Abstract
Aminobisphosphonates are drugs administered for the treatment of bone resorption. They can indirectly activate peripheral γδ T cells and render tumor cells susceptible to lysis by Vγ9Vδ2 T cells. We have investigated the molecules involved in conjugate formation and killing of aminobisphosphonate-treated MCF-7 breast tumor cells by Vγ9Vδ2 T cells. Lysis of aminobisphosphonate (Pamidronate and Zoledronate)-treated MCF-7 tumor cells by Vγ9Vδ2 T cells was assessed by chromium release assays and time-lapse video microscopy. MCF-7 breast cancer cells were chosen as aminobisphosphonates are employed to alleviate bone resorption in this malignancy. Cell cycle profile and expression of MICA, ICAM-I and FasL on aminobisphosphonate-sensitized MCF-7 breast tumor cells was confirmed by flow cytometry. Involvement of γδ TCR and NKG2D in mediating cytotoxicity of aminobisphosphonate-treated MCF-7 breast tumor cells by Vγ9Vδ2 T cells was assessed using blocking antibodies in chromium release assays. MCF-7 tumor cells pretreated with Pamidronate and Zoledronate were efficiently lysed by Vγ9Vδ2 T cells. Pamidronate and Zoledronate treatment of MCF-7 cells induced S phase arrest and did not alter expression of MICA, ICAM-I and FasL. Blocking γδ TCR and NKG2D on Vγ9Vδ2 T cells inhibited lysis of Pamidronate and Zoledronate-treated MCF-7 cells. Inhibiting the perforin-granzyme pathway in Vγ9Vδ2 T cells using concanamycin A reduced their ability to lyse aminobisphosphonate-treated MCF-7 cells. Vγ9Vδ2 T cells form strong conjugates with aminobisphosphonate-treated MCF-7 breast tumor cells. γδ TCR, NKG2D and perforin-granzyme pathway are involved in the lysis of MCF-7 breast tumor cells treated with aminobisphosphonates by Vγ9Vδ2 T cells.
Keywords: cultured tumor cells, breast cancer, aminobisphosphonate, T lymphocytes, immunologic cytotoxicity
Introduction
Human γδ T lymphocytes constitute approximately 1-5% of the total T cell population. Peripheral blood γδ (Vγ9Vδ2) T cells recognize non-peptide phosphoantigens such as isopentenyl pyrophosphate (IPP) and its structural analogs aminobisphosphonates (1). Bisphosphonates have direct anti-tumor effects via inhibition of tumor cell adhesion, invasion, proliferation and induction of apoptosis (2).
Studies in myeloma patients revealed that Pamidronate infusion can induce selective expansion of Vγ9Vδ2 T cells (3). In vivo administration of Zoledronate in cancer patients with bone metastases was reported to induce maturation of Vγ9Vδ2 T cells to an IFN-γ producing effector phenotype which could potentiate their anti-tumor responses (4). Recent studies have elucidated that Zoledronic acid can induce potent anti-tumor activity via activation of Vγ9Vδ2 T cells in colorectal and hepatocellular carcinomas (5), hormone-refractory prostate cancer (6) and multiple myeloma (7).
Substantial evidence indicates that aminobisphosphonate treatment of tumor cells augments the anti-tumor activity of Vγ9Vδ2 T cells (8-10). Aminobisphosphonates inhibit the farnesyl pyrophosphate synthase (FPPS) enzyme in the mammalian mevalonate pathway (11), allowing accumulation of endogenous IPP in tumor cells and resulting in activation of Vγ9Vδ2 T cells (12).
However, the mechanism(s) involved in the lysis of aminobisphosphonate-treated tumor cells by Vγ9Vδ2 T cells are incompletely understood. This study attempts to investigate the molecules involved in the lysis of aminobisphosphonate-treated MCF-7 breast tumor cells by Vγ9Vδ2 T cells. In the present study, representative cell lines of breast, prostate and bone cancers were chosen as aminobisphosphonates are a standard modality of treatment for skeletal metastasis seen frequently in such malignancies. We specifically analyzed the MCF-7 breast tumor cell line for our further studies as a high incidence of bone metastasis is reported in breast cancers and aminobisphosphonates are administered to inhibit bone resorption (13). We report that Pamidronate and Zoledronate treatment of MCF-7 tumor cells sensitizes them to efficient lysis by Vγ9Vδ2 T cells which is mediated by the γδ TCR and partially by the NKG2D receptor. In addition, the perforin-granzyme pathway is also involved in the lysis of aminobisphosphonate-treated tumor cells by Vγ9Vδ2 T cells.
Results
Pamidronate and Zoledronate treatment of MCF-7 tumor cells sensitizes them to increased lysis mediated by Vγ9Vδ2 T cells
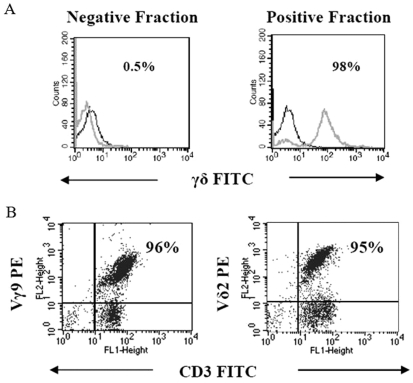
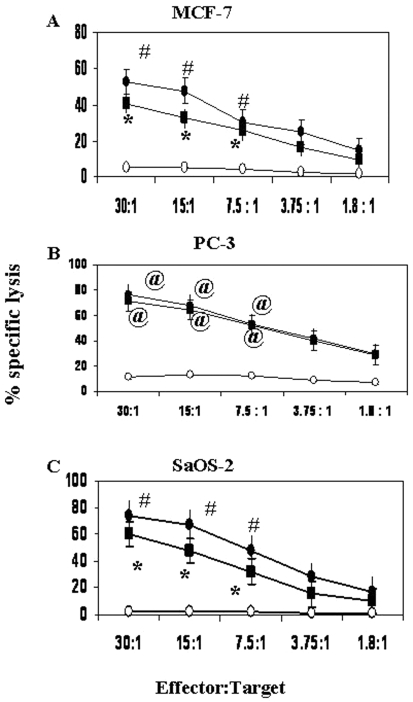
After immunomagnetic separation of γδ T cells, the purity of γδ T cells was assessed by flow cytometry (Figure 1A). The percentage of purified Vγ9 and Vδ2 T cells was 96% and 95% respectively (Figure 1B). The ability of purified Vγ9Vδ2 T cells to lyse breast tumor cell line MCF-7 cells before and after treatment with the aminobisphosphonates Pamidronate and Zoledronate (100 µM) was assessed using 51Cr release assay. Further, we analyzed lysis mediated by Vγ9Vδ2 T cells against Pamidronate- and Zoledronate-sensitized PC-3 prostate carcinoma cells and SaOS-2 osteosarcoma cells, other malignancies which report frequent bone metastasis. Treatment of tumor cells with Pamidronate and Zoledronate significantly augmented the lysis of MCF-7 and PC-3 cells mediated by Vγ9Vδ2 T cells when tested at E:T ratios ranging from 30:1 to 7.5:1 (Figure 2, panels A and B). It was observed that SaOS-2 osteosarcoma cells were also efficiently primed by Pamidronate and Zoledronate for lysis by Vγ9Vδ2 T cells (Figure 2C). The efficiency of lysis was reduced when tumor cells were treated with lower concentrations of Pamidronate and Zoledronate (10 µM and 50 µM, data not shown).
Figure 1.
Analysis of γδ T cells in the negative and positive fractions isolated by magnetic-activated cell sorting (MACS) using single color flow cytometry. (A) Each overlay represents γδ T cells stained with FITC-conjugated mAb against γδ TCR (grey) and FITC-conjugated mAb against isotype IgG (black) in negative and positive fractions. The positive fraction shows the presence of 98% γδ T cells. (B) Subset analysis of immunomagnetically purified γδ T cells by dual color flow cytometry. Purified γδ T cells were stained with fluorochrome (FITC or PE as indicated)-conjugated mAb against Vγ9, Vδ2 and CD3. The quadrants were established on the basis of staining of γδ T cells with IgG isotype control FITC- and PE-conjugated antibodies. Figures indicate the percentage of double positive cells.
Figure 2.
Pamidronate and Zoledronate treatment sensitizes tumor cells to lysis by Vγ9Vδ2 T cells. MCF-7 breast carcinoma (A), PC-3 prostate carcinoma (B) and SaOS-2 osteosarcoma (C) cells were treated with Pamidronate (black rectangles, 100 µM) and Zoledronate (black-filled circles, 100 µM) or left untreated (open circles) for 16-18 hours. 51Cr-labeled tumor cells were co-cultured with Vγ9Vδ2 T cells for 4 hours at E:T ratios ranging from 30:1 to 1.8:1. Error bars indicate % cytotoxicity±SE. Statistical significance: @, P < 0.005; *, P < 0.0001; #, P < 0.002. Shown are the means of three independent experiments performed using γδ T cells from 3 different donors against each target cell line.
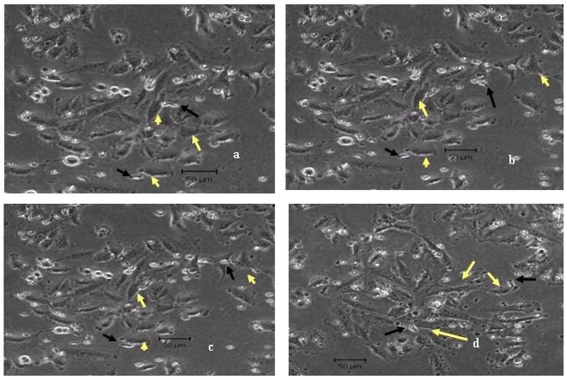
We corroborated our findings of augmented lysis of aminobisphosphonate-treated MCF-7 cells by Vγ9Vδ2 T cells using time-lapse video microscopy. When Vγ9Vδ2 T cells were co-cultured with untreated MCF-7 cells, it was observed that γδ T cells crawled on the surface of untreated tumor targets but were unable to form stable conjugates and lyse tumor cells (Figure 3, panels a-c, and Supplementary Video 1). Even after 4 hours of co-culture, there was no visible killing of the untreated MCF-7 cells by the Vγ9Vδ2 T cells (Figure 3d).
Figure 3.
Time-lapse imaging of untreated MCF-7 cells co-cultured with Vγ9Vδ2 T cells. Untreated MCF-7 cells were co-cultured with Vγ9Vδ2 T cells at a 1:2 ratio for 4 hours at 37˚C. Snapshots of continuous time-lapse imaging on a LSM510 Meta Zeiss confocal microscope taken during the last 1 hour are shown. The images were taken at 30 seconds interval (a-d). Vγ9Vδ2 T cells (black arrows) were unable to lyse the tumor cells (yellow arrows) even at the end of 4 hours (d). Representative images from one of four independent experiments are shown.
However, when Vγ9Vδ2 T cells were co-cultured with MCF-7 cells pretreated with Zoledronate (100 µM), it was observed that a number of γδ T cells surrounded the MCF-7 tumor cell forming tight conjugates, subsequently killing the tumor cell within 10 seconds. Vγ9Vδ2 T cells constantly scanned the surface of Zoledronate-treated MCF-7 tumor cells and lysed them (Figure 4, panels a-h, and Supplementary Videos 2 and 3) unlike the crawling movement of γδ T cells on untreated tumor cells. Similar observations were recorded with Pamidronate-treated tumor cells (data not shown).
Figure 4.
Time-lapse imaging of Zoledronate-treated MCF-7 cells co-cultured with Vγ9Vδ2 T cells. MCF-7 cells were pretreated with Zoledronate (100 µM) for 16-18 hours and co-cultured with Vγ9Vδ2 T cells at a 1:2 ratio for 4 hours at 37˚C. Snapshots from continuous time-lapse imaging taken during the last 1 hour are shown. The images were taken at 30 seconds interval (a-h). The yellow arrow indicates a single MCF-7 cell being targeted by Vγ9Vδ2 T cells (black arrow). Changes in tumor cell morphology are observed (e-h) finally leading to lysis of the tumor cell by Vγ9Vδ2 T cells (g-h). Representative images from one of four independent experiments are shown.
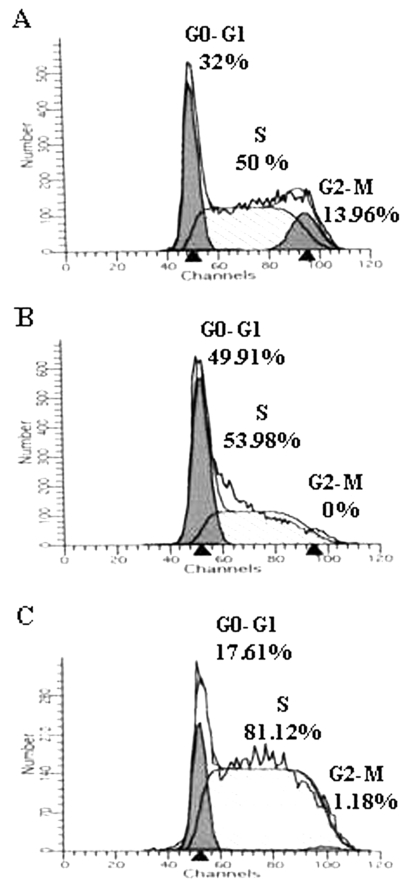
Pamidronate and Zoledronate treatment induces a cell cycle arrest in MCF-7 tumor cells
In order to understand the direct effects of Pamidronate and Zoledronate on MCF-7 tumor cells, cell cycle analysis was performed. It was observed that treatment of MCF-7 cells with Pamidronate and Zoledronate induced an S phase arrest wherein the percentage of cells in S phase increased from 50% in untreated (Figure 5A) to 53.9% in Pamidronate- (Figure 5B) and 81.1% in Zoledronate (Figure 5C)-treated MCF-7 cells. Zoledronate was more potent in inducing S phase arrest as compared to Pamidronate. In Pamidronate-treated MCF-7 cells, the percentage of cells in G0-G1 increased to 49.91% (Figure 5B) from 32% in untreated MCF-7 cells (Figure 5A). Interestingly, both Pamidronate and Zoledronate treatment of MCF-7 cells resulted in an absent G2-M phase. In addition, there was no apoptosis in Pamidronate- and Zoledronate-treated MCF-7 cells.
Figure 5.
Pamidronate and Zoledronate induce S-phase arrest in MCF-7 cells. Tumor cells were either left untreated (A) or pretreated with 100 µM Pamidronate (B) or 100 µM Zoledronate (C) for 16-18 hours. Cells were fixed in chilled 70% ethanol and stained with 5 µg/ml of propidium iodide. Ten thousand events were acquired on a FACS Calibur and cell cycle analysis was performed. Data representative of two independent experiments are shown.
Involvement of γδ TCR and NKG2D in cytotoxicity of aminobisphosphonate-treated tumor cells by Vγ9Vδ2 T cells
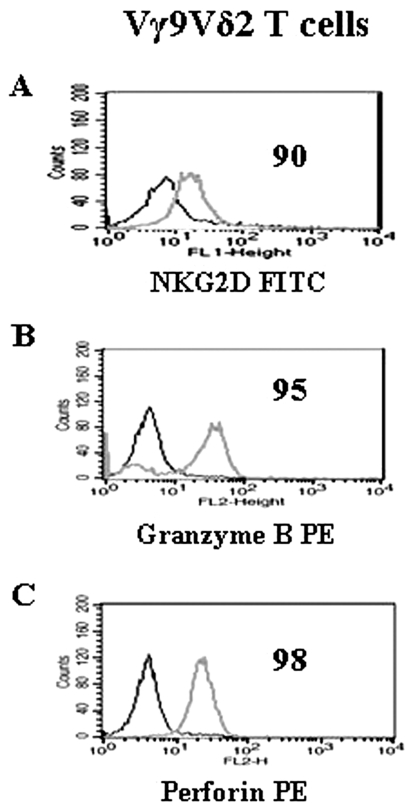
In order to identify the molecules involved in aminobisphosphonate-mediated tumor cell susceptibility to Vγ9Vδ2 T cell lysis, MCF-7 cells pretreated with Pamidronate and Zoledronate were co-cultured with Vγ9Vδ2 T cells treated with blocking antibodies against pan γδ TCR, Vγ9Vδ2 subset and NKG2D in a 4-hour 51Cr release assay. We observed that Vγ9Vδ2 T cells showed expression of the C-type lectin receptor, NKG2D (Figure 6A).
Figure 6.
Vγ9Vδ2 T cells express NKG2D and intracellular granzyme and perforin. Vγ9Vδ2 T cells were stained for NKG2D (A), intracellular granzyme B (B) and perforin (C) expression where isotype control (black) and positive staining (grey) is shown. Figures in the histograms indicate the percentage of positive cells. Data representative of three independent experiments are shown.
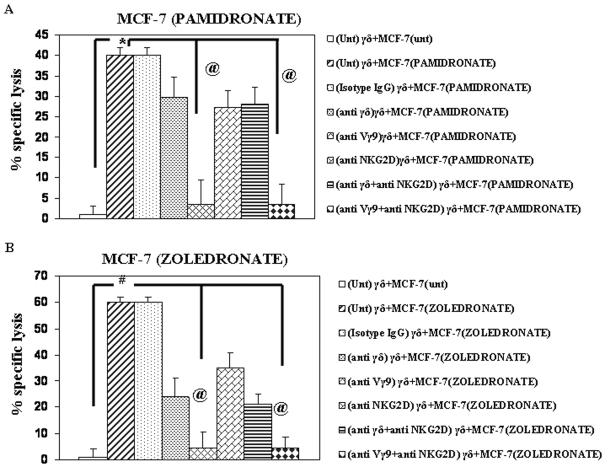
Lysis of Pamidronate-treated MCF-7 cells was inhibited by blocking Vγ9Vδ2 T cells with anti-γδ TCR mAb (29.7%±4% lysis, 41% inhibition), subset-specific anti-Vγ9 mAb (5%±0.5% lysis, 95% inhibition) and anti-NKG2D mAb (26%±7% lysis, 32% inhibition)(Figure 7A). Treatment of Vγ9Vδ2 T cells with a combination of either anti-γδ mAb and anti-NKG2D mAb or anti-Vγ9 mAb and anti-NKG2D did not reduce the cytotoxicity below that observed with only anti-γδ mAb or anti-Vγ9 mAb treatment (Figure 7A). Vγ9Vδ2 T cells incubated with isotype IgG control antibody did not inhibit lysis of Pamidronate-treated MCF-7 cells by Vγ9Vδ2 T cells (Figure 7A), indicating the specificity of the inhibition obtained with the mAbs used in the assay.
Figure 7.
Cytotoxicity of Vγ9Vδ2 T cells against Pamidronate- and Zoledronate-treated MCF-7 is mediated by γδ TCR and NKG2D. Pamidronate- (A) and Zoledronate (B)-treated MCF-7 cells were incubated with Vγ9Vδ2 T cells at an E:T ratio of 30:1 and the percentage of specific lysis determined by 51Cr release assay. Vγ9Vδ2 T cells were untreated (Unt) or treated with anti-γδ TCR (10 µg/ml), anti-Vγ9 (10 µg/ml) and anti-NKG2D (20 µg/ml) mAb or a combination of anti-γδ TCR (10 µg/ml) and anti-NKG2D (20 µg/ml) or anti-Vγ9 (10 µg/ml) and anti-NKG2D (20 µg/ml) mAbs. As control, Vγ9Vδ2 T cells treated with isotype specific antibodies were used as effectors and co-cultured with tumor targets. Error bars indicate % cytotoxicity±SE. Statistical significance: #, P < 0.002; @, P < 0.003; *, P < 0.0001. Data is representative of two independent experiments.
Similarly, cytotoxicity of Vγ9Vδ2 T cells against Zoledronate-treated MCF-7 cells was reduced when blocked with anti-γδ mAb (24%±3% lysis, 57% inhibition), anti-Vγ9 mAb (5%±1% lysis, 93% inhibition) and anti-NKG2D mAb (35%±2% lysis, 42% inhibition)(Figure 7B). Combination of mAbs against TCR and NKG2D could not further lower the cytotoxicity of Vγ9Vδ2 T cells against Zoledronate-treated MCF-7 tumor cells (Figure 7B). Thus, it can be inferred that lysis of Pamidronate- and Zoledronate-treated MCF-7 cells by Vγ9Vδ2 T cell is mediated by the γδ TCR and is partially dependent on the NKG2D receptor expressed on Vγ9Vδ2 T cells.
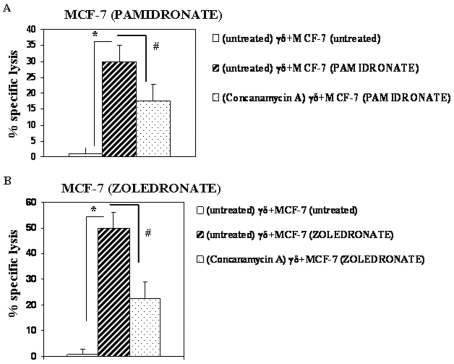
Lysis of aminobisphosphonate-treated MCF-7 cells by Vγ9Vδ2 T cells is dependent on the perforin-granzyme pathway
A high expression of granzyme B (95%, Figure 6B) and perforin (98%, Figure 6C) was observed in Vγ9Vδ2 T cells. In order to investigate the involvement of the perforin-granzyme pathway in the lysis of aminobisphosphonate-treated MCF-7 cells by Vγ9Vδ2 T cells, T cells were treated with concanamycin A (specific inhibitor of perforin release and lysosomal acidification). Vγ9Vδ2 T cells efficiently lysed Pamidronate- (30%±5% lysis, Figure 8A) and Zoledronate (50%±3% lysis, Figure 8B)-treated MCF-7 cells. Pretreatment of Vγ9Vδ2 T cells with concanamycin A significantly inhibited the cytotoxicity of Vγ9Vδ2 T cells against Pamidronate- (17%±4% lysis, 40% inhibition, Figure 8A) and Zoledronate (22%±3% lysis, 60% inhibition, Figure 8B)-treated MCF-7 cells.
Figure 8.
Cytotoxicity of Vγ9Vδ2 T cells against Pamidronate- and Zoledronate-treated MCF-7 cells is partially dependent on the perforin-granzyme pathway. Pamidronate- (A) and Zoledronate (B)-treated MCF-7 cells were incubated with Vγ9Vδ2 T cells at an E:T ratio of 30:1 and the percentage of specific lysis was determined by a 4-hour 51Cr release assay. Vγ9Vδ2 T cells were either untreated or pretreated with concanamycin A (200 nM) for 1 hour at 37˚C and then added to targets and further incubated for 4 hours at 37˚C. Error bars indicate % cytotoxicity±SE. Statistical significance: *, P < 0.001; #, P < 0.05. Shown are means of two independent experiments.
Discussion
Aminobisphosphonates are known to stimulate Vγ9Vδ2 T cells (1) and concomitantly have direct anti-tumor effects (14). In the present study, we have attempted to understand how treatment with aminobisphosphonates alters the conjugate formation between Vγ9Vδ2 T cells and MCF-7 breast tumor cells by time-lapse video microscopy and correlated these observations using cytotoxicity assays. We further demonstrated the role of molecules such as NKG2D, perforin and granzyme in the lysis of aminobisphosphonate-treated tumor cells by Vγ9Vδ2 T cells.
Our studies reveal that Vγ9Vδ2 T cells efficiently lysed Pamidronate- and Zoledronate-treated MCF-7 breast cancer cells, PC-3 prostate cancer cells, and SaOS-2 osteosarcoma cells. Other investigators have demonstrated that aminobisphosphonate treatment of tumor cells augments lysis of myeloma (15), non-small cell lung carcinoma (16), breast and prostate adenocarcinoma (17) and colon cancer stem cells (18) by Vγ9Vδ2 T cells. A comparison of cytotoxicity of Vγ9Vδ2 T cells against Pamidronate- and Zoledronate-treated THP-1 (myelomonocytic cell line) and AW8507 (oral carcinoma cell line) cell lines at E:T 30:1 revealed differential susceptibility to lysis by Vγ9Vδ2 T cells (data not shown). These data demonstrate that aminobisphosphonates can differentially prime tumor cells of diverse origin to lysis by Vγ9Vδ2 T cells.
We observed that Zoledronate treatment of tumor cells increased their susceptibility to lysis by Vγ9Vδ2 T cells greater than Pamidronate. This could be attributed to the fact that Zoledronate is a more potent aminobisphosphonate for inhibiting the FPPS enzyme in the mevalonate pathway with a half maximal inhibitory concentration (IC50) value of 3 nM, as compared to 200 nM for Pamidronate (19).
Cell cycle analysis of Pamidronate- and Zoledronate-treated MCF-7 cells showed that Zoledronate induced a prominent S phase arrest while Pamidronate treatment increased the proportion of G0-G1 cells. We have included the cell cycle arrest data (Figure 5) to emphasize the fact that after exposure to Zoledronate for 16-18 hours tumor cells do not undergo apoptosis in our experimental system. There are reports which have accounted for the phenomenon of apoptosis or at least cell cycle arrest in tumor cells treated with aminobisphosphonates (20, 21) although the exposure time to bisphosphonates and the cell types used varied. Our purpose to include the cell cycle arrest data was to highlight that in our experimental protocol, overnight incubation of MCF-7 cells with aminobisphosphonates does not induce apoptosis of the MCF-7 cells. This is to support our data that the bisphosphonate-treated tumor cells do not die of apoptosis but are killed by γδ T cells.
An earlier study has reported that the aminobisphosphonate Risedronate induced a G2 arrest in MCF-7 cells after 48 hours (22). On the other hand, Zoledronate induced a prominent S phase arrest in human osteosarcoma (23) and in cholangiocarcinoma cells (24), while a G2 arrest was reported in HCT-116 colon carcinoma cells (25). In addition, we observed that treatment of MCF-7 cells with Pamidronate and Zoledronate did not induce apoptosis as observed by Annexin V staining (data not shown). Although we do not have direct evidence that cell cycle arrest makes bisphosphonate-treated tumor cells susceptible to lysis by γδ T cells, we speculate that the cell cycle arrest induced by aminobisphosphonates may predispose tumor cells to apoptosis. A recent study by Li et al. (26) showed that murine lung adenocarcinoma cells were arrested at the S/G2/M phase of the cell cycle upon Zoledronate treatment but no apoptotic cells were detected. It was suggested that Zolendronate may be a good candidate to pre-sensitize tumor cells to cytotoxic agents and radiation.
To visualize the events involved in Vγ9Vδ2 T cell-mediated lysis of Pamidronate- and Zoledronate-treated MCF-7 tumor cells, we analyzed γδ T cell and MCF-7 tumor cell interaction by time-lapse confocal video microscopy, something which has not been reported to date. Vγ9Vδ2 T cells co-cultured with Zoledronate-treated MCF-7 cells clearly showed strong conjugate formation with numerous T cells moving on the surface of a single tumor cell. This culminated in membrane damage and subsequent lysis of the tumor cell within a 10 second time frame (data not shown), followed by the release of the attacking Vγ9Vδ2 T cell. γδ T cells are known to participate in synaptic transfer and membrane stripping of target tumor cells (27). Using video microscopy, we observed that once the target cell was lysed, γδ T cells moved out and continued scanning for other prospective tumor targets. This is reminiscent of an earlier study where CTLs were shown to maintain sustained synapses with targets and polarized the release of lytic granules (27).
On the other hand, Vγ9Vδ2 T cells continuously scanned the surface of untreated tumor cells but were unable to form conjugates and lyse the tumor cells even after 4 hours. These results support the possibility that aminobisphosphonate-sensitized tumor targets could allow efficient tethering of γδ T cells, either via interaction of a receptor-ligand complex or by altering the kinetics of known receptor-ligand interaction. For the video time-lapse microscopy experiments, the tumor cells (MCF-7) were treated overnight at 37˚C with Zoledronate at a concentration of 100 µM. On the next day the tumor cells were washed and co-incubated with γδ T cells. It is therefore unlikely that there is residual bisphosphonate left in the culture medium that could stimulate the γδ T cells in 4 hours. We would like to mention that purified γδ T cells do not respond to bisphosphonates unless the bisphosphonates are presented to them via an antigen-presenting cell. Several papers demonstrate the requirement of monocytes to present bisphosphonates to γδ T cells (10, 28). It is therefore unlikely that residual aminobisphononates in the medium can stimulate γδ T cells to form the conjugates with MCF-7 cells. Since we do not use monocytes in our assay system but only pretreat the tumor cells with bisphosphonates, we believe that the increased conjugate formation which occurs between bisphosphonate-coated tumor cells and γδ T cells, as compared to untreated tumor cells, is due to the binding of NKG2D expressed on γδ T cells with an unidentified ligand expressed on bisphosphonate-treated tumor cells. However, if the γδ T cells are independently stimulated with aminobisphosphonates using monocytes for presentation, the activated γδ T cells will form conjugates with tumor cells.
To elucidate the mechanism(s) involved in the increased lysis of aminobisphosphonate-treated tumor cells by Vγ9Vδ2 T cells, we analyzed the expression of surface molecules (MICA, ICAM-I and FasL) on MCF-7 cells and studied whether these are altered after aminobisphosphonate treatment (Supplementary Figure 1). Earlier reports have suggested an important role of the adhesion molecule ICAM-I expressed on tumor cells in modulating the cytotoxicity of Vγ9Vδ2 T cells by interacting with its ligand LFA-I on Vγ9Vδ2 T cells (29, 30). In addition, FasL expression on MCF-7 cells was shown to be upregulated by treatment with IFN-γ and TNF-α, thus facilitating CTL-mediated lysis of tumor cells (31). We observed low MICA, ICAM-I and FasL expression on MCF-7 cells which was not altered after treatment with Pamidronate and Zoledronate. This suggested that aminobisphosphonate treatment of MCF-7 cells did not quantitatively increase the expression of any of the molecules responsible for Vγ9Vδ2 T cell-mediated cytotoxicity against tumor cells known so far.
The optimal activation of Vγ9Vδ2 T cells is controlled by activating NK receptors (e.g. NKG2D) and inhibitory NK receptors (e.g. NKp44, NKG2A/C, etc.) which act as co-stimulatory molecules on Vγ9Vδ2 T cells (32). NKG2D, a C-type lectin receptor expressed on NK cells and γδ T cells and its interaction with its cognate receptors MICA/B, ULBP-1 etc. has been reported to play a prominent role in controlling cytotoxicity (33-36). In our studies we observed that Vγ9Vδ2 T cells showed high expression of NKG2D and therefore cytotoxicity assays were performed using blocking antibodies against γδ TCR and NKG2D.
Blocking the Vγ9Vδ2 T cells with subset-specific Vγ9 mAb showed 95% inhibition in Vγ9Vδ2 T cell lysis of Pamidronate- and Zoledronate-treated MCF-7 cells. On the other hand, blocking the NKG2D receptor on Vγ9Vδ2 T cells reduced their lytic efficiency by almost 50%. Thus, lysis of aminobisphosphonate-treated tumor targets by Vγ9Vδ2 T cells appears to be mediated by the γδ TCR and is partially dependent on the NKG2D receptor. However, unlike earlier studies where a synergy between the γδ TCR and the NKG2D receptor in controlling the lytic activity of Vγ9Vδ2 T cells was reported (5, 36), in our study we observed that blocking the Vγ9 receptor was sufficient to abolish the lytic activity of Vγ9Vδ2 T cells against Pamidronate- and Zoledronate-treated MCF-7 cells. A recent study has reported the involvement of DNAX accessory molecule-1 (DNAM-1) besides NKG2D in Vγ9Vδ2 T cell lysis of tumor cells (37). This explains the partial inhibition in cytotoxicity assays we observed upon blocking NKG2D.
We observed that treatment of Vγ9Vδ2 T cells with concanamycin A, a specific inhibitor of the perforin pathway abrogates the anti-tumor activity of Vγ9Vδ2 T cells against Pamidronate- and Zoledronate-treated MCF-7 tumor cells by almost 50%. Further, our results are in agreement with earlier reports demonstrating high expression of granzyme B and perforin in γδ T cells that are key molecules involved in granule-mediated cytotoxicity (38, 39). Thus, it appears that the lysis of aminobisphosphonate-treated tumor cells by Vγ9Vδ2 T cells also involves a functional perforin-granzyme pathway.
The physiological phosphoantigen recognized by Vγ9Vδ2 T cells is isopentenyl pyrophosphate (IPP), an intermediate of the mevalonate pathway. Investigations have shown that aminobisphosphonates, the chemical analogs of IPP, inhibit the FPPS enzyme in the mevalonate pathway and thereby cause indirect activation of Vγ9Vδ2 T cells. To this end, recent studies have revealed that Zoledronate exposure of MCF-7 cells leads to the formation of a cytotoxic analog ApppI [triphosphoric acid 1-adenosin-5-yl ester 3-(3-methylbut-3-enyl) ester], that can induce apoptosis in these tumor cells (40). However, we speculate that in addition to the above mechanism, aminobisphosphonates could also modulate the avidity of interaction between Vγ9Vδ2 T cells and tumor cells, thus culminating in the formation of a stable γδ T cell:tumor cell conjugate and eventual lysis of tumor cells. A proteomic analysis of aminobisphosphonate-treated tumor cells may help in identifying novel molecules that are involved in conjugate formation between γδ T cells and tumor cells and also provide important leads in understanding mechanisms involved in the lysis of aminobisphosphonate-sensitized tumor cells by Vγ9Vδ2 T cells.
Acknowledgements
S. Dhar was supported by a fellowship from the Lady Tata Memorial Trust, Mumbai, India.
References
- 1.Thedrez A, Sabourin C, Gertner J, Devilder MC, Allain-Maillet S, Fournie JJ, Scotet E, Bonneville M. Self/non-self discrimination by human gammadelta T cells: simple solutions for a complex issue? Immunol Rev. 2007;215:123–135. doi: 10.1111/j.1600-065X.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- 2.Green JR. Antitumor effects of bisphosphonates. Cancer. 2003;97:840–847. doi: 10.1002/cncr.11128. [DOI] [PubMed] [Google Scholar]
- 3.Kunzmann V, Bauer E, Wilhelm M. Gamma/delta T-cell stimulation by pamidronate. N Engl J Med. 1999;340:737–738. doi: 10.1056/NEJM199903043400914. [DOI] [PubMed] [Google Scholar]
- 4.Dieli F, Gebbia N, Poccia F, Caccamo N, Montesano C, Fulfaro F, Arcara C, Valerio MR, Meraviglia S, Di Sano C, Sireci G, Salerno A. Induction of gammadelta T-lymphocyte effector functions by bisphosphonate zoledronic acid in cancer patients in vivo. Blood. 2003;102:2310–2311. doi: 10.1182/blood-2003-05-1655. [DOI] [PubMed] [Google Scholar]
- 5.Bouet-Toussaint F, Cabillic F, Toutirais O, Le Gallo M, Thomas de la Pintière C, Daniel P, Genetet N, Meunier B, Dupont-Bierre E, Boudjema K, Catros V. Vgamma9Vdelta2 T cell-mediated recognition of human solid tumors. Potential for immunotherapy of hepatocellular and colorectal carcinomas. Cancer Immunol Immunother. 2008;57:531–539. doi: 10.1007/s00262-007-0391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dieli F, Vermijlen D, Fulfaro F, Caccamo N, Meraviglia S, Cicero G, Roberts A, Buccheri S, D'Asaro M, Gebbia N, Salerno A, Eberl M, Hayday AC. Targeting human {gamma}delta} T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007;67:7450–7457. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abe Y, Muto M, Nieda M, Nakagawa Y, Nicol A, Kaneko T, Goto S, Yokokawa K, Suzuki K. Clinical and immunological evaluation of zoledronate-activated Vgamma9gammadelta T-cell-based immunotherapy for patients with multiple myeloma. Exp Hematol. 2009;37:956–968. doi: 10.1016/j.exphem.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Kato Y, Tanaka Y, Miyagawa F, Yamashita S, Minato N. Targeting of tumor cells for human gammadelta T cells by nonpeptide antigens. J Immunol. 2001;167:5092–5098. doi: 10.4049/jimmunol.167.9.5092. [DOI] [PubMed] [Google Scholar]
- 9.Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96:384–392. [PubMed] [Google Scholar]
- 10.Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruediger T, Tony HP. Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood. 2003;102:200–206. doi: 10.1182/blood-2002-12-3665. [DOI] [PubMed] [Google Scholar]
- 11.van Beek E, Pieterman E, Cohen L, Lowik C, Papapoulos S. Farnesyl pyrophosphate synthase is the molecular target of nitrogen-containing bisphosphonates. Biochem Biophys Res Commun. 1999;264:108–111. doi: 10.1006/bbrc.1999.1499. [DOI] [PubMed] [Google Scholar]
- 12.Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–168. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross JR, Saunders Y, Edmonds PM, Patel S, Broadley KE, Johnston SR. Systematic review of role of bisphosphonates on skeletal morbidity in metastatic cancer. BMJ. 2003;327:469. doi: 10.1136/bmj.327.7413.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caraglia M, Santini D, Marra M, Vincenzi B, Tonini G, Budillon A. Emerging anti-cancer molecular mechanisms of aminobisphosphonates. Endocr Relat Cancer. 2006;13:7–26. doi: 10.1677/erc.1.01094. [DOI] [PubMed] [Google Scholar]
- 15.Mariani S, Muraro M, Pantaleoni F, Fiore F, Nuschak B, Peola S, Foglietta M, Palumbo A, Coscia M, Castella B, Bruno B, Bertieri R, Boano L, Boccadoro M, Massaia M. Effector gammadelta T cells and tumor cells as immune targets of zoledronic acid in multiple myeloma. Leukemia. 2005;19:664–670. doi: 10.1038/sj.leu.2403693. [DOI] [PubMed] [Google Scholar]
- 16.Sato K, Kimura S, Segawa H, Yokota A, Matsumoto S, Kuroda J, Nogawa M, Yuasa T, Kiyono Y, Wada H, Maekawa T. Cytotoxic effects of gammadelta T cells expanded ex vivo by a third generation bisphosphonate for cancer immunotherapy. Int J Cancer. 2005;116:94–99. doi: 10.1002/ijc.20987. [DOI] [PubMed] [Google Scholar]
- 17.Mattarollo SR, Kenna T, Nieda M, Nicol AJ. Chemotherapy and zoledronate sensitize solid tumour cells to Vgamma9Vdelta2 T cell cytotoxicity. Cancer Immunol Immunother. 2007;56:1285–1297. doi: 10.1007/s00262-007-0279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Todaro M, D'Asaro M, Caccamo N, Iovino F, Francipane MG, Meraviglia S, Orlando V, La Mendola C, Gulotta G, Salerno A, Dieli F, Stassi G. Efficient killing of human colon cancer stem cells by gammadelta T lymphocytes. J Immunol. 2009;182:7287–7296. doi: 10.4049/jimmunol.0804288. [DOI] [PubMed] [Google Scholar]
- 19.Rogers MJ. New insights into the molecular mechanisms of action of bisphosphonates. Curr Pharm Des. 2003;9:2643–2658. doi: 10.2174/1381612033453640. [DOI] [PubMed] [Google Scholar]
- 20.Pandha H, Birchall L, Meyer B, Wilson N, Relph K, Anderson C, Harrington K. Antitumor effects of aminobisphosphonates on renal cell carcinoma cell lines. J Urol. 2006;176:2255–2261. doi: 10.1016/j.juro.2006.07.053. [DOI] [PubMed] [Google Scholar]
- 21.Dumon JC, Journe F, Kheddoumi N, Lagneaux L, Body JJ. Cytostatic and apoptotic effects of bisphosphonates on prostate cancer cells. Eur Urol. 2004;45:521–528. doi: 10.1016/j.eururo.2003.12.012. Discussion 528-529. [DOI] [PubMed] [Google Scholar]
- 22.Suyama K, Noguchi Y, Tanaka T, Yoshida T, Shibata T, Saito Y, Tatsuno I. Isoprenoid-independent pathway is involved in apoptosis induced by risedronate, a bisphosphonate, in which Bim plays a critical role in breast cancer cell line MCF-7. Oncol Rep. 2007;18:1291–1298. [PubMed] [Google Scholar]
- 23.Iguchi T, Miyakawa Y, Saito K, Nakabayashi C, Nakanishi M, Saya H, Ikeda Y, Kizaki M. Zoledronate-induced S phase arrest and apoptosis accompanied by DNA damage and activation of the ATM/Chk1/cdc25 pathway in human osteosarcoma cells. Int J Oncol. 2007;31:285–291. [PubMed] [Google Scholar]
- 24.Romani AA, Desenzani S, Morganti MM, La Monica S, Borghetti AF, Soliani P. Zoledronic acid determines S-phase arrest but fails to induce apoptosis in cholangiocarcinoma cells. Biochem Pharmacol. 2009;78:133–141. doi: 10.1016/j.bcp.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Sewing L, Steinberg F, Schmidt H, Goke R. The bisphosphonate zoledronic acid inhibits the growth of HCT-116 colon carcinoma cells and induces tumor cell apoptosis. Apoptosis. 2008;13:782–789. doi: 10.1007/s10495-008-0211-z. [DOI] [PubMed] [Google Scholar]
- 26.Li YY, Chang JW, Chou WC, Liaw CC, Wang HM, Huang JS, Wang CH, Yeh KY. Zoledronic acid is unable to induce apoptosis, but slows tumor growth and prolongs survival for non-small-cell lung cancers. Lung Cancer. 2008;59:180–191. doi: 10.1016/j.lungcan.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 27.Gertner J, Wiedemann A, Poupot M, Fournié JJ. Human gammadelta T lymphocytes strip and kill tumor cells simultaneously. Immunol Lett. 2007;110:42–53. doi: 10.1016/j.imlet.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Miyagawa F, Tanaka Y, Yamashita S, Minato N. Essential requirement of antigen presentation by monocyte lineage cells for the activation of primary human gamma delta T cells by aminobisphosphonate antigen. J Immunol. 2001;166:5508–5514. doi: 10.4049/jimmunol.166.9.5508. [DOI] [PubMed] [Google Scholar]
- 29.Corvaisier M, Moreau-Aubry A, Diez E, Bennouna J, Mosnier JF, Scotet E, Bonneville M, Jotereau F. V gamma 9V delta 2 T cell response to colon carcinoma cells. J Immunol. 2005;175:5481–5488. doi: 10.4049/jimmunol.175.8.5481. [DOI] [PubMed] [Google Scholar]
- 30.Uchida R, Ashihara E, Sato K, Kimura S, Kuroda J, Takeuchi M, Kawata E, Taniguchi K, Okamoto M, Shimura K, Kiyono Y, Shimazaki C, Taniwaki M, Maekawa T. Gamma delta T cells kill myeloma cells by sensing mevalonate metabolites and ICAM-1 molecules on cell surface. Biochem Biophys Res Commun. 2007;354:613–618. doi: 10.1016/j.bbrc.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 31.Naujokat C, Sezer O, Possinger K. Tumor necrosis factor-alpha and interferon-gamma induce expression of functional Fas ligand on HT29 and MCF7 adenocarcinoma cells. Biochem Biophys Res Commun. 1999;264:813–819. doi: 10.1006/bbrc.1999.1500. [DOI] [PubMed] [Google Scholar]
- 32.Casetti R, Martino A. The plasticity of gamma delta T cells: innate immunity, antigen presentation and new immunotherapy. Cell Mol Immunol. 2008;5:161–170. doi: 10.1038/cmi.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pende D, Rivera P, Marcenaro S, Chang CC, Biassoni R, Conte R, Kubin M, Cosman D, Ferrone S, Moretta L, Moretta A. Major histocompatibility complex class I-related chain A and UL16-binding protein expression on tumor cell lines of different histotypes: analysis of tumor susceptibility to NKG2D-dependent natural killer cell cytotoxicity. Cancer Res. 2002;62:6178–6186. [PubMed] [Google Scholar]
- 34.Das H, Groh V, Kuijl C, Sugita M, Morita CT, Spies T, Bukowski JF. MICA engagement by human Vgamma2Vdelta2 T cells enhances their antigen-dependent effector function. Immunity. 2001;15:83–93. doi: 10.1016/s1074-7613(01)00168-6. [DOI] [PubMed] [Google Scholar]
- 35.Wrobel P, Shojaei H, Schittek B, Gieseler F, Wollenberg B, Kalthoff H, Kabelitz D, Wesch D. Lysis of a broad range of epithelial tumour cells by human gamma delta T cells: involvement of NKG2D ligands and T-cell receptor- versus NKG2D-dependent recognition. Scand J Immunol. 2007;66:320–328. doi: 10.1111/j.1365-3083.2007.01963.x. [DOI] [PubMed] [Google Scholar]
- 36.Rincon-Orozco B, Kunzmann V, Wrobel P, Kabelitz D, Steinle A, Herrmann T. Activation of V gamma 9V delta 2 T cells by NKG2D. J Immunol. 2005;175:2144–2151. doi: 10.4049/jimmunol.175.4.2144. [DOI] [PubMed] [Google Scholar]
- 37.Toutirais O, Cabillic F, Le Friec G, Salot S, Loyer P, Le Gallo M, Desille M, de La Pintière CT, Daniel P, Bouet F, Catros V. DNAX accessory molecule-1 (CD226) promotes human hepatocellular carcinoma cell lysis by Vgamma9Vdelta2 T cells. Eur J Immunol. 2009;39:1361–1368. doi: 10.1002/eji.200838409. [DOI] [PubMed] [Google Scholar]
- 38.Narazaki H, Watari E, Shimizu M, Owaki A, Das H, Fukunaga Y, Takahashi H, Sugita M. Perforin-dependent killing of tumor cells by Vgamma1Vdelta1-bearing T-cells. Immunol Lett. 2003;86:113–119. doi: 10.1016/s0165-2478(02)00292-4. [DOI] [PubMed] [Google Scholar]
- 39.Alexander AA, Maniar A, Cummings JS, Hebbeler AM, Schulze DH, Gastman BR, Pauza CD, Strome SE, Chapoval AI. Isopentenyl pyrophosphate-activated CD56+ {gamma}{delta} T lymphocytes display potent antitumor activity toward human squamous cell carcinoma. Clin Cancer Res. 2008;14:4232–4240. doi: 10.1158/1078-0432.CCR-07-4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Räikkönen J, Crockett JC, Rogers MJ, Mönkkönen H, Auriola S, Mönkkönen J. Zoledronic acid induces formation of a pro-apoptotic ATP analogue and isopentenyl pyrophosphate in osteoclasts in vivo and in MCF-7 cells in vitro. Br J Pharmacol. 2009;157:427–435. doi: 10.1111/j.1476-5381.2009.00160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamaguchi T, Fujimiya Y, Suzuki Y, Katakura R, Ebina T. A simple method for the propagation and purification of gamma delta T cells from the peripheral blood of glioblastoma patients using solid-phase anti-CD3 antibody and soluble IL-2. J Immunol Methods. 1997;205:19–28. doi: 10.1016/s0022-1759(97)00062-8. [DOI] [PubMed] [Google Scholar]
- 42.Atre N, Thomas L, Mistry R, Pathak K, Chiplunkar S. Role of nitric oxide in heat shock protein induced apoptosis of gammadeltaT cells. Int J Cancer. 2006;119:1368–1376. doi: 10.1002/ijc.21966. [DOI] [PubMed] [Google Scholar]
Materials and methods
Reagents
Pamidronate (Pamifos) was purchased from Dabur, India and Zoledronate (Zoldonat) was purchased from Natco, India. Concanamycin A was obtained from Sigma-Aldrich, USA.
Expansion of Vγ9Vδ2 T cells
Peripheral blood mononuclear cells (PBMCs) were isolated from healthy volunteers (n = 20) by Ficoll-Hypaque (Sigma-Aldrich, USA) density centrifugation. PBMCs were stimulated with anti-CD3 mAb (1 µg/ml) and rIL-2 (100 IU/ml) for 5 days in RPMI 1640 (Invitrogen Life-Technologies, USA) with 10% heat-inactivated human AB serum and further cultured for 12 days as described earlier (41, 42). γδ T cells were isolated by positive separation using MACS (Miltenyi Biotec, Germany) and showed >95% expression of Vγ9Vδ2 TCR. γδ T cells were left in culture medium without IL-2 for 24 hours before use in all functional assays. This study was approved by the Institutional Ethics Committee.
Tumor cell lines
MCF-7 (breast adenocarcinoma), PC-3 (prostatic carcinoma) and SaOS-2 (osteosarcoma) were obtained from the American Type Culture Collection, USA. MCF-7 cells were maintained in RPMI 1640 medium (Invitrogen Life-Technologies, USA), PC-3 cells were cultured in Ham's F-12K medium (Sigma-Aldrich, USA) and SaOS-2 cells were cultured in McCoy's 5A medium in 10% FBS (Invitrogen Life-Technologies, USA) with antibiotics.
Immunostaining
Purified Vγ9Vδ2 T cells (0.5-1 x 106) were washed in FACS buffer (1xPBS, 1% FCS, 0.01% sodium azide), fixed in 1% paraformaldehyde (Sigma-Aldrich, USA) and stained with the following FITC- or PE-conjugated antibodies: Vγ9 TCR (clone B3, IgG1, BD Pharmingen USA), Vδ2 TCR (clone B6, IgG1, BD Pharmingen USA), Granzyme B (clone 351927, IgG2A, R&D Systems, USA), Perforin (clone deltaG9, IgG2b, eBioscience, USA) and NKG2D (clone 149810, IgG1, R&D Systems). Prior to intracellular staining of granzyme B and perforin, Vγ9Vδ2 T cells were permeabilized by treating with 0.1% saponin (Sigma-Aldrich, USA) in FACS buffer for 10 minutes at room temperature. Depending on the primary antibody used, staining was detected by incubating cells with either polyclonal goat anti-mouse FITC-conjugated antibody (Sigma-Aldrich, USA) or polyclonal goat anti-rabbit PE-conjugated antibody (Sigma-Aldrich, USA). At least 10,000 events were acquired on a FACS Calibur (Becton Dickinson, USA) and the data analyzed using the Cell Quest software (Becton Dickinson, USA).
Cell cycle
Tumor cells were treated with Pamidronate and Zoledronate at 100 µM for 16-18 hours and fixed in 70% chilled ethanol. The cells were stained with 5 µg/ml propidium iodide (Sigma-Aldrich, USA) for 15 minutes at 37˚C, 5% CO2 in the dark and acquired on a FACS Calibur (Becton Dickinson, USA). The cell cycle profile was analyzed using ModFit software (Verity Inc., USA).
Cytotoxicity assay
In all experiments, tumor cells were treated with Pamidronate and Zoledronate (100 µM each) for 16-18 hours at 37˚C. Tumor cells (1 x 106) were labeled with 50 µCi of 51Cr (sodium chromate, Amersham, UK) for 1 hour at 37˚C. Purified Vγ9Vδ2 T cells were titrated at effector:target (γδ:tumor) ratios ranging from 30:1 to 1.8:1 in triplicates and added to 51Cr-labeled tumor targets (5 x 103). Tumor cells were co-cultured with Vγ9Vδ2 T cells for 4 hours at 37˚C in 96-well plates (Nunc, Denmark). After 4 hours, supernatants were collected and chromium release was quantitated in a Gamma counter (Packard, USA) and expressed as counts per minute (cpm). The maximum release of chromium was determined by lysing tumor cells with Triton X-100 while the spontaneous release was determined by incubating only tumor cells in medium for 4 hours. The % cytotoxicity of Vγ9Vδ2 T cells was calculated as follows: % cytotoxicity = [(mean experimental CPM - mean spontaneous CPM)/(mean maximum CPM - mean spontaneous CPM)]x100.
For blocking experiments, Vγ9Vδ2 T cells were incubated with anti-pan γδ mAb (10 µg/ml, clone B1, BD PharMingen, USA), anti-Vγ9 mAb (10 µg/ml, clone B3, BD Pharmingen), anti-NKG2D mAb (20 µg/ml, R&D Systems, USA) or a combination of TCR and NKG2D antibodies for 1 hour at 37˚C before adding to the targets at an E:T ratio of 30:1. To analyze the perforin-granzyme pathway, effectors were pretreated with concanamycin A (200 nM, Fluka, USA) for 1 hour at 37˚C before adding to targets at an E:T ratio of 30:1. The % inhibition of cytotoxicity was determined as follows: % inhibition of cytotoxicity = 1-(cytotoxicity in the presence of the mAb/cytotoxicity in the absence of the mAb)x100.
Time-lapse video microscopy
MCF-7 cells were left untreated or treated with Pamidronate and Zoledronate for 16-18 hours. Tumor targets were washed and co-cultured with Vγ9Vδ2 T cells an E:T ratio of 1:2 for 4 hours at 37˚C in 35-mm plates (Nunc, Denmark). The co-cultures were then monitored for the last 1 hour on a confocal microscope (LSM510 Meta, Zeiss, USA) at 37˚C. Selected areas were imaged every 30 seconds and videos were recorded.
Statistical analysis
All results were analyzed using SPSS software (Version 15.0, SPSS Inc., USA). Significance was assessed by unpaired Student's t test. The difference between groups was considered statistically significant when the P value was <0.05. All values in the figures or text are represented as either % cytotoxicity or mean fluorescence intensity (MFI) ± standard error of mean (SEM).
Supplemental data
Download from http://www.cancerimmunity.org/v10p10/100809_suppl_fig1.pdf (355 KB PDF file).
Download from http://www.cancerimmunity.org/v10p10/100809_suppl_vid1.avi (91.4 MB AVI file).
Download from http://www.cancerimmunity.org/v10p10/100809_suppl_vid2.avi (24.3 MB AVI file).
Download from http://www.cancerimmunity.org/v10p10/100809_suppl_vid3.avi (27.4 MB AVI file).
Download from http://www.cancerimmunity.org/v10p10/100809_suppl_data.zip (60.1 MB WinZip file).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Download from http://www.cancerimmunity.org/v10p10/100809_suppl_fig1.pdf (355 KB PDF file).
Download from http://www.cancerimmunity.org/v10p10/100809_suppl_vid1.avi (91.4 MB AVI file).
Download from http://www.cancerimmunity.org/v10p10/100809_suppl_vid2.avi (24.3 MB AVI file).
Download from http://www.cancerimmunity.org/v10p10/100809_suppl_vid3.avi (27.4 MB AVI file).
Download from http://www.cancerimmunity.org/v10p10/100809_suppl_data.zip (60.1 MB WinZip file).