Abstract
Neurosteroids play a crucial role in stress, alcohol dependence and withdrawal, and other physiological and pharmacological actions by potentiating or inhibiting neurotransmitter action. This review article focuses on data showing that the interaction among stress, ethanol, and neuroactive steroids may result in plastic molecular and functional changes of GABAergic inhibitory neurotransmission. The molecular mechanisms by which stress-ethanol-neuroactive steroids interactions can produce plastic changes in GABAA receptors have been studied using different experimental models in vivo and in vitro in order to provide useful evidence and new insights into the mechanisms through which acute and chronic ethanol and stress exposure modulate the activity of GABAergic synapses. We show detailed data on a) the effect of acute and chronic stress on peripheral and brain neurosteroid levels and GABAA receptor gene expression and function; b) ethanol-stimulated brain steroidogenesis; c) plasticity of GABAA receptor after acute and chronic ethanol exposure. The implications of these new mechanistic insights to our understanding of the effects of ethanol during stress are also discussed. The understanding of these neurochemical and molecular mechanisms may shed new light on the physiopathology of diseases, such as anxiety, in which GABAergic transmission play a pivotal role. These data may also lead to the need for new anxiolytic, hypnotic and anticonvulsant selective drugs devoid of side effects.
Keywords: neuroactive steroids, stress, ethanol, GABAA receptor plasticity, GABAA receptor function, rat
1. Introduction
In the last twenty years, the study of GABAergic transmission has become one of the most fascinating fields of research in neuropsychopharmacology. Thus, GABAA receptors, first indicated to be one of the major targets involved in the physiopathology of anxiety disorders are now suggested to play crucial role also in the modulation of those neuronal pathways involved in mental disorders such as depression, schizophrenia and drugs of abuse.
The more recent discovery that endogenous compounds such as steroid derivatives produced by both peripheral organs and brain have the capability to induce, through the activation of GABAA-mediated neurotransmission, behavioral changes indistinguishable from those elicited by anxiolytic drugs suggested that these hormones may play a physiological role in the etiology of some of the above mentioned mental disorders and more in general to stress-associated diseases.
All these findings have suggested that understanding the functional significance of the fluctations in the brain content of neuroactive steroids induced by physiological, pharmacological and pathological conditions makes an important contribution to regulate the threshold excitability and the functional properties of specific neuronal populations localized in brain areas involved in the modulation of the emotional and affective responses. Previous evidence that acute stress induces parallel but opposite changes in GABAA receptor function (reduction) and in brain content of neuroactive steroids (increase) together with the more recent data revealing that chronic stressful conditions reduce neuroactive steroid content in plasma and brain further indicate that these hormones may play a crucial role not only in the physiological modulation of brain homeostasis, but also under environmental, chemical, pharmacological and pathological stressful stimuli associated with changes in the function of different neurons. Consistent with this conclusion different laboratories have recently shown that, like stress, ethanol intake and withdrawal, changes the peripheral and central secretion of neuroactive steroids, an effect associated with parallel change in GABAA receptor function and gene expression of selective subunits assembled in synaptic and extrasynaptic GABAA receptors.
Given that stressful conditions able to change neurosteroid brain content and GABAA receptor function are often associated with long lasting increases in ethanol intake, we consider it of interest to report recent data obtained by our group and other authors showing the most relevant functional and molecular events involved in the interaction between neurosteroids and GABAA receptors during stressful conditions, acute and chronic ethanol intake and withdrawal. Mainly, the results reported have indicated that neuroactive steroids synthetized in peripheral and brain play a role in modulating the plastic and functional changes elicited by stress and ethanol on GABAA mediated neurotransmission in a way and through mechanisms similar to those associated to the changes of GABAA receptor gene expression and function elicited by physiological events such as pregnancy and delivery. Finally, the evidence that the plasma and brain content of neurosteroids is greater in females than in males and that in the former, at variance to the latter, there are physiological fluctuations in the synthesis and secretion of these hormones that result in functional changes of specific neuronal subpopulations in different brain areas, the data reported in this chapter may also open a new view to better understand some gender differences in the physiopathology of affective and emotional diseases.
2. Acute stress, neuroactive steroids, and GABAergic transmission
2.1. Acute stress and GABAergic transmission
The response of an animal or human being to stress is characterized by neuronal and hormonal changes that are triggered by the adverse stimulus and enable the organism to cope more effectively with the stressful situation before returning to normal homeostasis. Among the neurotransmitters that contribute to the rapid coordination of behavioral, emotional, neuroendocrine, and metabolic aspects of the response to acute stress, γ-aminobutyric acid (GABA) appears to play a central role. More than two decades ago, we showed that type A receptors for GABA (GABAA receptors) in the brain are affected by changes in the emotional state of rats elicited by handling. Stress induced by acute handling was thus found to reduce the density of low-affinity GABAA receptors in the cerebral cortex of naïve animals (handled just before killing) compared with that apparent in rats that were habituated for 5 to 7 days to the handling manipulation that precedes killing (Biggio et al., 1980; Biggio et al., 1981; Biggio, 1983). We concluded that the handling-habituated rats are in a relatively nonstressed condition whereas the acute handling of the naïve animals before killing constitutes an emotional stimulus responsible for the marked decrease in GABAA receptor density. Moreover, these data indicated that the emotional state of animals during an experimental procedure is of utmost importance in studies of the role of the GABAergic system in the physiological response to stress.
The notion that GABAA receptors are affected by stress was further supported by the observations that various acute stress paradigms, including mild foot shock, inhalation of CO2, forced swimming, and exposure to a new environment, all of which also elicit anxiety-related behavior, induced a rapid and reversible down-regulation of GABAergic transmission (Biggio et al., 1981; Concas et al., 1987; Concas et al., 1988; Drugan et al., 1989; Serra et al., 1989a; Biggio et al., 1990; Andrews et al., 1992; Sanna et al., 1992; File et al., 1993). The latter was assessed by measurement either of the binding to brain membranes of [3H]GABA, [3H]benzodiazepine ligands, or t-[35S]butylbicyclophosphorothionate (TBPS; which interacts with recognition sites associated with the GABAA receptor–operated Cl− channel) or of GABA-stimulated Cl− flux in synaptoneurosomes.
Important insight into the functional significance of the observed changes in biochemical parameters of GABAergic transmission elicited by stress was provided by the finding that inhibitors of GABAA receptor function or negative allosteric modulators of the GABAA receptor complex (anxiogenic β-carbolines) mimicked the effects of acute stress (Biggio et al., 1990). Isoniazid, FG 7142, and other drugs known to reduce GABAergic transmission as well as to induce both a proconflict effect in rats and experimental anxiety in primates, including humans (Ninan et al., 1982; Corda et al., 1983; Dorow et al., 1983; Corda & Biggio, 1986), were thus found to share with stress the ability to reduce Cl− flux and [3H]GABA binding and to enhance [35S]TBPS binding in rat brain (Biggio et al., 1990; Serra et al., 1991a). The evidence that the effects of stress are similar to those of anxiogenic drugs, which reduce the activity of the GABAA receptor complex, further suggested that stress might induce a down-regulation of GABAergic transmission. Furthermore, this conclusion was supported by the finding that anxiolytic drugs, which enhance GABAergic transmission and modulate the function of the GABA-gated Cl− channel in a manner opposite to that of anxiogenic β-carbolines, abolished, in a manner sensitive to the benzodiazepine antagonist flumazenil, the effects elicited in the brain by acute handling, foot shock, or CO2 inhalation in rats (Biggio et al., 1990; Sanna et al., 1992). Foot shock was also shown to potentiate the convulsant activity of isoniazid (Serra et al., 1991a).
2.2. Acute stress and the homeostatic action of neuroactive steroids
Among the various factors that have been proposed to modulate the down-regulation of GABAergic transmission induced by acute stress, neuroactive steroids feature prominently. Neuroactive steroids are pregnane steroids that induce rapid changes in neuronal excitability as well as elicit behavioral effects within seconds to minutes of their administration to experimental animals (Biggio & Purdy, 2001; Smith, 2004). Their mechanism of action thus differs from that of classical steroid hormones, which exert their effects at the level of gene transcription. Our understanding of the physiological role of endogenous neuroactive steroid derivatives was greatly increased by the findings that 3α-hydroxy-5α-pregnane-20-one (3α,5α-THP) and 3α,21-dihydroxy-5α-pregnane-20-one (3α,5α-THDOC) are among the most potent and efficacious positive allosteric modulators of GABAA receptor function (Majewska, 1992; Lambert et al., 1995) and that their administration to animals, either systemically or intracerebroventricularly, induces marked anxiolytic, sedative-hypnotic, and anticonvulsant effects (Kokate et al., 1994; Bitran et al., 1995; Concas et al., 1996). These observations thus suggested that changes in the brain concentrations of 3α,5α-THP and 3α,5α-THDOC may play an important role in the adaptive response to stress (Purdy et al., 1991; Barbaccia et al., 1994; Barbaccia et al., 1996a; Barbaccia et al., 1996b; Barbaccia et al., 1996c; Barbaccia et al., 1997; Barbaccia et al., 2001).
The first evidence that neuroactive steroid levels in the brain are affected by acute stress was provided by the observation that forced swimming induced rapid increases in the concentrations of progesterone and its metabolites 3α,5α-THP, and 3α,5α-THDOC in the cerebral cortex, hypothalamus, and plasma of rats (Purdy et al., 1991). The stress-induced increase in the concentration of 3α,5α-THP in plasma, but not that in the brain, was abolished by adrenalectomy, whereas the increases in 3α,5α-THDOC concentration in both plasma and the brain were not observed in adrenalectomized rats. The authors concluded that, in contrast to 3α,5α-THDOC, which is predominantly of adrenal origin, 3α,5α-THP is synthesized locally in the brain. This conclusion was consistent with observations that neurons and glial cells are able to synthesize steroids (Hu et al., 1987; Le Goascogne et al., 1987; Usui et al., 1995).
With the use of different stress paradigms, we confirmed the results of Purdy et al. (1991) and further showed that the brain concentrations of 3α,5α-THP and 3α,5α-THDOC as well as those of the precursors pregnenolone and progesterone correlate with the “emotional state” of experimental animals. We thus showed that the concentrations of these steroids were much lower in the brain of handling-habituated rats than in that of acutely handled naïve (stressed) rats (Barbaccia et al., 1994; Barbaccia et al., 1997). From this finding, we concluded that habituation to repeated handling prevents the increase in brain neuroactive steroid levels elicited by the acute stress of handling in naïve rats. This conclusion was further supported by the observation that an acute anxiogenic and stressful challenge such as CO2 inhalation or foot shock increased the concentrations of neuroactive steroids in the brain and plasma by a greater extent in handling-habituated rats than in acutely handled naïve (stressed) animals (Barbaccia et al., 2001).
2.2.1. CO2 inhalation
Inhalation of CO2 induces anxiety and panic attacks in humans (Woods et al., 1986) as well as proconflict behavior in rats, as revealed by a decrease in the number of licking periods during punishment in Vogel's test (Cuccheddu et al., 1995). Although the molecular mechanism responsible for these behavioral effects remains unknown, studies in rats have shown that inhalation of a gas mixture containing 35% CO2 and 65% O2 for 1 min reduces the level of GABAergic transmission, as reflected by a time-dependent increase in [35S]TBPS binding to brain membranes and a parallel decrease in GABA- or muscimol-stimulated Cl− uptake by synaptoneurosomes (Sanna et al., 1992). The CO2 inhalation–induced changes in GABAA receptor function were associated with time-dependent increases in the cerebral cortical and plasma concentrations of 3α,5α-THP and its precursors pregnenolone and progesterone (Barbaccia et al., 1996b). In contrast to the steroid concentrations in plasma, which peaked ∼30 min after the onset of stress, those in the brain manifested at least two distinct temporal patterns, with that of progesterone showing an immediate and marked increase that persisted for 10 min whereas those of pregnenolone and 3α,5α-THP exhibited slower and longer-lasting increases, reaching a peak at 30 min and remaining significantly higher than control values 60 min after CO2 inhalation.
We examined the possible relations among the changes in brain steroid concentrations, GABAA receptor function, and conflict behavior elicited by stress. Comparison of the respective time courses revealed that one of the first effects of acute stress is an increase in [35S]TBPS binding to cerebral cortical membranes (Concas et al., 1988), which is thought to reflect a decrease in GABAergic transmission. This latter interpretation is consistent with the observation that administration of anxiogenic β-carbolines (negative allosteric modulators of the GABAA receptor complex) to rats mimics, in a flumazenil-sensitive manner, the effect of stress on [35S]TBPS binding to brain membranes, whereas administration of anxiolytic drugs (positive allosteric modulators of the GABAA receptor) reduces the extent of such binding (Concas et al., 1988; Biggio et al., 1990). The effect of CO2 inhalation on [35S]TBPS binding was accompanied by anxiety-related behavior as measured by Vogel's test; exposure to CO2 thus reduced the number of licking periods during punishment in this test without affecting unpunished drinking periods (Cuccheddu et al., 1995). Both these effects of CO2 were maximal 5 min after CO2 inhalation and were no longer apparent after 60 min. Thus, at a time when the concentration of 3α,5α-THP in brain was maximal (30 min), the effects of stress on punished behavior and [35S]TBPS binding were already declining (Barbaccia et al., 2001).
The temporal pattern of the increase in brain steroid concentrations after CO2 inhalation may be of functional relevance in the central response to stress. The delayed increase in the concentration of 3α,5α-THP, one of the most potent positive modulators of GABAA receptors, may thus represent a homeostatic mechanism for restoration of basal GABAergic tone after its reduction by stress. This conclusion is consistent with the findings that both [35S]TBPS binding and the brain concentration of 3α,5α-THP had returned to baseline values 90 min after CO2 exposure (Barbaccia et al., 2001) and that intracerebroventricular injection of 3α,5α-THP in rats to achieve brain concentrations similar to those observed after CO2 inhalation resulted in a time- and dose-dependent decrease in the binding of [35S]TBPS to cerebral cortical membranes (Concas et al., 1996). One hour after CO2 inhalation, when the brain concentration of 3α,5α-THP was still significantly increased and [35S]TBPS binding was already declining, rats were no longer sensitized to electric shock in Vogel's test. The 24-hour water deprivation to which the animals are exposed for the Vogel test may constitute a stress sufficient per se to increase steroid concentrations in plasma and brain. However, we have shown that, although prior exposure of rats to a stressful stimulus (such as handling that precedes killing in naïve rats) increased the basal levels of neuroactive steroids in the brain, it did not prevent the CO2-induced increase in these steroid concentrations (Barbaccia et al., 1994). These results thus suggested a potential functional relation among the restoration of GABAergic transmission, the disappearance of conflict behavior, and the increase in the brain concentration of 3α,5α-THP.
2.2.2. Foot shock
Similar to the effects of CO2 inhalation, exposure of adult male rats to foot shock (one 0.2-mA, 500-ms pulse each second for 5 min) elicits both proconflict behavior and a reduction in the level of GABAergic transmission in the brain (Biggio et al., 1990) that are associated with a time-dependent increase in the cerebral cortical content of neuroactive steroids (Barbaccia et al., 2001). Foot shock stress increases the brain concentrations of pregnenolone, progesterone, and 3α,5α-THP, and, in contrast to other stress paradigms such as CO2 inhalation and handling, it also increases that of 3α,5α-THDOC (Barbaccia et al., 1996a; Barbaccia et al., 1997). The increases in the brain concentrations of 3α,5α-THP and 3α,5α-THDOC peak between 10 and 30 min after foot shock, persist for 1 h, and are paralleled by analogous increases in the plasma concentrations of these steroids. In contrast, the increase in the plasma concentration of corticosterone is maximal immediately after the foot shock session and then declines, consistent with the time course of the reduction in GABAA receptor function (Barbaccia et al., 1996a). Thus, whereas the increase in plasma corticosterone concentration after foot shock coincides with the decrease in GABAA receptor function, the increases in the brain and plasma concentrations of 3α,5α-THP and 3α,5α-THDOC coincide with the recovery of GABAA receptor function, similar to the changes observed in response to the acute stress of CO2 inhalation (Barbaccia et al., 1996b). These findings provided further support for the notion that endogenous 3α,5α-THP and 3α,5α-THDOC may contribute to an important homeostatic mechanism in the adaptation to stress by limiting the extent and duration of the reduction in GABAergic transmission elicited by acute stress.
In contrast to CO2 inhalation, foot shock induces a marked activation of the hypothalamic-pituitary-adrenal (HPA) axis, as demonstrated by a pronounced increase in the plasma concentration of corticosterone (+320 and +58% after foot shock or CO2 inhalation, respectively) (Barbaccia et al., 1996a; Barbaccia et al., 1996b). This difference in efficacy of activation of the HPA axis may explain the failure of CO2 inhalation to increase the brain concentration of 3α,5α-THDOC (Barbaccia et al., 1996a), given that, in contrast to 3α,5α-THP, which is synthesized de novo in the brain, 3α,5α-THDOC appears to be derived almost exclusively from the adrenal cortex (Reddy, 2003). This conclusion is consistent with data showing that the brain concentrations of 3α,5α-THP and 3α,5α-THDOC are differentially affected by other pharmacological treatments (Concas et al., 2000; Porcu et al., 2003; Strohle et al., 2003).
The HPA axis is under the control of multiple neurotransmitter systems (Plotsky et al., 1987), with GABA exerting inhibitory control on the secretion of corticotropin-releasing factor (CRF) (Calogero et al., 1988). The decrease in GABAergic tone elicited by acute stress (Biggio et al., 1990) may thus be responsible for activation of the HPA axis in response to such stress and for the consequent observed increases in the plasma and brain concentrations of neuroactive steroids. A role for the HPA axis in the effects of acute stress on neuroactive steroid concentrations is further supported by the observation that foot shock failed to increase 3α,5α-THP and 3α,5α-THDOC concentrations in the brain of rats whose major peripheral steroidogenic organs (adrenals and gonads) had been removed (Barbaccia et al., 1997; Barbaccia et al., 2001). The major proportion of the increase in the brain content of 3α,5α-THP and 3α,5α-THDOC induced by acute stress therefore appears to be derived from peripheral tissues and to reach the brain via the bloodstream. In the brain, these neuroactive steroids appear to play a role in restoration of GABAergic neurotransmission, and, through this mechanism, they may also contribute to feedback modulation of the HPA axis.
The failure of foot shock to increase the brain concentration of 3α,5α-THP in adrenalectomized and orchiectomized (ADX-ORX) rats contrasts with the observation that swim stress still increased the amount of 3α,5α-THP in the brain of ADX rats (Purdy et al., 1991). Possible reasons for this apparent discrepancy include the difference in the type of stressor (swim stress versus mild foot shock) and in the surgical procedure (adrenalectomy versus adrenalectomy-orchiectomy). We studied ADX/ORX rats because GABAergic transmission appears to modulate the hypothalamic-pituitary-gonadal axis (Masotto et al., 1989; Jackson & Kuehl, 2002) in addition to the HPA axis. However, in both ADX and ADX-ORX rats, the extent of the decrease in the concentration of 3α,5α-THP was greater in plasma than in brain, supporting the notion that most of the basal amount of 3α,5α-THP in the brain does not originate from peripheral steroidogenic tissues. The failure of foot shock to increase neuroactive steroid concentrations in ADX-ORX animals therefore suggests that steroidogenesis in the brain is not associated with changes in GABAA receptor function. Our observations indicate that acute stress increases the brain content of 3α,5α-THP by eliminating the negative GABAergic control of one or both of the HPA and hypothalamic-pituitary-gonadal axes. Consistent with this conclusion, potentiation of GABAA receptor function by abecarnil, an anxiolytic β-carboline derivative that acts as a positive allosteric modulator at GABAA receptors (Stephens et al., 1990), prevents the stress-induced increase in 3α,5α-THP concentration in the brain of rats, probably by attenuating the sensitivity of the HPA axis to stress (Barbaccia et al., 1996a).
2.3. Anxiogenic drugs and neuroactive steroids
Further insight into the putative role of GABAergic transmission in the stress-induced activation of the HPA axis and subsequent increases in the plasma and brain concentrations of neuroactive steroids was provided by the observations that pharmacological agents that selectively reduce the extent of GABAA receptor–mediated transmission in the brain mimic the effects of stress in rats. The systemic administration of isoniazid, a drug that depletes the brain content of GABA (Horton et al., 1979) and thereby induces proconflict behavior and convulsions in rats (Corda & Biggio, 1986; Serra et al., 1989b), was thus shown to induce marked increases in the concentrations of 3α,5α-THP and 3α,5α-THDOC as well of the precursors pregnenolone and progesterone in the brain and plasma (Barbaccia et al., 1996b; Barbaccia et al., 1997). The time course of GABA depletion induced by isoniazid was paralleled by those of the increases in the cerebrocortical concentrations of these steroids, with the effects being maximal 40 min after administration of the drug and no longer apparent at 300 min. Like acute stress, isoniazid also increased the concentration of corticosterone in plasma.
The finding that the action of acute stress on the brain and plasma concentrations of neuroactive steroids is mimicked by isoniazid suggested that central GABAA receptor–mediated transmission exerts a tonic inhibitory effect on the mechanism responsible for up-regulation of the concentrations of these steroids. This conclusion was further supported by the observation that the effects of acute stress and isoniazid were also mimicked by the β-carboline derivative FG 7142, a negative allosteric modulator of GABAA receptors. Treatment of rats with FG 7142, a proconvulsant that evokes a proconflict effect in rats and induces anxiety in both humans and nonhuman primates (Ninan et al., 1982; Corda et al., 1983; Dorow et al., 1983), thus also increased the concentrations of 3α,5α-THP and 3α,5α-THDOC in both the cerebral cortex and plasma with time courses similar to those of its GABAA receptor–mediated proconvulsant and anxiogenic actions (Corda et al., 1983).
The notion that down-regulation of GABA-mediated neurotransmission may contribute to the increases in neuroactive steroid concentrations after stress was also supported by the ability of abecarnil, a positive allosteric modulator at GABAA receptors with potent anxiolytic and anticonvulsant actions (Stephens et al., 1990), to antagonize the effects of both stress and isoniazid on the brain concentrations of neuroactive steroids (Barbaccia et al., 1996a; Barbaccia et al., 1997). Together, these data showing a functional relation between inhibition of GABAA receptor function and up-regulation of the amounts of 3α,5α-THP and 3α,5α-THDOC in the brain suggest that the latter effect is attributable, at least in part, to a reduction in the extent of GABA-mediated inhibition of the HPA axis. This conclusion is consistent with the hypothesis that these neuroactive steroids, which potentiate the function of GABAA receptors, play an important role in regulation of the stress-induced activation of the HPA axis (Calogero et al., 1988). Such an inhibitory effect of neuroactive steroids on the response of the HPA axis to acute stress may be beneficial in two ways: (1) by reducing the time to which neurons are exposed to the insulting action of excessive glucocorticoid concentrations (Sapolsky et al., 1983), and (2) by allowing a rapid resetting of the responsiveness of the system to subsequent stressful events. Disruption of this homeostatic mechanism might be expected to play a pathogenic role in certain psychiatric disorders, such as major depression, posttraumatic stress disorder, and panic disorder, that are thought to be related to stressful events and are often associated with dysregulation of glucocorticoid output from the adrenal glands. Indeed, the concentrations of neuroactive steroids have been found to be decreased in the plasma and cerebrospinal fluid of individuals with major depression (Uzunova et al., 1998).
3. Acute ethanol, neuroactive steroids, and GABAA receptor function
3.1. Ethanol and GABAA receptors: pharmacological and electrophysiological studies
A wide body of experimental evidence accumulated over the past two decades suggests that the GABAA receptor is an important and sensitive neurochemical target in the acute and chronic actions of ethanol (Faingold et al., 1998; Grobin et al., 1998; Harris, 1999; Ueno et al., 2001). GABAA receptors are ligand-gated Cl− channels that are responsible for mediating fast inhibitory synaptic transmission in the mammalian central nervous system (Barnard et al., 1998; Mehta & Ticku, 1999b; Vicini, 1999). They are also targets for several classes of clinically relevant drugs, including benzodiazepines, barbiturates, and general anesthetics, as well as for endogenous compounds such as neuroactive steroids, all of which allosterically modulate receptor function (Frye et al., 1981; Sieghart, 1995; Barnard et al., 1998; Mohler et al., 2002). Like these various GABAA receptor modulators, ethanol elicits, in a dose-dependent manner, an array of central depressant effects including anxiolytic, anticonvulsant, sedative-hypnotic, muscle relaxant, and general anesthetic actions (Frye et al., 1981; Deitrich et al., 1989).
GABAA receptors are heteromeric complexes formed by the assembly of five subunits that belong to various subunit classes (α1 to α6, β1 to β4, γ1 to γ3, δ, ε, π, θ, ρ1 to ρ3) (Barnard et al., 1998; Whiting et al., 1999; Kumar et al., 2002). Brain region–specific distribution and ontogeny-dependent expression of GABAA receptor subunit isoforms are responsible for the generation of a relatively large number of GABAA receptor subtypes, which differ not only in their subunit composition but also in their physiological and pharmacological properties (Kumar et al., 2002; Sieghart, 1995; Whiting et al., 1999).
A role for GABAA receptor–mediated neurotransmission in the effects of ethanol was suggested by the early observations that low concentrations (20 to 60 mM) of ethanol increased agonist-induced Cl− flux in brain synaptoneurosomes (Allan & Harris, 1986; Suzdak et al., 1986; Morrow et al., 1988) and cultured neurons (Ticku & Burch, 1980). In addition, certain behavioral effects of ethanol were found to be enhanced by GABAA receptor agonists or positive modulators and to be attenuated or blocked by receptor antagonists or negative modulators (Martz et al., 1983). Neurophysiological evidence that ethanol acutely modulates the function of GABAA receptors has been somewhat more elusive and controversial. Whereas some studies have demonstrated a potentiating effect of ethanol on GABAA receptor function, others have failed to do so (Faingold et al., 1998; Grobin et al., 1998). Potentiation of GABAA receptor function by ethanol has also been suggested to be region specific, with the hippocampus generally regarded as a relatively ethanol-insensitive brain area. However, ethanol potentiation of GABAA receptor function has been demonstrated in the hippocampus under specific experimental conditions, requiring, for example, blockade of presynaptic GABAB receptors (Ariwodola & Weiner, 2004; Wan et al., 1996), proximal versus distal stimulation (Weiner et al., 1997), or activation of β-adrenergic receptor signaling (Freund & Palmer, 1997).
More recent studies have contributed to unraveling additional mechanisms by which ethanol might influence the activity of GABAergic synapses (Criswell & Breese, 2005; Breese et al., 2006; Weiner & Valenzuela, 2006). In addition to affecting postsynaptic GABAA receptors, ethanol is now thought to exert a presynaptic action that results in an increased probability of GABA release. Ethanol has been shown to increase the frequency of GABAA receptor–mediated inhibitory postsynaptic currents (IPSCs) that are potential dependent or independent in hippocampal CA1 pyramidal neurons (Carta et al., 2003; Ariwodola & Weiner, 2004; Sanna et al., 2004), the amygdala (Roberto et al., 2003; Nie et al., 2004), cerebellar granule cells (Carta et al., 2004), and spinal motor neurons (Ziskind-Conhaim et al., 2003). This effect of ethanol might increase the extracellular concentration of GABA to a level sufficient to activate presynaptic GABAB receptors, which negatively regulate GABA release from presynaptic terminals (Ariwodola & Weiner, 2004). Consistent with this notion, blockade of presynaptic GABAB receptors with SCH 50911 was found to greatly increase the efficacy of ethanol in modulating GABAA receptor–mediated IPSCs in the CA1 region of the hippocampus (Ariwodola & Weiner, 2004), suggesting that ethanol may exert a self-limiting modulatory activity at GABAergic synapses via indirect activation of presynaptic GABAB receptors.
In the central amygdala, inhibitory GABAergic transmission has been suggested to play a role in the expression of emotionality, including behavioral states of fear and anxiety (Davis et al., 1994), as well as in mediating the behavioral effects of acute and chronic ethanol consumption (Rassnick et al., 1993; Hyytia & Koob, 1995). The amygdala formation has actually been implicated in various physiological functions including attention (McDonald, 1998) and memory (Henke, 1985; McDonald, 1998; Holland & Gallagher, 1999; Liubashina et al., 2000) in addition to emotion (Gentile et al., 1986; LeDoux, 1995; Goosens et al., 2000). Together with its many interconnections with other limbic structures, the amygdala is also thought to play a substantial role in drug addiction—and in alcoholism, in particular (Koob et al., 1998; McBride, 2002; Koob, 2003). Given that the goal of stress reduction has long been considered to contribute to ethanol-seeking behavior in humans, it was hypothesized that the central amygdala and its connections might be sites of the GABA-like actions of ethanol that mediate ethanol reinforcement. The role of GABAA receptors in the central amygdala in this process was examined in Wistar rats trained to obtain 10% ethanol or water in a two-lever, free-choice operant task (Hyytia & Koob, 1995). Bilateral microinjection of the competitive GABAA receptor antagonist SR95531 into the central amygdala resulted in a significant reduction in the number of activations of the ethanol lever with no effect on the number of activations of the control water lever, suggesting that activation of GABAA receptors in the central amygdala mediates ethanol self-administration behavior. In addition, lesion of the central amygdala with ibotenic acid was found to reduce voluntary alcohol consumption in rats (Moller et al., 1997), supporting the idea that the central amygdala plays a role in alcohol drinking in these animals. Furthermore, microinjection of the GABAA receptor agonist muscimol into the central amygdala reduced operant ethanol self-administration in dependent rats but had little effect in nondependent animals (Roberts et al., 1996).
In vitro electrophysiological studies have analyzed the effects of ethanol in rat amygdala slices (Roberto et al., 2003). A significant effect of 44 mM ethanol on the amplitude of GABAA receptor–mediated miniature IPSCs (mIPSCs) was detected in four out of six neurons tested in the central amygdala. Ethanol (44 mM) also potentiated GABAA receptor–mediated potentials evoked by local application of GABA in 11 out of 16 neurons tested. The same research group also showed that the effects of ethanol on GABAA receptors in the central amygdala were absent in mice that lack the CRF1 receptor (Nie et al., 2004), suggesting that CRF1 receptors mediate ethanol-induced enhancement of GABAergic synaptic transmission in this brain region.
3.2. Ethanol sensitivity and GABAA receptor subunit composition
Studies on the molecular mechanisms of ethanol action have also suggested that this drug at concentrations as low as 2 to 30 mM selectively potentiates the function of recombinant GABAA receptors containing α4, β3, and δ subunits or α6, β3, and δ subunits expressed in Xenopus oocytes. Such an effect was not observed at receptors in which the δ subunit was replaced with the γ2 subunit (Sundstrom-Poromaa et al., 2002; Wallner et al., 2003). In contrast to GABAA receptors that contain the γ2 subunit, those containing both α4 or α6 and δ subunits are located exclusively at extrasynaptic sites and are thought to mediate tonic inhibitory activity (Mody et al., 1994; Semyanov et al., 2004). Furthermore, δ-containing extrasynaptic GABAA receptors, which are expressed selectively in the dentate gyrus and thalamus (α4βδ) as well as cerebellar granule cells (α6βδ) (Semyanov et al., 2004), are characterized by a higher sensitivity for GABA (EC50 ∼0.5 μM), a slower desensitization rate, and a preferential sensitivity to neuroactive steroids (Semyanov et al., 2004). In addition, mouse hippocampal CA1 pyramidal neurons have been shown to express a subpopulation of α5-containing GABAA receptors (likely α5β3γ2 receptors) that is responsible for mediating tonic inhibition and is endowed with different properties, such as insensitivity to neuroactive steroids and sensitivity to the α5-selective inverse agonist L-655,708, with respect to α4βδ and α6βδ receptors (Caraiscos et al., 2004). GABAA receptor–mediated tonic activity has also been shown to be increased by a relatively low (30 mM) concentration of ethanol in granule cells of the dentate gyrus but not in CA1 pyramidal neurons (Wei et al., 2004). In addition, the effect of ethanol at α4β3δ or α6β3δ GABAA receptors was shown to be competitively antagonized by the benzodiazepine receptor inverse agonist Ro15-4513 (Hanchar et al., 2006). The benzodiazepine receptor antagonist flumazenil, which was ineffective in blocking the action of ethanol at these GABAA receptor subtypes, was found to antagonize the inhibitory effect of Ro15-4513 (Wallner et al., 2006). Interestingly, following chronic intermittent ethanol exposure in rats a decrease in sensitivity of the tonic current in dentate gyrus granule cells to the acute modulation by ethanol was demonstrated (Liang et al., 2006). Tolerance to ethanol in these hippocampal neurons was suggested to result from the reduction in the expression of the δ subunit and a parallel translocation of the α4 subunit from extrasynaptic to synaptic sites. In turn, newly formed synaptic α4-containing receptors showed an increased sensitivity to low concentrations of ethanol (Liang et al., 2006).
Very recently, in the interneurons of the dentate gyrus molecular layer, a novel subpopulation of GABAA receptors comprising the α1, βn and δ subunits has been identified that is capable of mediating tonic inhibition and that is sensitive to the acute modulation of 30 mM ethanol, suggesting that these receptors represent an additionally molecular target that may contribute to the overall sensitivity to this drug (Glykys et al., 2007).
Together, these various results suggest that, in view of the role of tonic inhibitory activity in the fine-tuning of neuronal excitability, extrasynaptic GABAA receptors may be important targets for the actions of ethanol at pharmacologically relevant concentrations. It should be mentioned, however, that other laboratories have failed to obtain similar results in this regard (Carta et al., 2004; Borghese et al., 2006; Casagrande et al., 2006; Yamashita et al., 2006), so the question as to whether there are selective subpopulations of GABAA endowed with high sensitivity to ethanol still remains a highly controversial issue. Furthermore, studies with mice that lack the δ subunit of the GABAA receptor have revealed that δ subunit–containing receptors may be important for some but not all behavioral actions of ethanol. These mice manifest reduced withdrawal hyperexcitability after chronic ethanol exposure, a reduced sensitivity to the anticonvulsant effect of ethanol, and a reduced preference for voluntary ethanol consumption compared with wild-type animals. On the other hand, other behavioral effects of ethanol, including the induction of anxiolysis, hypothermia, sedation, and tolerance, do not appear to be altered in the knockout mice (Mihalek et al., 2001). In another study, the discriminative stimulus effect of ethanol was not altered in mice lacking the δ subunit compared with that in wild-type mice (Shannon et al., 2004).
3.3. Ethanol pharmacology and neuroactive steroids
Recent studies have suggested that many of the acute pharmacological actions of ethanol are mediated by an increase in the brain levels of neuroactive steroids (Morrow et al., 1999). Acute systemic administration of ethanol in rats was thus found to result in marked increases in the concentrations of 3α,5α-THP in plasma, cerebral cortex, and hippocampus (Barbaccia et al., 1999; VanDoren et al., 2000; Mihalek et al., 2001). A role for neuroactive steroids in the pharmacological effects of ethanol is also supported by the observation that pretreatment of animals with finasteride, an inhibitor of the enzyme 5α-reductase and therefore of the biosynthesis of 3α,5α-THP (Azzolina et al., 1997), reduced the extent of the ethanol-induced increase in the cerebrocortical level of 3α,5α-THP and prevented certain neurochemical, electrophysiological, and behavioral actions of ethanol (VanDoren et al., 2000; Khisti et al., 2002b). Circulating 3α,5α-THP has also been suggested to influence ethanol reinforcement (Morrow et al., 2001). Indeed, female mice, in which the brain concentration of 3α,5α-THP is higher than that in male animals, consume greater amounts of ethanol that do males (Sinnott et al., 2002). In addition, ethanol consumption in male mice was increased by systemic administration of 3α,5α-THP. Administration of 3α,5α-THP also increased ethanol-reinforced operant responding in male rats (Janak et al., 1998). In contrast, self-administration of ethanol in female rats was found to be lower during estrus (Roberts et al., 1998), when the brain concentration of 3α,5α-THP is higher than in other phases of the estrous cycle, and socially isolated rats, in which the brain concentration of 3α,5α-THP is markedly reduced, consume increased amounts of ethanol (Schenk et al., 1990; Wolffgramm, 1990).
A stimulatory effect of ethanol on the HPA axis is thought to represent the main mechanism by which this drug increases 3α,5α-THP levels (Ellis, 1966; Rivier et al., 1984; Rivier, 1996; Ogilvie et al., 1997; Khisti et al., 2003). Indeed, ethanol failed to increase the plasma levels of 3α,5α-THP and to induce some of its pharmacological effects in ADX rats (Khisti et al., 2002a; Khisti et al., 2003). Together, these various observations suggest that neuroactive steroids produced by peripheral organs in response to activation of the HPA axis may be responsible for certain effects of ethanol on GABAA receptors. However, because neuroactive steroids are also produced de novo in the brain independently of peripheral organs (Hu et al., 1987; Mathur et al., 1993), it was important to determine whether ethanol is able to stimulate steroidogenesis directly in brain tissue.
3.4. Effect of ethanol on hippocampal neuroactive steroid content
We recently investigated the possibility that ethanol, in addition to stimulating the synthesis and secretion of neuroactive steroids from peripheral organs, might also exert a similar action on brain steroidogenesis (Sanna et al., 2004). Brain cells express steroidogenic enzymes, and neuroactive steroid formation has been shown to occur in the brain independently of peripheral sources (Hu et al., 1987; Mathur et al., 1993). We found that incubation of hippocampal slices from 3-week-old rats with ethanol resulted in a significant increase in the concentration of 3α,5α-THP relative to the basal level. This effect of ethanol was dependent both on its concentration, with 50 mM being the lowest effective concentration, and on time, with an apparent onset at ∼20 min and a further increase observed at 30 min. The effect of ethanol on neuroactive steroid content was mimicked by other drugs known to be capable of increasing, through different mechanisms, the production of 3α,5α-THP, including the steroid precursor progesterone, the selective agonist of the peripheral benzodiazepine receptor CB34 (Serra et al., 1999), and γ-hydroxybutyrate (GHB), which increases brain neuroactive steroid concentrations by activating GABAB receptors (Barbaccia et al., 2002).
3.5. Ethanol, neuroactive steroids, and hippocampal GABAA receptor function
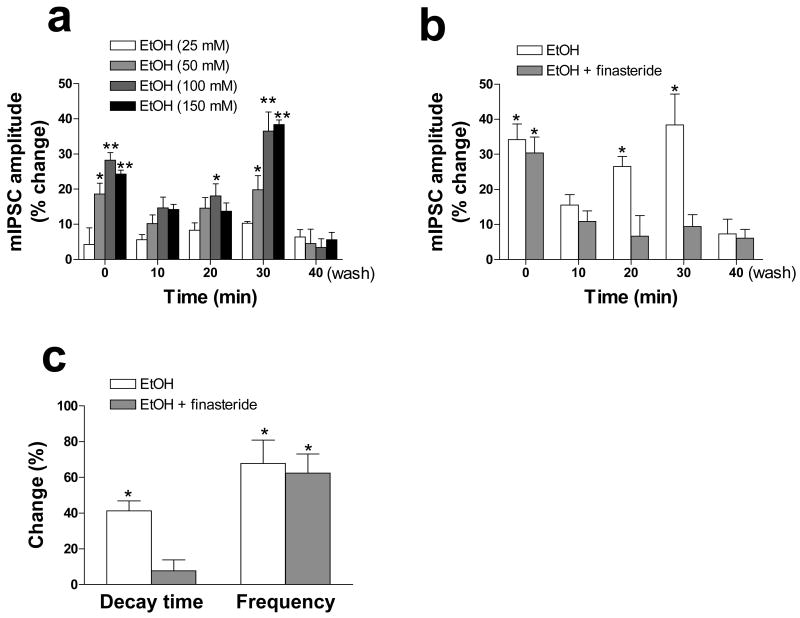
The possibility that the increase in the concentration of 3α,5α-THP in isolated hippocampal tissue induced by ethanol as well as by progesterone, CB34, or GHB might result in modulation of GABAA receptors, which are sensitive targets of neuroactive steroids (Lambert et al., 2001; Belelli & Lambert, 2005), was subsequently tested. By recording spontaneous GABAA receptor–mediated mIPSCs from pyramidal neurons of the CA1 region of hippocampal slices in the presence of tetrodotoxin, we found that continuous bath application of ethanol for 30 min induced a time- and concentration-dependent modulation of GABAA receptor function (Fig. 1). Ethanol was thus found to increase mIPSC amplitude in a biphasic manner, with the initial effect being apparent during the first 3 min of ethanol exposure and the secondary effect being observed at 20 and 30 min (Fig. 1a). Ethanol also increased the frequency and decay time of mIPSCs (Fig. 1c). To determine whether the ethanol-induced enhancement of GABAA receptor–mediated mIPSCs might be related to the increase in the tissue content of 3α,5α-THP, we treated hippocampal slices with the 5α-reductase inhibitor finasteride both before and during bath application of ethanol. Although it was devoid of any effect by itself on the kinetic characteristics of mIPSCs, finasteride prevented the delayed increase in mIPSC amplitude apparent between 10 and 30 min after the onset of ethanol exposure without affecting the immediate increase apparent during the initial 3 min (Fig. 1b). These observations suggest that the initial, finasteride-insensitive effect of ethanol may be due to direct modulation of postsynaptic GABAA receptors, whereas the delayed, finasteride-sensitive effect may be attributable to increased synaptic levels of neuroactive steroids. Finasteride also blocked the increase in mIPSC decay time induced by ethanol (Fig. 1c), consistent with the ability of neuroactive steroids to prolong mIPSC inactivation (Harrison et al., 1987; Zhu & Vicini, 1997). With a similar experimental protocol, other researchers showed that exposure of Purkinje neurons in cerebellar slices to ethanol induced an increase in GABAergic mIPSC decay time that was significant at 20 and 30 min and was prevented by coapplication of finasteride (Breese et al., 2006). However, the same study failed to detect an effect of ethanol on mIPSC amplitude. It should be mentioned that although generally most of the studies have reported an increase in current decay time with no effect on amplitude by neuroactive steroids, others have however shown a clear potentiation of 3α,5α-THP on mIPSC amplitude as well as decay time (Poibeau et al., 1997; Sullivan & Moenter, 2003). Difference in certain experimental conditions used (for example bath temperature or age of rats) may contribute to this variability.
Figure 1.
Effects of ethanol of GABAA receptor–mediated mIPSCs in CA1 pyramidal neurons of rat hippocampal slices. (a) CA1 pyramidal neurons in hippocampal slices were voltage-clamped at −60 mV and subjected to whole-cell recording in artificial cerebrospinal fluid containing 600 nM tetrodotoxin and 1 mM kynurenic acid. The slices were exposed to various concentrations of ethanol for the indicated times up to 30 min, after which the amplitude of GABAA receptor–mediated mIPSCs was determined. The time zero (0 min) recording was actually obtained during the initial 3 min of ethanol application. The values for the 40-min time point were obtained after washing for 10 min with medium not containing ethanol. (b) Neurons were exposed (or not) to 1 μM finasteride for 20 min before the additional application of 100 mM ethanol for the indicated times and measurement of mIPSC amplitude as in (a). (c) Neurons were exposed to 1 μM finasteride and to 100 mM ethanol for 30 min as in (b), and the decay time and frequency of mIPSCs were then determined. All data are expressed as percentage change in the measured parameter induced by bath application of ethanol and are means ± SEM of values from 5 to 25 cells. *P < 0.05, **P < 0.01 versus control value (ANOVA followed by Scheffè's post hoc test). Modified with permission from Sanna et al. (2004).
Finasteride failed to affect the ethanol-induced increase in mIPSC frequency in our studies (Fig. 1c), indicating that ethanol increased the probability of GABA release from presynaptic sites independently of neuroactive steroids. A presynaptic action of ethanol on GABAA receptor function has also been described by other groups (Roberto et al., 2003; Ziskind-Conhaim et al., 2003; Ariwodola & Weiner, 2004; Carta et al., 2004;) and is consistent with our result that ethanol also reduced and reversed the ratio of paired-pulse facilitation (Sanna et al., 2004).
Consistent with their abilities to increase the hippocampal concentration of 3α,5α-THP, we also found that progesterone, CB34, and GHB each enhanced GABAA receptor function in hippocampal slices. Indeed, bath application of each of these three drugs for 30 min resulted in significant increases in the amplitude and decay time, but not in the frequency, of GABAA receptor–mediated mIPSCs. Moreover, none of these three drugs affected mIPSC amplitude during the initial 3 min of application, consistent with their inability to interact directly with GABAA receptors (Serra et al., 1991b; Serra et al., 1999; Lambert et al., 2001). In addition, the finding that finasteride prevented the increases in both mIPSC amplitude and decay time further suggested that the modulatory action of these drugs on GABAA receptor function is mediated by an increase in the synaptic levels of neuroactive steroids. As a control, we also tested the effects of lorazepam, a positive allosteric modulator of GABAA receptors, and found that its application for 3 min resulted in significant increases in mIPSC amplitude and decay time and that these effects were no longer apparent after 30 min of its continuous bath application. As expected, the rapid modulatory effects of lorazepam were not affected by application of finasteride.
3.6. Ethanol and neuroactive steroids in ADX-CX animals
Our data showing that ethanol promotes the biosynthesis of 3α,5α-THP in isolated hippocampal tissue prompted us to explore further whether this action is independent of HPA axis activity. We thus studied adrenalectomized-castrated (ADX-CX) rats in order to determine whether this effect of ethanol was attributable to an increase in the rate of conversion of steroid precursors (such as progesterone) derived from peripheral sources to 3α,5α-THP by brain cells or whether it was due to an increase in the formation of this neuroactive steroid de novo from cholesterol. Indeed, the plasma and brain concentrations of progesterone and pregnenolone are markedly reduced in ADX-CX rats 1 week after surgery compared with those in sham-operated animals (Porcu et al., 2004). Adrenalectomy and castration reduced the basal levels of 3α,5α-THP in the hippocampus by only ∼40%, consistent with the notion that brain cells continue to produce neuroactive steroids independently from peripheral sources. Incubation of hippocampal slices from either ADX-CX or sham-operated rats with ethanol (100 mM) for 30 min resulted in an ∼70% increase in the content of 3α,5α-THP (Fig. 1a). Consistent with these results, patch-clamp recordings of GABAA receptor–mediated mIPSCs in CA1 pyramidal neurons showed that ethanol increased current amplitude, decay time, and frequency by similar extents in both groups of animals (Fig. 1b–d). As expected, finasteride prevented the ethanol-induced increases in mIPSC amplitude and decay time, but not that in mIPSC frequency, in both groups of rats. These results suggest that the effects of ethanol on both 3α,5α-THP concentration and mIPSC amplitude and decay time in hippocampal slices are independent of peripheral steroid precursors and are likely attributable to stimulation of local neurosteroidogenesis from cholesterol. Consistent with our findings, the increase in the brain content of 3α,5α-THP did not correlate with changes in the plasma concentrations of corticosterone, progesterone, or 3α,5α-THP in ethanol-treated rats, suggesting that brain and circulating steroid levels are regulated differently (VanDoren et al., 2000).
4. Chronic ethanol, neuroactive steroids, and GABAA receptor plasticity and function
Long-term treatment with positive allosteric modulators that act at various sites of the GABAA receptor results in changes in the biochemical and functional properties of the receptor that are accompanied by changes in the abundance of specific receptor subunits (Morrow et al., 1990; Roca et al., 1990a; Roca et al., 1990b; Montpied et al., 1991a; Mhatre & Ticku, 1992; Mhatre et al., 1993; Holt et al., 1996; Impagnatiello et al., 1996; Yu et al., 1996; Holt et al., 1997; Biggio et al., 2003; Wafford, 2005). Chronic administration and subsequent withdrawal of ethanol also elicit neurochemical and molecular effects in rat brain similar to those induced by drugs that potentiate GABAA receptor function (Majchrowicz, 1975; Morrow et al., 1990; Mhatre et al., 1993; Tseng et al., 1993; Devaud et al., 1997; Mahmoudi et al., 1997; Cagetti et al., 2003). Moreover, chronic ethanol administration induces functional tolerance of GABAA receptors to the effects of ethanol, an outcome that results from adaptive changes in GABAA receptor–mediated neurotransmission (Chandler et al., 1998; Faingold et al., 1998; Grobin et al., 1998). Indeed, altered GABAA receptor function, characterized by a decreased responsiveness to GABA, decreased sensitivity to ethanol, cross-tolerance to benzodiazepines and barbiturates, as well as increased sensitivity to neuroactive steroids and inverse agonists, is thought to be important in the development of overall tolerance to and dependence on ethanol (Ticku & Burch, 1980; Allan & Harris, 1987; Morrow et al., 1988; Sanna et al., 1993; Devaud et al., 1996).
Although the molecular mechanisms responsible for the changes in GABAA receptor function induced by persistent ethanol exposure remain unclear, it has been proposed that they include changes in receptor density and in posttranslational protein modification (Grobin et al., 1998; Kumar et al., 2004). It appears, however, that a single mechanism alone may not be sufficient to explain the observed changes in receptor function and plasticity. Attempts to characterize the molecular mechanisms that underlie the development of ethanol tolerance and dependence have focused on the effects of chronic ethanol administration and its abrupt withdrawal on GABAA receptor gene expression and plasticity in several brain regions.
4.1. In vivo studies
Many studies have shown that chronic ethanol administration alters the expression of various GABAA receptor subunits, suggesting that changes in GABAA receptor gene expression may contribute to changes in GABAA receptor function (Morrow et al., 1990; Buck et al., 1991a; Montpied et al., 1991b; Morrow et al., 1992; Mhatre et al., 1993; Mhatre & Ticku, 1994; Devaud et al., 1995). However, changes in GABAA receptor function and gene expression appear to differ among brain regions and may also depend on the treatment protocol including treatment duration (Faingold et al., 1998; Grobin et al., 1998; Kumar et al., 2004).
Long-term ethanol treatment in rats results in marked changes in expression of the genes for various GABAA receptor subunits, including a decrease in the abundance of α1, α2, α3, and α5 subunit mRNAs and proteins (Montpied et al., 1991b; Mhatre & Ticku, 1992; Mhatre et al., 1993; Devaud et al., 1995) and an increase in that of α4, γ1, and γ2S mRNAs (Devaud et al., 1995) as well as in that of β1, β2, and β3 mRNAs and proteins (Mhatre & Ticku, 1994) in the cerebral cortex. In the cerebellum, a decrease in the abundance of the α1 subunit was accompanied by an increase in that of the α6 subunit (Mhatre & Ticku, 1992). In addition, chronic ethanol exposure resulted in a decrease in the amount of the α1 subunit and an increase in that of the α4 subunit, without an effect on that of the γ2 subunit, in the hippocampus (Matthews et al., 1998). Chronic intermittent treatment with ethanol induced down-regulation of α1 and δ subunit gene expression and up-regulation of α4, γ1, and γ2 subunit gene expression in the hippocampus (Mahmoudi et al., 1997; Cagetti et al., 2003). These various observations thus support the notion that GABAA receptor gene expression is differentially regulated by ethanol in different regions of the brain.
Most, but not all, of the observed ethanol-induced changes in the abundance of GABAA receptor subunit mRNAs are associated with corresponding alterations in the amounts of the encoded proteins (Grobin et al., 1998; Kumar et al., 2004). In some instances, however, there is no correlation between such changes in subunit gene expression and other neurochemical parameters such as receptor density. For example, the abundance of GABAA receptor assemblies containing the α1 subunit in the cerebral cortex and cerebellum was not changed by chronic ethanol administration (Mehta & Ticku, 1999a). Moreover, it remains unclear whether the observed changes in GABAA receptor gene expression are directly correlated with changes in GABAA receptor function or pharmacological sensitivity that result from chronic exposure to and subsequent withdrawal of ethanol.
The use of well-defined experimental paradigms may provide insight into the nature of the relation between changes in GABAA receptor gene expression and those in receptor function as well as into the roles played by individual receptor subunits during chronic ethanol treatment and withdrawal. Studies with primary neurons in culture allow comparison of the effects of prolonged exposure to and abrupt withdrawal of ethanol on GABAA receptor function, expression, and responsiveness to ligands selective for different receptor subtypes in different neuronal cells derived from discrete brain regions. In contrast to studies with laboratory animals, those with cultured primary neurons also overcome the difficulty of establishing the precise onset of ethanol withdrawal.
4.2. Chronic ethanol and GABAA receptor gene expression
Prolonged exposure of cerebellar granule cells in culture to ethanol induced a decrease in the abundance of the mRNA for the γ2 subunit of the GABAA receptor (Table 1). The same treatment increased the abundance of the α3 and β3 subunit mRNAs but had no significant effect on that of the α1, α2, α4, α5, α6, β1, β2, and δ subunit mRNAs (Follesa et al., 2003; Follesa et al., 2004; Follesa et al., 2005; Follesa et al., 2006). In contrast, ethanol induced a significant decrease in the abundance of α1, α3, and γ2 subunit mRNAs and a marked increase in that of the δ subunit mRNA in cultured hippocampal neurons (Table 1). The same treatment did not significantly affect the amounts of the α2, α4, and α5 subunit mRNAs in these neurons (Sanna et al., 2003). In cultured cortical neurons (Table 1), long-term ethanol exposure induced a decrease in the abundance of α1, α2, and γ2 subunit mRNAs but did not affect that of α4, β2, and β3 mRNAs (Sheela Rani & Ticku, 2006). These data thus suggest that ethanol affects the expression of GABAA receptor genes in a manner dependent on neuronal type, consistent with the in vivo data showing that chronic ethanol treatment exerts differential effects on GABAA receptor gene expression in the cerebral cortex, hippocampus, and cerebellum (Grobin et al., 1998; Kumar et al., 2004).
Table 1.
Effects of long-term treatment with ethanol on GABAA receptor gene expression in different types of neurons in culture.
| GABAA receptor subunit mRNA | Cerebellar granule cells | Hippocampal neurons | Cortical neurons |
|---|---|---|---|
| α1 | ↔ | ↓ | ↓ |
| α2 | ↔ | ↔ | ↓ |
| α3 | ↑ | ↓ | Not measured |
| α4 | ↔ | ↔ | ↔ |
| α5 | ↔ | ↔ | Not measured |
| α6 | ↔ | Not expressed | Not expressed |
| δ | ↔ | ↑ | Not measured |
| γ2 | ↓ | ↓ | ↓ |
| β1 | ↔ | Not measured | Not measured |
| β2 | ↔ | Not measured | ↔ |
| β3 | ↑ | Not measured | ↔ |
Arrows indicate an increase, decrease, or no change in the abundance of the mRNA relative to control values. Data for β subunit mRNAs in cerebellar granule cells are original; all other data are derived with permission from previous studies (Follesa et al., 2003; Sanna et al., 2003; Follesa et al., 2004; Follesa et al., 2005; Follesa et al., 2006; Sheela Rani & Ticku, 2006).
4.3. Ethanol withdrawal and GABAA receptor gene expression
Discontinuation of chronic ethanol treatment induced a decrease in the abundance of mRNAs for the α1, α6, and δ subunits of the GABAA receptor in cultured cerebellar granule cells (Follesa et al., 2003; Follesa et al., 2005). The abundance of both γ2 subunit mRNAs (γ2L and γ2S) remained decreased after ethanol withdrawal (Follesa et al., 2003), whereas that of α2, α4, and α5 subunit was increased (Follesa et al., 2003; Follesa et al., 2004; Follesa et al., 2006). In hippocampal neurons, the abundance of α1 and γ2 subunit mRNAs remained decreased relative to control values after ethanol withdrawal, whereas that of the α5 subunit mRNA remained unchanged (Sanna et al., 2003). In contrast, the amounts of the α2, α3, and α4 subunit mRNAs as well as that of the α4 subunit peptide were markedly increased, relative to control values, in response to ethanol withdrawal (Sanna et al., 2003). The abundance of the δ subunit mRNA and peptide remained significantly increased (Follesa et al., 2005). In cortical neurons, ethanol withdrawal resulted in a return of the abundance of α1, α2, and γ2 subunit mRNAs to control levels whereas that of α4, β2, and β3 subunit mRNAs remained unaffected (Sheela Rani & Ticku, 2006). It should be pointed out, however, that the described effects of ethanol withdrawal on GABAA receptor gene expression in cortical neurons were measured 5 days after the onset of withdrawal whereas those in cerebellar granule cells and hippocampal neurons were measured after 3 to 6 hours. We showed that the effects of ethanol withdrawal on GABAA receptor gene expression were no longer apparent in cerebellar granule cells and hippocampal neurons as early as 24 hours after the onset of withdrawal (Follesa et al., 2003; Sanna et al., 2003; Follesa et al., 2004; Follesa et al., 2005).
These changes in the expression of specific GABAA receptor subunit genes might be expected to contribute to the development of tolerance to and dependence on ethanol as well as to the central hyperexcitability that follows abrupt discontinuation of prolonged exposure to this drug (Faingold et al., 1998; Grobin et al., 1998). This hypothesis is based in part on the fact that changes in the subunit composition of GABAA receptors have pronounced effects on their physiological and pharmacological properties (Barnard et al., 1998; Hevers & Luddens, 1998; Sieghart, 1995) and thus might be responsible for the reduced receptor function and altered pharmacological and behavioral sensitivity characteristic of ethanol tolerance and dependence. Given the diversity and heterogeneity of GABAA receptors expressed in different neuronal cell types, it is not surprising that the ethanol-dependent changes in the expression of individual subunit genes differ among specific neuronal populations.
4.4. Changes in GABAA receptor function induced by chronic ethanol exposure or withdrawal
Given that the pharmacology of benzodiazepine receptor ligands depends on the subunit composition of the GABAA receptor, especially with regard to the α and γ subunits (Pritchett et al., 1989; Barnard et al., 1998), it is important to examine the effects of the changes in receptor subunit composition induced by chronic exposure to and withdrawal of ethanol on GABAA receptor function and pharmacology. Patch-clamp electrophysiological recording from single neurons in culture allows dissection of the functional properties of GABAA receptors as well as discrimination among receptors containing different α subunits through evaluation of the effects of various modulators. Zaleplon, a selective ligand for receptors containing the α1 subunit, and the competitive benzodiazepine receptor antagonist flumazenil have thus been used to evaluate the function of GABAA receptors (Table 2). In addition, given that receptors containing the δ subunit (α4β3δ receptors) manifest a greater sensitivity to the partial agonist THIP (4,5,6,7-tetrahydroisoxazolo-pyridin-3-ol, or gaboxadol) than do those containing the γ2 subunit (α4β3γ2 receptors) (Adkins et al., 2001; Brown et al., 2002), we used this compound to evoke GABAA receptor-mediated Cl− currents and to detect changes in δ subunit–containing receptors (Table 2).
Table 2.
Effects of ethanol withdrawal on GABAA receptors: correlation between changes in gene expression and modulation of receptor function with selective ligands and differences between neuronal cell types.
| GABAA receptor subunit | Gene Expression | Modulation of receptor function by: |
|---|---|---|
| α1 | Zaleplon (α1 selective) | |
| ↓Cerebellar granule cells | ↓Cerebellar granule cells | |
| ↓Hippocampal neurons | ↓Hippocampal neurons | |
| α4 | Flumazenil (agonist at α4) | |
| ↑Cerebellar granule cells | ↑Cerebellar granule cells | |
| ↑Hippocampal neurons | ↑Hippocampal neurons | |
| δ | 3α,5α-THP (δ-preferentially acting steroid) | |
| ↓Cerebellar granule cells | ↓Cerebellar granule cells | |
| ↑Hippocampal neurons | ↑Hippocampal neurons | |
Direction of arrows indicates increases or decreases in subunit gene expression (of both mRNA and corresponding peptide) and receptor function measured with the subunit specific receptor modulator as indicated. Data are derived with permission from previous studies (Follesa et al., 2003; Sanna et al., 2003; Follesa et al., 2004; Follesa et al., 2005; Follesa et al., 2006).
4.4.1. Modulation of α4 subunit–containing GABAA receptors by flumazenil
The presence of the α4 or α6 subunits in recombinant GABAA receptors is associated with a reduced sensitivity to classical benzodiazepine agonists and to zolpidem as well as with a distinct pattern of regulation (positive rather than no allosteric modulation) by flumazenil (Wafford et al., 1996). With the use of the patch-clamp technique, we therefore examined the effects of flumazenil on cultured cerebellar granule and hippocampal neurons subjected to chronic treatment with and subsequent withdrawal of ethanol. The modulatory action of flumazenil in cerebellar granule cells subjected to chronic ethanol treatment was similar to that apparent in control cells (Follesa et al., 2003). Likewise and consistent with its pharmacological profile (antagonist devoid of intrinsic activity), flumazenil did not significantly affect GABA-evoked Cl− currents in hippocampal neurons subjected to long-term treatment with ethanol or in control cells (Sanna et al., 2003). In contrast, in granule cells or hippocampal neurons subjected to ethanol withdrawal, flumazenil markedly potentiated GABA-evoked Cl− currents (Table 2). These results are thus consistent with the observations that ethanol withdrawal induced up-regulation of α4 subunit gene expression in both cerebellar granule cells and hippocampal neurons (Table 2). An increase in α4 subunit gene expression is also induced by withdrawal of benzodiazepine receptor ligands or of neuroactive steroids both in vivo and in vitro (Holt et al., 1996; Holt et al., 1997; Smith et al., 1998b; Follesa et al., 2000; Follesa et al., 2001; Follesa et al., 2002), suggesting that up-regulation of α4 subunit expression might play an important role in the cellular hyperexcitability and anxiety-like behavior apparent in both animals and humans during withdrawal of these positive allosteric modulators of the GABAA receptor. Consistent with this notion, depletion of the α4 subunit with the use of antisense RNA prevented the development of withdrawal symptoms in a rat progesterone withdrawal paradigm (Smith et al., 1998a).
4.4.2. Modulation of α1 subunit–containing GABAA receptors by zaleplon
Zaleplon at low concentrations in vitro binds selectively to GABAA receptors containing the α1 subunit (Sanna et al., 2002). This agent can thus be used to discriminate between receptors containing the α1 subunit and those containing other α subunits. Zaleplon at a low concentration potentiated GABA-evoked Cl− currents to similar extents in cerebellar granule cells subjected to chronic ethanol treatment and in control cells (Follesa et al., 2006). In contrast, this effect was reduced by about one-half in cerebellar granule cells subjected to ethanol withdrawal. These results are thus consistent with the lack of effect of chronic ethanol treatment on the abundance of the α1 subunit and with the decrease in its abundance induced by ethanol withdrawal in cerebellar granule cells (Table 2). The potentiating effect of zaleplon on GABAA receptor function was reduced by ∼50% in hippocampal neurons subjected to chronic ethanol treatment or to withdrawal, compared with that apparent in control neurons (Sanna et al., 2003). These data are consistent with the decrease in the amount of the α1 subunit apparent in hippocampal neurons subjected to long-term ethanol treatment or ethanol withdrawal (Table 2).
The action of benzodiazepine receptor ligands is dependent on the specific α and γ subunit isoforms present in GABAA receptors (Barnard et al., 1998; Pritchett et al., 1989; Whiting et al., 1999). The reduced abundance of both the α1 and γ2 subunit mRNAs in both cerebellar granule cells and hippocampal neurons subjected to ethanol withdrawal may thus actually underlie the associated functional uncoupling between the neurotransmitter binding site and the modulatory benzodiazepine recognition site of the GABAA receptor. Given that GABAA receptor subtypes containing α1 or α2 subunits mediate the sedative and anxiolytic effects of benzodiazepines, respectively (Mohler et al., 2002), the effects of ethanol on GABAA receptor gene expression and function observed in cultured neurons, if will be observed also in vivo, could be related to the reduced sedative efficacy of benzodiazepines in human alcoholics as well as to their low efficacy in preventing the anxiogenic effect of ethanol withdrawal (Sellers et al., 1983; Lejoyeux et al., 1998). With the same in vitro model systems, we have shown that the changes in GABAA receptor gene expression induced by ethanol withdrawal are similar to those induced by withdrawal either of benzodiazepines (Follesa et al., 2001), imidazopyridines or pyrazolopyrimidines (Follesa et al., 2002), or neuroactive steroids (Yu et al., 1996; Follesa et al., 2000; Mascia et al., 2002), suggesting the existence of a common molecular mechanism by which positive modulators, including ethanol, trigger changes in receptor function that might account for the development of withdrawal symptoms in vivo.
4.4.3. Modulation of δ subunit–containing GABAA receptors by THIP or 3α,5α-THP
Given that GABAA receptors that contain the δ subunit manifest a greater sensitivity to the partial agonist THIP than do those containing the γ2 subunit (Adkins et al., 2001; Brown et al., 2002), the use of this compound to evoke GABAA receptor-mediated Cl− currents in cultured neurons would be expected to reveal changes in receptor function attributable to changes in the abundance of the δ subunit induced by chronic ethanol treatment or withdrawal. In cerebellar granule cells, chronic ethanol treatment did not significantly affect THIP potency compared with that apparent in control cells (Follesa et al., 2005). However, withdrawal of ethanol resulted in a significant decrease in THIP potency. These results are thus consistent both with the lack of effect of chronic ethanol exposure on the abundance of the δ subunit mRNA and peptide and with the decrease in the amount of this subunit induced by ethanol withdrawal in cerebellar granule neurons (Follesa et al., 2005). In hippocampal neurons, chronic exposure to ethanol induced a significant increase in the potency of THIP compared with that apparent in control cells (Follesa et al., 2005). Moreover, withdrawal of ethanol was accompanied by only a small (nonsignificant) decrease in THIP potency compared with that observed in cells chronically exposed to ethanol. Again, these results are consistent with the increase in the abundance of the δ subunit mRNA and peptide that is apparent in hippocampal neurons subjected to long-term ethanol treatment or withdrawal (Follesa et al., 2005).
The modulatory effect of several neuroactive steroids on GABAA receptor function is also markedly enhanced by the presence of the δ subunit (Adkins et al., 2001; Brown et al., 2002; Wohlfarth et al., 2002). This modulatory action is thus impaired in mice that lack the δ subunit (Mihalek et al., 1999; Spigelman et al., 2003). The changes in δ subunit gene expression elicited by chronic ethanol exposure and ethanol withdrawal in cerebellar granule cells and hippocampal neurons were associated with parallel changes in the effect of 3α,5α-THP on THIP-evoked Cl− currents. In cerebellar granule neurons, chronic ethanol exposure thus did not alter the modulatory effect of 3α,5α-THP whereas withdrawal of ethanol was associated with a significant decrease in 3α,5α-THP efficacy (Table 2). In contrast, the modulatory effect of 3α,5α-THP was significantly increased in hippocampal neurons subjected to chronic ethanol treatment or ethanol withdrawal (Table 2) compared with that apparent in control cells. Together, the results obtained with THIP and 3α,5α-THP suggest that the changes in the expression of the δ subunit gene induced by ethanol treatment and withdrawal are accompanied by corresponding changes in the functional properties of GABAA receptors in the two neuronal populations examined (Table 2).
4.5. Differential effects of ethanol and progesterone on δ subunit–containing GABAA receptors in cerebellar granule cells and hippocampal neurons in culture
Extrasynaptic GABAergic neurotransmission in the cerebellum, hippocampus, and thalamus is mediated in large part by GABAA receptors that contain the δ subunit (Nusser et al., 1998; Nusser & Mody, 2002; Wei et al., 2003). These receptor subtypes, which possess distinct pharmacological properties (Saxena & Macdonald, 1994; Hevers et al., 2000; Mody, 2001), are thus considered the major extrasynaptic targets of GABAA receptor modulators. Although other receptor subtypes may contribute to extrasynaptic inhibition in discrete brain regions (Brunig et al., 2002; Lindquist et al., 2003), those containing the δ subunit are of particular interest because of their ability to mediate the actions of neuroactive steroids and, possibly, those of low concentrations of ethanol (Mihalek et al., 1999; Belelli et al., 2002; Sundstrom-Poromaa et al., 2002; Spigelman et al., 2003; Wallner et al., 2003). The subunit composition of the GABAA receptor may thus determine both its pharmacological properties and subcellular localization (Nusser et al., 1998; Wei et al., 2003; Follesa et al., 2005).
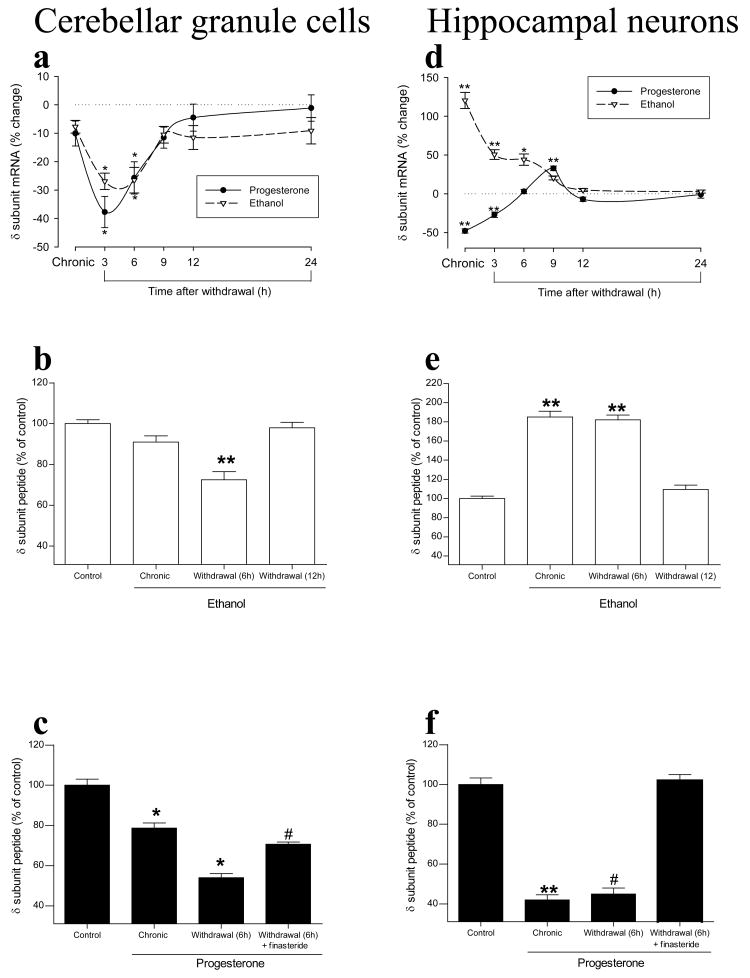
Given that δ subunit–containing GABAA receptors responsible for tonic inhibition are preferential targets for neuroactive steroids (Stell et al., 2003) and possibly for ethanol (Wallner et al., 2003), chronic exposure to or withdrawal of ethanol might be expected to induce plastic changes in δ subunit–containing receptors similar to those elicited by neuroactive steroids. Our studies with cerebellar granule cells (Follesa et al., 2005; Biggio et al., 2006) and hippocampal neurons (Follesa et al., 2005; Mostallino et al., 2006) in culture, however, showed that ethanol and neuroactive steroids have similar effects in cerebellar granule cells but opposite effects in hippocampal neurons. Chronic exposure of cerebellar granule cells to either ethanol or progesterone had no significant effect on the abundance of the δ subunit mRNA (Fig. 2a), whereas withdrawal of either modulator elicited a marked and time-dependent decrease in the amount of this mRNA (Fig. 2a) and corresponding peptide (Fig. 2b, c). In contrast, chronic exposure of hippocampal neurons to ethanol resulted in a marked increase in the abundance of the δ subunit mRNA and peptide (Fig. 2d, e) whereas chronic progesterone treatment induced a pronounced decrease (Fig. 2d, f). Ethanol withdrawal resulted in a gradual return of the increased level of the δ subunit to control values (Fig. 2d, e). On the other hand, progesterone withdrawal resulted in a gradual return of the reduced level of the δ subunit mRNA to control values (Fig. 2d) while the correspondin peptide was still low (Fig. 2f).
Figure 2.
Effects of prolonged exposure to and subsequent withdrawal of ethanol or progesterone on the abundance of the δ subunit, mRNA and peptide, of the GABAA receptor in rat cerebellar granule cells or hippocampal neurons in culture. Cells were incubated first for 5 days with 100 mM ethanol or 1 μM progesterone (chronic) and then for the indicated times in drug-free medium (withdrawal), after which the amount of the δ subunit mRNA was determined with an RNase protection assay and the peptide was measured by immunofluorescence analysis with antibodies to the δ subunit and with a confocal microscope. Data are expressed as percentage change relative to control for mRNA and as a percentage of the fluorescence intensity of control cell for the peptide. *P < 0.05, **P < 0.001 versus control cells; #P < 0.001 versus progesterone withdrawal (ANOVA followed by Scheffè's post hoc test). Modified with permission from Follesa et al. (2005), Biggio et al. (2006), and Mostallino et al. (2006).
The effects of progesterone on the abundance of the δ subunit in both cerebellar granule cells and hippocampal neurons were abolished by concomitant treatment with finasteride (Fig. 2e, f) and were identical to those of 3α,5α-THP or 3α,5α-THDOC (Biggio et al., 2006; Mostallino et al., 2006), consistent with the finding that neurons in culture express the enzymatic machinery necessary for the conversion of progesterone to the neuroactive steroids 3α,5α-THP and 3α,5α-THDOC (Follesa et al., 2000).
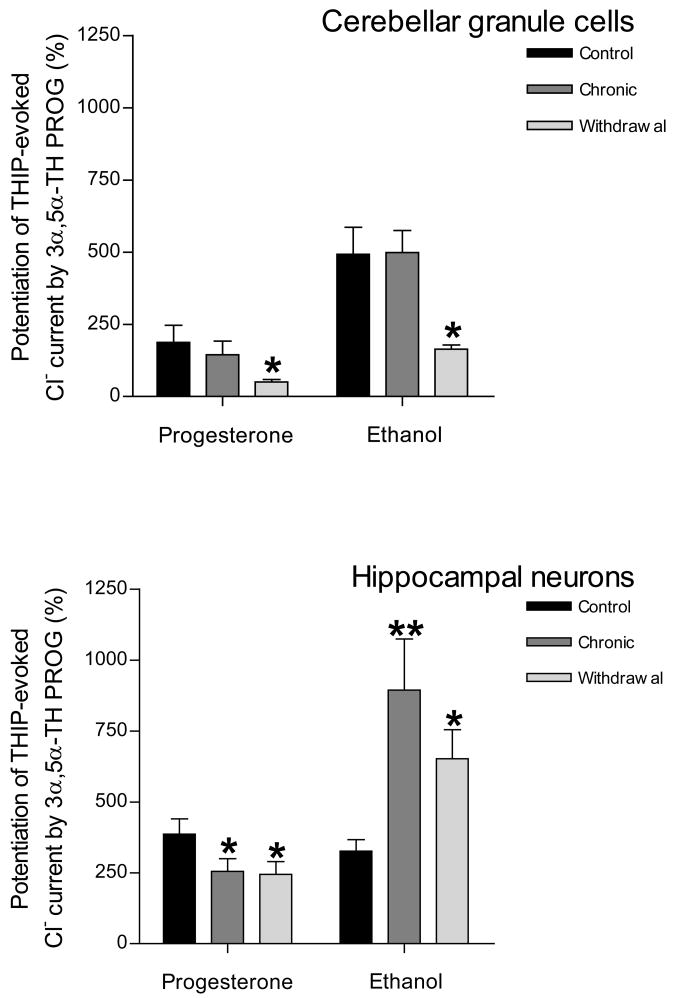
The differential changes in the expression of the δ subunit gene induced by chronic exposure to and withdrawal of ethanol or progesterone in cerebellar granule cells and hippocampal neurons were also accompanied by parallel changes in GABAA receptor function. Measurement of THIP-evoked Cl− currents in cerebellar granule cells revealed that chronic exposure to ethanol or progesterone did not have a significant effect on current modulation by 3α,5α-THP (Fig. 3). However, withdrawal of ethanol or progesterone resulted in a significant decrease in the efficacy of 3α,5α-THP, consistent with the down-regulation of δ subunit expression induced by withdrawal of either of these two agents. Chronic exposure of hippocampal neurons to progesterone resulted in a significant decrease in the modulatory effect of 3α,5α-THP on THIP-evoked Cl− current, compared with that apparent in control cells, and this reduced efficacy of 3α,5α-THP was still apparent after progesterone withdrawal (Fig. 3). In contrast, chronic exposure of hippocampal neurons to ethanol resulted in a significant increase in the efficacy of 3α,5α-THP and this effect remained apparent after ethanol withdrawal.
Figure 3.
Effects of chronic exposure to and subsequent withdrawal of progesterone or ethanol on the modulation of THIP-evoked Cl− currents by 3α,5α-THP in rat cerebellar granule cells or hippocampal neurons in culture. Cells were incubated first for 5 days with 100 mM ethanol or 1 μM progesterone (chronic) and then for 6 hours in drug-free medium (withdrawal), after which Cl− currents evoked by THIP (EC10) were measured in the absence or presence of 1 μM 3α,5α-THP. Data are expressed as percentage potentiation of the THIP response by 3α,5α-THP. *P < 0.05, **P < 0.01 versus control cells (ANOVA followed by Scheffè's post hoc test). Modified with permission from Follesa et al. (2005), Biggio et al. (2006), and Mostallino et al. (2006).
These various observations demonstrating opposite effects of progesterone and ethanol in hippocampal neurons but not in cerebellar granule cells suggest that, even though different GABAA receptor subtypes containing the δ subunit exhibit similar functional properties (Brown et al., 2002) as well as a sensitivity to ethanol (Wallner et al., 2003), different subunit compositions of δ subunit–containing extrasynaptic receptors, such as those expressed in hippocampal neurons and cerebellar granule cells, may confer distinct properties and explain the different plastic changes induced by ethanol or progesterone within or between these two neuronal cell populations.
5. Pregnancy: an in vivo model of long-term exposure to progesterone
Pregnancy is associated with marked changes in the hormonal milieu. Progesterone reaches its highest levels in a woman's life during pregnancy, and the concentrations of other steroids are also markedly increased. The serum levels of 3α,5α-THP and 3α,5α-THDOC are increased in pregnant women as a result of the high concentrations of their precursors, progesterone and deoxycorticosterone, respectively (Luisi et al., 2000; Pearson Murphy et al., 2001, Paoletti et al., 2006). In addition, the activities of the enzymes responsible for the synthesis of 3α,5α-THP and 3α,5α-THDOC are increased in both maternal (especially the placenta) and fetal tissue (Milewich et al., 1979; Buster, 1983). We have previously shown that the concentrations of these neuroactive steroids are also increased in the brain and plasma of rats during pregnancy, albeit with different time courses (Concas et al., 1998; Concas et al., 1999; Biggio et al., 2000; Biggio et al., 2001). The plasma concentrations of pregnenolone, progesterone, 3α,5α-THP, and 3α,5α-THDOC peak on days 15 or 19 of pregnancy, return to control values immediately before delivery (day 21), and then remain unchanged for 2 days after delivery. Whereas progesterone concentrations in both the brain and plasma are maximal on day 15 (∼12 and 10 times the values for estrus, respectively) and remain substantially increased on day 19, the cerebrocortical concentrations of pregnenolone, 3α,5α-THP, and 3α,5α-THDOC do not peak until day 19 of pregnancy, suggesting that the synthesis of 3α,5α-THP and 3α,5α-THDOC in the brain is not simply a function of the plasma concentration of progesterone. One possible explanation for this difference in kinetics is that steroid hormones such as estrogens or other agents may regulate the activity or expression of 5α-reductase or 3α-hydroxysteroid dehydrogenase in the brain during pregnancy. Indeed, estradiol has been shown to regulate 3α-hydroxysteroid dehydrogenase activity in rat brain (Penning et al., 1985).
Given that 3α,5α-THP and 3α,5α-THDOC each positively modulate GABAA receptors (Majewska, 1992; Lambert et al., 1995), several studies have been undertaken to determine whether the physiological fluctuations in the concentrations of these neuroactive steroids that occur during pregnancy and after delivery affect the plasticity and function of GABAA receptors in various regions of the brain. Measurements of [3H]GABA, [3H]flunitrazepam, and [35S]TBPS binding to cerebrocortical membranes revealed a progressive increase in the density of recognition sites associated with the GABAA receptor complex during pregnancy in rats (Concas et al., 1999). The maximal increase in receptor density was apparent on day 19 of pregnancy and was followed by a marked decrease to control values immediately before delivery (day 21). The density of the three binding sites was further decreased after delivery before returning to control values by 7 days after delivery. These data are consistent with changes in [3H]muscimol binding in rat forebrain during pregnancy and in the postpartum period (Majewska et al., 1989), but they differ from other results showing no modification of GABAA and central benzodiazepine receptors in the cerebral cortex during pregnancy (Weizman et al., 1997).
In addition, measurement of muscimol-stimulated 36Cl− uptake by cerebrocortical membrane vesicles revealed that the sensitivity of the GABAA receptor–associated Cl− channel to the action of this GABA agonist was reduced during the last week of pregnancy, whereas it was markedly potentiated 2 days after delivery (Concas et al., 1998; Concas et al., 1999). The reduced sensitivity of the Cl− channel to the action of muscimol during pregnancy is thus suggestive of a reduced activity of this channel in vivo. Consistent with these results, the abilities of diazepam and 3α,5α-THP to potentiate the effect of muscimol on 36Cl− uptake were decreased during pregnancy and increased after delivery (Follesa et al., 1998).
The temporal changes in the density and function of GABAA receptors during pregnancy and after delivery are similar to those in the concentrations of 3α,5α-THP and 3α,5α-THDOC. The cerebrocortical concentrations of these neuroactive steroids peak on day 19 of pregnancy, coincident with the maximal increase in the density of GABA, benzodiazepine, and TBPS recognition sites and the maximal reduction in Cl− channel function. Furthermore, the normalization of the plasma and cerebrocortical concentrations of neuroactive steroids that precedes delivery (day 21 of pregnancy) is paralleled by a return of the density and function of GABAA receptors to control values.
These observations suggested that neuroactive steroids in the cerebral cortex might play a role in the modulation of GABAA receptor density and activity during pregnancy and after delivery. This notion was further supported by the observations that administration of the specific 5α-reductase inhibitor finasteride to pregnant rats from day 12 to day 18 of pregnancy not only resulted in a marked reduction in the extent of the increases in the plasma and cerebrocortical concentrations of 3α,5α-THP and 3α,5α-THDOC normally apparent on day 19 of pregnancy (Concas et al., 1998; Concas et al., 1999; Biggio et al., 2000) but also prevented both the increases in the density and dissociation constant of [3H]flunitrazepam and [35S]TBPS binding sites as well as the decrease in the stimulatory effect of muscimol on 36Cl− uptake normally observed in the cerebral cortex (Concas et al., 1998; Concas et al., 1999).
These results demonstrated that the pregnancy-induced changes in the density and sensitivity of GABAA receptors are functionally related to the increases in the concentrations of 3α,5α-THP and 3α,5α-THDOC associated with this condition. Consistent with this conclusion, long term-treatment of rats wiTHPesterone induces up-regulation of GABA and benzodiazepine binding sites in specific brain regions (Gavish et al., 1987; Canonaco et al., 1989). Furthermore, steroid hormone deprivation by ovariectomy or adrenalectomy (or both) results in a decrease in GABAA receptor density in rat brain (Jussofie et al., 1995). The increase in the brain concentrations of neuroactive steroids during the first 19 days of pregnancy can thus be considered comparable to the long-term administration of high doses of these compounds, whereas the marked decrease in the concentrations of progesterone and its metabolites apparent at the end of pregnancy and after delivery is similar to a sudden discontinuation of such treatment.
The pregnancy-induced changes in the density and function of GABAA receptors are accompanied by changes in the expression of GABAA receptor subunit genes (Concas et al., 1998; Follesa et al., 1998). In particular, the amounts of the γ2L subunit mRNA and protein in rat cerebral cortex and hippocampus decrease progressively during pregnancy before returning to control values immediately before delivery (Follesa et al., 1998). Given that the γ2 subunit is required for benzodiazepine sensitivity of GABAA receptors (Pritchett et al., 1989), these molecular changes might be expected to affect the pharmacology of GABAA receptor–mediated neurotransmission. Indeed, diazepam fails to induce sedation or loss of the righting reflex in mice with a targeted disruption of the γ2 subunit gene (Günther et al., 1995). Moreover, the absence of the γ2 subunit in such mice results in a substantial impairment in the ability of GABAA receptors to form postsynaptic clusters and in a consequent marked reduction in receptor function (Essrich et al., 1998). Such a deficit might therefore explain the reduction in the activity of the GABAA receptor–associated Cl− channel as well as the reduced ability of diazepam to potentiate GABA-induced 36Cl− uptake observed during pregnancy (Concas et al., 1998; Follesa et al., 1998).
The amount of the mRNA for the α5 subunit of the GABAA receptor was also found to decrease in the cerebral cortex during pregnancy, with the maximal reduction apparent on day 21, before returning to control values after delivery (Follesa et al., 1998). Given that GABAA receptors containing the α5 subunit seems to be involved in the regulation of the acquisition and expression of memory (Crestani et al., 2002), the change in the abundance of the α5 subunit mRNA during pregnancy might be related to the memory disturbance apparent during this time (Brett and Baxendale, 2001). Moreover, our recent immunohistochemistry results (Mostallino et al., in preparation) have shown that the abundance of the α4 subunit peptide is increased in the hippocampus 2 days after delivery, consistent with the observations that withdrawal of 3α,5α-THP, either as a result of termination of intermittent administration of progesterone (Smith et al., 1998a) or in a model of pseudopregnancy (Smith et al., 1998b), results in an increase in the amounts of both the α4 subunit mRNA and peptide in the rat hippocampus. Moreover, progesterone withdrawal has been shown to increase expression of the α4 subunit in the amygdala (Guliniello et al., 2003). These various observations suggest that the time-dependent changes in the abundance of the γ2L, α5 and α4 subunit mRNAs and peptides associated with pregnancy and delivery are region specific. Only specific neurons containing specific populations of GABAA receptors may thus contribute to the changes in GABAA receptor function and expression observed during pregnancy and after delivery. Consistent with this notion, the abundance of the α1 subunit mRNA was found to increase during pregnancy before decreasing during the last 2 days prior to birth specifically in oxytocin neurons present within the supraoptic nucleus of the hypothalamus (Fenelon & Herbison, 1996). This observation, together with evidence that GABAA receptors in the supraoptic nucleus are more sensitive to the action of 3α,5α-THP during late pregnancy than at parturition (Brussaard et al., 1997), suggests that 3α,5α-THP modulation of GABAA receptor function in these oxytocin neurons represents an important physiological feedback mechanism through which progesterone represses the activity of these neurons in late pregnancy to prevent premature delivery (Herbison, 2001). Recent data suggest that functional synaptic diversity at the postsynaptic level in neurons of the supraoptic nucleus during pregnancy is correlated with a differential clustering of distinct GABAA receptor subtypes at individual synapses rather than with an increase in the expression level of the α1 subunit (Koksma et al., 2005).
Our studies have thus shown that fluctuations in the concentrations of 3α,5α-THP and 3α,5α-THDOC during pregnancy and after delivery are temporally correlated with changes in the expression of genes for the γ2 and α4 subunits of the GABAA receptor. This conclusion is supported by the observation that treatment of dams from day 12 to day 18 of pregnancy with finasteride prevented the decrease in the abundance of the γ2L subunit mRNA that is normally apparent in both the cerebral cortex and hippocampus on day 19 of pregnancy (Concas et al., 1998; Biggio et al., 2001). These data further support the existence of a functional correlation between changes in the cerebrocortical concentrations of neuroactive steroids and those in GABAA receptor subunit composition and activity during pregnancy and after delivery. The changes in GABAA receptors may thus reflect a plastic adaptation both to counteract the increased stimulation of GABAA receptors by neuroactive steroids during pregnancy and to compensate for the reduced inhibitory tone when neuroactive steroid concentrations are decreased immediately before and after delivery.
Given that GABAA receptors are implicated in a variety of neuropsychophysiologic phenomena, including anxiety, sleep, seizures, and depression, the available evidence suggests that fluctuations in the concentrations of neuroactive steroids that act at these receptors contribute, at least in part, to the cognitive and psychiatric changes associated with pregnancy and the postpartum period. Indeed, pregnancy in humans is characterized by pronounced alterations in mood and emotional state, including somnolence, feelings of elation, memory impairment, and elevation of nociceptive response thresholds, whereas puerperium is often associated with increased anxiety and depressive disorders (Russell et al., 2001). Moreover, neuroendocrine adaptations, including attenuation of the responsiveness of the HPA axis to a variety of external stimuli, are associated with pregnancy and lactation (Douglas et al., 2003). Exposure of mice to stressors during late pregnancy delays the onset of birth (Newton et al., 1968) and impairs the protective behavior of the dam (Pardon et al., 2000). Attenuation of CRF, adrenocorticotropic hormone (ACTH), and corticosterone secretory responses to a variety of stressors during pregnancy and lactation in the rat has been proposed as a means of minimizing exposure of the fetus and neonate to glucocorticoids in the peripartum period (McCormick et al., 1995; Johnstone et al., 2000). Given the important role of GABAergic transmission in regulation of HPA axis responsiveness to stress (Calogero et al., 1998), the marked increases in the concentrations of neuroactive steroids in plasma and the brain during pregnancy may contribute to tonic inhibition of the HPA axis mediated by GABA, whereas the rapid and substantial decreases in the concentrations of these compounds apparent immediately before delivery may confer predisposition to postpartum depression (Carter et al., 2001).
6. Chronic stress, neuroactive steroids, and GABAA receptor plasticity and function
In spite of evidence that chronic stress, as a consequence of dysregulation of the HPA axis, can induce maladaptive changes that have been postulated to contribute to mental disorders (McEwen & Wingfield, 2003; de Kloet et al., 2005), few studies have examined the effects of prolonged stress on the levels of neuroactive steroids and on GABAA receptor plasticity and function. One well-characterized animal model of chronic stress is social isolation in rats. Rats deprived of social contact with other rats at a young age experience a form of prolonged stress that leads to long-lasting changes in their behavioral profile (Einon & Morgan, 1977; Hall et al., 1998). The isolated rodents are thus aggressive, neophobic, and highly reactive to human handling. They display aggressive behavior—in particular, biting and boxing—when transferred to groups of animals (Wongwitdecha & Marsden, 1996), and they manifest increased exploratory and locomotor activity in response to novel situations (Hilakivi et al., 1989; Varty et al., 2000). Furthermore, in the elevated plus-maze test, light-dark test, and exploratory head-dipping test, social isolation is associated with high levels of fearlike behavior (Parker & Morinan, 1986; Hilakivi et al., 1989; Voikar et al., 2005). Social isolation also markedly increases the hyponeophagic response of rats and reduces the punished consumption of water in the Vogel conflict test (Parker & Morinan, 1986; Serra et al., 2000). This stress paradigm is thus thought to be anxiogenic for these normally gregarious animals, and their abnormal reactivity to environmental stimuli when reared under this condition is thought to be a product of prolonged stress.
6.1. Neuroactive steroids
Exposure of mice to chronic intermittent stress by daily restraint for 1-hour intervals induced repetitive and transient increases in the cerebrocortical concentrations of 3α,5α-THP and 3α,5α-THDOC, which returned to control values by 30 min after each exposure (Mele et al., 2004). This effect likely represents the expression of daily acute stress rather that an adaptive response to chronic stress. In contrast, mild chronic stress due to social isolation of rats for 30 days immediately after weaning, in the absence of any additional stressor, induced marked reductions in the basal cerebrocortical and plasma concentrations of progesterone (25 and 61%, respectively), 3α,5α-THP (42 and 34%, respectively), and 3α,5α-THDOC (39 and 37%, respectively), compared with the corresponding values for group-housed animals (Serra et al., 2000). In mice, social isolation also reduced the brain content of 3α,5α-THP and its precursor 3α-dihydroprogesterone but did not affect that of progesterone or pregnenolone (Matsumoto et al., 1999; Dong et al., 2001). The effects of social isolation on the activity or expression of enzymes involved in the synthesis of certain neuroactive steroids may thus be species specific.
The molecular mechanisms that underlie the persistent decrease in the abundance of neuroactive steroids induced by social isolation in rats remain unclear. Given that adrenal steroidogenesis plays an important role in maintaining the concentrations of neuroactive steroids in both plasma and the brain, as revealed by the observation that adrenalectomy results in a marked reduction in these concentrations (Purdy et al., 1991; Barbaccia et al., 1997), a reduced activity of the HPA axis may be responsible for the down-regulation of these steroids apparent in isolated animals. Changes in the activity of the HPA axis are associated with various types of chronic stress, including isolation rearing. However, the effects of social isolation on the HPA axis in rats are not consistent among studies, with differences in the duration of isolation or in animal age at its onset possibly accounting for the increase (Gamallo et al., 1986), no change (Holson et al., 1991), or decrease (Mar Sánchez et al., 1998) in HPA axis function described in these studies.
We found that the basal plasma concentration of ACTH in isolated rats (1023 ± 148 pg/ml, mean ± SEM) was decreased compared with that in group-housed animals (1495 ± 210 pg/ml) (Serra et al., 2004). A decrease in the plasma level of ACTH, despite the continuous presence of the stressor, has also been described for animals exposed to various chronic stressful stimuli, and several mechanisms for this effect, in addition to a reduction in pituitary responsiveness to modulators of ACTH secretion (CRF, arginine vasopressin), have been proposed (Keller-Wood & Dallman, 1984; Rivier & Vale, 1987; Hauger et al., 1988). Rivier and Vale (1987) suggested that both a decrease in the readily releasable pool of ACTH and the negative feedback exerted by corticosterone may account for the diminished responsiveness of the HPA axis of rats exposed to chronic intermittent electroshock. However, the relatively small difference in the plasma concentration of ACTH detected in our study (Serra et al., 2004), although significant (P < 0.05), is likely not entirely responsible for the decrease in the basal cerebrocortical and plasma concentrations of progesterone, 3α,5α-THP, and 3α,5α-THDOC induced by social isolation.
The biosynthesis of steroids begins with the conversion of the precursor cholesterol to pregnenolone, the rate-limiting step of which is the transport of cholesterol from cellular stores across the intermembrane space of mitochondria to the inner mitochondrial membrane. Steroidogenic acute regulatory protein (StAR) and the peripheral-type benzodiazepine receptor (PBR) function in a coordinated manner to mediate this transport of cholesterol (Krueger & Papadopoulos, 1990; Stocco, 2000; Casellas et al., 2002; Hauet et al., 2005). The 37-kDa precursor form of StAR is produced in the cytoplasm and is converted to a 32-kDa form during its incorporation into the inner mitochondrial membrane (Lin et al., 1995). We have found that the amounts of both StAR mRNA and the two forms of the protein (37 and 32 kDa) in the cerebral cortex or hippocampus did not differ between isolated and group-housed rats under basal conditions (Mostallino MC, Serra M, unpublished data), moreover, kinetic analysis of the binding of the specific PBR ligand [3H]PK 11195 to membranes prepared from the cerebral cortex, adrenal gland, or testis of rats revealed that social isolation induced an increase in receptor density (Bmax) in each tissue (cerebral cortex, + 16%; adrenal gland, + 30%; testis, + 24%), although these effects did not achieve statistical significance, and that the binding affinity (dissociation constant, or Kd) was unaffected (Serra et al., 2004) These results suggest that changes in the expression of StAR or PBRs are not responsible for the reduction in the basal levels of neuroactive steroids observed after social isolation.
However, the sensitivity of PBRs in isolated rats is increased, as demonstrated by the observation that a single injection of the PBR agonist CB34—a 2-phenyl-imidazo[1,2-a]pyridine derivative that possesses a high affinity for PBRs (Trapani et al., 1997) and markedly stimulates the synthesis of neuroactive steroids in the brain of nonstressed rats (Serra et al., 1999)—increased the concentrations of progesterone, 3α,5α-THP, and 3α,5α-THDOC in both the cerebral cortex and plasma of socially isolated rats to a greater extent than it did in those of group-housed animals (Serra et al., 2004). Such a change in the sensitivity of PBRs may result from an alteration in the synthesis or release of diazepam binding inhibitor (DBI), an endogenous ligand for these receptors. The abundance of DBI mRNA has thus been shown to be reduced in the brain of socially isolated mice (Dong et al., 1999). Given that DBI stimulates the production of neuroactive steroids (Papadopoulos, et al., 1997), down-regulation of the expression of DBI may contribute to the reduced basal levels of these compunds apparent after social isolation. In addition, the persistent decrease in the expression of this endogenous ligand may account for the increased sensitivity of PBRs to CB34 in isolated rats, consistent with the notion that a reduction in ligand concentration results in supersensitivity of the corresponding receptor. The observation that the steroidogenic response to injection of CB34 was enhanced in socially isolated rats indicates that, despite the decline in basal plasma and cerebrocortical concentrations of neuroactive steroids, full secretory capacity is maintained in these animals. This conclusion is in agreement with observations on the effects of a novel acute stressor on the cerebrocortical and plasma concentrations of neuroactive steroids in socially isolated rats.
Chronic stress induces both functional and structural adaptations within the HPA axis that are suggestive of long-term alterations in neuroendocrine reactivity to subsequent stressors. Evidence suggests that the response of the HPA axis to new stimuli depends on the type of chronic stressor (psychogenic or systemic), given that different chronic stressors affect homeostasis differently and are regulated by distinct neurocircuits (Herman & Cullinan 1997; Herman et al., 2003). In general, repeated experience with a homotypic stressor induces habituation or diminution of the HPA axis response to this stressor (Pitman et al., 1988; Gadek-Michalska & Bugajski, 2003) but potentiates the response to a novel stressor (Akana et al., 1992; Bhatnagar & Dallman, 1998; Hauger et al., 1990).
Abnormalities in the behavioral response of isolated rats to distinct challenges have been associated with functional changes in the endocrine response, although differences in social isolation procedures or test environments among studies have led to apparently discrepant results. For example, the basal level of corticosterone in plasma was found to be either unchanged (Morinan & Leonard, 1980; Viveros et al., 1988), increased (Rivier & Vale, 1987; Serra et al., 2000; Sandstrom & Hart, 2005), or decreased (Miachon et al., 1993; Sanchez et al., 1998; Chida et al., 2005) in socially isolated animals. We found that foot shock, used as a novel acute stressor, increased the cerebrocortical and plasma concentrations of progesterone, 3α,5α-THP, and 3α,5α-THDOC by a greater percentage in isolated rats than in group-housed animals (Serra et al., 2000), revealing a hyperresponsiveness of the HPA axis in isolated rats.
Neurons in the medial parvocellular portion of the hypothalamic paraventricular nucleus integrate excitatory and inhibitory signals and behavioral responses to stress and adjust the secretion of CRF, the main stimulator of ACTH release (Axelrod & Reisine, 1984; Carrasco & Van de Kar, 2003). GABA is an important negative regulator of neuronal excitability in the paraventricular nucleus and thus modulates the amplitude and duration of the stress response (Kovacs et al., 2004). GABAergic afferents to the paraventricular nucleus originate from various regions of the forebrain (Roland & Sawchenko, 1993; Miklos & Kovacs, 2002), GABAA receptors have been detected in this nucleus (Fenelon et al., 1995; Cullinan, 2000), and GABA inhibits the release of CRF from the hypothalamus (Calogero et al., 1988). The sensitivity and function of GABAA receptors in the brain are reduced in socially isolated rats compared with those in group-housed animals (Serra et al., 2000). A reduced ability of GABA to regulate the response of the HPA axis to stress may account for the increased responsiveness of socially isolated rats to acute stress. A subcutaneous injection of isoniazid—an inhibitor of glutamic acid decarboxylase (Horton et al., 1979) that induces a transient reduction in GABA-mediated inhibitory neurotransmission and increases the brain and plasma concentrations of steroids (Barbaccia et al., 1996c; Barbaccia et al., 1997)—also increased the abundance of progesterone, 3α,5α-THP, and 3α,5α-THDOC in the cerebral cortex and plasma to a greater extent in isolated rats than in group-housed animals (Serra et al., 2003). This enhanced response of isolated rats to isoniazid might reflect the preexisting reduced level of GABAergic transmission apparent in these animals before administration of the drug. Together, these various observations indirectly suggest that, in spite of the decreased basal plasma level of ACTH, the overall secretory capacity of the pituitary is maintained in isolated rats.
6.2. GABAA receptor plasticity
The expression of specific GABAA receptor subunit genes and the consequent subunit composition of the receptor are affected by chronic stress. Exposure of rats for 3 weeks to chronic unpredictable stress resulted in a small, nonsignificant, reduction in the amount of the α1, α3, γ1 and γ2 subunit mRNA and a significant increase and decrease in the α5 and δ subunit mRNAs, respectively, in the paraventricular nucleus (Verkuyl et al., 2004). In contrast, with the same stress protocol, Qin et al. (2004) detected no change in the expression of GABAA receptor subunit genes under basal corticosteroid conditions. However, the combination of chronic unpredictable stress and injection of a high dose of corticosterone induced a significant increase in the relative expression of the α1 subunit gene in the dentate gyrus. These results suggested that changes in GABAA receptor gene expression in response to a high dose of corticosterone, similar to the effect of acute stress, may be more pronounced in animals subjected to prolonged periods of stress than in naïve animals.
The level of α4 subunit immunoreactivity was also found to be increased throughout the hippocampus of socially isolated rats compared with that apparent in group-housed rats. The increase was significant in the strata oriens and radiatum of CA1 and CA3, in the stratum lacunosum-moleculare of CA1, in the molecular layer of the dentate gyrus, and in CA4, and it was most pronounced in granule cells of the dentate gyrus and in the pyramidal cell layers of CA1 and CA3 (Fig. 4). An increase in transcription of the gene for the α4 subunit in the hippocampus of isolated rats elicited by the prolonged decrease in the level of 3α,5α-THP might thus contribute to the increase in seizure susceptibility (see below) as well as to the anxiety-like behavior apparent in such animals (Serra et al., 2000). Increased expression of the α4 subunit is associated with many seizure-prone states, with such an increase being apparent in dentate granule cells of rats or mice subjected to kindling with pilocarpine (Brooks-Kayal et al., 1998; Peng et al., 2004). Social isolation is also associated with small increases in the amount of δ subunit immunoreactivity in the hippocampus of rats (Fig. 5). with the changes being statistically significant in pyramidal cells of CA1 and CA3, in the stratum oriens of CA3, and in the molecular layer and granular cells of the dentate gyrus. The increased expression of α4 and δ subunits in the hippocampus of isolated rats likely results in the formation of GABAA receptors that contain both of these subunits (Sur et al., 1999). Given that the δ subunit substitutes for the γ2 subunit and that the latter subunit is essential for synaptic localization of GABAA receptors (Essrich et al., 1998), receptors containing α4 and δ subunits would be expected to be extrasynaptic. Furthermore, given that extrasynaptic GABAA receptors are responsible for tonic inhibition in granule cells of the cerebellum and dentate gyrus (Nusser et al., 1998; Nusser & Mody, 2002; Stell and Mody, 2002), these changes in GABAA receptor subunit composition in the hippocampus of isolated rats likely result in alterations in tonic inhibition in this brain region (see below). The increased expression of GABAA receptors containing both α4 and δ subunits associated with social isolation might also be expected to have important consequences for the ability of ethanol to directly modulate GABAergic inhibitory transmission, given that recombinant GABAA receptors containing these subunits were suggest to have a high and selective sensitivity to low concentrations of ethanol (Sundstrom-Poromaa et al., 2002; Wallner et al., 2003), although Borghese et al. (2006) failed to reproduce these observations both in recombinant receptors and in dentate gyrus granule cells.
Figure 4.
Changes in immunoreactivity for the α4 subunit of the GABAA receptor in the hippocampal formation induced by social isolation. (A, B) Representative immunohistochemical analysis of the distribution of the α4 subunit in hippocampal slices from group-housed or isolated rats, respectively. Scale bar, 1 mm. Insets (A1–A3, B1–B3) show α4 subunit immunoreactivity in the pyramidal cell layer of CA3, the pyramidal cell layer of CA1, and the granule cell layer of the dentate gyrus, respectively. Scale bar, 50 μm. (C) Quantitation of α4 subunit immunoreactivity in the indicated regions of the hippocampus. Data are expressed as percentage change in gray intensity for isolated rats relative to the corresponding value for group-housed rats and are means ± SEM of values from six animals for each experimental group. aP < 0.05, bP < 0.001 versus the corresponding value for group-housed rats (ANOVA followed by Scheffè's post hoc test). Reproduced with permission from Serra et al. (2006)
Figure 5.
Changes in immunoreactivity for the δ subunit of the GABAA receptor in the hippocampal formation induced by social isolation. (A, B) Representative immunohistochemical analysis of the distribution of the δ subunit in hippocampal slices from group-housed or isolated rats, respectively. Scale bar, 1 mm. Insets (A1–A3, B1–B3) show δ subunit immunoreactivity in the pyramidal cell layer of CA3, the pyramidal cell layer of CA1, and the granule cell layer of the dentate gyrus, respectively. Scale bar, 50 μm. (C) Quantitation of α4 subunit immunoreactivity in the indicated regions of the hippocampus. Data are expressed as percentage change in gray intensity in isolated rats relative to the corresponding value for group-housed rats and are means ± SEM of values from six animals for each experimental group. aP < 0.05, bP < 0.001 versus the corresponding value for group-housed rats (ANOVA followed by Scheffè's post hoc test). Reproduced with permission from Serra et al. (2006).
6.3. GABAA receptor function
The persistent decreases in the concentrations of neuroactive steroids in socially isolated rats affect the functional coupling between the recognition site of GABAA receptors for GABA and those for allosteric modulators such as benzodiazepines. The efficacy, but not the potency, of diazepam in enhancement of GABA-evoked Cl− currents was thus markedly decreased in both the cerebral cortex and hippocampus of isolated rats (Serra et al., 2000). Responsiveness to the behavioral actions of pentobarbital and muscimol was also shown to be decreased in socially isolated mice (Matsumoto et al., 1999; Pinna et al., 2000). In contrast, at concentrations of 1 or 3 μM, 3α,5α-THP increased the total current associated with synaptically evoked GABAA receptor–mediated IPSCs by similar extents in CA1 pyramidal neurons of group-housed and isolated rats (Serra et al., 2006). Furthermore, the kinetic characteristics of spontaneous mIPSCs in these neurons under basal conditions did not differ significantly between group-housed and isolated animals.
Electrophysiological evidence suggests that the subunit composition of GABAA receptors at synapses differs from that of extrasynaptic receptors and that these two types of GABAA receptor mediate two different forms of inhibition: synaptic and tonic inhibition, respectively (Farrant & Nusser, 2005). Transient activation of synaptic GABAA receptors is thus responsible for conventional phasic inhibition, whereas specific GABAA receptors that possess a much higher affinity for GABA and which are located perisynaptically or extrasynaptically are activated by the nanomolar levels of GABA constantly present in the extracellular space, generating a constant current flow through the membrane that underlies tonic inhibition.
GABAA receptors that contain the δ subunit are restricted to extrasynaptic locations (Nusser et al., 1998) and have a high affinity for GABA (Saxena & Macdonald, 1996), making them likely mediators of the tonic GABAA receptor conductance recorded from granule cells of the cerebellum (Brickley et al., 1996) and dentate gyrus (Nusser & Mody, 2002; Stell & Mody, 2002). We found that the amplitude of GABAA receptor–mediated tonic inhibitory currents in granule cells of the dentate gyrus was markedly greater in hippocampal slices from socially isolated rats than in those from group-housed animals. The reduction in tonic current noise induced by bath application of the GABAA receptor antagonist bicuculline was thus significantly greater in hippocampal slices from isolated rats than in those from group-housed animals (Serra et al., 2006). In addition, the enhancement of tonic current noise induced by 3α,5α-THP (3 μM) was markedly greater in granule cells of the dentate gyrus from isolated rats than in those from group-housed animals (Serra et al., 2006). Given that enhancement of tonic inhibition is an effective means of reducing neuronal excitability (Stell & Mody, 2002), our data suggest that the augmented tonic inhibitory current mediated by δ subunit–containing extrasynaptic GABAA receptors in the hippocampus of socially isolated rats might reflect a compensatory mechanism to counteract the increased seizure susceptibility due to the increased expression of the α4 subunit. Consistent with this notion, mice that lack the δ subunit of the GABAA receptor develop epilepsy and show other signs of hyperexcitability (Mihalek et al., 1999; Spigelman et al., 2002). Moreover, the expression of this subunit is decreased in the hippocampal formation of a mouse model of temporal lobe epilepsy (Peng et al., 2004). Conversely, seizure susceptibility during the estrous cycle in rats is decreased during late diestrus, when expression of the δ subunit and, consequently, tonic inhibition are increased (Maguire et al., 2005).
7. Social isolation stress and ethanol sensitivity
The neurochemical correlates of the behavioral consequences of isolation rearing or housing of rats are complex and involve many neurotransmitters (Schenk et al., 1982; Jones et al., 1992; Heidbreder et al., 2001; Hall et al., 2002; Preece et al., 2004; Thorsell et al., 2005; Thorsell et al., 2006). Moreover, socially isolated rats respond differently from group-housed animals to a variety of psychoactive drugs including ethanol (Paivarinta et al, 1990; Lapiz et al., 2003). We have studied whether adult rats housed in isolation are more sensitive than are group-housed animals to the effects of acute ethanol administration on neuroactive steroid concentrations and GABAA receptor function.
7.1. Neuroactive steroids
Ethanol, like stress and pharmacological inhibitors of GABAA receptor–mediated neurotransmission (Barbaccia et al., 1997), increases the abundance of 3α,5α-THP and 3α,5α-THDOC in the brain and plasma (Barbaccia et al., 1999; VanDoren et al., 2000), and these effects have been thought to be largely dependent on stimulation of the HPA axis, given that they are largely abolished after adrenalectomy (Khisti et al., 2002a; O'Dell et al., 2004). We found that acute administration of ethanol increased the cerebrocortical and plasma concentrations of progesterone, 3α,5α-THP, and 3α,5α-THDOC to a markedly greater extent in socially isolated rats than in group-housed controls (Serra et al., 2003). This increased sensitivity of socially isolated rats to the effects of ethanol on the brain and plasma concentrations of neuroactive steroids is consistent with the notion that a “facilitatory trace,” characterized by hyperresponsiveness of the HPA axis to new stimuli, may develop during chronic stress (Akana et al., 1992). It is also in agreement with the increased functional response of the HPA axis of isolated rats to an acute stressful stimulus (Serra et al., 2000). Moreover, acute administration of ethanol in socially isolated rats increases the concentration of 3α,5α-THP to a substantially greater degree in the cerebral cortex than in plasma (Serra et al., 2003), consistent with our observation that ethanol increases local neurosteroid synthesis in the brain independently of the HPA axis (Sanna et al., 2004).
7.2. StAR
Consistent with the results of a previous study (Kim et al., 2003), we found that the acute administration of ethanol induced a rapid change in the expression of StAR in the brain (Serra et al., 2006). However, we further showed that ethanol induced a greater increase in the amount of StAR mRNA in the cerebral cortex of isolated rats (+38 ± 12%) than in that of group-housed rats (+24 ± 10%). Moreover, ethanol reduced the amount of the 37-kDa form of the StAR protein (−40 ± 6%) and increased that of the 32-kDa form (+55 ± 13 %) in the cerebral cortex of socially isolated rats, whereas it increased the abundance of both forms of StAR in the cortex of group-housed animals. Given that the molecular mechanism of StAR function remains unclear (Sierra, 2004), it is not yet possible to explain the opposite effects of ethanol on the abundance of the two forms of the protein in the cerebral cortex of isolated rats. An increase in the amount of the 32 (or 30)–kDa form of StAR has also been shown by other researchers to be accompanied by a decrease in the amount of the 37-kDa form (Kimoto et al., 2001; Shibuya et al., 2003). Given that the 37-kDa precursor form of StAR is produced in the cytoplasm and is converted to the 32-kDa form during its incorporation into the inner mitochondrial membrane (Lin et al., 1995), our data suggest that the rate of proteolytic conversion of the full-length StAR protein to the 32-kDa form in the cerebral cortex may be increased by ethanol in isolated rats. The increased sensitivity of neuroactive steroid production to ethanol in socially isolated rats may thus be attributable, at least in part, to an increase in the abundance of StAR in brain mitochondria.
7.3. GABAA receptor function
The potency of ethanol in increasing the amplitude of mIPSCs is markedly greater in hippocampal slices from isolated rats than in those from group-housed animals. Indeed, whereas ethanol at a concentration of 50 mM significantly increased mIPSC amplitude in neurons from isolated animals, it proved ineffective in those from group-housed rats (Serra et al., 2006). Moreover, the observation that the delayed effect of ethanol on mIPSC amplitude was inhibited by finasteride supports the idea that this action of ethanol is mediated by an increased production of 3α,5α-THP. Together, these observations suggest that a hyperresponsiveness of the HPA axis is not the only mechanism responsible for the enhanced effect of ethanol on neuroactive steroid concentrations in the brain of isolated rats. The increased sensitivity of neurons from isolated rats to ethanol is thus likely due to a greater production of 3α,5α-THP induced by ethanol in these animals rather than to a change in the responsiveness of postsynaptic GABAA receptors to neuroactive steroids. This conclusion is supported by the lack of a difference in the effect of 3α,5α-THP at concentrations of 1 or 3 μM on the amplitude of synaptically evoked IPSCs in CA1 pyramidal neurons between isolated and group-housed rats (Serra et al., 2006).
7.4. Behavioral studies
The ethanol-induced increase in the amount of 3α,5α-THP in the brain is thought to contribute to the anticonvulsant effect of this drug in a manner dependent on GABAA receptor function. Indeed, inhibition of neuroactive steroid synthesis by prior administration of finasteride prevented the anticonvulsant effect of ethanol (VanDoren et al., 2000). The greater efficacy of ethanol in increasing 3α,5α-THP synthesis in the brain of socially isolated rats is associated with an increase in the ability of ethanol to antagonize convulsions induced by isoniazid. Acute ethanol thus inhibited isoniazid-induced convulsions in isolated rats, as revealed by a greater delay in the onset of convulsions and by a reduced percentage of animals manifesting convulsions, but it had no effect on the pattern of convulsions induced by isoniazid in group-housed animals (Serra et al., 2006). The increase in the anticonvulsant efficacy of ethanol induced by social isolation was prevented by pretreatment with finasteride. Finasteride reduced the delay in the onset of isoniazid-induced seizures in group-housed animals but not in isolated rats, consistent with the fact that the basal level of 3α,5α-THP in the brain is reduced by social isolation. The reduction in the brain level of 3α,5α-THP induced by social isolation is thus likely responsible, at least in part, for the increased seizure vulnerability apparent in isolated animals. Isolated mice are also more susceptible to picrotoxin-induced seizures than are group-housed animals (Matsumoto et al., 2003). These observations are also consistent with the significant increase in susceptibility to seizure induction by kainic acid, pentylenetetrazol, picrotoxin, or the proconvulsant and convulsant β-carbolines apparent in animals subjected to withdrawal from 3α,5α-THP (Frye & Bayon, 1998; Moran & Smith, 1998; Frye & Bayon, 1999; Reddy et al., 2001).
In contrast with these various observations, socially isolated animals displayed reduced sensitivity to the effects of ethanol and 3α,5α-THP in the elevated plus-maze test. The effective dose of ethanol (2 g/kg, intraperitoneal) or 3α,5α-THP (2.5 μg per animal, intracerebroventricular) in group-housed animals thus failed to elicit an anxiolytic effect in socially isolated animals. Indeed, a higher dose of ethanol (3.0 to 3.5 g/kg) or 3α,5α-THP (3 μg per rat) was required to increase the time spent in and the number of entries into the open arms of the maze by isolated animals (Hirani et al., 2005). Moreover, social isolation was found to inhibit the hypnotic action of ethanol in mice (Matsumoto et al., 1996).
8. Effects of chronic ethanol in socially isolated rats
Adverse life experiences, family influences, and alcohol accessibility are the most common environmental factors implicated in increased risk for alcohol abuse (Prescott & Kendler, 1999; Averna & Hesselbrock, 2001). Animals have been used to model the effects of adverse life experiences on development of drinking behavior. For instance, separation of rats from their peers during adolescence and adulthood has been shown to increase voluntary ethanol consumption (Schenk et al., 1990; Wolffgramm, 1990; Juarez & Vazquez-Cortes, 2003; Thorsell et al., 2005). This finding, together with our results showing that socially isolated rats are more sensitive to the effects of ethanol on the brain concentrations of 3α,5α-THP and 3α,5α-THDOC (Serra et al., 2003), both of which posses anxiolytic properties (Engel & Grant, 2001) and potentiate the central actions of ethanol (Vanover et al., 1999), suggests that chronic stress may induce plastic adaptation of neuronal systems that contributes to a vulnerability to alcohol abuse. We have therefore recently examined whether chronic voluntary ethanol consumption modifies the effects of isolation on neuroactive steroid concentrations and on GABAA receptor gene expression and function.
8.1. Neuroactive steroids
Although several laboratories have examined the influence of chronic ethanol exposure on neuroactive steroid levels, most such studies have investigated the possible role of neuroactive steroids in the molecular mechanisms that underlie ethanol tolerance and dependence. Studies with different durations and types of ethanol treatment have thus indicated that chronic ethanol administration alters 3α,5α-THP concentrations. With the use of the chronic intermittent ethanol model of alcohol dependence, Cagetti et al. (2004) showed that rats exposed intermittently to intoxicating levels of ethanol manifested a reduction in the abundance of 3α,5α-THP in the hippocampus measured 2 days after withdrawal. The authors hypothesized that this decrease in 3α,5α-THP synthesis may contribute to the symptoms associated with alcohol withdrawal syndrome. Indeed, the plasma level of neuroactive steroids has been found to be reduced in alcoholic patients during withdrawal syndrome (Romeo et al., 1996).
The basal cerebrocortical level of 3α,5α-THP is significantly reduced in ethanol-dependent rats and mice (Janis et al., 1998). Devaud et al. (1996) found that the anticonvulsant effect of neuroactive steroids was increased, without a change in the plasma concentration of 3α,5α-THP, in rats after chronic ethanol treatment. Consistent with these results, we found that voluntary ethanol consumption did not affect the cerebrocortical level of 3α,5α-THP in group-housed rats (10.3 ± 3.1 versus 9.7 ± 2.0 ng per gram of protein (for rats exposed to ethanol and control animals, respectively), but reversed the effect of social isolation on the concentration of this steroid (10.8 ± 3.8 versus 5.6 ± 0.6 ng per gram of protein).
Given that social isolation represents a model of mild chronic stress, that 3α,5α-THP is among the most potent endogenous positive modulators of GABAA receptors (Majewska, 1992), and that the concentration of this steroid is reduced in socially isolated animals (Serra et al., 2000), the increased rate of ethanol intake in isolated rats may result from a rewarding effect of this drug that is attributable to the increase in the concentration of this endogenous anxiolytic steroid induced by ethanol consumption.
8.2. GABAA receptor plasticity and function
Social isolation increases expression of the α4 and δ subunits of the GABAA receptor in the hippocampus (Serra et al., 2006). We found that, whereas voluntary ethanol consumption failed to modify the level of α4 subunit immunoreactivity in the hippocampus of isolated or group-housed rats (Serra et al., in preparation), it was associated with small increases in the amount of δ subunit immunoreactivity throughout the hippocampus of both isolated and control rats (Table 3).
Table 3.
Effects of voluntary ethanol consumption on the abundance of the δ subunit of the GABAA receptor in various regions of the hippocampus in group-housed or socially isolated rats.
| Group-housed (% increase) | Isolated (% increase) | |
|---|---|---|
| CA1 | ||
| Stratum oriens | 46 ± 2a | 34 ± 2a |
| Pyramidal layer | 54 ± 3a | 43 ± 1a |
| Stratum radiatum | 33 ± 2a | 30 ± 2a |
| Stratum lacunosum-moleculare | 36 ± 3a | 22 ± 3a |
| CA3 | ||
| Stratum oriens | 51 ± 5a | 14 ± 2 |
| Pyramidal layer | 79 ± 4a | 27± 1a |
| Stratum radiatum | 42 ± 2a | 12 ± 3 |
| Dentate gyrus | ||
| Molecular layer | 33 ± 3a | 26± 2a |
| Granular layer | 46 ± 3a | 15 ± 1a |
| CA4 | 43 ± 6a | 30 ± 2a |
Rats were housed in groups or in isolation for 30 days immediately after weaning. Isolated or group-housed rats had 24-hour access for 30 days to two bottles, one containing water and the other containing either ethanol (6, 10, or 20%) or water. The hippocampus was then subjected to immunohistochemical staining for determination of the abundance of the δ subunit as described (Serra et al., 2006). Data are expressed as percentage increase in subunit expression for the animals with access to ethanol relative to the corresponding values for those exposed only to water and are means ± SEM of values from six animals for each experimental group.
P < 0.05 versus respective water-only group.
GABAA receptors that contain the δ subunit are restricted to extrasynaptic locations (Nusser et al., 1998) and are mediators of tonic GABAergic conductance (Nusser & Mody, 2002; Stell & Mody, 2002). The amplitude of GABAA receptor–mediated tonic inhibitory currents in granule cells of the dentate gyrus was greater in hippocampal slices from socially isolated rats than in those from group-housed animals (Serra et al., 2006) and was further increased in socially isolated animals after voluntary ethanol consumption, as demonstrated by a further reduction in tonic current noise induced by bath application of the GABAA receptor antagonist bicuculline at 20 μM (Serra et al., in preparation). These data suggest that voluntary ethanol consumption in isolated animals results in the formation of an increased number of α4 subunit–containing GABAA receptors that also contain the δ subunit.
Moreover, the responsiveness of synaptic GABAA receptors to ethanol is reduced in the hippocampus of isolated animals with free access to ethanol, as demonstrated by the observation that 50 mM ethanol increased the amplitude of GABAA receptor–mediated mIPSCs in CA1 pyramidal neurons of control isolated rats but not in those of isolated rats that had free access to ethanol for 30 days (Serra et al., in preparation). These data provide further support for the notion that the presence of the δ subunit is not an absolute requirement for sensitivity of GABAA receptors to low concentrations of ethanol (Carta et al., 2004; Borghese et al., 2006).
9. Conclusions
Since the discovery that GABAA receptors are the target for drugs such as benzodiazepines, barbiturates, ethanol etc. able to modulate the threshold of cell excitability and the emotional state, a great deal of effort has expended in attempting to define the putative endogenous compounds acting at these receptors. The finding that some steroid derivatives, such as 3α,5α–TH PROG and 3α,5α–THDOC are among the most efficacious and potent positive modulators of GABAA receptors has open a revolutionary view on the endogenous mechanisms able to modulate the function of those neuronal populations that in specific brain areas play a crucial role in the control of the affective and emotional behaviors which are strictly linked to the cognitive function. Moreover, the subsequent clinical evidences that the plasma content of these neuroactive steroids undergo to great fluctuations not only in physiological condition such as stress, menstrual cycle, pregnancy, menopause etc. but also during the course of disorders associated to altered emotional and affective states together with the finding that psychotropic drugs such as antidepressant, atypical antipsichotics, mood stabilisers etc. are able to increase the content of these hormones in plasma, cerebral spinal fluid and brain gave an important indications of the crucial role of these hormones in the modulation of brain functions.
This article reports a large amount of the most significative data from our and other laboratories on the role played by neuroactive steroids in the modulation of GABAA receptor gene expression and function in response to acute and chronic stress as well as to the acute and chronic exposure to ethanol. Thus, the previous evidence that natural fluctuations in neuroactive steroid level during acute stress and pregnancy are associated to changes of GABAA receptor activity and the expression of specific receptor subunit genes were more recently followed by findings showing that other physiological, experimental or pharmacological conditions such as menstrual cycle, chronic mild stress, chronic ethanol administration and its withdrawal are able to dramatically modify GABAA receptor gene expression and function and the associate behaviors through selective changes in the function of machinery able to control steroidogenesis in periphery and brain.
Taken together the results reported in this review further support the idea that neuroactive steroids play a crucial role the control of physiological and pharmacological modulation of the gene expression and function of GABAA receptors in the mammalian brain. Thus, perturbation in the synthesis and release of these hormones may be an important factor in the pathophysiology of certain mental disorders as well as in the rapid and long lasting capability of the central nervous system to adapt its plastic response to environmental changes.
Acknowledgments
This work was supported by grant Prot. 2005057519 from the Ministry of Instruction, Progetti di Ricerca di Interesse Nazionale (PRIN), and from the USPHS National Institute on Alcohol Abuse and Alcoholism (grant U01AA13641).
Abbreviations
- ACTH
adrenocorticotropic hormone
- ADX
adrenalectomized
- CRF
corticotropin-releasing factor
- CX
castrated
- DBI
diazepam binding inhibitor
- GABA
γ-aminobutyric acid
- GHB
γ-hydroxybutyrate
- HPA
hypothalamic-pituitary-adrenal
- IPSC
inhibitory postsynaptic current
- MAP2
microtubule-associated protein 2
- mIPSC
miniature IPSC
- ORX
orchiectomized
- PBR
peripheral benzodiazepine receptor
- StAR
steroidogenic acute regulatory protein
- TBPS
t-butylbicyclophosphorothionate
- THIP
4,5,6,7-tetrahydroisoxazolo-pyridin-3-ol
- 3α,5α-THDOC
3α,21-dihydroxy-5α-pregnane-20-one
- 3α,5α-THP
3α-hydroxy-5α-pregnane-20-one
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adkins CE, Pillai GV, Kerby J, Bonnert TP, Haldon C, McKernan RM, et al. α4β3δ GABAA receptors characterized by fluorescence resonance energy transfer-derived measurements of membrane potential. J Biol Chem. 2001;276:38934–38939. doi: 10.1074/jbc.M104318200. [DOI] [PubMed] [Google Scholar]
- Akana SF, Scribner KA, Bradbury MJ, Strack AM, Walker CD, Dallman MF. Feedback sensitivity of the rat hypothalamo-pituitary-adrenal axis and its capacity to adjust to exogenous corticosterone. Endocrinology. 1992;131:585–594. doi: 10.1210/endo.131.2.1322275. [DOI] [PubMed] [Google Scholar]
- Allan AM, Harris RA. Gamma-aminobutyric acid and alcohol actions: neurochemical studies of long sleep and short sleep mice. Life Sci. 1986;39:2005–2015. doi: 10.1016/0024-3205(86)90324-3. [DOI] [PubMed] [Google Scholar]
- Allan AM, Harris RA. Acute and chronic ethanol treatments alter GABA receptor-operated chloride channels. Pharmacol Biochem Behav. 1987;27:665–670. doi: 10.1016/0091-3057(87)90192-4. [DOI] [PubMed] [Google Scholar]
- Andrews N, Zharkovsky A, File SE. Acute handling stress down regulates benzodiazepine receptors: reversal by diazepam. Eur J Pharmacol. 1992;210:247–251. doi: 10.1016/0014-2999(92)90411-v. [DOI] [PubMed] [Google Scholar]
- Ariwodola OJ, Weiner JL. Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: role of presynaptic GABAB receptors. J Neurosci. 2004;24:10679–10686. doi: 10.1523/JNEUROSCI.1768-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averna S, Hesselbrock V. The relationship of perceived social support to substance use in offspring of alcoholics. Addict Behav. 2001;26:363–374. doi: 10.1016/s0306-4603(00)00112-x. [DOI] [PubMed] [Google Scholar]
- Axelrod J, Reisine TD. Stress hormones: their interaction and regulation. Science. 1984;224:452–459. doi: 10.1126/science.6143403. [DOI] [PubMed] [Google Scholar]
- Azzolina B, Ellsworth K, Andersson S, Geissler W, Bull HG, Harris GS. Inhibition of rat α-reductases by finasteride: evidence for isozyme differences in the mechanism of inhibition. J Steroid Biochem Mol Biol. 1997;61:55–64. doi: 10.1016/s0960-0760(97)00002-2. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Cuccheddu T, Concas A, Biggio G. Neurosteroids in the brain of handling-habituated and naive rats: effect of CO2 inhalation. Eur J Pharmacol. 1994;261:317–320. doi: 10.1016/0014-2999(94)90123-6. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Bolacchi F, Concas A, Mostallino MC, Purdy RH, et al. Stress-induced increase in brain neuroactive steroids: antagonism by abecarnil. Pharmacol Biochem Behav. 1996a;54:205–210. doi: 10.1016/0091-3057(95)02133-7. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Mostallino MC, Concas A, Purdy RH, et al. Time-dependent changes in rat brain neuroactive steroid concentrations and GABAA receptor function after acute stress. Neuroendocrinology. 1996b;63:166–172. doi: 10.1159/000126953. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Purdy RH, Mostallino MC, Perra C, et al. Isoniazid-induced inhibition of GABAergic transmission enhances neurosteroid content in the rat brain. Neuropharmacology. 1996c;35:1299–1305. doi: 10.1016/s0028-3908(96)00067-6. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Purdy RH, Mostallino MC, Concas A, et al. The effects of inhibitors of GABAergic transmission and stress on brain and plasma allopregnanolone concentrations. Br J Pharmacol. 1997;120:1582–1588. doi: 10.1038/sj.bjp.0701046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaccia ML, Affricano D, Trabucchi M, Purdy RH, Colombo G, Agabio R, et al. Ethanol markedly increases “GABAergic” neurosteroids in alcoholpreferring rats. Eur J Pharmacol. 1999;384:R1–R2. doi: 10.1016/s0014-2999(99)00678-0. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Serra M, Purdy RH, Biggio G. Stress and neuroactive steroids. Int Rev Neurobiol. 2001;46:243–272. doi: 10.1016/s0074-7742(01)46065-x. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Colombo G, Affricano D, Carai MA, Vacca G, Melis S, et al. GABAB receptor-mediated increase of neurosteroids by γ-hydroxybutyric acid. Neuropharmacology. 2002;42:782–791. doi: 10.1016/s0028-3908(02)00026-6. [DOI] [PubMed] [Google Scholar]
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, et al. International Union of Pharmacology. XV. Subtypes of γ-aminobutyric acid A receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABAA receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABAA receptors. Neuropharmacology. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- Biggio F, Gorini G, Caria S, Murru L, Mostallino MC, Sanna E, et al. Plastic neuronal changes in GABAA receptor gene expression induced by progesterone metabolites: in vitro molecular and functional studies. Pharmacol Biochem Behav. 2006;84:545–554. doi: 10.1016/j.pbb.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Biggio G. The action of stress, β-carbolines, diazepam, and Ro 15-1788 on GABA receptors in the rat brain. Adv Biochem Psychopharmacol. 1983;38:105–119. [PubMed] [Google Scholar]
- Biggio G, Purdy RH, editors. Neurosteroids and Brain Function Int Rev Neurobiol. Vol. 46. New York: Academic Press; 2001. [Google Scholar]
- Biggio G, Corda MG, Demontis G, Concas A, Gessa GL. Sudden decrease in cerebellar GABA binding induced by stress. Pharmacol Res Commun. 1980;12:489–493. doi: 10.1016/s0031-6989(80)80120-2. [DOI] [PubMed] [Google Scholar]
- Biggio G, Corda MG, Concas A, Demontis G, Rossetti Z, Gessa GL. Rapid changes in GABA binding induced by stress in different areas of the rat brain. Brain Res. 1981;229:363–369. doi: 10.1016/0006-8993(81)91000-3. [DOI] [PubMed] [Google Scholar]
- Biggio G, Concas A, Corda MG, Giorgi O, Sanna E, Serra M. GABAergic and dopaminergic transmission in the rat cerebral cortex: effect of stress, anxiolytic and anxiogenic drugs. Pharmacol Ther. 1990;48:121–142. doi: 10.1016/0163-7258(90)90077-f. [DOI] [PubMed] [Google Scholar]
- Biggio G, Barbaccia ML, Follesa P, Serra M, Purdy RH, Concas A. Neurosteroids and GABAA receptor plasticity. In: Martin DL, Olsen RW, editors. GABA in the Nervous System: The View at Fifty Years. New York: Lippincott Williams & Wilkins; 2000. pp. 207–232. [Google Scholar]
- Biggio G, Follesa P, Sanna E, Purdy RH, Concas A. GABAA-receptor plasticity during long-term exposure to and withdrawal from progesterone. Int Rev Neurobiol. 2001;46:207–241. doi: 10.1016/s0074-7742(01)46064-8. [DOI] [PubMed] [Google Scholar]
- Biggio G, Dazzi L, Biggio F, Mancuso L, Talani G, Busonero F, et al. Molecular mechanisms of tolerance to and withdrawal of GABAA receptor modulators. Eur Neuropsychopharmacol. 2003;13:411–423. doi: 10.1016/j.euroneuro.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Bitran D, Shiekh M, McLeod M. Anxiolytic effect of progesterone is mediated by neurosteroid allopregnanolone at brain GABAA receptors. J Neuroendocrinol. 1995;7:171–177. doi: 10.1111/j.1365-2826.1995.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Borghese CM, Storustovu S, Ebert B, Herd MB, Belelli D, Lambert JJ, et al. The δ subunit of γ-aminobutyric acid type A receptors does not confer sensitivity to low concentrations of ethanol. J Pharmacol Exp Ther. 2006;316:1360–1368. doi: 10.1124/jpet.105.092452. [DOI] [PubMed] [Google Scholar]
- Breese GR, Criswell HE, Carta M, Dodson PD, Hanchar HJ, Khisti RT, et al. Basis of the gabamimetic profile of ethanol. Alcohol Clin Exp Res. 2006;30:731–744. doi: 10.1111/j.0145-6008.2006.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Baxendale S. Motherhood and memory: a review. Psychoneuroendocrinology. 2002;27:299–302. doi: 10.1016/s0306-4530(01)00003-8. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol (Lond) 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABAA receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human α4β3δ GABAA receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunig I, Scotti E, Sidler C, Fritschy JM. Intact sorting, targeting, and clustering of γ-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J Comp Neurol. 2002;443:43–55. doi: 10.1002/cne.10102. [DOI] [PubMed] [Google Scholar]
- Brussaard AB, Kits KS, Baker RE, Willems WPA, Leyting-Vermeulen JW, Voorn P, et al. Plasticity in fast synaptic inhibition of adult oxytocin neurons caused by switch in GABAA receptor subunit expression. Neuron. 1997;19:1103–1114. doi: 10.1016/s0896-6273(00)80401-8. [DOI] [PubMed] [Google Scholar]
- Buck KJ, Hahner L, Sikela J, Harris RA. Chronic ethanol treatment alters brain levels of γ-aminobutyric acidA receptor subunit mRNAs: relationship to genetic differences in ethanol withdrawal seizure severity. J Neurochem. 1991;57:1452–1455. doi: 10.1111/j.1471-4159.1991.tb08313.x. [DOI] [PubMed] [Google Scholar]
- Buster JE. Gestational changes in steroid hormone biosynthesis, secretion, metabolism, and action. Clin Perinatol. 1983;10:527–552. [PubMed] [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- Cagetti E, Pinna G, Guidotti A, Baicy K, Olsen RW. Chronic intermittent ethanol (CIE) administration in rats decreases levels of neurosteroids in hippocampus, accompanied by altered behavioral responses to neurosteroids and memory function. Neuropharmacology. 2004;46:570–579. doi: 10.1016/j.neuropharm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Calogero AE, Gallucci WT, Chousos GP, Gold PW. Interaction between GABAergic neurotransmission and rat hypothalamic corticotropin releasing hormone secretion in vitro. Brain Res. 1988;463:223–228. doi: 10.1016/0006-8993(88)90523-9. [DOI] [PubMed] [Google Scholar]
- Canonaco M, O'Connor LH, Pfaff DW, McEwen BS. Longer term progesterone treatment induces changes of GABAA receptor levels in forebrain sites in the female hamster: quantitative autoradiography study. Exp Brain Res. 1989;77:407–411. doi: 10.1007/BF00274998. [DOI] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, et al. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by α5 subunit-containing γ-aminobutyric acid type A receptors. Proc Natl Acad Sci USA. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003;463:235–272. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- Carta M, Partridge LD, Savage DD, Valenzuela CF. Neurosteroid modulation of glutamate release in hippocampal neurons: lack of an effect of a chronic prenatal ethanol exposure paradigm. Alcohol Clin Exp Res. 2003;27:1194–1198. doi: 10.1097/01.ALC.0000075828.50697.70. [DOI] [PubMed] [Google Scholar]
- Carta M, Mameli M, Valenzuela CF. Alcohol enhances GABAergic transmission to cerebellar granule cells via an increase in Golgi cell excitability. J Neurosci. 2004;24:3746–3751. doi: 10.1523/JNEUROSCI.0067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Altemus M, Chrousos GP. Neuroendocrine and emotional changes in the post-partum period. Prog Brain Res. 2001;133:241–249. doi: 10.1016/s0079-6123(01)33018-2. [DOI] [PubMed] [Google Scholar]
- Casagrande S, Cupello A, Pellistri F, Robello M. Only high concentrations of ethanol affect GABAA receptors of rat cerebellum granule cells in culture. Neurosci Lett. 2006 Dec 27; doi: 10.1016/j.neulet.2006.12.024. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Casellas P, Galiegue S, Basile AS. Peripheral benzodiazepine receptors and mitochondrial function. Neurochem Int. 2002;40:475–486. doi: 10.1016/s0197-0186(01)00118-8. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Harris RA, Crews FT. Ethanol tolerance and synaptic plasticity. Trends Pharmacol Sci. 1998;19:491–495. doi: 10.1016/s0165-6147(98)01268-1. [DOI] [PubMed] [Google Scholar]
- Chida Y, Sudo N, Kubo C. Social isolation stress exacerbates autoimmune disease in MRL/Ipr mice. J Neuroimmunol. 2005;158:138–144. doi: 10.1016/j.jneuroim.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Concas A, Mele S, Biggio G. Foot shock stress decreases chloride efflux from rat brain synaptoneurosomes. Eur J Pharmacol. 1987;135:423–427. doi: 10.1016/0014-2999(87)90694-7. [DOI] [PubMed] [Google Scholar]
- Concas A, Serra M, Atsoggiu T, Biggio G. Foot-shock stress and anxiogenic β-carbolines increase t-[35S]butylbicyclophosphorothionate binding in the rat cerebral cortex, an effect opposite to anxiolytics and γ-aminobutyric acid mimetics. J Neurochem. 1988;51:1868–1876. doi: 10.1111/j.1471-4159.1988.tb01170.x. [DOI] [PubMed] [Google Scholar]
- Concas A, Mostallino MC, Perra C, Lener R, Roscetti G, Barbaccia ML, et al. Functional correlation between allopregnanolone and [35S]-TBPS binding in the brain of rats exposed to isoniazid, pentylenetetrazol or stress. Br J Pharmacol. 1996;118:839–846. doi: 10.1111/j.1476-5381.1996.tb15476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, et al. Role of brain allopregnanolone in the plasticity of γ-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci USA. 1998;95:13284–13289. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concas A, Follesa P, Barbaccia ML, Purdy RH, Biggio G. Physiological modulation of GABAA receptor plasticity by progesterone metabolites. Eur J Pharmacol. 1999;375:225–235. doi: 10.1016/s0014-2999(99)00232-0. [DOI] [PubMed] [Google Scholar]
- Concas A, Porcu P, Sogliano C, Serra M, Purdy RH, Biggio G. Caffeine-induced increases in the brain and plasma concentrations of neuroactive steroids in the rat. Pharmacol Biochem Behav. 2000;66:39–45. doi: 10.1016/s0091-3057(00)00237-9. [DOI] [PubMed] [Google Scholar]
- Corda MG, Biggio G. Proconflict effect of GABA receptor complex antagonists. Reversal by diazepam. Neuropharmacology. 1986;25:541–544. doi: 10.1016/0028-3908(86)90181-4. [DOI] [PubMed] [Google Scholar]
- Corda MG, Blaker WD, Mendelson WB, Guidotti A, Costa E. β-Carbolines enhance shock-induced suppression of drinking in rats. Proc Natl Acad Sci USA. 1983;80:2072–2076. doi: 10.1073/pnas.80.7.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, et al. Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proc Natl Acad Sci USA. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criswell HE, Breese GR. A conceptualization of integrated actions of ethanol contributing to its GABAmimetic profile: a commentary. Neuropsychopharmacology. 2005;30:1407–1425. doi: 10.1038/sj.npp.1300750. [DOI] [PubMed] [Google Scholar]
- Cuccheddu T, Floris S, Serra M, Porceddu ML, Sanna E, Biggio G. Proconflict effect of carbon dioxide inhalation in rats. Life Sci. 1995;56:321–324. doi: 10.1016/0024-3205(95)00093-3. [DOI] [PubMed] [Google Scholar]
- Cullinan WE. GABAA receptor subunit expression within hypophysiotropic CRH neurons: a dual hybridization histochemical study. J Comp Neurol. 2000;419:344–351. doi: 10.1002/(sici)1096-9861(20000410)419:3<344::aid-cne6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 1994;17:208–214. doi: 10.1016/0166-2236(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Deitrich RA, Dunwiddie TV, Harris RA, Erwin VG. Mechanism of action of ethanol: initial central nervous system actions. Pharmacol Rev. 1989;41:489–537. [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Smith FD, Grayson DR, Morrow AL. Chronic ethanol consumption differentially alters the expression of γ-aminobutyric acidA receptor subunit mRNAs in rat cerebral cortex: competitive, quantitative reverse transcriptase-polymerase chain reaction analysis. Mol Pharmacol. 1995;48:861–868. [PubMed] [Google Scholar]
- Devaud LL, Purdy RH, Finn DA, Morrow AL. Sensitization of γ-aminobutyric acidA receptors to neuroactive steroids in rats during ethanol withdrawal. J Pharmacol Exp Ther. 1996;278:510–517. [PubMed] [Google Scholar]
- Devaud LL, Fritschy JM, Sieghart W, Morrow AL. Bidirectional alterations of GABAA receptor subunit peptide levels in rat cortex during chronic ethanol consumption and withdrawal. J Neurochem. 1997;69:126–130. doi: 10.1046/j.1471-4159.1997.69010126.x. [DOI] [PubMed] [Google Scholar]
- Dong E, Matsumoto K, Tohda M, Kaneko Y, Watanabe H. Diazepam binding inhibitor (DBI) gene expression in the brain of socially isolated and group-housed mice. Neurosci Res. 1999;33:171–177. doi: 10.1016/s0168-0102(99)00010-3. [DOI] [PubMed] [Google Scholar]
- Dong E, Matsumoto K, Uzunova V, Sugaya I, Takahata H, Nomura H, et al. Brain 5α-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc Natl Acad Sci USA. 2001;98:2849–2854. doi: 10.1073/pnas.051628598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorow R, Horowski R, Paschelke G, Amin M. Severe anxiety induced by FG 7142, a β-carboline ligand for benzodiazepine receptors. Lancet. 1983;ii:298–299. doi: 10.1016/s0140-6736(83)90076-4. [DOI] [PubMed] [Google Scholar]
- Douglas AJ, Brunton PJ, Bosch OJ, Russell JA, Neumann ID. Neuroendocrine responses to stress in mice: hyporesponsiveness in pregnancy and parturition. Endocrinology. 2003;144:5268–5276. doi: 10.1210/en.2003-0461. [DOI] [PubMed] [Google Scholar]
- Drugan RC, Morrow AL, Weizman R, Weizman A, Deutsch SI, Crawley JN, et al. Stress-induced behavioral depression in the rat is associated with a decrease in GABA receptor-mediated chloride ion flux and brain benzodiazepine receptor occupancy. Brain Res. 1989;487:45–51. doi: 10.1016/0006-8993(89)90938-4. [DOI] [PubMed] [Google Scholar]
- Einon DF, Morgan MJ. A critical period for social isolation in the rat. Dev Psychobiol. 1977;10:123–132. doi: 10.1002/dev.420100205. [DOI] [PubMed] [Google Scholar]
- Ellis FW. Effect of ethanol on plasma corticosterone levels. J Pharmacol Exp Ther. 1966;153:121–127. [PubMed] [Google Scholar]
- Engel SR, Grant KA. Neurosteroids and behavior. Int Rev Neurobiol. 2001;46:321–348. doi: 10.1016/s0074-7742(01)46067-3. [DOI] [PubMed] [Google Scholar]
- Essrich C, Lopez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the γ2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Faingold CL, N'Gouemo P, Riaz A. Ethanol and neurotransmitter interactions—from molecular to integrative effects. Prog Neurobiol. 1998;55:509–535. doi: 10.1016/s0301-0082(98)00027-6. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fenelon VS, Herbison AE. Plasticity in GABAA receptor subunit mRNA expression by hypothalamic magnocellular neurons in the adult rat. J Neurosci. 1996;16:4872–4880. doi: 10.1523/JNEUROSCI.16-16-04872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenelon VS, Sieghart W, Herbison AE. Cellular localization and differential distribution of GABAA receptor subunit proteins and messenger RNAs within hypothalamic magnocellular neurons. Neuroscience. 1995;64:1129–1143. doi: 10.1016/0306-4522(94)00402-q. [DOI] [PubMed] [Google Scholar]
- File SE, Zangrossi H, Jr, Andrews N. Novel environment and cat odor change GABA and 5-HT release and uptake in the rat. Pharmacol Biochem Behav. 1993;45:931–934. doi: 10.1016/0091-3057(93)90142-g. [DOI] [PubMed] [Google Scholar]
- Follesa P, Floris S, Tuligi G, Mostallino MC, Concas A, Biggio G. Molecular and functional adaptation of the GABAA receptor complex during pregnancy and after delivery in the rat brain. Eur J Neurosci. 1998;10:2905–2912. doi: 10.1111/j.1460-9568.1998.00300.x. [DOI] [PubMed] [Google Scholar]
- Follesa P, Serra M, Cagetti E, Pisu MG, Porta S, Floris S, et al. Allopregnanolone synthesis in cerebellar granule cells: roles in regulation of GABAA receptor expression and function during progesterone treatment and withdrawal. Mol Pharmacol. 2000;57:1262–1270. [PubMed] [Google Scholar]
- Follesa P, Cagetti E, Mancuso L, Biggio F, Manca A, Maciocco E, et al. Increase in expression of the GABAA receptor α4 subunit gene induced by withdrawal of, but not by long-term treatment with, benzodiazepine full or partial agonists. Brain Res Mol Brain Res. 2001;92:138–148. doi: 10.1016/s0169-328x(01)00164-4. [DOI] [PubMed] [Google Scholar]
- Follesa P, Mancuso L, Biggio F, Cagetti E, Franco M, Trapani G, et al. Changes in GABAA receptor gene expression induced by withdrawal of, but not by long-term exposure to, zaleplon or zolpidem. Neuropharmacology. 2002;42:191–198. doi: 10.1016/s0028-3908(01)00167-8. [DOI] [PubMed] [Google Scholar]
- Follesa P, Mancuso L, Biggio F, Mostallino MC, Manca A, Mascia MP, et al. Gamma-hydroxybutyric acid and diazepam antagonize a rapid increase in GABAA receptor α4 subunit mRNA abundance induced by ethanol withdrawal in cerebellar granule cells. Mol Pharmacol. 2003;63:896–907. doi: 10.1124/mol.63.4.896. [DOI] [PubMed] [Google Scholar]
- Follesa P, Biggio F, Mancuso L, Cabras S, Caria S, Gorini G, et al. Ethanol withdrawal-induced up-regulation of the α2 subunit of the GABAA receptor and its prevention by diazepam or γ-hydroxybutyric acid. Brain Res Mol Brain Res. 2004;120:130–137. doi: 10.1016/j.molbrainres.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Follesa P, Mostallino MC, Biggio F, Gorini G, Caria S, Busonero F, et al. Distinct patterns of expression and regulation of GABA receptors containing the δ subunit in cerebellar granule and hippocampal neurons. J Neurochem. 2005;94:659–671. doi: 10.1111/j.1471-4159.2005.03303.x. [DOI] [PubMed] [Google Scholar]
- Follesa P, Biggio F, Talani G, Murru L, Serra M, Sanna E, et al. Neurosteroids, GABAA receptors, and ethanol dependence. Psychopharmacology (Berl) 2006;186:267–280. doi: 10.1007/s00213-005-0126-0. [DOI] [PubMed] [Google Scholar]
- Freund RK, Palmer MR. Beta adrenergic sensitization of γ-aminobutyric acid receptors to ethanol involves a cyclic AMP/protein kinase A second-messenger mechanism. J Pharmacol Exp Ther. 1997;280:1192–1200. [PubMed] [Google Scholar]
- Frye CA, Bayon LE. Seizure activity is increased in endocrine states characterized by decline in endogenous levels of the neurosteroid 3α,5α-THP. Neuroendocrinology. 1998;68:272–280. doi: 10.1159/000054375. [DOI] [PubMed] [Google Scholar]
- Frye CA, Bayon LE. Cyclic withdrawal from endogenous and exogenous progesterone increases kainic acid and perforant pathway induced seizures. Pharmacol Biochem Behav. 1999;62:315–321. doi: 10.1016/s0091-3057(98)00182-8. [DOI] [PubMed] [Google Scholar]
- Frye GD, Chapin RE, Vogel RA, Mailman RB, Kilts CD, Mueller RA, et al. Effects of acute and chronic 1,3-butanediol treatment on central nervous system function: a comparison with ethanol. J Pharmacol Exp Ther. 1981;216:306–314. [PubMed] [Google Scholar]
- Gadek-Michalska A, Bugajski J. Repeated handling, restraint, or chronic crowding impair the hypothalamic-pituitary-adrenocortical response to acute restraint stress. J Physiol Pharmacol. 2003;54:449–459. [PubMed] [Google Scholar]
- Gamallo A, Villanua A, Trancho G, Fraile A. Stress adaptation and adrenal activity in isolated and crowded rats. Physiol Behav. 1986;36:217–221. doi: 10.1016/0031-9384(86)90006-5. [DOI] [PubMed] [Google Scholar]
- Gavish M, Weizman A, Youdim MBH, Okun F. Regulation of central and peripheral benzodiazepine receptors in progesterone-treated rats. Brain Res. 1987;409:386–390. doi: 10.1016/0006-8993(87)90728-1. [DOI] [PubMed] [Google Scholar]
- Gentile CG, Jarrell TW, Teich A, McCabe PM, Schneiderman N. The role of amygdaloid central nucleus in the retention of differential pavlovian conditioning of bradycardia in rabbits. Behav Brain Res. 1986;20:263–273. doi: 10.1016/0166-4328(86)90226-3. [DOI] [PubMed] [Google Scholar]
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABAA receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Holt W, Maren S. A role for amygdaloid PKA and PKC in the acquisition of long-term conditional fear memories in rats. Behav Brain Res. 2000;114:145–152. doi: 10.1016/s0166-4328(00)00224-2. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABAA receptors in the acute and chronic effects of ethanol. Psychopharmacology (Berl) 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- Gulinello M, Orman R, Smith SS. Sex differences in anxiety, sensorimotor gating and expression of the α4 subunit of the GABAA receptor in the amygdala after progesterone withdrawal. Eur J Neurosci. 2003;17:641–648. doi: 10.1046/j.1460-9568.2003.02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther U, Benson JA, Benke D, Fritschy JM, Reyes G, Knoflach F, et al. Benzodiazepine-insensitive mice generated by targeted disruption of the γ-aminobutyric acid type A receptors. Proc Natl Acad Sci USA. 1995;92:7749–7753. doi: 10.1073/pnas.92.17.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS, Huang S, Fong GW, Pert A, Linnoila M. Effect of isolation-rearing on voluntary consumption of ethanol, sucrose and saccharin solutions in Fawn Hooded and Wistar rats. Psychopharmacology (Berl) 1998;139:210–216. doi: 10.1007/s002130050706. [DOI] [PubMed] [Google Scholar]
- Hall FS, Ghaed S, Pert A, Xing G. The effects of isolation rearing on glutamate receptor NMDAR1A mRNA expression determined by in situ hybridization in Fawn hooded and Wistar rats. Pharmacol Biochem Behav. 2002;73:185–191. doi: 10.1016/s0091-3057(02)00796-7. [DOI] [PubMed] [Google Scholar]
- Hanchar HJ, Chutsrinopkun P, Meera P, Supavilai P, Sieghart W, Wallner M, et al. Ethanol potently and competitively inhibits binding of the alcohol antagonist Ro15-4513 to α4/6β3δ GABAA receptors. Proc Natl Acad Sci USA. 2006;103:8546–8551. doi: 10.1073/pnas.0509903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RA. Ethanol actions on multiple ion channels: Which are important? Alcohol Clin Exp Res. 1999;23:1563–1570. [PubMed] [Google Scholar]
- Harrison NL, Vicini S, Barker JL. A steroid anesthetic prolongs inhibitory postsynaptic currents in cultured rat hippocampal neurons. J Neurosci. 1987;7:604–609. doi: 10.1523/JNEUROSCI.07-02-00604.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauet T, Yao ZX, Bose HS, Wall CT, Han Z, Li W, et al. Peripheral-type benzodiazepine receptor-mediated action of steroidogenic acute regulatory protein on cholesterol entry into Leydig cell mitochondria. Mol Endocrinol. 2005;19:540–554. doi: 10.1210/me.2004-0307. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Millan MA, Lorang M, Harwood JP, Aguilera G. Corticotropin-releasing factor receptors and pituitary adrenal responses during immobilization stress. Endocrinology. 1988;123:396–405. doi: 10.1210/endo-123-1-396. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Lorang M, Irwin M, Aguilera G. CRF receptor regulation and sensitization of ACTH responses to acute ether stress during chronic intermittent immobilization stress. Brain Res. 1990;532:34–40. doi: 10.1016/0006-8993(90)91738-3. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Foxton R, Cilia J, Hughes ZA, Shah AJ, Atkins A, et al. Increased responsiveness of dopamine to atypical, but not typical antipsychotics in the medial prefrontal cortex of rats reared in isolation. Psychopharmacology (Berl) 2001;156:338–351. doi: 10.1007/s002130100760. [DOI] [PubMed] [Google Scholar]
- Henke PG. The amygdala and forced immobilization of rats. Behav Brain Res. 1985;16:19–24. doi: 10.1016/0166-4328(85)90078-6. [DOI] [PubMed] [Google Scholar]
- Herbison AE. Physiological roles for the neurosteroid allopregnanolone in the modulation of brain function during pregnancy and parturition. Prog Brain Res. 2001;133:39–47. doi: 10.1016/s0079-6123(01)33003-0. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, et al. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Hevers W, Luddens H. The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol Neurobiol. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- Hevers W, Korpi ER, Luddens H. Assembly of functional α6β3γ2δ GABAA receptors in vitro. Neuroreport. 2000;11:4103–4106. doi: 10.1097/00001756-200012180-00038. [DOI] [PubMed] [Google Scholar]
- Hilakivi LA, Ota M, Lister RG. Effect of isolation on brain monoamines and the behavior of mice in tests of exploration, locomotion, anxiety and behavioral ‘despair’. Pharmacol Biochem Behav. 1989;3:371–374. doi: 10.1016/0091-3057(89)90516-9. [DOI] [PubMed] [Google Scholar]
- Hirani K, Sharma AN, Jain NS, Ugale RR, Chopde CT. Evaluation of GABAergic neuroactive steroid 3α-hydroxy-5α-pregnane-20-one as a neurobiological substrate for the anti-anxiety effect of ethanol in rats. Psychopharmacology (Berl) 2005;180:267–278. doi: 10.1007/s00213-005-2169-7. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends Cogn Sci. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- Holson RR, Scallet AC, Ali SF, Turner BB. “Isolation stress” revisited: Isolation-rearing effects depend on animal care methods. Physiol Behav. 1991;49:1107–1118. doi: 10.1016/0031-9384(91)90338-o. [DOI] [PubMed] [Google Scholar]
- Holt RA, Bateson AN, Martin IL. Chronic treatment with diazepam or abecarnil differently affects the expression of GABAA receptor subunit mRNAs in the rat cortex. Neuropharmacology. 1996;35:1457–1463. doi: 10.1016/s0028-3908(96)00064-0. [DOI] [PubMed] [Google Scholar]
- Holt RA, Martin IL, Bateson AN. Chronic diazepam exposure decreases transcription of the rat GABAA receptor γ2-subunit gene. Brain Res Mol Brain Res. 1997;48:164–166. doi: 10.1016/s0169-328x(97)00129-0. [DOI] [PubMed] [Google Scholar]
- Horton WR, Chapman G, Meldrum BS. Isoniazid as a glutamic acid decarboxylase inhibitor. J Neurochem. 1979;33:745–749. doi: 10.1111/j.1471-4159.1979.tb05220.x. [DOI] [PubMed] [Google Scholar]
- Hu ZY, Bourreau E, Jung-Testas I, Robel P, Baulieu EE. Neurosteroids: oligodendrocyte mitochondria convert cholesterol to pregnenolone. Proc Natl Acad Sci USA. 1987;84:8215–8219. doi: 10.1073/pnas.84.23.8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyytia P, Koob GF. GABAA receptor antagonism in the extended amygdala decreases ethanol self-administration in rats. Eur J Pharmacol. 1995;283:151–159. doi: 10.1016/0014-2999(95)00314-b. [DOI] [PubMed] [Google Scholar]
- Impagnatiello F, Pesold C, Longone P, Caruncho H, Fritschy JM, Costa E, et al. Modifications of γ-aminobutyric acidA receptor subunit expression in rat neocortex during tolerance to diazepam. Mol Pharmacol. 1996;49:822–831. [PubMed] [Google Scholar]
- Jackson GL, Kuehl D. Gamma-aminobutyric acid (GABA) regulation of GnRH secretion in sheep. Reproduction Suppl. 2002;59:15–24. [PubMed] [Google Scholar]
- Janak PH, Redfern JE, Samson HH. The reinforcing effects of ethanol are altered by the endogenous neurosteroid, allopregnanolone. Alcohol Clin Exp Res. 1998;22:1106–1112. [PubMed] [Google Scholar]
- Janis GC, Devaud LL, Mitsuyama H, Morrow AL. Effects of chronic ethanol consumption and withdrawal on the neuroactive steroid 3α-hydroxy-5α-pregnan-20-one in male and female rats. Alcohol Clin Exp Res. 1998;22:2055–2061. [PubMed] [Google Scholar]
- Johnstone HA, Wigger A, Douglas AJ, Neumann ID, Landgraf R, Seckl JR, et al. Attenuation of hypothalamic-pituitary-adrenal axis stress responses in late pregnancy: changes in feedforward and feedback mechanisms. J Neuroendocrinol. 2000;12:811–822. doi: 10.1046/j.1365-2826.2000.00525.x. [DOI] [PubMed] [Google Scholar]
- Jones GH, Hernandez TD, Kendall DA, Marsden CA, Robbins TW. Dopaminergic and serotonergic function following isolation rearing in rats: study of behavioural responses and postmortem and in vivo neurochemistry. Pharmacol Biochem Behav. 1992;43:17–35. doi: 10.1016/0091-3057(92)90635-s. [DOI] [PubMed] [Google Scholar]
- Juarez J, Vazquez-Cortes C. Alcohol intake in social housing and in isolation before puberty and its effects on voluntary alcohol consumption in adulthood. Dev Psychobiol. 2003;43:200–207. doi: 10.1002/dev.10133. [DOI] [PubMed] [Google Scholar]
- Jussofie A, Körner I, Schell C, Hiemke C. Time course of the effects of steroid hormone deprivation elicited by ovariectomy or ovariectomy plus adrenalectomy on the affinity and density of GABA binding sites in distinct rat brain areas. Exp Clin Endocrinol Diabetes. 1995;103:196–204. doi: 10.1055/s-0029-1211350. [DOI] [PubMed] [Google Scholar]
- Keller-Wood ME, Dallman MF. Corticosteroid inhibition of ACTH secretion. Endocr Rev. 1984;5:1–24. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- Khisti RT, Kralic JE, VanDoren MJ, Morrow AL. Adrenalectomy attenuates increase in cortical allopregnanolone and behavioral effects induced by acute ethanol administration. Alcohol Clin Exp Res. 2002a;26:103A. [Google Scholar]
- Khisti RT, Penland SN, Van Doren MJ, Grobin AC, Morrow AL. Neurosteroid modulation of ethanol action. World J Biol Psychiatry. 2002b;3:87–95. doi: 10.3109/15622970209150606. [DOI] [PubMed] [Google Scholar]
- Khisti RT, Kumar S, Morrow AL. Ethanol rapidly induces steroidogenic acute regulatory protein expression and translocation in rat adrenal gland. Eur J Pharmacol. 2003;473:225–227. doi: 10.1016/s0014-2999(03)01969-1. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Ha M, Park CH, Park SJ, Youn SM, Kang SS, et al. StAR and steroidogenic enzyme transcriptional regulation in the rat brain: effects of acute alcohol administration. Mol Brain Res. 2003;115:39–49. doi: 10.1016/s0169-328x(03)00177-3. [DOI] [PubMed] [Google Scholar]
- Kimoto T, Tsurugizawa T, Ohta Y, Makino J, Tamura H, Hojo Y, et al. Neurosteroid synthesis by cytochrome P450-containing systems localized in the rat brain hippocampal neurons: N-methyl-D-aspartate and calcium-dependent synthesis. Endocrinology. 2001;142:3578–3589. doi: 10.1210/endo.142.8.8327. [DOI] [PubMed] [Google Scholar]
- Kokate TG, Svensson BE, Rogawski MA. Anticonvulsant activity of neurosteroids: correlation with γ-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Ther. 1994;270:1223–1229. [PubMed] [Google Scholar]
- Koksma JJ, Fritschy JM, Mack V, Van Kesteren RE, Brussaard AB. Differential GABAA receptor clustering determines GABA synapse plasticity in rat oxytocin neurons around parturition and the onset of lactation. Mol Cell Neurosci. 2005;28:128–140. doi: 10.1016/j.mcn.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur Neuropsychopharmacol. 2003;13:442–452. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P, et al. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res. 1998;22:3–9. [PubMed] [Google Scholar]
- Kovacs KJ, Miklos IH, Bali B. GABAergic mechanisms constraining the activity of the hypothalamo-pituitary-adrenocortical axis. Annl NY Acad Sci. 2004;1018:466–476. doi: 10.1196/annals.1296.057. [DOI] [PubMed] [Google Scholar]
- Krueger KE, Papadopoulos V. Peripheral-type benzodiazepine receptors mediate translocation of cholesterol from outer to inner mitochondrial membranes in adrenocortical cells. J Biol Chem. 1990;265:15015–15022. [PubMed] [Google Scholar]
- Kumar S, Sieghart W, Morrow AL. Association of protein kinase C with GABAA receptors containing α1 and α4 subunits in the cerebral cortex: selective effects of chronic ethanol consumption. J Neurochem. 2002;82:110–117. doi: 10.1046/j.1471-4159.2002.00943.x. [DOI] [PubMed] [Google Scholar]
- Kumar S, Fleming RL, Morrow AL. Ethanol regulation of γ-aminobutyric acid A receptors: genomic and nongenomic mechanisms. Pharmacol Ther. 2004;101:211–226. doi: 10.1016/j.pharmthera.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Hill-Venning C, Peters JA. Neurosteroids and GABAA receptor function. Trends Pharmacol Sci. 1995;16:295–303. doi: 10.1016/s0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Harney SC, Peters JA, Frenguelli BG. Modulation of native and recombinant GABAA receptors by endogenous and synthetic neuroactive steroids. Brain Res Brain Res Rev. 2001;37:68–80. doi: 10.1016/s0165-0173(01)00124-2. [DOI] [PubMed] [Google Scholar]
- Lapiz MD, Fulford A, Muchimapura S, Mason R, Parker T, Marsden CA. Influence of postweaning social isolation in the rat on brain development, conditioned behaviour and neurotransmission. Ross Fiziol Zh Im I M Sechenova. 2001;87:730–751. [PubMed] [Google Scholar]
- LeDoux JE. Emotion: clues from the brain. Annu Rev Psychol. 1995;46:209–235. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- Le Goascogne C, Robel P, Gouezou M, Sananes N, Baulieu EE, Waterman M. Neurosteroids: cytochrome P-450scc in rat brain. Science. 1987;237:1212–1214. doi: 10.1126/science.3306919. [DOI] [PubMed] [Google Scholar]
- Lejoyeux M, Solomon J, Ades J. Benzodiazepine treatment for alcohol-dependent patients. Alcohol Alcohol. 1998;33:563–575. doi: 10.1093/alcalc/33.6.563. [DOI] [PubMed] [Google Scholar]
- Liang J, Zhang N, Cagetti E, Houser CR, Olsen RW, Spiegelman I. Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. J Neurosci. 2006;26:1749–1758. doi: 10.1523/JNEUROSCI.4702-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Sugawara T, Strauss JF, 3rd, Clark BJ, Stocco DM, Saenger P, et al. Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science. 1995;267:828–831. doi: 10.1126/science.7892608. [DOI] [PubMed] [Google Scholar]
- Lindquist CE, Ebert B, Birnir B. Extrasynaptic GABAA channels activated by THIP are modulated by diazepam in CA1 pyramidal neurons in the rat brain hippocampal slice. Mol Cell Neurosci. 2003;24:250–257. doi: 10.1016/s1044-7431(03)00128-3. [DOI] [PubMed] [Google Scholar]
- Liubashina O, Jolkkonen E, Pitkanen A. Projections from the central nucleus of the amygdala to the gastric related area of the dorsal vagal complex: a Phaseolus vulgaris-leucoagglutinin study in rat. Neurosci Lett. 2000;291:85–88. doi: 10.1016/s0304-3940(00)01392-6. [DOI] [PubMed] [Google Scholar]
- Luisi S, Petraglia F, Benedetto C, Nappi RE, Bernardi F, Fadalti M, et al. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. 2000;85:2429–2433. doi: 10.1210/jcem.85.7.6675. [DOI] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Mahmoudi M, Kang MH, Tillakaratne N, Tobin AJ, Olsen RW. Chronic intermittent ethanol treatment in rats increases GABAA receptor α4-subunit expression: possible relevance to alcohol dependence. J Neurochem. 1997;68:2485–2492. doi: 10.1046/j.1471-4159.1997.68062485.x. [DOI] [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia. 1975;43:245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- Majewska MD. Neurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog Neurobiol. 1992;38:379–395. doi: 10.1016/0301-0082(92)90025-a. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Ford-Rice F, Falkay G. Pregnancy-induced alterations of GABAA receptor sensitivity in maternal brain: an antecedent of post-partum “blues”? Brain Res. 1989;482:397–401. doi: 10.1016/0006-8993(89)91208-0. [DOI] [PubMed] [Google Scholar]
- Mar Sánchez M, Aguado F, Sánchez-Toscano F, Saphier D. Neuroendocrine and immunocytochemical demonstrations of decreased hypothalamo-pituitary-adrenal axis responsiveness to restraint stress after long-term social isolation. Endocrinology. 1998;139:579–587. doi: 10.1210/endo.139.2.5720. [DOI] [PubMed] [Google Scholar]
- Martz A, Deitrich RA, Harris RA. Behavioral evidence for the involvement of γ-aminobutyric acid in the actions of ethanol. Eur J Pharmacol. 1983;89:53–62. doi: 10.1016/0014-2999(83)90607-6. [DOI] [PubMed] [Google Scholar]
- Mascia MP, Biggio F, Mancuso L, Cabras S, Cocco PL, Gorini G, et al. Changes in GABAA receptor gene expression induced by withdrawal of, but not by long-term exposure to, ganaxolone in cultured rat cerebellar granule cells. J Pharmacol Exp Ther. 2002;303:1014–1020. doi: 10.1124/jpet.102.040063. [DOI] [PubMed] [Google Scholar]
- Masotto C, Wisniewski G, Negro-Vilar A. Different γ-aminobutyric acid receptor subtypes are involved in the regulation of opiate-dependent and independent luteinizing hormone-releasing hormone secretion. Endocrinology. 1989;125:548–553. doi: 10.1210/endo-125-1-548. [DOI] [PubMed] [Google Scholar]
- Mathur C, Prasad VV, Raju VS, Welch M, Lieberman S. Steroids and their conjugates in the mammalian brain. Proc Natl Acad Sci USA. 1993;90:85–88. doi: 10.1073/pnas.90.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Ojima K, Watanabe H. Neurosteroidal modulation of social isolation-induced decrease in pentobarbital sleep in mice. Brain Res. 1996;708:1–6. doi: 10.1016/0006-8993(95)01277-x. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Uzunova V, Pinna G, Taki K, Uzunov DP, Watanabe H, et al. Permissive role of brain allopregnanolone content in the regulation of pentobarbital-induced righting reflex loss. Neuropharmacology. 1999;38:955–963. doi: 10.1016/s0028-3908(99)00018-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Nomura H, Murakami Y, Taki K, Takahata H, Watanabe H. Long-term social isolation enhances picrotoxin seizure susceptibility in mice: up-regulatory role of endogenous brain allopregnanolone in GABAergic systems. Pharmacol Biochem Behav. 2003;75:831–835. doi: 10.1016/s0091-3057(03)00169-2. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Devaud LL, Fritschy JM, Sieghart W, Morrow AL. Differential regulation of GABAA receptor gene expression by ethanol in the rat hippocampus versus cerebral cortex. J Neurochem. 1998;70:1160–1166. doi: 10.1046/j.1471-4159.1998.70031160.x. [DOI] [PubMed] [Google Scholar]
- McBride WJ. Central nucleus of the amygdala and the effects of alcohol and alcohol-drinking behavior in rodents. Pharmacol Biochem Behav. 2002;71:509–515. doi: 10.1016/s0091-3057(01)00680-3. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smythe JW, Sharma S, Meaney MJ. Sex-specific effects of prenatal stress on hypothalamic-pituitary-adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Brain Res Dev Brain Res. 1995;84:55–61. doi: 10.1016/0165-3806(94)00153-q. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Wingfield BC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Mehta AK, Ticku MK. Prevalence of the GABAA receptor assemblies containing α1-subunit in the rat cerebellum and cerebral cortex as determined by immunoprecipitation: lack of modulation by chronic ethanol administration. Brain Res Mol Brain Res. 1999a;67:194–199. doi: 10.1016/s0169-328x(99)00020-0. [DOI] [PubMed] [Google Scholar]
- Mehta AK, Ticku MK. An update on GABAA receptors. Brain Res Brain Res Rev. 1999b;29:196–217. doi: 10.1016/s0165-0173(98)00052-6. [DOI] [PubMed] [Google Scholar]
- Mele P, Oberto A, Serra M, Pisu MG, Floris I, Biggio G, et al. Increased expression of the gene for the Y1 receptor of neuropeptide Y in the amygdala and paraventricular nucleus of Y1R/LacZ transgenic mice in response to restraint stress. J Neurochem. 2004;89:1471–1478. doi: 10.1111/j.1471-4159.2004.02444.x. [DOI] [PubMed] [Google Scholar]
- Mhatre MC, Ticku MK. Chronic ethanol administration alters γ-aminobutyric acidA receptor gene expression. Mol Pharmacol. 1992;42:415–422. [PubMed] [Google Scholar]
- Mhatre M, Ticku MK. Chronic ethanol treatment upregulates the GABA receptor β subunit expression. Brain Res Mol Brain Res. 1994;23:246–252. doi: 10.1016/0169-328x(94)90231-3. [DOI] [PubMed] [Google Scholar]
- Mhatre MC, Pena G, Sieghart W, Ticku MK. Antibodies specific for GABAA receptor α subunits reveal that chronic alcohol treatment down-regulates α-subunit expression in rat brain regions. J Neurochem. 1993;61:1620–1625. doi: 10.1111/j.1471-4159.1993.tb09795.x. [DOI] [PubMed] [Google Scholar]
- Miachon S, Rochet T, Mathian B, Barbagli B, Claustrat B. Long-term isolation of Wistar rats alters brain monoamine turnover, blood corticosterone, and ACTH. Brain Res Bull. 1993;32:611–614. doi: 10.1016/0361-9230(93)90162-5. [DOI] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, et al. Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor δ subunit knockout mice. Proc Natl Acad Sci USA. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalek RM, Bowers BJ, Wehner JM, Kralic JE, VanDoren MJ, Morrow AL, et al. GABAA-receptor δ subunit knockout mice have multiple defects in behavioral responses to ethanol. Alcohol Clin Exp Res. 2001;25:1708–1718. [PubMed] [Google Scholar]
- Miklos IH, Kovacs KJ. GABAergic innervation of corticotropin-releasing hormone (CRH)-secreting parvocellular neurons and its plasticity as demonstrated by quantitative immunoelectron microscopy. Neuroscience. 2002;113:581–592. doi: 10.1016/s0306-4522(02)00147-1. [DOI] [PubMed] [Google Scholar]
- Milewich L, Gant NF, Schwarz BE, Chen GT, MacDonald PC. 5α-Reductase activity in human placenta. Am J Obstetr Gynecol. 1979;133:611–617. doi: 10.1016/0002-9378(79)90006-1. [DOI] [PubMed] [Google Scholar]
- Mody I. Distinguishing between GABAA receptors responsible for tonic and phasic conductances. Neurochem Res. 2001;26:907–913. doi: 10.1023/a:1012376215967. [DOI] [PubMed] [Google Scholar]
- Mody I, De Koninck Y, Otis TS, Soltesz I. Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 1994;17:517–525. doi: 10.1016/0166-2236(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Mohler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- Moller C, Wiklund L, Sommer W, Thorsell A, Heilig M. Decreased experimental anxiety and voluntary ethanol consumption in rats following central but not basolateral amygdala lesions. Brain Res. 1997;760:94–101. doi: 10.1016/s0006-8993(97)00308-9. [DOI] [PubMed] [Google Scholar]
- Montpied P, Ginns EI, Martin BM, Roca D, Farb DH, Paul SM. γ-Aminobutyric acid (GABA) induces a receptor-mediated reduction in GABAA receptor α subunit messenger RNAs in embryonic chick neurons in culture. J Biol Chem. 1991a;266:6011–6014. [PubMed] [Google Scholar]
- Montpied P, Morrow AL, Karanian JW, Ginns EI, Martin BM, Paul SM. Prolonged ethanol inhalation decreases γ-aminobutyric acidA receptor α subunit mRNAs in the rat cerebral cortex. Mol Pharmacol. 1991b;39:157–163. [PubMed] [Google Scholar]
- Moran MH, Smith SS. Progesterone withdrawal I: pro-convulsant effects. Brain Res. 1998;807:84–90. doi: 10.1016/s0006-8993(98)00782-3. [DOI] [PubMed] [Google Scholar]
- Morinan A, Leonard BE. Some anatomical and physiological correlates of social isolation in the young rat. Physiol Behav. 1980;24:637–640. doi: 10.1016/0031-9384(80)90265-6. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Suzdak PD, Paul SM. Benzodiazepine, barbiturate, ethanol and hypnotic steroid hormone modulation of GABA-mediated chloride ion transport in rat brain synaptoneurosomes. Adv Biochem Psychopharmacol. 1988;45:247–261. [PubMed] [Google Scholar]
- Morrow AL, Montpied P, Lingford-Hughes A, Paul SM. Chronic ethanol and pentobarbital administration in the rat: effects on GABAA receptor function and expression in brain. Alcohol. 1990;7:237–244. doi: 10.1016/0741-8329(90)90012-2. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Herbert JS, Montpied P. Differential effects of chronic ethanol administration on GABAA receptor α1 and α6 subunit mRNA levels in rat cerebellum. Mol Cell Neurosci. 1992;3:251–258. doi: 10.1016/1044-7431(92)90045-4. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Janis GC, VanDoren MJ, Matthews DB, Samson HH, Janak PH, et al. Neurosteroids mediate pharmacological effects of ethanol: a new mechanism of ethanol action? Alcohol Clin Exp Res. 1999;23:1933–1940. doi: 10.1111/j.1530-0277.1999.tb04094.x. [DOI] [PubMed] [Google Scholar]
- Morrow AL, VanDoren MJ, Penland SN, Matthews DB. The role of GABAergic neuroactive steroids in ethanol action, tolerance and dependence. Brain Res Brain Res Rev. 2001;37:98–109. doi: 10.1016/s0165-0173(01)00127-8. [DOI] [PubMed] [Google Scholar]
- Mostallino MC, Mura ML, Maciocco E, Murru L, Sanna E, Biggio G. Changes in expression of the δ subunit of the GABAA receptor and in receptor function induced by progesterone exposure and withdrawal. J Neurochem. 2006;99:321–332. doi: 10.1111/j.1471-4159.2006.04055.x. [DOI] [PubMed] [Google Scholar]
- Newton N, Peeler D, Newton M. Effect of disturbance on labor. An experiment with 100 mice with dated pregnancies. Am J Obstetr Gynecol. 1968;101:1096–1102. doi: 10.1016/0002-9378(68)90355-4. [DOI] [PubMed] [Google Scholar]
- Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, Siggins GR. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science. 2004;303:1512–1514. doi: 10.1126/science.1092550. [DOI] [PubMed] [Google Scholar]
- Ninan PT, Insel TM, Cohen RM, Cook JM, Skolnick P, Paul SM. Benzodiazepine receptor-mediated experimental “anxiety” in primates. Science. 1982;218:1332–1334. doi: 10.1126/science.6293059. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell LE, Alomary AA, Vallee M, Koob GF, Fitzgerald RL, Purdy RH. Ethanol-induced increases in neuroactive steroids in the rat brain and plasma are absent in adrenalectomized and gonadectomized rats. Eur J Pharmacol. 2004;484:241–247. doi: 10.1016/j.ejphar.2003.11.031. [DOI] [PubMed] [Google Scholar]
- Ogilvie KM, Lee S, Rivier C. Role of arginine vasopressin and corticotropin-releasing factor in mediating alcohol-induced adrenocorticotropin and vasopressin secretion in male rats bearing lesions of the paraventricular nuclei. Brain Res. 1997;744:83–95. doi: 10.1016/s0006-8993(96)01082-7. [DOI] [PubMed] [Google Scholar]
- Paivarinta P. Social isolation increases the stimulatory effect of ethanol on locomotor activity. Pharmacol Biochem Behav. 1990;36:401–403. doi: 10.1016/0091-3057(90)90422-e. [DOI] [PubMed] [Google Scholar]
- Paoletti AM, Romagnino S, Contu R, Orru MM, Marotto MF, Zedda P, et al. Observational study on the stability of the psychological status during normal pregnancy and increased blood levels of neuroactive steroids with GABAA receptor agonist activity. Psychoneuroendocrinology. 2006;31:485–492. doi: 10.1016/j.psyneuen.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Amri H, Boujrad N, Cascio C, Culty M, Garnier M, et al. Peripheral benzodiazepine receptor in cholesterol transport and steroidogenesis. Steroids. 1997;62:21–28. doi: 10.1016/s0039-128x(96)00154-7. [DOI] [PubMed] [Google Scholar]
- Pardon MC, Gerardin P, Joubert C, Perez-Diaz F, Cohen-Salmon C. Influence of prepartum chronic ultramild stress on maternal pup care behaviour in mice. Biol Psychiatry. 2000;47:858–863. doi: 10.1016/s0006-3223(99)00253-x. [DOI] [PubMed] [Google Scholar]
- Parker V, Morinan A. The socially-isolated rat as a model for anxiety. Neuropharmacology. 1986;25:663–664. [Google Scholar]
- Pearson Murphy BE, Steinberg SI, Hu FY, Allison CM. Neuroactive ring A-reduced metabolites of progesterone in human plasma during pregnancy: elevated levels of 5α-dihydroprogesterone in depressed patients during the latter half of pregnancy. J Clin Endocrinol Metab. 2001;86:5981–5987. doi: 10.1210/jcem.86.12.8122. [DOI] [PubMed] [Google Scholar]
- Peng Z, Huang CS, Stell BM, Mody I, Houser CR. Altered expression of the δ subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J Neurosci. 2004;24:8629–8639. doi: 10.1523/JNEUROSCI.2877-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning TM, Sharp RB, Krieger NR. Purification and properties of 3α-hydroxysteroid dehydrogenase from rat brain cytosol. Inhibition by non-steroidal anti-inflammatory drugs and progestins. J Biol Chem. 1985;260:15266–15272. [PubMed] [Google Scholar]
- Pinna G, Uzunova V, Matsumoto K, Puia G, Mienville JM, Costa E, et al. Brain allopregnanolone regulates the potency of the GABAA receptor agonist muscimol. Neuropharmacology. 2000;28:440–448. doi: 10.1016/s0028-3908(99)00149-5. [DOI] [PubMed] [Google Scholar]
- Pitman DL, Ottenweller JE, Natelson BH. Plasma corticosterone levels during repeated presentation of two intensities of restraint stress: chronic stress and habituation. Physiol Behav. 1988;43:47–55. doi: 10.1016/0031-9384(88)90097-2. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Otto S, Sutton S. Neurotransmitter modulation of corticotropin releasing factor secretion into the hypophysial-portal circulation. Life Sci. 1987;41:1311–1317. doi: 10.1016/0024-3205(87)90211-6. [DOI] [PubMed] [Google Scholar]
- Poisbeau P, Feltz P, Schlichter R. Modulation of GABA-A receptor-mediated IPSCs by neuroactive steroids in a rat hypothalamo-hypohyseal coculture model. J Physiol. 1997;500:475–485. doi: 10.1113/jphysiol.1997.sp022034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P, Sogliano C, Cinus M, Purdy RH, Biggio G, Concas A. Nicotine-induced changes in cerebrocortical neuroactive steroids and plasma corticosterone concentrations in the rat. Pharmacol Biochem Behav. 2003;74:683–690. doi: 10.1016/s0091-3057(02)01065-1. [DOI] [PubMed] [Google Scholar]
- Porcu P, Sogliano C, Ibba C, Piredda M, Tocco S, Marra C, et al. Failure of γ-hydroxybutyric acid both to increase neuroactive steroid concentrations in adrenalectomized-orchiectomized rats and to induce tolerance to its steroidogenic effect in intact animals. Brain Res. 2004;1012:160–168. doi: 10.1016/j.brainres.2004.03.059. [DOI] [PubMed] [Google Scholar]
- Preece MA, Dalley JW, Theobald DE, Robbins TW, Reynolds GP. Region specific changes in forebrain 5-hydroxytryptamine1A and 5-hydroxytryptamine2A receptors in isolation-reared rats: an in vitro autoradiography study. Neuroscience. 2004;123:725–732. doi: 10.1016/j.neuroscience.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. Am J Psychiatry. 1999;156:34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- Pritchett DB, Sontheimer H, Shivers BD, Ymer S, Kettenmann H, Schofield PR, et al. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989;338:582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of γ-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci USA. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Karst H, Joels M. Chronic unpredictable stress alters gene expression in rat single dentate granule cells. J Neurochem. 2004;89:364–374. doi: 10.1111/j.1471-4159.2003.02332.x. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Is there a physiological role for the neurosteroid THDOC in stress-sensitive conditions? Trends Pharmacol Sci. 2003;24:103–106. doi: 10.1016/S0165-6147(03)00023-3. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kim HY, Rogawski MA. Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia. 2001;42:328–336. doi: 10.1046/j.1528-1157.2001.10100.x. [DOI] [PubMed] [Google Scholar]
- Rivier C. Alcohol stimulates ACTH secretion in the rat: mechanisms of action and interactions with other stimuli. Alcohol Clin Exp Res. 1996;20:240–254. doi: 10.1111/j.1530-0277.1996.tb01636.x. [DOI] [PubMed] [Google Scholar]
- Rivier C, Vale W. Diminished responsiveness of the hypothalamic-pituitary-adrenal axis of the rat during exposure to prolonged stress: a pituitary-mediated mechanism. Endocrinology. 1987;121:1320–1328. doi: 10.1210/endo-121-4-1320. [DOI] [PubMed] [Google Scholar]
- Rivier C, Bruhn T, Vale W. Effect of ethanol on the hypothalamic-pituitary-adrenal axis in the rat: role of corticotropin-releasing factor (CRF) J Pharmacol Exp Ther. 1984;229:127–131. [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci USA. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Smith AD, Weiss F, Rivier C, Koob GF. Estrous cycle effects on operant responding for ethanol in female rats. Alcohol Clin Exp Res. 1998;22:1564–1569. [PubMed] [Google Scholar]
- Roca DJ, Rozenberg I, Farrant M, Farb DH. Chronic agonist exposure induces down-regulation and allosteric uncoupling of the γ-aminobutyric acid/benzodiazepine receptor complex. Mol Pharmacol. 1990a;37:37–43. [PubMed] [Google Scholar]
- Roca DJ, Schiller GD, Friedman L, Rozenberg I, Gibbs TT, Farb DH. γ-Aminobutyric acidA receptor regulation in culture: altered allosteric interactions following prolonged exposure to benzodiazepines, barbiturates, and methylxanthines. Mol Pharmacol. 1990b;37:710–719. [PubMed] [Google Scholar]
- Roland BL, Sawchenko PE. Local origins of some GABAergic projections to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol. 1993;332:123–143. doi: 10.1002/cne.903320109. [DOI] [PubMed] [Google Scholar]
- Romeo E, Brancati A, De Lorenzo A, Fucci P, Furnari C, Pompili E, et al. Marked decrease of plasma neuroactive steroids during alcohol withdrawal. Clin Neuropharmacol. 1996;19:366–369. doi: 10.1097/00002826-199619040-00011. [DOI] [PubMed] [Google Scholar]
- Russell JA, Douglas AJ, Ingram CD. Brain preparations for maternity-adaptive changes in behavioral and neuroendocrine systems during pregnancy and lactation. An overview. Prog Brain Res. 2001;133:1–38. doi: 10.1016/s0079-6123(01)33002-9. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Aguado F, Sanchez-Toscano F, Saphier D. Neuroendocrine and immunocytochemical demonstrations of decreased hypothalamo-pituitary-adrenal axis responsiveness to restraint stress after long-term social isolation. Endocrinology. 1998;139:579–587. doi: 10.1210/endo.139.2.5720. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Hart SR. Isolation stress during the third postnatal week alters radial arm maze performance and corticosterone levels in adulthood. Behav Brain Res. 2005;56:289–296. doi: 10.1016/j.bbr.2004.05.033. [DOI] [PubMed] [Google Scholar]
- Sanna E, Cuccheddu T, Serra M, Concas A, Biggio G. Carbon dioxide inhalation reduces the function of GABAA receptors in the rat brain. Eur J Pharmacol. 1992;216:457–458. doi: 10.1016/0014-2999(92)90447-c. [DOI] [PubMed] [Google Scholar]
- Sanna E, Serra M, Cossu A, Colombo G, Follesa P, Cuccheddu T, et al. Chronic ethanol intoxication induces differential effects on GABAA and NMDA receptor function in the rat brain. Alcohol Clin Exp Res. 1993;17:115–123. doi: 10.1111/j.1530-0277.1993.tb00735.x. [DOI] [PubMed] [Google Scholar]
- Sanna E, Busonero F, Talani G, Carta M, Massa F, Peis M, et al. Comparison of the effects of zaleplon, zolpidem, and triazolam at various GABAA receptor subtypes. Eur J Pharmacol. 2002;451:103–110. doi: 10.1016/s0014-2999(02)02191-x. [DOI] [PubMed] [Google Scholar]
- Sanna E, Mostallino MC, Busonero F, Talani G, Tranquilli S, Mameli M, et al. Changes in GABAA receptor gene expression associated with selective alterations in receptor function and pharmacology after ethanol withdrawal. J Neurosci. 2003;23:11711–11724. doi: 10.1523/JNEUROSCI.23-37-11711.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, et al. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J Neurosci. 2004;24:6521–6530. doi: 10.1523/JNEUROSCI.0075-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Corticosterone receptors decline in a site-specific manner in the aged rat brain. Brain Res. 1983;289:235–240. doi: 10.1016/0006-8993(83)90024-0. [DOI] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. Assembly of GABAA receptor subunits: role of the δ subunit. J Neurosci. 1994;14:7077–7086. doi: 10.1523/JNEUROSCI.14-11-07077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena N, Macdonald R. Properties of putative cerebellar γ-aminobutyric acid A receptor isoforms. Mol Pharmacol. 1996;49:458–466. [PubMed] [Google Scholar]
- Schenk K, Gorman K, Amit Z. Age-dependent effects of isolation housing on the self-administration of ethanol in laboratory rats. Alcohol. 1990;7:321–326. doi: 10.1016/0741-8329(90)90090-y. [DOI] [PubMed] [Google Scholar]
- Schenk S, Britt MD, Atalay J, Charleson S. Isolation rearing decreases opiate receptor binding in rat brain. Pharmacol Biochem Behav. 1982;16:841–842. doi: 10.1016/0091-3057(82)90245-3. [DOI] [PubMed] [Google Scholar]
- Sellers EM, Naranjo CA, Harrison M, Devenyi P, Roach C, Sykora K. Diazepam loading: simplified treatment of alcohol withdrawal. Clin Pharmacol Ther. 1983;34:822–826. doi: 10.1038/clpt.1983.256. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Serra M, Concas A, Atsoggiu T, Biggio G. Stress like bicuculline and DMCM reduces the basal 36Cl− uptake in the rat cortical membrane vesicles: an effect reversed by GABA. Neurosci Res Commun. 1989a;4:41–50. [Google Scholar]
- Serra M, Sanna E, Biggio G. Isoniazid, an inhibitor of GABAergic transmission, enhances [35S]TBPS binding in rat cerebral cortex. Eur J Pharmacol. 1989b;164:385–388. doi: 10.1016/0014-2999(89)90484-6. [DOI] [PubMed] [Google Scholar]
- Serra M, Sanna E, Concas A, Foddi C, Biggio G. Foot-shock stress enhances the increase of [35S]TBPS binding in the rat cerebral cortex and the convulsions induced by isoniazid. Neurochem Res. 1991a;16:17–22. doi: 10.1007/BF00965822. [DOI] [PubMed] [Google Scholar]
- Serra M, Sanna E, Foddi C, Concas A, Biggio G. Failure of γ-hydroxybutyrate to alter the function of the GABAA receptor complex in the rat cerebral cortex. Psychopharmacology (Berl) 1991b;104:351–355. doi: 10.1007/BF02246035. [DOI] [PubMed] [Google Scholar]
- Serra M, Madau P, Chessa MF, Caddeo M, Sanna E, Trapani G, et al. 2-Phenyl-imidazo[1,2-a]pyridine derivatives as ligands for peripheral benzodiazepine receptors: stimulation of neurosteroid synthesis and anticonflict action in rats. Br J Pharmacol. 1999;127:177–187. doi: 10.1038/sj.bjp.0702530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Littera M, Papi G, Sanna E, Tuveri F, et al. Social isolation-induced decreases in both the abundance of neuroactive steroids and GABAA receptor function in rat brain. J Neurochem. 2000;75:732–740. doi: 10.1046/j.1471-4159.2000.0750732.x. [DOI] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Floris I, Cara V, Purdy RH, Biggio G. Social isolation-induced increase in the sensitivity of rats to the steroidogenic effect of ethanol. J Neurochem. 2003;85:257–263. doi: 10.1046/j.1471-4159.2003.01680.x. [DOI] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Floris I, Floris S, Cannas E, Mossa A, et al. Social isolation increases the response of peripheral benzodiazepine receptors in the rat. Neurochem Int. 2004;45:141–148. doi: 10.1016/j.neuint.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Serra M, Mostallino MC, Talani G, Pisu MG, Carta M, Mura ML, et al. Social isolation-induced increase in alpha and delta subunit gene expression is associated with a greater efficacy of ethanol on steroidogenesis and GABA receptor function. J Neurochem. 2006;98:122–133. doi: 10.1111/j.1471-4159.2006.03850.x. [DOI] [PubMed] [Google Scholar]
- Shannon EE, Shelton KL, Vivian JA, Yount I, Morgan AR, Homanics GE, et al. Discriminative stimulus effects of ethanol in mice lacking the γ-aminobutyric acid type A receptor δ subunit. Alcohol Clin Exp Res. 2004;28:906–913. doi: 10.1097/01.alc.0000128227.28794.42. [DOI] [PubMed] [Google Scholar]
- Sheela Rani CS, Ticku MK. Comparison of chronic ethanol and chronic intermittent ethanol treatments on the expression of GABAA and NMDA receptor subunits. Alcohol. 2006;38:89–97. doi: 10.1016/j.alcohol.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Shibuya K, Takata N, Hojo Y, Furukawa A, Yasumatsu N, Kimoto T, et al. Hippocampal cytochrome P450s synthesize brain neurosteroids which are paracrine neuromodulators of synaptic signal transduction. Biochim Biophys Acta. 2003;1619:301–316. doi: 10.1016/s0304-4165(02)00489-0. [DOI] [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of γ-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- Sierra A. Neurosteroids: the StAR protein in the brain. J Neuroendocrinol. 2004;6:787–793. doi: 10.1111/j.1365-2826.2004.01226.x. [DOI] [PubMed] [Google Scholar]
- Sinnott RS, Phillips TJ, Finn DA. Alteration of voluntary ethanol and saccharin consumption by the neurosteroid allopregnanolone in mice. Psychopharmacology (Berl) 2002;162:438–447. doi: 10.1007/s00213-002-1123-1. [DOI] [PubMed] [Google Scholar]
- Smith SS, editor. Methods & New Frontiers in Neuroscience. New York: CRC Press; 2004. Neurosteroid effects in the central nervous system. [Google Scholar]
- Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JM, Li X. GABAA receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998a;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Li X, Moran MH, Bitran D, Frye CA, et al. Withdrawal from 3α-OH-5α-pregnan-20-one using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABAA receptor α4 subunit in association with increased anxiety. J Neurosci. 1998b;18:5275–5284. doi: 10.1523/JNEUROSCI.18-14-05275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spigelman I, Li Z, Banerjee PK, Mihalek RM, Homanics GE, Olsen RW. Behavior and physiology of mice lacking the GABAA-receptor δ subunit. Epilepsia. 2002;43:3–8. doi: 10.1046/j.1528-1157.43.s.5.8.x. [DOI] [PubMed] [Google Scholar]
- Spigelman I, Li Z, Liang J, Cagetti E, Samzadeh S, Mihalek RM, et al. Reduced inhibition and sensitivity to neurosteroids in hippocampus of mice lacking the GABAA receptor δ subunit. J Neurophysiol. 2003;90:903–910. doi: 10.1152/jn.01022.2002. [DOI] [PubMed] [Google Scholar]
- Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABAA conductances in hippocampal neurons. J Neurosci. 2002;22:RC223. doi: 10.1523/JNEUROSCI.22-10-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc Natl Acad Sci USA. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DN, Schneider HH, Kehr W, Andrews JS, Rettig KJ, Turski L, et al. Abecarnil, a metabolically stable, anxioselective β-carboline acting at benzodiazepine receptors. J Pharmacol Exp Ther. 1990;253:334–343. [PubMed] [Google Scholar]
- Stocco DM. The role of the StAR protein in steroidogenesis: challenges for the future. Endocrinology. 2000;164:247–253. doi: 10.1677/joe.0.1640247. [DOI] [PubMed] [Google Scholar]
- Strohle A, Romeo E, di Michele F, Pasini A, Hermann B, Gajewsky G, et al. Induced panic attacks shift γ-aminobutyric acid type A receptor modulatory neuroactive steroid composition in patients with panic disorder: preliminary results. Arch Gen Psychiatry. 2003;60:161–168. doi: 10.1001/archpsyc.60.2.161. [DOI] [PubMed] [Google Scholar]
- Sullivan SD, Moenter SM. Neurosteroids alter γ-aminobutyric acid postsynaptic currents in gonadotropin-realising hormone neurons: a possible mechanism for direct steroidal control. Endocrinology. 2003;144:4366–4375. doi: 10.1210/en.2003-0634. [DOI] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, et al. Hormonally regulated α4β2δ GABAA receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur C, Farrar SJ, Kerby J, Whiting PJ, Atack JR, McKernan RM. Preferential coassembly of α4 and δ subunits of the γ-aminobutyric acidA receptor in rat thalamus. Mol Pharmacol. 1999;56:110–115. doi: 10.1124/mol.56.1.110. [DOI] [PubMed] [Google Scholar]
- Suzdak PD, Glowa JR, Crawley JN, Schwartz RD, Skolnick P, Paul SM. A selective imidazobenzodiazepine antagonist of ethanol in the rat. Science. 1986;234:1243–1247. doi: 10.1126/science.3022383. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Slawecki CJ, El Khoury A, Mathe AA, Ehlers CL. Effect of social isolation on ethanol consumption and substance P/neurokinin expression in Wistar rats. Alcohol. 2005;36:91–97. doi: 10.1016/j.alcohol.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Slawecki CJ, El Khoury A, Mathe AA, Ehlers CL. The effects of social isolation on neuropeptide Y levels, exploratory and anxiety-related behaviors in rats. Pharmacol Biochem Behav. 2006;83:28–34. doi: 10.1016/j.pbb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Ticku MK, Burch T. Alterations in γ-aminobutyric acid receptor sensitivity following acute and chronic ethanol treatments. J Neurochem. 1980;34:417–423. doi: 10.1111/j.1471-4159.1980.tb06612.x. [DOI] [PubMed] [Google Scholar]
- Trapani G, Franco M, Ricciardi L, Latrofa A, Genchi G, Sanna E, et al. Synthesis and binding affinity of 2-phenylimidazo[1,2-a]pyridine derivatives for both central and peripheral benzodiazepine receptors. A new series of high-affinity and selective ligands for peripheral type. J Med Chem. 1997;40:3109–3118. doi: 10.1021/jm970112+. [DOI] [PubMed] [Google Scholar]
- Tseng YT, Miyaoka T, Ho IK. Region-specific changes of GABAA receptors by tolerance to and dependence upon pentobarbital. Eur J Pharmacol. 1993;236:23–30. doi: 10.1016/0014-2999(93)90222-4. [DOI] [PubMed] [Google Scholar]
- Ueno S, Harris RA, Messing RO, Sanchez-Perez AM, Hodge CW, McMahon T, et al. Alcohol actions on GABAA receptors: from protein structure to mouse behavior. Alcohol Clin Exp Res. 2001;25(suppl 5):76S–81S. doi: 10.1097/00000374-200105051-00014. [DOI] [PubMed] [Google Scholar]
- Usui M, Yamazaki T, Kominami S, Tsutsui K. Avian neurosteroids. II. Localization of a cytochrome P450scc-like substance in the quail brain. Brain Res. 1995;678:10–20. doi: 10.1016/0006-8993(95)00117-9. [DOI] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, et al. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluovoxamine. Proc Natl Acad Sci USA. 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3α-hydroxy-5α-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci. 2000;20:1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanover KE, Suruki M, Robledo S, Huber M, Wieland S, Lan NC, et al. Positive allosteric modulators of the GABAA receptor: differential interaction of benzodiazepines and neuroactive steroids with ethanol. Psychopharmacology (Berl) 1999;141:77–82. doi: 10.1007/s002130050809. [DOI] [PubMed] [Google Scholar]
- Varty GB, Paulus MP, Braff DL, Geyer MA. Environmental enrichment and isolation rearing in the rat: effects on locomotor behavior and startle response plasticity. Biol Psychiatry. 2000;47:864–873. doi: 10.1016/s0006-3223(99)00269-3. [DOI] [PubMed] [Google Scholar]
- Verkuyl JM, Hemby SE, Joels M. Chronic stress attenuates GABAergic inhibition and alters gene expression of parvocellular neurons in rat hypothalamus. Eur J Neurosci. 2004;20:1665–1673. doi: 10.1111/j.1460-9568.2004.03568.x. [DOI] [PubMed] [Google Scholar]
- Vicini S. New perspectives in the functional role of GABAA channel heterogeneity. Mol Neurobiol. 1999;19:97–110. doi: 10.1007/BF02743656. [DOI] [PubMed] [Google Scholar]
- Viveros MP, Hernandez R, Martinez I, Gonzalez P. Effects of social isolation and crowding upon adrenocortical reactivity and behavior in the rat. Rev Esp Fisiol. 1988;44:315–321. [PubMed] [Google Scholar]
- Voikar V, Polus A, Vasar E, Rauvala H. Long-term individual housing in C57BL/6J and DBA/2 mice: assessment of behavioral consequences. Genes Brain Behav. 2005;4:240–252. doi: 10.1111/j.1601-183X.2004.00106.x. [DOI] [PubMed] [Google Scholar]
- Wafford KA. GABAA receptor subtypes: any clues to the mechanism of benzodiazepine dependence? Curr Opin Pharmacol. 2005;5:47–52. doi: 10.1016/j.coph.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Thompson SA, Thomas D, Sikela J, Wilcox AS, Whiting PJ. Functional characterization of human γ-aminobutyric acidA receptors containing the α4 subunit. Mol Pharmacol. 1996;50:670–678. [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances α4β3δ and α6β3δ γ-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci USA. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Low-dose alcohol actions on α4β3δ GABAA receptors are reversed by the behavioral alcohol antagonist Ro15-4513. Proc Natl Acad Sci USA. 2006;103:8540–8545. doi: 10.1073/pnas.0600194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan FJ, Berton F, Madamba SG, Francesconi W, Siggins GR. Low ethanol concentrations enhance GABAergic inhibitory postsynaptic potentials in hippocampal pyramidal neurons only after block of GABAB receptors. Proc Natl Acad Sci USA. 1996;93:5049–5054. doi: 10.1073/pnas.93.10.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Faria LC, Mody I. Low ethanol concentrations selectively augment the tonic inhibition mediated by δ subunit-containing GABAA receptors in hippocampal neurons. J Neurosci. 2004;24:8379–8382. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of δ subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Ther. 2006;111:533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Weiner JL, Gu C, Dunwiddie TV. Differential ethanol sensitivity of subpopulations of GABAA synapses onto rat hippocampal CA1 pyramidal neurons. J Neurophysiol. 1997;77:1306–1312. doi: 10.1152/jn.1997.77.3.1306. [DOI] [PubMed] [Google Scholar]
- Weizman R, Dagan E, Snyder SH, Gavish M. Impact of pregnancy and lactation on GABAA receptor and central-type and peripheral-type benzodiazepine receptors. Brain Res. 1997;752:307–314. doi: 10.1016/s0006-8993(96)01489-8. [DOI] [PubMed] [Google Scholar]
- Whiting PJ, Bonnert TP, McKernan RM, Farrar S, Le Bourdelles B, Heavens RP, et al. Molecular and functional diversity of the expanding GABA-A receptor gene family. Ann NY Acad Sci. 1999;868:645–653. doi: 10.1111/j.1749-6632.1999.tb11341.x. [DOI] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABAA receptors containing the δ subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffgramm J. Free choice ethanol intake of laboratory rats under different social conditions. Psychopharmacology (Berl) 1990;101:233–239. doi: 10.1007/BF02244132. [DOI] [PubMed] [Google Scholar]
- Wongwitdecha N, Marsden CA. Social isolation increases aggressive behaviour and alters the effects of diazepam in the rat social interaction test. Behav Brain Res. 1996;75:27–32. doi: 10.1016/0166-4328(96)00181-7. [DOI] [PubMed] [Google Scholar]
- Woods SW, Charney DS, Loke J, Goodman WK, Redmond DE, Jr, Heninger GR. Carbon dioxide sensitivity in panic anxiety. Ventilatory and anxiogenic response to carbon dioxide in healthy subjects and patients with panic anxiety before and after alprazolam treatment. Arch Gen Psychiatry. 1986;43:900–909. doi: 10.1001/archpsyc.1986.01800090090013. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Marszalec W, Yeh JZ, Narahashi T. Effects of ethanol on tonic GABA currents in cerebellar granule cells and mammalian cells recombinantly expressing GABAA receptors. J Pharmacol Exp Ther. 2006;319:431–438. doi: 10.1124/jpet.106.106260. [DOI] [PubMed] [Google Scholar]
- Yu R, Follesa P, Ticku MK. Down-regulation of the GABA receptor subunits mRNA levels in mammalian cultured cortical neurons following chronic neurosteroid treatment. Brain Res Mol Brain Res. 1996;41:163–168. doi: 10.1016/0169-328x(96)00087-3. [DOI] [PubMed] [Google Scholar]
- Zhu WJ, Vicini S. Neurosteroid prolongs GABAA channel deactivation by altering kinetics of desensitized states. J Neurosci. 1997;17:4022–4031. doi: 10.1523/JNEUROSCI.17-11-04022.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziskind-Conhaim L, Gao BX, Hinckley C. Ethanol dual modulatory actions on spontaneous postsynaptic currents in spinal motoneurons. J Neurophysiol. 2003;89:806–813. doi: 10.1152/jn.00614.2002. [DOI] [PubMed] [Google Scholar]