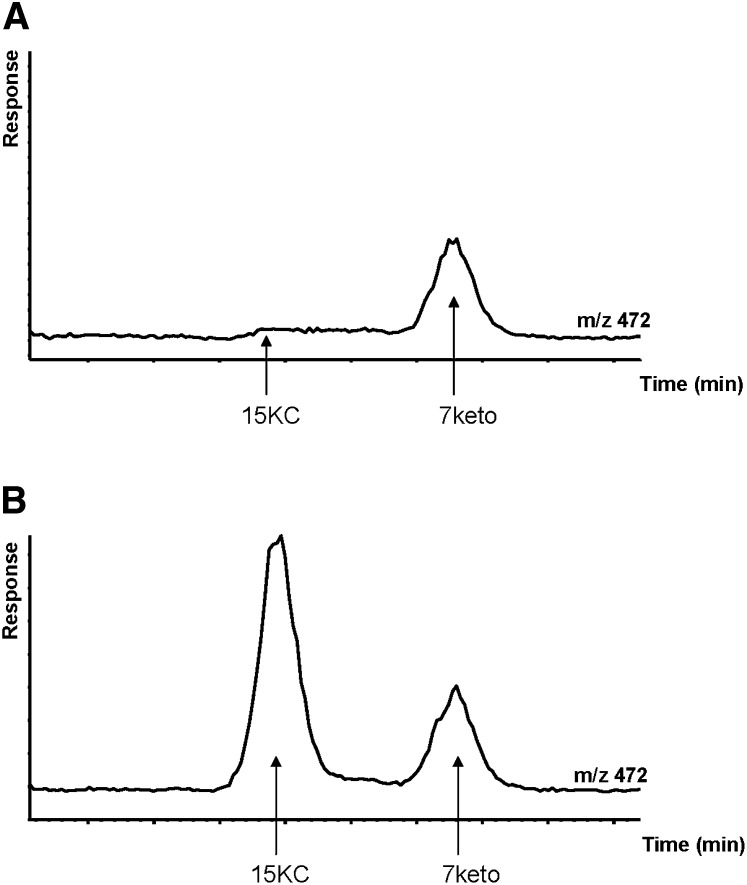
Fig. 4.
Ion chromatogram of trimethylsilyl ether of a purified extract of a plasma sample (A) and of the same plasma sample to which 90 ng/ml of 3β-hydroxy-5α-cholest-8(14)-en-15-one (15KC) had been added. 7keto: 7-ketocholesterol.
Autoxidation of LDL cholesterol
An LDL fraction isolated from EDTA plasma was oxidized with 5 μM CuSO4 under the conditions previously described (7). The formation of conjugated dienes was followed by the increase in absorbance at 234 nm. The oxysterol fraction was isolated and analyzed as above.
Statistical analysis
Differences in levels of oxysterols in plasma from patients with multiple sclerosis and controls were analyzed with the nonparametric Kruskal-Wallis and Dunn's posttest (GraphPad Prism 3.0, San Diego, CA).
RESULTS
Attempts to identify 15HC and 15KC in plasma from healthy subjects
Using our standard procedure for assay of oxysterols by combined GC-MS, the retention time of the trimethylsilyl ether of 15HC was found to be almost identical to that of the derivative of 7β-hydroxycholesterol, an oxysterol occurring naturally in human circulation. The retention time of the corresponding derivative of 15KC was found to be similar to that of 7-oxocholesterol, another naturally occurring oxysterol in human circulation. The difference in retention time, about 0.25 min under the conditions employed, was, however, sufficient to allow a separation. Attempts to change the chromatographic conditions in order to get a better separation between the derivative of 15HC and 7β-hydroxycholesterol failed or resulted in cochromatography with other oxysterols.
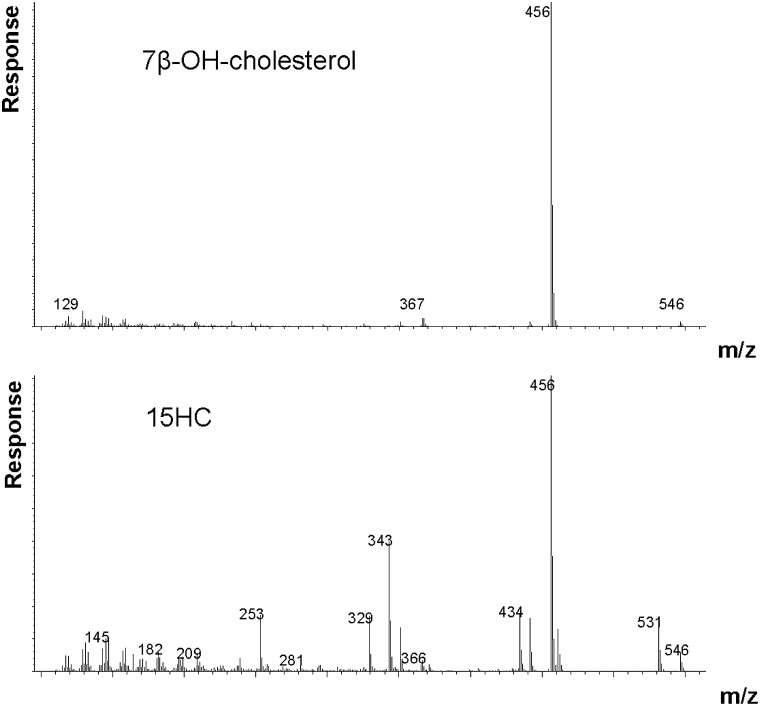
We found that the mass spectrum of the trimethylsilyl derivative of 15HC contains an ion at m/z 343 (M-90-side chain) and an ion at m/z 434 (M-side chain + H) that are practically absent in the mass spectrum of trimethylsilyl derivative of 7β-hydroxycholesterol (Fig. 2) . By using the ion at m/z 434 or m/z 343 in the assay of the derivative of 15HC and the ion at m/z 456 (M-90) in the assay of the derivative of 7β-hydroxycholesterol, it was possible to assay the two oxysterols. Because the mass spectrum of 15HC also contains m/z 456, a correction for this must be made in case of analysis of samples containing significant amounts of 15HC in addition to 7β-hydroxycholesterol.
Fig. 2.
Mass spectrum of trimethylsilyl ether of 7β-hydroxycholesterol (A), and 5α-cholest-8(14)ene-3β,15α-diol (B).
The mass spectrum of the trimethylsilyl derivative of 15KC was found to be practically identical to that of the corresponding derivative of 7-oxocholesterol. In view of the separation between these two steroids, the same ion, m/z 472 (M), could, however, be used in the assay of both these steroids.
Figure 3 shows an ion chromatogram of trimethylsilyl derivative of a purified extract of a plasma sample and of a plasma sample to which 90 ng /ml of 15HC had been added. In the unspiked sample, no peak occurred in the tracing of the ion at m/z 343 corresponding to presence of endogenous 15HC. In repeated experiments, the recovery of the added 15HC was found to be 101 ± 2% (mean ± SD, n = 3). The same results were obtained when using the ion at m/z 434 instead of m/z 343.
From the results of analyses of plasma samples from 20 healthy subjects, it could be estimated that if there is some 15HC in the circulation of these subjects, the level must be <2 ng/ml.
Figure 4 shows a chromatogram of derivative of a purified extract of a plasma sample and of a plasma sample to which 90 ng of 15KC had been added.
In the unspiked sample, no significant peak occurred in the tracing of the ion at m/z 472 corresponding to presence of endogenous 15KC. In repeated experiments, the recovery of the added 15KC was found to be 92 ± 6% (mean ± SD, n = 3).
In the analysis of some plasma samples a small peak sometimes occurred in the tracing of the ion at m/z 472 with a retention time similar to or identical to that of 15KC. Assuming that this endogenous compound is 15KC, the amount of it was always <5 ng/ml. Because of the very low amounts and presence of contaminants, it was not possible to establish the identity of this compound.
Assay of 15HC and 15KC and other oxysterols in plasma from patients with multiple sclerosis
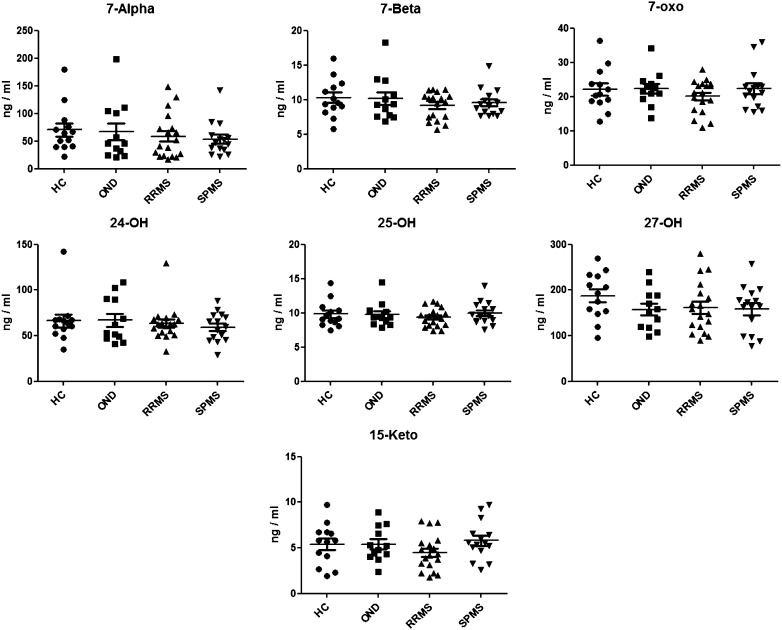
We could not find significant levels of 15HC in any of the patients with multiple sclerosis (levels < 3 ng/ml in all samples, results not shown). With respect to 15KC, the levels were <10 ng/ml in all subjects, with no significant difference between healthy controls, patients with other neurological diseases, and patients with the two different stages of multiple sclerosis (Fig. 5).
Fig. 5.
Levels of the different oxysterols in healthy controls (HC), patients with relapsing-remitting MS (RRMS), patients with secondary progressive MS (SPMS), and patients with other neurological diseases (OND).
The possibility must be considered that Farez et al. (3) may have measured some other oxysterol(s) than 15HC and 15KC that could increase in plasma of patients with multiple sclerosis. Because of this, we also measured the levels of 7α-,7β-, 7-oxo, 24S-, 25-, and 27-hydroxycholesterol in the two different groups of patients and the two different control groups. As shown in Fig. 5, there were no significant differences between the controls and the patients with multiple sclerosis.
Attempts to identify the 15-oxygenated oxysterols among autoxidation products of cholesterol
Autoxidation of cholesterol results in high conversion into products such as 7α-, 7β-, and 7-oxocholesterol. Such autoxidation may occur in connection with freezing and thawing biological samples or during extraction and work-up. In order to study the possibility that 15HC and/or 15KC are formed by autoxidation, we oxidized a human LDL fraction with Cu2+ under the conditions previously described (7). In accordance with the expectation, there was a very high conversion of cholesterol into the 7-oxygenated products. We failed to detect any 15HC among the products. Small amounts were formed of a compound with the same retention time as 15KC and having the ion at m/z 472 in its mass spectrum. The amount of this steroid was <4% of the amount of 7-oxocholesterol. The mass spectrum was not identical to that of the trimethylsilyl ether of 15KC and no attempts were made to identify this steroid. When oxidizing a serum sample with Cu2+ under the same condition as above, the conversion of cholesterol into the above unknown steroid was <0.1% of the conversion into 7-oxocholesterol.
DISCUSSION
The present work seems to exclude that there are high levels of 15HC and 15KC in human circulation. The possibility that these oxysterols may have been lost during hydrolysis, extraction, or chromatography was clearly excluded by the excellent recovery of the authentic steroids added to plasma.
After completion of this study, we tried to publish a short summary of it in Nature Immunology, the journal in which Farez et al. (3) published their findings. The manuscript was rejected. After pressure from the editorial board of Nature Immunology, Farez et al. presented to us an incomplete description of the method they had used to assay the 15-oxygenated steroids. According to this description, gas chromatography-mass spectrometry was used with the same equipment as we use here. Surprisingly, no hydrolysis and no derivatization were used prior to the chromatographic step. No information was included with respect to the ions used for the assay. It should be mentioned that this procedure is markedly different from the one they gave a reference to in the Nature Immunology paper. Attempts in our laboratory to analyze free oxysterols without previous derivatization failed when analyzing plasma samples because of a very low sensitivity in the measurements. It should be noted that the major oxysterols in the circulation are esterified to 60–90% (5) and if the hydrolysis step is omitted, only a fraction of the oxysterol is measured.
We were recently informed by two other groups with long experience with oxysterol analyses that they also have failed to detect significant levels of 15-oxygenated steroids in human circulation (unpublished observations).
Reports of extremely high levels of oxysterols in biological systems are not unique. In 2003, Diestel et al. (4) stated that cerebrospinal fluid of patients with multiple sclerosis contains 7-oxocholesterol at levels about 10 μg/ml. Extensive in vitro experiments were made, exposing cultured cells to the same high levels of 7-oxocholesterol as they had found in vivo. When we tried to confirm the high levels of 7-oxocholesterol in cerebrospinal fluid of patients with multiple sclerosis, we found levels of about 1 ng/ml. In similarity with our experience with Nature Immunology, attempts to publish a correction in the journal in which Diestel et al. reported their findings, the Journal of Experimental Medicine, failed. The results were however, published in this journal (8).
If the findings by Farez et al. (3) can be confirmed, that there are compounds in the circulation that are increased up to 3-fold in patients with multiple sclerosis in a progressive state, it is clearly of interest. It seems important that these compounds can be identified.
Acknowledgments
The skilful technical assistance of Inger Moberg is gratefully acknowledged.
Footnotes
Abbreviations:
- 15HC
- 5α-cholest-8(14)ene-3β,15α-diol
- 15KC
- 3β-hydroxy-5α-cholest-8(14)-en-15-one
- RRMS
- relapsing-remitting multiple sclerosis
- SPMS
- secondary progressive multiple sclerosis
This work was supported by grants from the Swedish Research Council and Karolinska Institutet.
REFERENCES
- 1.Björkhem I., Diczfalusy U. 2002. Oxysterols. Friends, foes or just fellow passengers? Arterioscler. Thromb. Vasc. Biol. 22: 734–742. [DOI] [PubMed] [Google Scholar]
- 2.Emmons G. T., Pyrek J., Dam R., Martin M., Kudo K., Schroepfer G. J., Jr 1988. 5α-cholest-8(14)-en-3β-ol-15-one, a potent regulator of cholesterol metabolism: occurrence in rat skin. J. Lipid Res. 29: 1039–1054. [PubMed] [Google Scholar]
- 3.Farez M. F., Quintana F. J., Gandhi R., Izquierdo G., Lucas M., Weiner H. L. 2009. Toll-like receptor 2 and poly(ADP-ribose) polymerase 1 promote central nervous system neuroinflammation in progressive EAE. Nat. Immun. 10: 958–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diestel A., Aktas O., Hackel D., Häke I., Meier S., Raine C. S., Nitsch R., Zipp F., Ullrich O. 2003. Activation of microglial poly(ADP-ribose)-polymerase by cholesterol breakdown products during neuroinflammation: a link between demyeliniation and neuronal damage. J. Exp. Med. 198: 1729–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dzeletovic S., Breuer O., Lund E., Diczfalusy U. 1995. Determination of cholesterol oxidation products in human plasma by isotope dilution mass spectrometry. Anal. Biochem. 225: 73–80. [DOI] [PubMed] [Google Scholar]
- 6.McDonald W. I., Compston A., Edan G., Goodkin D., Hartung H. P., Lublin F. D., McFarland H. F., Paty D. W., Polman C. H., Reingold S. C., et al. 2001. Recommended diagnostic criteria for multiple sclerosis: guidlines from the International Panel on the diagnosis of multiple sclerosis. Ann. Neurol. 50: 121–127. [DOI] [PubMed] [Google Scholar]
- 7.Dzeletovic S., Babiker A., Lund E., Diczfalusy U. 1995. Time course of oxysterol formation during in vitro oxidation of low density lipoprotein. Chem. Phys. Lipids. 78: 119–128. [DOI] [PubMed] [Google Scholar]
- 8.Leoni V., Lűtjohann D., Masterman T. 2005. Levels of 7-oxocholesterol in cerebrospinal fluid are more than one thousand times lower than reported in multiple sclerosis. J. Lipid Res. 46: 191–195. [DOI] [PubMed] [Google Scholar]