Abstract
Treatment with the peroxisome proliferator-activated receptor γ agonist rosiglitazone has been reported to increase HDL-cholesterol (HDL-C) levels, although the mechanism responsible for this is unknown. We sought to determine the effect of rosiglitazone on HDL apolipoprotein A-I (apoA-I) and apoA-II metabolism in subjects with metabolic syndrome and low HDL-C. Subjects were treated with placebo followed by rosiglitazone (8 mg) once daily. At the end of each 8 week treatment, subjects (n = 15) underwent a kinetic study to measure apoA-I and apoA-II production rate (PR) and fractional catabolic rate. Rosiglitazone significantly reduced fasting insulin and high-sensitivity C-reactive protein (hsCRP) and increased apoA-II levels. Mean apoA-I and HDL-C levels were unchanged following rosiglitazone treatment, although there was considerable individual variability in the HDL-C response. Rosiglitazone had no effect on apoA-I metabolism, whereas the apoA-II PR was increased by 23%. The change in HDL-C in response to rosiglitazone was significantly correlated with the change in apoA-II concentration but not to changes in apoA-I, measures of glucose homeostasis, or hsCRP. Treatment with rosiglitazone significantly increased apoA-II production in subjects with metabolic syndrome and low HDL-C but had no effect on apoA-I metabolism. The change in HDL-C in response to rosiglitazone treatment was unrelated to effects on apoA-I, instead being related to the change in the metabolism of apoA-II.
Keywords: apolipoprotein A-I, stable isotope, thiazolidinedione, lipoprotein kinetics, high density lipoprotein
The metabolic syndrome consists of a cluster of abnormalities that include hypertension, abdominal obesity, impaired fasting glucose, elevated fasting triglyceride levels, and low HDL-cholesterol (HDL-C). Patients with metabolic syndrome are at increased risk of cardiovascular disease and, as a result, recommendations have been made to reduce the risk in these patients. These recommendations include therapeutic lifestyle changes and, if treatment goals are not met, pharmacological intervention (1).
Currently, few drugs are available that are suitable for treating patients with two components of the metabolic syndrome, insulin resistance and low HDL-C. Among treatments available for improving insulin resistance are the thiazolidinediones (TZDs) rosiglitazone and pioglitazone, which activate the nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ). In addition to their insulin-sensitizing effects, TZDs have been shown to increase HDL-C levels in diabetic populations (2) by up to 14% for rosiglitazone and up to 19% for pioglitazone (3). These changes in HDL-C compare favorably to HDL-C changes achieved with other drugs currently approved to treat low HDL-C levels (4–7). Although rosiglitazone and pioglitazone both activate PPARγ, they each have a characteristic metabolic response in regard to plasma lipid levels, with rosiglitazone also increasing plasma triglyceride levels in the relatively short term, whereas pioglitazone does not (8).
The mechanisms responsible for the HDL-C-raising effects of TZDs are currently unknown. Studies that have measured apolipoprotein A-I (apoA-I) and apoA-II levels following rosiglitazone treatment have found that although HDL-C and apoA-II generally increase, levels of apoA-I remain unchanged (9–14). Weak, partial transactivation of PPARα by rosiglitazone (15, 16) and pioglitazone (16, 17) has been demonstrated in vitro and may result in upregulation of the PPARα targets apoA-I and apoA-II, leading to an increase in the production rates (PRs) of these proteins. Consistent with these in vitro findings, Carreon-Torres et al. (18) recently reported that rosiglitazone increased apoA-I production in rabbits. In contrast, a study in patients with type 2 diabetes demonstrated that treatment with pioglitazone had no effect on apoA-I kinetics (19). To date, no studies have measured apoA-I kinetics in humans treated with roziglitazone, and no study has examined the effect of TZDs on the kinetics of apoA-II in humans.
We conducted studies with rosiglitazone in subjects with metabolic syndrome and low HDL-C. These studies were conducted to test the hypothesis that rosiglitazone increases HDL-C by increasing apoA-I and apoA-II production in humans. Similar to what was reported for pioglitazone (19), the results show that the metabolism of apoA-I was unchanged in response to rosiglitazone treatment, whereas there was a significant increase in the PR of apoA-II. The change in HDL-C in response to rosiglitazone was associated with the change in apoA-II levels, with those subjects increasing the plasma concentration of apoA-II having the greatest increase in HDL-C in response to rosiglitazone.
MATERIALS AND METHODS
Subjects
Men and women between the ages of 18 and 75 years were recruited from the local area to participate in the study. All subjects had low HDL-C (<40 mg/dl for men, <50 mg/dl for women) and at least two additional risk factors for metabolic syndrome as defined by the Adult Treatment Panel III: abdominal obesity defined by increased waist circumference, hypertension or current treatment for hypertension, impaired fasting glucose without a diagnosis of diabetes, and fasting triglycerides >150 mg/dl and ≤800 mg/dl. Exclusion criteria included current treatment with statins or niacin; a history of cardiovascular disease including coronary artery disease or heart failure; a history of diabetes or recent treatment with diabetic medications; a history of renal disease or a serum creatinine level greater than 2.0 mg/dl; a history of testing HIV positive; being pregnant or lactating; a history of a major active rheumatologic, pulmonary, or dermatologic disease or an inflammatory condition; uncontrolled blood pressure (>180/100 mmHg); abnormal measures of thyroid function; levels of aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, or total bilirubin greater than 2.0 times the upper limit of normal. All subjects gave written, informed consent. All protocols and procedures were approved by the Human Investigational Review Board at the University of Pennsylvania.
Study design
This study was a single-site, single-blind, placebo-controlled, fixed-sequence study. A fixed-sequence design with placebo treatment followed by rosiglitazone treatment was selected to avoid potential carryover effects from rosiglitazone treatment. Eligible subjects visited the General Clinical Research Center (GCRC) at the University of Pennsylvania for baseline fasting blood collection. Prior to discharge, subjects were instructed to take placebo once daily for 8 weeks. At the end of the 8 week placebo treatment period, subjects were admitted to GCRC for an overnight kinetic study. Following the kinetic study, subjects were instructed to take rosiglitazone (8 mg) once daily for 8 weeks. At the end of the 8 week rosiglitazone treatment period, subjects returned to the GCRC for a second kinetic study. After this second kinetic study, the drug was discontinued and the subjects discharged.
Kinetic study
Apolipoprotein kinetics were measured as previously described (20). Briefly, subjects were fed a standardized meal representing 1/20th of their daily caloric intake hourly for 5 h. Participants were then given a bolus of [5,5,5-D3]leucine (10 µmol/kg) immediately followed by a constant infusion of [5,5,5-D3]leucine (10 µmol/kg/h). Hourly standardized meals were given throughout the kinetic study to maintain a constant rate of lipoprotein production. Blood samples were collected at various time points over a 15 h period, at which point the infusion was stopped. Lipoprotein fractions were isolated by sequential ultracentrifugation, and apolipoproteins were isolated by SDS-PAGE. Apolipoproteins were hydrolyzed, their amino acids derivatized, and their isotope enrichment analyzed by GC/MS. The fractional catabolic rates (FCRs) for HDL apoA-I and apoA-II were determined using the WinSAAM modeling program by fitting a rising monoexponential curve that incorporated a secretory delay to the HDL apoA-I and apoA-II [5,5,5-D3]leucine tracer data using a weighted least-squares approach (21). The apoA-I and apoA-II liver precursor pool enrichment was assumed to be equal to the maximal estimated VLDL apoB-100 enrichment (i.e., VLDL apoB-100 enrichment plateau). Pool sizes for apoA-I and apoA-II were calculated as plasma concentration (mg/dl) multiplied by the estimated plasma volume (0.45 dl/kg body weight). PRs were calculated by multiplying the FCR by the pool size using the formula PR (mg/kg/day) = FCR (pools/day) • pool size (mg/kg body weight/pool).
Biochemical measurements
Plasma lipids were measured from EDTA plasma collected after a 12 h fast in a Centers for Disease Control and Prevention-standardized laboratory. Plasma total cholesterol (TC), VLDL-C (d < 1.006 g/ml fraction), HDL-C, and triglycerides were measured enzymatically on a Cobas Fara II autoanalyzer (Roche Diagnostic Systems, Inc.; Basel, Switzerland) using Sigma reagents (Sigma Chemical Co.; St. Louis, MO). LDL-C levels were determined by subtracting VLDL-C and HDL-C from the TC measurement. ApoA-I, apoB, apoC-II, apoC-III, apoE, and lipoprotein [a] (Lp[a]) were measured with immunoturbidemtric assays using Wako reagents (Wako Chemicals USA, Inc.; Richmond, VA). HDL particle sizes were measured using NMR (LipoScience; Raleigh, NC). High-sensitivity C-reactive protein (hsCRP) was measured with an ultra high-sensitivity latex turbidimetric immunoassay (Wako Chemicals USA, Inc.), and glucose and nonesterified FAs were measured using enzymatic reagents (Wako Chemicals USA, Inc.) on a Hitachi 912 autoanalyzer (Roche Diagnostics). Insulin levels were measured by radioimmunoassay (Linco Research, Inc). Insulin resistance was estimated with the homeostasis model assessment of insulin resistance (HOMA-IR) calculated as [plasma insulin (µIU/ml) × plasma glucose (mmol/l)]/22.5.
Cellular cholesterol efflux studies
Cholesterol efflux from Fu5AH rat hepatoma cells to patient HDL was obtained following precipitation of apoB-containing lipoproteins with polyethylene glycol, as previously described (22). Fu5AH cells efflux cholesterol primarily via the scavenger receptor class B type I (SR-BI) and aqueous diffusion pathways (23). Efflux was measured over a 4 h period on samples collected at the end of the placebo and rosiglitazone treatment periods.
Statistical analysis
Differences between treatment groups were determined using a paired t-test. Differences for nonnormally-distributed data were determined using the Wilcoxon sign rank test. Analyses were performed using GraphPad Prism, Version 3.02 (GraphPad Software, Inc.). Stepwise multiple regression was conducted using Intercooled Stata, Version 9. A two-tailed P value of less than 0.05 was considered statistically significant.
RESULTS
Subjects
Seventeen subjects (nine male, eight female) enrolled in the study. The mean age of the subjects was 50.6 ± 10.5 years, with nine Caucasian and eight African-American subjects. Two subjects voluntarily withdrew from the study prior to initiating rosiglitazone treatment, and fifteen subjects completed all study-related visits. Baseline subject characteristics are shown in Table 1. Body mass index (BMI) ranged from 25.8 to 42.6 kg/m2, with the mean falling within the obesity range (>30.0 kg/m2). Five of the enrolled subjects were smokers.
TABLE 1.
Baseline characteristics of the subjects enrolled in the study
| Subject characteristics (n = 17) | |
|---|---|
| Age (years), mean ± SD | 50.6 ± 10.5 |
| Male sex, n (%) | 9 (53) |
| Race | |
| White, n (%) | 9 (53) |
| African-American, n (%) | 8 (47) |
| BMI (kg/m ), mean ± SD | 31.5 ± 4.0 |
| Current smoker, n (%) | 5 (29) |
BMI, body mass index.
Treatment with rosiglitazone (8 mg once daily) was well-tolerated and there were no serious adverse events reported. One subject was found to have trace edema in lower extremities after 8 weeks on rosiglitazone, which resolved within 2 weeks of drug discontinuation and was judged as possibly related to treatment. A second subject gained an unexplained 5.8 kg between baseline and 8 weeks on placebo and another 2.1 kg after being on rosiglitazone for 8 weeks that was judged as possibly related to treatment. Neither subject had symptoms of congestive heart failure or ischemic cardiovascular events. Because the second subject with unexplained weight gain was not in a steady state during the study period, the results for this subject were excluded from all analyses.
Effects on plasma lipoproteins
The plasma lipid levels at the end of the placebo and rosiglitazone phases are shown in Table 2. There was an 11% increase in plasma TC in response to rosiglitazone that was due to a significant increase in the VLDL cholesterol level. There were no significant changes in triglyceride, LDL-C, or HDL-C levels following rosiglitazone treatment. Plasma nonesterified FA levels were significantly decreased (−36%) following rosiglitazone treatment. ApoA-I and apoB levels in plasma were unchanged following rosiglitazone treatment, whereas apoA-II, apoE, apoC-III, and Lp[a] levels in plasma were all significantly increased.
TABLE 2.
Plasma lipid, apolipoprotein, glucose, insulin, and high-sensitivity C-reactive protein levels in subjects completing the placebo and rosiglitazone treatment phases (n = 14)
| Placebo | Rosiglitazone | % Change | P | |
|---|---|---|---|---|
| Total cholesterol (mg/dl) | 209 ± 51 | 231 ± 68 | 11% | 0.05 |
| Triglycerides (mg/dl) | 203 ± 98 | 244 ± 157 | 18% | 0.11 |
| VLDL-C (mg/dl) | 34 (24–39) | 41 (33–88) | 40%a | 0.01 |
| LDL-C (mg/dl) | 141 ± 37 | 138 ± 47 | −1% | 0.77 |
| HDL-C (mg/dl) | 34 ± 6 | 36 ± 7 | 4% | 0.22 |
| Lp[a] (mg/dl) | 25 (11–59) | 26 (12–63) | 34%a | 0.04 |
| Nonesterified FAs (mEq/l) | 0.22 ± 0.06 | 0.14 ± 0.06 | −36% | 0.0002 |
| ApoA-I (mg/dl) | 94 ± 14 | 86 ± 13 | −8% | 0.08 |
| ApoA-II (mg/dl) | 28 ± 4 | 33 ± 4 | 19% | <0.0001 |
| ApoB (mg/dl) | 100 ± 22 | 105 ± 32 | 5% | 0.76 |
| ApoE (mg/dl) | 5.3 ± 1.7 | 5.9 ± 2.0 | 12% | 0.009 |
| ApoC-II (mg/dl) | 5.8 ± 2.7 | 7.7 ± 3.9 | 37% | 0.0003 |
| ApoC-III (mg/dl) | 14.0 ± 6.2 | 16.7 ± 8.3 | 16% | 0.009 |
| Fasting glucose (mg/dl) | 86 ± 7 | 83 ± 8 | −3% | 0.30 |
| Fasting Insulin (mU/ml) | 17.6 ± 8.8 | 12.6 ± 4.6 | −22% | 0.02 |
| HOMA-IR | 3.7 ± 1.9 | 2.6 ± 1.0 | −23% | 0.02 |
| hsCRP (mg/l) | 1.9 (0.7–3.4) | 1.3 (0.3–3.4) | −40%a | 0.01 |
Data are expressed as mean ± SD except for Lp[a] and hsCRP, which are median (interquartile range). ApoA-I, apolipoprotein A-I; HDL-C, HDL-cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; hsCRP, high-sensitivity C-reactive protein; LDL-C, LDL cholesterol; Lp[a], lipoprotein [a].
Median change.
Effect on fasting glucose, insulin, and hsCRP
Fasting glucose, insulin, and hsCRP levels at the end of the placebo and rosiglitazone phases are shown in Table 2. There was no change in fasting plasma glucose, whereas there were significant reductions in fasting insulin (−22%) and the HOMA-IR in response to rosiglitazone treatment. Plasma levels of hsCRP were significantly reduced following rosiglitazone treatment.
Effects on HDL particle concentration, size, and composition
We assessed HDL particle concentrations and particle size by NMR (Table 3). Consistent with the lack of change in the HDL-C concentration, there was no change in HDL particle concentration. There was, however, a change in the HDL size distribution, with an increase in medium-sized HDL and a decrease in small HDL.
TABLE 3.
Effects of rosiglitazone on HDL particle concentration, size, and composition (n = 14)
| Placebo | Rosiglitazone | P | |
|---|---|---|---|
| Total HDL particles (µmol/l) | 25.9 ± 3.5 | 24.5 ± 2.9 | 0.21 |
| Large HDL particles (µmol/l) | 2.7 ± 1.6 | 2.3 ± 1.9 | 0.16 |
| Medium HDL particles (µmol/l) | 2.2 ± 2.1 | 5.8 ± 4.1 | 0.0002 |
| Small HDL particles (µmol/l) | 21.0 ± 3.1 | 16.4 ± 4.2 | 0.003 |
| HDL size (nm) | 8.5 ± 0.2 | 8.5 ± 0.2 | 0.91 |
| Lipid composition (% lipid) | |||
| Cholesterol | 28.1 ± 1.4 | 28.4 ± 2.4 | 0.66 |
| Free cholesterol | 5.3 ± 0.4 | 5.3 ± 0.7 | 0.89 |
| Cholesteryl ester | 22.8 ± 1.2 | 23.1 ± 2.1 | 0.62 |
| Triglyceride | 12.5 ± 2.4 | 11.2 ± 2.7 | 0.10 |
| Phospholipids | 59.4 ± 1.9 | 60.4 ± 2.3 | 0.04 |
| HDL apolipoproteins (mg/dl) | |||
| apoE | 0.5 ± 0.4 | 0.7 ± 0.5 | 0.004 |
| apoC-III | 2.2 ± 1.5 | 3.2 ± 2.4 | 0.12 |
Data are expressed as mean ± SD.
The lipid composition and apolipoprotein content of HDL isolated by ultracentrifugation was determined at the end of the placebo and rosiglitazone treatment periods (Table 3). Treatment with rosiglitazone significantly increased the percent phospholipid content and content of apoE in the HDL fraction. There was a trend toward the percent triglyceride content in HDL with rosiglitazone treatment being reduced.
Effects on serum cholesterol efflux capacity
Treatment with rosiglitazone significantly increased the ability of HDL to promote cholesterol efflux from Fu5AH cells in in vitro studies. After 8 weeks of treatment with rosiglitazone, there was a 30% increase in the ability of HDL to promote cholesterol efflux (1.17 ± 0.41% efflux/4 h vs. 1.45 ± 0.44% efflux/4 h, placebo vs. rosiglitazone; P = 0.03). The percent change in HDL levels in response to rosiglitazone treatment was significantly correlated with the percent change in cholesterol efflux (r = 0.79; P = 0.0008).
Effects on apoA-I and A-II metabolism
The results for changes in apoA-I and apoA-II metabolism in response to rosiglitazone are summarized in Table 4. There was no significant change in the apoA-I pool size following rosiglitazone treatment, as compared with placebo treatment, although there was a trend toward a lower pool size (−7%). There was also no significant change in the apoA-I PR or FCR in response to rosiglitazone treatment.
TABLE 4.
ApoA-I and apoA-II kinetic parameters measured at the end of the placebo and rosiglitazone treatment periods (n = 14)
| Placebo | Rosiglitazone | % Change | P | |
|---|---|---|---|---|
| ApoA-I pool size | 3,715 ± 817 | 3,426 ± 808 | −7% | 0.14 |
| ApoA-I PR | 9.81 ± 2.47 | 9.47 ± 4.04 | −3% | 0.69 |
| ApoA-I FCR | 0.238 ± 0.084 | 0.244 ± 0.095 | 4% | 0.70 |
| ApoA-II pool size | 1,105 ± 282 | 1,315 ± 350 | 19% | <0.0001 |
| ApoA-II PR | 2.33 ± 0.67 | 2.87 ± 0.94 | 23% | <0.0001 |
| ApoA-II FCR | 0.190 ± 0.060 | 0.197 ± 0.067 | 4% | 0.37 |
Data are expressed as mean ± SD. FCR, fractional catabolic rate; PR, production rate.
In contrast, the apoA-II pool size was significantly increased in subjects treated with rosiglitazone. There was a significant increase in the apoA-II PR (23%) following rosiglitazone treatment, whereas the apoA-II FCR showed no significant change, although the individual changes in this variable ranged from −24% to 32%. Interestingly, the correlation between the percent change in the apoA-II PR and the percent change in the apoA-II pool size in response to rosiglitazone was not statistically significant (r = −0.17; P = 0.55) due to a concomitant increase in the apoA-II FCR in many subjects. Instead, there was a significant inverse correlation between the percent change in the apoA-II FCR and the percent change in the apoA-II pool size (r = −0.72; P = 0.004), indicating that the subjects with reduced or unchanged apoA-II FCR had the greatest change in apoA-II pool size.
Determinants of the change in HDL-C levels
The HDL-C response to rosiglitazone treatment was heterogeneous, ranging from −20% to 35%. To attempt to identify the factors that determine the HDL-C response to rosiglitazone, correlations were conducted between the percent change in HDL-C and the percent change in the following variables: plasma apoA-I and apoA-II concentration, apoA-I and apoA-II FCR and PR, triglyceride, apoC-III, nonesterfied FAs, fasting insulin, fasting glucose, HOMA, and hsCRP (Table 5). Of these, the percent changes in the apoA-II concentration, the apoA-II FCR, the apoC-III concentration, and the nonesterfied FA concentration were significant correlates. When adjusting for BMI and smoking, only the percent changes in the apoA-II concentration and the nonesterfied FA concentration remained significant correlates with the change in HDL-C.
TABLE 5.
Correlations between the percent change in HDL-C and the percent changes in candidate HDL-C determinants in response to rosiglitazone treatment (n = 14)
| Correlation Coefficient,Unadjusted | Correlation Coefficient,Adjusted for BMI and Smoking | |
|---|---|---|
| ApoA-I concentration | 0.35 | 0.44 |
| ApoA-I FCR | −0.26 | −0.14 |
| ApoA-I PR | 0.00 | 0.18 |
| ApoA-II concentration | 0.80b | 0.83b |
| ApoA-II FCR | −0.53a | −0.51 |
| ApoA-II PR | −0.07 | −0.05 |
| Fasting insulin | 0.21 | 0.42 |
| Fasting glucose | −0.25 | −0.23 |
| HOMA | 0.08 | 0.27 |
| HsCRP | −0.11 | −0.14 |
| Triglyceride | −0.45 | −0.50 |
| ApoC-III | −0.60a | −0.50 |
| Nonesterified FAs | −0.66a | −0.68a |
P ≤ 0.05.
P ≤ 0.001.
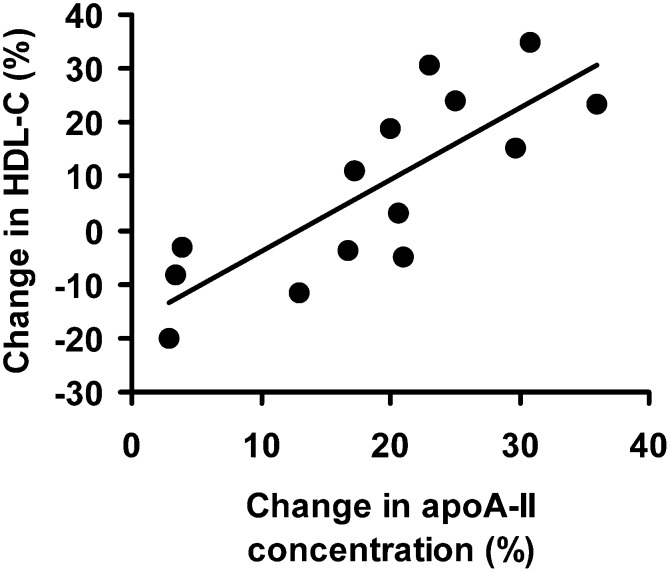
To determine whether the variables identified as significant correlates were independent predictors of the percent change in HDL-C levels, these variables were included in a stepwise regression model. When this was done, the percent change in the plasma apoA-II and apoC-III concentrations remained significant (P = 0.0001) and accounted for 75% of the change in the HDL-C concentration. When controlling for both BMI and smoking in the model, only the percent change in the plasma apoA-II remained a significant predictor of the percent change in HDL-C in response to rosiglitazone, accounting for 70% of the change, whereas apoC-III was of marginal significance in the model (P = 0.07). The relationship between the percent change in apoA-II concentration and the percent change in HDL-C is shown in Fig. 1.
Fig. 1.
The relationship between the percent change in HDL-cholesterol and percent change in apolipoprotein A-II concentration in plasma in response to rosiglitazone treatment.
DISCUSSION
Activation of the nuclear receptor PPARγ with rosiglitazone improves insulin sensitivity in patients with insulin resistance and type 2 diabetes. The mechanism by which this occurs is not completely understood but may involve reduction of hepatic glucose production and enhanced glucose uptake into adipose tissue following upregulation of the glucose transporter GLUT4 (24, 25). In addition to improving insulin sensitivity, rosiglitazone also reduces plasma nonesterified FAs and inflammatory cytokines and increases adiponectin, all of which are thought to contribute to improved insulin sensitivity (9).
In addition to improving insulin sensitivity, rosiglitazone may have beneficial effects on lipoprotein metabolism, possibly mediated through transactivation of PPARα in liver. Initial studies with rosiglitazone in rats examined the mechanisms responsible for its lipid-altering effects and concluded that there was no significant transactivation of PPARα in liver (26). However, subsequent reports have indicated that rosiglitazone increases apoA-I production in rabbits (18) and the human hepatoma cell line, HepG2 (16), via partial activation of PPARα. We found that there was no change in the PR of the PPARα target apoA-I following rosiglitazone treatment. Our results suggest that if there is activation of PPARα by rosiglitazone, it is relatively weak and insufficient to activate APOA1 transcription at doses given in humans, similar to what was reported in humans treated with pioglitazone (19).
In contrast to the lack of change in apoA-I production or FCR, we found a significant increase in the PR of apoA-II following rosiglitazone treatment. Although this could be due to transactivation of PPARα, it is also possible that there are direct or indirect effects of PPARγ activation that lead to increased transcription of APOA2. There was no evidence to suggest that the increase in the apoA-II PR was due to improvement in insulin sensitivity (not shown). Rosiglitazone has been shown to increase levels of the nuclear receptor hepatic nuclear factor (HNF)-4α in rat liver (27). A number of genes encoding proteins that are altered by rosiglitazone, including APOA2, APOC3, and LPA, have HNF-4α binding sequences in their promoter regions, suggesting that HNF-4α activation may have contributed to the increase in apoA-II production (28). Another possible mechanism by which rosiglitazone could increase the apoA-II PR is through reduction in circulating levels of inflammatory cytokines (9). ApoA-II mRNA levels and transcription (29) are decreased in rodents in response to acute inflammation (30), owing to both direct effects of cytokines on APOA2 gene expression (31) and indirect effects of NF-κB interfering with HNF4 activation of gene expression (32).
In general, treatment with rosiglitazone results in an increase in HDL-C (33). However, some studies have reported that HDL-C levels were unchanged or decreased following rosiglitazone treatment despite improvement in glucose homeostasis (13, 14). In the current study involving subjects with metabolic syndrome, there was no change in the mean HDL-C level in response to rosiglitazone. However, the response among individual subjects was variable, with some subjects increasing HDL-C levels, whereas others showed a decrease. A previous report suggests that the HDL-C response to PPARγ agonists is related to improvement in insulin sensitivity (34). However, we found that there was no significant correlation between changes in measures of glucose homeostasis and changes in HDL-C. Instead, the change in HDL-C was positively correlated with the change in apoA-II concentration in plasma. ApoA-II is known to influence HDL remodeling by cholesteryl ester transfer protein (CETP) and may inhibit cholesterol transfer between HDL and other lipoproteins, leading to an increase in HDL size and composition (35). Interestingly, we found that the change in apoA-II concentration significantly correlated with the change in the apoA-II FCR. Ng and coworkers (36) recently reported that the apoA-II FCR was influenced by changes in adiposity following weight loss. Because rosiglitazone promotes changes in adiposity, it is possible that it influences apoA-II metabolism, with effects on liver, to increase apoA-II production, and effects on adipose tissue that could influence the apoA-II FCR. Although speculative, effects on both apoA-II production and catabolism could contribute to the reported variability in the HDL response to rosiglitazone.
In addition to increasing HDL size, we also found that rosiglitazone altered the composition and apolipoprotein content of HDL. The change in HDL lipid composition may be related to effects on the CETP-mediated exchange of triglyceride between VLDL and HDL. There may also be changes in phospholipid transfer protein, whose expression increases in response to acute inflammatory stimulation and may be expected to decrease following rosiglitazone treatment (37). We also observed an increase in the apoE content of HDL during rosiglitazone treatment, an effect that should be interpreted with caution because there are significant losses of apoE from HDL during isolation by ultracentrifugation. These changes in lipid and apolipoprotein content were associated with a significant increase in the efflux of cholesterol from the hepatic cell line Fu5AH to HDL during the rosiglitazone treatment period, as compared with placebo, indicating a change in the functionality of HDL. Fu5AH cells efflux cholesterol primarily through SR-BI and aqueous diffusion (23). Increases in both the apoE content and percent phospholipid content of HDL have previously been shown to enhance the ability of HDL to accept cholesterol from cells via SR-BI (38–40). These changes in HDL composition and functionality would be expected to be antiatherogenic because they would be expected to promote reverse cholesterol transport. However, a recent report indicated that rosiglitazone has no effect on the progression of atherosclerosis, compared with placebo (41), similar to the results of the RECORD trial that showed no effect of rosiglitazone on cardiovascular risk (42). Thus, other metabolic effects of rosiglitazone may neutralize any beneficial effects of the drug on HDL levels and functionality. It is also possible that raising HDL enriched with apoA-II adversely influences HDL function and is of no cardiovascular benefit. The role of apoA-II in reducing cardiovascular risk is currently controversial and its benefits have yet to be established (43, 44).
There are a number of limitations to this study. These include the relatively small sample size, which was powered to detect a 10% change in the apoA-I- and apoA-II-associated metabolic parameters. The study is also of relatively short duration. It is possible that some metabolic effects of rosiglitazone treatment only become apparent with longer term treatment. Another limitation is the fixed-sequence design of the study. This design was selected to avoid residual drug effects that may have lingered beyond a washout period. This design does not control for temporal changes in lipoprotein metabolism that are unrelated to the study drug.
In conclusion, treatment of subjects with metabolic syndrome with rosiglitazone (8 mg) had no effect on apoA-I metabolism, whereas apoA-II production was significantly increased. Subjects in which apoA-II levels increased had the greatest rise in HDL, which was associated with significant stimulation of cholesterol efflux from cells in vitro. The results indicate that the change in HDL-C in response to rosiglitazone treatment is unrelated to changes in apoA-I metabolism, and instead is related primarily to changes in apoA-II levels.
Acknowledgments
The authors wish to acknowledge the technical expertise of Jennifer Dykhouse, Phyllis May, Anna DiFlorio, and Linda Morrell and the nursing staff and Investigational Drug Service at the GCRC at the University of Pennsylvania. The authors would also like to thank the study subjects for their participation.
Footnotes
Abbreviations:
- apoA-I
- apolipoprotein A-I
- BMI
- body mass index
- CETP
- cholesteryl ester transfer protein
- FCR
- fractional catabolic rate
- GCRC
- General Clinical Research Center
- HDL-C
- HDL cholesterol
- HNF
- hepatic nuclear factor
- HOMA-IR
- homeostasis model assessment of insulin resistance
- hsCRP
- high-sensitivity C-reactive protein
- LDL-C
- LDL cholesterol
- PR
- production rate
- PPARγ
- peroxisome proliferator-activated receptor γ
- SR-BI
- scavenger receptor class B type I
- TC
- total cholesterol
- TZD
- thiazolidinedione
This work was supported by an investigator-initiated grant from GlaxoSmithKline (D.J.R.). Additional support was provided by Public Health Services Research Grant M01-RR00040 from the National Institutes of Health and by Grant UL-1RR024134 from the National Center for Research Resources. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. 2001. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). J. Am. Med. Assoc. 285: 2486–2497. [DOI] [PubMed] [Google Scholar]
- 2.Chiquette E., Ramirez G., Defronzo R. 2004. A meta-analysis comparing the effect of thiazolidinediones on cardiovascular risk factors. Arch. Intern. Med. 164: 2097–2104. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg R. B., Kendall D. M., Deeg M. A., Buse J. B., Zagar A. J., Pinaire J. A., Tan M. H., Khan M. A., Perez A. T., Jacober S. J. 2005. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care. 28: 1547–1554. [DOI] [PubMed] [Google Scholar]
- 4.Capuzzi D. M., Guyton J. R., Morgan J. M., Goldberg A. C., Kreisberg R. A., Brusco O. A., Brody J. 1998. Efficacy and safety of an extended-release niacin (Niaspan): a long-term study. Am. J. Cardiol. 82: 74U–81U. [DOI] [PubMed] [Google Scholar]
- 5.Schaefer E. J., Asztalos B. F. 2006. The effects of statins on high-density lipoproteins. Curr. Atheroscler. Rep. 8: 41–49. [DOI] [PubMed] [Google Scholar]
- 6.Barter P. J., Rye K. A. 2008. Is there a role for fibrates in the management of dyslipidemia in the metabolic syndrome? Arterioscler. Thromb. Vasc. Biol. 28: 39–46. [DOI] [PubMed] [Google Scholar]
- 7.Hartweg J., Farmer A. J., Perera R., Holman R. R., Neil H. A. 2007. Meta-analysis of the effects of n-3 polyunsaturated fatty acids on lipoproteins and other emerging lipid cardiovascular risk markers in patients with type 2 diabetes. Diabetologia. 50: 1593–1602. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg R. B. 2006. Impact of thiazolidenediones on serum lipoprotein levels. Curr. Atheroscler. Rep. 8: 397–404. [DOI] [PubMed] [Google Scholar]
- 9.Samaha F. F., Szapary P. O., Iqbal N., Williams M. M., Bloedon L. T., Kochar A., Wolfe M. L., Rader D. J. 2006. Effects of rosiglitazone on lipids, adipokines, and inflammatory markers in nondiabetic patients with low high-density lipoprotein cholesterol and metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 26: 624–630. [DOI] [PubMed] [Google Scholar]
- 10.Freed M. I., Ratner R., Marcovina S. M., Kreider M. M., Biswas N., Cohen B. R., Brunzell J. D. 2002. Effects of rosiglitazone alone and in combination with atorvastatin on the metabolic abnormalities in type 2 diabetes mellitus. Am. J. Cardiol. 90: 947–952. [DOI] [PubMed] [Google Scholar]
- 11.Wagner J. A., Larson P. J., Weiss S., Miller J. L., Doebber T. W., Wu M. S., Moller D. E., Gottesdiener K. M. 2005. Individual and combined effects of peroxisome proliferator-activated receptor and {gamma} agonists, fenofibrate and rosiglitazone, on biomarkers of lipid and glucose metabolism in healthy nondiabetic volunteers. J. Clin. Pharmacol. 45: 504–513. [DOI] [PubMed] [Google Scholar]
- 12.Sarafidis P. A., Lasaridis A. N., Nilsson P. M., Mouslech T. F., Hitoglou-Makedou A. D., Stafylas P. C., Kazakos K. A., Yovos J. G., Tourkantonis A. A. 2005. The effect of rosiglitazone on novel atherosclerotic risk factors in patients with type 2 diabetes mellitus and hypertension. An open-label observational study. Metabolism. 54: 1236–1242. [DOI] [PubMed] [Google Scholar]
- 13.Derosa G., Cicero A. F., Gaddi A., Ragonesi P. D., Fogari E., Bertone G., Ciccarelli L., Piccinni M. N. 2004. Metabolic effects of pioglitazone and rosiglitazone in patients with diabetes and metabolic syndrome treated with glimepiride: a twelve-month, multicenter, double-blind, randomized, controlled, parallel-group trial. Clin. Ther. 26: 744–754. [DOI] [PubMed] [Google Scholar]
- 14.Derosa G., Gaddi A. V., Piccinni M. N., Salvadeo S., Ciccarelli L., Fogari E., Ghelfi M., Ferrari I., Cicero A. F. 2006. Differential effect of glimepiride and rosiglitazone on metabolic control of type 2 diabetic patients treated with metformin: a randomized, double-blind, clinical trial. Diabetes Obes. Metab. 8: 197–205. [DOI] [PubMed] [Google Scholar]
- 15.Wurch T., Junquero D., Delhon A., Pauwels J. 2002. Pharmacological analysis of wild-type alpha, gamma and delta subtypes of the human peroxisome proliferator-activated receptor. Naunyn Schmiedebergs Arch. Pharmacol. 365: 133–140. [DOI] [PubMed] [Google Scholar]
- 16.Sakamoto J., Kimura H., Moriyama S., Odaka H., Momose Y., Sugiyama Y., Sawada H. 2000. Activation of human peroxisome proliferator-activated receptor (PPAR) subtypes by pioglitazone. Biochem. Biophys. Res. Commun. 278: 704–711. [DOI] [PubMed] [Google Scholar]
- 17.Qin S., Liu T., Kamanna V. S., Kashyap M. L. 2007. Pioglitazone stimulates apolipoprotein A-I production without affecting HDL removal in HepG2 cells: involvement of PPAR-alpha. Arterioscler. Thromb. Vasc. Biol. 27: 2428–2434. [DOI] [PubMed] [Google Scholar]
- 18.Carreon-Torres E., Rendon-Sauer K., Monter-Garrido M., Toledo-Ibelles P., Gamboa R., Menjivar M., Lopez-Marure R., Luc G., Fievet C., Cruz D., et al. 2009. Rosiglitazone modifies HDL structure and increases HDL-apo AI synthesis and catabolic rates. Clin. Chim. Acta. 401: 37–41. [DOI] [PubMed] [Google Scholar]
- 19.Nagashima K., Lopez C., Donovan D., Ngai C., Fontanez N., Bensadoun A., Fruchart-Najib J., Holleran S., Cohn J. S., Ramakrishnan R., et al. 2005. Effects of the PPARgamma agonist pioglitazone on lipoprotein metabolism in patients with type 2 diabetes mellitus. J. Clin. Invest. 115: 1323–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Millar J. S., Brousseau M. E., Diffenderfer M. R., Barrett P. H., Welty F. K., Faruqi A., Wolfe M. L., Nartsupha C., Digenio A. G., Mancuso J. P., et al. 2006. Effects of the cholesteryl ester transfer protein inhibitor torcetrapib on apolipoprotein B100 metabolism in humans. Arterioscler. Thromb. Vasc. Biol. 26: 1350–1356. [DOI] [PubMed] [Google Scholar]
- 21.Ikewaki K., Rader D. J., Schaefer J. R., Fairwell T., Zech L. A., Brewer H. B., Jr 1993. Evaluation of apoA-I kinetics in humans using simultaneous endogenous stable isotope and exogenous radiotracer methods. J. Lipid Res. 34: 2207–2215. [PubMed] [Google Scholar]
- 22.Zimetti F., Weibel G. K., Duong M., Rothblat G. H. 2006. Measurement of cholesterol bidirectional flux between cells and lipoproteins. J. Lipid Res. 47: 605–613. [DOI] [PubMed] [Google Scholar]
- 23.Rothblat G. H., de la Llera-Moya M., Atger V., Kellner-Weibel G., Williams D. L., Phillips M. C. 1999. Cell cholesterol efflux: integration of old and new observations provides new insights. J. Lipid Res. 40: 781–796. [PubMed] [Google Scholar]
- 24.Hofmann C., Lorenz K., Colca J. R. 1991. Glucose transport deficiency in diabetic animals is corrected by treatment with the oral antihyperglycemic agent pioglitazone. Endocrinology. 129: 1915–1925. [DOI] [PubMed] [Google Scholar]
- 25.Armoni M., Kritz N., Harel C., Bar-Yoseph F., Chen H., Quon M. J., Karnieli E. 2003. Peroxisome proliferator-activated receptor-gamma represses GLUT4 promoter activity in primary adipocytes, and rosiglitazone alleviates this effect. J. Biol. Chem. 278: 30614–30623. [DOI] [PubMed] [Google Scholar]
- 26.Lefebvre A. M., Peinado-Onsurbe J., Leitersdorf I., Briggs M. R., Paterniti J. R., Fruchart J. C., Fievet C., Auwerx J., Staels B. 1997. Regulation of lipoprotein metabolism by thiazolidinediones occurs through a distinct but complementary mechanism relative to fibrates. Arterioscler. Thromb. Vasc. Biol. 17: 1756–1764. [DOI] [PubMed] [Google Scholar]
- 27.Sanguino E., Roglans N., Alegret M., Sanchez R. M., Vazquez-Carrera M., Laguna J. C. 2005. Different response of senescent female Sprague-Dawley rats to gemfibrozil and rosiglitazone administration. Exp. Gerontol. 40: 588–598. [DOI] [PubMed] [Google Scholar]
- 28.Shih D. Q., Dansky H. M., Fleisher M., Assmann G., Fajans S. S., Stoffel M. 2000. Genotype/phenotype relationships in HNF-4alpha/MODY1: haploinsufficiency is associated with reduced apolipoprotein (AII), apolipoprotein (CIII), lipoprotein(a), and triglyceride levels. Diabetes. 49: 832–837. [DOI] [PubMed] [Google Scholar]
- 29.Khovidhunkit W., Duchateau P. N., Medzihradszky K. F., Moser A. H., Naya-Vigne J., Shigenaga J. K., Kane J. P., Grunfeld C., Feingold K. R. 2004. Apolipoproteins A-IV and A-V are acute-phase proteins in mouse HDL. Atherosclerosis. 176: 37–44. [DOI] [PubMed] [Google Scholar]
- 30.Tu G. F., De Jong F., Apostolopoulos J., Nagashima M., Fidge N., Schreiber G., Howlett G. 1987. Effect of acute inflammation on rat apolipoprotein mRNA levels. Inflammation. 11: 241–251. [DOI] [PubMed] [Google Scholar]
- 31.Lacorte J. M., Beigneux A., Parant M., Chambaz J. 1997. Repression of apoC-III gene expression by TNFalpha involves C/EBPdelta/NF-IL6beta via an IL-1 independent pathway. FEBS Lett. 415: 217–220. [DOI] [PubMed] [Google Scholar]
- 32.Nikolaidou-Neokosmidou V., Zannis V. I., Kardassis D. 2006. Inhibition of hepatocyte nuclear factor 4 transcriptional activity by the nuclear factor kappaB pathway. Biochem. J. 398: 439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips L. S., Grunberger G., Miller E., Patwardhan R., Rappaport E. B., Salzman A. 2001. Once- and twice-daily dosing with rosiglitazone improves glycemic control in patients with type 2 diabetes. Diabetes Care. 24: 308–315. [DOI] [PubMed] [Google Scholar]
- 34.Rosenblatt S., Miskin B., Glazer N. B., Prince M. J., Robertson K. E. 2001. The impact of pioglitazone on glycemic control and atherogenic dyslipidemia in patients with type 2 diabetes mellitus. Coron. Artery Dis. 12: 413–423. [DOI] [PubMed] [Google Scholar]
- 35.Zhong S., Goldberg I. J., Bruce C., Rubin E., Breslow J. L., Tall A. 1994. Human ApoA-II inhibits the hydrolysis of HDL triglyceride and the decrease of HDL size induced by hypertriglyceridemia and cholesteryl ester transfer protein in transgenic mice. J. Clin. Invest. 94: 2457–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng T. W., Chan D. C., Barrett P. H., Watts G. F. 2009. Effect of weight loss on HDL-apoA-II kinetics in the metabolic syndrome. Clin. Sci. (Lond.). 118: 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levels J. H., Pajkrt D., Schultz M., Hoek F. J., van Tol A., Meijers J. C., van Deventer S. J. 2007. Alterations in lipoprotein homeostasis during human experimental endotoxemia and clinical sepsis. Biochim. Biophys. Acta. 1771: 1429–1438. [DOI] [PubMed] [Google Scholar]
- 38.Zhu Y., Bellosta S., Langer C., Bernini F., Pitas R. E., Mahley R. W., Assmann G., von Eckardstein A. 1998. Low-dose expression of a human apolipoprotein E transgene in macrophages restores cholesterol efflux capacity of apolipoprotein E-deficient mouse plasma. Proc. Natl. Acad. Sci. USA. 95: 7585–7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fournier N., Paul J. L., Atger V., Cogny A., Soni T., de la Llera-Moya M., Rothblat G., Moatti N. 1997. HDL phospholipid content and composition as a major factor determining cholesterol efflux capacity from Fu5AH cells to human serum. Arterioscler. Thromb. Vasc. Biol. 17: 2685–2691. [DOI] [PubMed] [Google Scholar]
- 40.Chroni A., Nieland T. J., Kypreos K. E., Krieger M., Zannis V. I. 2005. SR-BI mediates cholesterol efflux via its interactions with lipid-bound ApoE. Structural mutations in SR-BI diminish cholesterol efflux. Biochemistry. 44: 13132–13143. [DOI] [PubMed] [Google Scholar]
- 41.Varghese A., Yee M. S., Chan C. F., Crowe L. A., Keenan N. G., Johnston D. G., Pennell D. J. 2009. Effect of rosiglitazone on progression of atherosclerosis: insights using 3D carotid cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 11: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Home P. D., Pocock S. J., Beck-Nielsen H., Curtis P. S., Gomis R., Hanefeld M., Jones N. P., Komajda M., McMurray J. J. 2009. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 373: 2125–2135. [DOI] [PubMed] [Google Scholar]
- 43.Birjmohun R. S., Dallinga-Thie G. M., Kuivenhoven J. A., Stroes E. S., Otvos J. D., Wareham N. J., Luben R., Kastelein J. J., Khaw K. T., Boekholdt S. M. 2007. Apolipoprotein A-II is inversely associated with risk of future coronary artery disease. Circulation. 116: 2029–2035. [DOI] [PubMed] [Google Scholar]
- 44.Blanco-Vaca F., Escola-Gil J. C., Martin-Campos J. M., Julve J. 2001. Role of apoA-II in lipid metabolism and atherosclerosis: advances in the study of an enigmatic protein. J. Lipid Res. 42: 1727–1739. [PubMed] [Google Scholar]