Abstract
Purpose
To use regenerating Planaria Dugesia dorotocethala as a model to determine whether an intermittent modulated extremely low frequency electro-magnetic field (ELF-EMF) produces elevated levels of the heat shock protein hsp70 and stimulates intracellular pathways known to be involved in injury and repair. We focused on serum response element (SRE) binding through the extra-cellular signal-regulated kinase (ERK) cascade.
Materials and methods
Planaria were transected equidistant between the tip of the head and the tip of the tail. Individual head and tail portions from the same worm were exposed to a 60 Hertz 80 milliGauss ELF- EMF for one hour twice daily for 15 days post transection under carefully controlled exposure conditions. The regenerating heads and tails were photographed and the lengths measured at 3-day intervals. In other experiments, the timing of the appearance of pigmented eyes was monitored in the tail portion at 12 hour intervals following transection in both ELF-EMF exposed and sham control. In some experiments protein lysates were analyzed for hsp70 levels, doubly phosphorylated (pp)-ERK, Elk-1 kinase activity and serum response factor (SRF) -SRE binding.
Results
ELF-EMF exposure during the initial 3-days post surgery caused a significant increase in regeneration for both heads and tails, but especially tails. The first appearance of eyes occurred at day seven post-transection in tail portions exposed to ELF-EMF. In the sham control tail samples the initial appearance of eyes occurred 48 hours later. Concurrently, ELF-EMF-exposed heads and tails exhibited an elevation in the level of hsp70 protein, an activation of an ERK cascade, and an increase in SRF-SRE binding.
Conclusion
Exposures to a modulated sinusoidal ELF-EMF were delivered by a Helmholtz configuration at a frequency of 60Hz and 80mG twice a day for one hour. This is accompanied by an increase in hsp70 protein levels, activation of specific kinases and up-regulation of transcription factors that are generally associated with repair processes.
Keywords: Extremely low frequency-electromagnetic fields, hsp70, serum response element, ERKMAPK, transcription factor, regeneration
Introduction
Extremely low frequency electro-magnetic fields (ELF-EMF) have been used in clinical settings to prevent ischemia reperfusion injury (Albertini et al. 1999; DiCarlo, et al. 1999; Shallom et al. 2002; George et al. 2008), accelerate bone and wound healing, and to promote neural regeneration following injury (reviewed in Bassett 1995). Previous reports showed that exposure of cells to an ELF-EMF up-regulates the heat shock gene HSP70 and induces elevated levels of hsp70 protein (Goodman and Henderson 1988; Goodman et al. 1994; Goodman and Blank, 1998; Lin et al. 1998a; 1998b; 1999a; 2001; Han et al. 1998; Carmody et al. 2000).
Activation of the HSP70 occurs through the binding of heat shock factor 1 (HSF 1) to a heat shock element (HSE) in a region of the HSP70 promoter that contains three nCTCTn consensus sequences (referred to as electromagnetic field response elements; EMRE). The EMRE have a different consensus sequence from that which responds to elevated temperature in the heat shock domain (Lin et al. 1997; Morimoto et al. 1998). The energy of ELF-EMF required to induce elevated hsp70 protein levels is approximately fourteen orders of magnitude lower than the energy required to induce elevated hsp70 by thermal stress (Blank and Goodman 1997; Goodman and Blank 1998; Goodman and Blank 2002; Lin et al. 1997). Unlike thermal stress, ELF-EMF induction of hsp70 is neither sensitive to increased temperature nor does it turn off baseline protein synthesis as occurs with heat shock (Goodman and Henderson 1988; Goodman and Blank 1998). The hsp70 protein is cytoprotective and has been shown to act as a ‘chaperone’ to refold and restore the function of cellular proteins damaged by the stress of injury or inflammation (Morimoto 1998; Kultz 2005). The efficacy of applied ELF-EMF in regeneration protocols is probably due, at least in part, to increased levels of this cytoprotective stress response protein.
There is convincing evidence that ELF-EMF elevate the levels of hsp70 protein via the activation of the extracellular signal regulated kinases, ERK, members of the mitogen-activated protein kinase (MAPK) family at radio-frequency as well as at ELF-EMF (Jin et al. 2000; Leszczynski et al. 2002; Weisbrot et al. 2003; Friedman et al, 2007). Studies using a variety of cell types have shown that activated ERK can phosphorylate the transcription factor Elk-1 and that this ultimately leads to increased serum response factor- serum response element (SRF-SRE) binding and the initiation of transcription. This cascade, which is triggered by injury, has been shown to occur following transection of peripheral nerves in both invertebrates and rodents (Ambron et al. 1995; Lin et al. 2003). Given the ability of an ELF-EMF to upregulate HSP70 gene, levels of the stress response protein hsp70 and ERK were examined during regeneration of Planaria Dugesia dorotocethala. Because their tissues regenerate from pluripotent stem cells (neoblasts), Planaria are particularly well suited for studies of regeneration following injury. Additionally, many of the genes that govern events during regeneration, neural development, and specific stem cell regulation have been identified (Agata et al, 1998; Cebria et al. 2002a; 2002b; 2002c; Agata 2003;Sanchez Alvarado et al. 1999; 2002; Agata and Umesono 2008.).
Successful regeneration was accompanied by increased levels of hsp70 protein, activation of ERK, and an increase in the formation of a transcription complex that binds to the SRE. These results indicate that ELF-EMF can accelerate repair by activating pathways that normally participate in regeneration (Sanchez Alavarado, 2003; Newmark and Sanchez Alvarado, 2001).
Materials and methods
Planaria
Dugesia dorotocethala (Carolina Biological Supply Company; cat. # 132950) were shipped overnight and allowed to ‘recover’ for at least 24 hours in fresh oxygenated pond water (Carolina Biological Supply Co., Charlotte, NC, USA). Planaria were maintained in near darkness at 22–24°C (Precision Scientific incubator; Fisher Scientific, NJ, USA) throughout the experiments.
Electromagnetic field exposure system
The ELF-EMF exposure system uses Helmholtz coils (22 × 24cm with 10.5cm spacing) (designed and made by R. Cangialosi, Electro-Biology Inc, Parsippany, NJ, USA). The coils are composed of 19G copper wire bundles wound 164 times around a Plexiglas form and positioned vertically so that the oscillating magnetic field is generated in the horizontal plane inducing a relatively uniform electric field in the conductive medium. To minimize stray fields during ELF-EM field exposures, all Helmholtz coils containing samples were enclosed in individual 30cm high, 15cm diameter cylindrical mu metal containers (0.040″ thick, Amuneal Corp. Philadelphia, PA, USA) inside the incubator. The 60Hz shielding factor is Min. 90.1= 39.08dB. Experimental exposures were performed simultaneously with unexposed sham control samples in identical mu metal containers. All ELF-EMF field strength measurements were monitored with a Sypris triaxial magnetic field meter (Model 4080, Bell Laboratories, Orlando, FL, USA) before, during and after activation of the ELF-EMF within the mu metal containers in the incubator. ELF-EM field conditions were produced with a function generator (BK Precision 4011A 5MHz, Yorba Linda, CA, USA) and monitored with a digital multimeter (BK Precision 2706A, Yorba Linda, CA, USA). Field parameters were monitored with a Hitachi V-1065 100MHz oscilloscope and a calibrated (according to the National Institute of Standards and Technology) inductive 25X search coil. Parameters of the 60Hz field at a power setting of 5uV induced electric field and the estimated electric field measurements are described in Goodman et al. (1992). Detailed measurements of background magnetic fields in the incubator, harmonic distortion, DC magnetic fields and mean static magnetic fields in the incubators have been determined (Jin et al. 1997). The DC in these experiments was 0.95G (measured by Dr. Arthur Pilla).
Exposure protocol
The 60Hz frequency and 80mG field strength were selected based on previous experiments in which modulated sine waves were tested at a variety of different frequencies and field strengths (Goodman et al, 1989; Goodman and Bumann, 1991; Goodman and Blank 1998). In the experiments described here, Planaria were transected equidistant between the tip of the tail and the head (Figure 1). Each head and tail portion was photographed using a Nikon digital camera mounted on a Wilde dissecting microscope. Images were stored for subsequent measurements using ImageJ (see section on Quantitation). Head and tail portions were placed in separate Petri dishes (Falcon 351007 60 × 15mm; Fisher Scientific, NJ, USA) containing pond water approximately 0.6cm deep. Dishes were labeled so that heads and tails of the same worm could be identified for measurements. Dishes were placed on a Plexiglas stand between the Helmholtz coils so that the entire area of the dish was exposed to a uniform field. All exposures took place within an incubator and exposures were for 1 hour twice a day with a 4 hour interval between exposures. Control dishes were sham exposed. The temperature in the incubator and within the coils was maintained at 22–24°C and monitored with a thermocouple probe, with a sensitivity +/− 0.01°C (Physitemp, model BAT12, Hackensack, NJ, USA). Growth was assessed at three-day intervals from day 0 (immediately following transection and the onset of ELF-EMF exposure) to day 15 post-transection. In a series of separate experiments pigmented eye spot development was monitored at 12 hour intervals in transected tails exposed to ELF-EMF and sham control immediately following transection. In some experiments protein was extracted from the transected heads and tails, both experimental and sham , for analyses of hsp70 levels, Elk1 kinase activity and SRE-binding.
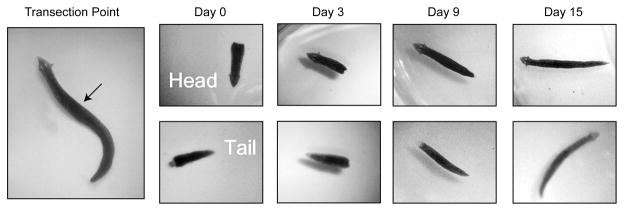
Figure 1. Transection of Planaria.
The image in the left panel shows an intact planarian with head facing up. The arrow indicates the transection point. The adjacent panels show representative images of heads and tails from transected Planaria at days 0, 3, 9 and 15. The completely regenerated Planaria at day 15 offers visible details of the resultant head and tail. The images have been enlarged 16X to show detail.
Protein lysates
Lysates were prepared from the heads and the tails of exposed and sham exposed Planaria for analyses of hsp70, Elk-1 kinase activity and SRF-SRE binding (Lin et al. 1997; 1999; 2001; 2003). Protein concentrations were determined by the Bradford assay (Bio-Rad Redmond, WA, USA).
Western blot
Lysed samples containing 30μg of protein were subjected to sodium dodecyl sulfate gel electrophoresis (Fisher Scientific, NJ, USA) on 10% polyacrylamide gels using appropriate molecular weight markers (Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA) and the polypeptides were transferred to polyvinylidene fluoride membrane for immunoblotting. Blots were probed with anti-hsp70 antibody (1:10,000). The blots were then stripped using SuperSignal West Pico Stable Peroxide Solution (Pierce, prod # 1859674, Fisher Scientific, NJ, USA) and SuperSignal West Pico Luminol Enhancer Solution (Pierce prod # 1859675 , from Fisher Scientific, NJ, USA) and reprobed with anti-actin (1:1000) to confirm equivalent loading. Visualization was by the enhanced chemiluminescent detection system (Fisher Scientific, NJ, USA) as previously described (Lin et al. 1998a).
Antibodies
Antibody to phosphorylated Elk-1 and AB91, was supplied by New England Biolabs, Beverly MA, USA. (Cat.# 9181). Antibodies to Elk-1, the SRF and the blocking phosphopeptide for Ab91 were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA), the anti-actin antibody from Sigma-Aldrich, St. Louis, MO, and the antibody to hsp70 was kindly provided by Dr. Richard Morimoto, Northwestern University.
The phosphorylation of Elk-1 was measured by the transfer of 32P-ATP , to a recombinant 32P-labeled Elk-1 that contains the ser38. The oligonucleotide containing the SRE sequence from the c-fos promoter, nCCATATn, was obtained from Santa Cruz Biotechnology Inc (Santa Cruz, CA, USA).
Protein kinase assays and electrophoretic mobility shift assays were performed as described (Lin et al. 1997; 1998a; 1998b; 2003).
Statistical analysis - Quantification of Planaria growth and bands on films from Western blots
For regeneration, multiple high-resolution digital pictures of Planaria heads and tails were taken at 3-day intervals and the rate of regeneration quantified using ImageJ v1.38 (http://rsb.info.nih.gov/ij). Length measurements were calibrated in millimeters and transposed to an Excel spreadsheet for statistical analysis. Lengths were normalized to the 0-time. Length differences over each 3-day interval were then subjected to 2-sample t-tests to assess significant differences in mean growth between the control and exposed conditions for both heads and tails. An additional analysis was conducted in SAS v9.1 (www.sas.com) to assess the significance of an interaction effect, i.e., whether the exposure had greater effect on either heads or tails. For this a ‘mixed effects’ analysis was performed which modeled the average length at 3-day intervals as a linear sum of the fixed effects of exposure (experimental vs. control), worm portion (heads vs. tails) and their interaction.
For quantification of hsp70, ERK, Elk-1 and SRE, the films from Western blot and electrophoretic mobility shift assay (EMSA) were scanned and saved as digital images. The densities of the bands were quantified using the histogram function in ImageJ and values transposed to Excel for statistical analysis (2 sample t-tests). A minimum of three replications of each assay were conducted.
Results
EMF accelerates rate of regeneration of transected Planaria
To assess the efficacy of the ELF-EMF on Planaria regeneration the length of ELF-EMF-exposed and sham control heads and tails were measured at three-day intervals starting immediately following transection (Figure 2). By day 3 the control regenerating head samples were significantly shorter than just after transection (day 0). This reduction in size was significantly attenuated by exposure to the ELF-EMF [exposed heads (HE) > control heads (HC): mean difference =1.33mm, t=5.95, p=6.8×10−8].
Figure 2. Regeneration of heads and tails following transection of Planaria.
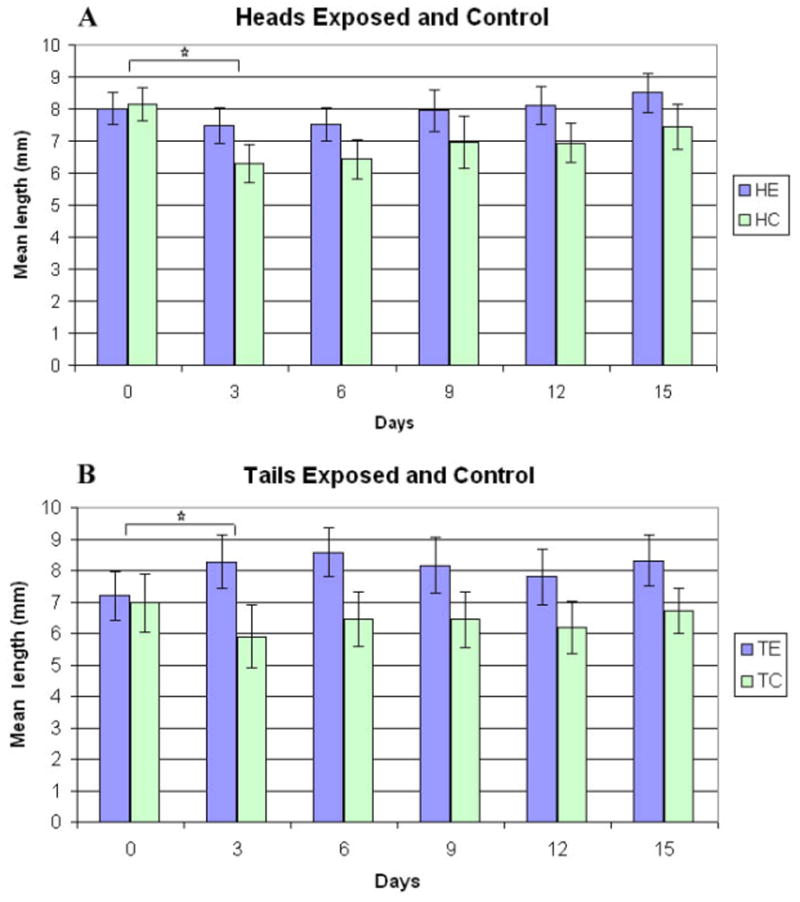
Histograms showing the mean length of control and ELF-EMF-exposed heads and tails immediately post-transection (0 time), and 3 to 15 days later. (A) Mean length ± 2 standard error of the mean (SEM) of exposed heads (HE, n=48) and sham exposed heads (HC, n=27) at each time. (B) Mean length ± 2SEM of exposed tails (TE, n=20) and sham exposed tails (TC, n=12) at each time. The asterisks indicate a significant difference in the rate of regeneration between exposed and sham exposed heads and tails assessed by 2-sample t-tests of mean difference in length between days 0 and 3.
ELF-EMF exposure also influenced the regeneration of the tails (Figure 2). The mean length of the tails on day 3 was greater than that at day 0 and was significantly greater than the length of the 3 day controls: [exposed tails (TE) > control tails (TC) mean difference on day 3 = 2.16mm, t=5.36, p=8.4×10−6]. Although ELF-EMF exposure promoted growth of both the heads and the tails, there was a significantly greater effect on the tails: [(TE > TC) > (HE > HC) mean difference after 3 days = 0.83mm, t=1.86, p < 0.07]. The tails must regenerate the more complex brain and eyes, whereas the heads regenerate a pharynx and peripheral nervous system. There was a tendency for the ELF-EMF-exposed heads and tails to be longer than the controls on day 15, but the difference was not statistically significant (Figure 2).
To monitor a specific aspect of nervous system regeneration, we assessed the initial appearance of the eyes in the tail portions and found a dramatic difference: the first appearance of eyes in animals exposed to the ELF-EMF occurred at day seven post-transection whereas they appeared 48 hours later in the sham controls. Three separate experiments were performed using ten tails for exposures and ten tails for sham control (n=30 E and 30 C).
ELF-EMF activation of biochemical markers associated with injury and repair
A 2- to 3-fold elevation in hsp70 levels was evident in both the head and tail portions that were exposed to the ELF-EMF (Figure 3A). The highest level of ELF-EMF-induced hsp70 was on day 3 post-transection and was particularly pronounced in the tail. The increase paralleled the accelerated regeneration noted above and is consistent with the role of hsp70 as a chaperone that monitors the folding of proteins during repair. ELF-EMF induction of the HSP70 gene may result from events triggered by the activation of ERK that utilize a unique consensus sequence (electromagnetic field response element; EMRE) upstream from the heat shock consensus sequence (Lin et al. 2001; Morimoto 1998). Active ERK is doubly phosphorylated (pp) and using an antibody that recognizes pp- ERK, a greater than 2-fold increase in the level of the active kinase in the ELF-EMF-exposed tail sample in comparison with the head samples and sham control (Figure 3B).
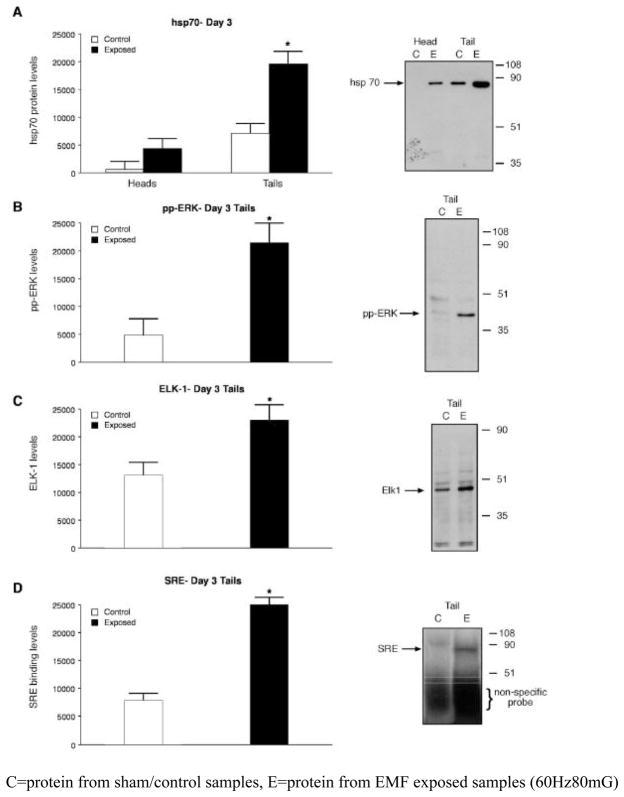
Figure 3. ELF-EMF activation of biochemical markers associated with injury and repair.
Hsp70, dual phosphorylated (activated) ERK, and Elk-1 were identified on Western blots and binding to a SRE was determined by EMSA. A representative image (one of three) is shown on the right of each panel. The images were scanned, the intensity of each band was determined and the mean intensity ± SEM was calculated and presented in a histogram. Statistically significant differences (p<0.05) between EMF-exposed and sham controls are indicated by asterisks.
A. Histogram showing a quantitative analysis of hsp70 protein levels from regenerating heads, tails, and sham controls. The relative protein levels are expressed in units based on the intensity of the protein on the Western blots.
B. Doubly phosphorylated ERKmapk: increased activation of the pp–ERK kinase in the ELF-EMF-exposed tail portions as compared with the sham exposed sample. The blot was probed with antibody that recognizes doubly phosphorylated (activated) ERKmapk (pp-ERK).
C. Electrophoretic mobility shift assay (EMSA). Phosphorylation of Elk1 was determined by measuring the transfer of 32P from 32P-ATP. Regenerating tail portion shows significantly greater Elk1 phosphorylation in the ELF-EMF - exposed tail portion than in the ELF-EMF- exposed head portion or control samples (data not shown).
D.Activation of SRE. Activation is initiated by a kinase cascade that leads to the dual phosphorylation of an ERK mapk. The mobility of this band corresponded to that of an Elk1 SRE transcription complex. [Mean quantitative differences in band density between exposed and sham exposed samples, for hsp70, ERK, Elk-1 and SRE, were assessed using 2-sample t-tests. n=3; asterisk denotes statistical significance p<0.05.].
Note: Analyses of EMF-induced hsp70 levels in the regenerating heads and tails were performed on day three (Figure 3A). The influence of ELF-EMF on molecular pathways in the regenerating tails was performed on day three post-transection (Figure 3B, C and D) coinciding with the time of maximal ELF-EMF-stimulated growth of the tail. C=protein from sham/control samples, E=protein from EMF exposed samples (60Hz80mG)
Studies have shown that the ERK influences growth by phosphorylating the transcription factor Elk-1, which then interacts with the SRF leading to the initiation of transcription via the SRE. (Marais et al. 1993; Gille et al. 1995). We found that this cascade was activated by the ELF-EMF. Thus, there was a greater Elk-1 phosphorylation in the tails exposed to the ELF-EMF compared to exposed heads or control samples (Figure 3C) and an EMSA using a 32P-labeled oligonucleotide containing the SRE sequence detected a retarded 32P-labeled band that was especially abundant in the ELF-EMF- exposed regenerating tails (Figure 3D). The activation of this ERK cascade observed in regenerating Planaria after ELF-EMF exposure is similar to that reported in Aplysia and the mouse following peripheral nerve transection (Ambron et al. 1995; Lin et al. 2003; Sung and Ambron 2004; Sung et al. 2006).
Discussion
EM fields promote specific effects in tissues
Exposure to electric or magnetic fields is reported to affect cell function in a variety of ways depending upon exposure and pulse design (Gordon, 2006; 2008; Jenrow et al. 1995 Jenrow et al. 1996; Novikov et al. 2008; Albertini et al. 1999; DiCarlo et al. 1999; Shallom et al. 2002; George et al. 2008). While generally efficacious, the most effective exposure conditions for a specific type of repair could depend on such variables as the magnetic and electric field components (dB/dt), signal modulation (sine waves versus pulsing fields) versus unmodulated sine waves, etc.
The fact that a variety of ELF-EMF conditions seem to facilitate nerve regeneration could mean that these fields are effective across a broad design parameter. A strong argument against this idea was the identification of a specific ELF-EMF-sensitive domain in the promoter of the HSP70 gene, nCTCTn (Lin et al. 1999; 2001; Goodman and Blank 2002). The current experiments focused on how ELF-EMF affects intracellular molecular pathways that were selected because they had been shown to be biologically effective in previous studies (Ambron et al. 1995; Lin et al. 2003; Sung and Ambron 2004; Sung et al. 2006).
EM field-stimulated head and tail regeneration
The extraordinary ability of Planaria to regenerate after injury is attributed to the presence of totipotent neoblasts capable of differentiating into all of the tissue types (Agata 2003;Baguna and Romero 1981). Perhaps most impressive is their ability to regenerate the nervous system. When transected the tail region forms a new head complete with bilateral optic nerves and eyes, a two-lobed brain, and a pair of ventral nerve cords (VNC) (Agata et al. 1998; Agata and Umesono,2008; Cebria et al. 2002a; 2002b; 2002c).
To determine the effects of an ELF-EMF on regeneration, Planaria were transected and heads and tails exposed to a 60Hz, 80mG ELF-EMF for one hour twice a day for 15 days while monitoring the ELF-EMF exposure and several other parameters to ensure reproducibility. As noted above, these field conditions have consistently upregulated HSP70 and induced elevated hsp70 levels in other systems including yeast (Weisbrot et al, 1993), Drosophila (Goodman et al, 1989; 1992 ) and cultured cells (Jin et al. 1997; Lin et al. 1997; Goodman and Blank 1998). Measurements showed that both heads and tails exposed to this ELF-EMF regenerated more rapidly relative to sham controls during the first 3 days of post-transection (Figure 2). The increased regeneration was especially notable in the tail (Figure 2). Moreover, the ELF-EMF specifically influenced the regeneration of the eyes in that they appeared two days earlier in the tails exposed to the ELF-EMF relative to sham controls (Figure 2).
As an additional approach to examining ELF-EMF on re-growth we carefully followed the initial appearance of the eyes in the tail portions. The first appearance of eyes in tails exposed to the ELF-EMF occurred at day seven post-transection whereas the eyes in the sham controls did not appear until 48 hours later. Three separate experiments were performed using ten tails for exposures and ten tails for sham control (n=30 ELF-EMF and 30 sham exposed).
To examine molecular pathways that are influenced by the ELF-EMF we first verified that the ELF-EMF conditions altered the levels of hsp70 in the regenerating heads and tails. This analysis was done on the third day after transection, which is the time of maximal ELF-EMF-stimulated growth of the tail (Figure 3A). During normal regeneration this is the developmental stage when the neural networks are actively forming (Cebria et al. 2002a,Cebria et al. 2002b 2000c) and the ventral nerve cords (VNC) are extending from the head into the newly formed tail. (Figure 3A).
The EMF activates the ERK cascade
The activation of ERK typically occurs when tissues are exposed to stress and appears to be an important pathway following injury in both vertebrates and invertebrates (Sung and Ambron 2004; Sung et al. 2006). Accelerated development of a new tail following ELF-EMF stimulation was accompanied by the activation of ERK, the consequent phosphorylation of Elk1, and the activation of the SRE (Figure 3B). Activation of the SRE is associated with regrowth in many cell types after injury including neurons (Lin et al. 2003). Elk-1 is an SRE accessory protein that contains a growth-regulated transcriptional activation domain (Marais et al. 1993). Although we do not yet know which mRNA is produced by activating Elk-1 in the regenerating Planaria, the phosphorylation of Elk-1 by ERK (Figure 3C) is known to potentiate tertiary complex formation and transactivation at the c-fos SRE (Figure 3D) (Gille et al. 1995). These findings are compatible with those of Rao and Henderson (1996) who reported that exposure to 60Hz 80mG sinusoidal fields significantly increased c-fos gene expression via the SRE site on the c-fos promoter, this might be expected since c-fos protein is elevated in cases of injury.
In summary, our data show that exposure to a designed ELF-EMF protocol facilitates regeneration in Planaria. This effect is accompanied by an increase in the level of the chaperone protein hsp70 and by the activation of the ERK cascade and downstream transcription factors. Regeneration is fostered because these events augment the synthesis of proteins that are necessary for the repair process. This study has established specific parameters that reproducibly modulate intracellular events in hope that it will encourage more extensive studies into the interaction between ELF-EMF and biological processes.
Acknowledgments
We thank Hana Lin for the EMSA assays, YiFan Liu for the Western blot assays. We are grateful to Eve Vagg for her invaluable contribution in preparing the figures. We are grateful to Ash Madkan for his invaluable help with editing. We thank Ms. Janet Wilson in Technical Support at Carolina Biological Supply Company. The Robert I. Goodman Fund provided partial support.
Abbreviations
- ELF
Extremely low frequency
- EMF
Electromagnetic field
- SRE
serum response element
- hsp
heat shock protein
- HSP
heat shock gene
- EMSA
electrophoretic mobility shift assay
- ERK
extracellular signal regulated kinases 42/44
- Hz
frequency
- G
Gauss
- SRF
serum response factor
- MAPK
mitogen-activated protein kinase
- VNC
ventral nerve cord
References
- Agata K. Regeneration and gene regulation in planarians. Current Opinion in Genetic Development. 2003;13:492–496. doi: 10.1016/j.gde.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Agata K, Umesono Y. Brain regeneration from pluripotent stem cells in planarian. Philosophila Transactions of the Royal Society B. 2008;363:2071–2078. doi: 10.1098/rstb.2008.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertini A, Zucchini P, Noera G, Cadossi R, Napoleone CP, Pierangeli A. Protective effect of low frequency low energy pulsing electromagnetic fields on actute experimental myocardial infarcts in rats. Bioelectromagnetics. 1999;20:372–377. doi: 10.1002/(sici)1521-186x(199909)20:6<372::aid-bem6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Agata K, Soejima Y, Kato K, Kobayashi C, Umesono Y, Watanabe K. Structure of the planarian central nervous system (CNS) revealed by neuronal cell markers. Zoological Society. 1998;15:433–440. doi: 10.2108/zsj.15.433. [DOI] [PubMed] [Google Scholar]
- Ambron RT, Dulin MF, Zhang XP, Schmied R, Walters ET. Axoplasm enriched in a protein mobilized by nerve injury induces memory-like alterations in Aplysia neurons. J Neuroscience. 1995;15:3440–3446. doi: 10.1523/JNEUROSCI.15-05-03440.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado Sanchez Alvarado A, Newmark PA. Double-stranded RNA specifically disrupts gene expression during planarian regeneration. Proceedings of the National Academy of Sciences, USA. 1999;96:5049–5054. doi: 10.1073/pnas.96.9.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado Sanchez A, Newmark PA, Robb SM, Juste R. The Schmidtea mediterranea database as a molecular resource for studying platyhelminths, stem cells and regeneration. Development. 2002;129:5659–5665. doi: 10.1242/dev.00167. [DOI] [PubMed] [Google Scholar]
- Alvarado Sanchez A. The fresh water planarian Schmidtea mediterranea: embryogenesis, stem cells and regeneration. Current Opinion in Genetics & Development. 2003;13:438–444. doi: 10.1016/s0959-437x(03)00082-0. [DOI] [PubMed] [Google Scholar]
- Alvarado Sanchez A, Newmark PA. Double-stranded RNA specifically disrupts gene expression during planarian regeneration. Proceedings of the National Academy of Sciences; USA. 1999. pp. 5049–5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado Sanchez A, Newmark PA, Robb SM, Juste R. The Schmidtea mediterranea database as a molecular resource for studying platyhelminths, stem cells and regeneration. Development. 2002;129:5659–5665. doi: 10.1242/dev.00167. [DOI] [PubMed] [Google Scholar]
- Baguna J, Romero R. Quantitative analysis of cell types during growth, regrowth and regeneration in the planarians Digesia mediterranea and Dugesia tigrina. Hydrobiologia. 1981;84:181–194. [Google Scholar]
- Bassett CAL. Bioelectromagnetics in the service of medicine. Advances in Chemistry. 1995;250:261–276. [Google Scholar]
- Blank M, Goodman R. Do electromagnetic fields interact directly with DNA? Bioelectromagnetics. 1997;18:111–115. doi: 10.1002/(sici)1521-186x(1997)18:2<111::aid-bem3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Carmody S, Wu XL, Lin H, Blank M, Skopicki H, Goodman R. Cytoprotection by electromagnetic field-induced hsp70: a model for clinical application. Journal of Cellular Biochemistry. 2000;79:453–459. doi: 10.1002/1097-4644(20001201)79:3<453::aid-jcb100>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Cebria F, Kudome T, Nakazawa M, Mineta K, Ikeo K, Gojobori T, Agata K. The expression of neural-specific genes reveals the structural and molecular complexity of the planarian central nervous system. Mechanistic Development. 2002a;116:199–204. doi: 10.1016/s0925-4773(02)00134-x. [DOI] [PubMed] [Google Scholar]
- Cebria F, Nakazawa M, Mineta K, Ikeo K, Gojobori T, Agata K. Dissecting planarian central nervous system regeneration by the expression of neural-specific genes. Developmental Growth & Differentiation. 2002b;44:135–146. doi: 10.1046/j.1440-169x.2002.00629.x. [DOI] [PubMed] [Google Scholar]
- Development Cebria F, Nakazawa M, Mineta K, Ikeo K, Gojobori T, Agata K. Dissecting planarian central nervous system regeneration by the expression of neural-specific genes. Developmental Growth & Differentiation. 2002c;44:135–146. doi: 10.1046/j.1440-169x.2002.00629.x. [DOI] [PubMed] [Google Scholar]
- DiCarlo AL, Farrell JM, Litovitz TA. Myocardial protection conferred by electromagnetic fields. Circulation. 1999;99:813–816. doi: 10.1161/01.cir.99.6.813. [DOI] [PubMed] [Google Scholar]
- Friedman J, Kraus S, Hauptman Y, Schiff Y, Seger R. Mechanism of short-term ERK activation by electromagnetic fields at mobile phone frequencies. Biochemistry Journal. 2007;405:559–568. doi: 10.1042/BJ20061653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George I, Geddis MS, Lill Z, Lin H, Gomez T, Blank M, Oz MC, Goodman R. Myocardial function improved by electromagnetic field induction of stress protein hsp70. Journal of Cellular Biochemistry. 2008 doi: 10.1002/jcp21461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille H, Kortenjann M, Thomae O, Moomaw C, Slaughter C, Cobb MH, Shaw PE. ERK phosphorylation potentiates Elk-1-mediated ternary complex formation and transactivation. EMBO Journal. 1995;14:951–962. doi: 10.1002/j.1460-2075.1995.tb07076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R, Wei L-X, Xu J-C, Henderson A. Exposure of human cells to low-frequency electromagnetic fields results in quantitative changes in transcripts. Biochim et Biophys Acta. 1989;1009:216–220. doi: 10.1016/0167-4781(89)90105-x. [DOI] [PubMed] [Google Scholar]
- Goodman R, Weisbrot D, Uluc A, Henderson A. Transcription in Drosophila melanogastersalivary gland cells is altered following exposure to low frequency electromagnetic fields: Analysis of chromosome 3R. Bioelectromagnetics. 1992;13:111–118. doi: 10.1002/bem.2250130205. [DOI] [PubMed] [Google Scholar]
- Goodman R, Bumann J. In: Electromagnetics in Biology and Medicine. Brighton CT, Pollack SR, editors. San Francisco Press., Inc; Box 6800, San Francisco CA 94101, USA: [Google Scholar]
- Goodman R, Blank M. Magnetic field stress induces expression of hsp70. Cell Stress & Chaperones. 1998;3:79–88. doi: 10.1379/1466-1268(1998)003<0079:mfsieo>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R, Blank M. Insights into electromagnetic interaction mechanisms. Journal Cellular Physiology. 2002;192:16–22. doi: 10.1002/jcp.10098. [DOI] [PubMed] [Google Scholar]
- Goodman R, Blank M, Lin H, Khorkova O, Soo L, Weisbrot D, Henderson A. Increased levels of hsp70 transcripts are induced when cells are exposed to low frequency electromagnetic fields. Bioelectrochemistry Bioenergetics. 1994;33:115–120. [Google Scholar]
- Goodman R, Henderson AS. Exposure of salivary gland cells to low-frequency electromagnetic fields alters polypeptide synthesis. Proceeding of the National Academy of Science (USA) 1988;85:3928–3932. doi: 10.1073/pnas.85.11.3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R, Bumann J, Wei L-X, Henderson AS. Exposure of human cells to electromagnetic fields: Effect of time and field strength on transcript levels. Electro-and Magnetobiology. 1992;11:19–28. [Google Scholar]
- Gordon G. Designed electromagnetoic pulsed therapy: clinical applications. Journal of Cellular Physiology. 2006 doi: 10.1002/JCP. [DOI] [PubMed] [Google Scholar]
- Gordon G. Extrinsic electromagnetic fields, low frequency (phonon) vibrations and control of cell function: A non-linear resonance system. Journal Biomedical Science and Engineering. 2008;1:152–156. [Google Scholar]
- Han L, Lin H, Head M, Jin M, Blank M, Goodman R. Application of magnetic field-induced heat shock protein 70 for presurgical cytoprotection. Journal of Cellular Biochemistry. 1998;71:577–583. doi: 10.1002/(sici)1097-4644(19981215)71:4<577::aid-jcb12>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Jenrow KA, Smith CH, Liboff AR. Weak extremely-low frequency magnetic fields and regeneration in the planarian Dugesia tigrina. Bioelectromagnetics. 1985;16:106–112. doi: 10.1002/bem.2250160206. [DOI] [PubMed] [Google Scholar]
- Jenrow KA, Smith CH, Liboff AR. Weak extremely-low frequency magnetic field-induced regeneration anomalies in the planarian Dugesia tigrina. Bioelectromagnetics. 1985;17:467–474. doi: 10.1002/(SICI)1521-186X(1996)17:6<467::AID-BEM6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Jin M, Blank M, Goodman R. ERK1/2 phosphorylation, induced by electromagnetic fields, diminishes during neoplastic transformation. Journal of Cellular Biochemistry. 2000;78:371–379. doi: 10.1002/1097-4644(20000901)78:3<371::aid-jcb3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Jin M, Lin H, Han L, Opler M, Maurer S, Blank M, Goodman R. Biological and technical variables in myc expression in HL60 cells exposed to 60 Hz electromagnetic fields. Bioelectrochemistry Bioenergetics. 1997;44:111–120. [Google Scholar]
- Kultz D. Molecular and evolutionary basis of the cellular stress response. Annual Review of Physiology. 2005;67:225–257. doi: 10.1146/annurev.physiol.67.040403.103635. [DOI] [PubMed] [Google Scholar]
- Lednev VV, Tiras Kh P, Belova NA, Ermakova ON, Ermakov AM. Biological effect of extremely weak industrial-frequency magnetic fields. Biophysics. 2005;50:S157–S162. [Google Scholar]
- Leszczynski D, Joenvaara S, Reivinen J, Kuokka R. Non-thermal activation of the hsp27/p38MAPK stress pathway by mobile phone radiation in human endothelial cells: molecular mechanism for cancer- and blood-brain barrier-related effects. Differentiation. 2002;70:120–129. doi: 10.1046/j.1432-0436.2002.700207.x. [DOI] [PubMed] [Google Scholar]
- Lin H, Bao J, Sung YJ, Walters ET, Ambron RT. Rapid electrical and delayed molecular signals regulate the serum response element after nerve injury: convergence of injury and learning signals. Journal of Neurobiology. 2003;57:204–220. doi: 10.1002/neu.10275. [DOI] [PubMed] [Google Scholar]
- Lin H, Blank M, Goodman R. A magnetic field-responsive domain in the human HSP70 promoter. Journal Cellular Biochemistry. 1999;75:170–176. doi: 10.1002/(sici)1097-4644(19991001)75:1<170::aid-jcb17>3.3.co;2-x. [DOI] [PubMed] [Google Scholar]
- Lin H, Blank M, Rossol-Haseroth K, Goodman R. Regulating genes with electromagnetic response elements. Journal of Cellular Biochemistry. 2001;81:143–148. doi: 10.1002/1097-4644(20010401)81:1<143::aid-jcb1030>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Lin H, Han L, Blank M, Head M, Goodman R. Magnetic field activation of protein-DNA binding. Journal of Cellular Biochemistry. 1998a;70:297–303. [PubMed] [Google Scholar]
- Lin H, Head M, Blank M, Han L, Jin M, Goodman R. Myc-mediated transactivation of HSP70 expression following exposure to magnetic fields. Journal of Cellular Biochemistry. 1998b;69:181–188. doi: 10.1002/(sici)1097-4644(19980501)69:2<181::aid-jcb8>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Lin H, Opler M, Head M, Blank M, Goodman R. Electromagnetic field exposure induces rapid, transitory heat shock factor activation in human cells. Journal of Cellular Biochemistry. 1997;66:482–488. doi: 10.1002/(sici)1097-4644(19970915)66:4<482::aid-jcb7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Newmark PA, Sanchez Alvarado A. Regeneration in Planaria. Encyclopedia of Life Sciences. 2001 www.els.net.
- Marais R, Wynne J, Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993;73:381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones and negative regulators. Genes& Development. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- Novikov VV, Sheiman IM, Fesenko EE. Effect of weak static and low-frequency alternating magnetic fields on the fission and regeneration of the planarian dugesia (Girardia) tigrina. Bioelectromagnetics. 2008 Jul;29(5):387–93. doi: 10.1002/bem.20407. [DOI] [PubMed] [Google Scholar]
- Rao S, Henderson AS. Regulation of c-fos is affected by electromagnetic fields. Journal of Cellular Biochemistry. 1996;63:358–365. doi: 10.1002/(SICI)1097-4644(19961201)63:3%3C358::AID-JCB11%3E3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Shallom JM, Di Carlo AL, Ko D, Penafiel LM, Nakai A, Litovitz TA. Microwave exposure induces Hsp70 and confers protection against hypoxia in chick embryos. Journal Cellular Biochemistry. 2002;86:490–496. doi: 10.1002/jcb.10243. [DOI] [PubMed] [Google Scholar]
- Sung YJ, Ambron RT. Pathways that elicit lomng-term changes in gene expression in noniceptive neuron following nerve injury contributions to neuropathic pain. Neurological Research. 2004;26:195–203. doi: 10.1179/016164104225013761. [DOI] [PubMed] [Google Scholar]
- Sung YJ, Chiu DTW, Ambron RT. Activation and retrograde transport of protein kinase G in rat noniceptive neurons after nerve injury and inflammation. Neuroscience. 2006;141:697–709. doi: 10.1016/j.neuroscience.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Weisbrot D, Khorkova O, Lin H, Henderson A, Goodman R. The effect of low frequency electric and magnetic fields on gene expression in Saccharomyces cerevisiae. Bioelectrochemistry Bioenergetics. 1993;31:167–177. [Google Scholar]
- Weisbrot D, Lin H, Ye L, Blank M, Goodman R. Effects of mobile phone radiation on reproduction and development in Drosophila melanogaster. Journal of Cellular Biochemistry. 2003;89:48–55. doi: 10.1002/jcb.10480. [DOI] [PubMed] [Google Scholar]