Abstract
FGF signaling is important for the formation of mesoderm in vertebrates, and when it is perturbed in Xenopus, most trunk and tail mesoderm fails to form. Here we have further dissected the activities of FGF in patterning the embryo by addressing its inductive and maintenance roles. We show that FGF signaling is necessary for the establishment of xbra expression in addition to its well-characterized role in maintaining xbra expression. The role of FGF signaling in organizer formation is not clear in Xenopus. We find that FGF signaling is essential for the initial specification of paraxial mesoderm but not for activation of several pan-mesodermal and most organizer genes; however, early FGF signaling is necessary for the maintenance of organizer gene expression into the neurula stage. Inhibition of FGF signaling prevents VegT activation of specific mesodermal transcripts. These findings illuminate how FGF signaling contributes to the establishment of distinct types of mesoderm.
Keywords: FGF, FGF8, FGF4, mesoderm, Xenopus, organizer, paraxial mesoderm, gastrulation
Introduction
In Xenopus, the three germ layers, endoderm, mesoderm, and ectoderm, are specified early in development after zygotic transcription by a complex interplay of signals and maternal determinants. The maternal determinant VegT is localized to the vegetal hemisphere of the embryo and can activate transcription of several TGFβ family members including derriere, Xnr1,2,4,5,6, and activin βB, VegT acts in concert with these nodal-like signals to induce endoderm and mesoderm (Lustig et al., 1996; Stennard et al., 1996; Zhang and King, 1996; Horb and Thomsen, 1997; Zhang et al., 1998; Clements et al., 1999; Kofron et al., 1999; Xanthos et al., 2001). Several studies have shown that these signals are necessary for mesoderm formation in Xenopus. Dominant negative Activin receptors or inhibitory smad7 mRNA inhibit mesoderm formation (Hemmati-Brivanlou and Melton, 1992; Chang et al., 1997; Bhushan et al., 1998; Casellas and Brivanlou, 1998). Similarly, inhibition of TGFβ ligands with non-cleavable precursors or by expression of nodal antagonists also prevent mesoderm formation (Sun et al., 1999; Agius et al., 2000; Cheng et al., 2000; Tanegashima et al., 2000; Eimon and Harland, 2002; White et al., 2002).
While nodal signaling is essential, FGF signaling also plays a crucial role in mesoderm formation, and an FGF ligand (FGF2) was the first identified mesoderm inducer (Kimelman and Kirschner, 1987; Slack et al., 1987; Slack et al., 1990) though since it lacks a signal sequence it may not be active in normal development (Thompson and Slack, 1992). Disruption of FGF signaling in the embryo with a dominant negative receptor causes a loss of trunk and tail tissues (Amaya et al., 1991; Amaya et al., 1993). In Xenopus explants, FGF signaling through MAPK is necessary for most mesoderm induction by activin (Cornell and Kimelman, 1994; LaBonne and Whitman, 1994; Cornell et al., 1995; LaBonne et al., 1995). In vivo, multiple FGF ligands are involved in regulating mesoderm formation, including FGF4 and FGF8, which are necessary for paraxial mesoderm formation (Isaacs et al., 1992; Isaacs et al., 1994; Isaacs et al., 1995; Standley et al., 2001; Fisher et al., 2002; Fletcher et al., 2006; Isaacs et al., 2007).
FGF signaling is essential for proper expression of the early mesodermal transcript, xbra. Disruption of FGF signaling causes a loss of xbra expression in embryos (Amaya et al., 1993; Delaune et al., 2005), and xbra itself can induce mesoderm in explants, but this activity is prevented if FGF signaling is disrupted (Isaacs et al., 1994; Schulte-Merker and Smith, 1995). Both xbra and FGF4 can induce expression of the other in explants (Isaacs et al., 1994; Schulte-Merker and Smith, 1995). Thus, xbra and FGF4 function in a positive feedback loop in explants, and a dominant-negative FGFR expressed after zygotic transcription reduces xbra expression more definitively as gastrulation proceeds (Isaacs et al., 1994; Schulte-Merker and Smith, 1995; Kroll and Amaya, 1996). For these reasons, FGF signaling has been argued to function in a maintenance role in mesoderm formation. However, it has not been determined if active FGF signaling is necessary for the initial establishment of xbra expression. One line of evidence supporting an early role of FGF signaling in mesoderm formation, in addition to a role in maintenance, is that at the blastula stage, MAPK is activated in the dorsal marginal zone in the same early time frame as SMAD2 (Schohl and Fagotto, 2002).
Though FGF signaling has clearly been shown to be necessary for proper xbra expression and formation of the paraxial mesoderm, its precise role in organizer formation is less clear. Not all types of mesoderm are sensitive to disruption of FGF signaling, as the organizer transcript, goosecoid (gsc), and the early mesodermal transcript, eomesodermin (eomes), are expressed when FGF signaling is disrupted (Amaya et al., 1993; Kumano and Smith, 2000; Delaune et al., 2005). A more systematic study of FGF signaling activity has shown that FGF signaling is not necessary for the initial expression of several organizer and anterior ectodermal transcripts suggesting that organizer formation is not dependent on FGF signaling (Delaune et al., 2005). However, for some organizer transcripts, different results have been reported, for example, chordin and noggin have been reported to be both sensitive and insensitive to FGF inhibition (Mitchell and Sheets, 2001; Delaune et al., 2005), and as these molecules are essential to organizer function (Khokha et al., 2005), the involvement of FGF signaling in their induction needs clarification. Further, although there is evidence that FGF signaling is not necessary for organizer formation, the involvement of FGF signaling in maintaining organizer gene transcription has not been addressed.
To address the necessity for FGF signaling to induce and maintain expression of different mesodermal transcripts, we used the FGFR inhibitor, SU5402, which can be applied at different times and washed out to allow recovery of signaling (Mohammadi et al., 1997; Delaune et al., 2005). To determine if FGF signaling is important for the initial zygotic transcription of xbra, we performed a time-course treatment of embryos. We analyzed a range of both organizer and non-organizer mesodermal gene expression patterns in the absence of FGF signaling to determine whether signaling inhibition affected organizer formation and maintenance and to understand how FGF signaling affected paraxial mesoderm development.
The way in which maternal determinants, and FGF and nodal pathways cooperate to induce mesoderm has been examined in several ways, including analysis on arrays (Kumano and Smith, 2000; Kumano and Smith, 2002; Chung et al., 2004). However, while a limited number of up- and down-regulated targets have been identified, we do not have a good understanding of how these signals cooperate to produce the final pattern of the embryo. We investigated interactions between VegT and FGF signaling by analyzing the ability of ectopic VegT to induce endoderm and mesoderm in the presence and absence of FGF signaling.
Experimental Procedures
Embryo culture
Xenopus laevis eggs were collected, fertilized and embryos cultured by standard procedures (Sive et al., 2000); embryos were staged according to Nieuwkoop and Faber (1994).
Whole mount RNA in situ hybridization
For RNA in situ hybridization multibasket containers were used (Sive et al. 2000). Nuclear localized β-galactosidase (nβgal-CS2+) mRNA was used to trace mRNAs. After fixation for 30 minutes in MEMFA and washing in PBS + 0.1% tween-20, tracer was visualized using Red-Gal (Research Organics) (Sive et al. 2000); after staining, embryos were refixed in MEMFA for an hour and dehydrated in methanol.
Embryos that were injected with the fluorescein-conjugated morpholino oligonucleotide as a lineage tracer were processed for in situ hybridization, then re-blocked, incubated with anti-fluorescein alkaline phosphatase conjugated secondary antibody, washed with MAB, and visualized with magenta phos and tetrazolium red histochemical substrates in a 10:1 ratio.
Anti-sense RNA probes were made for the following transcripts: xbra, (Smith et al., 1991); myoD, (Hopwood et al., 1989); sox2, (Grammer et al., 2000); otx2, (Lamb et al., 1993); otx5 (Vignali et al., 2000); hoxB9, (Sharpe et al., 1987); gsc, (Cho et al., 1991); myf5, (Hopwood et al., 1991); noggin, (Smith and Harland, 1992); chordin, (Sasai et al., 1995); xnr3, (Smith et al., 1995); sox17, (Hudson et al., 1997); eomes, (Ryan et al., 1996); VegT, (Stennard et al., 1996); edd, (Sasai et al., 1996); FGF4,(Isaacs et al., 1992); FGF8 (Fletcher et al., 2006).
mRNA synthesis and injection
Capped messenger RNA was synthesized using the SP6 mMessage mMachine kit (Ambion). Quantified mRNA was resuspended in RNAse-free H2O. The following constructs were linearized and used as templates for SP6 mediated in vitro mRNA synthesis: VegT (a version of antipodean expression cloned from a gastrula library by Wenge Zhang, unpublished); (Stennard et al., 1999); FGF8b (XLFGF8b-CS8) (Fletcher et al., 2006); FGF4 (Isaacs et al., 1994); nuclear β-galactosidase (Turner and Weintraub, 1994). Embryos were injected into one cell at the two-cell stage in 5 or 10 nL volumes.
RT-PCR
Proteinase K/SDS lysis, DNAse, and phenol/chloroform extraction were used to isolate RNA from embryos for reverse-transcriptase polymerase chain reaction (RT-PCR) (Wilson and Melton, 1994). To assay for DNA contamination in RT-PCR experiments, an uninjected control embryo was processed without reverse transcriptase and labeled as the RT minus lane in each experiment. EF1α and ornithine decarboxylase (ODC) were used as loading controls. RT-PCR primers for the following have been described: EF1α (Krieg et al., 1989); xbra (Isaacs et al., 1994); ODC, (Hudson et al., 1997); FGF8 (Fletcher et al., 2006); FGF4, (Isaacs et al., 1994); sox17beta, (Hudson et al., 1997); endodermin, (Sasai et al., 1996); xnr1, xnr2 (Kofron et al., 1999); xnr3, (Smith et al., 1995; Eimon and Harland, 2002).
Morpholino oligonucleotide (MO) design and injection
The morpholino oligonucleotide (MO) (Gene Tools, LLC) binds the translation initiation region of the FGF8 mRNA; the sequence of the X. laevis FGF8 translation blocking morpholino oligonucleotide (XlMOF8) is 5’-GGAGGTGATGTAGTTCATGTTGCTC-3’ (Fletcher et al., 2006). The Gene Tools, LLC standard control MO (5’-CCTCTTACCTCAGTTACAATTTATA-3’) conjugated to fluorescein or nuclear β-galactosidase mRNA was co-injected as a lineage tracer. The morpholino oligonucleotides were resuspended in RNAse-free 1/20 X MR. The injection volume was either 5 nL or 10 nL.
FGFR inhibitor SU5402 treatments
The FGFR inhibitor, SU5402 (Mohammadi et al., 1997), was resuspended in DMSO to make a master stock of 50 mM concentration and diluted in 1/3X MR in treatments to a final concentration of 90–100 micromolar. Although different batches have slightly different activities, we generally found that xbra expression was absent at an 80 micromolar dose; for all batches we used a higher dose than was needed to block Xbra expression to insure that FGF signaling was prevented.
Results
FGF signaling is necessary for initiation of xbra transcription
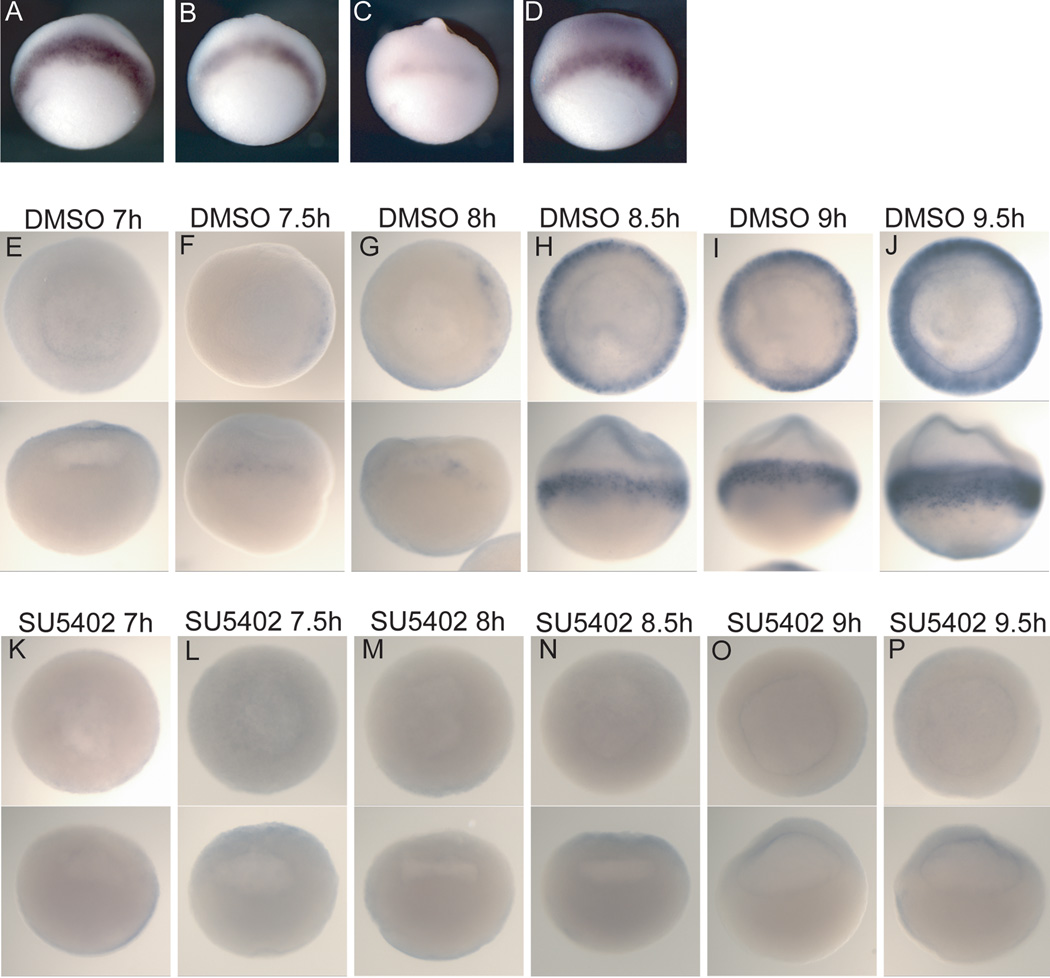
We used the FGFR inhibitor SU5402 to block FGF signaling (Mohammadi et al., 1997). Application of the inhibitor after zygotic transcription and the onset of xbra expression caused a reduction of xbra expression that was more severe the earlier the drug was administered (Figure 1B,C). This confirms the importance of FGF signaling in maintaining xbra expression (Isaacs et al., 1994; Schulte-Merker and Smith, 1995). Washing out the inhibitor before zygotic transcription begins can reverse its effects (Figure 1D). These results mirror the time-dependent effects that Delaune et al., (2005) found on subsequent expression of neural markers. Since it is well established that FGF signaling is necessary for xbra expression at gastrulation, we assayed a dose-response to the inhibitor SU5402 by analyzing gastrula stage embryos that had been treated since cleavage stages for xbra expression. Xbra expression was very strongly reduced but still detectable at the 60 micromolar dose (Supplemental Figure 1A). Additionally, we applied the inhibitor at a range of doses during gastrulation and observed the expected reduction in posterior neural tissue that was more severe at higher doses (Supplemental Figure 1B). Therefore, we used a dose of 90–100 micromolar for all drug-treatment experiments to ensure full inhibition of FGF signaling. This is about half the lethal dose.
Figure 1. FGF signaling is necessary for initiation of xbra expression.
All embryos were processed by RNA in situ hybridization for expression of xbra. Embryos were treated with SU5402 (90–100 uM) (A–D, K–P) or DMSO alone (E–J). (A–D) The bar represents time from fertilization until eleven hours post-fertilization with each hour represented by hash marks; the black region represents the time the embryos were in carrier (DMSO) only, and the blue region represents the time frame when embryos were treated with the inhibitor SU5402. (A–D) lateral views. (A) DMSO only; (B,C) initially treated with DMSO, then SU5402 was added at 10 and 9 hours post-fertilization, respectively. (D) Embryos were first treated with SU5402 then the inhibitor was washed out after 4 hours. (E–P) Embryos were treated with either DMSO alone or SU5402 from the 8–16-cell stage until the indicated time point post-fertilization and xbra expression was analyzed in these blastula stage embryos. Embryos were cleared in BB:BA (2:1). The two views in each panel are of the same embryo with an animal pole view above and a lateral view below.
To determine if FGF signaling is necessary for the initial activation of xbra expression or only for its maintenance, we examined closely spaced intervals of embryogenesis, after embryos were treated with SU5402 from the 8- to 16-cell stage. Embryos were collected and fixed at thirty-minute time intervals beginning at 6 hours post-fertilization during mid-blastulation. Xbra expression was not detected by in situ hybridization until 7.5 hours post-fertilization in a fraction of the carrier-treated embryos (Figure 1F). In the control embryos, xbra expression always first appeared on the dorsal side of the embryo in the marginal zone (Figure 1F,G), consistent with earlier work (Smith et al., 1991). Expression of xbra was not detected in the SU5402-treated embryos at any time-point (Figure 1K–P). This demonstrates that active FGF signaling is necessary for the initial establishment of xbra expression and not only for its maintenance through a positive feedback loop.
The effects of FGF signal inhibition on early endodermal and mesodermal gene expression
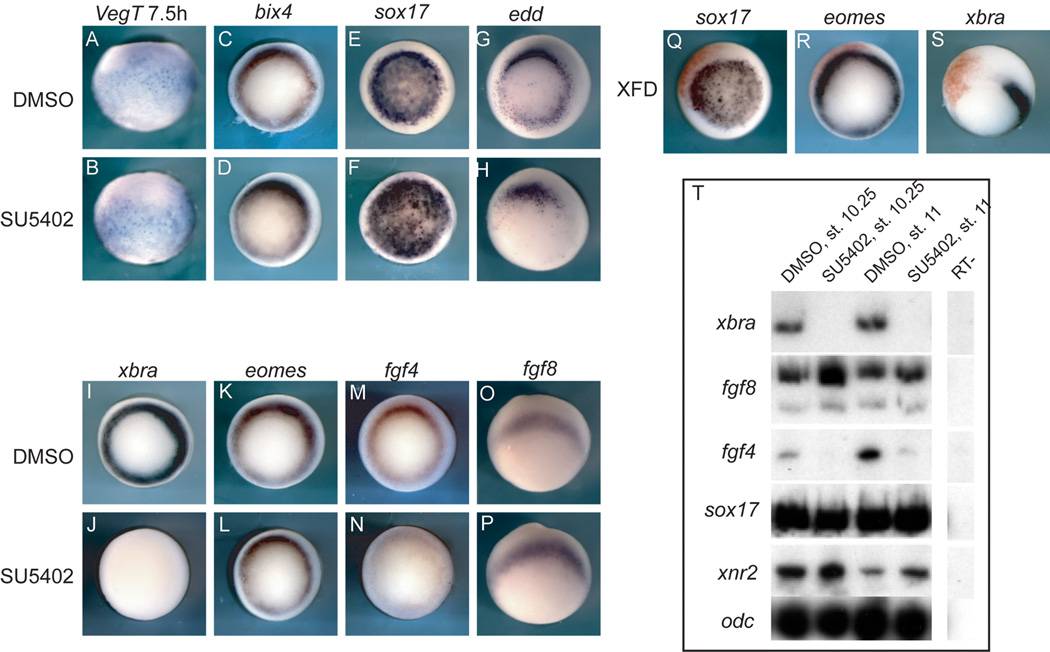
Consistent with the view that FGF signaling may be most important in patterning the animal part of the marginal zone (Kumano et al., 2001), FGF signaling does not induce endoderm (Cornell et al., 1995). However, it is necessary for proper expression of Mix1 in both the endoderm and mesoderm (Cornell et al., 1995; LaBonne and Whitman, 1997). To insure that the inhibitor was not arbitrarily causing general transcript degradation or unspecifically preventing transcription, expression of several endodermal transcripts was examined. Unlike xbra expression, the endodermal gene transcripts bix4 and sox17 are not reduced when FGF signaling is inhibited (Figure 2D,F,T; Table 1). Similarly, embryos injected with the dominant negative version of FGFR1 (XFD) do not show a decrease in sox17 expression on the injected side (Figure 2Q).
Figure 2. FGF signaling effects on endoderm and mesoderm.
These embryos have been processed by in situ hybridization for expression of the indicated transcript above each column. Embryos were treated with DMSO or SU5402 from the 8- to 16-cell stage as indicated on the side. (A,B) are lateral views; embryos were collected at the blastula stage, 7.5 hours post-fertilization; (C–S) gastrula stage; (C–N) are blastoporal views. (O,P) are lateral views with vegetal to the bottom. (Q–S) Embryos were injected into one cell at the two-cell stage with the dominant negative FGFR1 construct XFD (500 pg); the red staining is the lineage tracer nβgal. (T) Whole embryos were treated with either DMSO or SU5402, collected at the time indicated above the lanes, and analyzed by RT-PCR for expression of the indicated markers along the side. ODC was used as a loading control.
Table 1.
Summary of the effects of SU5402 treatment on endodermal and mesodermal marker gene expression.
| Markers/Gastrula stage | SU5402 (100 µM) | VegT (100–250 pg) | VegT/SU5402 |
|---|---|---|---|
| VegT | 8/8 (WT) | ||
| bix4 | 28/28 (WT) | ||
| edd | 7/7** (−) | ||
| eomes | 19/19 (WT) | ||
| sox17 | 32/32 (WT) | 38/38 (+++) | 12/12 (+++) |
| xbra | 107/107 (−−−) | 35/38 (+++) | 29/29 (−−−) |
| FGF4 | 23/23 (−−−) | 16/20 (++) | |
| FGF8 | 8/8 (WT) | 28/28 (++) | |
| gsc | 26/26** (−) | ||
| chordin | 24/24 (WT) | ||
| noggin | 44/44** (−−) | ||
| xnr3 | 19/19** (WT/+) | ||
| otx2 | 19/20 (WT) | ||
| otx5 | 6/7 (WT) | ||
| myoD | 11/11 (−−−) | ||
| myf5 | 11/11 (−−−) | ||
| sox2 | 28/28 (−−−) | ||
| Markers at early neurula | SU5402 (100 µM) 16-cell to EN | SU5402 (100 µM) gastrula to EN | |
| xbra | 36/36 (−−−) | 12/12 (−−−) | |
| myoD | 31/31 (−−−) | 12/12 (−−−) | |
| chordin | 21/21 (−−−) | 7/15 (−−) 8/15 (−) |
|
| noggin | 21/21 (−−−) | 3/15 (−−−) 5/15 (−−) 7/15 (−) |
|
| otx5 | 19/19** (−) | 16/16** (−) | |
| otx2 | 19/20** (−) | 14/14** (−) | |
| gsc | 22/22 (−−−) | 6/15 (−−) 9/15 (−) |
|
| edd | 19/19** (−) | 13/13** (−) | |
(WT), wild-type in appearance; (−−−), strong reduction to absence of marker expression; (−−), reduction of marker expression; (−), marker is noticeably still present but slightly weaker in expression or smaller in domain than WT; (+), is slightly higher than WT in intensity and/or area; (++), moderate increase in expression; (+++), very strong increase in expression;
refer to the text for a more precise description.
The endodermal transcript endodermin (edd) is still expressed in SU5402-treated embryos (Figure 2H), though its expression does not extend as far ventrally around the blastopore as in control embryos. This may be due to the reduction in proper cell movements in FGF inhibited embryos and to the apparent 30-minute delay in development that can be seen by observing the blastocoel in inhibited embryos (Figure 1H, O). There may also be a difference in regulation between endodermal transcripts which reflects the intricate network of gene regulation involved in endoderm formation reported in array experiments (Dickinson et al., 2006; Sinner et al., 2006).
When embryos are treated with the inhibitor SU5402 and analyzed at the early gastrula stage, xbra expression is absent (Figure 2J,T), but expression of eomes is not affected (Figure 2L,T). Likewise, XFD recapitulates these effects (Figure 2R,S), consistent with previous work showing that eomes is resistant to XFD’s effects (Kumano et al., 2001).
Since both FGF4 and FGF8b are important ligands in mesoderm formation in Xenopus, we analyzed the expression of both in embryos treated with SU5402. FGF4 expression is severely reduced but not absent (Figure 2N), while FGF8 expression is not reduced, indeed there is a slight increase in FGF8 expression (Figure 2P). This result suggests that the positive feedback loop between xbra and FGF4 is important in maintaining FGF4 expression, but there is a fraction of FGF4 expression that is not dependent upon the feedback loop and could be due to direct or indirect effects mediated by VegT. Conversely, FGF8 expression does not appear to be dependent on a positive feedback loop with xbra over this time scale.
FGF signaling is essential for establishment of paraxial mesoderm and is necessary for maintenance of axial mesoderm
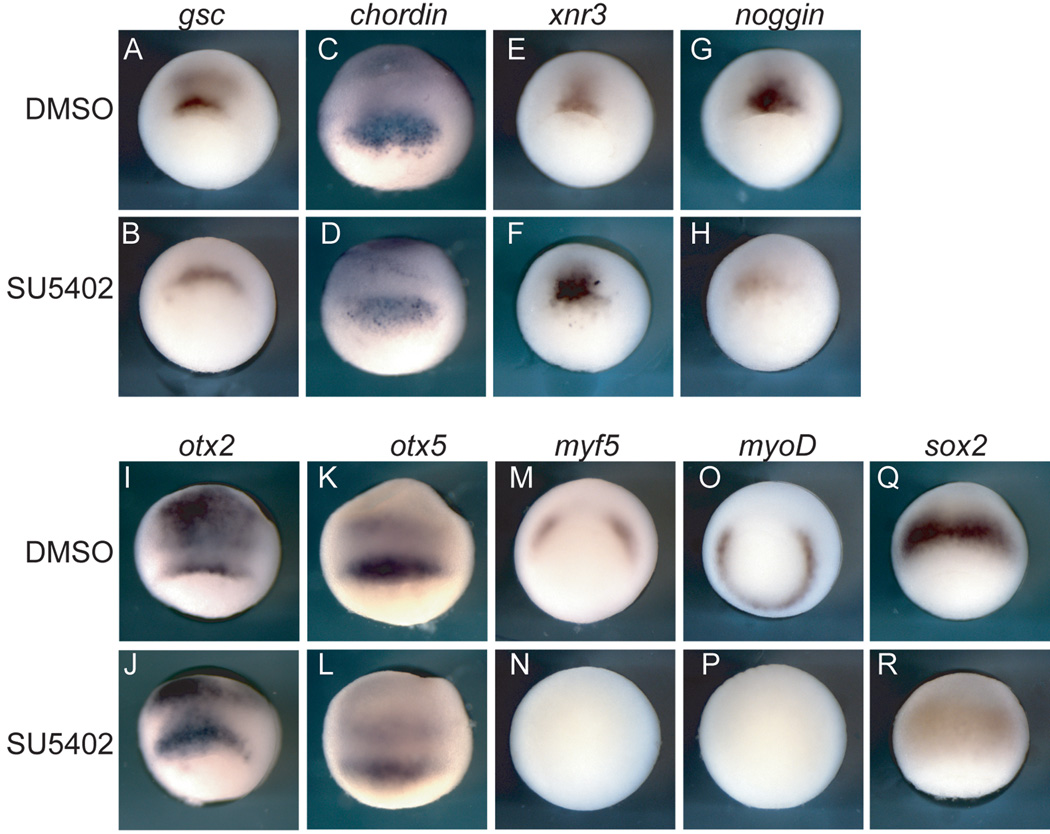
To help clarify how FGF signaling affects organizer formation, we analyzed expression of the organizer genes goosecoid (gsc), chordin, xnr3, and noggin (Cho et al., 1991; Smith and Harland, 1992; Sasai et al., 1995; Smith et al., 1995) as well as otx2 and otx5 (Lamb et al., 1993; Vignali et al., 2000) which are expressed in the dorsal mesendoderm prior to expression in the anterior neural plate. Embryos were analyzed at the early gastrula stage.
Gsc and chordin are expressed in SU5402-treated embryos though their expression domains are wider than in the control embryos due to the inhibition of convergence and extension movements (Figure 3B,D). Expression of gsc is reduced though still present in SU5402-treated embryos (Figure 3B). Xnr3 expression appears stronger in the SU5402-treated embryos, but this may also be due to delayed development, and inhibition of involution of the mesoderm, where Xnr3 normally turns off (Figure 3F). The expression of noggin is reduced in SU5402-treated embryos (Figure 3H); the effects were varied: the majority of embryos treated demonstrated the strong reduction shown while a few showed no noggin expression (not shown). Expression of both otx2 and otx5 is relatively unaffected in SU5402-treated embryos at the gastrula stage (Figure 3J,L). A reduction in FGF8 levels by antisense morpholino injection resulted in the same effects as inhibition with SU5402 but to a lesser degree; i.e. xbra and paraxial mesoderm is strongly reduced, but organizer gene transcripts are expressed (Supplemental Figure 2, Supplemental Table 1).
Figure 3. The involvement of FGF signaling in organizer and paraxial mesoderm development.
Embryos were treated with either DMSO or SU5402 from the 8- to 16-cell stage and analyzed at the gastrula stage for expression of the indicated transcripts. (A,B,E–H,M–P) embryos are displayed in lateral blastoporal view; (C,D,I–L,Q,R) embryos are displayed from lateral view with blastopore toward the bottom. Axial/organizer genes are present, but expression of paraxial mesodermal transcripts is absent in inhibitor treated embryos.
There has been a discrepancy in the findings on whether noggin and chordin expression require FGF signaling (Mitchell and Sheets, 2001; Delaune et al., 2005). In this study, we find that noggin expression is weaker or absent in a large percentage of treated embryos, while chordin expression remains at early gastrulation despite inhibition of FGF signaling. Overall, our results are consistent with a prior study showing that most axial, organizer genes are relatively independent of FGF signaling for initiation of transcription (Delaune et al., 2005). While the axial, organizer genes are largely unaffected by inhibition of FGF signaling, the paraxial mesodermal transcripts myoD and myf5 are absent in inhibitor-treated embryos (Figure 3N,P).
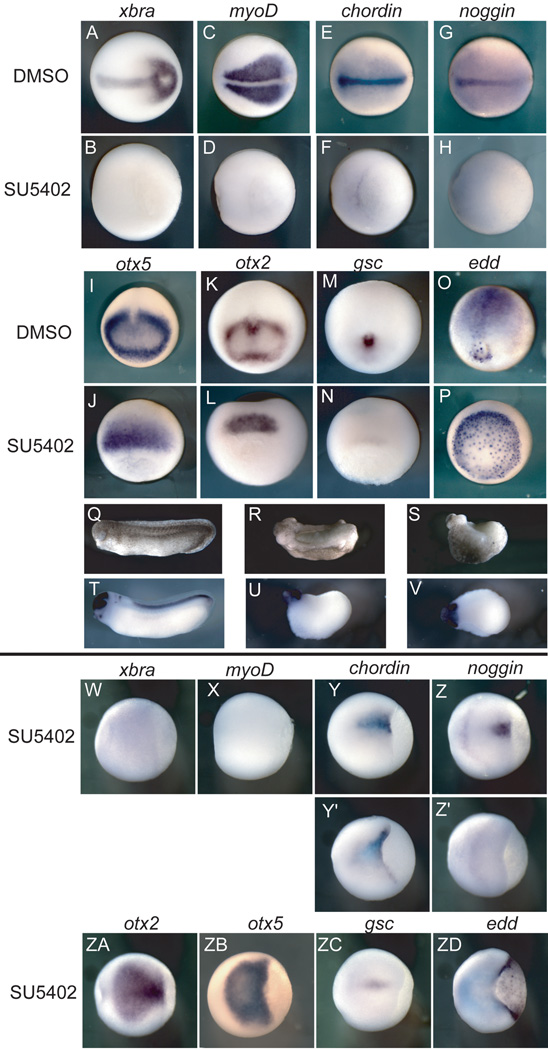
To determine subsequent effects of FGF inhibition, embryos were examined at the neurula stage. A wide range of disruptions to FGF signaling result in failure to form trunk and tail mesoderm (Amaya et al., 1993; Fisher et al., 2002; Delaune et al., 2005; Fletcher et al., 2006), and tadpoles that have strongly inhibited FGF signaling demonstrate a loss of some axial tissue as well based on morphology (Amaya et al., 1993; Delaune et al., 2005). Examination of early neurula embryos allowed us to address whether FGF signaling is necessary to maintain organizer gene transcription throughout gastrulation.
Although several organizer genes are activated in the absence of FGF signaling, their expression is not maintained when FGF signaling is continually inhibited through gastrulation. Thus, chordin and gsc are almost completely absent relative to controls (Figure 4F,N). Noggin expression is no longer detectable by the neurula stage (Figure 4H). Expression of otx5 and otx2 remains in inhibitor-treated embryos (Figure 4J,L) though it is restricted to an anterior domain and is extinguished in the mesoderm. As expected, expression of xbra and myoD remained absent (Figure 4B,D). Expression of the endodermal edd is maintained into the neurula stage, again illustrating the specificity of SU5402 treatment, though the expressing cells are not internalized in treated embryos (Figure 4P).
Figure 4. Early FGF signaling is necessary to maintain organizer gene expression.
Embryos were treated with either DMSO or SU5402 and analyzed at the early neurula stage for expression of a range of mesodermal, posterior and anterior, and endodermal genes, except for (Q–V) which were analyzed at stage 28. (A–H,W-ZD) are dorsal views with anterior to the left; (I–M) are anterior views; (O) is a posterior view; (J,L,N,P) are dorsal blastoporal views. (Q–U) are lateral views, anterior to the left; (V) is a dorsal view. (A–P) embryos were treated from the 8- to 16-cell stage until the early neurula stage; (R) was injected with XFD RNA at 250 pg into each blastomere at the 4-cell stage. (S,U,V) were treated from the 8- to 16-cell stage until stage 28; (W-ZD) embryos were treated from mid-gastrulation until the early neurula stage. (Y’,Z’) embryos demonstrating the range of effects from inhibitor treatment.
The morphology of early tadpoles that had been treated with an equivalent dose of the inhibitior were examined by in situ hybridization with a cocktail of probes to otx2, krox20, and hoxB9, which are normally expressed in the forebrain and midbrain, hindbrain, and spinal cord, respectively. These embryos show a more severe phenotype than that caused by injection of dominant-negative FGFR1 (XFD) RNA, but the effect is similar (Figure Q–S; Supplementary Figure 3). These tadpoles have some anterior head structures as well as a cement gland, and otx2 is expressed in the anterior region of these tadpoles (Figure 4Q–V; Supplemental Figure 3). The difference in the degree of head formation in embryos injected with the dominant negative construct RNA or treated with SU5402 could be because RNA injections are more localized and the inhibitor may provide a more thorough blockade of the entire signaling pathway over time. However, there is the possibility that the inhibitor has off-target effects, though the specificity of its effects on mesoderm and neural tissue is consistent with what is known about FGF signaling.
While early and continuous exposure to SU5402 has strong effects on organizer transcripts, inhibition of FGF signaling at stage 10.5 during gastrulation has much milder effects. Overall, organizer transcripts are weaker in expression, but gsc, chordin, and noggin expression persists in early neurula embryos (Figure 4Y–Z’, ZC). This contrast in the time-dependent severity of SU5402 effects suggests that FGF signaling is particularly critical before and at the onset of gastrulation for the maintenance of organizer gene transcription.
VegT induction of xbra expression depends on FGF signaling
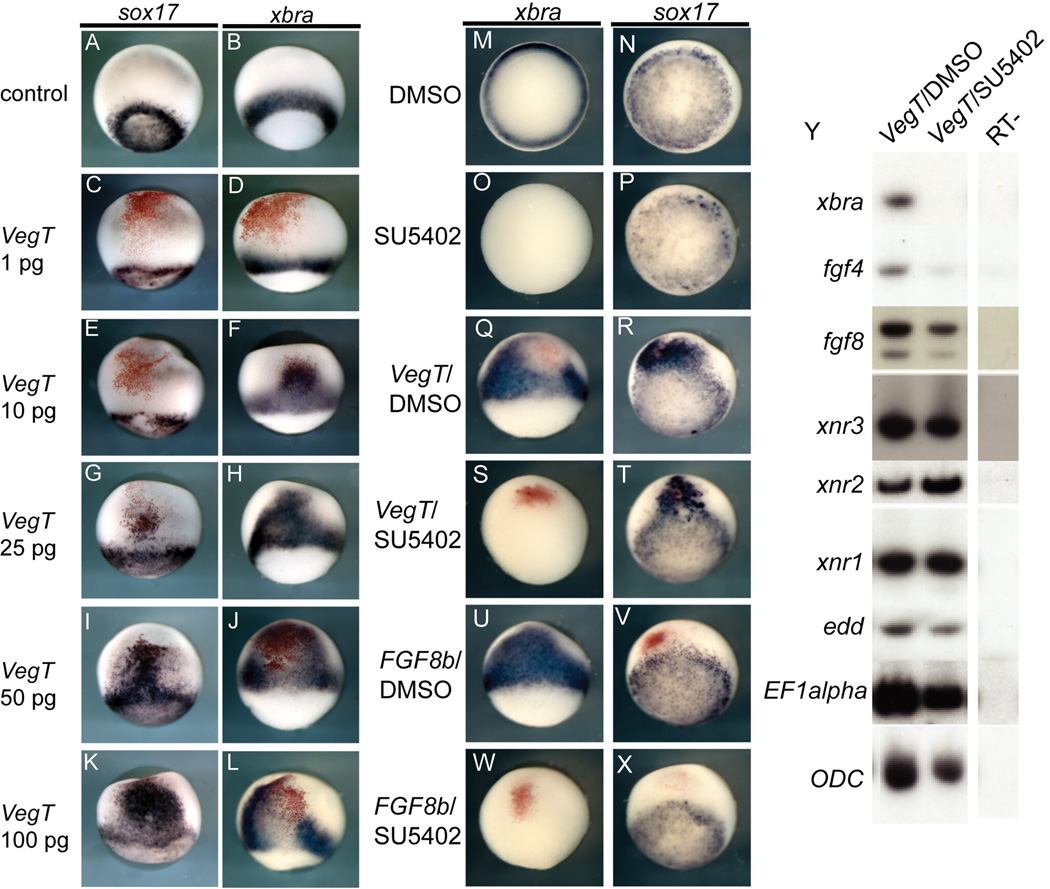
Several endodermal and mesodermal transcripts are direct targets of VegT, and many are also maintained or induced by the nodal or FGF targets of VegT. To examine the interactions between FGF signaling and VegT-mediated induction of mesoderm, we first determined the dose at which VegT overexpression would robustly induce xbra. VegT induction of both sox17 and xbra expression was dose dependent; lower VegT amounts induced xbra, while higher doses were required to induce sox17 (Figure 5A–L). At the 100 pg dose VegT robustly expanded xbra expression, but the most intense xbra expression was at the periphery of the injection site (Figure 5L), while endodermal sox17 expression was centered at the injection site (Figure 5K). The dose-dependent effects observed are consistent with one of the first studies of VegT, which documented that it had dose-dependent effects on the type of mesoderm induced in animal cap explants, inducing more dorsal mesoderm at higher doses and more ventral mesoderm at lower doses (Horb and Thomsen, 1997).
Figure 5. VegT activity in the absence of FGF signaling.
VegT has dose-specific effects in the whole embryo. (C–L) Embryos were injected with different doses of VegT mRNA into one cell at the two-cell stage marked by the lineage tracer nuclear β-gal in red and analyzed at the mid-gastrula stage. All embryos are displayed in lateral views with the blastopore toward the bottom. At a very low dose, VegT mRNA has no detectable effect (C,D). At the 10 pg dose, VegT induces ectopic xbra expression but not sox17 while higher doses induce ectopic sox17 at the focal point of injection and xbra at the periphery (E–L). (M–X) FGF signaling is necessary for VegT-mediated expansion of xbra but not sox17. Embryos were either uninjected, or injected with VegT mRNA (250pg) or FGF8b mRNA (10 pg) and treated with either DMSO or SU5402 as indicated (M–X). At the early gastrula stage embryos were analyzed for xbra and sox17 expression. (Y) is an RTPCR experiment on whole embryos treated as indicated along the top and analyzed for a range of markers. EF1α and ODC were used as loading controls.
To assay whether VegT-mediated induction of xbra was dependent upon FGF signaling, embryos were injected with 100 pg of VegT and treated with either the carrier DMSO or the FGFR inhibitor SU5402. Embryos were treated from early cleavage stages until the early gastrula stage and examined for marker gene expression by in situ hybridization or RT-PCR of whole embryos. VegT induction and expansion of the xbra expression domain is prevented by inhibition of FGF signaling (Figure 5S), while expansion of sox17 by VegT overexpression is not affected (Figure 5T). The effectiveness of the SU5402 inhibition of FGF signaling was assessed by overexpression of FGF8b, which is a strong mesoderm inducer and expands xbra expression into ectodermal domains (Figure 5U); in the presence of SU5402, all xbra expression is absent (Figure 5W). Notably, FGF8b overexpression does not induce sox17 expression nor does it appear to reduce the endogenous expression of sox17 (Figure 5V). These results are confirmed by analyzing whole embryo lysates by RT-PCR (Figure 5Y). Not only is xbra absent and sox17 unaffected, but also FGF4 expression is reduced in embryos injected with VegT and treated with the inhibitor relative to control, VegT-injected embryos (Figure 5Y). In contrast, FGF8 expression is much less affected by inhibition of FGF signaling (Figure 5Y), just as it is less affected in embryos treated only with SU5402 (Figure 2O,P). Replication of these experiments using an antisense morpholino targeted to FGF8 demonstrates similar results highlighting the importance of FGF8 in mediating some of the mesoderm inducing effects of VegT overexpression (Supplementary Figure 4).
Discussion
We have explored the role of FGF signaling in the induction and maintenance of mesodermal gene expression. We confirm that FGF signaling is required to maintain a positive feedback loop with xbra expression as previously reported (Isaacs et al., 1994; Schulte-Merker and Smith, 1995), but we also find that it is necessary for the initiation of xbra expression (Figure 1). We confirm that FGF signaling is necessary for the establishment of the paraxial mesoderm and that it is not needed for establishment of the axial, organizer mesoderm (Figure 3). In addition, we find that FGF signaling is required to maintain expression of several of the organizer transcripts into neurulation (Figure 4), providing insight into why development of the chordamesoderm fails in the absence of sustained FGF signaling (Amaya et al., 1991).
In contrast to the large effects FGF signaling has on mesoderm development, we did not observe much effect on endodermal gene expression. Our observation that inhibiting FGF signaling does not increase edd expression differs from other results in Xenopus, where RT-PCR of explants and whole embryos injected with the dominant negative FGFR1 (XFD) or treated with SU5402 showed an increase in endodermin expression (Cha et al., 2004); it could be that different degrees of pathway inhibition have different effects on endodermin expression. The relative similarity between expression of endodermal genes (endodermin, sox17, bix4) in control and SU5402-treated embryos in our work highlights a difference between Xenopus and zebrafish in the role of FGF signaling. In zebrafish, FGF signaling plays an important role in limiting endodermal development and promoting mesodermal fate (Mizoguchi et al., 2006; Poulain et al., 2006). However, in Xenopus, if FGF signaling limits endoderm development, it is to a much lesser degree than in zebrafish, at least with regard to the three endodermal transcripts analyzed.
The function of FGF signaling in Xenopus and zebrafish again diverges with regard to establishment of dorsal mesodermal fate. In zebrafish, FGF signaling functions to establish more dorsal cell fate in the embryo because a reduction in FGF8 enhances the ventralization seen in chordino mutants (Furthauer et al., 2004); furthermore, FGF signaling is necessary for proper establishment of dorsal mesoderm in the fish, since β-catenin mediated induction of both gsc and chordin is absent when FGF signaling is inhibited (Maegawa et al., 2006). In Xenopus, however, FGF overexpression does not induce axial, organizer gene expression though it does expand early non-axial mesodermal domains and posteriorize the embryo (Isaacs et al., 1994; Pownall et al., 1996; Christen and Slack, 1997; Lombardo et al., 1998; Pownall et al., 2003; Fletcher et al., 2006). In Xenopus, early FGF signaling is necessary to establish the paraxial mesoderm, to limit formation of more ventral mesoderm such as the blood islands, and to posteriorize the embryo (Amaya et al., 1991; Amaya et al., 1993; Kumano and Smith, 2000; Kumano et al., 2001; Kumano and Smith, 2002; Pownall et al., 2003; Fletcher et al., 2006; Isaacs et al., 2007).
VegT is the major maternal transcription factor that activates induction of the endoderm and mesoderm. To address which VegT targets depend upon FGF signaling, VegT-overexpressing embryos were treated with the FGF inhibitor SU5402 or a translation-blocking MO to FGF8. While many VegT targets are direct, VegT activation of xbra transcription is dependent on FGF signaling, and FGF4 expression is strongly reduced when FGF signaling is inhibited (Figure 5). Antisense morpholino knockdown of FGF8 leads to an early reduction in xbra expression and a loss of paraxial mesoderm (Fletcher et al., 2006), but inhibition of FGF signal transduction does not reduce FGF8 mRNA expression (Figure 2P). Further, VegT-mediated expansion of FGF4 expression is dependent upon FGF8 because when FGF8 is reduced, FGF4 expression is strongly diminished. When taken with the evidence that VegT is required for FGF8 expression (Xanthos et al., 2001), these results show that FGF8 is a more proximal target than FGF4 and serves to reinforce mesoderm development by activating FGF4.
There have been differing reports on the role of FGF signaling in the expression of organizer signaling molecules. The requirement for FGF in maintaining expression of organizer transcripts may account for some of these differences. For instance, Delaune et al. (2005) reported that noggin expression was absent at gastrulation in SU5402 treated embryos; we find that it initiates, but is very weak and diminishes to absence as gastrulation proceeds. On the other hand, our results differ from the findings of Mitchell and Sheets (2001) that expression of chordin at the onset of gastrulation is dependent upon FGF signaling. Thus our results lead us to conclude that the establishment of the organizer, or at least a field containing expression of genes with known organizer activities, occurs to a major extent in the absence of any FGF signaling. However, expression of these axial mesodermal transcripts diminishes as gastrulation proceeds.
FGF signaling can induce expression of xbra and myoD, but Activin and Nodals are capable of inducing mesoderm of different dorsal-ventral character depending on the dose in Xenopus (Green and Smith, 1990; Agius et al., 2000; Takahashi et al., 2000). It has been shown clearly that the ability of Activin to induce xbra and myoD, but not gsc, is dependent on active FGF signaling in explants (Cornell and Kimelman, 1994). This difference in the specification of axial and paraxial mesoderm is also apparent in the embryo. One early view defined FGF signaling as a competence factor for TGFβ signaling mediated induction of mesoderm. As more molecular interactions have been revealed over the last decade, we think the evidence has illuminated a more complex interaction: FGF and TGFβ signaling converge to activate and repress a range of genes to specify different types of mesoderm. While VegT activity and active TGFβ signaling is necessary for formation of all mesoderm, both ventral and dorsal, FGF signaling is necessary to induce expression of xbra and myoD, and in its absence paraxial mesoderm is not specified. On the other hand, FGF signaling is not necessary for the initial induction of most axial, organizer mesodermal gene expression.
This study therefore highlights the role of FGF signaling in the maintenance of organizer gene expression through gastrulation in addition to its established functions in paraxial mesodermal development. Prevention of FGF signaling during gastrulation has immediate effects on patterning of the embryo, resulting in posterior truncations and reductions in paraxial mesoderm and the spinal cord domain, where FGF signaling is essential for the onset of myoD, myf5, and hoxB9 expression (Pownall et al., 1996; Slack et al., 1996; Standley et al., 2001; Fisher et al., 2002; Fletcher et al., 2006; Isaacs et al., 2007).
In addition to the need for continued FGF signaling during gastrulation, there is also an early sensitive period for signaling beginning in the late blastula stage with respect to axial, organizer gene expression, since early and continuous exposure to a high dose of SU5402 leads to the loss of organizer transcripts by the end of gastrulation that is much more pronounced than the reduction of organizer transcripts that occurs if inhibition begins during gastrulation. These results parallel the findings of Delaune et al., (2005) who found that neural induction also fails after early exposure to SU5402. This finding is consistent with previous work that shows a continuing need for blockage of BMP signals during gastrulation to induce and maintain neural tissues (Hartley et al., 2001; Khokha et al., 2005). In addition to effects mediated through the organizer, the late tadpole phenotype is at least partly due to the involvement of FGF signaling in maintaining cell viability; embryos that have been treated with a sustained dose of SU5402 are smaller at the tadpole stages, and reduction of FGF signaling results in TUNEL-positive cell death during neurula and tailbud stages (not shown).
In the direct activity of FGF signaling in specification of paraxial mesoderm identity, the FGF signals must also cooperate with BMP antagonists, which are required to dorsalize mesoderm in order to specify muscle (Eimon and Harland, 1999; Khokha et al., 2005). Therefore, FGF signaling has multiple roles in gastrulation with regard to dorsal mesodermal development: FGF signals specify paraxial mesodermal identity, with a clear requirement for positive feedback, in part through FGF4, in maintaining FGF signaling and function; and, secondly, early FGF signaling is necessary to maintain axial, organizer gene expression, which is consistent with the lack of neural tissue in tadpoles observed after a short, early incubation with SU5402 (Delaune et al., 2005). At this level of FGF signaling inhibition the early expression of organizer transcripts is sufficient to specify anterior fates, in contrast to more severe suppression of organizer transcript function, which completely eliminates anterior development (Khokha et al., 2005).
Supplementary Material
Acknowledgments
We thank Harv Isaacs for discussions on FGF signaling and Anne-HÈlËne Monsoro-Burq for help with reagents. We also thank the members of the Harland lab for their comments and help along the way, especially Andrea Wills, James Walker, and Darwin Dichmann. This work was supported by NIH GM042341 to RMH.
Works Cited
- Agius E, Oelgeschlager M, Wessely O, Kemp C, De Robertis EM. Endodermal Nodal-related signals and mesoderm induction in Xenopus. Development. 2000;127:1173–1183. doi: 10.1242/dev.127.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaya E, Musci TJ, Kirschner MW. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991;66:257–270. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- Amaya E, Stein PA, Musci TJ, Kirschner MW. FGF signalling in the early specification of mesoderm in Xenopus. Development. 1993;118:477–487. doi: 10.1242/dev.118.2.477. [DOI] [PubMed] [Google Scholar]
- Bhushan A, Chen Y, Vale W. Smad7 inhibits mesoderm formation and promotes neural cell fate in Xenopus embryos. Dev Biol. 1998;200:260–268. doi: 10.1006/dbio.1998.8965. [DOI] [PubMed] [Google Scholar]
- Casellas R, Brivanlou AH. Xenopus Smad7 inhibits both the activin and BMP pathways and acts as a neural inducer. Dev Biol. 1998;198:1–12. doi: 10.1006/dbio.1998.8893. [DOI] [PubMed] [Google Scholar]
- Cha SW, Hwang YS, Chae JP, Lee SY, Lee HS, Daar I, Park MJ, Kim J. Inhibition of FGF signaling causes expansion of the endoderm in Xenopus. Biochem Biophys Res Commun. 2004;315:100–106. doi: 10.1016/j.bbrc.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Chang C, Wilson PA, Mathews LS, Hemmati-Brivanlou A. A Xenopus type I activin receptor mediates mesodermal but not neural specification during embryogenesis. Development. 1997;124:827–837. doi: 10.1242/dev.124.4.827. [DOI] [PubMed] [Google Scholar]
- Cheng AM, Thisse B, Thisse C, Wright CV. The lefty-related factor Xatv acts as a feedback inhibitor of nodal signaling in mesoderm induction and L-R axis development in xenopus. Development. 2000;127:1049–1061. doi: 10.1242/dev.127.5.1049. [DOI] [PubMed] [Google Scholar]
- Cho KW, Blumberg B, Steinbeisser H, De Robertis EM. Molecular nature of Spemann's organizer: the role of the Xenopus homeobox gene goosecoid. Cell. 1991;67:1111–1120. doi: 10.1016/0092-8674(91)90288-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen B, Slack JM. FGF-8 is associated with anteroposterior patterning and limb regeneration in Xenopus. Dev Biol. 1997;192:455–466. doi: 10.1006/dbio.1997.8732. [DOI] [PubMed] [Google Scholar]
- Chung HA, Hyodo-Miura J, Kitayama A, Terasaka C, Nagamune T, Ueno N. Screening of FGF target genes in Xenopus by microarray: temporal dissection of the signalling pathway using a chemical inhibitor. Genes Cells. 2004;9:749–761. doi: 10.1111/j.1356-9597.2004.00761.x. [DOI] [PubMed] [Google Scholar]
- Clements D, Friday RV, Woodland HR. Mode of action of VegT in mesoderm and endoderm formation. Development. 1999;126:4903–4911. doi: 10.1242/dev.126.21.4903. [DOI] [PubMed] [Google Scholar]
- Cornell RA, Kimelman D. Activin-mediated mesoderm induction requires FGF. Development. 1994;120:453–462. doi: 10.1242/dev.120.2.453. [DOI] [PubMed] [Google Scholar]
- Cornell RA, Musci TJ, Kimelman D. FGF is a prospective competence factor for early activin-type signals in Xenopus mesoderm induction. Development. 1995;121:2429–2437. doi: 10.1242/dev.121.8.2429. [DOI] [PubMed] [Google Scholar]
- Delaune E, Lemaire P, Kodjabachian L. Neural induction in Xenopus requires early FGF signalling in addition to BMP inhibition. Development. 2005;132:299–310. doi: 10.1242/dev.01582. [DOI] [PubMed] [Google Scholar]
- Dickinson K, Leonard J, Baker JC. Genomic profiling of mixer and Sox17beta targets during Xenopus endoderm development. Dev Dyn. 2006;235:368–381. doi: 10.1002/dvdy.20636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimon PM, Harland RM. In Xenopus embryos, BMP heterodimers are not required for mesoderm induction, but BMP activity is necessary for dorsal/ventral patterning. Dev Biol. 1999;216:29–40. doi: 10.1006/dbio.1999.9496. [DOI] [PubMed] [Google Scholar]
- Eimon PM, Harland RM. Effects of heterodimerization and proteolytic processing on Derriere and Nodal activity: implications for mesoderm induction in Xenopus. Development. 2002;129:3089–3103. doi: 10.1242/dev.129.13.3089. [DOI] [PubMed] [Google Scholar]
- Fisher ME, Isaacs HV, Pownall ME. eFGF is required for activation of XmyoD expression in the myogenic cell lineage of Xenopus laevis. Development. 2002;129:1307–1315. doi: 10.1242/dev.129.6.1307. [DOI] [PubMed] [Google Scholar]
- Fletcher RB, Baker JC, Harland RM. FGF8 spliceforms mediate early mesoderm and posterior neural tissue formation in Xenopus. Development. 2006;133:1703–1714. doi: 10.1242/dev.02342. [DOI] [PubMed] [Google Scholar]
- Furthauer M, Van Celst J, Thisse C, Thisse B. Fgf signalling controls the dorsoventral patterning of the zebrafish embryo. Development. 2004;131:2853–2864. doi: 10.1242/dev.01156. [DOI] [PubMed] [Google Scholar]
- Grammer TC, Liu KJ, Mariani FV, Harland RM. Use of large-scale expression cloning screens in the Xenopus laevis tadpole to identify gene function. Dev Biol. 2000;228:197–210. doi: 10.1006/dbio.2000.9945. [DOI] [PubMed] [Google Scholar]
- Green JB, Smith JC. Graded changes in dose of a Xenopus activin A homologue elicit stepwise transitions in embryonic cell fate. Nature. 1990;347:391–394. doi: 10.1038/347391a0. [DOI] [PubMed] [Google Scholar]
- Hartley KO, Hardcastle Z, Friday RV, Amaya E, Papalopulu N. Transgenic Xenopus embryos reveal that anterior neural development requires continued suppression of BMP signaling after gastrulation. Dev Biol. 2001;238:168–184. doi: 10.1006/dbio.2001.0398. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Melton DA. A truncated activin receptor inhibits mesoderm induction and formation of axial structures in Xenopus embryos. Nature. 1992;359:609–614. doi: 10.1038/359609a0. [DOI] [PubMed] [Google Scholar]
- Hopwood ND, Pluck A, Gurdon JB. MyoD expression in the forming somites is an early response to mesoderm induction in Xenopus embryos. Embo J. 1989;8:3409–3417. doi: 10.1002/j.1460-2075.1989.tb08505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood ND, Pluck A, Gurdon JB. Xenopus Myf-5 marks early muscle cells and can activate muscle genes ectopically in early embryos. Development. 1991;111:551–560. doi: 10.1242/dev.111.2.551. [DOI] [PubMed] [Google Scholar]
- Horb ME, Thomsen GH. A vegetally localized T-box transcription factor in Xenopus eggs specifies mesoderm and endoderm and is essential for embryonic mesoderm formation. Development. 1997;124:1689–1698. doi: 10.1242/dev.124.9.1689. [DOI] [PubMed] [Google Scholar]
- Hudson C, Clements D, Friday RV, Stott D, Woodland HR. Xsox17alpha and -beta mediate endoderm formation in Xenopus. Cell. 1997;91:397–405. doi: 10.1016/s0092-8674(00)80423-7. [DOI] [PubMed] [Google Scholar]
- Isaacs HV, Deconinck AE, Pownall ME. FGF4 regulates blood and muscle specification in Xenopus laevis. Biol Cell. 2007;99:165–173. doi: 10.1042/BC20060103. [DOI] [PubMed] [Google Scholar]
- Isaacs HV, Pownall ME, Slack JM. eFGF regulates Xbra expression during Xenopus gastrulation. Embo J. 1994;13:4469–4481. doi: 10.1002/j.1460-2075.1994.tb06769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs HV, Pownall ME, Slack JM. eFGF is expressed in the dorsal midline of Xenopus laevis. Int J Dev Biol. 1995;39:575–579. [PubMed] [Google Scholar]
- Isaacs HV, Tannahill D, Slack JM. Expression of a novel FGF in the Xenopus embryo. A new candidate inducing factor for mesoderm formation and anteroposterior specification. Development. 1992;114:711–720. doi: 10.1242/dev.114.3.711. [DOI] [PubMed] [Google Scholar]
- Khokha MK, Yeh J, Grammer TC, Harland RM. Depletion of Three BMP Antagonists from Spemann's Organizer Leads to a Catastrophic Loss of Dorsal Structures. Dev Cell. 2005;8:401–411. doi: 10.1016/j.devcel.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Kimelman D, Kirschner M. Synergistic induction of mesoderm by FGF and TGF-beta and the identification of an mRNA coding for FGF in the early Xenopus embryo. Cell. 1987;51:869–877. doi: 10.1016/0092-8674(87)90110-3. [DOI] [PubMed] [Google Scholar]
- Kofron M, Demel T, Xanthos J, Lohr J, Sun B, Sive H, Osada S, Wright C, Wylie C, Heasman J. Mesoderm induction in Xenopus is a zygotic event regulated by maternal VegT via TGFbeta growth factors. Development. 1999;126:5759–5770. doi: 10.1242/dev.126.24.5759. [DOI] [PubMed] [Google Scholar]
- Kroll KL, Amaya E. Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development. 1996;122:3173–3183. doi: 10.1242/dev.122.10.3173. [DOI] [PubMed] [Google Scholar]
- Kumano G, Ezal C, Smith WC. Boundaries and functional domains in the animal/vegetal axis of Xenopus gastrula mesoderm. Dev Biol. 2001;236:465–477. doi: 10.1006/dbio.2001.0341. [DOI] [PubMed] [Google Scholar]
- Kumano G, Smith WC. FGF signaling restricts the primary blood islands to ventral mesoderm. Dev Biol. 2000;228:304–314. doi: 10.1006/dbio.2000.9937. [DOI] [PubMed] [Google Scholar]
- Kumano G, Smith WC. The nodal target gene Xmenf is a component of an FGF-independent pathway of ventral mesoderm induction in Xenopus. Mech Dev. 2002;118:45–56. doi: 10.1016/s0925-4773(02)00186-7. [DOI] [PubMed] [Google Scholar]
- LaBonne C, Burke B, Whitman M. Role of MAP kinase in mesoderm induction and axial patterning during Xenopus development. Development. 1995;121:1475–1486. doi: 10.1242/dev.121.5.1475. [DOI] [PubMed] [Google Scholar]
- LaBonne C, Whitman M. Mesoderm induction by activin requires FGF-mediated intracellular signals. Development. 1994;120:463–472. doi: 10.1242/dev.120.2.463. [DOI] [PubMed] [Google Scholar]
- LaBonne C, Whitman M. Localization of MAP kinase activity in early Xenopus embryos: implications for endogenous FGF signaling. Dev Biol. 1997;183:9–20. doi: 10.1006/dbio.1996.8497. [DOI] [PubMed] [Google Scholar]
- Lamb TM, Knecht AK, Smith WC, Stachel SE, Economides AN, Stahl N, Yancopolous GD, Harland RM. Neural induction by the secreted polypeptide noggin. Science. 1993;262:713–718. doi: 10.1126/science.8235591. [DOI] [PubMed] [Google Scholar]
- Lombardo A, Isaacs HV, Slack JM. Expression and functions of FGF-3 in Xenopus development. Int J Dev Biol. 1998;42:1101–1107. [PubMed] [Google Scholar]
- Lustig KD, Kroll KL, Sun EE, Kirschner MW. Expression cloning of a Xenopus T-related gene (Xombi) involved in mesodermal patterning and blastopore lip formation. Development. 1996;122:4001–4012. doi: 10.1242/dev.122.12.4001. [DOI] [PubMed] [Google Scholar]
- Maegawa S, Varga M, Weinberg ES. FGF signaling is required for {beta}-catenin-mediated induction of the zebrafish organizer. Development. 2006;133:3265–3276. doi: 10.1242/dev.02483. [DOI] [PubMed] [Google Scholar]
- Mitchell TS, Sheets MD. The FGFR pathway is required for the trunk-inducing functions of Spemann's organizer. Dev Biol. 2001;237:295–305. doi: 10.1006/dbio.2001.0385. [DOI] [PubMed] [Google Scholar]
- Mizoguchi T, Izawa T, Kuroiwa A, Kikuchi Y. Fgf signaling negatively regulates Nodal-dependent endoderm induction in zebrafish. Dev Biol. 2006;300:612–622. doi: 10.1016/j.ydbio.2006.08.073. Epub 2006 Sep 2009. [DOI] [PubMed] [Google Scholar]
- Mohammadi M, McMahon G, Sun L, Tang C, Hirth P, Yeh BK, Hubbard SR, Schlessinger J. Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science. 1997;276:955–960. doi: 10.1126/science.276.5314.955. [DOI] [PubMed] [Google Scholar]
- Poulain M, Furthauer M, Thisse B, Thisse C, Lepage T. Zebrafish endoderm formation is regulated by combinatorial Nodal, FGF and BMP signalling. Development. 2006;133:2189–2200. doi: 10.1242/dev.02387. Epub 2006 May 2183. [DOI] [PubMed] [Google Scholar]
- Pownall ME, Tucker AS, Slack JM, Isaacs HV. eFGF, Xcad3 and Hox genes form a molecular pathway that establishes the anteroposterior axis in Xenopus. Development. 1996;122:3881–3892. doi: 10.1242/dev.122.12.3881. [DOI] [PubMed] [Google Scholar]
- Pownall ME, Welm BE, Freeman KW, Spencer DM, Rosen JM, Isaacs HV. An inducible system for the study of FGF signalling in early amphibian development. Dev Biol. 2003;256:89–99. doi: 10.1016/s0012-1606(02)00120-3. [DOI] [PubMed] [Google Scholar]
- Ryan K, Garrett N, Mitchell A, Gurdon JB. Eomesodermin, a key early gene in Xenopus mesoderm differentiation. Cell. 1996;87:989–1000. doi: 10.1016/s0092-8674(00)81794-8. [DOI] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Piccolo S, De Robertis EM. Endoderm induction by the organizer-secreted factors chordin and noggin in Xenopus animal caps. Embo J. 1996;15:4547–4555. [PMC free article] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, De Robertis EM. Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature. 1995;377:757. doi: 10.1038/377757a0. [DOI] [PubMed] [Google Scholar]
- Schohl A, Fagotto F. Beta-catenin, MAPK and Smad signaling during early Xenopus development. Development. 2002;129:37–52. doi: 10.1242/dev.129.1.37. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, Smith JC. Mesoderm formation in response to Brachyury requires FGF signalling. Curr Biol. 1995;5:62–67. doi: 10.1016/s0960-9822(95)00017-0. [DOI] [PubMed] [Google Scholar]
- Sharpe CR, Fritz A, De Robertis EM, Gurdon JB. A homeobox-containing marker of posterior neural differentiation shows the importance of predetermination in neural induction. Cell. 1987;50:749–758. doi: 10.1016/0092-8674(87)90333-3. [DOI] [PubMed] [Google Scholar]
- Sinner D, Kirilenko P, Rankin S, Wei E, Howard L, Kofron M, Heasman J, Woodland HR, Zorn AM. Global analysis of the transcriptional network controlling Xenopus endoderm formation. Development. 2006;133:1955–1966. doi: 10.1242/dev.02358. [DOI] [PubMed] [Google Scholar]
- Slack JM, Darlington BG, Gillespie LL, Godsave SF, Isaacs HV, Paterno GD. Mesoderm induction by fibroblast growth factor in early Xenopus development. Philos Trans R Soc Lond B Biol Sci. 1990;327:75–84. doi: 10.1098/rstb.1990.0044. [DOI] [PubMed] [Google Scholar]
- Slack JM, Darlington BG, Heath JK, Godsave SF. Mesoderm induction in early Xenopus embryos by heparin-binding growth factors. Nature. 1987;326:197–200. doi: 10.1038/326197a0. [DOI] [PubMed] [Google Scholar]
- Slack JM, Isaacs HV, Song J, Durbin L, Pownall ME. The role of fibroblast growth factors in early Xenopus development; Biochem Soc Symp; 1996. pp. 1–12. [PubMed] [Google Scholar]
- Smith JC, Price BM, Green JB, Weigel D, Herrmann BG. Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell. 1991;67:79–87. doi: 10.1016/0092-8674(91)90573-h. [DOI] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Smith WC, McKendry R, Ribisi S, Jr, Harland RM. A nodal-related gene defines a physical and functional domain within the Spemann organizer. Cell. 1995;82:37–46. doi: 10.1016/0092-8674(95)90050-0. [DOI] [PubMed] [Google Scholar]
- Standley HJ, Zorn AM, Gurdon JB. eFGF and its mode of action in the community effect during Xenopus myogenesis. Development. 2001;128:1347–1357. doi: 10.1242/dev.128.8.1347. [DOI] [PubMed] [Google Scholar]
- Stennard F, Carnac G, Gurdon JB. The Xenopus T-box gene, Antipodean, encodes a vegetally localised maternal mRNA and can trigger mesoderm formation. Development. 1996;122:4179–4188. doi: 10.1242/dev.122.12.4179. [DOI] [PubMed] [Google Scholar]
- Stennard F, Zorn AM, Ryan K, Garrett N, Gurdon JB. Differential expression of VegT and Antipodean protein isoforms in Xenopus. Mech Dev. 1999;86:87–98. doi: 10.1016/s0925-4773(99)00119-7. [DOI] [PubMed] [Google Scholar]
- Sun BI, Bush SM, Collins-Racie LA, LaVallie ER, DiBlasio-Smith EA, Wolfman NM, McCoy JM, Sive HL. derriere: a TGF-beta family member required for posterior development in Xenopus. Development. 1999;126:1467–1482. doi: 10.1242/dev.126.7.1467. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Yokota C, Takano K, Tanegashima K, Onuma Y, Goto J, Asashima M. Two novel nodal-related genes initiate early inductive events in Xenopus Nieuwkoop center. Development. 2000;127:5319–5329. doi: 10.1242/dev.127.24.5319. [DOI] [PubMed] [Google Scholar]
- Tanegashima K, Yokota C, Takahashi S, Asashima M. Expression cloning of Xantivin, a Xenopus lefty/antivin-related gene, involved in the regulation of activin signaling during mesoderm induction. Mech Dev. 2000;99:3–14. doi: 10.1016/s0925-4773(00)00465-2. [DOI] [PubMed] [Google Scholar]
- Thompson J, Slack JM. Over-expression of fibroblast growth factors in Xenopus embryos. Mech Dev. 1992;38:175–182. doi: 10.1016/0925-4773(92)90051-k. [DOI] [PubMed] [Google Scholar]
- Turner DL, Weintraub H. Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev. 1994;8:1434–1447. doi: 10.1101/gad.8.12.1434. [DOI] [PubMed] [Google Scholar]
- Vignali R, Colombetti S, Lupo G, Zhang W, Stachel S, Harland RM, Barsacchi G. Xotx5b, a new member of the Otx gene family, may be involved in anterior and eye development in Xenopus laevis. Mech Dev. 2000;96:3–13. doi: 10.1016/s0925-4773(00)00367-1. [DOI] [PubMed] [Google Scholar]
- White RJ, Sun BI, Sive HL, Smith JC. Direct and indirect regulation of derriere, a Xenopus mesoderm-inducing factor, by VegT. Development. 2002;129:4867–4876. doi: 10.1242/dev.129.20.4867. [DOI] [PubMed] [Google Scholar]
- Xanthos JB, Kofron M, Wylie C, Heasman J. Maternal VegT is the initiator of a molecular network specifying endoderm in Xenopus laevis. Development. 2001;128:167–180. doi: 10.1242/dev.128.2.167. [DOI] [PubMed] [Google Scholar]
- Zhang J, Houston DW, King ML, Payne C, Wylie C, Heasman J. The role of maternal VegT in establishing the primary germ layers in Xenopus embryos. Cell. 1998;94:515–524. doi: 10.1016/s0092-8674(00)81592-5. [DOI] [PubMed] [Google Scholar]
- Zhang J, King ML. Xenopus VegT RNA is localized to the vegetal cortex during oogenesis and encodes a novel T-box transcription factor involved in mesodermal patterning. Development. 1996;122:4119–4129. doi: 10.1242/dev.122.12.4119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.