Abstract
Context: Conflicting findings have been reported regarding the effect of menstrual cycle phase and sex hormones on insulin sensitivity.
Objective: The aim was to determine the pattern of insulin resistance over the menstrual cycle and whether variations in sex hormones explain these patterns.
Design: The BioCycle study is a longitudinal study that measured hormones at different phases of the menstrual cycle. Participants had up to eight visits per cycle; each visit was timed using fertility monitors to capture sensitive windows of hormonal changes.
Setting: The study was conducted in the general community of the University at Buffalo (Buffalo, NY).
Participants: A total of 257 healthy, premenopausal women (age, 27 ± 8 yr; body mass index, 24 ± 4 kg/m2) participated in the study.
Main Outcome Measures: We measured fasting insulin, glucose, and insulin resistance by the homeostasis model of insulin resistance (HOMA-IR).
Results: Significant changes in HOMA-IR were observed over the menstrual cycle; from a midfollicular phase level of 1.35, levels rose to 1.59 during the early luteal phase and decreased to 1.55 in the late-luteal phase. HOMA-IR levels primarily reflected changes in insulin and not glucose. After adjustment for age, race, cycle, and other sex hormones, HOMA-IR was positively associated with estradiol (β = 0.082; P < 0.001) and progesterone (β = 0.025; P < 0.001), and inversely associated with FSH (adjusted β = −0.040; P < 0.001) and SHBG (β = −0.085; P < 0.001). LH was not associated with HOMA-IR. Further adjustment for BMI weakened the association with SHBG (β = −0.057; P = 0.06) but did not affect other associations.
Conclusion: Insulin exhibited minor menstrual cycle variability. Estradiol and progesterone were positively associated with insulin resistance and should be considered in studies of insulin resistance among premenopausal women.
Among premenopausal women, estradiol and progesterone are positively associated with insulin resistance and should be considered in clinical studies of insulin resistance and related traits.
The obesity epidemic has increased the prevalence of metabolic disorders among young, premenopausal women (1), who are more subject to insulin resistance. However, little consideration is given to the effects of sex hormones that may work to influence insulin sensitivity. Ongoing debate has surrounded the role of sex hormones on insulin resistance (2,3). Although both oral contraceptive use and menopausal hormone therapy have been found to alter insulin sensitivity (4), the effects of variations in endogenous sex hormones across the menstrual cycle on insulin sensitivity have not been clearly demonstrated. Some evidence has suggested that menstrual cycle effects on insulin sensitivity are not trivial because premenopausal women with diabetes have been observed to have higher rates of hyperglycemia during the luteal phase (5). Longitudinal studies have also shown that sex hormones and SHBG levels are related to the development of type 2 diabetes (6). Thus, the variability of sex hormones and SHBG during a normal menstrual cycle may have an important impact on insulin resistance.
Conflicting findings have been reported from previous studies that evaluated the effects of menstrual cycle phase on insulin sensitivity (7,8,9,10,11,12,13,14,15,16). Intravenous glucose measures by hyperinsulinemic clamp (17) or other intensive techniques (18) have been treated as the “gold standard” methods for evaluating insulin sensitivity. However, due to the extensive testing required for determination of insulin sensitivity by these methods, they are, in general, not feasible in large epidemiological studies. Indeed, previous studies based on iv glucose measures to examine the effects of menstrual cycle phase on insulin sensitivity were generally small (n < 15), and some studies (9,10,11,14) compared only single measurements of hormone levels in each cycle phase (i.e. one follicular vs. one luteal measurement), timed by approximating calendar day. As such, they may have limited statistical power and inadequate sampling of hormone levels to tease apart the association between insulin resistance and changes in endogenous sex hormones and SHBG through the menstrual cycle. Understanding the physiological associations between sex hormones and insulin sensitivity during the menstrual cycle could have implications for understanding impaired glucose tolerance among premenopausal women. We therefore assessed these associations in a longitudinal cohort of young women in the BioCycle study. We hypothesized that insulin sensitivity, as estimated by homeostasis model of insulin resistance (HOMA-IR), varies significantly over the menstrual cycle and that its levels would be negatively associated with estradiol and SHBG while being positively associated with progesterone.
Subjects and Methods
Study population
The BioCycle study recruited 259 healthy, premenopausal women and followed them for one (n = 9) or two (n = 250) menstrual cycles for the original purpose of studying the effects of sex hormones on oxidative stress. The study design has been previously described (19). Briefly, healthy, regularly menstruating women were recruited from the Buffalo, New York, region. Women were included if they were 18–44 yr of age and reported having menstrual cycles between 21 and 35 d for each menstrual cycle for the past 6 months. Exclusions were also made based on factors that influence hormone levels such as current vitamin supplement use or oral contraceptive use in the past 3 months, breast-feeding in the past 6 months, certain medication use, ovulatory disorders, or any history of chronic diseases (e.g. heart disease, diabetes mellitus, etc.), polycystic ovary syndrome (PCOS), or gastrointestinal disease by self-report. Women with self-reported body mass index (BMI) of less than 18 kg/m2 or greater than 35 kg/m2 at baseline were not eligible. The University at Buffalo Health Sciences Institutional Review Board approved the study. All women provided signed informed consent.
Study visits
Participants attended up to eight visits per menstrual cycle corresponding to menstruation, mid and late follicular phase, LH/FSH surge, ovulation, and early, mid, and late luteal phase (20). Participants contacted the clinical center at the first sign of monthly bleeding and came in for the menstruation visit the following day. Subsequent visits were timed by fertility monitors (Clearblue Easy fertility monitor; Inverness Medical, Waltham, MA) that measured both urinary estrone-3-glucuronide and LH (20). Fertility monitoring by the participants began on calendar d 6 of the cycle. Monitor indications of low, high, and peak fertility were used to time midcycle and subsequent visits. Participants were asked to come in to the clinic on the day the monitor indicated “peak fertility” and the 2 d that followed (i.e. corresponding to ovulation). If no indication of peak fertility was given by calendar d 14, a visit was scheduled the next day, and monitoring continued for an additional 10 d. Dates of other visits were scheduled using an algorithm accounting for cycle length and adjusted according to fertility monitor readings of urinary estrone-3-glucuronide and LH levels. Ninety-four percent of women completed at least seven clinic visits per cycle, indicating high compliance to study protocol, with the main reason for fewer visits related to shorter cycle length (20).
Data collection
Extensive demographic and lifestyle information was collected. Information on age, race, education, income, marital status, family history, smoking, and alcohol consumption were self-reported (19). Anthropometric measures were taken by trained personnel. Weight was measured on a balance scale to the nearest quarter pound, and height by stadiometer to the nearest half centimeter. BMI was calculated by dividing weight in kilograms by height in meters squared. Physical activity was measured at baseline by the International Physical Activity Questionnaire (IPAQ), which assessed vigorous and moderate intensity activities related to work, transportation, housework, and leisure time (21). Metabolic equivalents in minutes per week were derived from the responses. Average caloric intake was assessed by four 24-h dietary recalls conducted during menses, the follicular phase, ovulation, and the mid-luteal phase. Total calories were derived using the Nutrition Data System for Research (NDSR; Nutrition Coordinating Center 2005, University of Minnesota, Minneapolis, MN) and were averaged over the four measures per cycle because intake did not significantly vary over the cycle.
Laboratory assays
Overnight 12-h fasting blood was drawn in the morning (0700–0830 h), processed immediately, and frozen until all samples from each cycle were collected (19). Serum tubes remained at room temperature for 20–30 min and were then centrifuged at 1500 × g for 10 min (19). Samples were sent to Kaleida Laboratories in Buffalo, New York, for analysis, with all samples from each participant’s cycle run together in one batch to control for interassay differences. Serum insulin, SHBG, estradiol, progesterone, LH, and FSH were measured using a competitive chemiluminescent enzymatic immunoassay (Immulite 2000; Siemens Medical Solutions Diagnostics, Deerfield, IL). Interassay coefficients of variation of analytes for three level quality control materials were less than 8% for insulin, less than 10% for SHBG, less than 10% for estradiol, less than 14% for progesterone, less than 4% for LH, and less than 4% for FSH at all levels. The corresponding limits of detection (LODs) were less than 2 μIU/ml, less than 2 nmol/liter, less than 20 pg/ml, less than 0.2 ng/ml, less than 0.1 ng/ml, and less than 0.1 mIU/ml, respectively. Fasting plasma glucose was assayed using a hexokinase-based methodology on a Beckman LX20 autoanalyzer (Beckman, Brea, CA), and the interassay coefficient of variation was less than 3%. Insulin resistance was calculated based on the HOMA-IR using the equation: fasting insulin (μU/ml) × fasting glucose (mmol/liter)/22.5 (22).
Statistical analysis
Hormone levels were log-transformed for normality. Data were examined for outliers. Two women were excluded from analysis due to having outlying levels of insulin at more than five visits (one with mostly nondetectable levels, the other with a 10-fold increase in insulin after the first three visits, suggesting nonadherence to the fasting protocol). Of the 4028 measured values, there were 126 (3%) below the LOD for insulin (<2 μIU/ml) and four (0.1%) below the LOD for SHBG (<2 nmol/liter). Descriptive statistics were computed for all study variables using data from the first cycle, and median and interquartile ranges are presented for non-normally distributed variables.
Variability of insulin, HOMA-IR, and glucose over the menstrual cycle was assessed using linear mixed models, taking into account the correlation of repeated measures throughout each cycle. Further adjustment for cycle was taken to account for differences in measures between cycles. Random intercepts were modeled to account for individual variability of baseline hormone levels. Time across the menstrual cycle was modeled using a quadratic term. The quadratic model was found to have the best model fit using the Akaike Information Criterion (AIC) criteria. Significant (P < 0.05) time and time-squared variables were considered as indicators of an effect of menstrual cycle on insulin levels. A model considering time as a categorical variable and adjusting for age, race, and cycle was used to test for day-specific differences, using measures at menses as the reference. Cycle differences in mean hormone values were tested and adjusted for by including cycle in all models. The timing of visits to specific cycle phase with fertility monitors helped standardize participants with different cycle lengths by design so that no additional adjustments for cycle length were made in statistical models. We tested for the interaction between cycle phase and each of the sex hormones and SHBG on their associations with insulin and HOMA-IR to determine whether the associations differed depending on the phase of the menstrual cycle. No significant interactions were detected. Analysis of progesterone was restricted to the luteal phase due to its low variability during the follicular phase.
Due to the time-dependent confounding nature of the sex hormones over the cycle, marginal structural models were used to test the association of each sex hormone (and SHBG) with insulin and HOMA-IR (23,24). Stabilized weights for each cycle visit were created using ordinary least-squares regression for the exposure of interest (e.g. estradiol) adjusting for established risk factors for insulin resistance (i.e. age, race, BMI) and sex hormones (e.g. progesterone, FSH, and LH) for each visit (23). These weights were then applied by inverse probability weighting to linear mixed effects models with random intercepts to provide estimates of the marginal structural model. In separate models, we adjusted for age, race, sex hormones, and SHBG in model 1; then additionally for BMI in model 2; and then adding total caloric intake and physical activity in model 3. Addition of BMI was included due to the strong associations previously documented between SHBG and insulin (25). Sensitivity analyses were conducted excluding 42 anovulatory cycles (by progesterone < 5 ng/ml and no observed serum LH peak on the mid or late luteal phase visit) and 97 cycles missing the last visit. Statistical analyses were conducted using SAS 9.1 (SAS Institute Inc., Cary, NC).
Results
Characteristics of the study population can be seen in Table 1. The women were young (mean age, 27 yr) and mostly Caucasian (60%). Because most of them were college students, 51% had some college education or less, and 75% were not married. Sixty-one percent had a normal BMI between 18.5 and 25 kg/m2, and 36% were overweight or obese with BMI of at least 25 kg/m2. Phase-specific median levels of sex hormones did not substantially differ by cycle (data not shown).
Table 1.
Baseline characteristics of 257 healthy, premenopausal women from the BioCycle study
| Characteristic | n = 257 |
|---|---|
| Age in years (range, 18–44 yr) | 27.3 (8) |
| Cycle length, mean from 2 cycles (d) | 28.8 (4.1) |
| Race, n (%) | |
| White | 153 (60%) |
| Black | 50 (20%) |
| Asian/Pacific Islander | 41 (16%) |
| Other race | 13 (4%) |
| Household income, n (%) | |
| Less than $19,999 | 55 (21%) |
| $20,000–$39,999 | 61 (24%) |
| $40,000–$74,999 | 72 (28%) |
| $75,000–$99,999 | 43 (17%) |
| $100,000 or over | 24 (9%) |
| Missing | 2 (1%) |
| Education, n (%) | |
| High school or less | 32 (12%) |
| Some college | 100 (39%) |
| Bachelor/associate degree | 97 (38%) |
| Graduate program | 28 (11%) |
| Married | 65 (25%) |
| Past smoker, n (%) | 43 (17%) |
| Current smoker, n (%) | 10 (4%) |
| Height (cm) | 164.1 (6) |
| Weight (kg) | 64.8 (11) |
| Body mass index (kg/m2) | 24.1 (4) |
| Underweight, BMI < 18.5 | 9 (3%) |
| Normal, 18.5 ≤ BMI < 25 | 157 (61%) |
| Overweight, 25 ≤ BMI < 30 | 66 (26%) |
| Obese, BMI ≥ 30 | 25 (10%) |
| Physical activity (METs/d) | 1281 (1907) |
| Mean caloric intake (kcal/d) | 1608 (405) |
| Glucose (mg/dl) | 87 (7) |
| Insulin (μU/ml)a | 6 (4–8) |
| HOMA-IRa | 1.3 (0.8–1.8) |
| Follicular SHBG (nmol/liter)a | 43.3 (30.9–61.5) |
All hormone values are from cycle 1. Values are expressed as mean (sd) and are from d 2 unless otherwise indicated. Conversion factors: Glucose, mg/dl × 0.0555 = mmol/liter; insulin, pIU/liter × 6.945 = pmol/liter; estradiol, pg/ml × 3.671 = pmol/liter; progesterone, ng/ml × 3.18 = nmol/liter; FSH mIU/ml × 1.0 = IU/liter; LH, mIU/ml × 1.0 = IU/liter. METs, Metabolic equivalents.
Median (25th–75th percentile) for cycle 1.
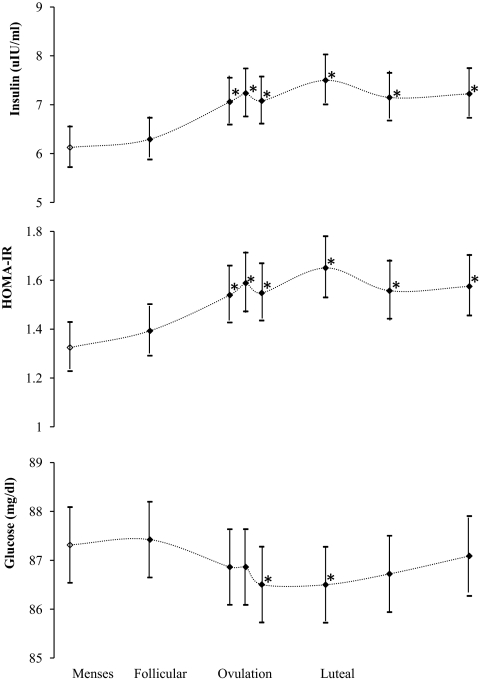
Statistically significant menstrual cycle variation was found for HOMA-IR and insulin levels (Fig. 1). The magnitudes of the variation were small, with maximum age-adjusted mean differences over the whole cycle of 0.3 U of HOMA-IR and 1.4 μIU/ml of insulin (Table 2). The patterns of HOMA-IR for cycles 1 and 2 were similar, although an overall significant increase (6%) in levels was observed during cycle 2. After adjusting for cycle in the statistical models, significant differences were found between the luteal and follicular phases of HOMA-IR primarily due to higher insulin levels in the luteal phase that increased after the onset of ovulation. Levels peaked during ovulation and in the early luteal phase. Glucose levels decreased slightly during ovulation and into the early luteal phase but were otherwise relatively constant throughout the cycle.
Figure 1.
Geometric mean (95% confidence interval) of insulin, HOMA-IR, and glucose levels over the menstrual cycle in the BioCycle Study (n = 257), adjusted for age, race, and cycle. *, Significantly different from reference visit during menstruation (○).
Table 2.
Phase-specific mean levels of insulin, HOMA-IR, glucose, and sex hormones in the BioCycle Study (n = 257)a
| Phase | No. of visits | Insulin (μIU/ml) | HOMA-IR | Glucose (mg/dl) | Mean
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Meana | Pb | Meana | Pb | Mean | Pb | E2 (pg/ml) | P (ng/ml) | LH (ng/ml) | FSH (mIU/ml) | ||
| Menses | 498 | 6.1 | Ref. | 1.3 | Ref. | 87.1 | Ref. | 42 | 0.8 | 4.3 | 6.9 |
| Mid-follicular | 493 | 6.3 | 0.24 | 1.4 | 0.24 | 87.0 | 0.67 | 68 | 0.6 | 5.2 | 7.1 |
| Late follicular | 496 | 7.1 | <0.0001 | 1.5 | <0.0001 | 86.7 | 0.19 | 159 | 1.0 | 13.7 | 7.5 |
| LH/FSH surge | 497 | 7.2 | <0.0001 | 1.5 | <0.0001 | 86.6 | 0.12 | 159 | 1.8 | 16.6 | 8.3 |
| Ovulation | 489 | 7.1 | <0.0001 | 1.5 | <0.0001 | 86.2 | 0.003 | 130 | 3.1 | 13.8 | 7.7 |
| Early luteal | 482 | 7.5 | <0.0001 | 1.6 | <0.0001 | 86.3 | 0.006 | 134 | 7.5 | 9.1 | 5.4 |
| Mid-luteal | 475 | 7.2 | <0.0001 | 1.5 | <0.0001 | 86.5 | 0.05 | 140 | 8.9 | 5.9 | 3.7 |
| Late luteal | 376 | 7.2 | <0.0001 | 1.6 | <0.0001 | 86.9 | 0.56 | 96 | 5.5 | 4.9 | 3.9 |
Phase-specific means are adjusted for age, race, and cycle. E2, Estradiol; P, progesterone.
Geometric means of insulin and HOMA-IR are given due to skewed distribution.
P value for levels at each visit compared to menses levels.
Because significant menstrual cycle variation was observed for insulin levels, we evaluated whether these variations were associated with variability in levels of estradiol, progesterone, LH, FSH, and SHBG (Table 3). In model 1, results were adjusted for age, race, sex hormones, and SHBG using marginal structural models. Estradiol and progesterone were positively associated with insulin and HOMA-IR, whereas FSH was negatively associated. SHBG was significantly inversely associated with insulin (β = −0.73; P < 0.001) and HOMA-IR (β = −0.85; P < 0.001). There was no association between LH and insulin or HOMA-IR. In model 2, additional adjustment for BMI resulted in little change in the associations between the sex hormones and insulin or HOMA-IR. However, the association between SHBG and insulin/HOMA-IR was greatly reduced, suggesting that their associations are predominantly explained by adiposity, due to the fact that HOMA-IR was positively and SHBG was negatively associated with BMI. Additional adjustment in model 3 for mean caloric intake for each cycle and for physical activity did not further change these associations. Glucose was negatively associated with estradiol alone. No significant interaction by BMI was detected (P > 0.05), and results were similar after excluding overweight women (data not shown). These estimates also remained unchanged in sensitivity analyses, excluding the potential anovulatory cycles or excluding cycles missing the last visit (data not shown).
Table 3.
β-coefficients (se) for associations for insulin, HOMA-IR, and glucose with sex hormones and SHBG over the menstrual cycle
| Estradiol | Luteal P | SHBG | LHa | FSHb | |
|---|---|---|---|---|---|
| Insulin | |||||
| Model 1 | 0.086 (0.009) | 0.025 (0.009) | −0.073 (0.03) | 0.011 (0.008) | −0.039 (0.01) |
| Model 2 | 0.088 (0.009) | 0.025 (0.008) | −0.049 (0.03) | 0.010 (0.008) | −0.038 (0.01) |
| Model 3 | 0.088 (0.009) | 0.024 (0.008) | −0.049 (0.03) | 0.007 (0.008) | −0.038 (0.01) |
| HOMA-IR | |||||
| Model 1 | 0.082 (0.009) | 0.025 (0.009) | −0.085 (0.03) | 0.009 (0.009) | −0.040 (0.01) |
| Model 2 | 0.084 (0.009) | 0.025 (0.009) | −0.057 (0.03) | 0.008 (0.009) | −0.038 (0.01) |
| Model 3 | 0.084 (0.009) | 0.024 (0.009) | −0.058 (0.03) | 0.006 (0.009) | −0.038 (0.01) |
| Glucose | |||||
| Model 1 | −0.40 (0.11) | −0.05 (0.13) | −0.65 (0.35) | −0.12 (0.10) | 0.12 (0.10) |
| Model 2 | −0.38 (0.11) | −0.05 (0.13) | 0.63 (0.35) | −0.11 (0.10) | 0.08 (0.15) |
| Model 3 | −0.36 (0.11) | −0.06 (0.13) | 0.64 (0.35) | −0.10 (0.10) | 0.10 (0.15) |
Boldface data correspond to a statistically significant relationship (P < 0.05). All values of insulin, HOMA-IR, sex hormones, and SHBG were log-transformed. P, progesterone. Model 1 = age, race, sex hormones, and SHBG (excluding the exposure of interest). Model 2 = age, race, BMI, sex hormones, and SHBG (excluding the exposure of interest). Model 3 = age, race, BMI, mean calories, physical activity, BMI, sex hormones, and SHBG (excluding the exposure of interest).
FSH excluded from model due to the strong correlation (r = 0.70) around time of ovulation with LH.
LH excluded from model due to the strong correlation (r = 0.70) around time of ovulation with FSH.
Discussion
Our findings demonstrate that insulin and a related measure of insulin resistance (HOMA-IR) change over the menstrual cycle, with levels beginning to rise before ovulation and reaching maximum levels during the luteal phase. These fluctuations across the cycle are most likely explained by the fact that insulin and HOMA-IR were positively associated with estradiol and progesterone, whereas negatively associated with FSH and SHBG. BMI partially accounted for the association between SHBG and insulin but had no influence on the other associations.
Menstrual cycle variability of insulin sensitivity has been tested in previous studies (7,8,9,10,11,12,13,14,15,16). All but one (7) of four studies using iv glucose tolerance testing (IVGTT) found a significant decrease in insulin sensitivity during the luteal phase of the menstrual cycle (7,10,11,14). None of the three studies using euglycemic hyperinsulinemic clamp found any significant differences in glucose metabolism (9,15,16). Two studies investigated the changes using HOMA-IR as an index for insulin resistance and found no differences across the cycle (7,8). Our results follow what has been found for IVGTT, which may suggest that the variability rests in insulin sensitivity. The variation in insulin and HOMA-IR across menstrual cycle was very small and may explain why previous studies of smaller size may not have detected significant change. HOMA-IR has previously been shown to be a valid measure of insulin resistance with strong correlations with measures by euglycemic clamp and by IVGTT (22).
The above studies on the association between sex hormones and insulin sensitivity were limited by the small number of participants and limited sampling of hormone measures to determine the association between estradiol and insulin throughout the menstrual cycle. We found a moderate positive association between insulin and estradiol that was consistent regardless of cycle phase, which is in line with two larger studies (26,27). A study of premenopausal women in Norway (ages, 25–35 yr) demonstrated a positive association between daily salivary free estradiol and insulin levels, but only among women who were taller than average (>170 cm) (26). We found no interaction with height and the positive association with insulin persisted regardless of height (data not shown). A cross-sectional study among postmenopausal women (ages, 45–65 yr) also demonstrated a strong association between insulin resistance and bioavailable estradiol that was independent of BMI and waist circumference (27). The study suggested that the association between estradiol and insulin resistance was not solely due to the shift in location of estradiol production from the ovaries to adipocytes after menopause (27). Our study supports this suggestion, that the positive biological association between estradiol and insulin may not be altered by the reduction of estrogen levels related to the occurrence of menopausal transition, but may rather be exacerbated, perhaps, by changes in adiposity after menopause.
As for the association between progesterone and insulin, our findings contradict a study among premenopausal women that observed a positive correlation between insulin sensitivity by IVGTT and luteal phase progesterone levels (r = 0.44; P = 0.07) (7). However, the association did not reach statistical significance, and evidence from oral contraceptive research has suggested contrary evidence that progesterone, in fact, decreases insulin sensitivity (28). It has been observed that peripheral glucose insensitivity occurs 3–6 months after incident oral contraceptive use (28). In terms of biological mechanism, some recent evidence has found that progesterone may induce insulin resistance through inhibition of insulin signaling in adipocytes (29).
Our findings of the positive associations for estradiol and progesterone with insulin resistance among premenopausal women are inconsistent with studies based on trials of hormone therapy (HT) among postmenopausal women (30). Although both the Heart and Estrogen/Progestin Replacement Study (HERS) and Women’s Health Initiative (WHI) trials of estrogen plus progesterone found that HT lowered the risk of diabetes, the cause for these relationships remains unclear (31,32). Postmenopausal hormone use can change the relationships between sex hormones and metabolic factors (33), which may explain the discrepancy between our findings and these large trials. Contrary to WHI and HERS, a smaller trial of estrogen and progesterone use among women early in the menopause transition found a 17% decrease in insulin sensitivity within 6 months of treatment that was restored 1 yr after treatment was stopped (34). In addition, women with premature ovarian failure placed on HT have observed decreases in insulin sensitivity, which has been attributed to progesterone (35). It has also been suggested that long-term effects of estrogen exposure differ from those of short-term use, with the former perhaps being able to preserve pancreatic function (2).
That the timing of the highest level of insulin resistance did not coincide exactly with levels of both estradiol and progesterone in the late luteal phase may indicate that the two major sex hormones do not lead to additive effects. A previous observation by Lindheim et al. (36) also observed a possible bimodal effect of estrogen. Although this may possibly explain our observation, when we statistically tested for whether the association between insulin and estradiol differed by phase of menstrual cycle, no significant interaction was found, suggesting that the association between insulin and estradiol is consistent. We also further took into account the simultaneous effects of other sex hormones on these measures.
The negative correlation between SHBG and insulin/insulin resistance has been observed in various epidemiological studies (25,37,38). The association between SHBG and insulin in our study was explained mostly by adiposity. That adjustment for the other sex hormones and in particular for estradiol and progesterone, which have significant menstrual cycle variation, did not affect the association between insulin and SHBG may indicate that SHBG may have actions independent of the steroidal sex hormones. However, we did not measure testosterone, whose binding by SHBG has been purported to explain its protective associations on insulin resistance and type 2 diabetes risk (6).
The negative association between endogenous FSH and insulin resistance during the menstrual cycle, to our knowledge, has not been previously observed. However, it follows the evidence that women with insulin resistance as seen in PCOS have lower FSH levels (39).
Our study had some limitations. We lacked information on levels of testosterone, which studies have demonstrated is associated with insulin resistance. However, the measures of SHBG indirectly account for testosterone because SHBG affects the bioavailability of testosterone (40). The use of a surrogate measure of insulin resistance by HOMA-IR was a limitation, but its use has been previously validated, with correlations to insulin sensitivity derived from euglycemic clamp ranging from 0.68 to 0.88 in studies that have included individuals with normal glucose tolerance (22). Nevertheless, these results can only be applied to the fasting state and cannot speak to postprandial glucose metabolism. We also observed small variation in insulin resistance in this cohort of young, healthy women, which may be of minor clinical relevance. Lastly, the pulsatility in the secretion of sex hormones LH and progesterone may have influenced the single day representativeness of our measures. However, we had multiple measures in each phase of the cycle, including three LH measures around the time of ovulation and three luteal phase measures of progesterone at a standardized time of day (i.e. between 0700 and 0900 h).
The strengths of our study include the unique study design with extensive data across two well-characterized menstrual cycles. BioCycle is the largest study to date with repeated measures of insulin and glucose over two menstrual cycles. Serum, rather than urinary, measures of sex hormones were also available. The study included normal menstruating women, rather than women whose insulin resistance may have already been altered by other factors such as PCOS or pregnancy. Moreover, all measurements were timed to the LH surge of the women’s menstrual cycles using fertility monitors, decreasing the possibility of misclassification of cycle phases (20).
In conclusion, our study presents, in the setting of the natural menstrual cycle, small yet significant variations of insulin and HOMA-IR that correspond, in part, to levels of estradiol and progesterone. Moreover, both estradiol and progesterone exhibited positive associations with insulin and insulin resistance, whereas FSH and SHBG exhibited inverse associations. However, the association with SHBG was attenuated and not significant after adjusting for BMI, suggesting that the association might be mediated by adiposity. Although the changes across the cycle may not be clinically meaningful, these findings do suggest that clinical research studies of insulin sensitivity among premenopausal women could be more optimally conducted by timing visits to menstrual cycle phase to reduce the overall variability in measures of insulin sensitivity. Our findings also suggest that in studies of insulin sensitivity or glucose homeostasis among young women, which have now become important due to the obesity epidemic, sex hormones should be considered in the roles they play in regulating insulin.
Acknowledgments
We acknowledge the BioCycle working group for their assistance.
Footnotes
This study was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Disclosure Summary: The authors have nothing to declare.
First Published Online September 15, 2010
Abbreviations: BMI, Body mass index; HOMA-IR, homeostasis model of insulin resistance; HT, hormone therapy; IVGTT, iv glucose tolerance testing; LOD, limit of detection; PCOS, polycystic ovary syndrome.
References
- Ramos RG, Olden K 2008 The prevalence of metabolic syndrome among US women of childbearing age. Am J Public Health 98:1122–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godsland IF 2005 Oestrogens and insulin secretion. Diabetologia 48:2213–2220 [DOI] [PubMed] [Google Scholar]
- Livingstone C, Collison M 2002 Sex steroids and insulin resistance. Clin Sci (Lond) 102:151–166 [DOI] [PubMed] [Google Scholar]
- Cagnacci A, Ferrari S, Tirelli A, Zanin R, Volpe A 2009 Insulin sensitivity and lipid metabolism with oral contraceptives containing chlormadinone acetate or desogestrel: a randomized trial. Contraception 79:111–116 [DOI] [PubMed] [Google Scholar]
- Widom B, Diamond MP, Simonson DC 1992 Alterations in glucose metabolism during menstrual cycle in women with IDDM. Diabetes Care 15:213–220 [DOI] [PubMed] [Google Scholar]
- Ding EL, Song Y, Malik VS, Liu S 2006 Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 295:1288–1299 [DOI] [PubMed] [Google Scholar]
- Bingley CA, Gitau R, Lovegrove JA 2008 Impact of menstrual cycle phase on insulin sensitivity measures and fasting lipids. Horm Metab Res 40:901–906 [DOI] [PubMed] [Google Scholar]
- Blum CA, Müller B, Huber P, Kraenzlin M, Schindler C, De Geyter C, Keller U, Puder JJ 2005 Low-grade inflammation and estimates of insulin resistance during the menstrual cycle in lean and overweight women. J Clin Endocrinol Metab 90:3230–3235 [DOI] [PubMed] [Google Scholar]
- Diamond MP, Jacob R, Connolly-Diamond M, DeFronzo RA 1993 Glucose metabolism during the menstrual cycle. Assessment with the euglycemic, hyperinsulinemic clamp. J Reprod Med 38:417–421 [PubMed] [Google Scholar]
- Escalante Pulido JM, Alpizar Salazar M 1999 Changes in insulin sensitivity, secretion and glucose effectiveness during menstrual cycle. Arch Med Res 30:19–22 [DOI] [PubMed] [Google Scholar]
- González-Ortiz M, Martínez-Abundis E, Lifshitz A 1998 Insulin sensitivity and sex steroid hormone levels during the menstrual cycle in healthy women with non-insulin-dependent diabetic parents. Gynecol Obstet Invest 46:187–190 [DOI] [PubMed] [Google Scholar]
- Jarrett RJ, Graver HJ 1968 Changes in oral glucose tolerance during the menstrual cycle. Br Med J 2:528–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott AR, Macdonald IA, Bowman CA, Jeffcoate WJ 1990 Effect of phase of menstrual cycle on insulin sensitivity, peripheral blood flow and cardiovascular responses to hyperinsulinaemia in young women with type 1 diabetes. Diabet Med 7:57–62 [DOI] [PubMed] [Google Scholar]
- Valdes CT, Elkind-Hirsch KE 1991 Intravenous glucose tolerance test-derived insulin sensitivity changes during the menstrual cycle. J Clin Endocrinol Metab 72:642–646 [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H 1984 Insulin sensitivity during the menstrual cycle. J Clin Endocrinol Metab 59:350–353 [DOI] [PubMed] [Google Scholar]
- Toth EL, Suthijumroon A, Crockford PM, Ryan EA 1987 Insulin action does not change during the menstrual cycle in normal women. J Clin Endocrinol Metab 64:74–80 [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Tobin JD, Andres R 1979 Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237:E214–E223 [DOI] [PubMed] [Google Scholar]
- Bergman RN 1989 Lilly lecture 1989. Toward physiological understanding of glucose tolerance. Minimal-model approach. Diabetes 38:1512–1527 [DOI] [PubMed] [Google Scholar]
- Wactawski-Wende J, Schisterman EF, Hovey KM, Howards PP, Browne RW, Hediger M, Liu A, Trevisan M 2009 BioCycle study: design of the longitudinal study of the oxidative stress and hormone variation during the menstrual cycle. Paediatr Perinat Epidemiol 23:171–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howards PP, Schisterman EF, Wactawski-Wende J, Reschke JE, Frazer AA, Hovey KM 2009 Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: the BioCycle Study. Am J Epidemiol 169:105–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P 2003 International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 35:1381–1395 [DOI] [PubMed] [Google Scholar]
- Wallace TM, Levy JC, Matthews DR 2004 Use and abuse of HOMA modeling. Diabetes Care 27:1487–1495 [DOI] [PubMed] [Google Scholar]
- Cole SR, Hernán MA 2008 Constructing inverse probability weights for marginal structural models. Am J Epidemiol 168:656–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins JM, Hernán MA, Brumback B 2000 Marginal structural models and causal inference in epidemiology. Epidemiology 11:550–560 [DOI] [PubMed] [Google Scholar]
- Bataille V, Perret B, Evans A, Amouyel P, Arveiler D, Ducimetière P, Bard JM, Ferrières J 2005 Sex hormone-binding globulin is a major determinant of the lipid profile: the PRIME study. Atherosclerosis 179:369–373 [DOI] [PubMed] [Google Scholar]
- Finstad SE, Emaus A, Tretli S, Jasienska G, Ellison PT, Furberg AS, Wist EA, Thune I 2009 Adult height, insulin, and 17β-estradiol in young women. Cancer Epidemiol Biomarkers Prev 18:1477–1483 [DOI] [PubMed] [Google Scholar]
- Kalish GM, Barrett-Connor E, Laughlin GA, Gulanski BI 2003 Association of endogenous sex hormones and insulin resistance among postmenopausal women: results from the Postmenopausal Estrogen/Progestin Intervention Trial. J Clin Endocrinol Metab 88:1646–1652 [DOI] [PubMed] [Google Scholar]
- Kasdorf G, Kalkhoff RK 1988 Prospective studies of insulin sensitivity in normal women receiving oral contraceptive agents. J Clin Endocrinol Metab 66:846–852 [DOI] [PubMed] [Google Scholar]
- Wada T, Hori S, Sugiyama M, Fujisawa E, Nakano T, Tsuneki H, Nagira K, Saito S, Sasaoka T 2010 Progesterone inhibits glucose uptake by affecting diverse steps of insulin signaling in 3T3–L1 adipocytes. Am J Physiol Endocrinol Metab 298:E881–E888 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Howard BV, Cowan LD, Yeh J, Schaefer CF, Wild RA, Wang W, Lee ET 2002 The effect of estrogen use on levels of glucose and insulin and the risk of type 2 diabetes in American Indian postmenopausal women: the Strong Heart Study. Diabetes Care 25:500–504 [DOI] [PubMed] [Google Scholar]
- Kanaya AM, Herrington D, Vittinghoff E, Lin F, Grady D, Bittner V, Cauley JA, Barrett-Connor E; Heart and Estrogen/progestin Replacement Study 2003 Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/progestin Replacement Study. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 138:1–9 [DOI] [PubMed] [Google Scholar]
- Margolis KL, Bonds DE, Rodabough RJ, Tinker L, Phillips LS, Allen C, Bassford T, Burke G, Torrens J, Howard BV; Women’s Health Initiative Investigators 2004 Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia 47:1175–1187 [DOI] [PubMed] [Google Scholar]
- Sowers MR, Randolph Jr J, Jannausch M, Lasley B, Jackson E, McConnell D 2008 Levels of sex steroid and cardiovascular disease measures in premenopausal and hormone-treated women at midlife: implications for the “timing hypothesis.” Arch Intern Med 168:2146–2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sites CK, L'Hommedieu GD, Toth MJ, Brochu M, Cooper BC, Fairhurst PA 2005 The effect of hormone replacement therapy on body composition, body fat distribution, and insulin sensitivity in menopausal women: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab 90:2701–2707 [DOI] [PubMed] [Google Scholar]
- Elkind-Hirsch KE, Sherman LD, Malinak R 1993 Hormone replacement therapy alters insulin sensitivity in young women with premature ovarian failure. J Clin Endocrinol Metab 76:472–475 [DOI] [PubMed] [Google Scholar]
- Lindheim SR, Presser SC, Ditkoff EC, Vijod MA, Stanczyk FZ, Lobo RA 1993 A possible bimodal effect of estrogen on insulin sensitivity in postmenopausal women and the attenuating effect of added progestin. Fertil Steril 60:664–667 [DOI] [PubMed] [Google Scholar]
- Haffner SM, Katz MS, Stern MP, Dunn JF 1989 Association of decreased sex hormone binding globulin and cardiovascular risk factors. Arteriosclerosis 9:136–143 [DOI] [PubMed] [Google Scholar]
- Golden SH, Dobs AS, Vaidya D, Szklo M, Gapstur S, Kopp P, Liu K, Ouyang P 2007 Endogenous sex hormones and glucose tolerance status in postmenopausal women. J Clin Endocrinol Metab 92:1289–1295 [DOI] [PubMed] [Google Scholar]
- Stener-Victorin E, Holm G, Labrie F, Nilsson L, Janson PO, Ohlsson C 2010 Are there any sensitive and specific sex steroid markers for polycystic ovary syndrome? J Clin Endocrinol Metab 95:810–819 [DOI] [PubMed] [Google Scholar]
- Corbould A 2008 Effects of androgens on insulin action in women: is androgen excess a component of female metabolic syndrome? Diabetes Metab Res Rev 24:520–532 [DOI] [PubMed] [Google Scholar]