Abstract
Better childhood nutrition is associated with earlier physical maturation during adolescence and increased schooling attainment. However, as earlier onset of puberty and increased schooling can have opposing effects on fertility, the net effect of improvements in childhood nutrition on a woman’s fertility are uncertain. Using path analysis, we estimate the strength of the pathways between childhood growth and subsequent fertility outcomes in Guatemalan women studied prospectively since birth. Height for age z score at 24 months was positively related to body mass index (BMI kg/m2) and height (cm) in adolescence and to schooling attainment. BMI was negatively associated (−0.23 ± 0.09 years per kg/m2; p < .05) and schooling was positively associated (0.38 ± 0.06 years per grade; p < .001) with age at first birth. Total associations with the number of children born were positive from BMI (0.07 ± 0.02 per kg/m2; p < .05) and negative from schooling (−0.18 ± 0.02 per grade; p < .01). Height was not related to age at first birth or the number of children born. Taken together, childhood nutrition, as reflected by height at 2 years, was positively associated with delayed age at first birth and fewer children born. If schooling is available for girls, increased growth during childhood will most likely result in a net decrease in fertility.
Childhood nutrition—usually measured as stature for age using a reference population— is related positively to nutritional status in adolescence (Khan et al. 1995; Martorell et al. 1998), inversely to the onset of puberty (Ellis 2004; Ellison 1982), and positively to cognitive development (Grantham-McGregor and Baker-Henningham 2005; Walker et al. 2000) and educational achievement (Jukes 2005; Li et al. 2004; Pollitt et al. 1995). Schooling attainment is associated with delayed age at first birth and lower fertility across several countries and socioeconomic strata (Bongaarts 2003; Castro Martin 1995). Yet, earlier menarche and physical maturation are associated with earlier ages at first marriage and first birth (Chowdhury et al. 2000; Gupta and da Costa Leite 1999), which can lead to higher fertility (Department Economic and Social Affairs 2004). If more schooling tends to decrease fertility and earlier physical maturation tends to increase fertility, the net effect of improved child nutrition on fertility is uncertain.
Our study compares the joint associations of childhood and pre-pregnant nutritional status and schooling on fertility outcomes. Implementing a path analysis with data collected from Ladino (mixed Spanish-Mayan heritage) Guatemalan women during childhood, adolescence, and adulthood, we investigate the net effects of improved childhood nutrition on women’s fertility (measured as age at first birth and children born to date) by examining the relative strengths of two mediating factors: (1) adolescent growth and maturation and (2) schooling. We use a remarkable data set for this analysis, namely a series of studies carried out in rural Guatemala by investigators at the Institute of Nutrition of Central America and Panama (INCAP) and in the United States. A supplementation study of growth and development, carried out from 1969 to 1977, initiated the series of studies in which measures of physical, intellectual, and reproductive health have been collected during early childhood, adolescence, and adulthood (Habicht and Martorell 1993; Martorell et al. 2005).
BACKGROUND
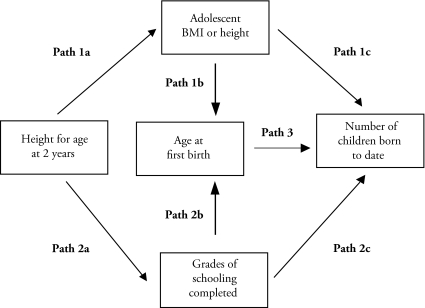
As the basis for this study, we adopt a biosocial model of women’s fertility-related behavior. A woman’s fertility-related behavior—and especially her age at first birth and cumulative fertility—is expected to result from two competing biological and social processes (Figure 1). On the one hand, improvements in a woman’s nutritional status—or growth—in childhood should improve her nutritional status in adolescence (path 1a), and thereby reduce her age at first birth (path 1b) and increase her cumulative fertility (paths 1c and 3) by enhancing her physiological capacity to bear children. On the other hand, improvements in a woman’s nutritional status—or growth—in childhood should enhance her cognitive abilities, and thereby improve her readiness for and performance in school (paths 2a). A woman’s exposure to more schooling also will alter her childbearing preferences, leading to a later age at first birth (path 2b) and lower cumulative fertility (paths 2c and 3).
Figure 1.
Conceptual Tested Path Models From Early Childhood Nutrition to Age at First Birth and Number of Children Born to Date Through Adolescent Pre-pregnant Nutrition Status (BMI/height) and Grades of Schooling Completed
In the current study, we investigate the association of height at age 2 years—a measure of childhood nutrition status—with a woman’s age at first birth and number of children ever born, mediated through two competing paths: her adolescent pre-pregnant nutrition status (BMI or height as indicators of physical growth and maturation) and her schooling (indicated by the number of grades completed). We address three questions. First, compared with their peers, are women who are taller at age 2 years more likely to begin child bearing at an earlier age and have more children because they were taller and larger during adolescence/young adulthood? Second, compared with their peers, are women who are taller at 2 years more likely to begin childbearing at a later age and have fewer children because they acquired more schooling during childhood and adolescence/young adulthood? Finally, considering these two sets of pathways, what is the net effect of being taller at 2 years on a woman’s age at first birth and number of children born?
Childhood Nutrition Status, Maturation, and Fertility
Undernutrition early in life results in delayed physical and pubertal development (Ellis 2004; Ellison 1982; Victora et al. 2008; see Path 1a in Figure 1). In the cohort under study here, nutritional supplementation during the first three years of life resulted in increased height and weight during childhood (Schroeder et al. 1995) and was associated with increased height and weight in adolescence (Rivera et al. 1995). Similarly, stunting at age 2 years was related to lower BMI in Jamaican adolescents (Walker, Chang, and Powell 2007), and stunting at age 3 years was associated with shorter height in adolescence in the cohort under study (Ruel et al. 1995).
In recent years, the mean age at menarche has decreased in many lower-income countries (Chowdhury et al. 2000), whereas height and weight-for-age during adolescence have increased (da Veiga, da Cunha, and Sichieri 2004; Turconi et al. 2006). These changes are attributed to improvements in nutrition and health through increased access to and availability of food, sanitation, and healthcare (Dick et al. 2006; Rees 1993).
Age at menarche is inversely associated with adolescent height (Ellison 1982), weight or fat (Frisch 1981), or both (Bini et al. 2000; Kaplowitz 2006). Girls with increased BMI are more likely to enter puberty earlier and have earlier breast development, menarche, and other secondary sexual maturation characteristics (Kaplowitz 2006). Among well-nourished adolescents, girls who mature earlier tend to gain height faster before menarche, whereas girls who mature later take longer to attain a similar height (Tanner 1972). Taken together, girls who are taller or larger at any age are likely to reach stages of sexual maturation earlier than their shorter, same-aged peers.
Earlier physical and sexual maturation may lead to an earlier age at first sex, first union, and first birth (Path 1b in Figure 1). In rural Bangladesh and the Sudan, for example, girls who were larger, taller, or more sexually mature at earlier ages were younger at first marriage and first pregnancy (Chowdhury et al. 2000; Otor and Pandey 1999).
In the cohort under investigation here, women who were not severely stunted at 2 years (height for age z score > −3) were seven months younger at menarche (Khan et al. 1995) and one year younger at first birth than women who were severely stunted at 2 years, which corroborates the idea that improved nutritional status in childhood may result in an earlier pregnancy through earlier maturation in adolescence. Earlier maturation and first birth will subsequently lengthen the time that a woman has to bear children, often leading to higher total fertility (Paths 1c and 3 in Figure 1).
Childhood Nutrition Status, Education, and Fertility
Childhood nutrition is positively associated with cognitive development (Path 2a in Figure 1). Stunting at age 2 years has been associated with lower cognitive and IQ test scores, poorer schooling attainment, and irreversible intellectual impairment in adolescence (Grantham-McGregor and Baker-Henningham 2005; Walker et al. 2000). In the Guatemalan cohort under study here, early postnatal nutrition and height at two years in girls were associated with improved intellectual functioning in adolescence and adulthood (Li et al. 2004; Stein et al. 2008). Thus, the first two years of life are a critical period for cognitive and intellectual development.
Studies over the past decade have also pointed to a strong positive relationship between early cognitive functioning, tests of intelligence, and scholastic performance and achievement (Currie 2005; Ramey and Ramey 2004). When cognitive functioning is impaired or hindered in early childhood as a result of poor health, children are more likely to begin school at a later age, perform poorly on grade achievement tests, repeat a grade, or need special education (Currie and Stabile 2004; Lorntz et al. 2006). Furthermore, parents are more likely to invest in the schooling of their children who demonstrate higher cognitive functioning (Hess and Holloway 1984).
Increased schooling has been associated with a later age at first marriage and first birth (Gupta and da Costa Leite 1999; MSPAS 2003; see Path 2b in Figure 1), and lower total fertility (Bongaarts 2003; Castro Martin 1995; see Paths 2c and 3 in Figure 1). Researchers have proposed various mechanisms by which women’s schooling influences their fertility. Schooling teaches women skills, such as literacy and problem-solving, that increase their opportunities in the labor market (Duryea, Edwards, and Ureta 2001) and reduce the time available for childbearing and child rearing. Schooling also increases aspirations for improved economic status (Caldwell 1980). Women with more schooling invest more time and money in themselves and their children (Behrman et al. 1999). Schooling can provide a stronger sense of control over personal choices, so that women feel freer to use contraception to control their fertility (Tountas et al. 2004).
Summary and Hypotheses
Synthesizing the above discussion, Figure 1 illustrates the pathways that we will test in this analysis. Hypotheses of the process relations, which correspond to our three research questions, are as follows:
H1: A woman’s nutrition in childhood, as measured by her height for age z score at 24 months (z24), will be positively associated with her adolescent pre-pregnant nutritional status (measured as BMI and height at an age during adolescence before her first pregnancy) and with schooling attainment (measured as grades of schooling completed).
H2a: A woman’s schooling attainment will be positively associated with her age at first pregnancy and negatively associated with her number of children born to date.
H2b: A woman’s pre-pregnant nutritional status will be negatively associated with her age at first pregnancy and positively associated with her number of children born to date.
H3: The negative associations of schooling attainment with these fertility outcomes will be larger in absolute magnitude than will the positive associations of pre-pregnant nutritional status with the same outcomes. As a result, childhood nutrition will have a net negative association with cumulative fertility.
DATA AND METHODS
Sample Description
The women in this analysis participated as children in a nutritional supplementation study conducted between 1969 and 1977 (Habicht and Martorell 1993) in four Ladino, Spanish-speaking villages located in the Department of El Progreso, Guatemala, about 100 km northeast of Guatemala City. Extensive measurements were obtained on women and their offspring, including serial measures of child length and weight (Martorell, Habicht, and Rivera 1995). Several follow-up studies have been conducted. Data for the present analysis were derived from information collected during the supplementation study in 1969–1977 and follow-up studies in 1988–1989 (Martorell 1995), and 2002–2004 (Martorell et al. 2005). All study protocols were approved by institutional review boards at INCAP, Emory University, and the International Food Policy Research Institute (IFPRI). Informed consent was obtained from all participants for each study.
The villages originally were chosen because of their similar population size, social isolation, levels of schooling (30% literacy among residents aged 8 years and older), and high prevalences of malnutrition and gastrointestinal and respiratory infections. Improvements in transportation to and from the villages over the past 30 years have provided better access to schooling, health care, food, goods and services, and labor markets for men and women.
Accompanying these improvements is a higher standard of living among village residents, as measured by living situation and durable goods (Maluccio et al. 2005). Between 1967 and 2002, rates of literacy among village women aged 15 and older rose from 35% to 79%, and the mean number of completed grades of primary school increased from one to three or four years. Comparatively, women who were born in the villages and later moved to Guatemala City had, on average, four to five years of schooling (Stein et al. 2005). Today, health centers in the four villages provide birth control, iron supplements, immunizations, and prenatal check-ups, but due to a lack of resources, primary health care is limited. Consequently, many residents turn to local traditional birth attendants and healers or health clinics in larger towns and cities as well as in Guatemala City. Village markets provide staples such as corn, beans, bread, tortillas, eggs, meats, cheese, and some fresh fruits and vegetables. The villagers also have access to processed foods such as chips, candies, and soda. Larger markets and grocery stores are located in cities.
In-depth descriptions of the socioeconomic and demographic characteristics of the villages and the changes in these characteristics between 1967 and 2002 are reported elsewhere (Bergeron 1992; Maluccio et al. 2005). In general, the changes have provided women in the current study with more opportunities for schooling, increased access to food, and improved healthcare, giving them more control over their childbearing practices.
Selection Criteria
The data for this analysis come from 692 women who had height-for-age z scores taken as children between 1969 and 1977, were surveyed again in 2002–2004, and had been pregnant at least once. Of these, 304 women had measures of height and weight taken during adolescence from the follow-up study in 1988–1989. From this sample, observed measures of z24 were available for 177 women. We imputed z24 for another 47 women for whom height-for-age z scores were taken at other ages. The procedures for imputation are described below and elsewhere (Li et al. 2003). The path analysis is based on a final sample size of 224 women.
Measurement and Variables
Fertility outcomes. We consider two fertility milestones; the age at first birth and the number of children born to date. Women recalled the dates and outcomes of each pregnancy during the 2002–2004 survey. Age at first birth was calculated by subtracting the reported date when the first pregnancy ended (as a live birth or not) from the woman’s date of birth. Since 93% of first pregnancies resulted in a live birth, there was little difference between the age at the end of the first pregnancy and the age at first live birth. In this population, the timing of first intercourse, first marriage, and the end of the first pregnancy are highly correlated (Pearson r > .65), generally occurring within one year of each other. Therefore, the outcome age at first birth refers to the age at which the first pregnancy ended, regardless of whether it ended in a live birth, and to the multiple, highly correlated events of first intercourse, marriage, and pregnancy. The total number of children born to date was derived from the dates and outcomes of each pregnancy.
Nutritional status in childhood. As described above, z24 was used to indicate nutrition status in childhood. Height-for-age z score at 24 months is among the preferred indicators of childhood nutrition and is a strong predictor of human capital (Victora et al. 2008). In our study sample, height at 24 months reflects the influences of supplementation, home diet, infection, and other factors, and thus captures overall nutrition and health in the prior two years of life (Martorell et al. 1995; Schroeder et al. 1995). Length was measured to the nearest millimeter every 3 months through the first 24 months, then every 6 months to 60 months, and again at 72 and 84 months. Lengths were scaled as z scores relative to the median length for the WHO/NCHS reference population and according to the 2000 CDC Growth Charts (CDC 2000).
Intermediate outcomes. Height and BMI during adolescence and prior to first pregnancy (adolescent pre-pregnant height and BMI) were used to measure pre-pregnant nutritional status. As described elsewhere (Martorell 1995), height and weight were taken at age 12–20 years during the 1988–1989 follow-up. BMI was calculated as weight in kg/(height in m)2. Because adolescent measures are age-specific, we adjust for the age at measurement in all analyses.
Achievement in schooling is measured as the number of grades of schooling completed. During the 2002–2004 survey, interviewers administered an extensive schooling history in which they gathered data on the highest grade completed (0–14 grades) in local rural or urban schools.
Control variables. We considered eight variables that, according to the literature, may confound the relationships of interest. These variables were village of origin, socioeconomic status (SES) in childhood, parental schooling, the number of or experience of spontaneous abortions, the mean duration of postpartum amenorrhea, age at adolescent height measurement or BMI calculation, age at menarche, and age at interview (Bongaarts 1978; Jukes 2005; Pollitt et al. 1993; Rosen 2004). The first five of these measures warrant some clarification.
Dummy variables for three of the four villages of origin were considered to control for time-invariant attributes of the study villages and the woman’s original assignment of nutritional supplement. A composite score for household socioeconomic status during childhood, computed using data collected in 1975, was considered to capture family economic status during each woman’s childhood. To derive this score, indicators of housing quality (e.g., type of walls, roof, and floor; number of rooms), mother’s education (literacy and years of schooling), and family occupation ranked by income were standardized and summed (Pollitt et al. 1993). The score has been validated against income and other data (Pollitt et al. 1995). Paternal schooling attainment, as measured by the number of grades completed, was considered to control for parental schooling because the levels of maternal schooling were too low. Of the 224 eligible women who had observed or imputed z24, 217 had data on grades of paternal schooling. For seven women with missing data on paternal grades of schooling, maternal grades of schooling were used instead.
Measures of abortion and postpartum amenorrhea were considered as controls only in the paths to fertility. Data on abortions and postpartum amenorrhea were available from reproductive histories that were obtained in 2002–2004. The survey did not differentiate between spontaneous and induced abortions. Twenty percent of women reported having at least one abortion. Because induced abortions are illegal in Guatemala, women may have underreported the total number of abortions. Still, the number of induced abortions is presumed to be low because in 2002, less than 5% of women reported that they did not desire their last child. The reported outcomes of each pregnancy were used to calculate the number of abortions for each woman. We adjusted separately for the number and experience of any abortions (at least one abortion versus none) in the paths to age at first birth and the number of children born, but the results (not shown but available upon request) did not differ appreciably. Postpartum amenorrhea—or the mean interval in months between each pregnancy and the resumption of menses—was considered in the paths to number of children born; however, the model estimates for variables of interest were robust to its inclusion or exclusion.
Thus, in the final analyses, control variables for each intervening outcome and fertility outcome were as follows: (1) paths to grades of schooling completed included village of birth, socioeconomic status in childhood, and parental schooling; (2) paths to adolescent pre-pregnant BMI or height included village of birth, socioeconomic status in childhood, and age when BMI was calculated or height was measured; (3) paths to age at first birth included the logarithm of the age when BMI was calculated or height was measured; and (4) paths to number of children born to date included the logarithm of age at interview.
Approaches to Missing Data
Using a regression model and multiple imputation techniques (Little and Rubin 1987; Schafer 1997), we imputed z24 for 47 women from highly correlated (r ≥ .8) height for age z score measurements taken when the women were ages 15, 18, 21, 30, 36, 42, or 48 months. Further details are described in Li et al. (2003). To reflect the uncertainty of the imputed data and to reduce type I errors, the imputation process was repeated five times, generating five sets of imputed z24. Values for socioeconomic status in childhood for four women were imputed five times using a mean-plus-random-noise approach, with the imputed values constrained to fall within the range of the observed values (Little and Rubin 1987; Schafer 1997). Path analyses were performed using each of these five data sets. Coefficient estimates were averaged, and the total variance was computed using Rubin’s method (1987) for multiple imputation inferences.
Data Analysis
We used path analysis with observed variables and assumed a recursive model to analyze the pathways from early childhood growth to our fertility outcomes through pre-pregnant nutritional status and schooling attainment. Using MPlus Statistical Analysis Software (Los Angeles, CA), direct associations and standard errors were generated for each pathway. Indirect associations are calculated as the product of the parameter estimates (path coefficients) along a given path. Standard errors for indirect effects with two path coefficients (e.g., p1 × p2) were calculated as . Direct and indirect associations were considered significant at p < .05. All estimated standard errors and chi-square tests of model fit account for the nonindependence of responses among related women (Muthén and Muthén 2001). Standardized path coefficients that are less than |0.05| are considered trivial (Hatcher 1994). Total associations of the number of children born to date with schooling attainment (grades of schooling completed) or adolescent pre-pregnant nutrition status (BMI or height) are shown.
Part of the evaluation of a path model involves assessing how well it accounts for variation in the data. With 61 linearly independent equations and 42 parameters, the models were overidentified (Hatcher 1994), and the sample size of 224 is adequate, providing more than five observations per parameter (Kline 1998). All measures of model fit have their own strengths and weaknesses (Bollen and Long 1993). Our interpretations of the adequacy of model fit are based on three recommended measures for goodness-of-fit: the chi-square (χ2) statistic, the Comparative Fit Index (CFI), and the Standardized Root Mean Square Residual (SRMR) (Kline 1998). We also use the Satorra-Bentler chi-square difference test (Satorra 2000) to compare the goodness-of-fit indices for our full models and nested models, in which at least one path from the full model was constrained to equal zero. A significant chi-square difference test implies that the nested model provides a poorer fit for the data than the full model.
RESULTS
Characteristics of the Sample
Table 1 shows selected characteristics for women in the sample. Stunting was common during early childhood, as evidenced by the mean z24, of −2.42. The women had more schooling (4.9 grades), on average, than their parents (1.9 grades). In 2002, a majority of the women (73.7%) still lived in the villages or nearby small towns. Twenty-three percent had moved to Guatemala City, and 3% had moved to other areas of Guatemala.
Table 1.
Selected Characteristics of Ladino Guatemalan Women Who Were Born in One of Four Rural Guatemalan Villages
| Variable | N | Mean or % | SD |
|---|---|---|---|
| Main Exogenous Variable | |||
| Height for age z score at 24 months | 224 | −2.42 | 1.02 |
| Intermediate Outcomes | |||
| Grades of school completed | 224 | 4.9 | 3.1 |
| Adolescent pre-pregnant BMI (kg/m2) | 224 | 20.3 | 2.7 |
| Adolescent pre-pregnant height (cm) | 224 | 147.7 | 6.2 |
| Final Outcomes | |||
| Age at first birth (years) | 224 | 21.3 | 3.6 |
| Number of children born to date | 224 | 2.6 | 1.3 |
| Control Variables (and other descriptive variables) | |||
| Socioeconomic status in childhood (1975) | 224 | –0.19 | 1.6 |
| Parental schooling (grades) | 224 | 1.9 | 2.1 |
| Age at menarche | 224 | 13.5 | 1.3 |
| Age at first marriage (years) | 213 | 20.3 | 3.6 |
| Age at adolescent pre-pregnant BMI and height (years) | 224 | 15.7 | 2.2 |
| First pregnancy resulted in a live birth (%) | 224 | 92.8 | |
| Postpartum amenorrhea (months) | 224 | 7.7 | 3.4 |
| Abortion (% who had at least one) | 224 | 16.5 | |
| Contraceptive use in the past 12 months (%) | 224 | 74.1 | |
| Reside in or around village of residence in 2002 (%) | 224 | 73.7 | |
| Age at interview (years) | 224 | 30.3 | 2.2 |
The average age at first birth was 21.3 years, and 92.8% of these pregnancies resulted in a live birth. Based on adolescent pre-pregnant height and BMI taken between the ages of 12 and 19 years, women were relatively short (148 cm, range 127 cm to 163 cm), but most were of adequate weight according to their height (20.6 kg/m2, range 13.7 kg/m2 to 27.3 kg/m2). Seven-and-one-half percent of women were overweight, and 5.3% were underweight (WHO Multicentre Growth Reference Study Group 2006). At the average age at interview of 30 years, women had delivered an average of three children, and 16.5% of women had experienced at least one abortion. Use of contraception in the year before the interview was high, at 74.1%.
Path Analysis of Child Nutrition and Fertility
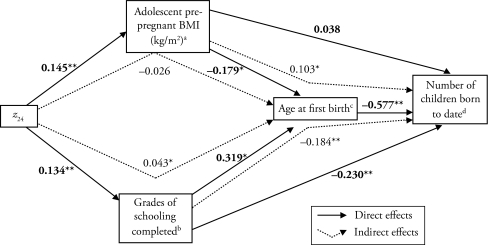
For the model represented in Figure 2 and Table 2, in which the mediators are adolescent pre-pregnant BMI and grades of schooling completed, each unit increase in z24 was associated with an increase of 0.41 grades completed and of 0.38 kg/m2 in BMI (Table 2). Subsequently, the standardized direct association of grades completed with age at first birth, 0.319, was significant and larger in absolute magnitude than the opposing direct association of adolescent pre-pregnant BMI, −0.179 (Figure 2). Expressed in original units (Table 2), each completed grade was associated with a 0.36 ± 0.06-year increase in the age at first birth, whereas each BMI unit (kg/m2) was associated with a 0.23 ± 0.10-year decrease in the age at first birth. The indirect associations of z24 with age at first birth through grades of schooling completed (0.043 or 0.15 ± 0.04 years per grade) and adolescent pre-pregnant BMI (−0.026 or −0.09 ± 0.05 years per kg/m2) are considered trivial. Still, the total decrease in age at first birth that is mediated through BMI (−0.32 ± 0.10 years per kg/m2) is more than compensated for by the total increase in age at first birth that is mediated through schooling (0.51 ± 0.06 years per grade). The net result from each unit increase in z24 is an increase of 0.19 ± 0.10 years in age at first birth when mediated through BMI and schooling.
Figure 2.
Diagram of Direct and Indirect Standardized Path Coefficients From Height for Age z Score at 24 Months (z24) to Adolescent Pre-pregnant BMI, or Grades of Schooling Completed to Age at First Live Birth and Number of Children Born to Date (N = 224)
Note: Model fit statistics: χ2 =50.0; df = 111; p = 1.0; SRMR = 0.025; and CFI = 1.0
aModel is adjusted for village, socioeconomic status in 1975, and the log of age when BMI taken.
bModel is adjusted for village, socioeconomic status in 1975, and parental schooling.
cModel is adjusted for the log of age when BMI taken.
dModel is adjusted for the log of age at interview.
*p < .05; **p < .01
Table 2.
Nonstandardized Path Coefficients for Direct, Indirect, and (where applicable) Total Effects of Height for Age z Score at 24 Months (z24) Th rough Adolescent Pre-pregnant BMI or Grades of Schooling Completed on Age at First Birth and Number of Children Born to Date (N = 224)
| Path and Dependent Outcomes | Predictor Variables | Direct Effect | Indirect Effect | Total Effect |
|---|---|---|---|---|
| Path 1: Adolescent Pre-pregnant BMI (kg/m2) | z24 | 0.38** (0.14) | ||
| Path 1: Grades of Schooling Completed | z24 | 0.41** (0.12) | ||
| Path 2: Age at First Birth (years) | Adolescent pre-pregnant BMI | −0.23* (0.10) | ||
| Grades of schooling completed | 0.36** (0.06) | |||
| Through adolescent pre-pregnant BMI (kg/m2) | z24 | −0.09 (0.05) | −0.32** (0.10) | |
| Through grades of schooling completed | z24 | 0.15** (0.05) | 0.51** (0.06) | |
| Path 3: Number of Children Born to Date | Age at first birth | −0.22** (0.02) | ||
| Adolescent pre-pregnant BMI | 0.02 (0.03) | 0.05* (0.02) | 0.07* (0.03) | |
| Grades of schooling completed | −0.10** (0.02) | −0.08** (0.01) | −0.18** (0.02) |
Notes: Numbers in parentheses are standard errors. For the path 1: pre-pregnant BMI outcome, the model is adjusted for the log of age when BMI was taken, village, and socioeconomic status in 1975. For the path 1: grades of schooling outcome, the model is adjusted for parental schooling, village, and socioeconomic status in 1975. For path 2, the model is adjusted for the log of age when BMI was taken. For path 3, the model is adjusted for the log of age at interview.
p < .05;
p < .01
The associations of grades of schooling completed with the number of children born to date directly (−0.230) or indirectly (−0.184) through the age at first birth were larger in absolute magnitude than the opposing direct (0.038) and indirect (0.103) associations of adolescent pre-pregnant BMI with this outcome. As a result, the total association of z24 with the number of children born to date was 0.141 mediated through BMI and −0.414 mediated through schooling, for a net change of −0.273. Expressed in original units, each unit increase in BMI is associated with a 0.07 ± 0.03 increase in the number of children born to date, and each additional grade of schooling is associated with a 0.18 ± 0.02 decrease in the number of children born to date. Thus, for every unit increase in z24, the net change in the number of children born to date is −0.11 ± 0.03.
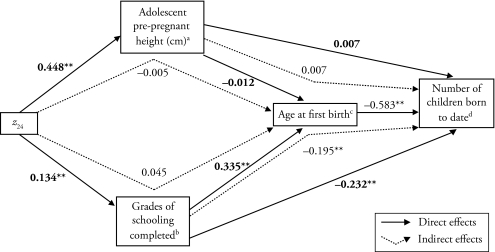
Turning to the model in which we used adolescent pre-pregnant height as a mediating variable (Figure 3 and Table 3), each unit increase in z24 was associated with an increase of 2.69 ± 0.34 cm in adolescent pre-pregnant height and of 0.41 ± 0.12 in grades of schooling completed. There was little direct association between age at first birth and adolescent pre-pregnant height (−0.012 or −0.01 ± 0.03 years per cm) or indirect association between age at first birth and z24 through adolescent pre-pregnant height (−0.005 or −0.02 ± 0.07 years per cm per unit z score). Yet, as in the models with adolescent pre-pregnant BMI, the direct association of grades of schooling completed with the age at first birth (0.335 or 0.38 ± 0.06 years per grade) and indirect association of each unit increase in z24 through schooling (0.045 or 0.15 ± 0.05 years per grade) were larger. Each unit increase in z24 resulted in a net increase of 0.50 ± 0.07 years in age at first birth when mediated through height and schooling.
Figure 3.
Diagram of Direct and Indirect Standardized Path Coefficients From Height for Age z Score at 24 Months (z24) to Adolescent Pre-pregnant Height, or Grades of Schooling Completed to Age at First Birth and Number of Children Born to Date (N = 224)
Note: Model fit statistics: χ2 =50.0; df = 111; p = 1.0; SRMR = 0.02; and CFI = 1.0
aModel is adjusted for log of age when height taken, village, and socioeconomic status in 1975.
bModel is adjusted for parental schooling, village, and socioeconomic status in 1975.
cModel is adjusted for the log of age when height taken.
dModel is adjusted for the log of age at interview.
*p < .05; **p < .01
Table 3.
Nonstandardized Path Coefficients for Direct, Indirect, and (where applicable) Total Effects of Height for Age z Score at 24 Months (z24) Through Adolescent Pre-pregnant Height or Grades of Schooling Completed on Age at First Birth and Number of Children Born to Date (N = 224)
| Path and Dependent Outcomes | Predictor Variables | Direct Effect | Indirect Effect | Total Effect |
|---|---|---|---|---|
| Path 1: Adolescent Pre-pregnant Height (cm) | z24 | 2.69** (0.34) | ||
| Path 1: Grades of Schooling Completed | z24 | 0.41** (0.12) | ||
| Path 2: Age at First Birth (years) | Adolescent pre-pregnant height | −0.01 (0.03) | ||
| Grades of schooling completed | 0.38** (0.06) | |||
| Through adolescent pre-pregnant height (cm) | z24 | −0.02 (0.07) | −0.03 (0.07) | |
| Through grades of schooling completed | z24 | 0.15** (0.05) | 0.53** (0.06) | |
| Path 3: Number of Children Born to Date | Age at first birth | −0.22** (0.02) | ||
| Adolescent pre-pregnant height | 0.001 (0.01) | 0.002 (0.01) | 0.003 (0.01) | |
| Grades of schooling completed | −0.10** (0.02) | −0.08* (0.01) | −0.18** (0.02) |
Notes: Numbers in parentheses are standard errors. For the path 1: pre-pregnant height outcome, the model is adjusted for the log of age when height was taken, village, and socioeconomic status in 1975. For the path 1: grades of schooling outcome, the model is adjusted for parental schooling, village, and socioeconomic status in 1975. For path 2, the model is adjusted for the log of age when height was taken. For path 3, the model was adjusted for the log of age at interview.
p < .05;
p < .01
The direct association of grades of schooling completed with the number of children born to date, −0.232, and the indirect association of grades of schooling completed, through age at first birth, with the number of children born to date, −0.195, were both significant. There was no direct association between the number of children born to date and adolescent pre-pregnant height, 0.007, or indirect association between the number of children born to date and adolescent pre-pregnant height through age at first birth, 0.007. The net association of adolescent pre-pregnant height and grades of schooling completed with the number of children born to date (−0.225 or −0.18 ± 0.02 in the number of children born per grade of schooling completed and cm of height) was mainly a result of schooling’s association with the number of children born.
As expected, in both models, age at first birth was negatively associated with the number of children born to date, resulting in a −0.22 ± 0.02 decrease in the number of children born per one-year increase in age at first birth.
Tests of Alternative Nested Models
The full model depicted in Figure 2 was compared to four nested models (Table 4, 2a–2d): nested models 2a and 2b exclude paths from adolescent pre-pregnant BMI or grades of schooling completed to the fertility outcomes, respectively; nested models 2c and 2d exclude paths from z24 to adolescent pre-pregnant BMI or grades of schooling completed, respectively. The same exercise was performed for the full model depicted in Figure 3 with schooling and height (Table 4: Models 3a, 3b, 3c, and 3d).
Table 4.
| Model | Chi-squarea | df | p Value | Scaled Correction Factor (c) for MLMb | SRMRc | CFId | Chi-Square Difference Test TRde (df) | |
|---|---|---|---|---|---|---|---|---|
| Model in Figure 2: Paths mediated through adolescent pre-pregnancy BMI and schooling | 50.0 | 111 | 1.0 | 2.8 | 0.025 | 1.0 | Full model | |
| Nested model 2a: Path mediated through BMI (excludes paths from schooling to fertility outcomes) | 110.2 | 116 | .6 | 2.75 | 0.06 | 1.0 | 99.6* | (5) |
| Nested model 2b: Path mediated through schooling (excludes paths from BMI to fertility outcomes) | 129.9 | 121 | .3 | 2.7 | 0.08 | .9 | 132.7* | (5) |
| Nested model 2c: Path mediated through BMI (excludes path from z24 to schooling) | 60.0 | 116 | .0 | 2.7 | 0.03 | 1.0 | 46.4* | (10) |
| Nested model 2d: Path mediated through schooling (excludes paths from z24 BMI) | 62.7 | 116 | .0 | 2.7 | 0.03 | 1.0 | 61.6* | (5) |
| Model in Figure 3: Paths through adolescent pre-pregnant height and schooling | 50.0 | 111 | 1.0 | 2.9 | 0.02 | 1.0 | Full model | |
| Nested model 3a: Path mediated through height (excludes path from schooling to fertility outcomes) | 116.6 | 116 | .5 | 2.8 | 0.06 | 1.0 | 312.9* | (5) |
| Nested model 3b: Path mediated through grades of schooling (excludes path from height to fertility outcomes) | 117.7 | 121 | .6 | 2.7 | 0.08 | .9 | 360.0* | (10) |
| Nested model 3c: Path mediated through height (excludes path from z24 to schooling) | 59.9 | 116 | 1.0 | 2.8 | 0.03 | 1.0 | 39.2* | (5) |
| Nested model 3d: Path mediated through schooling (excludes path from z24 to height) | 174.2 | 116 | < .01 | 2.8 | 0.05 | 0.9 | 591.0* | (5) |
Chi-square is based on MLM estimator.
The scaled correction factor (c) is multiplied with the chi-square for the MLM estimator (above) to get chi-square for ML estimator.
Standardized root mean square residual (SRMR) is the average difference between the predicted and observed variances and covariances in the model, based on standardized residuals. SRMR is 0 when the model fit is perfect; SRMR < 0.05 is considered a good fit.
Comparative fit index (CFI) compares the existing model fit with a null model to gauge the percent of lack of fit which is accounted for by going from the null model to the researcher’s SEM model. CFI is among the measures least affected by sample size (Fan, Thompson, and Wang 1999). CFI varies from 0 to 1. A CFI close to 1 indicates a very good fit. CFI should be equal to or greater than .90 to accept the model.
Satorra-Bentler scaled chi-square difference test comparing models depicted in Figures 2 and 3 with nested models. TRd = (ML chi-square for nested model – ML chi-square for full model) / cd, where cd (chi-square difference scaling correction) = (df for nested model × c for nested model – df for full model × c for full model) / df for nested model – df for full model.
p < .01
The goodness-of-fit statistics in Table 4 illustrate that all models except for nested model 3d provide a reasonable fit for the data. The Satorra-Bentler chi-square difference test between the full models represented in Figures 2 and 3 and each of the nested models were significant, implying that the full models, which include paths mediated through schooling and an indicator of adolescent pre-pregnant nutrition status, provide a better fit for the data as compared to the nested models. Path estimates from the alternative nested models were not appreciably different from those in the full models, except where paths were deleted in each of the nested models.
DISCUSSION
Our study uniquely compares, in a single model, the relative influence of biological (prepregnant nutritional status during adolescence or early adulthood) and social (grades of schooling completed) mediators between childhood nutritional status and women’s fertility outcomes. As expected, height at 2 years was associated with grades of schooling and with both adolescent BMI and height. Also, schooling attainment and, to a lesser extent, adolescent BMI were associated with at least one of the fertility outcomes under study. Compared with pre-pregnant nutritional status, however, schooling attainment was a stronger mediator of childhood nutritional status and the fertility outcomes. As a result, the net association of childhood nutrition with cumulative fertility was negative.
Specific findings are notable regarding the pathway from early childhood nutrition to adolescent nutritional status, and ultimately to fertility. Regarding the first component of this pathway of effect, the height for age z score at 24 months was positively related not only to adolescent height but also to adolescent BMI, and these associations were similar in magnitude. These findings corroborate prior research with this cohort on the associations of early childhood growth and nutrition with body size and composition in adolescence and adulthood. Namely, nutritional supplementation during the first three years of life has resulted in increased height and weight during childhood (Schroeder et al. 1995), and was associated with increased height and weight in adolescence (Rivera et al. 1995). Stunting at age 3 years also was associated with shorter height in adolescence (Ruel et al. 1995). Two other studies have assessed the relative effects of birth size, early childhood growth, or length at 2 years on subsequent nutritional status. Observed effect sizes of length at 2 years on adult height, weight, fat-free mass, and fat mass were 0.50, 0.43, 0.47, and 0.34, respectively (Li et al. 2003). Also, increases in BMI and length during the first year, as well as from the first to third years of life, each were associated with higher BMI in adulthood (Corvalan et al. 2007). Based on this set of findings, it is reasonable to expect that girls who are taller at 2 years also have higher BMI in adolescence.
Regarding the second component of this pathway of effect, BMI was inversely associated with age at first birth, whereas height was not associated with this fertility outcome. This finding suggests that attaining a certain weight for height (BMI), rather than height alone, is a better indicator of physical maturation status. Although both height and weight are associated with age at menarche (Bini et al. 2000, Ellison 1982; Frisch 1981; Kahl, Schaffrath Rosario, and Schlaud 2007; Kaplowitz et al. 2006), BMI is a better predictor of other secondary sexual characteristics (Kaplowitz et al. 2006; Zukauskaite et al. 2005) that are strongly associated with the timing of marriage and first birth and is perceived to be related to fertility and health (Tovée et al. 1998). Girls who are larger at an earlier age also physically mature earlier (Hoffman et al. 2005; Rosen 2004) and therefore tend to be younger at their first birth (Chowdhury et al. 2000; Gupta and da Costa Leite 1999). Thus, if schooling is not a countervailing factor, then achieving a higher BMI at an earlier age may increase the probability of an earlier first birth.
Moreover, both path analyses and qualitative findings corroborate the importance of the mediating paths from height for age z score at 24 months to fertility through grades of schooling completed and adolescent pre-pregnant BMI. Namely, nested path models that delete at least one of these paths showed a poorer fit to the data than did the full model (Figure 2), suggesting statistically that both grades of schooling completed and adolescent pre-pregnant BMI are independent mediators. Data from focus group discussions that were conducted with village women in 2004 also suggest that both mediators are relevant.1 Women, for example, underscored the importance of a woman’s mental and physical maturity for healthy childbearing. Women who had completed primary schooling also preferred to delay childbearing out of a concern for physical maturity, whereas women with less schooling cautioned against “excessive” delays.
Thus, adolescent BMI is one reasonable measure of size and physical maturation prior to first birth, with a higher BMI in adolescence implying earlier physical maturation, earlier menarche, and an earlier acquisition of secondary sex characteristics. Other measures of body size and composition, however, may be reasonable candidates for future work on the biological pathways to fertility. Four measures are noteworthy. First, the waist-hip ratio (WHR) has been used to determine pubertal and physical development as well as ideal body size and mating quality (Yu and Shepard 1998). WHR, as well as BMI, are associated positively with perceived attractiveness (Tovée et al. 1998; Yu and Shepard 1998) and negatively with age at first intercourse (Hughes and Gallup 2002) and hence fecundity. Second, peak height velocity, which measures the rate of growth in units per year, is typically attained at least one year before menarche and so reflects a maturation landmark (Rosen 2004). Factor analysis of anthropometric variables of girls in the National Heart, Lung, and Blood Institute Growth and Health Study showed larger loadings for weight and relative weight at menarche (R2 = .178) and height velocity (R2 = .167) than for height at menarche (R2 = .084) on age at menarche (Biro et al. 2005). Third, neither BMI nor height during adolescence was associated with the number of children born to date. Since a woman’s BMI is sensitive to reproductive events (Kac et al. 2003), pregnancy weight gain or weight retention may be important to consider in path models of cumulative fertility (Reifsnider and Gill 2000). Finally, adult height, rather than adolescent height, may be a better predictor of the number of children a woman bears. In a supplemental analysis, we divided women into quartiles by adult height and found that being in the highest quartile (154–167 cm) compared with the lowest quartile (135–146 cm) was associated with having 0.44 ± 0.14 fewer children born after controlling for schooling, age at first pregnancy, and current age. One explanation for this finding is that women who began childbearing earlier had not achieved their maximum height and did not grow further due to the energy demands of pregnancy and lactation (Casanueva et al. 2006). Furthermore, schooling not only may increase a woman’s age at first birth and reduce the number of children she bears but also may provide time for her to attain additional height.2
The relatively large total association of schooling attainment versus pre-pregnant nutritional status on the number of children born to date is equally notable. Schooling has been strongly associated with the use of contraception, which may influence the timing of first birth and total family size (Castro-Martin and Juarez 1995). Focus group discussions with village women revealed that, although all women had tried at least one form of contraception, more-schooled women described using a greater variety of methods with greater frequency than less-schooled women. In addition, more-schooled women considered not only the financial costs of clothing and feeding their children but also the costs of schooling them, which may have influenced them to have fewer children.
Regarding the other findings in this analysis, the large negative direct association of age at first birth with the number of children born to date not surprisingly suggests that a woman’s age at first childbearing strongly influences her cumulative fertility. Because the women in this cohort have not completed their reproductive years, many are likely to have more children. If women who began childbearing at a later age also ended childbearing at a later age, then the association of schooling with cumulative fertility could diminish. During focus group discussions, women who had completed primary school desired smaller family sizes and described the ideal family size as three children, whereas women who had three or fewer years of schooling considered four to five children to be ideal.
In addition, it is possible that the negative direct association of age at first birth on the number of children born to date was underestimated. Given the sensitivity of the topic, women were not asked about voluntary abortions. This number, however, is presumed to be small because less than 5% of women said they did not desire their last child. Still, the total number of induced abortions may be underreported. Gender preference also could influence the number of children born, if women continue bearing children until they have a certain number of males or females. Given the levels of fertility in this setting, gender preference is unlikely to elevate fertility further because most families will likely achieve their desired gender composition within their desired number (Yount, Langsten, and Hill 2000). Also, of the women who said they did not desire their last child, 48% had a girl and 52% had a boy, suggesting that gender preferences are approximately balanced in this population.
Certain analytical issues and their solutions also merit comment. First, missing data for selected variables were imputed using established methods that assume that data are missing at random (Allison 2002). If these assumptions are reasonable for these data, then such methods have the benefits of increasing analytic power and of preserving useful information on other variables (Little and Rubin 1987). Second, this analysis did not assess the influence of the quality of schooling received. Historically, the quality of schooling in the study villages has been low (Bergeron 1992), but schools in two of the four study villages had fewer teachers and were in exceptionally poor condition. The average grades of schooling also were lower in these villages, so the quantity of schooling completed partially reflects the quality received. Women who attended school in Guatemala City or in the larger municipal towns most likely received higher-quality schooling than did women in the villages. However, adjustment for residence and village of birth accounted for some of the difference in the quality of schooling that was not captured in the number of grades completed. Disentangling the effects the quantity and quality of schooling on fertility may be an important avenue for future research. Third, for reasons of parsimony and data availability, a limited number of pathways were tested in this study. Future research may well explore the roles of other mediating influences—such as induced abortions, birth spacing, and the mechanisms by which schooling affects fertility. Fourth, path analyses alone cannot determine which of two equally well-fitting models is preferred—in this case, a reliance on theory is required. Finally, although the available sample sizes met the minimal requirement of five subjects per parameter estimated, the optimal sample size is 10–20 subjects per parameter (Hatcher 1994; Kline 1998), or 420–840 women in this analysis. A larger sample size would increase the stability of parameter estimates and the power of the paths that are tested.
Even with these limitations, this study adds to prior research on early childhood nutrition and fertility milestones in this cohort (Ramakrishnan et al. 1999) and elsewhere by testing in an integrated model how biological and social variables mediate the relationship of childhood growth to fertility outcomes. The negative mediating role of schooling outweighs the net positive mediating role of adolescent pre-pregnant nutritional status, resulting in a net increase in the age at first birth and a net decrease in the number of children born. Schooling may encourage women to consider the importance of physical maturation, to communicate with their spouse about the timing of childbearing and family size, and to plan financially for the basic needs and schooling of each child. If women live in a setting where schooling is a real option, then improvements in growth and health during childhood and adolescence should improve nutrition in adulthood, increase schooling, and lower fertility. Yet, if nutrition and growth among girls in Guatemala improve dramatically without parallel improvements in schooling, the net effect could be an earlier age at first childbearing. Concurrent investments in women’s schooling and nutrition thus represent a viable strategy to improve health and to reduce fertility.
Acknowledgments
We gratefully acknowledge the financial support of the U.S. National Institutes of Health (R01 TW-05598: PI Martorell; R01 HD-046125: PI Stein) and the U.S. National Science Foundation (SES0136616: PI Behrman) for present activities and the many organizations (U.S. National Institutes of Health, Thrasher Research Fund, Nestle Foundation) that have funded the work of the INCAP Longitudinal Study since inception.
Footnotes
In the villages where study participants were born, three focus group discussions were held with more-schooled women (≥6 years of schooling) and three were held with less-schooled women (0–3 years). Focus group transcripts were analyzed for differences in perceived beliefs regarding events of childbearing held by more-schooled women compared with less-schooled women (Graff 2006).
In our sample, each year of schooling completed was associated with an additional 0.39 ± 0.07 cm of adult height, and completion of primary school was associated with an additional 2.02 ± 0.47 cm of adult height.
REFERENCES
- Allison P. Missing Data. Thousand Oaks: Sage Publications; 2002. [Google Scholar]
- Behrman J, Foster A, Rosenzweig M, Vashishtha P. “Women’s Schooling, Home Teaching, and Economic Growth”. Journal of Political Economy. 1999;107:682–714. [Google Scholar]
- Bergeron G. “Social and Economic Development in Four Ladino Communities of Eastern Guatemala: A Comparative Description”. Food & Nutrition Bulletin. 1992;14:221–36. [Google Scholar]
- Bini V, Celi F, Berioli MG, Bacosi ML, Stella P, Giglio P, Tosti L, Falorni A. “Body Mass Index in Children and Adolescents According to Age and Pubertal Age”. European Journal of Clinical Nutrition. 2000;54:214–18. doi: 10.1038/sj.ejcn.1600922. [DOI] [PubMed] [Google Scholar]
- Biro FM, Huang BJ, Dorn L, Grumbach MM, Rogol AD, Daniels S. “Anthropometric Factors Associated With Age of Menarche: An Analysis of Two Cohorts Across Five Decades”. Journal of Adolescent Health. 2005;36:145. [Google Scholar]
- Bollen KA, Long JS, editors. Testing Structural Equation Models. Newbury Park, CA: Sage Publications; 1993. [Google Scholar]
- Bongaarts J. “A Framework for Analyzing the Proximate Determinants of Fertility”. Population and Development Review. 1978;4:105–32. [Google Scholar]
- Bongaarts J.2003“Completing the Fertility Transition in the Developing World: The Role of Education Differences and Fertility Preferences” Working Paper No 177. Population Council; New York: [DOI] [PubMed] [Google Scholar]
- Caldwell J. “Mass Education as a Determinant of the Timing of Fertility Decline”. Population and Development Review. 1980;6:225–55. [Google Scholar]
- Casanueva E, Roselló-Soberón ME, De-Regil LM, Argüelles M, Céspedes MI. “Adolescents With Adequate Birth Weight Newborns Diminish Energy Expenditure and Cease Growth”. Journal of Nutrition. 2006;136:2498–501. doi: 10.1093/jn/136.10.2498. [DOI] [PubMed] [Google Scholar]
- Castro Martin T. “Women’s Education and Fertility: Results From 26 Demographic and Health Surveys”. Studies in Family Planning. 1995;26:187–202. [PubMed] [Google Scholar]
- Castro Martin T, Juarez F.1995“The Impact of Education on Women’s Fertility in Latin America: Searching for Explanations.” International Family Planning Perspectives 2152–57. and 80 [Google Scholar]
- Centers for Disease Control and Prevention (CDC) 2000“NCHS/CDC Growth Charts: United States” CDC/National Center for Health Statistics.
- Chowdhury S, Shahabuddin A, Seal A, Talukder K, Hassan Q, Begum R, Rahman Q, Tomkins A, Costello A, Talukder M. “Nutritional Status and Age at Menarche in a Rural Area of Bangladesh”. Annals of Human Biology. 2000;27:249–56. doi: 10.1080/030144600282136. [DOI] [PubMed] [Google Scholar]
- Corvalan C, Gregory CO, Ramirez-Zea M, Martorell R, Stein AD. “Size at Birth, Infant, Early and Later Childhood Growth and Adult Body Composition: A Prospective Study in a Stunted Population”. International Journal of Epidemiology. 2007;36:550–57. doi: 10.1093/ije/dym010. [DOI] [PubMed] [Google Scholar]
- Currie J. “Health Disparities and Gaps in School Readiness”. The Future of Children. 2005;15:117–38. doi: 10.1353/foc.2005.0002. [DOI] [PubMed] [Google Scholar]
- Currie J, Stabile M.2004“Child Mental Health and Human Capital Accumulation: The Case of ADHD.” Working Paper. Department of Economics, University of California at Los Angeles. [DOI] [PubMed]
- da Veiga GV, da Cunha AS, Sichieri R. “Trends in Overweight Among Adolescents Living in the Poorest and Richest Regions of Brazil”. American Journal of Public Health. 2004;94:1544–48. doi: 10.2105/ajph.94.9.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department Economic and Social Affairs . World Population Monitoring 2002. New York: United Nations; Population Division: 2004. “Reproductive Behavior.”; pp. 28–46. [Google Scholar]
- Dick B, Ferguson J, Chandra-Mouli V, Brabin L, Chatterjee S, Ross DA.2006“Review of the Evidence for Interventions to Increase Young People’s Use of Health Services in Developing Countries.” 151–204.Preventing HIV/AIDS in Young People A Systematic Review of the Evidence From Developing Countries Ross DA, Dick B, Ferguson J. World Health Organization (WHO) Technical Report Series 938. Geneva: WHO; [PubMed] [Google Scholar]
- Duryea S, Edwards AC, Ureta M. “Women in the Latin American Labor Market: The Remarkable 1990s.”. Inter-American Development Bank: Labor Markets Policy Briefs Series 2001 [Google Scholar]
- Ellis B. “Timing of Pubertal Maturation in Girls: An Integrated Life History Approach”. Psychological Bulletin. 2004;130:920–58. doi: 10.1037/0033-2909.130.6.920. [DOI] [PubMed] [Google Scholar]
- Ellison P. “Skeletal Growth, Fatness, and Menarcheal Age: A Comparison of Two Hypotheses”. Human Biology. 1982;54:269–81. [PubMed] [Google Scholar]
- Fan X, Thompson B, Wang L. “The Effects of Sample Size, Estimation Methods, and Model Specification on SEM Fit Indices”. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6:56–83. [Google Scholar]
- Frisch R. “Nutrition, Fatness, Puberty, and Fertility”. Comprehensive Therapy. 1981;7:15–23. [PubMed] [Google Scholar]
- Graff M.2006“The Role of Childhood Growth, Pre-pregnant Nutrition Status, and Education on Fertility in Ladino Guatemalan Women.” Unpublished Ph.D. dissertation. Nutrition and Health Sciences, Emory University.
- Grantham-McGregor S, Baker-Henningham H. “Review of the Evidence Linking Protein and Energy to Mental Development”. Public Health Nutrition. 2005;8:1191–201. doi: 10.1079/phn2005805. [DOI] [PubMed] [Google Scholar]
- Gupta N, da Costa Leite I. “Adolescent Fertility Behavior: Trends and Determinants in Northeastern Brazil”. International Family Planning Perspectives. 1999;25:125–30. [Google Scholar]
- Habicht J, Martorell R. “Objectives, Research Design and Implementation of the INCAP Longitudinal Study”. Food & Nutrition Bulletin. 1993;14:176–90. [Google Scholar]
- Hatcher L. A Step-by-Step Approach to Using the SAS System for Factor Analysis and Structural Equation Modeling. Cary, NC: SAS Institute, Inc; 1994. [Google Scholar]
- Hess RD, Holloway SD. “Family and School as Educational Institutions.”. In: Parke RD, Emde RM, McAdoo HP, Sackett GP, editors. Review of Child Development Research. Vol. 7. Chicago: University of Chicago Press; 1984. pp. 179–222. [Google Scholar]
- Hoffman WH, Barbeau P, Litaker MS, Johnson MH, Howe CA, Gutin B. “Tanner Staging of Secondary Sexual Characteristics and Body Composition, Blood Pressure, and Insulin in Black Girls”. Obesity Research. 2005;13:2195–201. doi: 10.1038/oby.2005.272. [DOI] [PubMed] [Google Scholar]
- Hughes SM, Gallup GG., Jr “Sex Differences in Morphological Predictors of Sexual Behavior: Shoulder to Hip and Waist to Hip Ratios”. Evolution and Human Behavior. 2002;24:173–78. [Google Scholar]
- Jukes M. “The Long-Term Impact of Preschool Health and Nutrition on Education”. Food & Nutrition Bulletin. 2005;26(Suppl. 2):S193–S201. doi: 10.1177/15648265050262S210. [DOI] [PubMed] [Google Scholar]
- Kac G, Benicio MH, Velasquez-Melendez G, Valente J. “Nine Months Postpartum Weight Retention Predictors for Brazilian Women”. Public Health Nutrition. 2003;7:621–28. doi: 10.1079/PHN2003579. [DOI] [PubMed] [Google Scholar]
- Kahl H, Schaffrath Rosario A, Schlaud M. “Sexual Maturation of Children and Adolescents in Germany”. Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz. 2007;50:677–85. doi: 10.1007/s00103-007-0229-3. [DOI] [PubMed] [Google Scholar]
- Kaplowitz P. “BMI and the Onset of Puberty.”. In: Pescovitz OH, Walvoord EC, editors. When Puberty Is Precocious: Scientific and Clinical Aspects, (Contemporary Endocrinology) Totowa, NJ: Humana Press; 2006. pp. 137–50. [Google Scholar]
- Khan AD, Schroeder DG, Martorell R, Haas JD, Rivera J. “Early Childhood Determinants of Age at Menarche in Rural Guatemala”. American Journal of Human Biology. 1995;8:717–23. doi: 10.1002/(SICI)1520-6300(1996)8:6<717::AID-AJHB3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Kline R. Principles and Practice of Structural Equation Modeling. New York: Guilford Press; 1998. [Google Scholar]
- Li H, DiGirolamo AM, Barnhart H, Stein AD, Martorell R. “Relative Importance of Birth Size and Postnatal Growth for Women’s Educational Achievement”. Early Human Development. 2004;76:1–16. doi: 10.1016/j.earlhumdev.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Li H, Stein AD, Barnhart H, Ramakrishnan U, Martorell R. “Associations Between Prenatal and Postnatal Growth and Adult Body Size Composition”. American Journal of Clinical Nutrition. 2003;77:1498–505. doi: 10.1093/ajcn/77.6.1498. [DOI] [PubMed] [Google Scholar]
- Little R, Rubin DB. Statistical Analysis With Missing Data. New York: John Wiley & Sons; 1987. [Google Scholar]
- Lorntz B, Soares AM, Moore SR, Pinkerton R, Gansneder B, Bovbjerg VE, Guyatt H, Lima AMG, Richard L. “Early Childhood Diarrhea Predicts Impaired School Performance”. Pediatric Infectious Disease Journal. 2006;25:513–20. doi: 10.1097/01.inf.0000219524.64448.90. [DOI] [PubMed] [Google Scholar]
- Maluccio J, Melgar P, Méndez H, Murphy A, Yount K. “Social and Economic Development and Change in Four Guatemalan Villages: Demographic, Schooling, Occupation, and Assets”. Food & Nutrition Bulletin. 2005;26(Suppl.):S25–S45. doi: 10.1177/15648265050262S104. [DOI] [PubMed] [Google Scholar]
- Martorell R. “Results and Implications of the INCAP Follow-up Study”. Journal of Nutrition. 1995;125:1127S–38S. doi: 10.1093/jn/125.suppl_4.1127S. [DOI] [PubMed] [Google Scholar]
- Martorell R, Behrman JR, Flores R, Stein AD. “Rationale for a Follow-up Study Focusing on Economic Productivity”. Food and Nutrition Bulletin. 2005;26:S5–S14. doi: 10.1177/15648265050262S102. [DOI] [PubMed] [Google Scholar]
- Martorell R, Habicht J-P, Rivera JA. “History and Design of the INCAP Longitudinal Study (1969–77) and Its Follow-up (1988–89)”. Journal of Nutrition. 1995;125:1027S–41S. doi: 10.1093/jn/125.suppl_4.1027S. [DOI] [PubMed] [Google Scholar]
- Martorell R, Ramakrishnan U, Schroeder DG, Melgar P, Nenfeld L. “Intrauterine Growth Retardation, Body Size, Body Composition and Physical Performance in Adolescence”. European Journal of Clinical Nutrition. 1998;52(Suppl.):S43–S52. [PubMed] [Google Scholar]
- Ministry of Public Health and Social Action (MSPAS) Guatemala City: CARE; 2003. “Encuesta Nacional de Salud Materno Infantil (ENSMI) 2002.”. [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. 5th ed. Los Angeles, CA: Muthén & Muthén; 2001. [Google Scholar]
- Otor CJ, Pandey A. “Adolescent Transition to Coitus and Premarital Child Bearing in Sudan: A Biosocial Context”. Journal of Biosocial Science. 1999;31:361–74. doi: 10.1017/s0021932099003612. [DOI] [PubMed] [Google Scholar]
- Pollitt E, Gorman KS, Engle PL, Martorell R, Rivera J. “Early Supplementary Feeding and Cognition: Effects Over Two Decades”. Monographs of the Society for Research in Child Development. 1993;58(7):1–99. [PubMed] [Google Scholar]
- Pollitt E, Gorman KS, Engle PL, Rivera JA, Martorell R. “Nutrition in Early Life and the Fulfillment of Intellectual Potential”. Journal of Nutrition. 1995;125(Suppl.):S1111–18. doi: 10.1093/jn/125.suppl_4.1111S. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan U, Barnhart H, Schroeder DG, Stein AD, Martorell R. “Early Childhood Nutrition, Education and Fertility Milestones in Guatemala”. Journal of Nutrition. 1999;129:2199–202. doi: 10.1093/jn/129.12.2196. [DOI] [PubMed] [Google Scholar]
- Ramey CT, Ramey SL. “Early Learning and School Readiness: Can Early Intervention Make a Difference?”. Merrill-Palmer Quarterly. 2004;50:471–91. [Google Scholar]
- Rees M. “Menarche When and Why?”. Lancet. 1993;342:1375–76. doi: 10.1016/0140-6736(93)92746-g. [DOI] [PubMed] [Google Scholar]
- Reifsnider E, Gill S. “Nutrition for the Childbearing Years”. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 2000;29:43–55. doi: 10.1111/j.1552-6909.2000.tb02755.x. [DOI] [PubMed] [Google Scholar]
- Rivera JA, Martorell R, Ruel MT, Habicht JP, Haas JD. “Nutritional Supplementation During the Preschool Years Influences Body Size and Composition of Guatemalan Adolescents”. Journal of Nutrition. 1995;125(Suppl.):1068S–77S. doi: 10.1093/jn/125.suppl_4.1068S. [DOI] [PubMed] [Google Scholar]
- Rosen DS. “Physiologic Growth and Development During Adolescence”. Pediatrics in Review. 2004;25:194–99. doi: 10.1542/pir.25-6-194. [DOI] [PubMed] [Google Scholar]
- Rubin D. Multivariate Imputation for Non-response in Surveys. New York: John Wiley & Sons, Inc; 1987. [Google Scholar]
- Ruel MT, Rivera J, Habicht JP, Martorell R. “Differential Response to Early Nutrition Supplementation: Long-Term Effects on Height at Adolescence”. International Journal of Epidemiology. 1995;24:404–12. doi: 10.1093/ije/24.2.404. [DOI] [PubMed] [Google Scholar]
- Satorra A. “Scaled and Adjusted Restricted Tests in Multi-Sample Analysis of Moment Structures.”. In: Heijmans RDH, Pollak DSG, Satorra A, editors. Innovations in Multivariate Statistical Analysis: A Festschrift for Heinz Neudecker. London: Kluwer Academic Publishers; 2000. pp. 233–47. [Google Scholar]
- Schafer JL. Analysis of Incomplete Multivariate Data. London: Chapman & Hall; 1997. [Google Scholar]
- Schroeder DG, Martorell R, Rivera JA, Ruel MT, Habicht J-P. “Age Differences in the Impact of Nutritional Supplementation on Growth”. Journal of Nutrition. 1995;125(Suppl.):1060S–67S. doi: 10.1093/jn/125.suppl_4.1051S. [DOI] [PubMed] [Google Scholar]
- Stein A, Behrman J, DiGirolamo AM, Grajeda R, Martorell R, Quisumbing A, Ramakrishnan U. “Schooling, Educational Achievement, and Cognitive Functioning Among Young Guatemalan Adults”. Food and Nutrition Bulletin. 2005;26:S46–S54. doi: 10.1177/15648265050262S105. [DOI] [PubMed] [Google Scholar]
- Stein AD, Wang M, DiGirolamo A, Grajeda R, Ramakrishnan U, Ramirez-Zea M, Yount K, Martorell R. “Nutritional Supplementation in Early Childhood, Schooling, and Intellectual Functioning in Adulthood: A Prospective Study in Guatemala”. Archives of Pediatric and Adolescent Medicine. 2008;162:612–18. doi: 10.1001/archpedi.162.7.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner JM. Growth at Adolescence. 2nd ed. Oxford: Blackwell Scientific Publications; 1972. [Google Scholar]
- Tountas Y, Dimitrakaki C, Antoniou A, Boulamatsis D, Creatsas G. “Attitudes and Behavior Towards Contraception Among Greek Women During Reproductive Age: A Country-Wide Survey”. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2004;116:190–95. doi: 10.1016/j.ejogrb.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Tovée MJ, Reinhardt S, Emery JL, Cornelissen PL. “Optimum Body-Mass Index and Maximum Sexual Attractiveness”. Lancet. 1998;352:548. doi: 10.1016/s0140-6736(05)79257-6. [DOI] [PubMed] [Google Scholar]
- Turconi G, Guarcello M, Maccarini L, Bazzano R, Zaccardo A, Roggi C. “BMI Values and Other Anthropometric and Functional Measurements as Predictors of Obesity in a Selected Group of Adolescents”. European Journal of Nutrition. 2006;45:136–43. doi: 10.1007/s00394-005-0571-x. [DOI] [PubMed] [Google Scholar]
- Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, Singh Sachdev H. “Maternal and Child Undernutrition: Consequences for Adult Health and Human Capital”. Lancet. 2008;371:340–57. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SP, Chang SM, Powell CA. “The Association Between Early Childhood Stunting and Weight Status in Late Adolescence”. International Journal of Obesity. 2007;31:347–52. doi: 10.1038/sj.ijo.0803383. [DOI] [PubMed] [Google Scholar]
- Walker SP, Grantham-McGregor SM, Powell CA, Chang SM. “Effects of Growth Restriction in Early Childhood on Growth, IQ, Cognition at Age 11 to 12 Years and the Benefits of Nutritional Supplementation and Psychosocial Stimulation”. Journal of Pediatrics. 2000;137:36–41. doi: 10.1067/mpd.2000.106227. [DOI] [PubMed] [Google Scholar]
- WHO Multicentre Growth Reference Study Group . WHO Child Growth Standards: Methods and Development. Geneva: World Health Organization; 2006. [Google Scholar]
- Yount KM, Langsten R, Hill K. “The Effect of Gender Preference on Contraceptive Use and Fertility in Rural Egypt”. Studies in Family Planning. 2000;31:290–300. doi: 10.1111/j.1728-4465.2000.00290.x. [DOI] [PubMed] [Google Scholar]
- Yu D, Shepard G. “Is Beauty in the Eye of the Beholder?”. Nature. 1998;396:321–22. doi: 10.1038/24512. [DOI] [PubMed] [Google Scholar]
- Zukauskaite S, Lasiene D, Lasas L, Urbonaite B, Hindmarsh P. “Onset of Breast and Pubic Hair Development in 1,231 Preadolescent Lithuanian Schoolgirls”. Archives of Disease in Childhood. 2005;90:932–36. doi: 10.1136/adc.2004.057612. [DOI] [PMC free article] [PubMed] [Google Scholar]