Abstract
Despite the increasing number of population-based surveys in sub-Saharan Africa that provide testing and counseling for HIV over the past decade, understanding the nature of nonresponse in these surveys, especially panel HIV surveys, is still limited. This article uses longitudinal HIV data collected from rural Malawi in 2004 and 2006 to examine nonresponse in repeat population-based testing. It shows that nonresponse in repeat testing led to significant bias in the estimates of HIV prevalence and to inconsistent conclusions about the predictors of HIV status. In contrast, previous cross-sectional analyses found that nonresponse does not significantly bias the estimates of HIV prevalence. The difference in conclusions from cross-sectional and longitudinal analyses of nonresponse can be attributed to two factors: the different definitions of what constitutes nonresponse in both contexts, and the risk profiles of the missed populations. In particular, although refusal and temporary absence are the major sources of nonresponse in the cross-sectional contexts, attrition attributable to mortality and out-migration are additional sources of nonresponse in repeat testing. Evidence shows that out-migrants have higher HIV prevalence than nonmigrants, which could account for significant bias in the estimates of prevalence among participants in both tests observed in this study.
Sub-Saharan Africa remains the region most affected by the HIV/AIDS epidemic, with the region recording the highest HIV prevalence compared with the other regions of the world. Prevalence among adults aged 15–49 years in the region (5%) is five times higher than that of the Caribbean (1%), which has the second-highest prevalence (UNAIDS and WHO 2007). However, the estimate for sub-Saharan Africa masks the wide variations in prevalence among countries in the region with national prevalence ranging from <1% in the Comoros to 33% in Swaziland (UNAIDS 2006). Besides abstinence, being faithful/partner reduction, and condom use (popularly known as the “ABC of HIV prevention”), the other strategy being promoted to stem further spread of the epidemic in the region is voluntary counseling and testing (VCT). One aim of this strategy is to promote behavior change by encouraging those who are already infected to avoid transmitting the virus to others and encouraging those who are not infected to avoid high-risk behavior (Family Health International 2006; Matovu et al. 2007).1
The provision of VCT services in much of the region is done in stand-alone VCT clinics and in selected prenatal clinics, health centers, and hospitals (Boerma, Ghys, and Walker 2003; Family Health International 2005; WHO and UNAIDS 2003). The past decade has also witnessed an increasing number of population-based initiatives (at the community and national levels) providing testing and counseling for HIV. Unlike VCT programs provided within fixed facilities, these initiatives have the advantage that they (1) cover a much wider segment of the population, particularly in the rural areas of sub-Saharan Africa, because the clinics and health facilities are disproportionately located in urban and peri-urban areas (Boerma et al. 2003; Nyblade et al. 2001), and (2) collect information on the segment of the population that is at risk of HIV infection but chooses not to participate in the VCT program (Nyblade et al. 2001). This allows for a better understanding of the characteristics associated with participation in the programs, which is essential for gauging the acceptability and effectiveness of the programs.
The population-based initiatives, however, face challenges that can adversely affect their effectiveness. One of these is nonresponse due to refusal or absence, and due to loss to follow-up in longitudinal studies. The other challenges include logistical difficulties of testing in the field rather than a clinic setting, the cost associated with conducting a survey, ethical issues, and sample representativeness (Boerma, Holt, and Black 2001; Fisher, Pappas, and Limb 1996; WHO and UNAIDS 2003). This article focuses on nonresponse in repeat, community-level population-based VCT for HIV in rural Malawi, one of the countries in sub-Saharan Africa with generalized epidemics (national HIV prevalence of more than 10%). Specifically, this article (1) compares the characteristics of participants and nonparticipants in the repeat HIV testing program to determine whether they significantly differ; and (2) examines the impact of nonresponse in the HIV prevalence estimates and on the predictors of HIV status.
Nonresponse arises when individuals who are selected to participate in a survey fail to do so because of outright refusal for various reasons, because of physical and mental impairment, or by failure of the research team to contact respondents who are temporarily away from home (Groves 2004; Groves and Couper 1998). Nonresponse is important in population-based HIV surveys because it can lead to significant bias in the HIV prevalence estimates and inconsistent estimates of the predictors of HIV status. These, in turn, lead to questionable conclusions about the state of the HIV/AIDS epidemic in the population and about the risk factors associated with it. Furthermore, the growing number of population-based HIV surveys in the past decade have been accompanied by warnings that the prevalence estimates obtained from these surveys may not be the gold standard because of the potential for nonresponse bias (e.g., by Boerma et al. 2003; WHO and UNAIDS 2003).2 However, the literature on nonresponse bias for this type of surveys is still limited, with much of the available evidence coming from cross-sectional surveys, most notably the Demographic and Health Surveys (DHS) (e.g., Bignami-Van Assche, Salomon, and Murray 2005; Mishra et al. 2006). Thus, the literature on nonresponse bias in population-based longitudinal HIV surveys is even more limited.
CONTEXT
Malawi had a total of 28 administrative districts as of 2004 (National Statistics Office [NSO] and ORC Macro 2005). Six of the districts were in the Northern region, nine in the Central region, and thirteen in the Southern region. With a population close to 14 million by mid-2008, approximately 83% lived in the rural areas (Population Reference Bureau 2008). The Northern region is less densely populated but is wealthier and has a higher proportion of individuals with some education (primary and higher) than the Central or the Southern regions (NSO and ORC Macro 2005). The three regions are also characterized by distinctive marriage patterns: the Southern region is predominantly matrilineal and matrilocal, the Northern region is predominantly patrilineal and patrilocal, and the Central region is characterized by mixed marriage patterns (Zulu 1996; Zulu and Chepng’eno 2003). These distinctive marriage patterns have been found to play a role in defining the mechanisms through which social interactions shape attitudes toward HIV/AIDS (Helleringer and Kohler 2005).
As of 2004, the national HIV prevalence among adults aged 15–49 years was 12% (NSO and ORC Macro 2005). Prevalence was more than twice as high in the Southern region (18%) than in the Northern (8%) or the Central regions (7%). According to estimates by UNAIDS (2006), between 480,000 and 1.4 million people (including adults and children) were living with HIV in Malawi as of 2005. By the end of 2005, slightly more than 37,000 patients were on anti-retroviral treatment (ART) in 60 public health facilities (Harries et al. 2006). Plans to scale up ART were started in 2004 (Libamba et al. 2006), but even with scale-up, it was projected that only about 50% of those who are eligible for treatment would receive it each year (Harries et al. 2006). Efforts to cover all eligible HIV/AIDS patients with ART are hampered by the inadequacy of the health care capacity to offer treatment, logistical difficulties with drug supplies, drug resistance, and financial limitations. The availability of ART is one of the factors that influence the decision to be tested for HIV. Knowing that one is HIV-positive is much more relevant if he/she can get ART. For instance, Reniers et al. (2009) found that consent for testing in Ethiopia increased after the introduction of ART in the hospital where the study was based.
The percentage of respondents in the 2004 Malawi Demographic and Health Survey (MDHS) that were tested for HIV was higher in the Northern region (78%) than in the Southern (66%) or Central regions (64%) (NSO and ORC Macro 2005). The proportion that refused the test was higher in the Central region (26%) than in the Southern or the Northern regions (21% and 14%, respectively), while the percentage that was temporarily away was higher in the Southern region (11%) than in the Central (9%) or Northern regions (8%) (NSO and ORC Macro 2005). Individuals can refuse to be tested for HIV for various reasons: for example, if they suspect that they are already infected based on their assessment of prior sexual or other risky behavior (such as injecting drug use). Mobility, which comprises temporary absence and out-migration, has been associated with increased risk of HIV infection in parts of sub-Saharan Africa (e.g., Crampin et al. 2003; Anglewicz 2007). This is because when those in sexual relationships are separated from each other, they are likely to seek other sexual partners at points of destination, thereby increasing their chances of being infected with HIV; otherwise, mobility unto itself does not matter for the risk of HIV infection.
DATA
This study uses data from a longitudinal survey, the Malawi Diffusion and Ideational Change Project (MDICP), conducted in three rural sites in Malawi. The three sites are located in Balaka District in the Southern region, Mchinji District in the Central region, and Rumphi District in the Northern region (hereafter referred to as the South, Center, and North, respectively). The aim of the project is to examine the role of social networks in changing attitudes and behavior regarding family size, family planning, and HIV/AIDS in rural Malawi. The initial sample was not designed to represent the national population of rural Malawi, but the sample characteristics closely matched those of the rural sample in the 1996 Malawi Demographic and Health Survey (Watkins et al. 2003). The first and second waves (1998 and 2001) involved survey data collection only. The 1998 sample targeted 1,500 ever-married women and their husbands (Watkins et al. 2003); in 2001, new spouses of those already in the sample were added. The third and fourth waves of the project were conducted in 2004 and 2006, respectively, and included a biomarker component in addition to the main survey data collection. The target sample in 2004 was the 2001 sample, new spouses to those already in the sample, and a new sample of about 1,000 adolescents (married and unmarried) aged 15–24 years. The 2006 sample also included spouses of married adolescents.
In 2004, trained nurses collected saliva specimens (using OraSure oral swab) from consenting respondents for HIV testing about two to three days after the visit by the survey team (Anglewicz et al. 2005; Bignami-Van Assche et al. 2004). The specimens were then analyzed at the laboratory in the capital city (Lilongwe) in Malawi, using enzyme-linked immunosorbent assay (ELISA) and confirmatory Western blot tests (Bignami-Van Assche et al. 2004). The test results and posttest counseling were provided by team nurses in mobile VCT centers set up by the project (Thornton 2008). In 2006, consenting survey respondents provided blood spots for rapid HIV tests in their homes. HIV testing was done by trained VCT counselors using parallel Determine (Abbott Laboratories, Abbott Park, Illinois) and reactive samples confirmed by UniGold (Trinity Biotech, Bray, Ireland). Respondents were given the option to receive their test results either in their homes or at mobile clinics, which were to be set up by the project at the end of the survey. However, virtually all respondents opted for obtaining their test results immediately in their homes.
Conditional upon contact, acceptance of HIV testing in the MDICP was high and remained fairly stable in both surveys. Of the 3,282 individuals successfully contacted for HIV test in 2004, 91% (2,983) consented to be tested. Similarly, a total of 2,987 individuals were successfully contacted in 2006, and 92% (2,758) consented to be tested. Nonetheless, the response rate for HIV testing among those ever interviewed in previous waves was much lower than the acceptance rate (Table 1). The percentage of respondents who obtained their test results and posttest counseling was also lower in the first survey than in the second survey. Of those whose test results were available in 2004, only about two-thirds (67%) obtained their test results. In contrast, 98% of those who were tested in 2006 obtained their test results. With respect to acceptance of repeat HIV testing, of those who were tested in 2004, 68% (2,031 individuals) were also tested in 2006; another 110 individuals refused the test; 365 individuals were either temporarily away or had out-migrated from the study sites by 2006; and the remaining individuals were lost to follow-up either because of mortality or because they could not be traced (Table 1).
Table 1.
Distribution of Study Participants by HIV Test Outcome in 2004 for Those Ever Interviewed in Previous Waves, and in 2006 for Those Ever Interviewed in Previous Waves and Those Tested in 2004, MDICP 2004–2006
| Distribution by Test Outcome in 2004 for Those Ever Interviewed in Previous Waves (1998 and 2001)a |
||||||
|---|---|---|---|---|---|---|
| Men |
Women |
Both Sexes |
||||
| Test Outcome | Number | % | Number | % | Number | % |
| Tested | 697 | 54.2 | 1,130 | 61.1 | 1,827 | 58.3 |
| Refused | 81 | 6.3 | 100 | 5.4 | 181 | 5.8 |
| Absent/Moved | 176 | 13.7 | 220 | 11.9 | 396 | 12.6 |
| Dead | 33 | 2.6 | 52 | 2.8 | 85 | 2.7 |
| Other/Missing | 299 | 23.3 | 347 | 18.8 | 646 | 20.6 |
| Total | 1,286 | 100.0 | 1,849 | 100.0 | 3,135 | 100.0 |
| Distribution by Test Outcome in 2006 for Those Ever Interviewed in Previous Waves (1998, 2001, and 2004)b |
||||||
| Tested | 1,105 | 52.9 | 1,353 | 55.7 | 2,458 | 54.4 |
| Refused | 91 | 4.4 | 107 | 4.4 | 198 | 4.4 |
| Absent/Moved | 376 | 18.0 | 438 | 18.0 | 814 | 18.0 |
| Dead | 63 | 3.0 | 66 | 2.7 | 129 | 2.9 |
| Other/Missing | 456 | 21.8 | 467 | 19.2 | 923 | 20.4 |
| Total | 2,091 | 100.0 | 2,431 | 100.0 | 4,522 | 100.0 |
| Distribution by Test Outcome in 2006 for Those Tested in 2004 |
||||||
| Tested | 903 | 65.6 | 1,128 | 70.2 | 2,031 | 68.1 |
| Refused | 51 | 3.7 | 59 | 3.7 | 110 | 3.7 |
| Absent/Moved | 184 | 13.4 | 181 | 11.3 | 365 | 12.2 |
| Dead | 19 | 1.4 | 15 | 0.9 | 34 | 1.1 |
| Other/Not Known/Missing | 219 | 15.9 | 224 | 13.9 | 443 | 14.9 |
| Total | 1,376 | 100.0 | 1,607 | 100.0 | 2,983 | 100.0 |
Note: Percentages may not add up to exactly 100 in some cases because of rounding error.
Source: From the author’s calculations of data from the Malawi Diffusion and Ideational Change Project.
Excludes the adolescent sample that was added in 2004.
Includes the 2004 adolescent sample.
METHODS
Descriptive Analysis
The first part of descriptive analysis involves a comparison of participants in both tests and nonparticipants in the second test who participated in the first test based on their background characteristics and HIV-related behaviors. It also entails testing whether the two groups significantly differ in terms of these characteristics. The purpose is to examine whether nonparticipation in the second testing was associated with either HIV status or standard measures of risky behavior in 2004. If nonparticipants systematically exhibit characteristics that suggest that they were more likely to be HIV-positive, this should be an indication that the HIV prevalence estimate for the longitudinal participants is significantly biased downward. The opposite (significant upward bias) should be the case if nonparticipants’ characteristics show that they were systematically less likely to be HIV-positive than participants.
The background characteristics considered in the comparisons include age, sex, study site, highest educational attainment, and current marital status. Prevalence estimates from the two surveys indicate that HIV prevalence was highest among those aged 25–44 years, among women, in the South, among those with less than secondary education, and among those who were formerly married (separated, divorced, and widowed). One should therefore expect significant downward bias in the prevalence estimates for the longitudinal participants if the percentage of nonparticipants with these characteristics was significantly higher than the participants with similar characteristics. The HIV-related behaviors include HIV status in 2004, number of marital unions, number of lifetime sexual partners, suspicion of the spouse’s/partner’s infidelity, level of worry about getting HIV/AIDS, perceived risk of current infection, and knowledge of someone who was living with or who had died of AIDS. Multiple unions and sexual partnerships increase chances of HIV infection. One might expect that suspicion of the partner’s infidelity, the level of worry about getting HIV/AIDS, and perceived risk of current infection are reflections of prior or current high-risk sexual behavior of the partner or the individual. Knowledge of persons living with or who had died of AIDS is an indicator of the disease’s prevalence in the community. One should therefore expect significant bias in the prevalence estimates for the longitudinal participants if characteristics associated with high prevalence or high perceived likelihood of infection are also associated with nonparticipation in testing over time.
The second part (of descriptive analysis) is a sensitivity analysis that involves assuming a prevalence of, say, 0%, 5%, 10%, and so on to 50% among nonparticipants in the second testing who participated in the first testing, estimating a new prevalence on the basis of these assumptions,3 and testing whether the new estimate is significantly different from the observed estimate among longitudinal participants. The question of interest here is, If in 2006 the MDICP had only tested those who were also tested in 2004, would non-response have significantly biased the prevalence estimate for that year?4 The expectation is that if nonresponse was a significant source of bias, there should be significant differences between the observed and the estimated prevalence at plausible levels of assumed prevalence among nonparticipants. The implication then is that the observed prevalence in 2006 among the longitudinal participants was an underestimate of the trends in rural prevalence for the districts included in the MDICP.
Multivariate Probit Analysis
The multivariate probit analysis aims at examining whether the predictors of HIV status among longitudinal participants in the testing program differed between the first and second testing. If that is the case, this should be an indication that nonparticipation in the second testing by those who participated in the first results in inconsistent estimates of the predictors of HIV status. The empirical model posits that each individual has an underlying risk of being infected with HIV, denoted by Y*i, and that there is some threshold, θ, beyond which an individual is observed to be HIV-positive if the underlying risk exceeds this threshold, or to be HIV-negative if the underlying risk is at or below the threshold:
| (1) |
Yi in Eq. (1) is the observed HIV status of individual i, which is a function of measured individual background and behavioral characteristics and a residual term (ɛi) that accounts for the unmeasured characteristics. The model formulation is as follows:
| (2) |
where β0 is the intercept. I estimate Eq. (2) by means of a probit model, since it is appropriate for modeling latent responses (Allison 1999). The potential predictors of HIV status include age, sex of the respondent, study site, highest education level, religious affiliation, current marital status, whether the respondent had stayed outside the district for six months or more since age 15, (if ever married) the number of times the respondent had ever been married, whether the spouse usually stayed outside the village, number of lifetime sexual partners, and perceived risk of current infection.
RESULTS
Comparison of Participants’ and Nonparticipants’ Characteristics
Tables 2 and 3 present the results of the comparison of participants and nonparticipants in the MDICP HIV testing program in the longitudinal context. Participants in this case are those who were tested for HIV in both surveys; nonparticipants are those who were tested in 2004 but not in 2006 because they refused, were temporarily away, had moved out of the study sites, or could not be tracked down by the survey despite having been previously tested (see Table 1, bottom panel).5 Table 2 presents the results of these comparisons based on individual background characteristics. The results suggest that a significant downward bias in the prevalence estimates for the longitudinal participants could result from (1) the significant difference in the percentage of individuals who were tested in 2004 but refused the test in 2006 and the percentage of those who were tested in both surveys among those from the South, the region with the highest HIV prevalence in the country; and (2) the significant difference in the percentage of individuals who were tested in 2004 but were temporarily away or had out-migrated from the study sites in 2006 and the percentage of those who were tested in both surveys among those from the South and those with primary education.
Table 2.
Comparison of Participants in Both HIV Testing Programs With Nonparticipants in 2006 Who Were Tested in 2004, by Selected Background Characteristics, MDICP 2004–2006
| Tested in 2004 but Not in 2006 (%) |
Significance Tests of Differencesa |
||||||
|---|---|---|---|---|---|---|---|
| Characteristics | % Tested in Both Surveys (1) | Refused (2) | Temporarily Away/Out- Migrated (3) | Other Outcomes/Missing (4) | (1) vs. (2) | (1) vs. (3) | (1) vs. (4) |
| Age Group | |||||||
| 15–24 | 23.9 | 23.6 | 52.1 | 35.4 | ns | ** | ** |
| 25–34 | 25.2 | 28.2 | 20.0 | 20.1 | ns | * | * |
| 35–44 | 24.4 | 20.0 | 13.7 | 17.4 | ns | ** | ** |
| 45+ | 26.6 | 28.2 | 14.3 | 26.2 | ns | ** | ns |
| Missing | 0.0 | 0.0 | 0.0 | 0.8 | ns | ns | ** |
| Sex | |||||||
| Male | 44.5 | 46.4 | 50.4 | 49.9 | ns | * | * |
| Female | 55.5 | 53.6 | 49.6 | 50.1 | ns | * | * |
| Study Site | |||||||
| South | 33.4 | 60.0 | 43.6 | 35.6 | ** | ** | ns |
| Center | 30.2 | 32.7 | 25.2 | 33.3 | ns | ns | ns |
| North | 36.4 | 7.3 | 31.2 | 31.0 | ** | ns | * |
| Highest Educational Level | |||||||
| No schooling | 25.6 | 35.5 | 11.8 | 20.3 | * | ** | * |
| Primary schooling | 60.5 | 50.9 | 67.4 | 57.4 | * | * | ns |
| Secondary and higher | 13.9 | 13.6 | 19.2 | 16.1 | ns | ** | ns |
| Missing | 0.1 | 0.0 | 1.6 | 6.1 | ns | ** | ** |
| Current Marital Status | |||||||
| Never married | 12.3 | 10.9 | 32.3 | 14.5 | ns | ** | ns |
| Currently married | 82.1 | 81.8 | 57.3 | 73.0 | ns | ** | ** |
| Formerly marriedb | 5.6 | 7.3 | 4.7 | 6.7 | ns | ns | ns |
| Missing | 0.0 | 0.0 | 5.8 | 5.9 | ns | ** | ** |
| Total | 100.0 | 100.0 | 100.0 | 100.0 | |||
| Number of Respondents | 2,031 | 110 | 365 | 477 | |||
Note: Percentages may not add up to exactly 100 in some cases because of rounding error.
Source: From the author’s calculations of data from the Malawi Diffusion and Ideational Change Project.
Significance tests of proportions; ns = not statistically significant.
Formerly married refers to separated, divorced, and widowed respondents.
p < .05;
p < .01
Table 3.
Comparison of Participants in Both HIV Testing Programs With Nonparticipants in 2006 Who Were Tested in 2004, by Selected HIV-Related Behaviors, MDICP 2004–2006
| Tested in 2004 but Not in 2006 (%) |
Significance Tests of Differencesa |
||||||
|---|---|---|---|---|---|---|---|
| Characteristics | % Tested in Both Surveys (1) | Refused (2) | Temporarily Away/Out- Migrated (3) | Other Outcomes/Missing (4) | (1) vs. (2) | (1) vs. (3) | (1) vs. (4) |
| HIV Status in 2004 | |||||||
| Negative/indeterminate | 95.6 | 84.5 | 82.9 | 87.0 | ** | ** | ** |
| Positive | 4.4b | 15.5 | 9.9 | 13.0 | ** | ** | ** |
| Missing | 0.0 | 0.0 | 0.3 | 0.0 | ns | * | ns |
| Number of Unions | |||||||
| Never married | 12.3 | 10.9 | 32.3 | 14.5 | ns | ** | ns |
| Married once | 54.3 | 49.1 | 41.4 | 50.5 | ns | ** | ns |
| More than once | 33.5 | 40.0 | 20.6 | 29.1 | ns | ** | ns |
| Missing | 0.0 | 0.0 | 5.8 | 5.9 | ns | ** | ** |
| Number of Sexual Partners | |||||||
| Never had sex | 4.0 | 4.6 | 11.5 | 5.7 | ns | ** | ns |
| One partner | 32.2 | 31.8 | 23.6 | 22.4 | ns | ** | ** |
| 2+ partners | 63.0 | 62.7 | 49.6 | 49.9 | ns | ** | ** |
| Missing | 0.8 | 0.9 | 15.3 | 22.0 | ns | ** | ** |
| Suspects Partner of Infidelity | |||||||
| No/don’t know | 68.1 | 65.5 | 38.9 | 48.9 | ns | ** | ** |
| Yes | 18.3 | 22.7 | 31.0 | 20.3 | ns | ** | ns |
| No spouse/partner | 13.2 | 11.8 | 19.7 | 14.7 | ns | ** | ns |
| Missing | 0.3 | 0.0 | 10.4 | 16.1 | ns | ** | ** |
| Worried About Getting HIV/AIDS | |||||||
| No/don’t know | 57.4 | 53.6 | 34.8 | 46.8 | ns | ** | ** |
| Yes | 42.6 | 46.4 | 64.9 | 51.8 | ns | ** | ** |
| Missing | 0.0 | 0.0 | 0.3 | 1.5 | ns | * | ** |
| Perceived Risk of Current Infection | |||||||
| No/low risk/don’t know | 90.4 | 94.5 | 84.4 | 88.1 | ns | ns | * |
| Medium/high risk | 8.9 | 5.5 | 12.0 | 12.0 | ns | ns | * |
| Knows Someone With AIDS/Died of AIDS | |||||||
| No one/don’t know | 3.9 | 3.6 | 9.0 | 5.5 | ns | ** | ns |
| At least one | 95.8 | 96.4 | 81.4 | 78.2 | ns | ** | ** |
| Missing | 0.3 | 0.0 | 9.6 | 16.4 | ns | ** | ** |
| Total | 100.0 | 100.0 | 100.0 | 100.0 | |||
| Number of Respondents | 2,031 | 110 | 365 | 477 | |||
Note: Percentages may not add up to exactly 100 in some cases because of rounding error.
Source: From the author’s calculations of data from the Malawi Diffusion and Ideational Change Project.
Significance tests are tests of proportions; ns = not statistically significant.
Prevalence in 2006 among those tested in both surveys was 5% (men: 4%; women: 6%).
p < .05;
p < .01
Table 3 presents the results of the comparisons based on HIV status in 2004 and other HIV-related behaviors. Again, instances when a significant downward bias in the prevalence estimates for the longitudinal participants could occur include (1) the significant difference in the percentage of individuals who were tested in 2004 but refused the test in 2006 and the percentage of those who were tested in both surveys among those who were HIV-positive in 2004; (2) the significant difference in the percentage of individuals who were tested in 2004 but were temporarily away or had out-migrated from the study sites by the second survey, and the percentage of those who were tested in both surveys among those who were HIV-positive in 2004, those who suspected their spouses/partners of infidelity, and those who were worried about getting HIV/AIDS; and (3) the significant difference in the percentage of individuals who were tested in 2004 but could not be tracked down by the survey team in 2006 and the percentage of those who were tested in both surveys according to the following characteristics: being HIV-positive in 2004, being worried about getting HIV/AIDS, and perceiving medium or high risk of being infected with HIV.
The observed patterns are indicative of the potential for nonresponse to significantly bias downward the HIV prevalence estimates for the longitudinal participants. They are also consistent with the results from the logistic regression analysis predicting attrition for repeat testing in 2006 among those ever interviewed in previous waves (results presented in Table S1, available online at Demography’s Web site: http://www.populationassociation.org/publications/demography). In particular, the likelihood of attrition for repeat testing was significantly higher among those who tested HIV-positive in 2004, those who suspected or knew of their spouses’ or partners’ infidelity, and those who were worried about getting HIV/AIDS.
Impact of Nonresponse on the HIV Prevalence Estimates
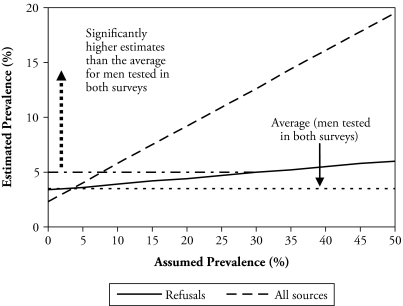
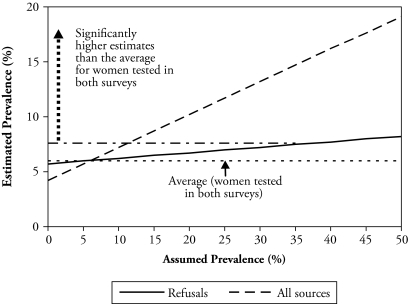
The results shown in Figures 1 and 2 are based on sensitivity analysis aimed at determining whether the HIV prevalence estimates obtained are significantly higher than what was observed among the longitudinal participants by assuming plausible prevalence among non-participants. Both figures indicate that significantly higher estimates are obtained than what was observed among individuals tested in both surveys by assuming plausible prevalence among all nonparticipants in 2006 who were tested in 2004. For men (Figure 1), a prevalence of about 8% or higher among nonparticipants must be assumed in order to obtain a significantly higher estimate (of at least 5.0%) than that observed among men tested in both surveys (i.e., 3.5%). For female nonparticipants (Figure 2), a prevalence of 12% or higher must be assumed in order to obtain a significantly higher estimate (of at least 7.6%) than that for women tested in both surveys (i.e., 6.0%).
Figure 1.
Estimated HIV Prevalence by Assumed Prevalence Among Men Who Were Tested in 2004 but Not in 2006, MDICP 2004–2006
Figure 2.
Estimated HIV Prevalence by Assumed Prevalence Among Women Who Were Tested in 2004 but Not in 2006, MDICP 2004–2006
The prevalence estimates that must be assumed for the nonparticipants are plausible because a test of whether they are significantly different from those of the 2004 MDHS for rural men and women shows that they are not (p = .14 for men; p = .68 for women). Similarly, a test of whether they are significantly different from the 2004 estimates for these men and women (who were nonparticipants in 2006 but were tested in 2004) shows that they are not (p = .30 for men; p = .42 for women).6 This suggests that nonresponse significantly biased downward the HIV prevalence estimates for the longitudinal participants.7 It further implies that if the 2006 survey restricted HIV testing to those who were tested in 2004, the cross-sectional 2006 prevalence estimates would have been significantly biased downward because of nonresponse.
Impact of Nonresponse on Predictors of HIV Status
Table 4 compares the coefficient estimates from the model predicting HIV status in 2004 among those who participated in the first testing with those from the model predicting HIV status in 2006 for the longitudinal participants. The results show differences between most of the estimates from the two sets of models in terms of significance and, in some cases, in the absolute magnitude of the coefficients. For instance, based on the model predicting HIV status among those who participated in the first test, those from the Center or the North were significantly less likely to be HIV-positive compared with those from the South. However, a different conclusion is reached when this is considered with the model predicting HIV status among those who participated in both tests (Table 4).
Table 4.
Coefficient Estimates From the Probit Models Predicting HIV Status in 2004 Among Those Who Participated in the First Test, and in 2006 Among Those Who Participated in Both Tests, MDICP 2004–2006
| Characteristics | Coefficient Estimates (β) for 2004 | Coefficient Estimates (β) for 2006 | Absolute Ratio (larger/smaller estimate) |
|---|---|---|---|
| Age Group (ref. = 35+ years) | −0.08 (0.09) | 0.04 (0.12) | 2.00 |
| Sex (female = 1) | 0.19* (0.09) | 0.16 (0.12) | 1.19 |
| Study Site (ref. = South) | |||
| Center | −0.26* (0.12) | −0.21 (0.17) | 1.24 |
| North | −0.42** (0.13) | −0.21 (0.17) | 2.00 |
| Highest Educational Level (ref. = no schooling) | |||
| Primary schooling | 0.17 (0.13) | 0.17 (0.14) | 1.00 |
| Secondary and higher | 0.25 (0.18) | −0.01 (0.23) | 25.00 |
| Religious Affiliation (ref. = Christian) | |||
| Muslim | −0.24 (0.13) | −0.18 (0.17) | 1.33 |
| Other | −0.03 (0.14) | 0.25 (0.21) | 8.33 |
| Current Marital Status (formerly marrieda = 1) | 0.43** (0.16) | 0.66** (0.17) | 1.53 |
| Stayed Outside District 6+ Months (ref. = no/can’t remember)b | 0.15 (0.08) | 0.23* (0.11) | 1.53 |
| Partner Usually Stays Outside Village (ref. = no/not married) | 0.22 (0.13) | 0.52** (0.19) | 2.36 |
| Perceived Risk of Current Infection (ref. = no/low/don’t know) | 0.25* (0.10) | 0.78** (0.13) | 3.12 |
| Number of Sexual Partners (ref. = one/never had sex) | 0.18 (0.12) | 0.32* (0.16) | 1.78 |
| Number of Times Married (ref. = once/never married) | 0.37** (0.11) | 0.39** (0.13) | 1.05 |
| N | 2,440 | 1,898 | |
Notes: Robust standard errors are in parentheses. All models are based on Eq. (2).
Source: From the author’s calculations of data from the Malawi Diffusion and Ideational Change Project.
Formerly married refers to separated, divorced, and widowed respondents.
The question asked whether the respondent had stayed outside the district for six months or more since age 15.
p < .05;
p < .01
Despite the different sets of conclusions arising from the two models, the direction of association between HIV status and the related factors (where this is noted to be significant) is in the expected direction. For instance, the regional differences are consistent with the estimates from the 2004 MDHS (NSO and ORC Macro 2005); partner mobility implies temporary partner separation, which is likely to raise the possibility of having other sexual partners, thereby increasing the chances of being infected with HIV; and multiple sexual partnerships and unions imply an increase in the chances of HIV infection.
DISCUSSION AND CONCLUSION
This article examined nonresponse for repeat population-based VCT for HIV in rural Malawi. This is relevant for gauging the acceptability and cost-effectiveness of providing VCT services in sub-Saharan Africa as a strategy for combating the spread of HIV/AIDS in the region. The findings show that nonresponse led to significant bias in the estimates of HIV prevalence and in inconsistent conclusions about the predictors of HIV status. Three findings inform this conclusion. First, a simple comparison of the characteristics of participants in repeat testing and those who participated in the first testing but not the second testing (non-participants in repeat testing) shows that the nonparticipants differed from the participants in ways that suggest significant bias. Second, the HIV prevalence estimates that were obtained were significantly higher than what was observed among individuals who were tested in both surveys by assuming plausible prevalence among all nonparticipants in repeat testing. Third, the models predicting HIV status among individuals who participated in the first test and those who participated in both tests result in different conclusions regarding some of the factors that are significantly associated with the likelihood of being HIV-positive.
Contrary to the conclusion of this study, previous cross-sectional analysis of the impact of nonresponse on either the 2004 and 2006 HIV prevalence estimates found that nonresponse did not significantly bias the estimates (Obare 2007). This was also found to be consistent with findings from previous similar (cross-sectional) analyses based on the Demographic and Health Surveys (e.g., Bignami-Van Assche et al. 2005; Mishra et al. 2006), which found that although nonrespondents tended to have higher HIV prevalence compared with those who consented to be tested, the overall bias on the prevalence estimates was generally small. The difference in conclusions from the analysis of nonresponse in repeat and cross-sectional testing could be attributed to two factors: (1) the different definitions of what constitutes nonresponse in both contexts, and (2) the risk profiles of the missed populations. In particular, refusal and temporary absence are the major sources of nonresponse in the cross-sectional analyses, while attrition attributable to out-migration, mortality, or inability to trace the respondents is an additional source of nonresponse in repeat testing. As noted earlier, out-migrants have been found to have higher HIV prevalence than the nonmigrants (Anglewicz 2007; Crampin et al. 2003), which could account for significant bias in the estimates of prevalence among nonmigrants.
The preceding conclusion has implications for the value of population-based longitudinal HIV surveys. Compared with cross-sectional surveys, longitudinal studies have several advantages that include the ability to study behaviors that change over time, how past behaviors influence current behaviors, and the effect of time-varying exogenous variables on endogenous behaviors while controlling for unobserved fixed characteristics (Alderman et al. 2001). With respect to HIV, longitudinal studies are useful for understanding incidence rates, the factors driving these rates, and trends in prevalence at the community level. Nonetheless, as the preceding finding suggests, these advantages are overshadowed by the fact that loss to follow-up—which remains “the Achilles heel” of longitudinal studies (Thomas, Frankenberg, and Smith 2001:590)—leads to significant bias in the estimates of prevalence, and can result in significant bias in the estimates of incidence as well. This is compounded by the fact that longitudinal studies are generally costly to undertake. It is therefore unlikely that governments and programs would rely on them to understand trends in HIV incidence and prevalence.
These findings should be viewed in the context of this article’s limitations. First, the contention that the different conclusions regarding the significant predictors of HIV status reflect the effect of nonresponse could be partly true. The differences could also be due to the changing patterns of HIV infection between the two waves. Nonetheless, this is not likely to be the case in the present study because of the short duration between the two waves, which could not have witnessed significant changes in HIV prevalence. Indeed, the HIV prevalence among the MDICP study participants remained stable at 7% in both waves (Obare et al. 2009). Second, the study does not consider changes in program contexts between 2004 and 2006, which could also affect the uptake of HIV testing: hence, the extent of nonresponse and its impact on the estimates. For instance, VCT services were available at the local health clinics in all the study sites in 2006; this was not the case in two of the study sites (North and South) in 2004. Moreover, in 2006, free antiretroviral treatment (ART) was available at the local health clinics in all the study sites. In contrast, in 2004, ART was available for a fee at the health center in only one study site (Center). The limited availability of VCT and ART services in 2004 could, therefore, have an impact on the extent of nonresponse, especially on the refusal to be tested.
Acknowledgments
The data for this article were collected through NIH/NICHD Grants RO1-HD372-276, RO1-HD41713, and RO1 HD044228-01. Work on the article started when the author was a doctoral candidate at the Population Studies Center, University of Pennsylvania. Hans-Peter Kohler, Susan Watkins, Jere Behrman, and anonymous reviewers provided valuable comments that greatly improved the content of the article. The views, however, remain those of the author.
Footnotes
The other aim of the strategy is to identify those who qualify for antiretroviral therapy (ART) and care (Family Health International 2006), but this is subject to the availability of such treatment.
Previous estimates of HIV prevalence have been derived from an extrapolation of the estimates from pregnant women attending selected prenatal clinics (which form the sentinel or surveillance sites) to the entire adult population. However, this method may also suffer from bias arising from three sources. The first is the selective location of the clinics that carry out testing, whereby smaller and more remote rural clinics are underrepresented. Second, those who attend the clinics are self-selected. That is, pregnant women attending clinics may not be representative of all pregnant women, of all adult women, or of the entire adult population (men and women); they also represent a group that is sexually active and mostly have sex without protection against sexually transmitted infections including HIV. The third source is the algorithm used to extrapolate the data to the national adult population; that is, fitting epidemic curves to the prevalence data from the sentinel site and sometimes relying on subjective judgment in cases in which the curve fails to provide a good fit to the data (Allen 2006; Boerma et al. 2003; National AIDS Commission 2004; WHO and UNAIDS 2003).
The choice of the cutoff of 50% is based on the fact that in almost all cases, the results showed that we achieve estimates that are significantly higher than the observed at this level and below.
Note that in 2006, new spouses to married adolescents and to adult sample members were included in the study (see Data section). There were also nonparticipants in the 2004 HIV testing program who were tested in 2006.
Temporary absentees were those who were reported to be away from the household or family for a few days (for instance, to visit relatives or on a business trip) and were expected back soon. Out-migrants, on the other hand, were those who moved either to settle in areas outside the study sites or to work in major towns or out of the country, especially South Africa and Mozambique. Whereas family/household members and/or neighbors reported whether an individual was temporarily away or had moved, the project employed the services of additional research assistants, referred to as “scouts,” who were selected from each village to assist with the identification of respondents (Anglewicz et al. 2005). These scouts confirmed for the interviewers the status of respondents from the reports by family/household members and/or neighbors.
The 2004 HIV prevalence for male nonparticipants in 2006 who were tested in 2004 was 9.4%, and that for women was 13.2%; the 2004 MDHS rural HIV prevalence was 8.8% among men and 12.5% among women. In addition, including those who were HIV-positive in 2004 but were nonparticipants in 2006 in the analysis (because their HIV status is already known) did not change these results and conclusions.
Analyses considering temporary absence as distinct from out-migration (two aspects of mobility) show that the prevalence estimates that must be assumed among those who participated in the first HIV testing (in 2004) but were temporarily away during the second testing (in 2006) are significantly higher than the estimates observed in the 2004 Malawi Demographic and Health Survey or among the same individuals in 2004. This suggests that temporary absence might not result in significant bias in the HIV prevalence estimates over time. A similar conclusion is reached for those who participated in the 2004 HIV testing but refused to participate in the subsequent test. The assumed prevalence among nonparticipants in repeat HIV testing becomes plausible when out-migrants and those with other or missing outcomes are included in the analysis.
REFERENCES
- Alderman H, Behrman JR, Kohler H-P, Maluccio JA, Watkins SC. Attrition in Longitudinal Household Survey Data. Demographic Research. 2001;5:79–124. article 4. Available online at http://www.demographic-research.org/Volumes/Vol5/4/default.htm. [Google Scholar]
- Allen T. AIDS and Evidence: Interrogating Some Ugandan Myths. Journal of Biosocial Science. 2006;38:7–28. doi: 10.1017/S0021932005001008. [DOI] [PubMed] [Google Scholar]
- Allison PD. Logistic Regression Using the SAS System: Theory and Application. Cary, NC: SAS Institute Inc; 1999. [Google Scholar]
- Anglewicz P. Unpublished PhD dissertation. Population Studies Center, University of Pennsylvania; 2007. Migration, HIV Infection, and Risk Perception in Malawi. [Google Scholar]
- Anglewicz P, Bignami-Van Assche S, Chao LW, Chilongozi D, Hoffman I, Kohler H-P, Martinson F, Onyango F, Reniers G, Smith K. The Collection of Biomarkers in Rural Malawi: What Did We Learn?. Paper presented at the Meeting of the International Union for the Scientific Study of Population (IUSSP); Tours, France. July 18–24.2005. [Google Scholar]
- Bignami-Van Assche S, Chao LW, Hoffman I, Kohler H-P, Reniers G, Smith K, Watkins S, Weinreb A. Protocol for Biomarker Testing in the 2004 Malawi Diffusion and Ideational Change Project. Philadelphia: University of Pennsylvania; 2004. SNP Working Paper No. 7. Available online at http://www.malawi.pop.upenn.edu. [Google Scholar]
- Bignami-Van Assche S, Salomon JA, Murray CJL. Evidence From National Population-Based Surveys on Bias in Antenatal Clinic-Based Estimates of HIV Prevalence. Paper presented at the annual meeting of the Population Association of America; Philadelphia. March 31–April 2.2005. [Google Scholar]
- Boerma JT, Ghys PD, Walker N. Estimates of HIV-1 Prevalence From National Population-Based Survey as a New Gold Standard. Lancet. 2003;362:1929–31. doi: 10.1016/S0140-6736(03)14967-7. [DOI] [PubMed] [Google Scholar]
- Boerma JT, Holt E, Black R. Measurement of Biomarkers in Surveys in Developing Countries: Opportunities and Problems. Population and Development Review. 2001;27:303–14. [Google Scholar]
- Crampin AC, Glynn JR, Ngwira BMM, Mwaungulu FD, Pönnighaus JM, Warndorff DK, Fine PEM. Trends and Measurement of HIV Prevalence in Northern Malawi. AIDS. 2003;17:1817–25. doi: 10.1097/00002030-200308150-00011. [DOI] [PubMed] [Google Scholar]
- Family Health International. Service Delivery Models for HIV Counseling and Testing. 2005. Available online at http://www.fhi.org/en/HIVAIDS/pub/fact/ctmodels.htm.
- Family Health International. Counseling and Testing for HIV. 2006. Available online at http://www.fhi.org/en/hivaids/pub/fact/vctforhiv.htm.
- Fisher G, Pappas G, Limb M. Prospects, Problems, and Prerequisites for National Health Examination Surveys in Developing Countries. Social Science and Medicine. 1996;42:1639–50. doi: 10.1016/0277-9536(95)00319-3. [DOI] [PubMed] [Google Scholar]
- Groves RM. Survey Errors and Survey Costs. Hoboken, NJ: John Wiley & Sons, Inc; 2004. [Google Scholar]
- Groves RM, Couper MP. Nonresponse in Household Interview Surveys. New York: John Wiley & Sons, Inc; 1998. [Google Scholar]
- Harries A, Makombe S, Libamba E, Schouten E, Lungu D. 5-Year Plan for Antiretroviral Therapy Scale-Up in Malawi: 2006–2010. Paper presented at The XVI International AIDS Conference; Toronto. August 13–18.2006. [Google Scholar]
- Helleringer S, Kohler H-P. Social Networks, Perceptions of Risk, and Changing Attitudes Towards HIV/AIDS: New Evidence From a Longitudinal Study Using Fixed-Effects Analysis. Population Studies. 2005;59:265–82. doi: 10.1080/00324720500212230. [DOI] [PubMed] [Google Scholar]
- Joint United Nations Programme on HIV/AIDS (UNAIDS) 2006 Report on the Global HIV/AIDS Epidemic. Geneva: UNAIDS; 2006. [Google Scholar]
- Joint United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization (WHO) AIDS Epidemic Update 2007. Geneva: UNAIDS and WHO; 2007. [Google Scholar]
- Libamba E, Makombe S, Harries A, Schouten E, Lungu D. National Scale-up of Antiretroviral Therapy in Malawi. Paper presented at The XVI International AIDS Conference; Toronto. August 13–18.2006. [Google Scholar]
- Matovu JKB, Gray RH, Kiwanuka N, Wabwire-Mangen F, Nalugoda F, Serwadda D, Sewankambo NK, Wawer MJ. Repeat Voluntary HIV Counseling and Testing (VCT), Sexual Risk Behavior and HIV Incidence in Rakai, Uganda. AIDS and Behavior. 2007;11:71–78. doi: 10.1007/s10461-006-9170-y. [DOI] [PubMed] [Google Scholar]
- Mishra V, Boerma T, Way A, Barrere B, Arnold F, Cross AR, Hong R, Johnson K, Khan S. Evaluating HIV Estimates From National Population-Based Surveys for Bias Due to Non-response. Paper presented at the annual meeting of the Population Association of America; Los Angeles. March 30–April 1.2006. [Google Scholar]
- National AIDS Commission (NAC) [Malawi] Malawi National HIV/AIDS Estimates 2003: Technical Report. Malawi: National AIDS Commission; 2004. [Google Scholar]
- National Statistics Office (NSO) [Malawi] and ORC Macro. Malawi Demographic and Health Survey 2004. Calverton, MD: NSO and ORC Macro; 2005. [Google Scholar]
- Nyblade L, Menken J, Wawer MJ, Sewankambo NK, Serwadda D, Makumbi F, Lutalo T, Gray RH. Population-Based HIV Testing and Counseling in Rural Uganda: Participation and Risk Characteristics. Journal of Acquired Immune Deficiency Syndrome. 2001;28:463–70. doi: 10.1097/00042560-200112150-00010. [DOI] [PubMed] [Google Scholar]
- Obare F. Unpublished PhD dissertation. Population Studies Center, University of Pennsylvania; 2007. Response to Population-Based Voluntary Counseling and Testing for HIV in Rural Malawi. [Google Scholar]
- Obare F, Fleming P, Anglewicz P, Thornton R, Martinson F, Kapatuka A, Poulin M, Watkins S, Kohler H-P. Acceptance of Repeat Population-Based Voluntary Counseling and Testing for HIV in Rural Malawi. Sexually Transmitted Infections. 2009;85:139–44. doi: 10.1136/sti.2008.030320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Population Reference Bureau. 2008 World Population Data Sheet. Washington, DC: Population Reference Bureau; 2008. [Google Scholar]
- Reniers G, Araya T, Berhane Y, Davey G, Sanders EJ. Implications of the HIV Testing Protocol for Refusal Bias in Seroprevalence Surveys. BMC Public Health. 2009;9:163. doi: 10.1186/1471-2458-9-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D, Frankenberg E, Smith JP. Lost But Not Forgotten: Attrition and Follow-up in the Indonesia Family Life Survey. Journal of Human Resources. 2001;36:556–92. [Google Scholar]
- Thornton RL. The Demand for Learning HIV Status and the Impact on Sexual Behavior: Evidence From a Field Experiment. American Economic Review. 2008;98:1829–63. doi: 10.1257/aer.98.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins SC, Zulu EM, Kohler H-P, Behrman JR. Introduction to: Social Interactions and HIV/AIDS in Rural Africa. Demographic Research. 2003. pp. 2–29. Special Collection 1, article 1. Available online at http://www.demographic-research.org/special/1/default.htm.
- World Health Organization (WHO) and Joint United Nations Programme on HIV/AIDS (UNAIDS) Reconciling Antenatal Clinic-Based Surveillance and Population-Based Survey Estimates of HIV Prevalence in Sub-Saharan Africa. Geneva: WHO and UNAIDS; 2003. [Google Scholar]
- Zulu EM. Unpublished PhD dissertation. Graduate Group in Demography, University of Pennsylvania; 1996. Social, Cultural and Biological Factors Affecting Reproductive Behavior in Malawi. [Google Scholar]
- Zulu EM, Chepng’eno G. Spousal Communication About the Risk of Contracting HIV/AIDS in Rural Malawi. Demographic Research. 2003. pp. 248–77. Special Collection 1, article 8. Available online at http://www.demographic-research.org/special/1/default.htm.