Abstract
Nelarabine is a nucleoside analog indicated for the treatment of adult and pediatric patients with T-cell acute lymphoblastic leukemia (T-ALL) or T-cell lymphoblastic lymphoma (T-LBL) that is refractory or has relapsed after treatment with at least two chemotherapy regimens. After being first synthesized in the late 1970s and receiving FDA approval in 2005, the appropriate use of nelarabine for refractory hematologic malignancies is still being elucidated. Nelarabine is the prodrug of 9-β-D-arabinofuranosylguanine (ara-G) which when phosphorylated intracellularly to ara-G triphosphate (ara-GTP), preferentially accumulates in cancerous T-cells. Dose-dependent toxicities, including neurotoxicity and myelosuppression, have been documented and may, in turn, limit the ability to appropriately treat the diagnosed malignancy. This article will summarize the pharmacologic properties of nelarabine and will address the current place in therapy nelarabine holds based upon the results of the available clinical trials to date.
Keywords: nelarabine, Arranon, Atriance, T-cell, leukemia, lymphoma
Introduction
According to the 2010 statistics from the American Cancer Society, there are 5,330 expected cases with 1,420 expected deaths from acute lymphoblastic leukemia (ALL).1 Diagnosis peaks at ages 2–5 and approximately two-thirds of ALL cases are within this age population.2 ALL continues to be the most common malignancy in children less than 15 years old.1 Complete remissions within this population is upward of 95% with 5-year disease-free survival (DFS) rates greater that 80%. And while the response and survival rates in the pediatric population are promising, the treatment of ALL in the adult population is much more difficult and shows much lower DFS rates, even with more aggressive chemotherapeutic regimens.2
A subset of ALL includes T-cell lymphoblastic leukemia (T-ALL), which has shown to have a poorer prognosis due to a lesser response to initial chemotherapy and a high relapse rate.2 Treatment of T-ALL in the adult population has been the subject of recent study, especially in the patient that shows either refractoriness or extensive disease. The current recommended regimens in the treatment of adult T-ALL include cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or a slight alteration on this regimen with the addition of etoposide (EPOCH) that includes some dose variation from CHOP.3 Some protocols suggest the use of a similar regimen of cyclophosphamide, vicristine, doxorubicin, and prednisone (termed hyper-CVAD in this case) alternating with high-dose methotrexate and cytarabine. This regimen may be preferred in patients with CNS involvement or to prevent relapse within the CNS.3 Even with successful remission subsequent to the above regimens, adult T-ALL patients show short times to relapse or recurrence and must often consider a hematopoietic stem cell transplant, which has significant side effects and can itself lessen the patient’s life span.2,3
A related malignancy to ALL is lymphoblastic lymphoma (LBL) which is considered an aggressive form of non-Hodgkin’s lymphoma. The majority of LBL is of T-cell origin (T-LBL) and is treated similarly to T-ALL in regard to systemic chemotherapy. The risk of relapse and recurrence following appropriate therapy is high, with prognosis worsening in these patients.4 With the problems associated with recurrent T-ALL and T-LBL, it is imperative that additional options for prolongation of response and survival be elucidated.
Nelarabine is a potential option to be included in the treatment of recurrent and/or relapsed T-ALL/T-LBL. Nelarabine is an antimetabolite based on a guanine nucleoside base residue. It is the prodrug of 9-β-D-arabinofuranosyl guanine (ara-G) and was approved by the United States Food and Drug Administration in October of 2005. The approved indications for nelarabine are T-ALL and T-LBL in pediatric and adults patients that have refractory or progressive disease following two or more previous chemotherapeutic regimens.5 The role of nelarabine in treating refractory hematologic malignancies is still being reviewed and studied. We will look to address what is known regarding nelarabine’s pharmacologic properties and the results from the clinical trials to date to further explain where nelarabine might be utilized for a disease state with limited options after previous treatment failure.
Mechanism of Action and Pharmacology
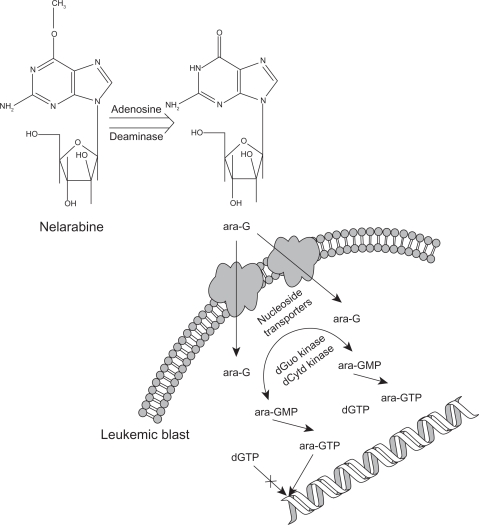
Nelarabine is a prodrug that after demethoxylation via adenosine deaminase, is converted to the compound ara-G.6 In turn, ara-G is triphosphorylated by multiple kinases intracellularly to the active nucleotide, ara-GTP, which actively competes in leukemic blast cells for incorporation into cellular DNA.7,8 The resultant incorporation into leukemic cell DNA inhibits further DNA synthesis, although the specific location of insertion has not been fully elucidated. This process prompts chain termination and programmed cell death leading to the clinical response (Fig. 1).6–8
Figure 1.
Nelarabine is converted systemically to ara-G via adenosine deaminase. ara-G is transported into the leukemic blast by two different transporters. One is a nitrobenzylthioinosoine sensitive (NBMPR+) transporter and the other is a nitrobenzylthioinosine insensitive (NBMPR−) transporter. The rate limiting step in the formation of ara-GTP is the initial phosphorylation of ara-G to ara-GMP via both deoxyguanosine (dGuo) kinase and deoxycytidine (dCytd) kinase. Upon subsequent phosphorylation, ara-GTP competes with deoxyguanosine triphosphate (dGTP) for incorporation into DNA. Upon incorporation of ara-GTP into DNA, apoptosis occurs as formation is terminated.6–9
The preferential accumulation of ara-GTP in malignant T-cells helps to explain nelarabine’s clinical activity in T-ALL and T-LBL. Although B-cells will accumulate ara-GTP, it is to a much lesser extent than with T-cells. This is explained partially by the reduced half-life of ara-GTP in B-cells when compared to that of T-cells.10–12 The cellular toxicity induced by nelarabine provides for its efficacy as well as explains some of the adverse drug reactions associated with its use.
Pharmacokinetics
Conversion of nelarabine to ara-G
The pharmacokinetics of nelarabine have been evaluated in refractory hematologic malignancies in both pediatric and adult patients.13,14 As nelarabine, a pro-drug, is typically administered as an intravenous infusion, the maximum concentration (Cmax) is observed at the end of the infusion. The Cmax and area under the curve (AUC) of nelarabine are linearly related to the nelarabine dose over the range of 5 to 75 mg/kg.13 Conversion of nelarabine to ara-G, catalyzed by adenosine deaminase, is required to produce effective concentrations of ara-G which is converted intracellularly to the active nucleotide ara-GTP. As one mole of ara-G is converted from one mole of nelarabine, the elimination rate of nelarabine represents the formation rate of ara-G (Fig. 1).13 The half-life (t½) of nelarabine is less than 20 minutes in most patients15 and is related to the efficient conversion to ara-G. Nelarabine is typically administered as a one or two-hour infusion. After one hour, approximately 94% of nelarabine is converted to ara-G and after two hours, more than 99% of nelarabine is converted to ara-G.16 Table 1 summarizes the pharmacokinetics of nelarabine.
Table 1.
| Pediatric | Adult | ||
|---|---|---|---|
| Nelarabine | |||
| Vd (L) | 8.511 ± 11.518 | 4.197 ± 5.802 | NSa |
| CL (L/kg/hr) | 9.259 ± 12.794 | 5.875 ± 8.434 | NSa |
| t½ (minutes) | 14.1b | 16.5b | NSa |
| ara-G | |||
| Vd (L) | 1.023 ± 0.345 | 0.923 ± 0.231 | NSa |
| CL (L/kg/hr) | 0.312 ± 0.112 | 0.213 ± 0.072 | P < 0.0001 |
| t½ (hrs) | 2.1b | 3.0b | P < 0.01 |
| ara-GTP | |||
| Mean ara-GTP (μM) | 435 ± 519 | ||
| 746 ± 872c | |||
| t½ (hrs) | >24bd |
Notes:
No statistical difference;
Harmonic mean value;
Nelarabine following fludarabine (combination);
Patient with T-cell disease.
The combination of nelarabine and fludarabine was examined to explore the potential of the clinical utility of the combination. The intent was to examine if fludarabine would modulate ara-G concentrations.16 Nelarabine at the dose level of 1.2 g/m2 was given as an intravenous infusion on days one, three and five. On day one of treatment, nelarabine was administered alone. On days three and five, fludarabine (30 mg/m2) was administered and nelarabine was given four hours later. Fludarabine had no influence on the pharmacokinetics of the parent compound (nelarabine) as the pharmacokinetics of nelarabine when administered alone or after fludarabine were similar.16 Fludarabine is not an inhibitor of or substrate for adenosine deaminase, thus, it was expected that the pharmacokinetics of nelarabine would not be altered by prior administration of fludarabine.
Pharmacokinetics of ara-G
With efficient conversion of nelarabine to ara-G, the Cmax of ara-G also occurs at the end of the infusion of nelarabine and is proportional to the administered dose of nelarabine. The AUC of ara-G is also related in a linear fashion to the dose of nelarabine.13 As stated earlier, one mole of nelarabine would produce one mole of ara-G upon the demethoxylation of nelarabine by adenosine deaminase. Therefore, a linear relationship between the dose of nelarabine and the concentration of ara-G is expected. The plasma concentrations of ara-G decline with time in a mono-exponential fashion in both adult and pediatric patients.13 The t½ of ara-G in pediatric patients (2.1 hrs) was shorter than in adult patients (3 hrs) as pediatric patients exhibit a higher clearance of ara-G relative to adult patients (Table 1).13 A regression analysis of ara-G renal clearance and creatinine clearance resulted in an r2 value of 0.25, indicating that the clearance of ara-G was weakly associated with creatinine clearance.13 The clearance of nelarabine was lower (7%) in patients with creatinine clearances of 50 to 80 mL/min.15 Although the pharmacokinetics of ara-G have not been studied in patients with severe renal dysfunction, it has been recommended that close monitoring for toxicity be performed in patients with creatinine clearances less than 50 mL/min.15 Mechanistically, ara-G is metabolized to guanine by purine nucleoside phosphorylase (PNP). Guanine is then converted via deamination to xanthine, which is then oxidized to form uric acid. With respect to the volume of distribution, no statistically significant differences were noted in the comparing pediatric to adult patients.13 Finally, the pharmacokinetics of ara-G are similar when comparing patients with various hematologic malignancies.13
As stated previously, the pharmacokinetics of ara-G were evaluated in the face of combination therapy with nelarabine and fludarabine. The pharmacokinetics of ara-G were determined on the first day of treatment (nelarabine alone), and on at least one subsequent day when nelarabine followed fludarabine administration by four hours. The t½ of ara-G when nelarabine was administered alone or in combination with fludarabine was similar.16 When comparing the Cmax and AUC of ara-G on day one (nelarabine alone) to day three values (nelarabine following fludarabine), the Cmax and AUC were slightly, but not statistically significantly higher with the combination.16 Thus, fludarabine administration four hours prior to nelarabine dosing has no effect on the pharmacokinetics of ara-G.16
Pharmacokinetics of ara-GTP
Intracellular leukemic ara-GTP concentrations were evaluated in 19 patients with various hematologic malignancies. The median Cmax value of ara-GTP was 23 μM/L, 42 μM/L, 85 μM/L, and 93 μM/L, with nelarabine doses 20 mg/kg, 30 mg/kg (1.2 g/m2 in adult patients), 40 mg/kg (1.2 g/m2 in pediatric patients), and 60 mg/kg, respectively.12 The intracellular concentration of ara-GTP was greater in T-lymphoblastic disease patients (n = 7; median 140 μM/L) as compared to other diagnoses (n = 9; 50 μM/L).14 It was noted that the leukemic intracellular concentrations were higher than concentrations measured in normal mono-nuclear cells (median 30 μM/L; n = 3). Additionally, ara-GTP accumulated to a greater extent in leukemic T-cells and was retained for a longer time in leukemic T-cells from patients as compared to other types of leukemia cells.12
The combination of fludarabine and nelarabine was intended to increase ara-GTP concentrations in leukemia cells that otherwise would not accumulate therapeutic concentrations of this active triphosphate (malignant non-T-cells). A previous response to nelarabine in a patient with B-cell chronic lymphocytic leukemia (CLL) supported the examination of the combination of fludarabine and nelarabine.14 As stated earlier, nelarabine was administered intravenously (1.2 g/m2) on days one, three, and five of a five day regimen. Fludarabine (30 mg/m2) was administered intravenously on days three and five over 30 minutes four hours prior to nelarabine administration.16 It was proposed that fludarabine would inhibit ribonucleotide reductase and result in increased ara-GTP concentration.17 Accumulation of fludarabine triphosphate (F-ara-ATP) results in a decrease in deoxynucleotides, including deoxycytidine triphosphate (dCTP) and deoxyguanosine triphosphate (dGTP).17 The decrease in deoxynucleotides results in decreased feed-back inhibition deoxynucleotide kinases18,19 resulting in an increase in the phosphorylation of ara-G to ara-GMP. The rate-limiting step in the formation of ara-GTP is the conversion of ara-G to ara-GMP. Therefore, combining fludarabine and nelarabine was proposed as a way to increase cellular concentrations of ara-GTP thus improving efficacy in the treatment of certain non-T-cell hematologic malignancies. Investigators noted a direct relationship between cellular F-ara-ATP (via fludarabine administration) and ara-GTP indicating modulation of the active nucleotide triphosphate. The half-life of ara-GTP in this study was similar to earlier findings with the value in most patients being greater than 24 hours (median 35 hours).16
Clinical Efficacy
Treatment of refractory hematologic malignancies with nelarabine has been evaluated in a number of clinical trials. In the initial trial in both pediatric and adult leukemia and lymphoma patients, a 5 day treatment course involving daily infusions was evaluated for efficacy.14 A dose escalation approach was utilized to address both treatment response as well as tolerability. Toxicity occurred at an unacceptable rate at doses of 75 mg/kg and above and the observed maximal tolerated dose of nelarabine in the adult population was 40 mg/kg and in the pediatric population was 60 mg/kg.14
The trial involved patients of multiple cell lineages with 39 of 93 patients enrolled in the study showing either T-ALL or T-LBL. In this population, a complete response (CR) was achieved in 9 of the 39 patients with the majority of those responses occurring in the pediatric population (7 CRs in 26 pediatric patients and 2 CRs in 13 adult patients).14 In addition to the CRs observed here, a partial response (PR) was achieved in another 12 of 39 patients resulting in a total of 53.8% (21/39) of patients showing either a CR or PR. 15 of the 21 patients that had a response to nelarabine therapy were heavily treatment experienced showing at least two prior relapses and many had failed prior hematopoietic stem cell transplant.14 Total response was not limited solely to the T-cell malignancies, but only 1 CR and 7 PRs occurred outside the T-ALL and T-LBL patient groups. Regardless the specific diagnosis in this study, patient response occurred independent of the specific nelarabine dose.14
The CALGB 19801 study specifically studied patients with T-cell malignancies. The study enrolled 26 patients with T-ALL and 13 patients with T-LBL with all participants having either a relapse after a CR with prior therapy or showing refractoriness to at least one induction chemotherapy regimen.20,21 This study was predominantly in the adult population with the age range of 16 to 66 years in the trial. Dosing in the CALGB 19801 study utilized 1.5 g/m2 per dose for a total of three doses given on alternate days. Patients that achieved a CR following one course were given the option of continuing for two additional courses.20,21 Thirty-nine patients were evaluable with 16 showing either a CR or PR after nelarabine therapy. Thirty-one percent (8/26) of the T-ALL patients and 31% (4/13) of the T-LBL patients achieved a CR with a median DFS of 20 weeks subsequent to treatment.20,21
A National Cancer Institute-sponsored phase II study of nelarabine in non-Hodgkin’s lymphoma was completed and evaluated by Goy, et al.22 The trial evaluated 23 patients with 13 of those diagnosed with T-LBL and all patients failing at least one previous chemotherapy regimen. The same dosing regimen from the CALGB 19801 trial was utilized here, although six cycles of nelarabine were administered with each cycle repeated every 28 days. Only 17 of 23 patients were evaluable and two and six patients achieved a CR or PR, respectively. The individual responses were not linked directly to diagnosis in this trial.22
Berg, et al evaluated nelarabine response in pediatric patients with T-ALL and T-LBL.23 Toxicities in this trial limited the initial 153 patients in the trial to 122 patients receiving the defined trial dosage level. Of those 122 patients, 106 patients were evaluable. This trial classified the study participants into four groups; 1) Patients refractory to initial induction therapy or showing greater than 25% blasts in first relapse, 2) Patients showing greater than 25% blasts in a relapse subsequent to primary relapse, 3) Patients with CSF involvement and showing greater than 5% blasts, and 4) Patients with extramedullary relapse and showing less than 25% blasts.23 Groups 1 and 2 received nelarabine 650 mg/m2 and groups 3 and 4 received nelarabine 400 mg/m2 adjusted for neurotoxic adverse events in the individual groups. Group 1 consisted of 33 subjects, of which 16 achieved a CR and two achieved a PR. Group 2 had 30 subjects with seven CRs and one PR. Of the 21 subjects in group 3, five achieved a CR and three achieved a PR. In the final group, only PRs were seen with three of the 22 subjects showing this response.23
Demonstrated responses to nelarabine were seen in this trial in patients with refractory T-cell malignancies, especially those with T-ALL in their first relapse. Further, this study showed that of those patients with positive CSF involvement, eight of 22 patients achieved negative CSF cytology seven days subsequent to nelarabine treatment.23 Nelarabine resulted in a response (either CR or PR) in 37 of 106 patients and was the impetus for future trials in both the pediatric and adult populations.23
The CALGB 59901 trial further evaluated nelarabine in cutaneous and peripheral T-LBL. This trial enrolled 19 patients with an age range of 33 to 69 years. Eleven patients were diagnosed with cutaneous T-LBL and eight had peripheral T-LBL and all but five of the cutaneous T-LBL patients had prior local or systemic therapy.24 Study subjects received nelarabine 1.5 g/m2 on days one, three, and five and repeated every 21 days, which is similar to previous protocols. Results from this study showed only two subjects achieving a PR with no CR in any subject. This trial showed limited efficacy in the adult population with T-LBL, which is in stark contrast to trials in pediatric patients, albeit in a small population with varying histologic subtypes of the disease.24
Gandhi, et al evaluated nelarabine in 35 patients with indolent leukemias in three different dosing protocols.25 Schedule A in this trial was daily weight-based dosing of nelarabine for five consecutive days; schedule B consisted of alternate day dosing on days one, three, and five based on body surface area (BSA); schedule C consisted of a slightly lower BSA-based dose than schedule B given on alternate days for five days but included fludarabine infusions prior to the second and third doses of nelarabine per cycle. The subjects in this trial were heavily treated with multiple chemotherapeutic regimens. Overall and complete responses were 20% and 0%, 15% and 5%, and 63% and 13% for schedules A, B, and C, respectively.25 This study exhibited a statistically significant benefit with nelarabine with fludarabine as compared to nelarabine monotherapy. Responders in this study showed a median time to treatment failure of 17 months, and that response was not different between the three treatment schedules. This study further demonstrated that nelarabine can show activity in mature T- and B-cell leukemias, especially in the presence of concomitant fludarabine.25
In a recently published study by Commander, et al, nelarabine was evaluated in combination as salvage therapy in seven pediatric patients with advanced T-ALL and T-LBL.26 Nelarabine was given at a dose of 650 mg/m2 per day for five days either before or after a dose of both etoposide and cyclophosphamide. Intrathecal prophylaxis was provided to all patients due to the high risk of CNS relapse in patients with T-ALL.26 All five of the T-ALL patients achieved a CR and four of five went on to receive a hematopoietic stem cell transplant. The two T-LBL patients both had a PR with therapy and this is consistent with previous studies illustrating the difficulty in inducing a second remission in T-LBL patients.26 This study shows the need for further evaluation of nelarabine in combination therapy for these advanced hematologic malignancies. Studies describing patients with a T-cell malignancy that have been treated with nelarabine have been summarized in Table 2.
Table 2.
Summary of clinical trials with nelarabine administration in T-cell malignancies.
| Trial | Kurzberg et al 200514 | DeAngelo et al 200721 | Goy et al 200322 | Berg et al 200523 | Czuczman et al 200724 | Gandhi et al 200825 | Commander et al 201026 |
|---|---|---|---|---|---|---|---|
| Evaluable patientsa | 39 | 39 | 17 | 106 | 19 | 11 | 7 |
| Diagnosis | T-ALL, T-LBL | T-ALL, T-LBL | T-LBL | T-ALL, T-LBL | Cutaneous and peripheral T-LBL | T-PLL | T-ALL, T-LBL |
| Outcomes (%)b | |||||||
| CR | 23.1% | 30.8% | 11.8% | 26.4% | 0% | 5.7%c | 71.4% |
| PR | 30.8% | 10.3% | 35.3% | 8.5% | 10.5% | 28.6%c | 28.6% |
Notes:
With T-cell malignancy receiving nelarabine;
Includes all diagnoses and trial doses;
Includes patients with B-cell lymphocytic leukemia as numbers were not reported per diagnosis.
Abbreviations: T-ALL, T-cell acute lymphoblastic leukemia; T-LBL, T-cell lymphoblastic lymphoma; T-PLL, T-cell prolymphocytic leukemia; CR, complete response; PR, partial response.
Safety and Toxicity Profile
Central and peripheral neurotoxicity has been the most identifiable toxicity associated with nelarabine administration. At the recommended adult dose of 1.5 g/m2, the incidence of motor and/or sensory peripheral neuropathy was 21%.27 Multiple studies show varying degrees of peripheral neuropathy. Kurtzberg, et al showed that where overall incidence of reversible neurotoxicity occurred more readily in adult patients than pediatric patients, incidence of grade 3 and 4 neurotoxicity was more common in the pediatric population.14 The most frequently reported central neurotoxicities are somnolence, malaise, and fatigue with onset of symptomatology occurring most often within six to eight days following administration of nelarabine. Other neurotoxicities include paresthesias, ataxia, tremor, neuropathy, amnesia, balance abnormalities, and sensory loss.27 The neurotoxicity associated with nelarabine is dose related with higher incidence with corresponding larger doses of the medication resulting in the dose-limiting toxicity often associated with regimens containing nelarabine.14
In the CALGB 19801 trial, authors reported grade 3 fatigue and weakness in 18% and 11% of subjects, respectively.20,21 Lower grade peripheral sensory neuropathy was also seen in this trial. The most common grade 3 and 4 adverse events in this trial were blood dyscrasias. Neutropenia, thrombocytopenia, and anemia were all reported in the CALGB 19801 trial, but are difficult to assess because many patients with T-ALL and T-LBL will have concomitant bone marrow involvement which could confound the causative agent responsible for these effects.20,21
Commander, et al described neurotoxicity in six of seven subjects that all resolved subsequent to completion of nelarabine therapy. Due to the postulated additive neurotoxicity in the study, as long as administration of nelarabine administration and intrathecal chemotherapy were separated, there was no increased incidence of neurotoxicity.26 Czuczman, et al described grade 3 and 4 adverse events in 50% and 28% of patients, respectively. The most common of these were neurologic toxicities, although single episodes of grade 3 and 4 hematologic toxicities occurred and were self-limiting.24 A recent case report describes complete paraplegia in a T-ALL patient treated with nelarabine. Ischemic, hemorrhagic, and leukemic infiltration were all ruled out as potential etiologies and given the rapid loss of autonomic sensory and motor functions, the most plausible etiology was cumulative drug toxicity attributed to nelarabine.28
Of importance to consider within the scope of nelarabine use is the concomitant risks of neurotoxicity seen in patients with these malignancies. Many patients have previously been treated with neurotoxic systemic chemotherapy, intrathecal chemotherapy, radiation therapy, and multiple combinations of these.14 It is difficult to individually assess a patient’s risk for neurotoxicity or if it is potentiated with nelarabine administration. It is certain that increased monitoring for multiple adverse effects must be done when nelarabine treatment is being considered.
Conclusion
Nelarabine is FDA-approved for the treatment of T-cell leukemias and lymphomas in adult and pediatric patients that have previously failed at least two chemotherapeutic regimens. It is a prodrug of ara-G that requires triphosphorylation prior to incorporation into T-cell DNA, which subsequently leads to programmed cellular death of these malignant cells.6–8 Given the difficulty in treating these refractory malignancies, data suggest that nelarabine as monotherapy can induce both complete and partial responses in patients with these cancers.14,20–23 The durability of these responses is still a topic that needs further elucidation. Further, the risk of neurotoxicity as a dose-limiting toxicity with nelarabine continues to hinder widespread utility outside the scope of advanced T-ALL and T-LBL.14,20,21,26 The use of nelarabine as part of a combination regimen may show increased benefit,25,26 but more research is needed to define nelarabine’s role in treating these very resistant and refractory cancers.
Footnotes
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.American Cancer Society . Cancer facts and figures 2010. Atlanta (GA): American Cancer Society; 2010. [Google Scholar]
- 2.Pui CH, Robison LL, Look AT. Acute lymphoblastic leukemia. Lancet. 2008;371:1030–43. doi: 10.1016/S0140-6736(08)60457-2. [DOI] [PubMed] [Google Scholar]
- 3.Zelenetz AD, Abramson JS, Advani RH, et al. National Comprehensive Cancer Network, Inc; 2010. Oct, NCCN practice guidelines in oncology – non-hodgkin’s lymphomas. Available at: http://www.nccn.org/professionals/physician_gls/PDF/nhl.pdf. Accessed 2010 Oct 27. [DOI] [PubMed] [Google Scholar]
- 4.Getting the facts: T-cell lymphomas. Lymphoma Research Foundation. 2008. Mar, pp. 1–4.
- 5.National PBM monograph: nelarabine (Arranon®). 2007. Jan, Available at: http://www.pbm.va.gov/clinical%20guidance/drug%20monographs/nelarabine.pdf. Accessed 2010 Oct 27.
- 6.Rodriguez CO, Gandhi V. Arabinosylguanine-induced apoptosis of T-lymphoblastic cells: incorporation into DNA is a necessary step. Cancer Res. 1999;59:4937–43. [PubMed] [Google Scholar]
- 7.Rodriguez CO, Mitchell BS, Ayres M, Eriksson S, Gandhi V. Arabinosylguanine is phosphorylated by both cytoplasmic deoxycytidine kinase and mitochondrial deoxyguanosine kinase. Cancer Res. 2002;62:3000–5. [PubMed] [Google Scholar]
- 8.Gandhi V, Mineishi S, Huang P, et al. Cytotoxicity, metabolism, and mechanism of action of 2’,2’-difluorodeoxyguanosine in Chinese hamster ovary cells. Cancer Res. 1995;55:1517–24. [PubMed] [Google Scholar]
- 9.Prus KL, Averett DR, Zimmerman TP. Transport and metabolism of 9-β-D-arabinofuranosylguanine in a human T-lymphoblastoid cell line: nitrobenzylthioinosine-sensitive and -insensitive influx. Cancer Res. 1990;50:1817–21. [PubMed] [Google Scholar]
- 10.Scharenberg JG, Spaapen LJ, Rijkers GT, et al. Mechanism of deoxyguanosine toxicity for human T and B lymphocytes. Adv Exp Med Bio. 1986;195B:191–9. doi: 10.1007/978-1-4684-1248-2_29. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez CO, Stellrecht CM, Gandhi V. Mechanisms for T-cell selective cytotoxicity of arabinosylguanine. Blood. 2003;102:1842–8. doi: 10.1182/blood-2003-01-0317. [DOI] [PubMed] [Google Scholar]
- 12.Gandhi V, Plunkett W, Rodriguez CO, et al. Compound GW506U78 in refractory hematologic malignancies: relationship between cellular Pharmacokinetics and clinical response. J Clin Oncol. 1998;16:3607–15. doi: 10.1200/JCO.1998.16.11.3607. [DOI] [PubMed] [Google Scholar]
- 13.Kisor DF, Plunkett W, Kurtzberg J, et al. Pharmacokinetics of nelarabine and 9-beta-D-arabinofuranosyl guanine in pediatric and adult patients during a phase I study of nelarabine for the treatment of refractory hematologic malignancies. J Clin Oncol. 2000;18:995–1003. doi: 10.1200/JCO.2000.18.5.995. [DOI] [PubMed] [Google Scholar]
- 14.Kurtzberg J, Ernst TJ, Keating MJ, et al. A phase I study of 2-amino-9-β-D-arabinofuranosyl-6-methoxy-9Hpurine (506U78 [nelarabine]) administered on a consecutive five day schedule in children and adults with refractory hematologic malignancies. J Clin Oncol. 2005;23:3396–403. doi: 10.1200/JCO.2005.03.199. [DOI] [PubMed] [Google Scholar]
- 15.European Medicines Agency Atriance (nelarabine): summary 2007 Jun 15;109(12):5136–42 of product information [online] Available at: http://www.emea.europa.eu/humandocs/PDFs/EPAR/atriance/. Accessed 2010 Oct 27.
- 16.Gandhi V, Plunkett W, Weller S, et al. Evaluation of the combination of nelarabine and fludarabine in leukemias: clinical response, pharmacokinetics and pharmacodynamics in leukemia cells. J Clin Oncol. 2001;19:2142–52. doi: 10.1200/JCO.2001.19.8.2142. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez COR, Legha JK, Estey E, Keating MJ, Gandhi V. Pharmacological and biochemical strategies to increase accumulation of arabinofuranosyl-guanine triphosphate in primary human leukemia cells. Clin Cancer Res. 1997;3:2107–13. [PubMed] [Google Scholar]
- 18.Gandhi V, Plunkett W. Interaction of arabinosyl nucleotides in K562 human leukemia cells. Biochem Pharmacol. 1989;38:3551–8. doi: 10.1016/0006-2952(89)90127-5. [DOI] [PubMed] [Google Scholar]
- 19.Gandhi V, Plunkett W. Modulation arabinosylnucleoside metabolism by ara-binosylnucleotides in human leukemia cells. Cancer Res. 1988;48:329–34. [PubMed] [Google Scholar]
- 20.DeAngelo DJ, Yu D, Dodge RK, et al. A phase II study of 2-amino-9-β-D-arabinosyl-6-methoxy-9H-purine (506U78) in patients with relapse of refractory T-lineage acute lymphoblastic leukemias (ALL) of lymphoblastic lymphoma (LBL) (abstract): CALGB Study 19801. Blood. 2002;100:742. [Google Scholar]
- 21.DeAngelo DJ, Yu D, Johnson JL, et al. Nelarabine induces complete remissions in adults with relapsed or refractory T-lineage acute lymphoblastic leukemia or lymphoblastic lymphoma: Cancer and Leukemia Group B study 19801. Blood. 2007;109:5136–42. doi: 10.1182/blood-2006-11-056754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goy A, Bleyer A, Hagemeister F, et al. Phase II study of compound GW506U78 (araG) for patients with indolent B-cell or peripheral T-cell lymphoma previously treated with chemotherapy. 45th Annual Meeting American Society of Hematology; San Diego, USA. 2003. (Abstract 2359). [Google Scholar]
- 23.Berg SL, Blaney SM, Devidas M, et al. Phase II study of nelarabine (compound 506U78) in children and young adults with refractory T-cell malignancies: a report from the Children’s Oncology Group. J Clin Oncol. 2005;23:3376–82. doi: 10.1200/JCO.2005.03.426. [DOI] [PubMed] [Google Scholar]
- 24.Czuczman MS, Porcu P, Johnson J, et al. Results of a phase II study of 506U78 in cutaneous T-cell lymphoma and peripheral T-cell lymphoma: CALGB 59901. Leuk Lymphoma. 2007;48(1):97–103. doi: 10.1080/10428190600961058. [DOI] [PubMed] [Google Scholar]
- 25.Gandhi V, Tam C, O’Brien S, et al. Phase I trial of nelarabine in indolent leukemias. J Clin Oncol. 2008;26(7):1098–1105. doi: 10.1200/JCO.2007.14.1986. [DOI] [PubMed] [Google Scholar]
- 26.Commander LA, Seif AE, Insogna IG, Rheingold SR. Salvage therapy with nelarabine, etoposide, and cyclophosphamide in relapsed/refractory pediatric T-cell lymphoblastic leukemia and lymphoma. Brit J Hematol. 2010;150:345–51. doi: 10.1111/j.1365-2141.2010.08236.x. [DOI] [PubMed] [Google Scholar]
- 27.Research Triangle Park; NC, USA: 2005. GlaxoSmithKline: Arranon® (nelarabine) injection for intravenous use (product information) [Google Scholar]
- 28.Papayannidis C, Iacobucci I, Abbenante MC, et al. Complete paraplegia after nelarabine treatment in a T-cell acute lymphoblastic leukemia adult patient. Am J Hematol. 2010;85:608. doi: 10.1002/ajh.21719. [DOI] [PubMed] [Google Scholar]