Abstract
Context: Vitamin D deficiency and frailty are common with aging, but the association between these conditions is uncertain.
Objective: To determine the association between 25-hydroxyvitamin D (25(OH)D) levels and prevalent and incident frailty status among older women.
Design: Cross-sectional and longitudinal analyses of a prospective cohort study.
Setting: Four U.S. centers.
Participants: 6307 women aged ≥69 years.
Main Outcome Measures: Frailty status classified as robust, intermediate stage, or frail at baseline; and robust, intermediate stage, frail, or dead (all-cause mortality) at follow-up an average of 4.5 years later.
Results: At baseline, there was a U-shaped association between 25(OH)D level and odds of frailty with the lowest risk among women with levels 20.0–29.9 ng/ml (referent group). Compared with this group, the odds of frailty were higher among those with levels <15.0 ng/ml [multivariable odds ratio (MOR) 1.47, 95% confidence interval (CI), 1.19–1.82], those with levels 15.0–19.9 ng/ml (MOR 1.24, 95% CI 0.99–1.54), and those with levels ≥30 ng/ml (MOR 1.32, 95% CI 1.06–1.63). Among 4551 nonfrail women at baseline, the odds of frailty/death (vs. robust/intermediate) at follow-up appeared higher among those with levels 15.0–19.9 ng/ml (MOR 1.21, 95% CI 0.99–1.49), but the CI overlapped 1.0. The odds of death (vs. robust/intermediate/frail at follow-up) was higher among those with levels <15.0 ng/ml (MOR 1.40, 95% CI 1.04–1.88) and those with levels 15.0–19.9 ng/ml (MOR 1.30, 95% CI 0.97–1.75), although the latter association did not quite reach significance.
Conclusion: Lower (<20 ng/ml) and higher (≥30 ng/ml) levels of 25(OH)D among older women were moderately associated with a higher odds of frailty at baseline. Among nonfrail women at baseline, lower levels (<20 ng/ml) were modestly associated with an increased risk of incident frailty or death at follow-up.
Lower and higher 25(OH)D levels among older women were associated with higher odds of frailty at baseline, and lower levels among non-frail women at baseline were associated with increased risk of incident frailty or death at 4.5 years.
Lower levels of circulating 25-hydroxyvitamin D (25(OH)D) and frailty (a term indicating multisystem impairment and expanding vulnerability) are increasingly common with advancing age. Several previous studies have reported that frailty independently predicts risks of adverse health outcomes including incident disability, falls, fractures, and mortality (1,2,3,4). Dimensions of frailty including weakness and slowness are potential outcomes of vitamin D deficiency, and lower 25(OH)D levels have been inconsistently associated with poorer physical performance and increased risks of falls, fractures, and death in postmenopausal women and older men (5,6).
Previous cross-sectional studies have inconsistently reported that older adults with lower 25(OH)D levels are more likely to be classified as frail as compared with not frail (7,8). However, only one previous study (9) expressed frailty status as a continuum ranging from robust to intermediate stage to frail; this study reported an association between lower 25(OH)D levels and greater frailty status among men but not women. Among individuals classified as not frail at baseline, three studies (8,10) (Ensrud, K. E., T. L. Blackwell, J. A. Cauley, S. R. Cummings, E. Barrett-Connor, T. L. Dam, A. R. Hoffman, J. M. Shikany, N. E. Lane, M. L. Stefanick, E. S. Orwoll, and P. M. Cawthon, submitted for publication) have examined the association between 25(OH)D levels and risk of becoming frail during follow-up and reported conflicting results.
To test the hypothesis that lower 25(OH)D levels at baseline were associated with greater prevalent frailty status, we measured 25(OH)D and assessed frailty status in a cohort of 6307 community-dwelling women aged ≥69 years enrolled in the Study of Osteoporotic Fractures (SOF). To determine whether lower 25(OH)D levels at baseline were associated with an increased risk of greater frailty status at follow-up, 4551 women classified as nonfrail (robust or intermediate) at baseline had frailty status reassessed an average of 4.5 years later.
Materials and Methods
Participants
From September 1986 to October 1988, 9704 women who were ≥65 years were recruited for participation in the baseline examination of the prospective SOF (11). Women were recruited from population-based listings in four regions of the United States. Black women were originally excluded from SOF because they have a low incidence of hip fracture. In addition, women were excluded if they had a history of bilateral hip replacement or were unable to walk without the assistance of another person.
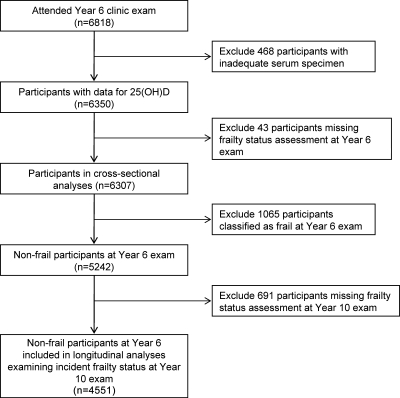
All surviving participants were invited to attend a Year 6 examination between August 1992 and July 1994. A total of 6818 women (78% of survivors as of July 31, 1994) completed the Year 6 examination and 6350 provided sufficient sera for measurement of 25(OH)D level. Of these, 6307 women (99.3%) provided adequate data for assessment of frailty status and comprised the analytical cohort for the cross-sectional analysis (Fig. 1). A total of 5242 of the 6307 women were classified as nonfrail (robust or intermediate stage) at baseline and were eligible for the longitudinal analysis. After excluding 691 women (Fig. 1), the final longitudinal cohort was comprised of 4551 women including 4119 who had repeat assessment of frailty status at a Year 10 examination an average of 4.5 years later and 432 who died before this follow-up examination. The institutional review board at each center approved the study protocol, and written informed consent was obtained from all subjects.
Figure 1.
Cohort for cross-sectional and longitudinal analyses.
Measurement of 25(OH)D
Fasting morning blood was collected, serum was prepared immediately after phlebotomy, and then was stored at −70 C. Measures for 25(OH)D2 (derived from ergocalciferol) and 25(OH)D3 (derived from cholecalciferol) were performed at the Mayo Clinic using liquid chromatography tandem mass spectroscopy (LC-MS/MS) as previously described (12). Deuterated stable isotope (d3-25-hydroxyvitamin D) was added to a 0.2 ml serum sample as internal standard. 25(OH)D2, 25(OH)D3, and the internal standard were extracted using acetonitrile precipitation The extracts were then further purified online and analyzed by LC-MS/MS using multiple reaction monitoring. 25(OH)D2 and 25(OH)D3 were quantified and summed for total 25(OH)D. The minimum detectable limit for 25(OH)D2 was 4 ng/ml and for 25(OH)D3 was 2 ng/ml. For 25(OH)D2, the interassay coefficient of variation (CV) ranged from 4.7% to 6.2% and the intraassay CV ranged from 3.3% to 4.4%. For 25(OH)D3, the interassay CV ranged from 5.0% to 6.8% and the intraassay CV ranged from 2.4% to 4.7%.
Other measurements
Participants completed a questionnaire and were interviewed at the examinations and asked about health status, educational achievement, smoking status, and alcohol intake. A selected medical history was obtained that included a history of a physician diagnosis of fracture since the age of 50 years, stroke, cancer excluding skin cancer, dementia, hypertension, parkinsonism, diabetes mellitus, coronary heart disease, and chronic obstructive lung disease. Participants were asked to bring all medications including nonprescription supplements to clinic for verification of use. Total calcium intake (mg/d) was calculated by summing dietary calcium intake (13,14) and daily dosage of calcium supplements. Physical activity (15) was assessed using a modified version of the Harvard Alumni Questionnaire (16,17) and expressed as kilocalories expended per week from walking. Depressive symptoms were evaluated using the 15-item Geriatric Depression Scale (18). Cognitive function was assessed with a modified version of the Mini-Mental State Examination (19) with a maximum score of 26. Tests of physical function included grip strength (using a hand-held Jamar dynamometer) and walk speed (time in seconds to walk 6 m at usual pace). Body weight and height measurements were used to calculate a standard body mass index (BMI).
Frailty status
Frailty status at the Year 6 examination (baseline for this analysis) was defined using the following criteria similar to those proposed by Fried and colleagues (1) using data collected in the Cardiovascular Health Study (CHS):
Shrinking/Sarcopenia as identified by weight loss of 5% or more between the Year 3.5 and Year 6 examinations [mean (sd) years between examinations, 2.0 (0.3)];
Weakness as identified by a grip strength in the lowest quintile stratified by BMI (quartiles);
Exhaustion as identified by an answer of “no” to the question “Do you feel full of energy?” from the Geriatric Depression Scale (19);
Slowness as identified by a walk speed in the lowest quintile stratified by standing height (median); and
Low physical activity level, as identified by kilocalories expended per week from walking in the lowest quintile.
Women with none of the above components were considered to be robust, those with one or two components were considered to be in an intermediate stage, and those with ≥3 components were considered to be frail.
Frailty status at the Year 10 (follow-up) examination was defined using criteria cut points from the baseline examination (shrinking/sarcopenia) was identified by weight loss of 5% or more between the Year 8 and Year 10 examinations [mean (sd) years between examinations, 2.3 (0.5)]. To jointly analyze the outcome of frailty status at the follow-up exam and mortality between baseline and the follow-up exam, four levels of frailty status were considered at the follow-up examination: robust, intermediate stage, frail, or dead (died between Year 6 and 10 examinations).
Statistical analysis
Characteristics of participants at the baseline (Year 6) examination according to category of total 25(OH)D level were compared using ANOVA for normally distributed continuous data, Kruskal–Wallis tests for skewed continuous data, and χ2 tests for categorical data.
We used restricted cubic splines to determine whether the association between 25(OH)D level and frailty status at baseline was nonlinear (21,22). Knots were placed at the 5th, 25th, 50th, 75th, and 95th percentiles of 25(OH)D and three cubic terms were calculated for each participant. The spline curve for the log odds ratio of frailty vs. 25(OH)D level was plotted, and this graph suggested the presence of a nonlinear (U-shaped) association between 25(OH)D level and odds of frailty (vs. robust/intermediate) with the lowest risk among women with levels 20.0–29.9 ng/ml and increasing odds of frailty at lower and higher values of 25(OH)D. Similarly, spline longitudinal analyses (results not shown) indicated the presence of a nonlinear association between baseline 25(OH)D level among nonfrail women at baseline and frailty status (robust, intermediate stage, frail, or dead) at follow-up with increasing odds of greater frailty status at lower and higher levels of 25(OH)D. Thus, for the primary cross-sectional and longitudinal analyses, the predictor variable, 25(OH)D level, was expressed as a four-category variable using the following cut points: <15.0 ng/ml, 15.0–19.9 ng/ml, 20.0–29.9 ng/ml (referent group), and ≥30.0 ng/ml. In addition, secondary analyses were conducted expressing 25(OH)D level as quartiles (cut points 15.9, 21.9, and 28.9 ng/ml) using the 3rd quartile as the referent group. Because higher total 25(OH) levels may be a marker of supplemental vitamin D use and supplemental vitamin D during the study period (1992–1998) was largely reflected in 25(OH)D2 [not 25(OH)D3] levels, we also performed an analysis substituting 25(OH)D3 level for total 25(OH)D level.
The association between 25(OH)D level and the ordinal frailty status outcome was initially examined using proportional odds models. We evaluated the assumption of homogeneity of effect of 25(OH)D across all levels of the frailty outcome (23). However, the proportionality assumption was not met in the longitudinal models. Thus for consistency in presenting cross-sectional and longitudinal results, two separate logistic regression models were performed for the cross-sectional analyses [dichotomizing the outcome as intermediate stage/frail (vs. robust) and frail (vs. robust/intermediate stage)] and three separate logistic regression models were performed for the longitudinal analyses [dichotomizing the outcome as intermediate stage/frail/dead (vs. robust), frail/dead (vs. robust/intermediate), and dead (vs. robust/intermediate stage/frail)].
Initial models were adjusted for age, clinic site, season of blood draw, and BMI, then for multiple potential confounders (multivariable model). Factors associated with frailty status at the baseline examination independent of age and those related to 25(OH)D level at P ≤ 0.10 at baseline or follow-up examinations were considered for inclusion in the multivariable model. Variables used to define each of the individual frailty components (such as physical activity) were not included in the covariate selection process. Covariates in the cross-sectional multivariable model included age, site, season, BMI, health status, education, smoking status, alcohol intake, number of comorbid medical conditions, and cognitive function. The longitudinal multivariable model was additionally adjusted for baseline frailty status (intermediate vs. robust).
Statistical analyses were completed using SAS v9.2 (SAS Inc., Cary, NC) and Stata v11.0 (Stata Corporation, College Station, TX).
Results
Of the 6307 women in the cohort at baseline, 2195 (34.8%) were classified as robust, 3047 (48.3%) were in the intermediate stage, and 1065 (16.9%) were classified as frail. The mean (sd) 25(OH)D level was 23.2 (11.6) ng/ml. Among the 2632 women reporting use of vitamin D supplementation, 86% were taking ≤400 IU/d. Characteristics of participants by category of 25(OH)D level are shown in Table 1. The proportion of women classified as frail was 23.5% among women with levels <15.0 ng/ml, 17.6% among women with levels 15.0–19.9 ng/ml, 13.6% women with levels 20.0–29.9 ng/ml, and 16.0% among the women with levels ≥30.0 ng/ml (P ≤ 0.001). A similar U-shaped pattern in the prevalence of frailty across 25(OH)D levels was observed when the cohort was restricted to 3675 women not taking vitamin D supplements at baseline (P < 0.001). In addition, the prevalence of four individual frailty components (shrinking/sarcopenia, weakness, slowness, low activity level) exhibited U-shaped patterns across category of 25(OH)D level.
Table 1.
Characteristics of 6307 participants by category of serum 25(OH)D level
| Variable | Overall cohort (n = 6307) | Category of serum 25(OH) D
|
P value | |||
|---|---|---|---|---|---|---|
| <15.0 ng/ml (n = 1280) | 15.0–19.9 ng/ml (n = 1233) | 20.0–29.9 ng/ml (n = 2428) | ≥30.0 ng/ml (n = 1366) | |||
| Age, years, mean (sd) | 76.7 (4.8) | 77.5 (5.0) | 76.8 (4.7) | 76.3 (4.7) | 76.3 (4.7) | <0.001 |
| Excellent or good health status, n (%) | 5117 (81.2) | 991 (77.6) | 994 (80.6) | 2001 (82.4) | 1131 (82.8) | 0.001 |
| Education, years, mean (sd) | 12.8 (2.8) | 12.6 (2.8) | 12.5 (2.9) | 12.9 (2.8) | 13.0 (2.7) | <0.001 |
| Current smoker, n (%) | 340 (5.5) | 97 (7.7) | 70 (5.7) | 110 (4.6) | 63 (4.7) | 0.001 |
| Alcohol intake, drinks per week, mean (sd) | 1.3 (3.1) | 1.5 (3.5) | 1.2 (2.9) | 1.2 (2.7) | 1.6 (3.5) | 0.05 |
| Vitamin D supplement use, n (%) | 2632 (41.7) | 190 (14.8) | 323 (26.2) | 1184 (48.8) | 935 (68.5) | <0.001 |
| Total calcium intake, mg/d, mean (sd) | 986 (790) | 741 (700) | 855 (682) | 1052 (795) | 1223 (866) | <0.001 |
| Co-morbidity score (range 0–9), mean (sd) | 1.5 (1.1) | 1.6 (1.2) | 1.5 (1.1) | 1.4 (1.1) | 1.4 (1.1) | <0.001 |
| Short MMSE score (range 0–26), mean (sd) | 24.4 (1.9) | 24.2 (2.3) | 24.4 (2.0) | 24.6 (1.8) | 24.5 (1.7) | 0.002 |
| Body mass index, kg/m2, mean (sd) | 26.4 (4.7) | 27.1 (5.2) | 27.2 (4.8) | 26.3 (4.5) | 25.5 (4.2) | <0.001 |
| Frailty criteria, n (%) | ||||||
| Shrinking/sarcopenia | 939 (15.7) | 223 (18.6) | 182 (15.6) | 325 (14.0) | 209 (16.1) | 0.006 |
| Weakness | 1445 (23.4) | 345 (27.7) | 305 (25.3) | 479 (20.1) | 316 (23.6) | <0.001 |
| Exhaustion | 2667 (43.0) | 617 (49.2) | 523 (43.2) | 983 (41.2) | 544 (40.3) | <0.001 |
| Slowness | 1297 (20.9) | 344 (27.6) | 267 (22.1) | 432 (18.0) | 254 (18.9) | <0.001 |
| Low activity level | 1149 (18.3) | 305 (23.9) | 232 (18.9) | 387 (16.0) | 225 (16.6) | <0.001 |
| Frailty classification at year 6 exam, n (%) | <0.001 | |||||
| Robust | 2195 (34.8) | 353 (27.6) | 414 (33.6) | 927 (38.2) | 501 (36.7) | |
| Intermediate stage | 3047 (48.3) | 626 (48.9) | 602 (48.8) | 1172 (48.3) | 647 (47.4) | |
| Frail | 1065 (16.9) | 301 (23.5) | 217 (17.6) | 329 (13.6) | 218 (16.0) | |
MMSE, mini-mental state examination.
Cross-sectional association between 25(OH)D level and frailty status
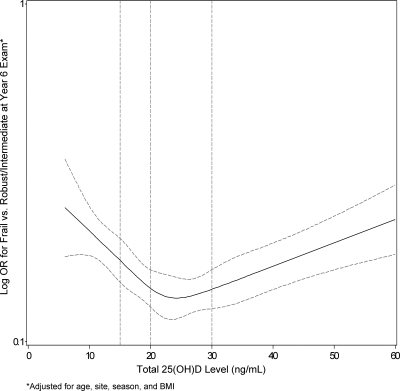
A nonlinear U-shaped pattern between 25(OH)D level and odds of frailty was confirmed in an analysis using restricted cubic spline models that indicated a nadir of risk among women with levels 20.0–29.9 ng/ml and increasing odds of frailty at lower and higher values of 25(OH)D (Fig. 2). The equality of slopes assumption failed for 25(OH)D <15.0 ng/ml vs. ≥30.0 ng/ml (P = 0.02) and for 25(OH)D 20.0–29.9 ng/ml vs. ≥30 ng/ml (P < 0.001) indicating that the association between 25(OH)D and frailty differed above and below this cut point. Findings were similar when 25(OH)D3 level was substituted for total 25(OH)D in the analysis.
Figure 2.
Restricted cubic spline plot of log odds ratios for greater frailty status at baseline by level of 25(OH)D.
Compared with women with 25(OH)D levels 20.0–29.9 ng/ml (referent group), the odds of frailty (vs. robust/intermediate) were higher among those with levels <15.0 ng/ml [multivariable odds ratio (MOR) 1.47, 95% confidence interval (CI), 1.19–1.82], those with levels 15.0–19.9 ng/ml (MOR 1.24, 95% CI 0.99–1.54), and those with levels ≥30.0 ng/ml (MOR 1.32, 95% CI 1.06–1.63) (Table 2). The odds ratio of intermediate/frail status (vs. robust) was higher among women with levels <15.0 ng/ml (MOR 1.30, 95% CI 1.10–1.53) compared with those with levels 20.0–29.9 ng/ml, but odds ratios were smaller in magnitude and not significant for those with levels 15.0-19.9 ng/ml and those with levels ≥30.0 ng/ml. When 25(OH)D3 was substituted for total 25(OH)D in the analyses, results were unchanged (results not shown). In addition, findings were in general agreement when 25(OH)D level was expressed in quartiles (cut points 16.0, 22.0, and 29.0 ng/ml) using quartile 3 as the referent group, but the odds ratios reached significance only for women in quartile 1.
Table 2.
Cross-sectional association between 25(OH)D level and odds of greater frailty status
| Odds ratio (95% CI) by category of 25(OH)D level
|
Odds ratio (95% CI) by quartilesc of 25(OH)D level
|
|||||||
|---|---|---|---|---|---|---|---|---|
| <15.0 ng/ml (n = 1280) | 15.0–19.9 ng/ml (n = 1233) | 20.0–29.9 ng/ml (n = 2428) | ≥30.0 ng/ml (n = 1366) | Q1 (n = 1496) | Q2 (n = 1534) | Q3 (n = 1699) | Q4 (n = 1578) | |
| Base modela | ||||||||
| Intermediate/frail vs. robust | 1.39 (1.19–1.63) | 1.15 (0.99–1.34) | 1.00 (referent) | 1.10 (0.95–1.27) | 1.28 (1.09–1.50) | 1.05 (0.90–1.22) | 1.00 (referent) | 1.01 (0.87–1.17) |
| Frail vs. robust/intermediate | 1.60 (1.33–1.94) | 1.25 (1.02–1.53) | 1.00 (referent) | 1.24 (1.02–1.51) | 1.46 (1.20–1.78) | 1.10 (0.90–1.35) | 1.00 (referent) | 1.13 (0.92–1.39) |
| Mutivariate modelb | ||||||||
| Intermediate/frail vs. robust | 1.30 (1.10–1.53) | 1.13 (0.96–1.32) | 1.00 (referent) | 1.13 (0.97–1.31) | 1.21 (1.02–1.43) | 1.05 (0.90–1.23) | 1.00 (referent) | 1.04 (0.89–1.21) |
| Frail vs. robust/intermediate | 1.47 (1.19–1.82) | 1.24 (0.99–1.54) | 1.00 (referent) | 1.32 (1.06–1.63) | 1.34 (1.08–1.67) | 1.09 (0.87–1.36) | 1.00 (referent) | 1.17 (0.94–1.47) |
Adjusted for age, site, season of blood draw, and body mass index.
Adjusted for age, site, season of blood draw, body mass index, self-reported health status, education, smoking status, alcohol intake, comorbidity score, and short MMSE score.
Quartile cut points: 15.9, 21.9, 28.9 ng/ml.
After adjustment for multiple potential confounders, the odds of each individual frailty component (shrinking/sarcopenia, weakness, exhaustion, slowness, low activity level) were higher among women with 25(OH)D levels <15.0 ng/ml compared with women in the referent group (25(OH)D level 20.0–29.9 ng/ml) (Table 3). Women with 25(OH)D 15.0–19.9 ng/ml and those with levels ≥30 ng/ml had higher odds of weakness. In addition, women with levels 15.0–19.9 ng/ml and those with levels ≥30 ng/ml appeared to have higher odds of shrinking/sarcopenia, slowness, and low activity level, though none of these associations reached significance.
Table 3.
Cross-sectional association between 25(OH)D level and odds of individual frailty components
| Frailty component | Odds ratio (95% CI) by category of 25(OH)D level
|
|||
|---|---|---|---|---|
| <15.0 ng/ml (n = 1280) | 15.0–19.9 ng/ml (n = 1233) | 20.0–29.9 ng/ml (n = 2428) | ≥30.0 ng/ml (n = 1366) | |
| Shrinking/sarcopenia | ||||
| Base modela | 1.36 (1.12–1.66) | 1.18 (0.96–1.44) | 1.00 (referent) | 1.10 (0.90–1.33) |
| Mutivariate modelb | 1.30 (1.05–1.60) | 1.19 (0.97–1.48) | 1.00 (referent) | 1.14 (0.93–1.39) |
| Weakness | ||||
| Base modela | 1.29 (1.09–1.53) | 1.31 (1.11–1.56) | 1.00 (referent) | 1.24 (1.05–1.47) |
| Mutivariate modelb | 1.20 (1.01–1.44) | 1.31 (1.10–1.57) | 1.00 (referent) | 1.25 (1.05–1.49) |
| Exhaustion | ||||
| Base modela | 1.22 (1.05–1.41) | 1.01 (0.88–1.17) | 1.00 (referent) | 0.98 (0.85–1.12) |
| Mutivariate modelb | 1.18 (1.01–1.38) | 0.99 (0.85–1.15) | 1.00 (referent) | 0.97 (0.83–1.13) |
| Slowness | ||||
| Base modela | 1.41 (1.18–1.68) | 1.20 (1.00–1.44) | 1.00 (referent) | 1.13 (0.94–1.36) |
| Mutivariate modelb | 1.27 (1.04–1.54) | 1.17 (0.96–1.42) | 1.00 (referent) | 1.17 (0.96–1.42) |
| Low activity level | ||||
| Base modela | 1.42 (1.18–1.70) | 1.10 (0.91–1.33) | 1.00 (referent) | 1.15 (0.95–1.39) |
| Mutivariate modelb | 1.30 (1.07–1.58) | 1.09 (0.89–1.33) | 1.00 (referent) | 1.21 (0.99–1.47) |
Adjusted for age, site, season of blood draw, and body mass index.
Adjusted for age, site, season of blood draw, body mass index, self-reported health status, education, smoking status, alcohol intake, comorbidity score, and short MMSE score.
Longitudinal association between 25(OH)D level and frailty status
A total of 4551 women classified as nonfrail (robust or intermediate) at baseline constituted the longitudinal cohort. At the follow-up examination an average of 4.5 years later, 1041 women (22.9%) were classified as robust, 2330 (51.2%) were in the intermediate stage, 748 (16.4%) were classified as frail, and 432 (9.5%) had died in the interim period. Compared with the longitudinal cohort, the 691 surviving women classified as nonfrail at baseline who did not provide enough data for frailty status assessment at follow-up were, on average, older (77.2 vs. 75.9 years, P < 0.001) and more likely to be classified as intermediate stage (65.4% vs. 57.0%, P < 0.001), but were no more likely to report poor to fair health status (14.6% vs. 14.1%, P = 0.70) and had similar 25(OH)D levels (23.0 vs. 23.2 ng/ml, P = 0.20).
Compared with women in the referent group [baseline 25(OH)D levels 20.0–29.9 ng/ml], the odds of shrinking/sarcopenia at follow-up were higher among women with levels 15.0–19.9 ng/ml (MOR 1.29, 95% CI 1.03–1.62, P = 0.02) and appeared higher among women with levels ≥30 ng/ml (MOR 1.22, 95% CI 0.98–1.52, P = 0.07). There was no association between baseline 25(OH)D level and odds of the other individual frailty components at follow-up (data not shown).
In base and multivariable models, there was no evidence of an association between 25(OH)D level at baseline and odds of being classified as intermediate/frail/dead (vs. robust) at follow-up (Table 4). Compared with women with 25(OH)D levels 20.0–29.9 ng/ml (referent group), women with levels 15.0–19.9 ng/ML (MOR 1.21, 95% CI 0.99–1.49) (P = 0.06), but not those with levels <15.0 ng/ml or those with levels ≥30.0 ng/ml, appeared to have a higher odds of being classified as frail/dead (vs. robust/intermediate) at follow-up. The odds of death in the interim (vs. robust/intermediate/frail at follow-up) were higher among those with levels <15.0 ng/ml (MOR 1.40, 95% CI 1.04–1.88) and those with levels 15.0–19.9 ng/ml (MOR 1.30, 95% CI 0.97–1.75), though the latter association did not reach significance (P = 0.08). Results were similar when 25(OH)D was expressed as quartiles (cut points 16.0, 22.0, and 29.0 ng/ml, referent group quartile 3). There was higher odds of becoming frail or dying among women in quartile 2 (MOR 1.38, 95% CI 1.13–1.70) and higher odds of death among women in quartile 1 (MOR 1.49, 95% CI 1.09–2.04) and those in quartile 2 (MOR 1.41, 95% CI 1.04–1.92).
Table 4.
Longitudinal association between 25(OH) D level and odds of greater frailty status
| Odds ratio (95% CI) by category of 25(OH)D level
|
Odds ratio (95% CI) by quartilesc of 25(OH)D level
|
|||||||
|---|---|---|---|---|---|---|---|---|
| <15.0 ng/ml (n = 851) | 15.0–19.9 ng/ml (n = 864) | 20.0–29.9 ng/ml (n = 1840) | ≥30.0 ng/ml (n = 996) | Q1 (n = 993) | Q2 (n = 1120) | Q3 (n = 1271) | Q4 (n = 1167) | |
| Base modela | ||||||||
| Intermediate/frail/dead vs. robust | 1.13 (0.90–1.42) | 0.96 (0.78–1.19) | 1.00 (referent) | 1.02 (0.84–1.24) | 1.21 (0.96–1.52) | 1.05 (0.85–1.29) | 1.00 (referent) | 1.04 (0.85–1.27) |
| Frail/dead vs. robust/intermediate | 1.09 (0.89–1.33) | 1.21 (0.99–1.47) | 1.00 (referent) | 0.93 (0.76–1.14) | 1.19 (0.96–1.46) | 1.37 (1.12–1.67) | 1.00 (referent) | 1.05 (0.85–1.29) |
| Dead vs. robust/intermediate/frail | 1.45 (1.10–1.93) | 1.24 (0.93–1.65) | 1.00 (referent) | 1.06 (0.79–1.42) | 1.56 (1.15–2.10) | 1.41 (1.04–1.90) | 1.00 (referent) | 1.22 (0.90–1.65) |
| Mutivariate modelb | ||||||||
| Intermediate/frail/dead vs. robust | 1.12 (0.88–1.41) | 0.94 (0.76–1.16) | 1.00 (referent) | 1.01 (0.83–1.24) | 1.19 (0.94–1.50) | 1.02 (0.83–1.26) | 1.00 (referent) | 1.03 (0.84–1.26) |
| Frail/dead vs. robust/intermediate | 1.03 (0.84–1.28) | 1.21 (0.99–1.49) | 1.00 (referent) | 0.95 (0.77–1.16) | 1.13 (0.91–1.41) | 1.38 (1.13–1.70) | 1.00 (referent) | 1.05 (0.85–1.30) |
| Dead vs. robust/intermediate/frail | 1.40 (1.04–1.88) | 1.30 (0.97–1.75) | 1.00 (referent) | 1.14 (0.85–1.54) | 1.49 (1.09–2.04) | 1.41 (1.04–1.92) | 1.00 (referent) | 1.26 (0.92–1.73) |
Adjusted for age, site, season of blood draw, body mass index, and baseline frailty status.
Adjusted for age, site, season of blood draw, body mass index, baseline frailty status, self-reported health status, education, smoking status, alcohol intake, comorbidity score, and short MMSE score.
Quartile cut points: 15.9, 21.9, 28.9 ng/ml.a
Discussion
Among this cohort of older women, lower (<20 ng/ml) and higher (≥30 ng/ml) 25(OH)D levels were moderately associated with a higher odds of frailty at baseline. In addition, lower 25(OH)D levels in nonfrail women at baseline were modestly associated with an increased risk of incident frailty or death at follow-up an average of 4.5 years later.
Previous smaller cross-sectional studies including or limited to older women have inconsistently observed an independent association between lower 25(OH)D levels and frailty. The Longitudinal Aging Study Amsterdam (LASA) of 1321 adults ≥65 years old (8) measured serum 25(OH)D using a competitive binding protein assay, defined frailty as the presence of three out of nine frailty indicators, and reported that the odds of being classified as frail (vs. non-frail) was 1.7-fold higher among those with levels between 10 and 20 ng/ml and 2.6-fold higher among those with levels <10 ng/ml, compared with that among the referent group (>20 ng/ml). The Women’s Heath and Aging Studies (7) measured serum 25(OH)D using a competitive binding protein assay, defined frailty using similar criteria to those used in the CHS index, and found an age-adjusted 1.7-fold higher odds of being classified as frail (vs. non-frail) among women with levels in the lowest quartile (cut point not reported) compared with that among women with levels in the upper three quartiles, but the association did not persist after further adjustment. Finally, the InCHIANTI study of older Italian men and women (9) measured serum 25(OH)D levels using a RIA, defined frailty with a modified CHS index, and reported that lower levels (<20 ng/ml) were associated with frailty in men, but not in women. While the InCHIANTI study found an independent association between lower 25(OH)D levels (<20 ng/ml) among women and the presence of only one of five individual frailty components (low activity level), the current study using a similar frailty definition observed independent associations between lower 25(OH)D levels (<15 ng/ml or in the case of weakness <20 ng/ml) and the presence of each of five individual frailty dimensions (weakness, shrinking/sarcopenia, exhaustion, slowness, low activity level).
Among women classified as nonfrail (robust or intermediate) at baseline, we found some evidence of an independent association between lower 25(OH)D levels (<20 ng/ml) at baseline and a higher odds of incident frailty or death at follow-up 4.5 years later. The LASA study (8) reported that among 885 nonfrail men and women at baseline, those with 25(OH)D levels <10 ng/ml, but not those with levels between 10–20 ng/ml, had increased odds of being classified as frail (vs. nonfrail) at the 3-yr follow-up exam, compared with that among the referent group (>20 ng/ml). In contrast, an analysis of 463 women in the Women’s Health and Aging Study I (10) classified as nonfrail at baseline reported that the odds of becoming frail at follow-up did not differ between women in the lowest quartile of 25(OH)D as compared with that among women in the top three quartiles. In addition, among a sample of 1267 nonfrail men enrolled in the baseline examination of Osteoporotic Fractures in Men (MrOS) study, there was no association between lower baseline 25(OH)D level (<20 ng/ml) and odds of greater frailty status at a 4.6-year follow-up examination (Ensrud, K. E., T. L. Blackwell, J. A. Cauley, S. R. Cummings, E. Barrett-Connor, T. L. Dam, A. R. Hoffman, J. M. Shikany, N. E. Lane, M. L. Stefanick, E. S. Orwoll, and P. M. Cawthon, submitted for publication).
While previous studies have assumed a linear inverse association between 25(OH)D levels and frailty, our findings suggest that the association between 25(OH)D and frailty status may have a U-shaped pattern with increasing odds of frailty at lower (<20 ng/ml) and higher (≥30 ng/ml) levels. In addition, we found no evidence that higher 25(OH)D levels among nonfrail women were associated with lower risks of incident frailty or death. The cross-sectional association we observed between higher 25(OH)D levels and frailty may at least in part reflect confounding by indication as frail women might be preferentially prescribed vitamin D supplements and thus have higher levels. To address this issue, we examined the association between 25(OH)D3 levels and frailty status because supplemental vitamin D during the study period (1992–1998) was largely reflected in 25(OH)D2 [not 25(OH)D3] levels. While our finding that higher 25(OH)D3 levels were associated with frailty makes confounding by indication less likely, the possibility cannot be eliminated due to the observational design of our study.
Inconsistencies between the findings of studies examining the association between 25(OH)D level and frailty status may in part be explained by differences in study populations, sample size, methods to measure 25(OH)D, cut points used to define 25(OH)D status, definitions of frailty syndrome, or adequacy of adjustment for potential confounders. Strengths of this study include its large sample size, prospective analysis, measurement of total 25(OH)D using the highly accurate LC-MS/MS method, use of a validated definition of frailty status, and adjustment for multiple potential confounders. However, this study has several limitations. Participants were older Caucasian women, and results may not apply to other populations. Power was insufficient to examine the association between severe vitamin D deficiency [25(OH)D level <10 ng/ml] and frailty status. Analyses were adjusted for multiple factors, but the possibility of residual confounding cannot be eliminated. Thus, while vitamin D supplementation has been proposed as a potential therapy for the prevention and treatment of frailty (23), the effect of vitamin D supplementation on incidence and progression of frailty, including among a target population defined by 25(OH)D status, can only be definitively addressed in a study with a randomized trial design. Surviving women not returning to clinic for repeat assessment of frailty status were slightly older at baseline but had similar health status and 25(OH)D levels. Thus, it is unlikely that missing data markedly biased our longitudinal findings toward the null hypothesis of no association. Longitudinal analyses incorporated death as a level in the ordinal frailty status outcome because death is a competing event. While our analyses are unable to determine whether the association between lower 25(OH)D levels and increased risk of incident frailty or death is due solely to the higher risk of mortality among those with lower levels, this is a plausible explanation as we found evidence of an association between baseline 25(OH)D levels and only one individual frailty component (shrinking/sarcopenia) at follow-up. Finally, measurement of 25(OH)D was performed at the baseline examination only. Thus, it was not possible to examine whether change in 25(OH)D levels was associated with incident frailty status.
Lower (<20 ng/ml) and higher (≥30 ng/ml) 25(OH)D levels were associated with greater evidence of frailty among older women and lower 25(OH)D levels among nonfrail women at baseline were associated with an increased risk of incident frailty or death at 4.5 years. These findings suggest that frailty status should be considered as a potential outcome in trials of vitamin D supplementation in older adults.
Footnotes
The Study of Osteoporotic Fractures (SOF) is supported by National Institutes of Health funding. The National Institute on Aging (NIA) provides support under the following grant numbers: AG05407, AR35582, AG05394, AR35584, AR35583, AG005407, AG027576, AG005394, and AG027574.
Disclosure Summary: The authors have nothing to declare.
For editorial see page 5210
Abbreviations: BMI, Body mass index; CHS, Cardiovascular Health Study; CI, confidence interval; CV, coefficient of variation; LC-MS/MS, liquid chromatography tandem mass spectroscopy; MOR, multivariable odds ratio; SOF, Study of Osteoporotic Fractures.
References
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA 2001 Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56:M146–M156 [DOI] [PubMed] [Google Scholar]
- Ensrud KE, Ewing SK, Taylor BC, Fink HA, Stone KL, Cauley JA, Tracy JK, Hochberg MC, Rodondi N, Cawthon PM 2007 Frailty and risk of falls, fracture, and mortality in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci 62:744–751 [DOI] [PubMed] [Google Scholar]
- Cawthon PM, Marshall LM, Michael Y, Dam TT, Ensrud KE, Barrett-Connor E, Orwoll ES 2007 Frailty in older men: prevalence, progression, and relationship with mortality. J Am Geriatr Soc 55:1216–1223 [DOI] [PubMed] [Google Scholar]
- Rockwood K, Howlett SE, MacKnight C, Beattie BL, Bergman H, Hebert R, Hogan DB, Wolfson C, McDowell I 2004 Prevalence, attributes, and outcomes of fitness and frailty in community-dwelling older adults: report from the Canadian study of health and aging. J Gerontol A Biol Sci Med Sci 59:1310–1317 [DOI] [PubMed] [Google Scholar]
- Effectiveness and safety of vitamin D in relation to bone health. Agency for Healthcare Research and Quality 2007. http://www.ahrq.gov/clinic/tp/vitadtp.htm. (Accessed April 19, 2010) [Google Scholar]
- Vitamin D and calcium: a systematic review of health outcomes. Agency for Healthcare Research and Quality 2009. http://www.ahrq.gov/clinic/tp/vitadcaltp.htm. (Accessed April 19, 2010) [Google Scholar]
- Michelon E, Blaum C, Semba RD, Xue QL, Ricks MO, Fried LP 2006 Vitamin and carotenoid status in older women: associations with the frailty syndrome. J Gerontol A Biol Sci Med Sci 61:600–607 [DOI] [PubMed] [Google Scholar]
- Puts MT, Visser M, Twisk JW, Deeg DJ, Lips P 2005 Endocrine and inflammatory markers as predictors of frailty. Clin Endocrinol 63:403–411 [DOI] [PubMed] [Google Scholar]
- Shardell M, Hicks GE, Miller RR, Kritchevsky S, Andersen D, Bandinelli S, Cherubini A, Ferrucci L 2009 Association of low vitamin D levels with the frailty syndrome in men and women. J Gerontol A Biol Sci Med Sci 64:69–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba RD, Bartali B, Zhou J, Blaum C, Ko CW, Fried LP 2006 Low serum micronutrient concentrations predict frailty among older women living in the community. J Gerontol A Biol Sci Med Sci 61:594–599 [DOI] [PubMed] [Google Scholar]
- Cummings SR, Black DM, Nevitt MC, Browner WS, Cauley JA, Genant HK, Mascioli SR, Scott JC, Seeley DG, Steiger P 1990 Appendicular bone density and age predict hip fracture in women. The Study of Osteoporotic Fractures Research Group. JAMA 263:665–668 [PubMed] [Google Scholar]
- Singh RJ, Taylor RL, Reddy GS, Grebe SK 2006 C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J Clin Endocrinol Metab 91:3055–3061 [DOI] [PubMed] [Google Scholar]
- Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L 1986 A data-based approach to diet questionnaire design and testing. Am J Epidemiol 124:453–469 [DOI] [PubMed] [Google Scholar]
- Cummings SR, Block G, McHenry K, Baron RB 1987 Evaluation of two food frequency methods of measuring dietary calcium intake. Am J Epidemiol 126:796–802 [DOI] [PubMed] [Google Scholar]
- Ensrud KE, Lui LY, Taylor BC, Schousboe JT, Donaldson MG, Fink HA, Cauley JA, Hillier TA, Browner WS, Cummings SR 2009 A comparison of prediction models for fractures in older women: is more better? Arch Intern Med 169:2087–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paffenbarger Jr RS, Wing AL, Hyde RT 1978 Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol 108:161–175 [DOI] [PubMed] [Google Scholar]
- Gregg EW, Cauley JA, Stone K, Thompson TJ, Bauer DC, Cummings SR, Ensrud KE 2003 Relationship of changes in physical activity and mortality among older women. JAMA 289:2379–2386 [DOI] [PubMed] [Google Scholar]
- Sheikh JI, Yesavage JA 1986 Geriatric depression scale (GDS): recent evidence and development of a shorter version. Clin Gerontol 5:165–173 [Google Scholar]
- Folstein MF, Robins LN, Helzer JE 1983 The Mini-Mental State Examination. Arch Gen Psychiatry 40:812 [DOI] [PubMed] [Google Scholar]
- Devlin TF, Weeks BJ 1986 Spline functions for logistic regression modeling. Proceedings of the 11th Annual SAS Users Group International Conference. Cary, NC: SAS Institute, Inc.; 646–651 [Google Scholar]
- Durrleman S, Simon R 1989 Flexible regression models with cubic splines. Stat Med 8:551–561 [DOI] [PubMed] [Google Scholar]
- Scott SC, Goldberg MS, Mayo NE 1997 Statistical assessment of ordinal outcomes in comparative studies. J Clin Epidemiol 50:45–55 [DOI] [PubMed] [Google Scholar]
- Cherniack EP, Florez HJ, Troen BR 2007 Emerging therapies to treat frailty syndrome in the elderly. Altern Med Rev 12:246–258 [PubMed] [Google Scholar]