Abstract
Context: ATP-sensitive potassium (KATP) channels regulate insulin secretion by coupling glucose metabolism to β-cell membrane potential. Gain-of-function mutations in the sulfonylurea receptor 1 (SUR1) or Kir6.2 channel subunit underlie neonatal diabetes.
Objective: The objective of the study was to determine the mechanisms by which two SUR1 mutations, E208K and V324M, associated with transient neonatal diabetes affect KATP channel function.
Design: E208K or V324M mutant SUR1 was coexpressed with Kir6.2 in COS cells, and expression and gating properties of the resulting channels were assessed biochemically and electrophysiologically.
Results: Both E208K and V324M augment channel response to MgADP stimulation without altering sensitivity to ATP4− or sulfonylureas. Surprisingly, whereas E208K causes only a small increase in MgADP response consistent with the mild transient diabetes phenotype, V324M causes a severe activating gating defect. Unlike E208K, V324M also impairs channel expression at the cell surface, which is expected to dampen its functional impact on β-cells. When either mutation was combined with a mutation in the second nucleotide binding domain of SUR1 previously shown to abolish Mg-nucleotide response, the activating effect of E208K and V324M was also abolished. Moreover, combination of E208K and V324M results in channels with Mg-nucleotide sensitivity greater than that seen in individual mutations alone.
Conclusion: The results demonstrate that E208K and V324M, located in distinct domains of SUR1, enhance transduction of Mg-nucleotide stimulation from the SUR1 nucleotide binding folds to Kir6.2. Furthermore, they suggest that diabetes severity is determined by interplay between effects of a mutation on channel expression and channel gating.
Characterization of E208K and V324M mutations in sulfonylurea receptor 1 suggests diabetes severity is determined by interplay between effects of mutations on channel expression and channel gating.
ATP-sensitive potassium (KATP) channels regulate insulin secretion by coupling intracellular ATP and ADP concentrations reflecting glucose levels to membrane excitability. The channel consists of four sulfonylurea receptor (SUR) 1 subunits (ABCC8) and four pore-forming Kir6.2 subunits (KCNJ11) (1). ATP inhibits the channel by interacting with Kir6.2, whereas MgATP/MgADP stimulates the channel via SUR1 (2). Gain-of-function SUR1 or Kir6.2 mutations underlie a range of neonatal diabetes (ND), from transient, relapsing to permanent (3). A gating property often affected by SUR1 mutations is channel sensitivity to Mg-nucleotides. SUR1, an ABC transporter, has three transmembrane domains (TMD0, TMD1, and TMD2) and two cytoplasmic nucleotide binding folds (NBF1 and NBF2) (Supplemental Fig. 1, published on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org) (1). Channel stimulation by Mg-nucleotides involves nucleotide binding/hydrolysis at NBFs, which induces a conformational change in SUR1 to open Kir6.2 (2). How conformational change at SUR1-NBFs is transduced to Kir6.2 is incompletely understood, although TMD0 and L0 (cytoplasmic loop between TMD0 and TMD1) of SUR1 is proposed as a transduction module (4,5,6).
We conducted functional analyses of two SUR1 mutations, E208K and V324M, identified in transient ND (7,8). E208K and V324M located in L0 and TMD1, respectively, cause channel overactivity by enhancing MgADP responsivity, establishing their causal role in ND. The enhancement effect on MgADP responsivity is greater in V324M than E208K; however, surface expression of the V324M mutant is significantly reduced, suggesting that the greater gain-of-function gating defect caused by V324M is offset by lower surface expression. When combined with a SUR1-NBF2 mutation known to abolish MgADP responsivity, effects of E208K and V324M were also abolished, indicating that these residues are involved in transducing the effect of Mg-nucleotides to channel gating.
Patients and Methods
Clinical analyses
Clinical analyses were performed as described previously (7). The study was approved by the Institutional Review Board of the Hospital de Cruces and Hospital Niño Jesus, and written consent was given by patients or parents.
Molecular biology and KATP channel expression
FLAG-tagged wild-type (WT) and mutant hamster-SUR1 in pECE vector were constructed using the QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) and confirmed by DNA sequencing; rat Kir6.2 is in pCDNA (9). Hamster SUR1 and rat Kir6.2 are commonly used for KATP channel reconstitution. They are highly homologous to human SUR1 and Kir6.2, and residues that have been mutated in disease are all conserved (10). KATP channels were expressed in COSm6 cells by cotransfection with fSUR1 (FLAG-tagged SUR1) and Kir6.2 cDNAs using FuGene6 and analyzed 48–72 h after transfection. COSm6 cells were chosen because they do not express endogenous KATP channels that complicate mutant analysis.
Biochemical and functional analyses of KATP channels
Biochemical and functional analyses of KATP channels, including 86Rb+ efflux assays, immunoblotting, immunostaining, chemiluminescence assays, and inside-out patch-clamp recordings were conducted as described previously (9,11). Detailed protocols are provided in the Supplemental Materials and Methods.
Results
Mutations and clinical data
E208K and V324M are SUR1 mutations identified in patients diagnosed with ND (7,8,12) (Supplemental Table 1). The most relevant data of the patient with a V324M mutation, not previously reported, is that at 1 month and 17 d of age she was admitted to the hospital due to general hypotonia with severe hyperglycemia (88.4 mmol/liter) and positive ketonemia. She was treated initially with regular insulin iv and subsequently with sc administration of a short-acting insulin analog after every feeding (0.1 to 0.3 IU according to capillary glycemic determinations). When sodium, calcium, and glycemia levels were normal, she had two episodes of generalized seizures that needed treatment with phenobarbital and phenitoine. Insulin requirements decreased and were discontinued when she was 5 months old. She is now 7 yr and 2 months old, and diabetes has not relapsed. No functional studies have been conducted on either E208K or V324M.
Functional analysis of mutant channels
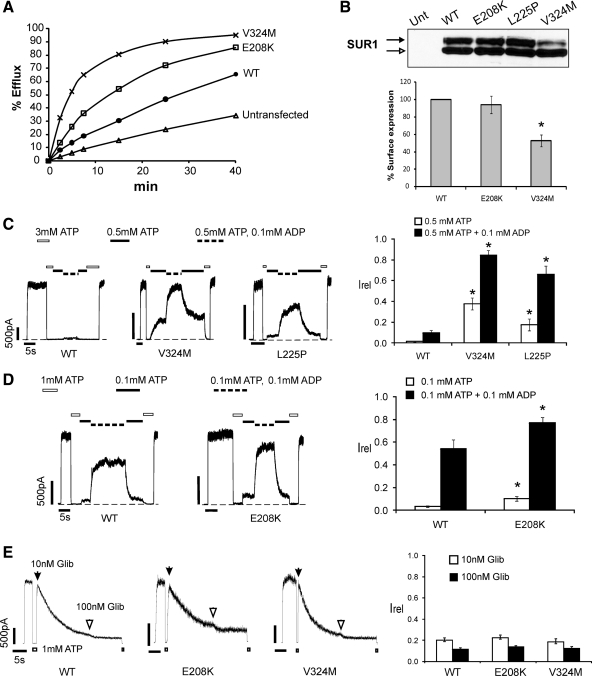
Because not all carriers are symptomatic (7,8,12), it is important to determine whether E208K and V324M affect KATP channel function. In 86Rb+ efflux assays, whereas WT channels exhibited the expected low activity due to inhibition by high intracellular ATP, both E208K and V324M channels yielded greater efflux, with V324M being the most active (Fig. 1A). Thus, both mutations cause gain of channel function, confirming their causal role in ND. To determine the mechanisms underlying increased channel activity, we first assessed surface expression of mutant channels. In Western blots, WT-SUR1 coexpressed with Kir6.2 is normally seen as a core-glycosylated lower band corresponding to SUR1 in the endoplasmic reticulum (ER) and a complex-glycosylated upper band corresponding to SUR1 that has trafficked past medial Golgi (13). Because exit of SUR1 from the ER requires coassembly with Kir6.2, abundance of the upper band correlates with the amount of SUR1 at the cell surface that has passed ER quality control (9,14). Figure 1B shows that whereas in E208K-SUR1 both bands were similar in intensity to WT-SUR1, V324M-SUR1 had a clearly reduced upper band, suggesting that V324M may impair channel trafficking from ER to the plasma membrane. Immunofluorescence staining of surface channels showed that V324M is indeed expressed at a reduced level compared with WT or E208K, although staining of permeabilized cells indicated that total V324M SUR1 protein levels were not significantly reduced (Supplemental Fig. 2). Surface expression was further quantified by chemiluminescence assays that confirmed markedly reduced surface expression of V324M, in contrast to E208K (56.3 ± 6.3 vs. 93.9 ± 10.1% of WT channels, respectively; Fig. 1B).
Figure 1.
Functional characterization of E208K- and V324M-SUR1 mutations. A, 86Rb+ efflux profile from COS cells transiently expressing WT or mutant channel subunits. Efflux was measured in Ringer’s solution without metabolic inhibitors. Both E208K and V324M exhibited higher efflux than WT, with the V324M mutant showing the highest activity, confirming that E208K- and V324M-SUR1 are activating KATP channel mutations. B, top, Western blot of SUR1 in COSm6 cells expressing Kir6.2 and WT or mutant SUR1. The empty arrow indicates the core-glycosylated immature band, and the solid arrow indicates the complex-glycosylated mature SUR1. The mature form of SUR1 is clearly reduced in V324M compared with WT, E208K, or L225P. Bottom, Quantification of surface expression of WT or mutant KATP channels using the chemiluminescence assay. Expression was normalized to that of WT channels. The expression level of V324M is significantly reduced compared with WT (n = 3; *, P < 0.05 by Student’s t test). C, Mg-nucleotide response was monitored by exposing channels to varying concentrations of ATP and ADP, with free [Mg2+] of approximately 1 mm. Representative recordings of WT, V324M, and L225P channels are shown. ATP and ADP concentrations are as indicated by the bars above the recordings. Bar graph shows quantification of currents relative to that seen in K-INT (140 mm KCl, 10 mm K-HEPES, 1 mm K-EGTA, pH 7.3) immediately after patch excision (n = 5–7; *, P < 0.05 by ANOVA with Dunnett’s post hoc test). D, Similar to panel C, except that the concentrations of ATP and ADP were different, and comparisons between WT and E208K were made. Bar graph is as in panel C (n = 5–7; *, P < 0.05 by Student’s t test). E, Channel sensitivity to glibenclamide (Glib). Shown are representative recordings of WT, E208K, and V324M channels in response to 10 or 100 nm glibenclamide. Because inhibition by glibenclamide is irreversible, channels were first exposed to 10 nm until the currents stabilized, followed by exposure to 100 nm glibenclamide. Bar graph shows quantification of near steady-state currents in 10 or 100 nm glibenclamide relative to currents in K-INT just before exposure to glibenclamide. Each bar is the mean ± sem of 7–10 patches. No statistical differences were observed between WT and the mutants.
Unaltered or reduced surface expression of E208K or V324M suggests that overactivity results from abnormal channel gating. Hence, we examined mutant channel sensitivities to ATP4− and MgADP by inside-out patch-clamp recordings. ATP dose-response studies showed that E208K and V324M do not affect channel ATP4− sensitivity (Supplemental Fig. 3). Next, Mg-nucleotide response was assessed. Different ATP/ADP ratios (0.1/0.1, 0.1/0.5, or 0.5/0.1 mm; with free [Mg2+] of ∼1 mm) were employed to reveal the difference between WT and the mutant that may otherwise be masked due to too much ATP inhibition (compare WT response in Fig. 1, C and D) or saturation of the MgADP stimulatory effects (compare Figs. 1D and 2B). These experiments showed that V324M markedly increases the Mg-nucleotide response (most evident at the 0.5/0.1 mm ATP/ADP ratio; Fig. 1C), even more so than L225P, another SUR1 mutation reported to cause permanent ND by a similar gating abnormality (15). In contrast, E208K subtly increased Mg-nucleotide sensitivity that was statistically significant only in the 0.1/0.1 mm ATP/ADP ratio (compare Figs. 1D and 2B). These results indicate that E208K and V324M cause ND by hypersensitizing channels to Mg-nucleotide stimulation.
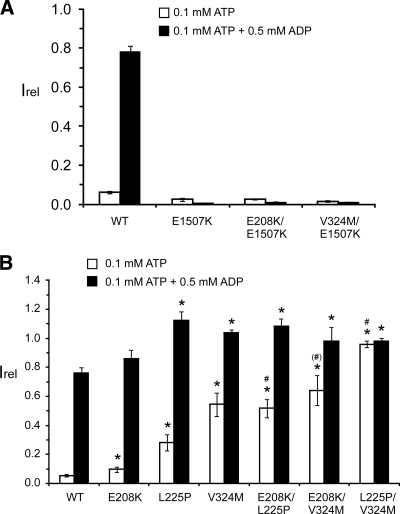
Figure 2.
E208K and V324M enhance channel response to MgADP by affecting transduction of the MgADP effect to Kir6.2. Inside-out patch recordings were performed similar to that shown in Fig. 1, C and D, by exposing channels to 0.1 mm ATP or 0.1 mm ATP+0.5 mm ADP (free [Mg2+] ∼1 mm). The currents relative to those observed in K-INT solution immediately after patch excision were quantified. A, The E1507K inactivating mutation in NBF2 completely abolished the activating effects of E208K or V324M. Each bar is the mean ± sem of three or four patches. B, Effects of the activating mutations in TMD0-L0 and TMD1 are additive. Each bar is the mean ± sem of 30 (WT), eight (E208K), six (L225P), five (V324M), nine (E208K/L225P), six (E208K/V324M), and six (L225P/V324M) patches. *, P < 0.05 for comparison between mutant and WT by one-way ANOVA with Dunnett’s post hoc test. #, P < 0.05 for comparison between each double mutant and its respective single mutants. (#), P < 0.05 between E208K/V324M and E208K but the difference between E208K/V324M and V324M is not significant.
Overactivity of KATP channels caused by some mutations can be suppressed by sulfonylureas (3,16). To determine whether E208K or V324M affects channel sensitivity to sulfonylureas, we tested channel response to 10 or 100 nm glibenclamide. E208K and V324M were inhibited by glibenclamide similar to WT channels at both concentrations (Fig. 1E). The effectiveness of glibenclamide in inhibiting V324M channels is consistent with the clinical observation reported previously that recurrent diabetes in a patient bearing this mutation was successfully treated with glibenclamide (8).
E208K and V324M enhance transduction of MgADP stimulation
Stimulation of KATP channels by Mg-nucleotides originates from Mg-nucleotide interactions with NBFs of SUR1. E208K and V324M are outside the NBFs, in the TMD0-L0 and TMD1 domains, respectively (Supplemental Fig. 1). We tested whether they affect the downstream transduction steps to augment Mg-nucleotide stimulation. E1507K is a congenital hyperinsulinism-causing NBF2 mutation previously shown to abolish MgADP responsivity (11,17). Combining E208K or V324M with E1507K completely abrogated the enhancement effects of E208K or V324M on Mg-nucleotide responses (Fig. 2A).
To determine the relationship between the activating effect caused by V324M in TMD1 and that caused by E208K or L225P in TMD0-L0, we compared Mg-nucleotide responsivity in channels harboring a combination of these mutations. Currents measured in 0.1 mm ATP (free [Mg2+] ∼1 mm) showed that channels harboring double mutations were more active than those with only one mutation such that the effects of E208K, L225P, or V324M are additive. Currents measured in 0.1 mm ATP plus 0.5 mm ADP (free [Mg2+] ∼1 mm) showed no such additive effects, likely because channel activity under this condition has reached the maximum. Taken together, the results demonstrate that in addition to TMD0-L0, TMD1 also has a distinct role in transducing the action of Mg-nucleotides at the NBFs into channel opening.
Discussion
We show that two heterozygous SUR1 mutations, E208K and V324M, identified in patients with transient ND cause KATP channel overactivity by enhancing channel responsivity to Mg-nucleotides without affecting ATP4− or glibenclamide sensitivity. These results demonstrate a causal role of these mutations in ND and effectiveness of sulfonylureas in correcting their activating defects. Although V324M renders a marked increase in Mg-nucleotide response, even more so than L225P previously reported to cause permanent ND (15), the effect of E208K is subtle, only statistically significant at 0.1 mm ATP/0.1 mm ADP; yet both E208K and V324M are associated with transient ND. One possible explanation for the lack of correlation between the severity of the gating defect and the severity of diabetes is the differential cell surface expression of these mutants. We previously proposed that in ND surface expression efficiency of a mutant modifies the extent of manifestation of the gating defect to determine disease outcome (18,19). Supporting this, whereas E208K (and also L225P; Fig. 1B) exhibited surface expression similar to WT, V324M was expressed at only approximately 50% that of WT (Fig. 1B). The severe gating defect caused by V324M is likely negated by low expression of the mutant on the β-cell surface, resulting in a less severe diabetes phenotype than one would predict based solely on gating defects. Of note, asymptomatic carriers have been described for both E208K and V324M(7,12). Moreover, in one V324M patient, seizure, developmental delay, and muscle weakness were also observed. What environmental and genetic factors underlie the wide variations in disease presentation for V324M is an important question to address in the future.
The activating effects of E208K and V324M were abrogated by a NBF2 mutation that abolishes the MgADP response. This suggests that similar to L225P (15), the two mutations affect transduction of MgATP binding/hydrolysis signal from the SUR1-NBFs to Kir6.2. E208K is in the TMD0-L0 domain that has been proposed to serve as a coupling module to transduce effects of Mg-nucleotide stimulation to Kir6.2 (4,5,6). Our results reveal an important role of TMD1 as an additional domain involved in structural rearrangements that occur upon Mg-nucleotide interactions with NBFs to gate the channel. That the effect of E208K or L225P and of V324M are additive suggests that there may be multiple transduction pathways to regulate Kir6.2 gating. Finally, V324M has normal glibenclamide sensitivity despite a markedly increased Mg-nucleotide response. High-affinity sulfonylurea block is proposed to occur by abolishing Mg-nucleotide stimulation of KATP channels (20). Sulfonylureas may block the activating effect of V324M by preventing the initial steps in the NBFs. Alternatively, transduction of the glibenclamide blocking effect may employ a pathway separate from that affected by V324M, as suggested for the L225P mutation (15). Future studies will distinguish these possibilities.
Supplementary Material
Acknowledgments
We thank Joel Gay and Dr. Cathrin Bruederle from Oregon Health & Science University for help with molecular biology and confocal imaging.
Footnotes
This work was supported by National Institutes of Health Grants R01DK57699 and DK66485 (to S.-L.S.) and Grant PI06/0690 from the Instituto de Salud Carlos III of the Spanish Ministry of Health (to G.P.d.N.). I.G. has received a grant from the Basque Department of Education (BFI06.266). G.P.d.N. is cofunded by the Instituto de Salud Carlos III I3SNS Program (CP03/0064; SIVI 1395/09). I.G., L.C., and G.P.d.N. are also supported by the Centro de Investigación Biomédica en Red de Enfermedades Raras, ISCIII, and Centro de Investigación Biomédica en Red de Diabetes y Enfermedades Metabólicas, ISCIII. J.A. and M.T.M.-C. are currently investigators of the CIBERobn from Instituto de Salud Carlos III from Madrid, Spain.
Disclosure Summary: The authors have nothing to disclose.
Abbreviations: ER, Endoplasmic reticulum; KATP, ATP-sensitive potassium; L0, cytoplasmic loop between TMD0 and TMD1; NBF, nucleotide binding fold; ND, neonatal diabetes; SUR, sulfonylurea receptor; TMD, transmembrane domain; WT, wild-type.
References
- Aguilar-Bryan L, Bryan J 1999 Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr Rev 20:101–135 [DOI] [PubMed] [Google Scholar]
- Nichols CG 2006 KATP channels as molecular sensors of cellular metabolism. Nature 440:470–476 [DOI] [PubMed] [Google Scholar]
- Ashcroft FM 2007 The Walter B. Cannon Physiology in Perspective Lecture, 2007. ATP-sensitive K+ channels and disease: from molecule to malady. Am J Physiol Endocrinol Metab 293:E880–E889 [DOI] [PubMed] [Google Scholar]
- Babenko AP 2005 K(ATP) channels “vingt ans apres”: ATG to PDB to mechanism. J Mol Cell Cardiol 39:79–98 [DOI] [PubMed] [Google Scholar]
- Babenko AP, Bryan J 2003 SUR domains that associate with and gate KATP pores define a novel gatekeeper. J Biol Chem 278:41577–41580 [DOI] [PubMed] [Google Scholar]
- Chan KW, Zhang H, Logothetis DE 2003 N-terminal transmembrane domain of the SUR controls trafficking and gating of Kir6 channel subunits. EMBO J 22:3833–3843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan SE, Patch AM, Mackay DJ, Edghill EL, Gloyn AL, Robinson D, Shield JP, Temple K, Ellard S, Hattersley AT 2007 Mutations in ATP-sensitive K+ channel genes cause transient neonatal diabetes and permanent diabetes in childhood or adulthood. Diabetes 56:1930–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaxillaire M, Dechaume A, Busiah K, Cavé H, Pereira S, Scharfmann R, de Nanclares GP, Castano L, Froguel P, Polak M 2007 New ABCC8 mutations in relapsing neonatal diabetes and clinical features. Diabetes 56:1737–1741 [DOI] [PubMed] [Google Scholar]
- Yan FF, Lin YW, MacMullen C, Ganguly A, Stanley CA, Shyng SL 2007 Congenital hyperinsulinism associated ABCC8 mutations that cause defective trafficking of ATP-sensitive K+ channels: identification and rescue. Diabetes 56:2339–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan SE, Clauin S, Bellanné-Chantelot C, de Lonlay P, Harries LW, Gloyn AL, Ellard S 2009 Update of mutations in the genes encoding the pancreatic beta-cell K(ATP) channel subunits Kir6.2 (KCNJ11) and sulfonylurea receptor 1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum Mutat 30:170–180 [DOI] [PubMed] [Google Scholar]
- Pinney SE, MacMullen C, Becker S, Lin YW, Hanna C, Thornton P, Ganguly A, Shyng SL, Stanley CA 2008 Clinical characteristics and biochemical mechanisms of congenital hyperinsulinism associated with dominant KATP channel mutations. J Clin Invest 118:2877–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellard S, Flanagan SE, Girard CA, Patch AM, Harries LW, Parrish A, Edghill EL, Mackay DJ, Proks P, Shimomura K, Haberland H, Carson DJ, Shield JP, Hattersley AT, Ashcroft FM 2007 Permanent neonatal diabetes caused by dominant, recessive, or compound heterozygous SUR1 mutations with opposite functional effects. Am J Hum Genet 81:375–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerangue N, Schwappach B, Jan YN, Jan LY 1999 A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron 22:537–548 [DOI] [PubMed] [Google Scholar]
- Cartier EA, Conti LR, Vandenberg CA, Shyng SL 2001 Defective trafficking and function of KATP channels caused by a sulfonylurea receptor 1 mutation associated with persistent hyperinsulinemic hypoglycemia of infancy. Proc Natl Acad Sci USA 98:2882–2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masia R, De Leon DD, MacMullen C, McKnight H, Stanley CA, Nichols CG 2007 A mutation in the TMD0-L0 region of sulfonylurea receptor-1 (L225P) causes permanent neonatal diabetes mellitus (PNDM). Diabetes 56:1357–1362 [DOI] [PubMed] [Google Scholar]
- Koster JC, Remedi MS, Dao C, Nichols CG 2005 ATP and sulfonylurea sensitivity of mutant ATP-sensitive K+ channels in neonatal diabetes: implications for pharmacogenomic therapy. Diabetes 54:2645–2654 [DOI] [PubMed] [Google Scholar]
- Huopio H, Reimann F, Ashfield R, Komulainen J, Lenko HL, Rahier J, Vauhkonen I, Kere J, Laakso M, Ashcroft F, Otonkoski T 2000 Dominantly inherited hyperinsulinism caused by a mutation in the sulfonylurea receptor type 1. J Clin Invest 106:897–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CW, Lin YW, Yan FF, Casey J, Kochhar M, Pratt EB, Shyng SL 2006 Kir6.2 mutations associated with neonatal diabetes reduce expression of ATP-sensitive K+ channels: implications in disease mechanism and sulfonylurea therapy. Diabetes 55:1738–1746 [DOI] [PubMed] [Google Scholar]
- Pratt EB, Yan FF, Gay JW, Stanley CA, Shyng SL 2009 Sulfonylurea receptor 1 mutations that cause opposite insulin secretion defects with chemical chaperone exposure. J Biol Chem 284:7951–7959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble FM, Tucker SJ, Ashcroft FM 1997 The interaction of nucleotides with the tolbutamide block of cloned ATP-sensitive K+ channel currents expressed in Xenopus oocytes: a reinterpretation. J Physiol 504:35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.