Abstract
Objective. To present the usefulness of a centralized system of data collection for the development of an international multicentre registry of SpA.
Method. The originality of this registry consists in the creation of a virtual network of researchers in a computerized Internet database. From its conception, the registry was meant to be a dynamic acquiring system.
Results. REGISPONSER has two developing phases (Conception and Universalization) and gathers several evolving secondary projects (REGISPONSER-EARLY, REGISPONSER-AS, ESPERANZA and RESPONDIA). Each sub-project answered the necessity of having more specific and complete data of the patients even from the onset of the disease so, in the end, obtaining a well-defined picture of SpAs spectrum in the Spanish population.
Conclusion. REGISPONSER is the first dynamic SpA database composed of cohorts with a significant number of patients distributed by specific diagnosis, which provides basic specific information of the sub-cohorts useful for patients’ evaluation in rheumatology ambulatory consulting.
Keywords: Multicentre registry, Spondyloarthropathies, REGISPONSER
Introduction
Health-care managers need reliable instruments to help them distribute and allocate health and social resources objectively and fairly. These instruments should be flexible and provide real-time data, and they should easily incorporate any change in practice and scientific knowledge. Disease registries are most suitable for this task, as they provide real-time data on the frequency, geographic and temporary distribution, as well as on the pattern of the disease [1]. They inform about the case mix in different locations and provide an enlightening tool for assessing the impact of the disease and clinical practice variability. Furthermore, disease registries are an ideal source of random samples for cohort studies or for case–control studies, the correct setting to test medical hypothesis [2]. Our experience from the registries developed over the past few years has shown us their usefulness in describing the epidemiological aspects, clinical pattern, disease activity, structural damage, response to therapy, impairment degree in quality of life and socio-economic impact associated with inflammatory rheumatic diseases, in our case focused on patients with SpAs.
REGISPONSER, which is a dynamic database registry, was initiated in April 2004, by SpAs study group of the Spanish Society of Rheumatology (GRESSER). Thereby, REGISPONSER (Registro Español de Espondiloartritis de la Sociedad Española de Reumatologia), that is, The Spanish National Registry of SpAs, is composed of a large enough cohort of patients to enable us to determine a well-defined picture of patient characteristics and the progress of their diseases, even from onset.
Methods
The originality of this registry has been the creation of a virtual network of researchers set upon a computerized Internet database accessible to all participating members, irrespective of which city or country they belong to (http://regisponser.ser.es/). Each centre has individual access to the registry, either for investigation or for managing purposes. The online application is easily accessible via a standard Internet browser. No additional software is needed to be installed on the user’s computer. Once the self-administered patients’ validated questionnaires are achieved, the data are introduced in the electronic clinical research form (e-CRF), under strict quality control by filters that detect data inconsistencies, for assuring the uniformity of data collection. The information contained in these e-CRFs was agreed with all the investigators before starting the database. The e-CRF provides the basic minimum data set required for the complete definition of the patient and his illness, according to the recommendations of ASAS (Assessment of Ankylosing Spondylitis International Society) [3] (Table 1). A specific code (login and password) is assigned to each investigator to access the e-CRF and random codes are given to each introduced case. In this way, the system meets the rules of the protection of personal data. The system performs an automatic log out after a predefined period of the user’s inactivity. This function prevents abuse of the system if the user forgets to log off. All submitted data are encrypted and collected in a central computer, where they are safely stored in the database. The data can be exported for authorized users as a local database for further processing. This study was approved by the Committee of Ethical and Sanitary Investigation of Reina Sofia University Hospital. Each patient has signed an informed consent at inclusion in REGISPONSER, according to the fundamental principles established in the Declaration of Human Rights in Helsinki.
Table 1.
Data collected by the e-CRF
| Socio-demographic data | Clinical data |
Variables of disease assessment | Treatment in the last 2 weeks | Working conditions | ||
|---|---|---|---|---|---|---|
| First signs and symptoms | Comorbidities | Diagnostic data | ||||
| Date of birth | BASDAI | NSAIDs | Fully employed | |||
| Gender | Date of first symptoms | Hypertension | Year of diagnostic | BASFI | DMARDs | Work disability |
| Race | Articular symptoms | Diabetes | Clinic form | Mander enthesis index | anti-TNF-α | Unemployed |
| Marital status | Extra-articular symptoms related to the disease (uveitis, IBD, psoriasis) | Ischaemic heart disease | HLA-B27 | Metrological index: mod.Schober occiput/tragus to wall distance, fingers to floor distance, cervical rotation, lateral spinal flexion, chest expansion | ||
| Dislipidaemia | ||||||
| Gastro-duodenal ulcer | ||||||
| Profession | Associated infection (urethritis, balanitis, cervicitis) | Cerebral-vascular desease | ESSG criteria | BASRI-total m-SASSS | ||
| Periphery vascular disease | ||||||
| Study degree | Trauma associated | Chronic obstructive bronchopneumopathy | Amor criteria | DNA bank for selected groups | ||
| Average income | Family history of SpA | Infections | Hip involvement | NPJ/NSJ | ||
| Living conditions | Treatment before the inclusion in the registry | Neoplasm | VAS-patient | |||
| Liver disease | VAS-physician | |||||
| Depression | VAS-night back pain | |||||
| Cytopenias | ASQoL | |||||
| Renal failure | SF-12 | |||||
| Amyloidosis | ESR | |||||
| Atlanto-axoidal subluxation | CRP | |||||
| Demyelinating diseases | MRI SI joint and spine for selected groups | |||||
| Drinking | ||||||
| Smoking | ||||||
| Fibromyalgia | ||||||
m-SASSS: modified stoke AS spine score; NPJ: number of painful joints; NSJ: number of swollen joints; VAS: visual analogue scale; ASQoL: AS quality of life; SF-12: short form health survey.
Results
Phase I
At its first beginning (2004–05) the registry (REGISPONSER I) was comprised of 12 reference Spanish rheumatology departments selected from all the centres that agreed to participate in the project, based on their experience in treating SpA patients. These centres, from eight different cities, represent a broad socio-demographic spectrum of the population attended at the Spanish Health System. The average population covered by the participating hospitals is 800 000 (range 300 000–1 100 000), and includes urban and rural zones. The participating rheumatologists were asked to include all consecutive patients fulfilling the inclusion criteria up to a minimum of 100 per centre.
The main purposes of REGISPONSER I were to create, develop and exploit the Spanish national registry of patients with SpA and its specific objective was to determine the characteristics of the Spanish patients with SpA (socio-demographic, clinical, radiological, laboratory and treatment features) in a cross-sectional study (photography). REGISPONSER I included 1379 patients fulfilling European Spondyloarthropathy Study Group (ESSG) classification criteria and the results were already published [4]. The results of this project were: (i) development of a useful computer application; (ii) user-friendly patient data processing obtained from different centres; and (iii) direct information of the characteristics of the Spanish SpA patients, although the most important goal achieved by REGISPONSER I has been the growing interest of the Spanish rheumatologists in SpA and so, thereby ‘increasing the visibility of SpA in Spain’.
Phase II
After the data-acquiring system was validated, an invitation was sent to all those Spanish rheumatology centres that met the minimum requirement criteria, and hence other 19 centres joined the project. Thus, by the end of March 2007, 2367 patients were included in the registry. This phase of the project is known as Universalization. REGISPONSER II helped to increase knowledge of SpAs in the Spanish population and to incite the development of new projects. REGISPONSER II collected information from 31 Spanish rheumatologic departments, from 31 different hospitals belonging to 19 provinces that cover a wide spectrum of the Spanish habitants with different social, economic and occupational conditions.
Continuous development
REGISPONSER II is a dynamic project that collects standardized socio-demographic, clinical, biological, radiological, genetic and treatment data relevant to SpAs spectrum. The authenticity of this project consists in the requirement that all the patients included to fulfil both ESSG and Amor criteria for SpA. Several studies were developed from REGISPONSER II and the results have already been published [5–7]. In addition to these published results, REGISPOSER II revealed some interesting facts: first, by increasing the sample, clinical and demographic outcomes obtained in REGISPONSER I did not change significantly [8]; and secondly, there was an unacceptable delay of 8.6 years in SpA diagnosis in Spain. Therefore, we created an ambitious project that involves both general practitioners and rheumatologists called ESPERANZA, with the purpose of early detection of SpA. The goals were to create and establish units for the early management of patients with SpAs and to develop a communication system between general practitioners and rheumatologists for early referral of patients with suspected spondylitis, according to the following criteria: patients <45 years of age with >3 and <24 months of evolution of one of the three following syndromes: (i) inflammatory back pain, defined in the specific case of the project as back pain with at least two out of three from the following: insidious onset, morning stiffness >30 min and improvement with exercise; (ii) asymmetrical oligoarthritis of the lower limbs (at least one and maximum four big joints in the lower limbs affected asymmetrically); and (iii) other suspicious criteria as: rachialgia plus at least one of following: psoriasis; IBD; acute anterior uveitis; family history of SpA, psoriasis, IBD or anterior uveitis; Rx sacroiliitis; and HLA-B27 positivity. At present there are 25 rheumatologic centres and 2099 general practitioners involved in the programme that has derived 898 new patients. Parts of the results have been reported during the 2009 EULAR Congress [9].
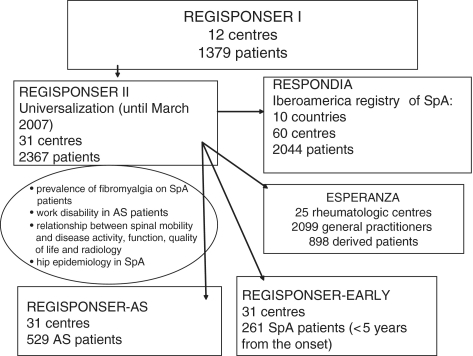
The main advantage of REGISPONSER II is that it provides a source of well-characterized patients treated in Spanish rheumatology centres, which may be used as database for new prospective projects (Fig. 1). Thus, a number of such new prospective secondary projects are now in progress: (i) REGISPONSER-EARLY that is comprised of a cohort of patients with SpAs with <5 years of disease evolution (150 patients) [10]; and (ii) REGISPONSER-AS, designed for the cohort of patients with AS, which contains 529 patients. In this cohort, we are also evaluating some of the most important genetic biomarkers known to be related with the disease susceptibility and pathogenesis. For these two sub-projects, screens and appropriate collection filters to alert that a specific patient was selected were created. A system for tracking and monitoring the patients was established. It was required that each selected patient fulfilled five visits (one every year). For the statistical analysis, statistical programs and SPSS (SPSS PASW Statistics v.18 spanish version, Chicago, IL) are used. The analysis plan varies, depending on the sub-studies.
Fig. 1.
Evolution of REGISPONSER registry.
Furthermore, due to our collaboration with the Latin American countries interested in SpAs, we also started a sub-project in which 10 Latin American countries (Argentina, Brazil, Costa Rica, Chile, Mexico, Peru, Uruguay and Venezuela) and over 100 rheumatologists were included. They were accepted initially to use the electronic platform of REGISPONSER II for introducing the same standardized data following the Spanish protocol. In this way, during 2005–06 RESPONDIA was formed as part of REGISPONSER.
RESPONDIA is now an independent project, gathering almost 2000 patients fulfilling the ESSG criteria of SpAs and has its own general coordinator. Results from this registry were published [11], and, moreover, the epidemiology and risk factors associated with hip involvement in AS [12] were confirmed.
Discussions
REGISPONSER is an ambitious programme that currently involves, as a whole, more than 500 Spanish-speaking rheumatologists (and Portuguese) and contains data from more than 6000 SpAs patients. One of the most important achievements was the incorporation of a self-administered questionnaire into daily clinical practice leading to a uniformity of data collection that further enable the comparison and analysis of data obtained from different sources [3]. REGISPONSER is providing rheumatologists, on one hand, demographic, socio-economic and clinical data and, on the other, information about evolution of the disease in each patient by means of clinical, biological and radiographic assessments. The efficiency of the treatment administrated to the patients can also be evaluated, as can the implication of genetic factors in disease evolution and in response to different therapies. Furthermore, implementation of this project has helped raise the profile of SpA, by increasing the knowledge and interest of the participating rheumatologists in these interesting conditions.
Conclusion
REGISPONSER is the first dynamic SpA database composed of cohorts with a significant number of patients distributed by specific diagnosis, which provides basic specific information of the sub-cohorts useful for patients’ evaluation in rheumatology ambulatory consulting.

Acknowledgements
Funding: This work was supported by The Spanish Foundation of Rheumatology (FER), by an unrestricted grant thanks to the financial collaboration of three pharmaceutical companies that participated equally in the development of REGISPONSER, and we are very grateful to Abbott, Schering-Plough and Wyeth for their huge contribution. ESPERANZA Program has been financed through an unrestricted grant by the FER, thanks to the financial collaboration of Wyeth Pharmaceuticals. Funding to pay the Open Access publication charges for this article was provided by Universidad de Córdoba.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Lynge E. Implication for epidemiology of disease registers. Public Health Rev. 1993–94;21:263–70. [PubMed] [Google Scholar]
- 2.Zink A, Listing J, Klindworth C, Zeidler H. The National database of the German Collaborative Arthriris Centres: I. Structure, aims, and patients. Ann Rheum Dis. 2001;60:199–206. doi: 10.1136/ard.60.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Heijde D, Calin A, Dougados M, Khan MA, van der Linden S, Bellamy N. Selection of instruments in the core set for DC-ART, SMARD, physical therapy, and clinical record keeping in ankylosing spondylitis. Progress report of the ASAS Working Group. Assessments in Ankylosing Spondylitis. J Rheumatol. 1999;26:951–4. [PubMed] [Google Scholar]
- 4.Collantes E, Zarco P, Muñoz E, et al. Disease pattern of spondyloarthropathies in Spain: description of the first national registry (REGISPONSER) extended report. Rheumatology. 2007;46:1309–15. doi: 10.1093/rheumatology/kem084. [DOI] [PubMed] [Google Scholar]
- 5.Ariza-Ariza R, Hernandez-Cruz B, Collantes E, et al. Work disability in patients with ankylosing spondylitis. J Rheumatol. 2009;36:2512–6. doi: 10.3899/jrheum.090481. [DOI] [PubMed] [Google Scholar]
- 6.Almodóvar R, Zarco P, Collantes E, et al. Relationship between spinal mobility and disease activity, function, quality of life and radiology in a cross-sectional Spanish registry of spondyloarthropathies (REGISPONSER) Clin Exp Rheumatol. 2009;27:439–45. [PubMed] [Google Scholar]
- 7.Almodovar R, Carmona L, Zarco P, et al. Fybromyalgia in patients with ankylosing spondylitis: prevalence and utility of the measures of activity, function and radiological damage. Clin Exp Rheumatol. (in press) [PubMed] [Google Scholar]
- 8.Miranda García MD, Font Ugalde P, Muñoz Gomariz E, Collantes E, por Regisponser The National Spondiloarthropathies Registry of the Spanish Society of Rheumatology (REGISPONSER). Descriptive Study of 2367 Spanish Patients. Reumatología Clinica. 2008;4(Suppl. 4):48–55. [Google Scholar]
- 9.Almodóvar R, Zarco P, Collantes E, et al. Agreement between primary care physicians and rheumatologist regarding predefined referral criteria in patients with spondiloarthropaties. Ann Rheum Dis. 2009;68(Suppl. 3):647. [Google Scholar]
- 10.Rojas-Vargas M, Muñoz-Gomariz E, Escudero A, et al. on behalf REGISPOSER working group First signs and symptoms of spondyloarthritis. Data from an inception cohort with a disease course of two years or less (REGISPONSER- Early) Rheumatology. 2009;48:404–9. doi: 10.1093/rheumatology/ken506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vazquez-Mellado Cervantes J, Font Ugalde P, Muñoz Gomariz E, Collantes E. RESPONDIA. Iberoamerican Spondyloarthritis: Registry What Is It, Who Are We, What We Do? General Methods. Reumatología Clinica. 2008;4(Suppl. 4):S17–22. [Google Scholar]
- 12.Vander Cruyssens B, Muñoz-Gomariz E, Font P, et al. Hip involvement in ankylosing spondylitis: epidemiology and risk factors associated with hip replacement surgery. Rheumatology. 2010;49:73–81. doi: 10.1093/rheumatology/kep174. [DOI] [PubMed] [Google Scholar]