Abstract
Context: Bisphosphonates are the mainstay of anti-osteoporotic treatment and are commonly used for a longer duration than in the placebo-controlled trials. A link to development of atypical subtrochanteric or diaphyseal fragility fractures of the femur has been proposed, and these fractures are currently the subject of a U.S. Food and Drug Administration review.
Objective: Our objective was to examine the risk of subtrochanteric/diaphyseal femur fractures in long term users of alendronate.
Design: We conducted an age- and gender-matched cohort study using national healthcare data.
Patients: Patients were alendronate users, without previous hip fracture, who began treatment between January 1, 1996, and December 31, 2005 (n = 39,567) and untreated controls, (n = 158,268).
Main outcome measures: Subtrochanteric or diaphyseal femur fractures were evaluated.
Results: Subtrochanteric and diaphyseal fractures occurred at a rate of 13 per 10,000 patient-years in untreated women and 31 per 10,000 patient-years in women receiving alendronate [adjusted hazard ratio (HR) = 1.88; 95% confidence interval (CI) = 1.62–2.17]. Rates for men were six and 31 per 10,000 patient-years, respectively (HR = 3.98; 95% CI = 2.62–6.05). The HR for hip fracture was 1.37 (95% CI = 1.30–1.46)) in women and 2.47 (95% CI = 2.07–2.95) in men. Risks of subtrochanteric/diaphyseal fracture were similar in patients who had received 9 yr of treatment (highest quartile) and patients who had stopped therapy after the equivalent of 3 months of treatment (lowest quartile).
Conclusions: Alendronate-treated patients are at higher risk of hip and subtrochanteric/diaphyseal fracture than matched control subjects. However, large cumulative doses of alendronate were not associated with a greater absolute risk of subtrochanteric/diaphyseal fractures than small cumulative doses, suggesting that these fractures could be due to osteoporosis rather than to alendronate.
While alendronate-treated patients experience more fractures, large cumulative doses of alendronate are not associated with greater risk of subtrochanteric/diaphyseal fractures than minimal exposure.
On March 20, 2010, the U.S. Food and Drug Administration issued a drug safety communication, announcing an ongoing review of oral bisphosphonates and atypical subtrochanteric femur fractures (1) and calling on health professionals to be aware of the possible risk of these fractures in patients taking bisphosphonates. In clinical trials, typically of a duration of less than 5 yr, antiresorptives decrease the risk of classical osteoporotic fractures, but it is unclear whether retention of old, less mechanically competent bone tissue could affect mechanical properties in the long term (2,3,4). In some cases, atypical fractures were seen in patients treated for less than 2 yr and thus well within the duration of the original phase III trials (5,6,7). A working definition for atypical femur fractures is currently being developed under the auspices of the American Society of Bone and Mineral Research. Radiological features of atypical femoral fractures reported in the literature have often included a transverse or short oblique fracture line, cortical thickening, and a subtrochanteric or diaphyseal location. Fractures may occur spontaneously, sometimes preceded by prodromal pain, and they may be bilateral or display delayed healing. Recently, Black and co-workers (8) reassessed 284 records of hip and femur fractures that had occurred among just over 14,000 women in three large bisphosphonate clinical trials (9,10,11,12). In this study, which included 51,000 patient-years of follow-up, no significant increase was seen for subtrochanteric/diaphyseal fractures, but confidence intervals (CI) were wide, and only a small number of patients had been followed for more than 5 yr. Similarly, we have previously conducted a relatively small register study in fracture patients who began or did not begin oral alendronate (13). This study, which included just over 5000 patients on alendronate and twice as many control subjects, did not reveal any shift away from classical hip fractures and toward subtrochanteric or diaphyseal fractures. However, fewer than 200 patients had been treated for 6 yr or more with high adherence to treatment. In total, the analysis covered 39,000 patient-years.
Taken together, these studies confirm that the short-term skeletal safety profile for alendronate (2–5 yr) is good but provide scarce information regarding long-term events (5–10 yr or more). Because most patients who begin alendronate have not suffered a previous fracture, we conducted a large national cohort study comprising a total of 679,500 patient-years, including 132,500 patient-years in the 39,567 alendronate users, along with a long-term analysis of patterns of proximal femur fractures in patients exposed to large cumulative doses.
Subjects and Methods
Study design
We conducted a national health register-based, restricted cohort study comparing incident fractures of the hip vs. subtrochanteric or diaphyseal femur fracture between patients exposed to alendronate and matched control subjects. This design simulates the patient selection process in clinical trials (14).
Data sources
Since 1977, the National Hospital Discharge Register has maintained a record of all hospitalizations in Denmark with diagnoses and dates linked to civil registry numbers and from 1995 also outpatient diagnoses. All prescriptions dispensed from pharmacies have been collected in the National Prescription Database since 1995.
Study cohort
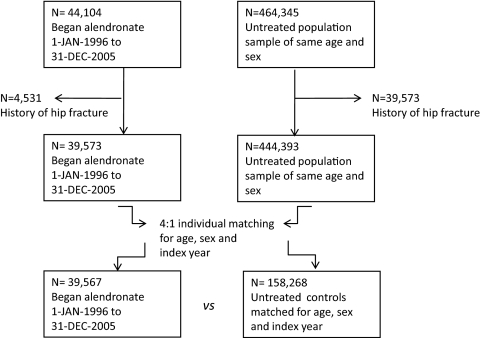
We identified all prescriptions of alendronate (M05BA04 and M05BB03) filled in Denmark between January 1, 1995, and December 31, 2005, using 1995 as a run-in year to identify incident (i.e. new) alendronate users. Patients who began alendronate without having redeemed a prescription for osteoporosis drugs in the preceding years, back to January 1, 1995, were considered incident users and the year of their first prescription the index year. From a large untreated population sample of subjects who were of the same gender, born in the same year, and alive at the time when the proband filled the first prescription for alendronate, we randomly selected four matching subjects (Fig. 1). We excluded persons with previous hip fractures to avoid the risk of misclassifying admissions due to fracture complications as new incident fractures. Six patients (0.015%) were excluded from the analysis because they could not be assigned four matched control subjects.
Figure 1.
STROBE (STrengthening the Reporting of OBservational studies in Epidemiology; http://www.strobe-statement.org/) Study chart for the matched cohort analysis. Please refer to text for details.
Statistical analyses
The primary endpoint was incident fracture of the subtrochanteric femur (S72.2) and the femoral diaphysis (S72.3). Because of the suspected pathophysiology, we also prespecified an analysis in which fractures, in both arms of the cohort, were not counted if sustained less than 6 months after the index date, and here we excluded patients and their matched control subjects if the exposure to alendronate had been less than 180 defined daily doses (DDD) in the first 12 months of treatment (medication possession ratio <50%).
Demographics were compared by t tests and χ2 analysis. We used Cox proportional hazards models incorporating time to fracture, death, or end of study (December 31, 2006) to obtain crude and adjusted hazard ratios (HR). We used SPSS version 18.0 and SAS version 9.1. A propensity score-matched analysis was undertaken as a sensitivity analysis. Propensity score matching was done in SAS using the Gmatch macro http://mayoresearch.mayo.edu/mayo/research/biostat/upload/gmatch.sas.
Ethics
Permission was obtained to access data from the hospital discharge register and the prescriptions database from the National Board of Health, The Danish Medicines Agency, and Statistics Denmark (reference 702538) pursuant to guidelines approved by the Data Protection Agency. The study was not a clinical trial. We did not have access to patient notes, x-rays, patient names, addresses, or civil registry numbers. Ethics committee approval was not needed.
Results
Main analyses
The analysis covered 132,500 patient-years in the alendronate cohort and 547,000 patient-years in the control cohort (Table 1). The mean follow-up time was 3.4 yr, because most subjects had begun alendronate toward the end of the inclusion period. However, among the 197,835 subjects who made up the study population, 34,204 were included early enough to provide 5–10 yr of follow-up, and 1920 subjects provided more than 10 yr of follow-up time. Three percent of control subjects and 3.1% of alendronate-treated subjects emigrated (χ2, P = 0.25); repeating the analyses without these persons did not alter the results.
Table 1.
Baseline demographics for subjects in the matched cohort analysis
| Controls (n = 158,268) | Alendronate (n = 39,567) | P value | |
|---|---|---|---|
| Age (yr) | 69.8 ± 11.6 | 69.8 ± 11.6 | Matched |
| Female (%) | 82.8 | 82.8 | Matched |
| Follow-up (yr) | 3.4 ± 2.2 | 3.4 ± 2.3 | <0.001 |
| Medications | |||
| Hormone therapy baseline (any, last 12 months) (%) | 8.3 | 7.4 | <0.001 |
| Prednisolone at baseline (any, last 12 months) (%) | 3.9% | 26.0% | <0.001 |
| Prednisolone (mg in the last 12 months) | 60.6 ± 420 | 791.3 ± 1772 | <0.001 |
| Number of comedications | 5.2 ± 4.7 | 8.6 ± 6.1 | <0.001 |
| Fracture history (%) | |||
| Any fracture after age 50 | 16.1 | 29.7 | <0.001 |
| Previous spine fracture | 0.6 | 4.7 | <0.001 |
| Previous forearm fracture | 6.5 | 12.1 | <0.001 |
| Previous humerus fracture | 2.3 | 5.2 | <0.001 |
| Previous hip fracture | 0.0 | 0.0 | Exclusion |
| More than one previous fracture | 2.2 | 6.7 | <0.001 |
| Comorbid conditions (%) | |||
| Charlson index, mean | 0.5 ± 1.2 | 0.8 ± 1.5 | <0.001 |
| 0 | 81.3 | 70.7 | <0.001 |
| 1–3 | 15.5 | 23.6 | |
| >3 | 3.2 | 5.7 | |
| Cardiac failure | 7.0 | 10.2 | <0.001 |
| Malignancy | 4.1 | 5.7 | <0.001 |
| Pulmonary disease | 3.3 | 12.5 | <0.001 |
| Cerebrovascular disease | 3.2 | 4.0 | <0.001 |
| Diabetes, w/o complications | 2.9 | 3.2 | <0.001 |
| Diabetes, with complications | 0.8 | 0.8 | 0.90 |
| Myocardial infarction | 2.0 | 2.6 | <0.001 |
| Peripheral vascular disease | 1.6 | 2.6 | <0.001 |
| Ulcer disease | 1.1 | 2.4 | <0.001 |
| Rheumatic or collagen disorder | 1.0 | 7.5 | <0.001 |
| Dementia | 0.9 | 0.9 | 0.44 |
| Renal failure | 0.4 | 0.4 | 0.24 |
| Mild liver disease | 0.3 | 1.1 | <0.001 |
| Severe liver disease | 0.0 | 0.2 | <0.001 |
| Hemiplegia | 0.1 | 0.3 | <0.001 |
| Solid metastatic tumor | 0.3 | 0.6 | <0.001 |
Treatment-naive patients without previous hip fracture beginning alendronate in Denmark between January 1, 1996, and December 31, 2005, with 4:1 matched bisphosphonate-unexposed control subjects, matched for age, sex, and index year.
Patients who began alendronate had significantly more comorbid conditions, in particular pulmonary or rheumatic diseases, were more likely to have a history of fractures and received more medications than the bisphosphonate unexposed controls (Table 1).
Subtrochanteric and diaphyseal fractures
The incidence rate was 13 per 10,000 patient-years in untreated women and 31 per 10,000 patient-years in women receiving alendronate (Table 2), with a mean time to fracture of 2.5 yr in the untreated group and 2.6 yr in the treated group. The rates for men were 6 and 31 per 10,000 patient-years, respectively. In untreated subjects, the risk of both fractures increased significantly with age, Charlson index, the number of comedications used, previous fracture history, and female gender, with no significant impact of glucocorticoid use. In patients on alendronate, the risk increased with age, the number of comedications, and previous fracture history, with no effect of Charlson index or glucocorticoid use.
Table 2.
Fracture rates in 39,567 patients who began alendronate 1996–2005, compared (1:4) with 158,268 age- and sex-matched control subjects from the background population
| n | Patient-years | n | Hip, rate per 10,000 patient-years | n | Subtrochanteric or diaphyseal, rate per 10,000 patient-years, total (subtrochanteric/diaphyseal) | Subtrochanteric or diaphyseal fractures (% of all proximal femur fractures) | |
|---|---|---|---|---|---|---|---|
| Men | |||||||
| Untreated | 27276 | 95826 | 469 | 49 | 59 | 6 (4.0/2.6) | 10.9 |
| Alendronate | 6819 | 19806 | 264 | 133 | 61 | 31 (19.2/13.1) | 18.9 |
| Women | |||||||
| Untreated | 130992 | 451333 | 4446 | 99 | 578 | 13 (7.9/5.7) | 11.6 |
| Alendronate | 32748 | 112748 | 1852 | 164 | 351 | 31 (19.4/13.3) | 15.9 |
Test for difference in proportion of subtrochanteric/diaphyseal fractures between alendronate-exposed and unexposed persons: χ2 = 33.4; P < 0.0001.
The difference in fracture rates between the alendronate and control cohorts is reflected in the crude HR of 2.66 (95% CI = 2.35–3.02) in the Cox proportional hazards analysis (Table 3). Treating subtrochanteric and diaphyseal fractures as a joint outcome in the analysis was supported by the almost identical risk estimates found for these two fracture subtypes. In the analysis where fractures occurring in the first 6 months after the index date did not qualify as outcomes, and subjects who took fewer than 180 DDD of alendronate in the first year of treatment were excluded along with their matched controls, the fully adjusted HR for subtrochanteric fractures was 1.89 (95% CI = 1.59–2.25). A statistically significant interaction with gender was noted, demonstrating a greater risk increase with alendronate treatment in men than in women, especially for diaphyseal fractures. Although the risk of subtrochanteric fractures was higher in the alendronate group, the risk was significantly lower with greater cumulative amounts of alendronate. Introducing a covariate to capture the incremental effect of taking more than 1825 DDD of alendronate (equivalent to 5 yr of use) showed a 30% risk decrease with the higher cumulative dose over lower amounts of alendronate (HR = 0.70; 95% CI = 0.54–0.92; Table 4).
Table 3.
Cox proportional hazards analysis
| Outcome | Fractures occurring at any time after initiation of alendronate (first exposure carried forward)a
|
Fractures occurring more than 6 months after initiation of alendronate and requiring intake of at least 180 DDD of alendronate in the first year for subjects to be considered alendronate exposed (per protocol)b
|
||
|---|---|---|---|---|
| HR crude | HR adjustedc | HR crude | HR adjustedc | |
| Any proximal femur | 1.89 (1.80–1.98) | 1.51 (1.43–1.59) | 1.64 (1.54–1.75) | 1.30 (1.21–1.39) |
| Women | 1.76 (1.67–1.85) | 1.42 (1.35–1.51) | 1.53 (1.43–1.64) | 1.23 (1.14–1–32) |
| Men | 3.01 (2.61–3.48) | 2.66 (2.25–3.14) | 2.71 (2.23–3.29) | 2.29 (1.83–2.87) |
| Hip (neck or pertrochanteric) | 1.81 (1.71–1.90) | 1.45 (1.37–1.53) | 1.57 (1.47–1.68) | 1.25 (1.16–1.34) |
| Women | 1.69 (1.60–1.79) | 1.37 (1.30–1.46) | 1.48 (1.38–1.59) | 1.19 (1.11–1.29) |
| Men | 2.78 (2.39–3.24) | 2.47 (2.07–2.95) | 2.41 (1.96–2.96) | 2.03 (1.60–2.57) |
| Subtrochanteric/diaphyseal femur | 2.66 (2.35–3.02) | 2.02 (1.77–2.32) | 2.48 (2.12–2.92) | 1.89 (1.59–2.25) |
| Women | 2.43 (2.13–2.78) | 1.88 (1.62–2.17) | 2.21 (1.86–2.63) | 1.70 (1.41–2.05) |
| Men | 4.89 (3.39–7.05) | 3.98 (2.62–6.05) | 5.87 (3.60–9.55) | 5.02 (2.89–8.72) |
| Subtrochanteric femur | 2.67 (2.28–3.13) | 2.06 (1.74–2.45) | 2.50 (2.03–3.07) | 1.88 (1.51–2.35) |
| Women | 2.44 (2.06–2.90) | 1.90 (1.58–2.28) | 2.22 (1.78–2.77) | 1.68 (1.32–2.13) |
| Men | 4.78 (3.02–7.56) | 4.02 (2.40–6.75) | 5.26 (2.98–9.30) | 4.48 (2.35–8.57) |
| Diaphyseal femur | 2.59 (2.14–3.13) | 1.95 (1.59–2.40) | 2.40 (1.88–3.07) | 1.87 (1.43–2.44) |
| Women | 2.36 (1.93–2.90) | 1.83 (1.47–2.27) | 2.13 (1.64–2.76) | 1.19 (1.10–1.30) |
| Men | 4.84 (2.75–8.50) | 3.79 (1.97–7.31) | 7.93 (3.27–19.21) | 7.57 (2.82–20.32) |
All cells, P < 0.001. The population included treatment-naive patients without previous hip fracture beginning alendronate in Denmark between January 1, 1996, and December 31, 2005. Outcomes were followed to December 31, 2006. DDD was 10 mg of alendronate. Subjects were matched for age, sex, and index year (Fig. 1).
For alendronate-exposed, subjects, n = 39,567 (132,500 patient-years); for controls, n = 158,268 (547,000 patient-years).
For alendronate-exposed, subjects, n = 29,706 (101,000 patient-years); for controls, n = 118,824 (400,800 patient-years).
Adjusted for sex, Charlson index, hormone therapy in the last year, prednisolone use in the last year, fracture after age 50, and number of comedications.
Table 4.
Cox proportional hazards analysis (per protocol)
| Fracture outcome | HR crude | HR adjusteda |
|---|---|---|
| Subtrochanteric/diaphyseal femur | ||
| Aln < 1825 DDD | 3.12 (2.59–3.79) | 2.19 (1.79–2.69) |
| Aln ≥ 1825 DDD | 1.83 (1.45–2.31) | 1.54 (1.21–1.97) |
| Hip (neck or pertrochanteric) | ||
| Aln < 1825 DDD | 2.01 (1.93–2.26) | 1.51 (1.39–1.64) |
| Aln ≥ 1825 DDD | 1.02 (0.91–1.13) | 0.91 (0.81–1.02) |
All cells, P < 0.001. The population included treatment-naive patients without previous hip fracture beginning alendronate in Denmark between January 1, 1996, and December 31, 2005. Outcomes followed to December 31, 2006. DDD was 10 mg of alendronate. The threshold of 1825 DDD was chosen because it corresponds to 5 yr of alendronate (Aln) treatment in a patient with complete refill compliance. Subjects were matched for age, sex, and index year (Fig. 1). Fractures had to occur more than 6 months after initiation of alendronate and require intake of at least 180 DDD of alendronate in the first year for subjects to be considered alendronate exposed. Comparisons between higher cumulative dose (≥1825 DDD) and lower amounts of alendronate: risk of subtrochanteric/diaphyseal fracture HR = 0.70 (95% CI = 0.54–0.92); hip fracture HR = 0.60 (95% CI = 0.53–0.68).
Adjusted for sex, Charlson index, hormone therapy in the last year, prednisolone use in the last year, fracture after age 50 and number of comedications.
Hip fractures
The incidence was 164 and 99 per 10,000 patient-years in women receiving vs. not receiving alendronate (Table 2). For men, the corresponding rates were 133 and 49. Risks in untreated subjects increased with age, glucocorticoid use, Charlson index, the number of comedications used, previous fracture history, and female gender. In alendronate-treated patients, hip fracture risk depended on age, the number of comedications, and glucocorticoids, with only a borderline significant effect of Charlson index. There was no effect of gender (P = 0.39). The crude HR for hip fractures with alendronate use was 1.81 (1.71–1.90), with higher HR in men than in women (Table 3), with a positive test for interaction between gender and alendronate. The risk increase remained significant after adjustment for key covariates. In the main analysis of fractures occurring more than 6 months after beginning therapy, the HR remained lower than that seen for subtrochanteric/diaphyseal fractures but still significantly above 1.0 (HR = 1.25; 95% CI = 1.16–1.34). Similar to the finding for subtrochanteric/diaphyseal fractures, the risk was lower in patients who filled prescriptions for more than 1825 DDD (equivalent to 5 yr) (HR = 0.60; 95% CI = 0.53–0.68; P < 0.001; Table 4).
Proximal femur fractures
This is the pooled hip, subtrochanteric, and diaphyseal fracture rate. Results were similar to those found for hip fracture alone, but results are provided in Table 2 for completeness.
Long-term dose-response analysis
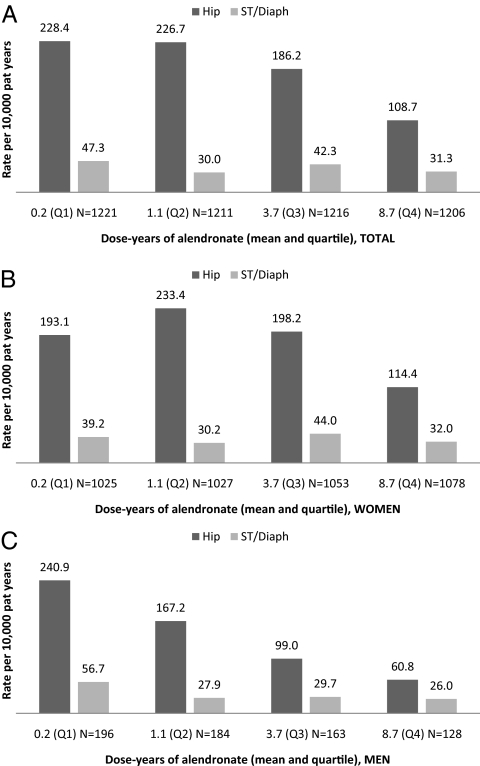
In this analysis, we considered only patients who had begun alendronate in the first years after introduction on the market, i.e. between January 1, 1996, and December 31, 1999, to assess whether the risk of subtrochanteric/diaphyseal fractures was higher or lower with large cumulative doses and long duration of therapy. We identified 4854 treatment-naive patients who began alendronate and divided this study population into quartiles of cumulative alendronate exposure. The mean follow-up was 9.2 yr in survivors and 6.6 yr in total. The mean intake of alendronate in the lowest quartile (Q1) was equivalent to only 2 months, whereas the highest quartile (Q4) had a mean intake equivalent to having taken alendronate at 100% medication possession ratio for 8.7 yr (Fig. 2). The rate of hip fractures was significantly lower with high cumulative exposure (trend test P < 0.001). A similar trend was observed for subtrochanteric/diaphyseal fracture, although this was not statistically significant (P = 0.22). Subtrochanteric and diaphyseal fractures accounted for 17% of proximal femur fractures in Q1 of exposure and 22% in Q4, but this was accounted for by a decrease in classical hip fractures with a smaller, nonsignificant, decline in the rate of subtrochanteric/diaphyseal fractures. Subdividing the subtrochanteric/diaphyseal fracture category into it subtrochanteric vs. diaphyseal fractures did not alter the results (not shown). Due to inclusion of patients with long-term data only, the dose-response analysis had less resolving power than the matched fracture-free survival analyses, but patient numbers were sufficient to provide 90% power for detecting a doubling of risk between Q1 and Q4 of cumulative exposure.
Figure 2.
Analysis of fracture incidence as a function of cumulative alendronate dose [quartiles (Q)]. This study population consisted of alendronate users with long-term observational data: treatment-naive patients without previous hip fracture, who began alendronate between January 1, 1996, and December 31, 1999 (n = 4854). There were 2466 deaths, the mean follow-up was 9.2 yr in survivors and 6.6 yr in the total cohort. The mean cumulative doses were 65 (Q1), 389 (Q2), 1357 (Q3), and 3172 (Q4) DDD. This corresponds to the amount of alendronate taken in 2 months, 1.1 yr, 3.7 yr, and 8.7 yr in a subject with 100% refill compliance or 2.5 months, 1.4 yr, 4.6 yr, and 10.9 yr at 80% refill compliance.
Sensitivity analyses
Matching procedure
An alternative, 2:1 propensity score-matched analysis was also undertaken, comprising 31,824 alendronate users and 63,668 propensity score-matched controls. Propensity scores for beginning alendronate were based on a logistic regression analysis with 41 covariates including age, gender, index year, the number of comedications, and dummy variables coding for glucocorticoid use, hormone therapy (systemic estrogens), previous fracture by anatomical location, and each individual Charlson index component. This analysis, which is provided in the Supplemental Data (published on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org), showed results for hip fracture risk that closely followed those of the main analysis (HR = 1.23; 95% CI = 1.14–1.34), but the HR for subtrochanteric/diaphyseal fracture was slightly lower, 1.68 (95% CI = 1.45–1.95).
Role of changing therapy to other bisphosphonates
We addressed the potential impact on our results if cumulative dose of all bisphosphonates was entered into the analyses instead of cumulative alendronate use. The dose-response relationships shown in Table 4 showed unaltered HR and CI, whether bisphosphonates other than alendronate were included in the cumulative dose or not. Adding use of bisphosphonates other than alendronate to the long-term subanalyses added only 2% to the cumulative exposure and did not alter the conclusions. Thus, subtrochanteric/diaphyseal fracture rates were 45, 31, 44, and 31 per 10,000 patient-years, going from the lowest to the highest quartile of cumulative bisphosphonate exposure.
Forearm fracture
As was the case for hip fracture, the risk of forearm fracture was higher in alendronate-treated subjects compared with population controls (HR = 1.39; 95% CI = 1.25–1.54). Long-term fracture rates in alendronate users were 128 (Q1), 104 (Q2), 96 (Q3), and 100 (Q4) per 10,000 patient-years (trend P = 0.09), indicating a nonsignificant decline in risk with increasing cumulative exposure.
Systemic glucocorticoid use
Repeating the analyses without subjects who were exposed to systemic glucocorticoids confirmed the results of the main analysis.
Discussion
This study confirms that patients who begin alendronate have an increased prevalence of most chronic comorbid conditions compared with the background population. It also shows, contrary to our findings in a smaller population of fracture patients (13), that the relative increase in subtrochanteric and diaphyseal fractures in an alendronate-treated cohort is greater than the relative increase in classical hip fractures. Absolute risks were low, however, and the dose-response analyses suggest that there is not an increasing risk of these fractures with growing amounts of alendronate exposure. Rather, alendronate may be less efficacious in preventing fractures at these locations, as suggested by the recent patient level analysis of bisphosphonate trials (8). Thus, patients taking alendronate for less than 5 yr were at greater risk of both classical hip fractures and subtrochanteric fractures than patients who received longer treatment. Among alendronate-exposed patients with long-term data, subtrochanteric/diaphyseal fracture rates were not higher in those who stayed on therapy and received on average just under 9 yr of treatment (top quartile) than in patients who had stopped therapy after having taken the equivalent of 3 months of treatment (bottom quartile). In accordance with the known antifracture efficacy of alendronate, the rate of hip fracture in this group was half of that seen in the low-exposure group. Repeating the analysis while taking into account shifts in therapy from alendronate to other bisphosphonates produced identical results because few patients who stopped alendronate went on to become persistent users of another bisphosphonate. Furthermore, as can be ascertained from the baseline demographics table, bisphosphonate users differ from their age- and gender-matched population controls in terms of major comorbidities and risk factors for fracture. Our preference was to present the results in the form of simple risk estimates with and without adjustment for these measured confounders. In this analysis, matching was by gender and age only, whereas other risk factors in the database (glucocorticoids, previous fractures, and comorbid conditions) were addressed within the Cox proportional hazards analysis itself. We did, however, also provide a more closely matched study cohort using propensity score matching that confirmed the findings. It is important to bear in mind in both analyses that we could take into account only information captured by health registers. Bone mineral density, body mass index, smoking, and family history are all well-established risk factors that cannot be captured from national registers at the present time. Because patients with low bone mineral density or family history of fracture are more likely to receive bisphosphonates, this could inflate the risk estimates, but this would affect both classical hip fractures and subtrochanteric/diaphyseal fractures. Our findings support recent U.S. epidemiological findings. Using MarketScan and public data from the National Hospital Discharge Survey, Nieves et al. (15) examined time trends for femur fractures and described the epidemiology of the distinct subtypes. There was a significant decline in the age-specific incidence rates of hip fractures but no change in subtrochanteric/diaphyseal fractures despite increasing use of bisphosphonates. Furthermore, a comparison of the medical and prescription history between noncases and hip, subtrochanteric, femoral shaft, and lower femur fracture cases revealed little if any observable differences between patients who suffered subtrochanteric fractures compared with patients with hip fractures.
The most important limitation to the present study is that we could not assess radiographs or patient notes to verify fracture locations and examine signs of atypia. In addition, information on whether fractures were bilateral or caused by low-energy trauma could often not be determined from the data record, so we could not include these features in the outcome definition. A recent review of the operation registry of the hospitals in two Swedish healthcare districts (16) found that patients receiving bisphosphonates were 46 times more likely to develop atypical fragility fractures of the femur, but the rates of atypical fragility fractures of the femur were low, 10 per 10,000 patient-years. CI on these estimates were wide, due to the rarity of such fractures. Interestingly, these numbers are in excellent agreement with those found in the present study, which used a different methodology and studied a different population. In the present study, subtrochanteric/diaphyseal fractures as such occurred at a rate of 31 per 10,000 patient-years, not only in the total treated cohort but also in patients exposed to high cumulative doses (mean 8.7 yr at 100% refill compliance). Assuming that fewer than one third of subtrochanteric fractures are atypical in x-ray appearance (7), a rate of 10 per 10,000 patient-years in alendronate-treated patients is fully compatible with our results. Certainly, not all transverse fractures of the femoral diaphysis are atypical. Thus, a Finnish population-based study reported that 40% of all femoral shaft fractures in adults treated in their area were transverse and that another 17% of fractures were oblique-transverse (17). The American Society for Bone and Mineral Research is currently developing diagnostic criteria for atypical fragility fractures of the femur.
In conclusion, this large national health database study confirms that subtrochanteric and diaphyseal fractures are more common in alendronate users than can be explained by age, sex, and available information on comorbidity and comedications, but there seems to be no relationship to the cumulative amount of alendronate used. The first finding establishes the presence of an association, whereas the second finding suggests that this association may be driven by patient factors rather than alendronate. In a worst-case scenario, one of 100% causality and one where all subtrochanteric and diaphyseal fractures had the hallmark of atypia, the benefits of alendronate would still greatly outweigh the risks.
Supplementary Material
Footnotes
The study received grant support from Kaptajnløjtnant Harald Jensen og Hustrus Fond, Denmark. The funding source had no role in any part of the design, execution, interpretation, or presentation of this study.
Disclosure Summary: B.A. received consultancy fees from Nycomed, Amgen, and Novartis; research grants from Roche; and speaker’s fees from Servier, Eli Lilly, and MSD. P.E. received consultancy fees from Nycomed and Amgen and speaker’s fees from Roche and Eli Lilly. R.E. received consulting or advisory-board fees from Amgen, Novartis, Procter & Gamble, Servier, Ono Pharmaceutical, and GlaxoSmithKline; lecture fees from Eli Lilly; and grant support from AstraZeneca, Procter & Gamble, and Novartis. R.E. was partly funded by the National Institute for Health Research (NIHR) via its Biomedical Research Units funding scheme for musculoskeletal health. The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the NIHR, or the Department of Health. B.A. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. B.A., P.E., and R.E. jointly designed the study, interpreted the results, and wrote the manuscript.
First Published Online September 15, 2010
For editorial see page 5207
Abbreviations: CI, Confidence interval; DDD, defined daily dose; HR, hazard ratio.
References
- US Food and Drug Administration 2010 Oral bisphosphonates: ongoing safety review of atypical subtrochanteric femur fractures. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm204127.htm [Google Scholar]
- Chapurlat RD, Arlot M, Burt-Pichat B, Chavassieux P, Roux JP, Portero-Muzy N, Delmas PD 2007 Microcrack frequency and bone remodeling in postmenopausal osteoporotic women on long-term bisphosphonates: a bone biopsy study. J Bone Miner Res 22:1502–1509 [DOI] [PubMed] [Google Scholar]
- Stepan JJ, Burr DB, Pavo I, Sipos A, Michalska D, Li J, Fahrleitner-Pammer A, Petto H, Westmore M, Michalsky D, Sato M, Dobnig H 2007 Low bone mineral density is associated with bone microdamage accumulation in postmenopausal women with osteoporosis. Bone 41:378–385 [DOI] [PubMed] [Google Scholar]
- Allen MR, Burr DB 2008 Skeletal microdamage: less about biomechanics and more about remodeling. Clin Rev Bone Miner Metab 6:24–30 [Google Scholar]
- Ing-Lorenzini K, Desmeules J, Plachta O, Suva D, Dayer P, Peter R 2009 Low-energy femoral fractures associated with the long-term use of bisphosphonates: a case series from a Swiss university hospital. Drug Saf 32:775–785 [DOI] [PubMed] [Google Scholar]
- Lee P, van der Wall H, Seibel MJ 2007 Looking beyond low bone mineral density: multiple insufficiency fractures in a woman with post-menopausal osteoporosis on alendronate therapy. J Endocrinol Invest 30:590–597 [DOI] [PubMed] [Google Scholar]
- Neviaser AS, Lane JM, Lenart BA, Edobor-Osula F, Lorich DG 2008 Low-energy femoral shaft fractures associated with alendronate use. J Orthop Trauma 22:346–350 [DOI] [PubMed] [Google Scholar]
- Black DM, Kelly MP, Genant HK, Palermo L, Eastell R, Bucci-Rechtweg C, Cauley J, Leung PC, Boonen S, Santora A, de Papp A, Bauer DC; Fracture Intervention Trial Steering Committee; HORIZON Pivotal Fracture Trial Steering Committee 2010 Bisphosphonates and fractures of the subtrochanteric or diaphyseal femur. N Engl J Med 362:1761–1771 [DOI] [PubMed] [Google Scholar]
- Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE 1996 Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 348:1535–1541 [DOI] [PubMed] [Google Scholar]
- Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, Satterfield S, Wallace RB, Bauer DC, Palermo L, Wehren LE, Lombardi A, Santora AC, Cummings SR 2006 Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 296:2927–2938 [DOI] [PubMed] [Google Scholar]
- Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R, Rubin SM, Scott JC, Vogt T, Wallace R, Yates AJ, LaCroix AZ 1998 Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 280:2077–2082 [DOI] [PubMed] [Google Scholar]
- Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR 2007 Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356:1809–1822 [DOI] [PubMed] [Google Scholar]
- Abrahamsen B, Eiken P, Eastell R 2009 Subtrochanteric and diaphyseal femur fractures in patients treated with alendronate: a register-based national cohort study. J Bone Miner Res 24:1095–1102 [DOI] [PubMed] [Google Scholar]
- Concato J, Shah N, Horwitz RI 2000 Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med 342:1887–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieves JW, Bilezikian JP, Lane JM, Einhorn TA, Wang Y, Steinbuch M, Cosman F 2010 Fragility fractures of the hip and femur: incidence and patient characteristics. Osteoporos Int 21:399–408 [DOI] [PubMed] [Google Scholar]
- Schilcher J, Aspenberg P 2009 Incidence of stress fractures of the femoral shaft in women treated with bisphosphonate. Acta Orthop 80:413–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen ST, Pihlajamäki HK, Avikainen VJ, Böstman OM 2000 Population based epidemiologic and morphologic study of femoral shaft fractures. Clin Orthop Relat Res 372:241–249 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.