Abstract
Context: Hemoglobin A1c (A1c) was recently added to the diagnostic criteria for diabetes and prediabetes.
Objective: Our objective was to examine performance of A1c in comparison with fasting plasma glucose (FPG) in diagnosing dysglycemia in older adults.
Design and Setting: We conducted a cross-sectional analysis of data from the Health, Aging, and Body Composition study at yr 4 (2000–2001) when FPG and standardized A1c measurements were available.
Participants: Of 3075 persons (aged 70–79 yr, 48% men, 42% Black) at study entry, 1865 participants without known diabetes who had appropriate measures were included.
Main outcome measures: Sensitivity and specificity of A1c-based diagnoses were compared with those based on FPG and the proportion of participants identified with dysglycemia by each measure.
Results: Of all participants, 2.7 and 3.1% had undiagnosed diabetes by FPG ≥126 mg/dl and A1c ≥6.5%, respectively. Among the remaining participants, 21.1% had prediabetes by impaired fasting glucose (≥100 mg/dl) and 22.2% by A1c ≥5.7%. Roughly one third of individuals with diabetes and prediabetes were identified by either FPG or A1c alone and by both tests simultaneously. Sensitivities and specificities of A1c compared with FPG were 56.9 and 98.4% for diabetes and 47.0 and 84.5% for prediabetes, respectively. Blacks and women were more likely to be identified with dysglycemia by A1c than FPG.
Conclusions: In this older population, we found considerable discordance between FPG- and A1c-based diagnosis of diabetes and prediabetes, with differences accentuated by race and gender. Broad implementation of A1c to diagnose dysglycemic states may substantially alter the epidemiology of these conditions in older Americans.
There is considerable discordance between fasting plasma glucose and A1c-based diagnoses of diabetes and pre-diabetes in older adults with differences accentuated by race and sex.
After careful consideration of the International Expert Committee Report (1), the American Diabetes Association (ADA) recently endorsed new diagnostic criteria for diabetes mellitus and prediabetes (2). The ADA now recommends the use of either fasting plasma glucose (FPG) of 126 mg/dl or higher, 2-h plasma glucose (PG) during an oral glucose tolerance test (OGTT) of 200 mg/dl or higher, or the new criterion of hemoglobin A1c (A1c) of 6.5% or higher for diagnosis of diabetes. To identify individuals at risk for diabetes (referred to hereafter as prediabetes), the ADA’s revised criteria are similarly based upon milder abnormalities in FPG of 100–125 mg/dl [impaired fasting glucose (IFG)], OGTT 2-h PG of 140–199 mg/dl [impaired glucose tolerance (IGT)], or A1c of 5.7–6.4%.
Although A1c is an integrated measure of average glycemia and has some practical and technical advantages over direct measures of glucose, it may identify different individuals with and at risk for diabetes. As a result, if widely implemented, it may to some degree alter the current epidemiological landscape of these dysglycemic states. The diagnostic use of A1c may have a particularly significant impact on older adults, especially those of non-Caucasian race. For example, data from the National Health and Nutrition Examination Survey and the Framingham Offspring Study suggest that A1c values increase with age even after adjustments for demographic and glycemic variables among nondiabetic subjects (3). A1c values have also been reported to be significantly higher among Blacks with IGT even after adjustment for various factors known to correlate with ambient glycemia (4). These differences may reflect higher underlying glucose levels, such as in the postprandial setting, differences in actual glycation rates at a given glucose concentration, or differences in the duration of hemoglobin exposure to glucose.
Given the expected increased use of the more convenient A1c as a screening tool to identify patients with diabetes and prediabetes, we examined a well-established biracial cohort of older Americans to assess how well A1c performs, using the recently proposed cut-points, as compared with FPG, which has essentially been the practice standard for diagnosing these conditions in the United States. To our knowledge, this is the first time this question has been specifically addressed in the elderly using a National Glycohemoglobin Standardization Program (NGSP)-certified A1c assay.
Subjects and Methods
Participants were from the Health ABC Study, an ongoing prospective cohort investigation of changes in body composition as a common pathway by which multiple diseases contribute to disability. Participants (n = 3075, 48.4% male, 41.6% Black, aged 70–79 yr) were recruited in 1997–1998 from Pittsburgh, PA, and Memphis, TN, using procedures described in an earlier report (5). A telephone interview determined eligibility using the following inclusion criteria: no difficulty in walking a quarter of a mile (400 m), climbing 10 steps, or performing mobility activities of daily living; no current diagnosis of life-threatening cancers requiring active treatment within the past 3 yr; and plans to remain within the study area for at least 3 yr. Participants provided informed consent before examinations, and the study was approved by institutional review boards at the University of Pittsburgh and the University of Tennessee Health Science Center.
An NGSP-certified A1c assay using modern chromatographic separation techniques was assessed for the first time at the 2000–2001 follow-up visit. Of the 3075 participants at baseline, 99 missed the 2000–2001 visit, 14 withdrew, and 187 had died. Individuals with home, telephone, or proxy visits (n = 370) were excluded because fasting blood work was not available. Furthermore, 439 persons were excluded based on a self-reported diagnosis of diabetes or use of antihyperglycemic medication (based on coding of medications from the previous 2 wk that were brought to the clinic for inspection). Of the 1966 available participants without known diabetes, 101 participants had missing FPG or A1c or missing information with respect to a previous diagnosis of diabetes, leaving 1865 participants (94.9%) in the analytic sample, which included 612 White men, 263 Black men, 621 White women, and 369 Black women.
Laboratory measurement of FPG and A1c
A1c was measured using Tosoh 2.2 Plus (Tosoh Bioscience Inc., Tokyo, Japan), a fully automated glycosylated hemoglobin analyzer that uses nonporous ion-exchange HPLC for separation of A1c, with any labile glycohemoglobin subfractions chromatographically separated from the Schiff base. Hemoglobin was analyzed for the presence of hemoglobin S and F variants; performance of the A1c assay was affected only in the presence of sickle cell disease (not trait), in which case the A1c measure was reported as missing. The A1c assay was NGSP certified and standardized to the Diabetes Control and Complications Trial (DCCT) assay method. FPG was measured after at least 8 h of fasting using the automated glucose oxidase method (VITROS system; Ortho Clinical Diagnostics, Rochester, NY).
Other measures
In addition to age, race, and sex, self-reported comorbidities, including hypertension and cardiovascular disease, were assessed at the 2000–2001 visit. Metabolic syndrome was based upon standard National Cholesterol Education Program Adult Treatment Panel III criteria (6) using data from the baseline (1997–1998) assessment, because the relevant information was not collected at the subsequent 2000–2001 visit.
Definition of diabetes and prediabetes
Participants were classified according to two measures used to define diabetes, namely FPG ≥126 mg/dl, the current practice standard, and A1c ≥6.5%, as now endorsed by the ADA (2). For identification of prediabetes, the range of increased glycohemoglobin (IGH) (A1c 5.7–6.4%) as recommended by the ADA was compared with the traditional FPG-based diagnosis of impaired fasting glucose (FPG 100–125 mg/dl).
Statistical analysis
Median A1c and FPG levels were compared across groups defined on the basis of race and gender using nonparametric Wilcoxon rank sum tests because the data were not normally distributed. To test whether levels of A1c and FPG were correlated with age and body mass index, we used the nonparametric Spearman’s ρ correlation coefficient.
Participants were classified according to FPG- and A1c-based cut-points for diabetes, thus defining four mutually exclusive groups (participants meeting both, neither, and only one of the criteria). This process was repeated for the prediabetes cut-points. Sensitivity, specificity, and positive and negative predictive values were calculated for abnormal A1c results, as compared with abnormal FPG results, using the respective diagnostic thresholds for diabetes and prediabetes. Venn diagrams were then constructed as a visual display of concordance/discordance between FPG- and A1c-based classification of participants. Receiver operator characteristics (ROC) curves were generated to further analyze the relationships between the diagnostic tests.
To determine whether the association between FPG- and A1c-based diagnoses for diabetes and prediabetes differed across race and sex, we performed stratified analyses using the Breslow-Day statistic to test for homogeneity of effects. Finally, to assess how participants diagnosed with diabetes solely on the basis of A1c (but not FPG) differ from those traditionally identified by FPG-based standards, we performed one-sample χ2 tests. Specifically, we calculated the proportions of Black and White participants in the sample of participants identified by the FPG-based criterion. We used these proportions to specify the null hypothesis in a one-sample χ2 test applied to the participants identified by A1c-based criterion alone. We repeated this procedure for men and women and then performed analogous analyses for prediabetes. In this manner, we could compare participants who would be newly identified by A1c testing vs. those currently being identified using the conventional screening approach of FPG. All analyses were performed using SAS statistical software (version 9.2; SAS Institute, Cary, NC).
Results
Table 1 provides information on demographic characteristics, relevant comorbid conditions, and A1c levels for all participants, including those with normoglycemia, those with prediabetes by either measure, and similarly those with diabetes by either measure. Overall, the median A1c and the interquartile range (IQR) was 5.3% (IQR 5.1–5.7), with higher levels in Blacks (median A1c 5.5%, IQR 5.2–5.8) than Whites (5.3%, IQR 5.1–5.5; P < 0.001), but comparable levels among men and women (5.3%, IQR 5.1–5.7 and 5.3%, IQR 5.1–5.6, respectively; P = 0.84). Median FPG level was 92.0 mg/dl (IQR 87–99) and did not differ across race [median FPG 92.0 mg/dl (IQR 86–99) in Whites, 92.0 (IQR 87–99) in Blacks; P = 0.63] but was higher in men (94.0 mg/dl, IQR 88–102) than in women (90.0 mg/dl, IQR 85–97; P < 0.001). Both A1c and FPG increased with body mass index (Spearman ρ = 0.19 and 0.25, respectively, P < 0.001) but not with age (Spearman ρ = 0.04 and 0.02, P = 0.40 and 0.09, respectively) within this group of individuals whose age range was relatively narrow (sd <3 yr).
Table 1.
Participant characteristics at the 2000–2001 Health ABC examination
| All participants | Normoglycemia by FPG and A1c (FPG <100 mg/dl and A1c <5.7%) | Prediabetes by FPG or A1c (FPG 100–125 mg/dl or A1c 5.7–6.4%) | Diabetes by FPG or A1c (FPG ≥126 mg/dl or A1c ≥6.5%) | |
|---|---|---|---|---|
| n | 1865 | 1189 | 596 | 80 |
| Demographics | ||||
| Age (mean ± sd) | 76.5 ± 2.9 | 76.4 ± 2.8 | 76.7 ± 2.9 | 76.4 ± 3.0 |
| Sex (% female) | 53.1 | 56.9 | 46.6 | 45.0 |
| Race (% White) | 66.1 | 71.9 | 57.4 | 45.0 |
| Body mass index (kg/m2) (mean ± sd) | 27.1 ± 4.8 | 26.3 ± 4.5 | 28.2 ± 5.0 | 30.3 ± 5.6 |
| Comorbidities (%) | ||||
| Hypertension | 59.2 | 54.0 | 67.3 | 76.3 |
| Metabolic syndrome | 30.1a | 24.6a | 37.1a | 60.0a |
| CVD (stroke, CHD) | 21.2 | 18.3 | 26.9 | 22.5 |
| Laboratory measures | ||||
| A1c (%) | ||||
| Median (IQR) | 5.3 (5.1–5.7) | 5.2 (5.0–5.4) | 5.7 (5.5–5.9) | 6.7 (6.3–7.2) |
| Mean ± sd | 5.4 ± 0.6 | 5.2 ± 0.3 | 5.7 ± 0.4 | 7.0 ± 1.2 |
| FPG (mg/dl) | ||||
| Median (IQR) | 92.0 (87–99) | 89.0 (84–93) | 102.0 (96–107) | 129 (121–140) |
| Mean ± sd | 94.6 ± 14.7 | 88.4 ± 6.1 | 101.5 ± 9.0 | 135.5 ± 35.5 |
CHD, Coronary heart disease; CVD, cardiovascular disease.
Evaluated at 1997–1998 baseline evaluation only.
Diagnosis of diabetes
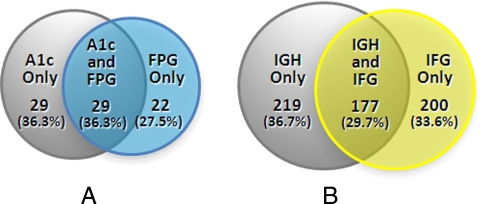
Of the 1865 participants without a history of diabetes, 1785 (95.7%) had both A1c below 6.5% and FPG below 126 mg/dl; 51 (2.7%) had undiagnosed diabetes based upon FPG ≥126 mg/dl, and 58 (3.1%) had undiagnosed diabetes based upon A1c ≥6.5%. Of those found to have diabetes by either FPG or A1c (n = 80), roughly equal numbers were identified solely by one method or simultaneously by both: 27.5% (n = 22) only by FPG, 36.3% (n = 29) only by A1c, and 36.3% (n = 29) by both methods (Fig. 1A).
Figure 1.
Venn Diagrams for diabetes (A) and prediabetes (B). A, Individuals meeting criteria for diabetes (A1c ≥6.5%, FPG ≥126 mg/dl); B, individuals meeting criteria for prediabetes (IGH: A1c 5.7 to <6.5%; IFG: FPG 100 to <126 mg/dl).
Table 2 shows that, when using FPG ≥126 mg/dl as the reference standard for diabetes, an A1c ≥6.5% demonstrated a sensitivity of 56.9%, specificity of 98.4%, positive predictive value (PPV) of 50.0%, and negative predictive value (NPV) of 98.8%. The area under the curve (AUC) for the ROC analysis with A1c as a continuous variable was 0.93 (P < 0.001); the AUC corresponding to the 6.5% cut-point was 0.78 (P < 0.001). The cut-point that optimized both sensitivity (84.3%) and specificity (91.7%) yielded an AUC of 0.88 (P < 0.001) and corresponded to an A1c of 6.0%, well below the newly endorsed target. Because of the relatively low overall prevalence of undiagnosed diabetes in this cohort, the PPV at this threshold was very low at 22.2%.
Table 2.
Diagnostic test characteristics for A1c cut-points using FPG as the gold standard
| % | 95% Confidence intervals (%) | |
|---|---|---|
| Diabetes (A1c ≥6.5%) | ||
| Sensitivity | 56.9 | 42.2–70.7 |
| Specificity | 98.4 | 97.7–98.9 |
| PPV | 50.0 | 36.6–63.4 |
| NPV | 98.8 | 98.2–99.2 |
| Prediabetes (A1c ≥5.7 and <6.5%) | ||
| Sensitivity | 47.0 | 41.8–52.1 |
| Specificity | 84.5 | 82.5–86.3 |
| PPV | 44.7 | 39.7–49.7 |
| NPV | 85.6 | 83.6–87.4 |
Gold standard for diabetes is defined as FPG ≥126 mg/dl and for prediabetes as FPG ≥100 and less than 126 mg/dl.
Analyses stratified by race revealed that Black participants were more likely to be diagnosed with diabetes by A1c than by FPG (5.7% by A1c and 3.5% by FPG) as compared with White participants (1.8% by A1c and 2.4% by FPG; Breslow-Day test P = 0.05). Analyses stratified by sex revealed a similar proportion of men identified by the A1c- and FPG-based criterion (3.5 and 3.8%, respectively) but a slightly higher proportion of women diagnosed by A1c than FPG (2.7% by A1c and 1.8% by FPG). The association between A1c- and FPG-based cut-points did not significantly differ according to gender, however (Breslow-Day test P = 0.26). Among participants identified as having diabetes based on FPG (i.e. the conventional approach), 56.9% were White and 64.7% were male. In contrast, participants identified as having diabetes based on A1c (but not FPG), i.e. those to be newly identified if A1c becomes the new standard test, were 75.9% Black and 62.1% female (P < 0.001 and P = 0.002 for one-sample χ2 tests, respectively).
Identification of prediabetes
There were 1785 participants without a history of diabetes who also did not meet either diagnostic criterion for diabetes (FPG <126 mg/dl and A1c <6.5%). Of these, 377 (21.1%) had IFG and 396 (22.2%) had IGH. Figure 1B shows that among 596 participants with IFG or IGH, roughly comparable proportions were identified by FPG only (33.6%, n = 200), A1c only (36.7%, n = 219), and by both tests (29.7%, n = 177).
IGH demonstrated sensitivity of 47.0%, specificity of 84.5%, PPV of 44.7%, and NPV of 85.6% in identifying IFG (Table 2). ROC curve analysis with A1c as a continuous variable resulted in an AUC of 0.72 (P < 0.001.) An A1c cut-point of 5.7% yielded an AUC of 0.66 (P < 0.001). The optimal cut-point fell at A1c of 5.6%, with an AUC of 0.67 (P < 0.001), but this corresponded to a relatively low PPV of 40.6%.
Stratified analyses by race showed interesting trends. Black participants were more likely to meet the diagnostic criteria for prediabetes based upon A1c, whereas White participants were more likely to meet them based upon FPG. Specifically, among Blacks, 36.6% met criteria for IGH and 19.1% for IFG; among Whites, 15.1% had IGH and 22.1% had IFG (Breslow-Day test P = 0.08). Similar trends were noted in the stratified analyses by gender; 22.1% of men were identified with prediabetes based upon A1c and 27.6% by FPG, and among women, 22.2% were identified by A1c and 15.5% by FPG (Breslow-Day test P = 0.08). When considering only the participants identified with IFG, we found they were 70.3% White and 60.7% male. In contrast, participants identified with prediabetes by A1c (i.e. with IGH but not IFG), constituting a group newly identified to be at risk if A1c testing becomes the standard, were 64.8% Black and 59.4% female (P < 0.001 for both one-sample χ2 tests).
Discussion
In this study, we found significant discordance in identifying older individuals with diabetes and prediabetes using the newly endorsed screening test of A1c and the traditional practice standard of FPG. Although the overall number of individuals identified with new diabetes was similar when the A1c or FPG criterion was applied, appreciably different groups of individuals were classified using each method. Similarly, the number of older persons identified with prediabetes was comparable when either IFG or the new A1c range (5.7–6.4%) endorsed by the ADA was applied, but again somewhat different groups were identified by each test. Upon further analysis, older Americans classified with diabetes and, to a lesser extent, prediabetes on the basis of A1c were more likely to be female and Black than those identified by the FPG-based criterion.
Obtaining more than one type of screening test simultaneously for diabetes is not currently recommended (indeed, the International Expert Committee specifically counseled against such an approach). However, given the pervasiveness of comprehensive metabolic panels, which are often conducted in the fasting state, it is likely that clinicians will have ready access to both FPG and A1c in a substantial fraction of their patients. These practitioners will be faced with an interpretive challenge, however, if the results of A1c and FPG testing happen to be discordant. Based on the recently updated ADA guidelines, in the context of a diabetic A1c but a nondiagnostic FPG (or vice versa), the diagnosis of diabetes remains established (so long as the abnormal test is repeated and confirmed) (2). In this context, and based upon our results, the diagnostic rate for diabetes may increase by approximately 50% in an older population if both FPG and A1c are analyzed. Notably, older persons already bear a disproportionate burden of this disease (7). However, because there are few studies on the optimal management of hyperglycemia in this group, the impact of diagnosing more elders with diabetes is not known, particularly in those with relatively mild glucose elevations. These concerns are heightened by recent large, randomized clinical trials indicating a lack of overall mortality benefit when older adults are submitted to aggressive antihyperglycemic drug regimens (8,9,10).
In general, the identification of individuals at risk for developing diabetes is important because lifestyle interventions clearly prevent or delay the onset of frank hyperglycemia and, potentially, related morbidities (11). The risk for diabetes increases gradually and linearly with rising FPG and A1c without a clear threshold effect (12,13). Therefore, any diagnostic cut-point for prediabetes will necessarily be arbitrary, as might also be said for the accepted lower boundaries for those measures predicated on glucose itself. The 5.7% cut-point for A1c was chosen in part to maximize overlap with the standard definitions of prediabetes, namely IGT and IFG. However, our results suggest that the switch to an A1c-based criterion will result in different individuals being identified as high risk for progression to diabetes, particularly with regards to race. Based on current evidence, the optimal approach to (and, moreover, the need to) identify older adults most likely to develop diabetes remains unclear.
Although A1c has many advantages over fasting glucose (including greater convenience, greater preanalytical stability, and equal if not superior correlation with microvascular complications) (1,2), there are certain limitations to its use, including its less than perfect correlation with recently prevalent glycemia (14). Indeed, A1c reflects the nonenzymatic modification of the amino acids on hemoglobin and depends not only on ambient glucose concentrations but also upon red blood cell turnover as well as glycation rates. There are multiple situations in which glucose concentration may not be correctly reflected in the A1c, such as hemoglobinopathies, iron-deficiency anemia, and chronic kidney disease (15). In addition, and germane to this report, several investigations have demonstrated that A1c concentration increases with age (16,17,18,19,20,21,22) and is higher in Blacks (4,23,24), even after adjustments for other glycemic measures (4,24). The reasons for these discrepancies are not known, but heritable differences in intra-erythrocytic glycation as well as red cell survival due to hemoglobin variants, blood disorders, and renal disease may play important roles (24,25,26). Other factors, such as socioeconomic status and analytic variation in the A1c assay are also possible mediators, although adjustment for these in some studies did not eliminate the discrepancies (4,24). Potential concerns have therefore been raised about whether A1c can accurately reflect mean plasma glucose in ethnically diverse and aging populations.
Because A1c may be higher in older, non-Caucasian individuals independent of their FPG (4,16,17,18,19,20,21,22,23,24), it is not surprising that we found discordance of these two measures in the diagnosis of diabetes among the Health ABC participants. Several recent reports have compared the performance of A1c to FPG in various populations and have found significant discordance (27,28,29,30,31). Although the studies included older individuals, none specifically focused on this group. Together, these studies add to the concerns about the sensitivity of the A1c compared with glucose-based standards that were raised even before ADA adopted this criterion (32). The only available data specifically examining the diagnostic performance of A1c in older adults come from the Rancho Bernardo study (33), where 85% of participants with A1C of 6.5% or higher were not classified as having diabetes by the conventional ADA criteria, despite having both FPG and 2-h PG data available. Although it also raised concerns regarding the use of A1c in older adults, it was initiated in 1984 and therefore did not use an NGSP-certified, DCCT-standardized A1c assay. Accordingly, it is possible that at least part of the discrepancy may be inherent in this older diagnostic test.
Our study has several strengths including a well-established cohort of older adults, a biracial sample allowing for optimal examination of racial differences in A1c performance, and rigorously standardized A1c measures. We also benefited from Health ABC’s standardized protocols and strict quality control of data collection and laboratory procedures. Important limitations include the availability of only single measures of FPG and A1c, which may have led us to overestimate the prevalence of undiagnosed diabetes and prediabetes. Our relatively small sample size limited our ability to perform multivariable analyses to examine the differences in characteristics of participants meeting the different diabetes criteria. Also, because OGTT results were not available, we could not evaluate how well A1c performed compared with 2-h PG. However, this test is not routinely performed in the United States, and any comparison with A1c may have less clinical relevance than the more clinically relevant comparison addressed in this report. Finally, our results may not be generalizable to Hispanic- and Asian-Americans, because they were not enrolled in the Health ABC Study.
This is the first report of the diagnostic performance of A1c testing in comparison with the practice standard of FPG in older persons using an NGSP-certified, DCCT-standardized A1c assay. We conclude that, in older adults, significant discordance exists between A1c- and FPG-based diagnoses of diabetes and the identification of prediabetes. Moreover, women and Blacks are more likely diagnosed with diabetes or prediabetes by A1c as compared with FPG. Sole reliance on A1c and even combined interpretation of concurrent A1c and FPG tests will likely have a significant impact on the landscape of dysglycemic states among the elderly.
Finally, the optimal screening test for diabetes (and prediabetes) remains uncertain. A recent report suggests that A1c is similarly associated with the risk for diabetes as FPG but outperforms the latter in its prediction of future cardiovascular death (34). To settle this controversy, additional long-term longitudinal studies are needed, especially those that include a large sample of older adults. These investigations will need to carefully assess the comparative ability of individual tests to predict the risk of progressive hyperglycemia in prediabetes and the risk of microvascular and macrovascular complications once diabetes is established. The overall importance of identifying dysglycemic states within an aging population remains to be clarified.
Footnotes
The Health ABC Study has been supported by the National Institute on Aging (NIA), contract numbers N01-AG-6-2106, N01-AG-6-2101, and N01-AG-6-2103. This work was also supported in part by funding from the Claude D. Pepper Older Americans Independence Center at the Yale University School of Medicine (2P30AG021342) and in part by the Intramural Research Program of the National Institutes of Health NIA. T.M.G. is the recipient of an NIA Midcareer Investigator Award in Patient-Oriented Research (K24AG021507).
Disclosure Summary: S.E.I. served on the Advisory Panel for Amylin Pharmaceuticals, Merck, and Takeda; received research support from Medtronic MiniMed and honoraria from Novo Nordisk; consulted for Daiichi-Sankyo; and was an expert witness for Eli Lilly. Other authors report no potential conflicts of interests.
First Published Online September 22, 2010
For editorial see page 5203
Abbreviations: A1c, Hemoglobin A1c; AUC, area under the curve; DCCT, Diabetes Control and Complications Trial; FPG, fasting plasma glucose; IFG, impaired fasting glucose; IGH, increased glycohemoglobin; IGT, impaired glucose tolerance; IQR, interquartile range; NGSP, National Glycohemoglobin Standardization Program; NPV, negative predictive value; OGTT, oral glucose tolerance test; PG, plasma glucose; PPV, positive predictive value; ROC, receiver operator characteristics.
References
- International Expert Committee 2009 International Expert Committee report on the role of the A1c assay in the diagnosis of diabetes. Diabetes Care 32:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association 2010 Diagnosis and classification of diabetes mellitus. Diabetes Care 33(Suppl 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani LN, Korenda L, Meigs JB, Driver C, Chamany S, Fox CS, Sullivan L, D'Agostino RB, Nathan DM 2008 Effect of aging on A1c levels in individuals without diabetes. Diabetes Care 31:1991–1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman WH, Ma Y, Uwaifo G, Haffner S, Kahn SE, Horton ES, Lachin JM, Montez MG, Brenneman T, Barrett-Connor E 2007 Diabetes Prevention Program Research Group. Differences in A1c by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care 30:2453–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strotmeyer ES, de Rekeneire N, Schwartz AV, Faulkner KA, Resnick HE, Goodpaster BH, Shorr RI, Vinik AI, Harris TB, Newman AB 2008 The relationship of reduced peripheral nerve function and diabetes with physical performance in older white and Black adults. Diabetes Care 31:1767–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford ES, Giles WH, Dietz WH 2002 Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 287:356–359 [DOI] [PubMed] [Google Scholar]
- Selvin E, Coresh J, Brancati FL 2006 The burden and treatment of diabetes in elderly individuals in the U.S. Diabetes Care 29:2415–2419 [DOI] [PubMed] [Google Scholar]
- Gerstein HC, Miller ME, Byington RP, Goff Jr DC, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm Jr RH, Probstfield JL, Simons-Morton DG, Friedewald WT 2008 Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F 2008 Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358:2560–2572 [Google Scholar]
- Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD 2009 Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 360:129–139 [DOI] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM; Diabetes Prevention Program Research Group 2002 Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droumaguet C, Balkau B, Simon D, Caces E, Tichet J, Charles MA, Eschwege E, the DESIR Study Group 2006 Use of HbA1c in predicting progression to diabetes in French men and women: data from an Epidemiological Study on the Insulin Resistance Syndrome (DESIR). Diabetes Care 29:1619–1625 [DOI] [PubMed] [Google Scholar]
- Edelman D, Olsen MK, Dudley TK, Harris AC, Oddone EZ 2004 Utility of hemoglobin A1c in predicting diabetes risk. J Gen Intern Med 19:1175–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomgarden ZT, Inzucchi SE, Karnieli E, Le Roith D 2008 The proposed terminology ’A(1c)-derived average glucose’ is inherently imprecise and should not be adopted. Diabetologia 51:1111–1114 [DOI] [PubMed] [Google Scholar]
- Saudek CD, Derr RL, Kalyani RR 2006 Assessing glycemia in diabetes using self-monitoring blood glucose and hemoglobin A1c. JAMA 295:1688–1697 [DOI] [PubMed] [Google Scholar]
- Carrera T, Bonamusa L, Almirall L, Navarro JM 1998 Should age and sex be taken into account in the determination of HbA1c reference range? Diabetes Care 21:2193–2194 [DOI] [PubMed] [Google Scholar]
- Simon D, Senan C, Garnier P, Saint-Paul M, Papoz L 1989 Epidemiological features of glycated haemoglobinA1c distribution in a healthy population: the Telecom Study. Diabetologia 32:864–869 [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Futamura A, Ikushima M 1995 Effect of aging on HbA1c in a working male Japanese population. Diabetes Care 18:1337–1340 [DOI] [PubMed] [Google Scholar]
- Yang YC, Lu FH, Wu JS, Chang CJ 1997 Age and sex effects on HbA1c: a study in a healthy Chinese population. Diabetes Care 20:988–991 [DOI] [PubMed] [Google Scholar]
- Nuttall FQ 1999 Effect of age on percentage of hemoglobin A1c and the percentage of total glycohemoglobin in non-diabetic persons. J Lab Clin Med 134:451–453 [DOI] [PubMed] [Google Scholar]
- Nakashima K, Nishizaki O, Andoh Y 1993 Acceleration of hemoglobin glycation with aging. Clin Chim Acta 215:111–118 [DOI] [PubMed] [Google Scholar]
- Kilpatrick ES, Dominiczak MH, Small M 1996 The effects of ageing on glycation and the interpretation of glycaemic control in type 2 diabetes. QJM 89:307–312 [DOI] [PubMed] [Google Scholar]
- Kirk JK, D'Agostino Jr RB, Bell RA, Passmore LV, Bonds DE, Karter AJ, Narayan KM 2006 Disparities in HbA1c levels between African Americans and non-Hispanic white adults with diabetes: a meta-analysis. Diabetes Care 29:2130–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman WH, Dungan KM, Wolffenbuttel BH, Buse JB, Fahrbach JL, Jiang H, Martin S 2009 Racial and ethnic differences in mean plasma glucose, hemoglobin A1c, and 1,5-anhydroglucitol in over 2000 patients with type 2 diabetes. J Clin Endocrinol Metab 94:1689–1694 [DOI] [PubMed] [Google Scholar]
- Cohen RM 2007 A1C: does one size fit all? Diabetes Care 30:2756–2758 [DOI] [PubMed] [Google Scholar]
- Cohen RM, Snieder H, Lindsell CJ, Beyan H, Hawa MI, Blinko S, Edwards R, Spector TD, Leslie RD 2006 Evidence for independent heritability of the glycosylation gap fraction of HbA1c in non-diabetic twins. Diabetes Care 29:1739–1743 [DOI] [PubMed] [Google Scholar]
- Selvin E, Zhu H, Brancati FL 2009 Elevated A1C in adults without a history of diabetes in the U.S. Diabetes Care 32:828–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson AP, Reynolds K, Fonseca VA, Muntner P 2010 Comparison of A1C and fasting glucose criteria to diagnose diabetes among U.S. adults. Diabetes Care 33:95–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie CC, Rust KF, Byrd-Holt DD, Gregg EW, Ford ES, Geiss LS, Bainbridge KE, Fradkin JE 2010 Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988–2006. Diabetes Care 33:562–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan V, Vijayachandrika V, Gokulakrishnan K, Anjana RM, Ganesan A, Weber MB, Narayan KM 2010 A1C cut points to define various glucose intolerance groups in Asian Indians. Diabetes Care 33:515–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van 't Riet E, Alssema M, Rijkelijkhuizen JM, Kostense PJ, Nijpels G, Dekker JM 2010 Relationship between A1C and glucose levels in the general Dutch population: The New Hoorn Study. Diabetes Care 33:61–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudek CD, Herman WH, Sacks DB, Bergenstal RM, Edelman D, Davidson MB 2008 A new look at screening and diagnosing diabetes mellitus. J Clin Endocrinol Metab 93:2447–2453 [DOI] [PubMed] [Google Scholar]
- Kramer CK, Araneta MR, Barrett-Connor E 2010 A1C and diabetes diagnosis: the Rancho Bernardo Study. Diabetes Care 33:101–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, Coresh J, Brancati FL 2010 Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 362:800–811 [DOI] [PMC free article] [PubMed] [Google Scholar]