Abstract
Mounting evidence has demonstrated that the autonomic system plays a role in the morbidity and mortality of certain cardiovascular disease states. Ventricular arrhythmias have been associated with the level of sympathetic activation. We attempted to determine if the presence of fibrosis, a marker for previous ischemic events, correlates with an increase in the number of left stellate ganglion nerve cell bodies which is indicative of hypersympathetic stimulation to the myocardial tissue. Left stellate ganglia were removed, sectioned and prepared using hematoxylin and eosin and Masson’s trichrome stain. The interventricular septum of the heart corresponding to the stellate ganglion samples were removed, serially sectioned, and stained with hematoxylin and eosin and Masson’s trichrome stain. The samples were described using a grading scale to quantify the percentage of fibrosis. Ganglion nerve cell bodies were then individually counted in three separate high-powered fields. A student’s T-test was used to statistically evaluate the data. Stellate ganglions were sampled from 32 cadavers. Fibrosis was present within 72% (23/32) of the interventricular septums that were sampled. Nine interventricular septums were found to be free of fibrosis. For those interventricular septums that were positive for the presence of fibrosis, the mean left stellate ganglion nerve cell bodies was 39.8 (Range: 26–51). For those interventricular septums that were negative for the presence of fibrosis, the mean left stellate ganglion nerve cell bodies was 34.3 (Range: 27–46). The difference between the mean nerve cell bodies for interventricular septums with fibrosis and without fibrosis was found to be statistically significant (P = 0.048). Histological changes in terms of the number of left stellate ganglion nerve cell bodies seem to be dependent upon the presence of fibrosis within the interventricular septum. Considering fibrosis of the interventricular septum is a marker for previous ischemic events, an increase in the number of nerve cell bodies of the left stellate ganglion in the presence of fibrosis suggests an association does exist between hypersympathetic stimulation to the myocardial tissue and myocardial infarction. Further research into this association is warranted in order to determine if left stellate ganglion blockade is a viable treatment option for arrhythmias following myocardial infarctions.
Keywords: stellate ganglion, sympathetic hyperinnervation, nerve sprouting, cardiopulmonary
Introduction
Mounting evidence has demonstrated that the autonomic system may play a role in the morbidity and mortality of certain cardiovascular disease states. Ventricular arrhythmias have been associated with the level of sympathetic activation.1,2 Increased sympathetic hyperinnervation following a myocardial infarction has been associated with the production of ventricular arrhythmias leading to sudden cardiac death3 and also with the production of atrial fibrillation.4,5
The hyperinnervation associated with increased arrhythmias is believed to be caused by sympathethic regeneration or nerve sprouting which occurs following ischemic injuries to the myocardial tissue.4 It is theorized that an elevation of nerve growth factor following a myocardial infarction plays a critical role in the production of hyperinnervation to the viable myocardium.6,5 It is theorized that an elevation of nerve growth factor following an acute myocardial infarction may play a role in the observed hyperinnervation.6,5 Previous studies have demonstrated an immediate elevation in the concentration of transcardiac NGF thought to be released by dying myocardial cells following an acute myocardial infarction.7
Nerve growth factor is a neurotropin that enhances the differentiation of sympathetic neurons, enhances innervation, and correlates with the density of innervation of the sympathetic nervous system.8,9 It is also theorized that NGF enhances nerve sprouting within the left stellate ganglion which increases the cardiac nerve density throughout the still viable myocardium which enhances the sympathethic signal.10
Literature has suggested an association between post-MI sympathetic nerve density and an increased susceptibility to life-threatening arrhythmias.6 The density of S100-immunoreactive nerve fibers was found to be significantly higher in the periphery of injured myocardium in individuals who have experienced arrhythmias when compared to those with no history of arrhythmias.11 In a canine model, injection of NGF into the left stellate ganglia caused an increase in nerve sprouting and resulted in an increased incidence of ventricular tachycardia and sudden cardiac death.11
The left stellate ganglion has consistently been shown to play a vital role in cardiac arrhythmias12 (Schwartz, 1980). Previous studies have linked the left stellate ganglion to arrhythmias such as ventricular fibrillation and sudden cardiac death. Left stellate ganglion ablation has reduced arrhythmias associated with coronary occlusive disease19 and shown to increase the threshold for ventricular fibrillation.20 In another study, sudden cardiac death following a first myocardial infarction was significantly decreased following left cardiac sympathetic denervation by a left stellate ganglion resection.13 Thus, due to the association between the left stellate ganglion and cardiac disease, he present study was conducted in order to demonstrate histologically a relationship between an increased number of left stellate ganglia and the presence of fibrosis within the myocardial septum. An increase in left stellate ganglia nerve cell bodies may be indicative of hypersympathetic innervation and the presence of fibrosis may signify previous myocardial tissue infarction.
Materials and Methods
Sample collection methods
Left stellate ganglia were removed from human cadavers of a New York state donor population at the New York College of Osteopathic Medicine. Those cadavers in which a left stellate ganglion could not be localized were excluded from the study. Age range of the cadavers was 60 to 96 with a mean of 83. Cadavers were placed in the supine position with their necks positioned in extension. Initial incisions were made from the angle of the mandible to the sternal notch and to the distal portion of the clavicle. Muscles such as the platysma muscle and sternocleidomastoid muscle were removed or reflected. Once the vasculature was exposed, the left stellate ganglia could be found on the sympathetic chain deep to the point at which the left internal thoracic artery branches from the left subclavian artery. The left stellate ganglion is formed by the fusion of the inferior cervical ganglion with the first thoracic ganglion(Pather et al 2006). Left stellate ganglia (n = 32) were detached from the sympathethic chain using a scalpel and forceps and then stored in three times the specimen volume in 10% buffered formalin.
The interventricular septum of the heart corresponding to the stellate ganglion sample was also removed from the human cadavers. Cadavers were placed in the supine position and the skin and muscles of the pectoral region were reflected laterally. The anterior thoracic wall was then removed to expose the underlying pericardium. A longitudinal incision was made midline and transverse incisions were made near the central tendon of the diaphragm and the great vessels to open the pericardium. The heart was detached from the great vessels and removed from the body. Cuts were made to open the right and left ventricle, exposing the interventricular septum. Using a scalpel and forceps, a 3-cm long section of the interventricular septum was removed in order to obtain a random sampling of the myocardium and endocardium. The interventricular septums were stored in 10% buffered formalin at three times the specimen volume.
Histological methods
Ganglia were serially sliced along the vertical plane into 2 mm thick sections and placed into cutting blocks. The interventricular septums were serially sliced along the horizontal plane into 2 mm thick sections, trimmed, and placed into cutting blocks. The specimens were placed in 10% buffered formalin for 24 hours followed by treatment with 70%–100% ethanol and xylene. Specimens were embedded in paraffin and sectioned at a thickness of 6 μm via a microtome. Two slides were stained for each specimen, one with hematoxylin and eosin and the other with Masson’s trichrome stain. Slides were observed with an Olympus BX41 multihead microscope and digitally photographed.
Each interventricular septum was examined in order to determine the extent of fibrosis. A pathologist examined samples from each interventricular septum and classifed the samples into one of two groups: Fibrosis or no fibrosis. Those cadavers in which interventricular septums demonstrated no fibrosis acted as the control group. Each ganglion slide associated with the same interventricular septum sample was examined in order to estimate the amount of nerve cell bodies per ganglion. Changes in nerve cell bodies were determined by counting the number of nerve cell bodies present in three separate high-powered fields at a 40× magnification. The number of nerve cell bodies calculated in each of the three fields was then averaged.
Statistical analysis
Student’s t test was used to compare the means between two groups. The number of ganglion cells per three high powered fields was average and this mean was used in the analysis of this variable. The t-test was used to compare the means of nerve cell bodies average of cardiovascular samples which showed fibrosis vs. nerve cell bodies average of cardiovascular samples which did not show fibrosis. A P-value ≤ 0.05 was considered significant.
Results
Nerve cell body changes of the left stellate ganglia
Stellate ganglions were sampled from 32 cadavers. Fibrosis was present within 72% (23/32) of the interventricular septums that were sampled. Nine interventricular septums were found to be free of fibrosis. For those interventricular septums that were positive for the presence of fibrosis, the mean left stellate ganglion nerve cell bodies was 39.8 (Range: 26–51). For those interventricular septums that were negative for the presence of fibrosis, the mean left stellate ganglion nerve cell bodies was 34.3 (Range: 27–46). The difference between the mean nerve cell bodies for interventricular septums with fibrosis and without fibrosis was found to be statistically significant (P = 0.048). Table 1 demonstrates the data from the histological analysis of each stellate ganglion and interventricular septum sample.
Table 1.
Results of the histological analysis of each ganglion and interventricular septum sample for each cadaver.
| Cadaver | Interventricular septum samples | Left stellate ganglion samples | |||
|---|---|---|---|---|---|
| Presence of fibrosis in the interventricular septum? | Number of nerve cell bodies in field 1 | Number of nerve cell bodies in field 2 | Number of nerve cell bodies in field 3 | Average number of nerve cell bodies | |
| 1 | Yes | 40 | 45 | 42 | 42 |
| 2 | Yes | 33 | 30 | 32 | 32 |
| 3 | Yes | 40 | 41 | 36 | 39 |
| 4 | Yes | 36 | 36 | 40 | 37 |
| 5 | Yes | 35 | 41 | 37 | 38 |
| 6 | Yes | 33 | 38 | 30 | 34 |
| 7 | None | 38 | 45 | 38 | 40 |
| 8 | Yes | 36 | 45 | 36 | 39 |
| 9 | Yes | 24 | 35 | 25 | 28 |
| 10 | None | 38 | 27 | 25 | 30 |
| 11 | Yes | 52 | 41 | 46 | 46 |
| 12 | Yes | 50 | 54 | 48 | 51 |
| 13 | None | 45 | 48 | 46 | 46 |
| 14 | None | 30 | 33 | 23 | 29 |
| 15 | Yes | 26 | 26 | 27 | 26 |
| 16 | Yes | 54 | 49 | 42 | 48 |
| 17 | Yes | 33 | 31 | 40 | 35 |
| 18 | None | 27 | 24 | 29 | 27 |
| 19 | None | 22 | 26 | 36 | 28 |
| 20 | Yes | 45 | 31 | 34 | 37 |
| 21 | Yes | 39 | 38 | 40 | 39 |
| 22 | Yes | 42 | 50 | 41 | 44 |
| 23 | None | 33 | 37 | 28 | 33 |
| 24 | Yes | 47 | 48 | 46 | 47 |
| 25 | Yes | 44 | 37 | 41 | 41 |
| 26 | None | 39 | 45 | 46 | 43 |
| 27 | Yes | 56 | 43 | 50 | 50 |
| 28 | Yes | 50 | 38 | 48 | 45 |
| 29 | Yes | 38 | 33 | 27 | 33 |
| 30 | Yes | 38 | 43 | 48 | 43 |
| 31 | Yes | 48 | 39 | 36 | 41 |
| 32 | None | 27 | 37 | 36 | 33 |
Discussion
Our data support the hypothesis that sympathetic hyperinnervation plays a role in the initiation of cardiopulmonary pathologies such as sudden cardiac death (SCD) and arrhythmias following myocardial infarctions. Our previous research14 demonstrated an increased number of nerve cell bodies within the left stellate ganglia of cadavers with cardiopulmonary disease, in comparison to other pathologies. In the present study, we attempted to present a definite link between an increased number of nerve cell bodies and evidence of previous myocardial infarction. Such a finding would offer a link between sympathetic regeneration through nerve sprouting and sympathetic hyperinnervation which is believed to cause arrhythmias and sudden cardiac death following myocardiacal infarctions.
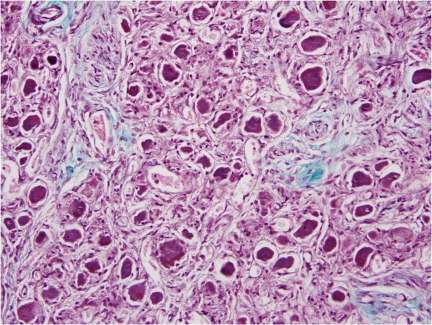
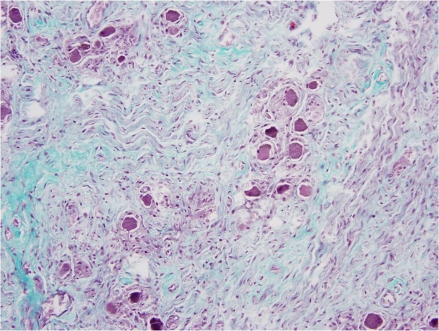
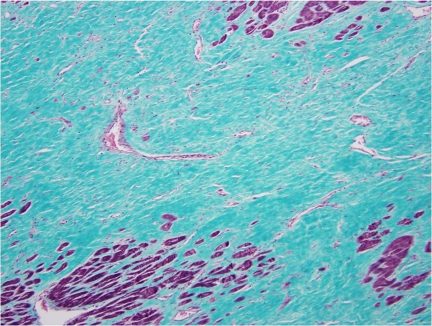
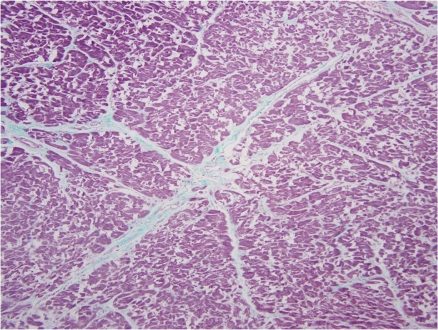
Previous literature analyzing histological sections of a left stellate ganglion from an individual with coronary artery disease and atrial fibrillation demonstrated an increased number of nerve cell bodies as compared to a specimen from an individual without cardiovascular diasease.14 The increased number of nerve cell bodies observed in cadavers with cardiovasucular pathology suggests NGF may initiate the generation of new ganglion cells which in-turn would cause an increased sympathetic tone to the distal organs.14 Figure 1 demonstrates a section of a stellate ganglion from a cadaver with the presence of fibrosis suggestive of cardiovascular pathology. Whereas in cadaveric heart samples with no fibrosis suggestive of cardiovascular pathology, the number of stellate ganglion were significally lower (Fig. 3). Figure 2 demonstrates the presence of fibrosis suggestive of cardiac pathology. Figure 4 demonstrates a lack of fibrosis which may be indicitive of a decreased amount of cardiac pathology.
Figure 1.
Stellate ganglion section: Evidence of cardiovascular disease. Histological section of the left stellate ganglion stained with Masson’s trichrome stain. Stellate ganglion sample was removed from a cadaver in which a sample of cardiac septum was found to demonstrate fibrosis suggestive of cardiovascular disease.
Figure 3.
Stellate Ganglion section: No evidence of cardiovascular disease. Histological section of the left stellate ganglion stained with Masson’s trichrome stain. Stellate ganglion sample was removed from a cadaver in which a sample of cardiac septum was not found to demonstrate fibrosis which suggests a lack of cardiovascular disease.
Figure 2.
Cardiac Septum section associated with an increase in nerve cell bodies. Histological section of the cardiac septum removed from a cadaver in which a sample of the stellate ganglion demonstrated an increased number of nerve cell bodies.
Figure 4.
Cardiac Septum section associated with a decrease in nerve cell bodies. Histological section of the cardiac septum removed from a cadaver in which a sample of the stellate ganglion demonstrated an increased number of nerve cell bodies.
Research suggests an increase in post-MI systemic NGF results in a retrograde transport of NGF to LSG which triggers nerve sprouting and sympathetic hyperinnervation—creating an environment for arrhythmia. The increase in NGF and GAP43 protein within the LSG without a concomitant increase in mRNA levels suggest a retrograde axonal transport of NGF from the infarcted site to the LSG.9,16 A possible mechanism is the release of NGF following a MI results in retrograde axonal transport of NGF to the left stellate ganglion resulting in nerve sprouting.14
An increased release of NGF may initiate an increase in nerve cell bodies of the LSG via a retrograde axonal transport causing sympathetic hyperinnervation to the respiratory system. A pathological change of the pulmonary system may cause a release of NGF, which may then return to the LSG via a retrograde axonal transport. The NGF within the LSG may cause sympathetic hyperinnervation to the pulmonary system which may subsequently cause an increase in morbidity and mortality of these individuals.
Our results are clinically significant considering the use of stellate ganglion blockade may provide protective benefits against post-MI arrhythmogenesis, SCD, and assist in such disease states as COPD and pulmonary hypertension. Considering infusion of NGF directly into the left stellate ganglion induced nerve sprouting and a higher incidence of ventricular tachycardia in dogs,17 it would seem feasible that a left stellate block could reduce the sympathethic hyperinnervation noted in cardiovascular pathologies and prevent further morbidity and mortality. With the onset of thorascopy, the magnified view of the sympathetic chain can allow for precise surgical resection of the stellate ganglion and also reduce costs and length of hospital stays.18
Acknowledgments
The authors would like to thank Alice O’Connor (NYCOM) for histological preparation of all slides and Dr. Robert Hill (NYCOM) and Ryan Palevsky (NYCOM) for thier assistance during the removal of samples from the NYCOM cadavers. Research funding was supported by the NYCOM Office of Research and Academic Medicine Research Fund.
Footnotes
Disclosures
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Jayachandran J, Sih H, Winkle W, Zipes D, Hutchins G, Olgin J. Atrial fibrillation produced by prolonged rapid atrial pacing is associated with heterogeneous changes in atrial sympathetic innervation. Circulation. 2000;101:1185–91. doi: 10.1161/01.cir.101.10.1185. [DOI] [PubMed] [Google Scholar]
- 2.Gould P, Esler M, Kaye D. Chronic atrial fibrillation does not influence the Magnitude of sympathetic overactivity in patients with heart failure. European Heart Journal. 2003;24:1657–62. doi: 10.1016/s0195-668x(03)00393-2. [DOI] [PubMed] [Google Scholar]
- 3.Swissa M, Zhou S, Gonzalez-Gomez I, et al. Long-term subthreshold electrical stimulation of the left stellate ganglion and a canine model of sudden cardiac death. J Am Coll Cardiol. 2004;43:858–64. doi: 10.1016/j.jacc.2003.07.053. [DOI] [PubMed] [Google Scholar]
- 4.Miyauchi Y, Zhou S, Okuyama Y, et al. Altered atrial electrical restitution and heterogeneous sympathetic hyperinnervation in hearts with chronic left ventricular myocardial infarction: implications for atrial fibrillation. Circulation. 2003;108:360–6. doi: 10.1161/01.CIR.0000080327.32573.7C. [DOI] [PubMed] [Google Scholar]
- 5.Swissa M, Zhou S, Paz O, Fishbein M, Chen L, Chen P. Canine model of Paroxysmal atrial fibrillation and paroxysmal atrial tachycardia. Am J Physiol Heart Circ Physiol. Nov;289(5):H1851–7. doi: 10.1152/ajpheart.00083.2005. Epub 2005 Jul 8. [DOI] [PubMed] [Google Scholar]
- 6.Verrier R, Kwaku K. Frayed nerves in myocardial infarction: the important of rewiring. Circ Res. 2004;94:5–6. doi: 10.1161/01.RES.0000136343.06161.ec. [DOI] [PubMed] [Google Scholar]
- 7.Heumann R, Korsching S, Scott J, Thoenen H. Relationship between levels of nerve growth factor (NGF) and its messenger RNA in sympathetic ganglia and peripheral target tissues. EMBO J. 1984;3:3183–9. doi: 10.1002/j.1460-2075.1984.tb02277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levi-Montalcini R. The nerve growth factor: its role in growth, differentiation and function of the sympathetic adrenergic neuron. Progress in Brain Research. 1976;45:235–58. doi: 10.1016/S0079-6123(08)60993-0. [DOI] [PubMed] [Google Scholar]
- 9.Korsching S, Thoenen H. Nerve growth factor in sympathetic ganglia and corresponding target organst of the rat: correlation with density of sympathetic innervation. Proc Natl Acad Sci U S A. 1983;80:3513–6. doi: 10.1073/pnas.80.11.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou S, Chen L, Miyauchi Y, et al. Mechanisms of cardiac nerve sprouting after myocardial infarction in dogs. Circ Res. 2004;95:76–83. doi: 10.1161/01.RES.0000133678.22968.e3. [DOI] [PubMed] [Google Scholar]
- 11.Cao J, Chen L, KenKnight B, et al. Nerve sprouting and sudden cardiac death. Circ Res. 2000;86:816–21. doi: 10.1161/01.res.86.7.816. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz PJ, Stone HL. Left stellectomy in the prevention of ventricular fibrillation caused by acute myocardial ishcmia in conscious dogs with anterior myocardial infarction. Circulation. 1980;62:1256–65. doi: 10.1161/01.cir.62.6.1256. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz PJ, Motolese M, Pollavini G, et al. Italian Sudden Death Prevention Group. Prevention of sudden cardiac death after a first myocardial infarction by pharmacologic or surgical antiadrenergic interventions. J Cardiovas Electorphysiol. 1992;3:2–16. [Google Scholar]
- 14.Docimo S, Jr, Piccolo C, Van Arsdale D, Elkowitz DE. Pathology-Dependent Histological Changes of the Left Stellate Ganglia: A Cadaveric Study. Clinical Medicine. Pathology. 2008;1:105–13. doi: 10.4137/cpath.s979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korsching S, Thoenen H. Developmental changes of nerve growth factor levels in sympathetic ganglia and their target organs. Dev Biol. 1988;126:40–6. doi: 10.1016/0012-1606(88)90236-9. [DOI] [PubMed] [Google Scholar]
- 16.Li JY, Kling-Petersen A, Dahlstrom A. GAP 43-like immunoreactivity in normal Adult rat sciatic nerve, spinal cord, and motoneurons: axonal transport and effect of spinal cord transaction. Neuroscience. 1993;57:759–76. doi: 10.1016/0306-4522(93)90022-8. [DOI] [PubMed] [Google Scholar]
- 17.Swissa M, Zhou S, Gonzalez-Gomez I, et al. Long-term subthreshold electrical stimulation of the left stellate ganglion and a canine model of sudden cardiac death. J Am Coll Cardiol. 2004;43:858–64. doi: 10.1016/j.jacc.2003.07.053. [DOI] [PubMed] [Google Scholar]
- 18.Pather N, Partab P, Singh B. Cerico-thoracic ganglion: its clinical implications. Clin Anat. 2006;9:323–6. doi: 10.1002/ca.20214. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz PJ, Stone HL, Brown AM. Effects of unilateral stellate ganglion blockade on the arrhythmias associated with coronary occlusion. Am Heart J. 1976;92:589. doi: 10.1016/s0002-8703(76)80078-6. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz PJ, Snebold NG, Brown AM. Effects of unilateral cardiac sympathetic denervation on the ventricular fibrillation threshold. Am J Cardiol. 1976;37:1034. doi: 10.1016/0002-9149(76)90420-3. [DOI] [PubMed] [Google Scholar]