Abstract
Aggression is regulated by pheromones in many animal species1,2,3. However in no system have aggression pheromones, their cognate receptors and corresponding sensory neurons been identified. Here we show that 11-cis-vaccenyl acetate (cVA), a male-specific volatile pheromone, robustly promotes male-male aggression in the vinegar fly Drosophila melanogaster. The aggression-promoting effect of synthetic cVA requires olfactory sensory neurons (OSNs) expressing the receptor Or67d4,5,6, as well as the receptor itself. Activation of Or67d-expressing OSNs, either by genetic manipulation of their excitability or by exposure to male pheromones in the absence of other classes of OSNs, is sufficient to promote aggression. High densities of male flies can promote aggression through release of volatile cVA. In turn, cVA-promoted aggression can promote male fly dispersal from a food resource, in a manner dependent upon Or67d-expressing OSNs. These data suggest that cVA may mediate negative feedback control of male population density, through its effect on aggression. Identification of a pheromone-OSN pair controlling aggression in a genetic organism opens the way to unraveling the neurobiology of this evolutionarily conserved behavior.
Male-male aggression (hereafter referred to as “aggression”) in the vinegar fly Drosophila melanogaster was first described almost a century ago7. Since then, considerable progress has been made in understanding its regulation8,9,10,11,12,13,14,15. Nevertheless, little is known about how this behavior is controlled by sensory stimuli, in particular by pheromones. Recently, we showed that Cyp6a20, a cytochrome P450 gene previously identified by genetic selection for aggressiveness11, also mediates the influence of social experience on aggression13. We found that Cyp6a20 is expressed in pheromone-sensitive trichoid sensilla5 by support cells that co-express LUSH13, an odorant binding protein required for detection of cVA16,17, a male-specific volatile pheromone6,16,18,19. These observations raised the question of whether cVA is involved in the pheromonal regulation of aggression in Drosophila.
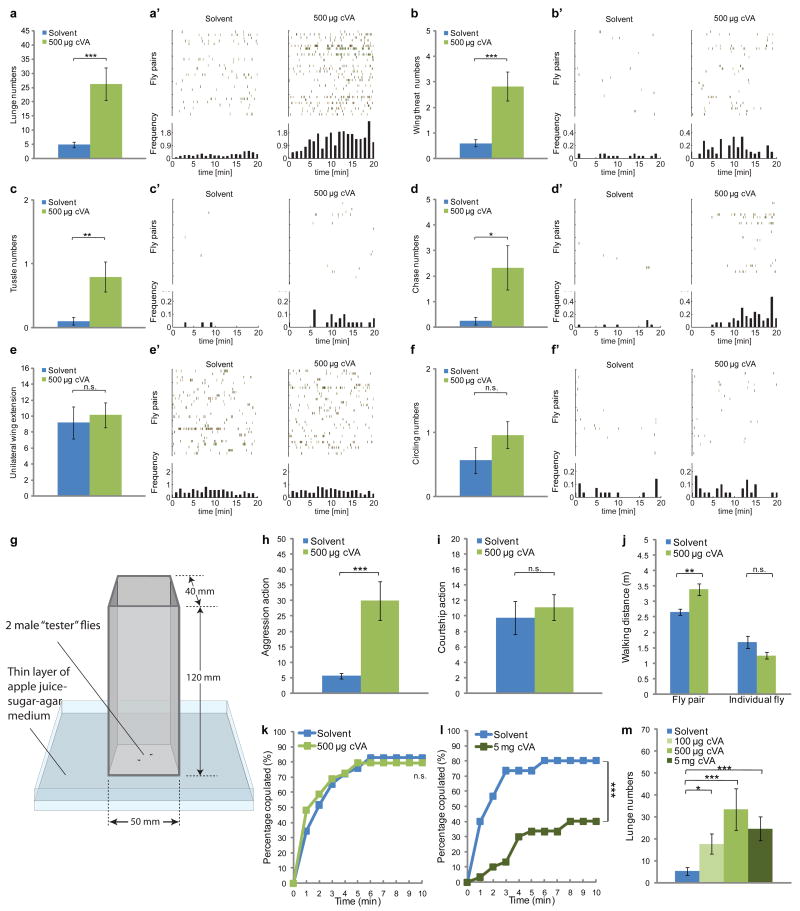
We used CADABRA software20 to assess the influence of cVA on the behavioral interactions between pairs of Canton-S male flies. When 500 μg synthetic cVA was provided on a piece of filter paper in the behavior chamber14 (Fig. 1g), a significantly higher number of lunges, the predominant aggressive behavior8,14,20, was observed (Fig. 1a,a′). The effect of synthetic cVA to promote aggression was dose-dependent (Fig. 1m). Other aggressive behaviors, including wing threat (Fig. 1b,b′), tussling (Fig. 1c,c′) and chasing (Fig. 1d,d′), were also up-regulated by addition of synthetic cVA (see ethograms in Supplementary Fig. S1). The total walking distance of the fly pair was only modestly increased by cVA, and was unaltered if only a single fly was present (Fig. 1j), suggesting that the aggression-promoting effect of the pheromone is not due to an increase in locomotor activity14.
Figure 1. Synthetic cVA promotes aggression.
(a-f) Number of (a) lunges, (b) wing threats, (c) tussles, (d) chases, (e) unilateral wing extensions and (f) circling behaviors (per 20 minutes) performed by pairs of Canton-S (CS) males in the presence of solvent alone or 500 μg cVA (n=28-30). (a′-f′) The temporal distribution of behaviors is shown in raster plots. Each row of spikes represents one fly pair, and each spike represents one occurrence of the behavior. The histogram integrates the occurrences of each behavior in 1-minute bins. (g) Illustration (to scale) of the behavior chamber used for experiments shown in Fig. 1-3. (h,i) Number of all aggressive (h) and courtship (i) actions, based on the data in (a-f). (j) Walking distance of pairs of (n=28-30) or individual (n=16) CS males in the presence of solvent alone or 500 μg cVA. (k,l) Cumulative latency of CS males to copulate with virgin CS females in the presence of solvent (acetone) alone or 500 μg cVA (k; n=29), or solvent alone or 5 mg cVA (l; n=30). (m) Number of lunges performed by pairs of C-S males in the presence of solvent alone, 100 μg cVA, 500 μg cVA or 5 mg cVA (n=20-24). * P<0.05, ** P<0.01 and *** P<0.001. Error Bars are s.e.m. in this and all figures.
Since courtship and aggression are opponent social behaviors that may reciprocally inhibit each other21, we tested whether the stimulatory effect of synthetic cVA on aggression is associated with any influence on male-male courtship. No change in male-male courtship was observed in response to 500 μg of synthetic cVA, as measured by the occurrence of unilateral wing extension (Fig. 1e,e′), or circling behavior (Fig. 1f,f′). Thus cVA promotes aggression without altering the frequency of male-male courtship behaviors (Fig. 1h,i, and Supplementary Fig. S1).
cVA has also been shown to suppress male mating behavior towards females6,19. However, under our conditions 500 μg of synthetic cVA was insufficient to suppress such behavior, as measured by cumulative latency to copulation (Fig. 1k). The effect of cVA to promote aggression can, therefore, be observed under conditions where the pheromone does not affect male sexual behaviors. Nevertheless, 5 mg of synthetic cVA was sufficient to suppress male-female mating (Fig. 1l), while no further increase in lunging was observed using this higher amount of cVA (Fig. 1m). Thus, synthetic cVA can regulate two different male social behaviors, aggression and mating, in opposite directions with different dosage requirements.
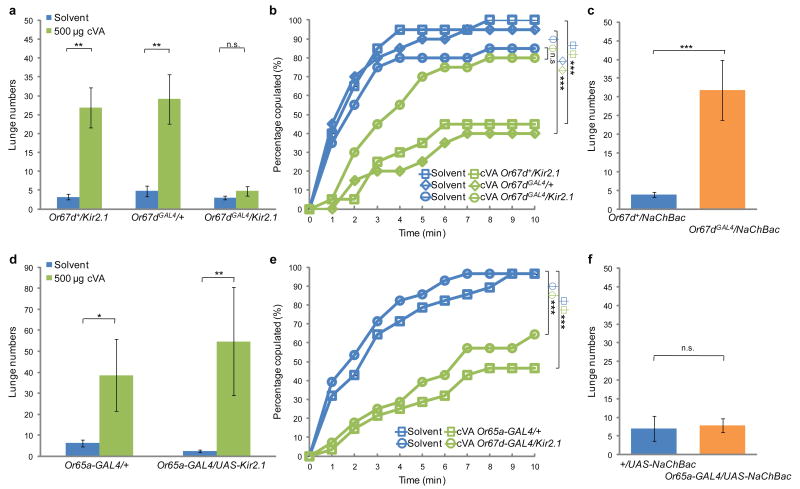
Two different olfactory receptors, Or67d and Or65a, have been identified as cVA receptors4,5,6,19. Silencing Or67d-expressing OSNs by expressing the inwardly rectifying potassium channel Kir2.122 blocked the effect of synthetic cVA to promote aggression (Fig. 2a). This effect of cVA was also eliminated in Or67dGAL4/GAL4 mutant flies6 (Supplementary Fig. S2a), indicating that Or67d receptors, as well as Or67d-expressing OSNs, are required. Consistent with a previous report that the Or67d gene is required for the mating-suppressing effect of synthetic cVA6, silencing Or67d-expressing OSNs blocked the effect of cVA to suppress male mating towards females (Fig. 2b). In contrast, silencing Or65a-expressing OSNs did not impair either promotion of aggression or suppression of male-female mating by cVA (Fig. 2d,e). These data suggest that synthetic cVA exerts its aggression-promoting effect, as well as its mating-suppressing effect, via Or67d-expressing OSNs.
Figure 2. Or67d-expressing OSNs mediate the aggression-promoting effect of synthetic cVA.
(a,c,d,f) Number of lunges (per 20 minutes) performed by pairs of males of the indicated genotype, in the presence of solvent alone or 500 μg cVA (a,d; n=18-20), or with no added pheromone (c,f; n=20-26). * P<0.05, ** P<0.01 and *** P<0.001. (b,e) Cumulative latency of males of the indicated genotype to copulate with virgin females in the presence of solvent alone or 5 mg cVA (n=20-28). Note that silencing of Or67d-expressing OSNs impairs the suppression of mating by cVA (green open circles in b). *** P<0.001.
We then asked whether increasing the excitability of Or67d-expressing OSNs is sufficient to promote aggression, in the absence of added cVA, by expressing a bacterially-derived sodium channel (“NaChBac”)23 in Or67d-expressing OSNs. Pairs of Or67dGAL4/UAS-NaChBac male flies exhibited a significantly increased number of lunges in comparison to Or67d+/UAS-NaChBac controls (Fig. 2c). These data indicate that increasing the excitability of Or67d-expressing OSNs can enhance aggression, and that the magnitude of this effect is similar to that obtained by addition of synthetic cVA. Activation of Or65a-expressing OSNs, in contrast, did not promote aggression (Fig. 2f). Negative results obtained using the Or65a-GAL4 driver should be interpreted with caution, however, because its strength may not be equivalent to that of Or67dGAL4 driver19.
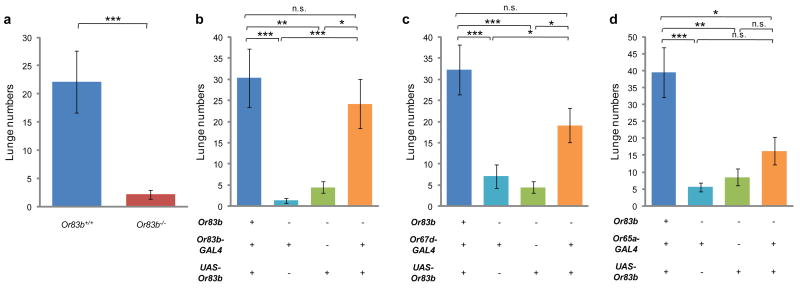
As endogenously produced cVA is able to activate Or67d-expressing OSNs17, we next asked whether these neurons are sufficient to mediate the effect of endogenously produced cVA to promote aggression. Male flies bearing a null mutation in Or83b, which encodes an obligatory co-receptor for olfactory receptors expressed in ∼70-80% of OSNs24, exhibited a significant reduction in lunging behavior (Fig. 3a). This aggression deficit could be rescued by expressing an Or83b cDNA under the control of an Or83b-GAL4 driver (Fig. 3b). These data indicate an essential role for one or more classes of Or83b-expressing OSNs, and thereby implicate one or more volatile pheromones released by male flies, in aggression.
Figure 3. Or67d-expressing OSNs are sufficient to mediate the aggression-promoting effect of endogenously produced cVA.
In all graphs, the number of lunges (per 20 minutes) performed by pairs of males of the indicated genotype is shown (n=20-34). For this experiment, single-housed male flies, which exhibit a higher baseline level of aggression13 were used in order to more readily detect the decreased aggression caused by the Or83b mutation. Note that restoration of Or83b expression to Or67d-expressing neurons rescues the loss of aggressiveness in Or83b-/- flies (c). * P<0.05, ** P<0.01 and *** P<0.001.
We then tested whether restoring Or83b expression selectively in Or67d-expressing OSNs could also rescue the aggression deficit of Or83b-/- mutant males. Indeed, this manipulation produced a significant rescue of the reduced aggression phenotype of Or83b-/- mutants, to a level ∼80% of that obtained using the O83b-GAL4 driver (Fig. 3c). Or83b-/- mutant males expressing Or83b under the control of Or65a-GAL4, in contrast, did not exhibit a significant rescue of the aggression phenotype, although there was a slight trend in this direction (Fig. 3d). As Or67d-expressing OSNs respond essentially exclusively to cVA25, these results indicate that activation of Or67d OSNs by endogenously produced cVA is sufficient to promote aggression, when all other classes of Or83b-expressing OSNs are inactive. Taken together, these gain-of-function data indicate that activation of Or67d-expressing OSNs (using NaChBac) is sufficient to promote aggression, and that these neurons are sufficient to mediate the aggression-promoting effect of endogenous cVA.
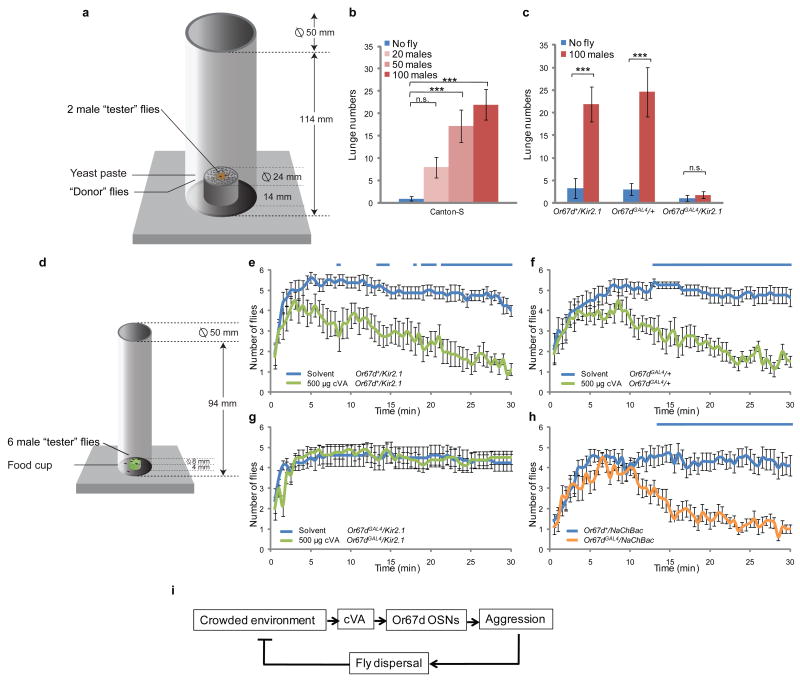
As cVA is a volatile pheromone, its concentration should be proportional to the number of male flies in a given environment. If so, then increased levels of aggression might be observed in a setting containing a high density of male flies, in a cVA-dependent manner. To eliminate the confound that a higher density of male flies could produce a higher number of lunges per fly pair simply because of an increased frequency of interaction, we developed an assay to examine the effect of a high density of “caged” male flies on the aggressiveness of a single pair of neighboring “tester” males. The “donor” caged male flies were separated by a meshed compartment from the “tester” males, permitting transmission of volatile odorants while preventing physical interactions between the “donor” and “tester” males (Fig. 4a). The “tester” males indeed performed a higher number of lunges in the presence of “donor” males, in a manner proportional to the number of these caged donors (Fig. 4b). Importantly, the ability of the “donor” males to enhance aggression was eliminated by silencing Or67d-expressing OSNs in the “tester” males (Fig. 4c), or by eliminating the Or67d gene (Supplementary Fig. S2b). These data indicate that proximity to a high density of male flies can increase the level of aggression, and that this increase is mediated predominantly, if not exclusively, by release and detection of endogenous cVA.
Figure 4. cVA promotes aggression at high fly densities and dispersal of male flies from a food resource.
(a) Illustration (to scale) of the behavior chamber used for the experiments shown in (b,c). (b) Number of lunges (per 20 minutes) performed by pairs of “tester” Canton-S males together with the indicated number of caged male “donor” flies (n=15); (c) Number of lunges (per 20 minutes) performed by pairs of “tester” males of the indicated genotype, in the presence or absence of 100 male “donor” flies (n=15). *** P<0.001. (d) Illustration (to scale) of the behavior chamber used for the experiments shown in (e-h). (e-h) Number of flies of the indicated genotype on the food cup in the presence of solvent only (blue line) or 500 μg cVA (green line) (n=8). Note gradual dispersal of flies from the food cup in the presence of cVA. Blue bars represent data sets that are significantly different (P<0.05). (h) Flies of the indicated genotype tested in the absence of solvent or cVA (n=10). (i) Model illustrating hypothetical negative feedback regulation of fly population density by cVA-promoted aggression.
The observation that aggression is enhanced by proximity to a high density of male flies raised the question of whether aggressive behavior might, in turn, regulate population density. Aggressive males chase competing males8 from a resource as part of their territorial behavior26, and thereby disperse them. If a high fly density promotes aggression via elevated levels of cVA, and if increased aggression in turn enhances dispersal, then cVA-promoted aggression might ultimately limit the density of male flies on a given resource. To test this hypothesis, we first examined whether synthetic cVA promotes fly dispersal in a setting where multiple (six) male flies compete for a limited food resource territory (Fig. 4d). In the absence of synthetic cVA, control Or67d+/UAS-Kir2.1 and Or67dGAL4/+ male flies quickly congregated on the food resource and remained there for at least 30 minutes after introduction into the chamber (Fig. 4e,f, blue line). In the presence of synthetic cVA, however, the number of these control male flies on the food cup declined following their initial attraction to the resource, indicating dispersal (Fig. 4e,f, green line). cVA did not promote dispersal from the food resource if individual male flies, instead of 6 flies, were introduced into the behavior chamber (data not shown), suggesting that the dispersal observed in the 6-fly assays is due to aggression. Indeed, under these conditions cVA also robustly promoted aggression (Supplementary Fig. S3a). In contrast, Or67dGAL4/UAS-Kir2.1 male flies exhibited neither increased dispersal (Fig. 4g), nor increased aggression (Supplementary Fig. S3a), in response to synthetic cVA. These data suggest that cVA promotes dispersal of male flies through Or67d-expressing OSNs. Consistent with this interpretation, increasing the excitability of Or67d-expressing OSNs (using “NaChBac”) promoted dispersal and aggression in a manner similar to that of exogenously added cVA (Fig. 4h and Supplementary Figure S3b).
These data indicate that activation of cVA-responsive OSNs can reduce the number of male flies on a food resource, by promoting aggression. cVA has also been reported to function as an aggregation pheromone in Drosophila16,18. Taken together, these data suggest that cVA may play a role in the homeostatic control of male fly population density on a food resource: at low population densities, cVA causes more flies to accumulate on the resource via its aggregation-promoting function; once the population density of male flies increases above some threshold, the increased levels of cVA promote aggression and dispersal, thereby reducing the population density to a level that achieves an optimal balance between feeding, reproduction and competition (Fig. 4i). How the different behavioral functions of cVA are exerted through a common population of OSNs is an interesting question for future study27.
The control of aggression by pheromones and other semiochemicals in insects has been extensively studied1,28,29. Such studies have established correlations between the quantity of certain pheromones in tissue extracts and the intensity of aggressive behavior29, and in some cases have demonstrated the ability of such pheromones, in pure or synthetic form, to promote aggression28. However, with few exceptions3, it has been difficult to establish whether these pheromones actually play a physiological role in regulating aggression. The genetic tools available in Drosophila have permitted us to establish that cVA, both in synthetic form and when released endogenously by male flies, promotes aggression in this species, via Or67d-expressing OSNs and the Or67d receptor itself. Further dissection of the circuits engaged by these OSNs30 should facilitate our understanding of the neurobiology of this evolutionarily conserved, innate social behavior.
Methods Summary
Behavioral assays in most experiments were performed using 5-6 day-old male flies that were raised after eclosion in groups of 10 flies/vial prior to testing. Single-housed flies were used in the experiments shown in Fig. 3, in order to provide a higher level of baseline aggression13 against which to evaluate decreases in aggression caused by the Or83b mutations. In all experiments involving genetic manipulations, comparisons between genotypes were made on equivalent genetic backgrounds. Or67dGAL4/GAL4 flies contain an insertion of GAL4 into the chromosomal Or67d gene that produces a null mutation; the genetic control for this allele, Or67d+/+, is derived by excision of the GAL4 insertion, reverting Or67d to a functional gene6.
Most experiments (Fig. 1-3) were performed in a behavior chamber similar to that described previously14 except that the floor was uniformly filled with apple juice-sugar-agar medium (Fig. 1a). Two males (taken from different housing vials) were introduced into the chamber by gentle aspiration without anesthesia, videotaped for 20 minutes and behavioral data extracted from the videotape using CADABRA20 software. For mating assays between males and virgin females, the latency to copulation was scored manually. Where indicated, synthetic cVA dissolved in acetone (or acetone alone as a control) was delivered by spotting onto a small piece of filter paper placed in one corner of the arena.
Different chamber designs were used for the experiments involving caged “donor” males (Fig. 4a), or males competing for a food resource (Fig. 4d), and the number of lunges (Fig. 4b-c) or the number of flies on the food cup (Fig. 4e-h) were scored manually for these experiments. Detailed descriptions of fly stocks, experimental designs and statistical analysis are provided in the Supplementary Methods.
Supplementary Material
Acknowledgments
We thank B. Dickson, M. Heisenberg and L. Vosshall for providing fly stocks, R. Axel, C. Bargmann, J. Levine and L. Vosshall for thoughtful comments on the manuscript and L. Zipursky for helpful discussions. This work was supported in part by NSF grants EF-0623527 and MCB-0418479. D.J.A is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Supplementary Information accompanies the paper on www.nature.com/nature.
Author Contributions: L.W. carried out all the experiments and performed the data analysis. L.W. and D.J.A. together conceived the research and wrote the manuscript.
References
- 1.Shorey HH. Behavioral responses to insect pheromones. Annu Rev Entomol. 1973;18:349. doi: 10.1146/annurev.en.18.010173.002025. [DOI] [PubMed] [Google Scholar]
- 2.Keverne EB. Mammalian pheromones: From genes to behaviour. Curr Biol. 2002;12:R807–R809. doi: 10.1016/s0960-9822(02)01314-3. [DOI] [PubMed] [Google Scholar]
- 3.Chamero P, et al. Identification of protein pheromones that promote aggressive behaviour. Nature. 2007;450:899–902. doi: 10.1038/nature05997. [DOI] [PubMed] [Google Scholar]
- 4.Ha TS, Smith DP. A pheromone receptor mediates 11-cis-vaccenyl acetate-induced responses in Drosophila. J Neurosci. 2006;26:8727–8733. doi: 10.1523/JNEUROSCI.0876-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Goes van Naters W, Carlson JR. Receptors and neurons for fly odors in Drosophila. Curr Biol. 2007;17:606–612. doi: 10.1016/j.cub.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446:542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- 7.Sturtevant AH. Experiments on sex recognition and the problem of sexual selection in Drosophila. Anim Behav. 1915;5:351–366. [Google Scholar]
- 8.Chen S, Lee AY, Bowens NM, Huber R, Kravitz EA. Fighting fruit flies: a model system for the study of aggression. Proc Natl Acad Sci USA. 2002;99:5664–5668. doi: 10.1073/pnas.082102599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vrontou E, Nilsen SP, Demir E, Kravitz EA, Dickson BJ. fruitless regulates aggression and dominance in Drosophila. Nat Neurosci. 2006;9:1469–1471. doi: 10.1038/nn1809. [DOI] [PubMed] [Google Scholar]
- 10.Baier A, Wittek B, Brembs B. Drosophila as a new model organism for the neurobiology of aggression? J Exp Biol. 2002;205:1233–1240. doi: 10.1242/jeb.205.9.1233. [DOI] [PubMed] [Google Scholar]
- 11.Dierick HA, Greenspan RJ. Molecular analysis of flies selected for aggressive behavior. Nat Genet. 2006;38:1023–1031. doi: 10.1038/ng1864. [DOI] [PubMed] [Google Scholar]
- 12.Dierick HA, Greenspan RJ. Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat Genet. 2007;39:678–682. doi: 10.1038/ng2029. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Dankert H, Perona P, Anderson DJ. A common genetic target for environmental and heritable influences on aggressiveness in Drosophila. Proc Natl Acad Sci USA. 2008;105:5657–5663. doi: 10.1073/pnas.0801327105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoyer SC, et al. Octopamine in male aggression of Drosophila. Curr Biol. 2008;18:159–167. doi: 10.1016/j.cub.2007.12.052. [DOI] [PubMed] [Google Scholar]
- 15.Zhou C, Rao Y, Rao Y. A subset of octopaminergic neurons are important for Drosophila aggression. Nat Neurosci. 2008;11:1059–1067. doi: 10.1038/nn.2164. [DOI] [PubMed] [Google Scholar]
- 16.Xu P, Atkinson R, Jones DNM, Smith DP. Drosophila OBP LUSH is required for activity of pheromone-sensitive neurons. Neuron. 2005;45:193–200. doi: 10.1016/j.neuron.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 17.Laughlin JD, Ha TS, Jones DNM, Smith DP. Activation of pheromone-sensitive neurons is mediated by conformational activation of pheromone-binding protein. Cell. 2008;133:1255–1265. doi: 10.1016/j.cell.2008.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartelt RJ, Schaner AM, Jackson LL. cis-vaccenyl acetate as an aggregation pheromone in Drosophila melanogaster. J Chem Ecol. 1985;11:1747–1756. doi: 10.1007/BF01012124. [DOI] [PubMed] [Google Scholar]
- 19.Ejima A, et al. Generalization of courtship learning in Drosophila is mediated by cis-vaccenyl acetate. Curr Biol. 2007;17:599–605. doi: 10.1016/j.cub.2007.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dankert H, Wang L, Hoopfer ED, Anderson DJ, Perona P. Automated monitoring and analysis of social behavior in Drosophila. Nat Methods. 2009;6:297–303. doi: 10.1038/nmeth.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Certel SJ, Savella MG, Schlegel DCF, Kravitz EA. Modulation of Drosophila male behavioral choice. Proc Natl Acad Sci USA. 2007;104:4706–4711. doi: 10.1073/pnas.0700328104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baines RA, Uhler JP, Thompson A, Sweeney ST, Bate M. Altered electrical properties in Drosophila neurons developing without synaptic transmission. J Neurosci. 2001;21:1523–1531. doi: 10.1523/JNEUROSCI.21-05-01523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren D, et al. A prokaryotic voltage-gated sodium channel. Science. 2001;294:2372–2375. doi: 10.1126/science.1065635. [DOI] [PubMed] [Google Scholar]
- 24.Larsson MC, et al. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 25.Schlief ML, Wilson RI. Olfactory processing and behavior downstream from highly selective receptor neurons. Nat Neurosci. 2007;10:623–630. doi: 10.1038/nn1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffmann AA. A laboratory study of male territoriality in the sibling species Drosophila melanogaster and Drosophila simulans. Anim Behav. 1987;35:807–818. [Google Scholar]
- 27.Benton R. Sensitivity and specificity in Drosophila pheromone perception. Trends Neurosci. 2007;30:512–519. doi: 10.1016/j.tins.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Ono M, Terabe H, Hori H, Sasaki M. Insect signalling: Components of giant hornet alarm pheromone. Nature. 2003;424:637–638. doi: 10.1038/424637a. [DOI] [PubMed] [Google Scholar]
- 29.Kou R, Chen SC, Chen YR, Ho HY. 3-Hydroxy-2-butanone and the first encounter fight in the male lobster cockroach, Nauphoeta cinerea. Naturwissenschaften. 2006;93:286–291. doi: 10.1007/s00114-006-0095-0. [DOI] [PubMed] [Google Scholar]
- 30.Datta SR, et al. The Drosophila pheromone cVA activates a sexually dimorphic neural circuit. Nature. 2008;452:473–477. doi: 10.1038/nature06808. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.