Abstract
Objectives/Hypothesis
To characterize the activation of cyclooxygenase (COX)-2/prostaglandin (PG) E2 signaling during airway mucosal repair and its subsequent role during the wound healing process.
Study Design
Prospective animal study.
Methods
The subglottis was approached via cricothyroidotomy. Sham airways were closed, and wounded airways were subjected to laser injury and closed. Subglottic tissue was harvested at 12 hours, 24 hours, 48 hours, and 72 hours postinjury. Secretions were collected preoperatively and at time of sacrifice. Inflammatory gene expression was analyzed using quantitative reverse transcriptase polymerase chain reaction. Subglottic/tracheal explants were exposed to exogenous IL-1β in the presence or absence of COX inhibitors. Explant-produced PGE2 levels were assayed using enzyme linked immunoassays. Human airway fibroblast migration and collagen contraction were assayed in the presence or absence of prostaglandin E2.
Results
Laser injury triggers a rapid, dose-dependent increase in mucosal IL-1β and COX-2 gene expression, with an anatomical distribution proportional to the distance from the site of injury. Gene up-regulation correlates with dose-dependent increases in PGE2 mucosal secretion levels. Ex vivo analysis indicates IL-1β is responsible for the activation of the COX-2/PGE2 pathway. Prostaglandin E2 differentially inhibits airway fibroblast migration and contraction in a specific, dose-dependent manner.
Conclusions
PGE2 is activated during mucosal inflammation and acts to decrease fibroplastic activity in the mucosal wound bed. During subglottic stenosis (SGS) development, the levels of PGE2 generated in response to injury may be insufficient to blunt the intrinsically fibroplastic phenotype of SGS fibroblasts, resulting in excessive scarring.
Keywords: Subglottis, fibroblast, mucosa, inflammation, scarring, wound healing
INTRODUCTION
A clear link between an altered inflammatory response to injury and aberrant subsequent fibroplastic events culminating in scar formation has been demonstrated in multiple tissue types.1–3 Although the development of fibrosis in other tissue types often results in functional impairment, excessive scarring in the upper airway can be life-threatening. Circumferential scarring of the upper airway mucosa, as encountered in subglottic stenosis (SGS) or tracheal stenosis, can interfere with requisite airflow and necessitate major surgical intervention.4–6 It is thus particularly troubling that the precise cellular and molecular processes underlying fundamental aspects of upper airway injury, inflammation, and fibrosis remain poorly understood to date. Throughout this article, fibroplastic events will be defined as those cellular and molecular processes, which have been demonstrated in one or more experimental systems to lead to the development of fibrosis or scar formation, including, but not limited to, fibroblast migration, proliferation, synthesis, contraction, and remodeling of extracellular matrix components.
Following injury, inflammatory cells invade the wound and secrete an array of primary inflammatory mediators including interleukin (IL)-1β. These primary (or first tier) mediators can subsequently activate downstream (secondary or second tier) inflammatory signals including IL-6, IL-8 and prostaglandin E2 (PGE2) as part of a well integrated response that coordinates cellular activity in the wound bed. It has been demonstrated that dual-purpose mediators, such as PGE2, participate not only in the coordination of the inflammatory response, but also in subsequent fibroplastic events via direct regulation of fibroblast proliferation, migration, contraction, synthesis, and remodeling of extracellular matrix components.7,8 PGE2 levels are closely regulated through the activity of synthetic cyclooxygenases (COX) and synthases (PGES) and degradative dehydrogenases (PGDH). PGE2 modulates inflammatory and mesenchymal cell activity via four E prostanoid (EP) receptors (EP1–EP4) coupled to intracellular calcium and/or cyclic adenosine monophosphate (cAMP) signaling pathways. PGE2 production (via COX-2, mPGES1), degradation (via15-PGDH) and reception (via EP1–EP4 receptors), are subject to regulation by various mediators, including IL-1β.9–11
Although the inflammatory response to injury and the role of PGE2 have been well studied in the lower airway, they remain less well characterized in the upper airway, including the subglottic and tracheal mucosa. Our study of the involvement of PGE2 in the inflammatory and fibroplastic phases of upper airway mucosal repair has led to several conclusions. First, activation of IL-1β and COX-2 expression occurs early following injury, is relatively localized to the injury site, and partially resolves over the duration of the repair process. Second, this activation correlates with changes in the secretion profile of inflammatory mediators (IL-1β, PGE2), in a manner partially dependent on the extent of mucosal injury. Third, mucosal fibroblasts represent a putative source of PGE2 present in mucosal secretions following injury. In contrast to their lower airway counterparts (fibrotic lung fibroblasts), mucosal fibroblasts from fibrotic (SGS) specimens do not appear to have an altered PGE2 synthetic mechanism.12–15
The current study is a continuation of previous work, combining in vivo approaches using the New Zealand white rabbit upper airway mucosal injury model with in vitro techniques aimed at characterizing the migration and contraction of upper airway mucosal fibroblasts of different phenotypes. We hypothesize that PGE2 activation is IL-1β dependent, accompanies the early inflammatory response to injury, and persists into the intermediate stages of repair. Through its presence at this transitional nexus, PGE2 likely plays an important role in the link between inflammation and subsequent fibroplastic events. We further speculate that deficient PGE2 responsiveness is key to development of SGS.
MATERIALS AND METHODS
Animals
The New Zealand white rabbit mucosal injury model has been previously shown to be applicable for the study of SGS and mucosal airway wound healing.13 A total of 56 animals were utilized for this study as follows: 1) two animals were used to provide uninjured control mucosal tissue, 2) six animals were used to generate tracheal explants, and 3) 48 animals (12 animals/time point) were used in the laser injury model as described below.
Airway Wounding
All animal experiments were conducted under protocols approved by the Children’s Hospital of Pittsburgh Institutional Animal Care and Use Committee, in compliance with federal animal welfare regulations, as previously described.13 Briefly, under general anesthesia the subglottis was entered via a mid-line cricothyroidotomy. The posterior subglottis was injured using a CO2 laser at the following settings: 1 second continuous pulse, beam diameter 2 mm, power settings of 2 W or 5 W delivered in 4 pulses/airway. Two-tier controls were utilized: 1) uninjured, normal airways, and 2) sham operated airways of animals at 12 hours, 24 hours, 48 hours, and 72 hours post-sham surgery.
Secretion Collection
Upper airway secretions were collected throughout the duration of study as previously described.12 IL-1β and PGE2 levels in mucosal secretions were determined using enzyme-linked immunoabsorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN). Levels of inflammatory mediators were standardized to total secretion weight and compared among the various treatment and time groups.
Tissue Analysis
Mucosal specimens were obtained from four regions of the upper airway: 1) injured subglottis (SG), 2) immediately inferior tracheal mucosa, 3) distal tracheal mucosa (approximately 5 mm inferior to injured subglottic mucosa), and 4) mucosa immediately superior to the injury, from the inferior aspect of the vocal fold (VF). The mRNA was extracted, reverse transcribed into cDNA, and quantitatively amplified (quantitative reverse-transcriptase polymerase chain reaction) as previously described using rabbit-specific primers for 18S rRNA, IL-1β, and COX-2.12 Relative quantitation (fold difference) of the expression levels of each transcript for each group was calculated using the 2-ΔΔCt method (compared to the 18S rRNA housekeeping gene), which produces a value inversely related to the relative abundance of the mRNA. Values were then standardized to mRNA levels derived from either control tissue or sham operated airways, and expressed as fold increase.
Tracheal Explant Cultures
Uninjured rabbit tracheas were removed immediately following euthanasia, placed in sterile saline solution, and dissected into explants measuring approximately 1 cm2. Each explant (including mucosa, lamina propria and cartilage layers) was placed, for 24 hours, in either incomplete medium or medium supplemented with one of the following: IL-1β, 10 ng/mL (Sigma-Aldrich, St. Louis, MO); indomethacin, 500 μM (nonspecific COX inhibitor) (Cayman Chemical, Ann Arbor, MI); NS-398, 30 μM; nimesulide, and 30 μM (specific COX-2 inhibitors) (Cayman Chemical). For experiments utilizing COX inhibitors, explants were pretreated for 1 hour prior to stimulation with IL-1β. Media were collected and analyzed for soluble PGE2 levels using commercial ELISAs as detailed above. PGE2 levels were standardized to explant weight. All tracheal explant and cell culture experiments were performed in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with the various indicated mediators.
Cell Culture
Human upper airway (subglottic/tracheal) fibroblasts previously characterized in our laboratory were utilized.12 A total of five upper airway fibroblast cultures were utilized: two fibroblast populations derived from normal adult subglottis/trachea (NAF) and three fibroblast populations derived from pathologic specimens of subglottic stenosis (SGS-F). Cells were cultured using previously described techniques and used prior to passage 10 (approximately 15 total population doublings).
Chemicals
PGE2, 11-deoxy-prostaglandin E1 (11-DEOXY), butaprost (BUTA, Cayman Chemical) and forskolin (FSK, Calbiochem, San Diego, CA) were reagent grade.
Cell Imaging
Fibroblasts were grown on glass coverslips, fixed using paraformaldehyde, and permeabilized using Triton-X 100. The actin cytoskeleton was visualized using Texas Red-X phalloidin (Invitrogen, Carlsbad, CA). Focal adhesion complexes were visualized using a mouse antihuman FITC conjugated antipaxillin antibody (BD Biosciences, San Jose, CA). Digital images were captured using an inverted confocal microscope (Olympus Fluoview 1000 Confocal Microscope 40X, Olympus Corporation, Tokyo, Japan), prepared and labeled using Adobe Photoshop version 7.0 software (Adobe Systems Inc., San Jose, CA).
Directional Migration Assay
All directional migration assays were performed using a two-dimensional scratch assay as previously described.7,8
Collagen Gel Contraction
A modification of the standard fibroblast populated collagen lattice (FPCL) method was used to measure collagen gel contraction by fibroblasts of different phenotypes, as previously described.7,8
Statistical Analysis
Statistical significance throughout the study was determined using Student t test, with significance attributed for P < .05. Throughout the manuscript and legends, CNT refers to the control value for that data set.
RESULTS
Laser-Induced Upper Airway Injury Resulted in Activation of Il-1β and Cox-2 Gene Expression With a Specific Temporal and Regional Profile
Because surgical interventions release systemic signals in additional to local ones, sham operated airways were used as controls for baseline measurements. In this study we first compared the expression pattern of IL-1β and COX-2 in sham operated airway mucosa with that present in normal, nonmanipulated airways. Our data indicate that sham surgery alone, consisting of the anterior cricothyroidotomy resulted in detectable upregulation of both genes compared to baseline levels (data not shown). Specifically, IL-1β values were significantly elevated at 12 hours, 24 hours, 48 hours, and 72 hours postoperatively, whereas COX-2 values were significantly elevated at 24 hours, 48 hours, and 72 hours postinjury. Interestingly, IL-1β and COX-2 values did not change substantially over time in the case of sham operated airways. For all subsequent comparisons, changes in gene expression and secretion levels were determined with respect to sham values.
Laser injury to the posterior subglottic mucosa triggered substantial upregulation of IL-1β and COX-2 expression compared to sham surgery alone. At the lower laser setting of 2 W, IL-1β and COX-2 expression trended above the sham baseline for the first 24 hours postinjury, achieving statistical significance for IL-1β at the 24-hour time point (Fig. 1A). At the higher 5 W laser setting, IL-1β levels were significantly elevated over the sham baseline at 12 hours, 24 hours, and 72 hours post-injury. COX-2 levels were significantly elevated at 24 hours postinjury, and also approached significance at the other time points.
Fig. 1.
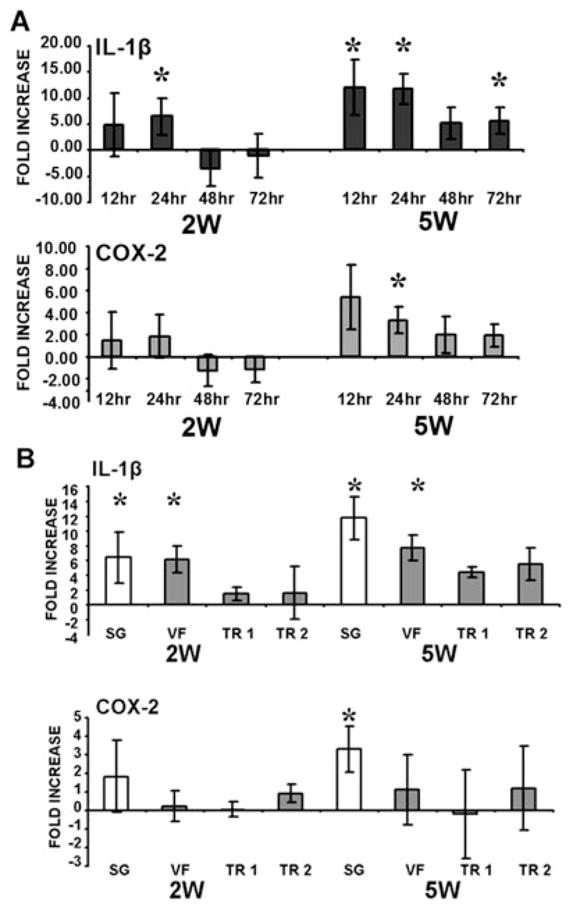
Interleukin (IL)-1β and cyclooxygenase (COX)-2 gene expression in the upper airway mucosa following injury. (A) CO2 laser injury triggers a time-dependent upregulation of both genes in the subglottic mucosa. (B) CO2 laser injury triggers the upregulation of both inflammatory genes in mucosal regions adjacent to area of direct injury Statistical significance is indicated by * representing P <.05 compared to sham values. SG = subglottis, VF = vocal fold, TR1 = proximal trachea, TR2 = distal trachea.
We have previously shown that upregulation of IL-1β and COX-2 gene expression is localized to the primary area of injury. Here we demonstrate that although upregulation of these two inflammatory genes is indeed maximal in the subglottic mucosa at 24 hours postinjury, there is substantial and significant upregulation above sham levels in adjacent areas, primarily in the case of IL-1β (Fig. 1B). Of particular interest is the significant upregulation in gene expression in the VF mucosa, directly superior to the injured mucosal region. At 24 hours postinjury, IL-1β expression was upregulated six-and eight-fold above sham levels with 2 W and 5 W laser settings, respectively, in the VF mucosa compared to six- and 12-fold in the SG mucosa.
Prostaglandin E2 Increases in Mucosal Secretions Early Following Laser-Induced Upper Airway Injury
CO2 laser settings used to injure the posterior subglottic mucosa were varied with respect to power. We have previously demonstrated that the extent of CO2 laser-induced injury correlates directly with the power delivered to the tissue bed.12,13,16 At both the 2- and 5-W power settings, PGE2 levels in mucosal secretions increased beginning at 12 hours postinjury and remained elevated through 72 hours (Fig. 2). Consistent with our previous data, this increase was significantly different from that associated with sham surgery alone. Preoperatively, the average value for all rabbit airways was found to be 10.1 ng/g of secretions, with a standard error of mean of ± 2.2 ng/g. Postoperatively, maximal values were as follows: 22.7 ng/g ± 5.8 (sham, 12 hours), 36.6 ng/g ± 3.8 ng/g (2 W, 12 hours) and 45.4 ng/g ± 5.7 ng/g (5 W, 24 hours). As such, at the maximal point within the defined time period there is an approximately four-fold increase above baseline levels of PGE2 present in mucosal secretions. Statistical significance was not achieved at all time points tested, likely due to both interanimal variability and a limited number of animals per tested time point/condition. Nevertheless, the trends are consistent with our previous data and are encouraging given the minimal laser power used to injure the posterior subglottic mucosa in this study.
Fig. 2.
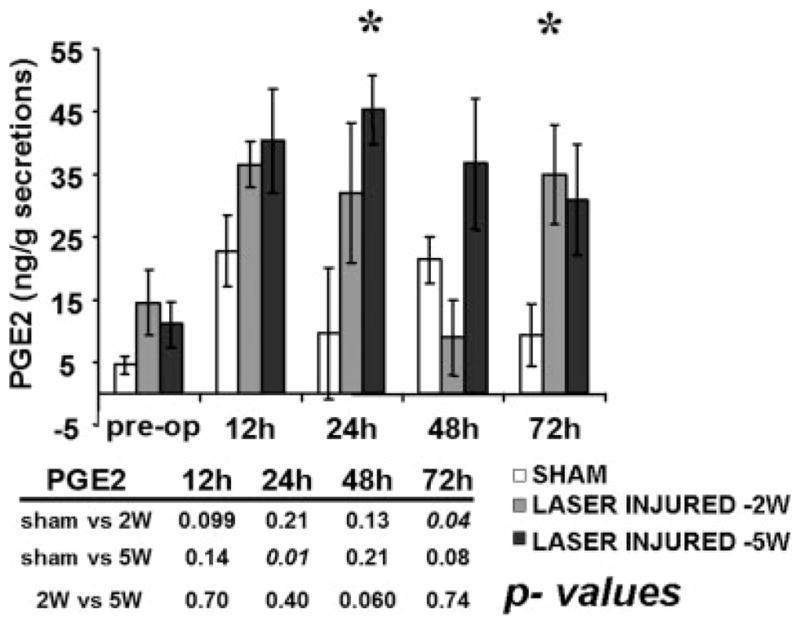
CO2 laser injury triggers a time and injury dependent up-regulation in soluble prostaglandin E2 (PGE2) levels. Secretions were collected from the airways of sham-operated and laser-injured airways at 12 hours, 24 hours, 48 hours, and 72 hours postinjury. PGE2 levels were assayed and compared across the various groups. Statistical significance is indicated by * representing P < .05 compared to sham PGE2 values for the specific time point.
We have previously analyzed the levels of both PGE2 and IL-1β present in upper airway mucosal secretions.12 In this current study our data showed an upregulation in IL-1β levels above sham operated levels, which displayed a similar trend compared to PGE2 levels, but one that did not reach a statistically significance difference at the tested time points. IL-1β triggers a dose-dependent, COX-2-dependent upregulation of PGE2 synthesis by tracheal explants.
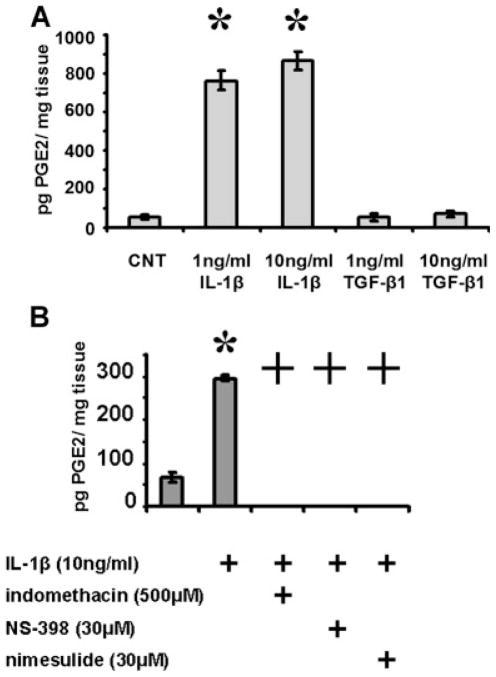
Rabbit tracheal explants were set up as described above. Following treatment with the acute proinflammatory cytokine IL-1β for 24 hours, media aliquots were assayed for PGE2 levels, which were found to be substantially elevated over control values in a dose-dependent manner (Fig. 3A). In contrast, treatment with TGF-β1, another ubiquitous soluble mediator in the wound bed, did not result in a substantial elevation of secreted PGE2 levels. TGF-β1 was chosen as a point of comparison for two reasons. First, it is considered to act as both a growth factor and cytokine. Second, cross talk between the IL-1β/COX-2/PGE2 and TGF-β1 pathways has been established in other experimental models.7,9 These data demonstrate that in tracheal explants, activation of PGE2 signaling is dependent on IL-1β stimulation, rather than representing a nonspecific response. Previous analysis has demonstrated that activation of PGE2 by IL-1β is dependent on upregulation of COX-2 activity.7,12 As illustrated in Figure 3B, inhibition of COX-2 activity using two specific pharmacologic antagonists (NS-398 or nimesulide) results in substantial abrogation of IL-1β induced PGE2 synthesis by tracheal explants.
Fig. 3.
Interleukin-1β (IL-1β) induces a cyclooxygenase-2 (COX-2) dependent upregulation in prostaglandin E2 (PGE2) levels produced by tracheal explants. (A) Tracheal explants were treated with either IL-1β or transforming growth factor (TGF)-β1 for 24 hours in incomplete media. (B) Tracheal explants were treated with IL-1β for 24 hours in incomplete media in the presence or absence of a nonspecific COX inhibitor (indomethacin) or specific COX-2 inhibitors (NS-398, nimesulide). Statistical significance is indicated by * representing P < .05, compared to control PGE2 levels and + representing P value < .05, compared to IL-1β-induced PGE2 levels. Note: PGE2 values for tracheal explants treated with IL-1β in the presence of COX inhibitors were below the minimum detecting threshold of the enzyme linked immunosorbent assay employed. As such, all three groups were assigned values of 0.
Upper Airway Fibroblast Migration and Contraction
A standardized two-dimensional migration assay was used to confirm and extend previous findings using human upper airway derived mucosal fibroblasts. As illustrated in Figure 4A, NAFs migrate with a speed of 3.6 μm/hr compared to 4.6 μm/hr for SGS fibroblasts (SGS-F). These values are comparable to those we have previously described for normal skin and keloid dermal fibroblasts,7 reflecting a consistent pattern for fibrotic fibroblasts from two different tissues.
Fig. 4.
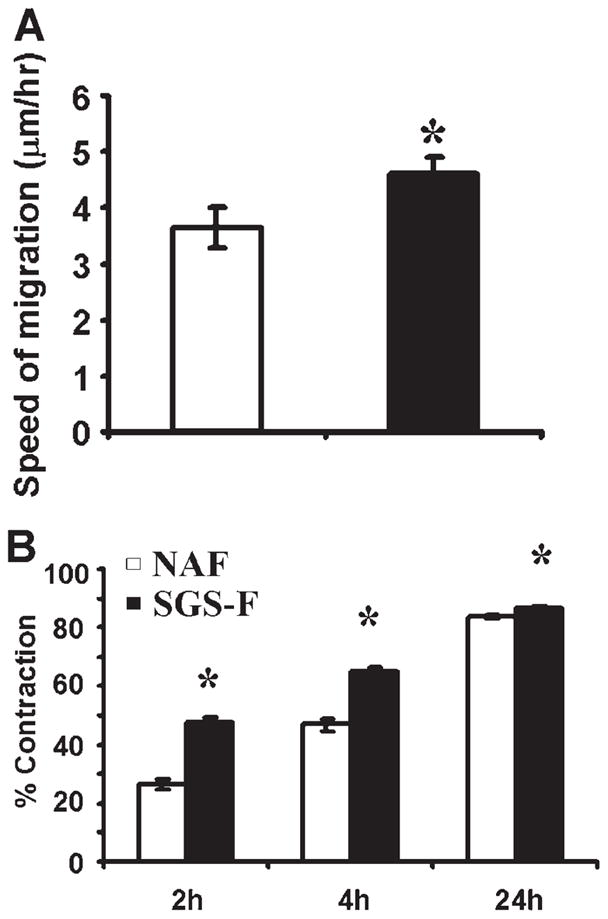
Subglottic stenosis fibroblast (SGS-F) migration and contraction rates are elevated compared to their normal adult counterpart (NAF). (A) All migration assays were performed using confluent cell monolayers in regular fibroblast media (DMEM) with no additional supplementation. (B) All contraction assays were performed in DMEM supplemented with 2% fetal bovine serum. Data points differing from the control value are indicated by * representing P <.05 using Student t test.
At early time points (2 hours and 4 hours), SGS-Fs contracted free floating collagen gels faster than their normal adult counterparts (NAFs), as shown in Figure 4B. At 24 hours into the contraction assay, both cell types achieved greater than 80% contraction, with SGS-Fs maintaining a significantly greater rate of contraction than NAFs.
Prostaglandin E2 Inhibits Upper Airway Fibroblast Migration and Contraction in a Dose-Dependent Manner Via Activation of Ep2/Ep4–Camp Signaling
PGE2 was found to inhibit both NAF and SGS-F migration in a dose-dependent manner (Fig. 5). As illustrated in Figure 6, several pharmacological agents were used in subsequent experiments to inhibit NAF and SGS-F migration: 11-DEOXY PGE1 (1 μm) (EP2/EP4 agonist), butaprost (1 μm) (EP2 agonist), and forskolin (25 μm) (direct activator of adenylate cyclase).
Fig. 5.
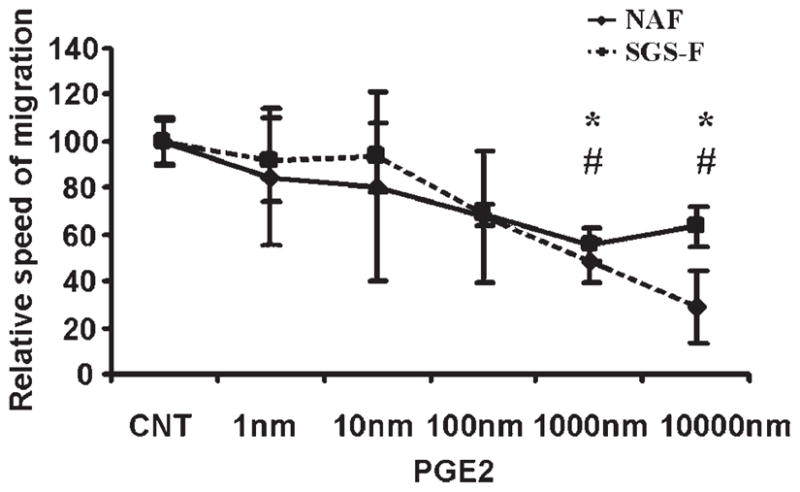
Prostaglandin E2 (PGE2) inhibits upper airway fibroblast migration in a dose-dependent manner. All scratch assays were performed using confluent cell monolayers in regular fibroblast medium (DMEM) with no additional supplementation. Data are presented as means with error bars representing standard error of the mean. Data points differing from the control value are indicated by: * representing normal adult counterpart (NAF), P < .05; # representing subglottic stenosis fibroblast (SGS-F), P < .05 using Student t test.
Fig. 6.
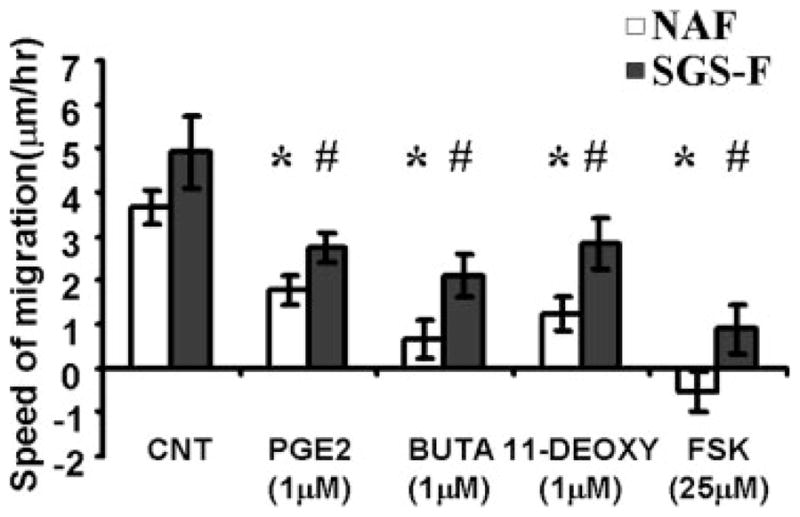
Prostaglandin E2 (PGE2) signaling via E prostanoid (EP) receptors EP2/EP4 inhibits fibroblast migration. Stimulation of normal adult airway fibroblasts (NAF) and subglottic stenosis fibroblast (SGS-F) without agents (CNT) or with prostaglandin E2 (PGE2, 1 μm), butaprost (EP2 agonist) (BUTA, 1 μm), 11-deoxy-prostaglandin E1 (EP2/EP4 agonist) (11-DEOXY, 1 μm), or forskolin (FSK, 25 μm) resulted in significant inhibition of fibroblast migration. All wounding assays were performed using confluent cell monolayers in regular fibroblast medium (DMEM) with no additional supplementation. Data points differing from the control value are indicated by: * representing normal adult counterpart (NAF), P < .05; # representing subglottic stenosis fibroblast (SGS-F), P <.05 using Student t test.
Contraction of fibroblast populated FPCLs was used to ascertain the effects of PGE2 on fibroblast migration on a bioactive substrate. Administration of exogenous PGE2 (1 μM) or forskolin (25 μM) significantly inhibited NAF contraction at 2 hours and 4 hours. In contrast, SGS-F appeared to be relatively refractory to both chemicals in the early phases of collagen gel contraction (Fig. 7).
Fig. 7.
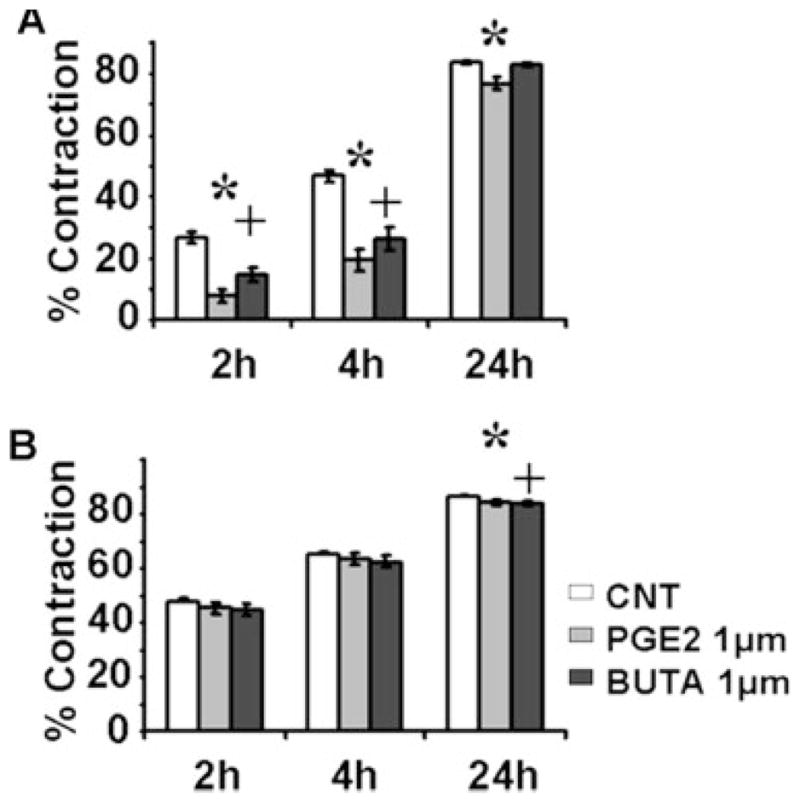
Prostaglandin E2 (PGE2) signaling via E prostanoid (EP) receptors EP2/EP4 inhibits fibroblast collagen gel contraction. (A) normal adult counterpart, and (B) subglottic stenosis fibroblast contraction was assayed in the presence or absence of PGE2 and/or butaprost (BUTA) over a 24-hour time period. All contraction assays were performed in regular fibroblast media (DMEM) supplemented with 2% fetal bovine serum, without agents (CNT) or with PGE2 (PGE2, 1 μm) or butaprost (EP2 agonist) (BUTA, 1 μm). Data points differing from the control value are indicated by: * PGE2, P <.05; + BUTA, P <.05 using Student t test.
Prostaglandin E2 Triggers Rearrangement of the Actin Cytoskeleton Via Activation of Camp Signaling
Administration of PGE2 (1 μm) or forskolin (25 μm) induced a disruption of actin filament in both NAF and SGS-F, as illustrated in Fig. 8. This demonstrates that PGE2 inhibits cell migration by disrupting the cytoskeletal organization, and that both normal and fibrotic airway fibroblasts are affected.
Fig. 8.
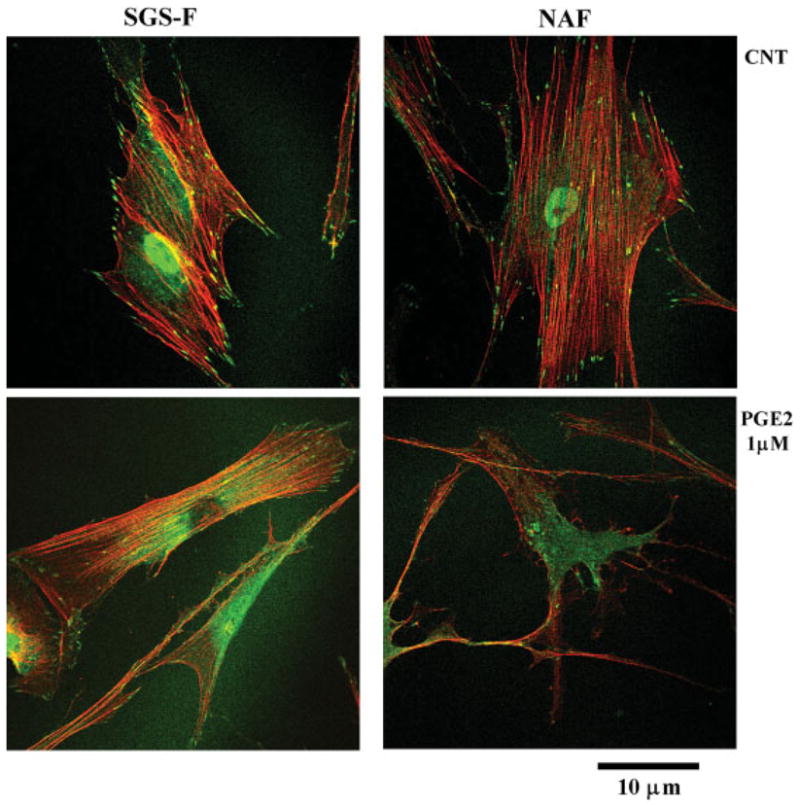
Prostaglandin E2 (PGE2) and forskolin induce rearrangement of the actin cytoskeleton of upper airway fibroblasts. Fibroblasts were exposed to regular (DMEM) media without (CNT) or with PGE2 (1 μm). Fibroblasts were fixed using para-formaldehyde and permeabilized using Triton-X 100. The cytoskeleton was visualized using phalloidin to highlight the actin fibers and an antipaxillin antibody to label focal adhesion complexes. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
Upper airway (UA) injury is not an infrequent occurrence, particularly in the pediatric population. Although UA damage generally resolves with restoration of tissue structure and function, certain injury modalities, through unclear mechanisms, trigger an aberrant repair process resulting in chronic scar formation. Upper airway (subglottis and tracheal) stenosis has been shown, in both animal models and clinical settings, to cause significant morbidity and mortality, and thus represents an important focus for clinically relevant laboratory investigation.4–6,13 Our work to date has been driven by a desire to elucidate the precise cellular and molecular processes underlying the development of excessive UA scarring/stenosis and an interest in developing clinical tools for improved monitoring and pharmacological manipulation of the scarring process.
Our study of SGS development focuses on two aspects of aberrant repair: 1) activation of specific inflammatory pathways (IL-1β, COX-2, and PGE2), and 2) the effects of a dual purpose inflammatory mediator (PGE2) on putative fibroplastic activities (migration, contraction, and cytoskeletal rearrangement). Both issues are being addressed in the context of a well-established animal model of SGS and previously described human upper airway mucosal fibroblasts derived from normal and pathologic specimens.13,16,17 The experimental approach employed was largely guided by work conducted by our lab and others in the dermis and the lower airway. These studies have clearly demonstrated an important role for activation of COX-2/PGE2 during the repair process, and more specifically, a link between altered PGE2 signaling and aberrant fibroplasias.1,2,7,10,12,15 Indeed, data from the lower airway demonstrate that PGE2 acts largely as an antifibroplastic mediator, whose activity is impaired during excessive scarring. Our own work in the dermis has demonstrated that although PGE2 inhibits normal and keloid fibroblast migration, contraction and collagen synthesis (processes essential to fibroplasia), activation of the COX-2/PGE2 pathway in keloid fibroblasts, is diminished secondary to impaired nuclear factor kappa beta nuclear translocation in response to IL-1β.7 This has led us to postulate, that although keloid fibroblasts respond appropriately to exogenous PGE2, endogenous fibroblast production of PGE2 is impaired in keloid fibroblasts, likely resulting in diminished tissue-wide levels and an overall imbalance in favor of profibrotic signals. In light of these findings, we focused our investigation of SGS on whether local activation on COX-2/PGE2 during mucosal inflammation might lead to altered fibroblast activity in the subsequent repair of the mucosal wound bed.
The effects of PGE2 during tissue repair are not limited to fibroplastic events. Indeed, it is our hypothesis that PGE2 plays an important role at the junction between inflammation and fibrosis during mucosal repair. Through its complex signaling pathways, PGE2 has been shown to regulate inflammatory cell activity and vascular permeability, both essential components of tissue edema. However, whether PGE2 acts to increase or decrease edema in response to injury appears to depend largely on the timing of its release, the properties of the underlying tissue, and the associated cascade of soluble mediators present at the time. Recent work by Shiraishi et al. (2008)18 indicates that stromal cell-produced PGE2 actually suppresses inflammatory cytokine production by dendritic cells, an effect which would be consistent with an overall anti-inflammatory effect. In contrast, White et al. (2008)19 demonstrated that PGE2 is an important contributor to IL-1β mediated pulmonary edema and subsequent fibro-proliferative events.
In this study, IL-1β gene expression in injured mucosa correlated with both the degree and regional extent of injury and was maximal in the early phase of inflammation (12 hours–48 hours). Changes in IL-1β gene expression directly correlated with COX-2, the main regulatory enzyme in the PGE2 synthetic pathway and with soluble PGE2 levels in mucosal secretions.12 The regional pattern of gene expression suggests that PGE2 in mucosal secretions is indicative of localized injury and not a generalized reaction of the upper airway. This finding lends credence to the view of inflammation as a relatively localized phenomenon in the upper airway, during which the injured tissue itself plays a crucial role in the production of soluble inflammatory mediators. It also lends additional importance to the interaction between specific inflammatory cascades and endogenous fibroblast activity in the wound bed.
It is well established that inflammatory mediators can act as regulators of fibroblast activity. A clear example is TGF-β1, which though known to be a potent cytokine, also plays a crucial role in regulating fibroplastic activity within the wound bed. It is thus not very surprising or unique that the inflammatory mediator PGE2 would possess fibroblast modulatory potential. Indeed, using dermal fibroblasts we have shown that PGE2 regulates important fibroplastic activities such as migration, contraction, and collagen synthesis, and plays an important role in guiding fibroplastic activity during both physiologic and pathologic states.7,8 Here we extended this analysis to the mucosal tissue of the upper airway to more fully characterize the dual role of this soluble mediator in regulating mucosal fibroplasia. Mucosal fibroblasts displayed a phenotype similar to their dermal counterparts, with SGS fibroblasts displaying elevated rates of migration and contraction when compared to normal adult airway fibroblasts. Although PGE2 inhibited migration in both cell phenotypes, in absolute terms, SGS fibroblasts were relatively refractory to its effects. Inhibition of migration was dependent on EP2/EP4 signaling coupled to cAMP.7,8 In contrast to migration, the elevated contractile rate of SGS fibroblasts was largely refractory to the effects of PGE2. Both migration and contraction are largely governed by the dynamics of the actin cytoskeleton and connections formed via focal adhesions to the underlying substrate.7,8,17,20 Our data suggest that cytoskeletal dynamics are retained in SGS fibroblasts, with PGE2 resulting in a profound disruption of the cytoskeleton and associated focal adhesions. As such, we hypothesize that the intrinsic differences in migration and contraction are a result of subtle alterations in cytoskeletal dynamics, in contrast to data generated in the dermis, which showed substantial correlation between the anti-migratory, anticontractile properties of PGE2 and its ability to severely disrupt the actin network.7,8
As for dermal fibroblasts, the in vitro effects of PGE2 on mucosal fibroblasts appear to be antifibrotic, blunting migration and contraction through altered actin cytoskeletal dynamics. However, in contrast to the dermal fibrotic fibroblast phenotype (keloid), SGS fibroblasts were found to be relatively refractory to these effects, suggesting a role for altered PGE2 signaling during excessive upper airway scarring and fibrosis. Although keloid formation is characterized by normal fibroblast responsiveness to, but diminished endogenous production of PGE2, SGS development appears to be characterized by appropriate endogenous production of, but diminished fibroblast responsiveness to PGE2. The precise cellular and molecular mechanisms underlying altered SGS fibroblast responses to PGE2 remain to be elucidated and will be addressed in subsequent studies. The data from our skin and airway studies suggest that measuring absolute levels of specific inflammatory mediators in a wound environment may not provide a complete picture of their effects on the overall tissue repair process. Whereas keloid formation is accompanied by diminished PGE2 synthesis and unchanged responsiveness by keloid fibroblasts to its effects, the situation is reversed during SGS formation, in which absolute levels of PGE2 are in the normal range, but perhaps insufficient to overcome the intrinsically diminished responsiveness of SGS fibroblasts. Both mechanisms would result in overall decreased PGE2 antifibroplastic activity, albeit in complementary fashion.
Exogenous manipulation of the inflammatory cascade can be accomplished through multiple approaches. One common therapeutic approach is the use of exogenous steroids (delivered systemically or locally) to reduce wholesale the inflammatory response to injury through downregulation of cell proliferation and soluble mediator production. The interaction between corticosteroids and the PGE2 signaling pathway is complex, consisting of multiple feed-back and feed-forward loops, and has only been extensively studied in the reproductive system and development. As previously demonstrated, corticosteroids can regulate the expression of EP receptors during various phases of gestation and can regulate PGE2 production by macrophages and other inflammatory cells.21–23 PGE2 can, in turn regulate synthesis and processing of specific glucocorticoids such as cortisol. The precise interaction between corticosteroids and PGE2 during upper airway repair remains to be fully characterized and may present an interesting approach for additional studies.24
Mitomycin-C has been used extensively as a chemotherapeutic agent, relying on its DNA cross-linking potential to result in subsequent inhibition of cell proliferation. It has been used in the airway alone or in combination with other treatment modalities to reduce scarring and fibrosis by reducing fibroblast proliferation. We expect that its effects would be overall synergistic with the antifibroplastic effects of PGE2 in so far as reducing scarring, but rather minimal with respect to cytoskeletal dynamics given its mechanism of action.
From a clinical perspective, the data presented previously hold important promise for future clinical applications by identifying COX-2/PGE2 as a putative target for pharmacological manipulation of the mucosal wound-healing response aimed at minimizing associated scarring/stenosis. Additional studies will continue to utilize the rabbit animal model to characterize specific pharmacological approaches aimed at modulating COX-2 activity in the mucosal wound bed and measuring healing outcomes.
CONCLUSION
Upper airway mucosal injury triggers a significant upregulation of IL-1β and COX-2 gene expression, which corresponds to increased PGE2 levels in mucosal secretions. Inflammatory gene upregulation is exquisitely sensitive to mucosal injury, varying proportionally with the extent of, and distance from, site of injury. The correlation between mRNA and protein levels in the affected tissue with mediator concentrations in mucosal secretions suggests that secretion analysis of key inflammatory markers correlate with tissue levels and thus may constitute a useful noninvasive diagnostic tool for monitoring purposes in the context of both SGS development and preventive treatments.
Combined with previous studies, in vitro data support the following paradigm. PGE2 is released shortly after injury and contributes to the acute inflammatory response, but later in the healing cascade counteracts the role of profibrotic soluble mediators during the fibroplastic phase of tissue repair. In cases of disregulated wound healing, PGE2 levels are insufficient to overcome the activity of other, profibrotic mediators, resulting in excessive scarring and SGS development. The mechanism underlying this exquisite regulatory system warrants further investigation and may provide therapeutic targets for optimizing wound healing in the upper airway mucosa.
Acknowledgments
This work was supported by a grant from the National Institutes of Health Grant #R01 DC007437 (PAH); Children’s Hospital of Pittsburgh.
The authors thank Ryan Branski, PhD for extensive discussion and interpretation of results.
Footnotes
The authors have no financial or other conflict of interest regarding the content of this paper.
BIBLIOGRAPHY
- 1.Talwar M, Moyana TN, Bharadwaj B, Tan LK. The effect of a synthetic analogue of prostaglandin E2 on wound healing in rats. Ann Clin Lab Sci. 1996;26:451–457. [PubMed] [Google Scholar]
- 2.Wilgus TA, Bergdall VK, Tober KL, et al. The impact of cyclooxygenase-2 mediated inflammation on scarless fetal wound healing. Am J Pathol. 2004;165:753–761. doi: 10.1016/S0002-9440(10)63338-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liechty KW, Kim HB, Adzick NS, Crombleholme TM. Fetal wound repair results in scar formation in interleukin-10-deficient mice in a syngeneic murine model of scarless fetal wound repair. J Pediatr Surg. 2000;35:866–872. doi: 10.1053/jpsu.2000.6868. discussion 872–863. [DOI] [PubMed] [Google Scholar]
- 4.Cotton RT, Tewfik TL. Laryngeal stenosis following carbon dioxide laser in subglottic hemangioma. Report of three cases. Ann Otol Rhinol Laryngol. 1985;94:494–497. doi: 10.1177/000348948509400516. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Volk MS, Shapshay SM. Endoscopic laryngotracheoplasty and graft soldering with the carbon dioxide laser. An animal study. Ann Otol Rhinol Laryngol. 1997;106:989–994. doi: 10.1177/000348949710601201. [DOI] [PubMed] [Google Scholar]
- 6.Whitehead E, Salam MA. Use of the carbon dioxide laser with the Montgomery T-tube in the management of extensive subglottic stenosis. J Laryngol Otol. 1992;106:829–831. doi: 10.1017/s0022215100121000. [DOI] [PubMed] [Google Scholar]
- 7.Sandulache VC, Parekh A, Li-Korotky H, Dohar JE, Hebda PA. Prostaglandin E2 inhibition of keloid fibroblast migration, contraction, and transforming growth factor (TGF)-beta1-induced collagen synthesis. Wound Repair Regen. 2007;15:122–133. doi: 10.1111/j.1524-475X.2006.00193.x. [DOI] [PubMed] [Google Scholar]
- 8.Sandulache VC, Parekh A, Li-Korotky HS, Dohar JE, Hebda PA. Prostaglandin E2 differentially modulates human fetal and adult dermal fibroblast migration and contraction: implication for wound healing. Wound Repair Regen. 2006;14:633–643. doi: 10.1111/j.1743-6109.2006.00156.x. [DOI] [PubMed] [Google Scholar]
- 9.Yan M, Rerko RM, Platzer P, et al. 15-Hydroxyprostaglandin dehydrogenase, a COX-2 oncogene antagonist, is a TGF-beta-induced suppressor of human gastrointestinal cancers. Proc Natl Acad Sci U S A. 2004;101:17468–17473. doi: 10.1073/pnas.0406142101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li HS, Hebda PA, Kelly LA, Ehrlich GD, Whitcomb DC, Dohar JE. Up-regulation of prostaglandin EP4 receptor messenger RNA in fetal rabbit skin wound. Arch Otolaryngol Head Neck Surg. 2000;126:1337–1343. doi: 10.1001/archotol.126.11.1337. [DOI] [PubMed] [Google Scholar]
- 11.Harizi H, Grosset C, Gualde N. Prostaglandin E2 modulates dendritic cell function via EP2 and EP4 receptor subtypes. J Leukoc Biol. 2003;73:756–763. doi: 10.1189/jlb.1002483. [DOI] [PubMed] [Google Scholar]
- 12.Sandulache VC, Chafin JB, Li-Korotky HS, Otteson TD, Dohar JE, Hebda PA. Elucidating the role of interleukin 1beta and prostaglandin E2 in upper airway mucosal wound healing. Arch Otolaryngol Head Neck Surg. 2007;133:365–374. doi: 10.1001/archotol.133.4.365. [DOI] [PubMed] [Google Scholar]
- 13.Chafin JB, Sandulache VC, Dunklebarger JL, et al. Graded carbon dioxide laser-induced subglottic injury in the rabbit model. Arch Otolaryngol Head Neck Surg. 2007;133:358–364. doi: 10.1001/archotol.133.4.358. [DOI] [PubMed] [Google Scholar]
- 14.Petkova DK, Clelland CA, Ronan JE, Lewis S, Knox AJ. Reduced expression of cyclooxygenase (COX) in idiopathic pulmonary fibrosis and sarcoidosis. Histopathology. 2003;43:381–386. doi: 10.1046/j.1365-2559.2003.01718.x. [DOI] [PubMed] [Google Scholar]
- 15.Wilborn J, Crofford LJ, Burdick MD, Kunkel SL, Strieter RM, Peters-Golden M. Cultured lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis have a diminished capacity to synthesize prostaglandin E2 and to express cyclooxygenase-2. J Clin Invest. 1995;95:1861–1868. doi: 10.1172/JCI117866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otteson TD, Sandulache VC, Barsic M, Disilvio GM, Hebda PA, Dohar JE. Acute and chronic changes in the subglottis induced by graded carbon dioxide laser injury in the rabbit airway. Arch Otolaryngol Head Neck Surg. 2008;134:694–702. doi: 10.1001/archotol.134.7.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sandulache VC, Parekh A, Dohar JE, Hebda PA. Fetal dermal fibroblasts retain a hyperactive migratory and contractile phenotype under 2-and 3-dimensional constraints compared to normal adult fibroblasts. Tissue Eng. 2007;13:2791–2801. doi: 10.1089/ten.2006.0412. [DOI] [PubMed] [Google Scholar]
- 18.Shiraishi H, Yoshida H, Saeki K, et al. Prostaglandin E2 is a major soluble factor produced by stromal cells for preventing inflammatory cytokine production from dendritic cells. Int Immunol. 2008;20:1219–1229. doi: 10.1093/intimm/dxn078. [DOI] [PubMed] [Google Scholar]
- 19.White KE, Ding Q, Moore BB, et al. Prostaglandin E2 mediates IL-1beta- related fibroblast mitogenic effects in acute lung injury through differential utilization of prostanoid receptors. J Immunol. 2008;180:637–646. doi: 10.4049/jimmunol.180.1.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ehrlich HP, Rajaratnam JB. Cell locomotion forces versus cell contraction forces for collagen lattice contraction: an in vitro model of wound contraction. Tissue Cell. 1990;22:407–417. doi: 10.1016/0040-8166(90)90070-p. [DOI] [PubMed] [Google Scholar]
- 21.Smith GC, Wu WX, Nijland MJ, Koenen SV, Nathanielsz PW. Effect of gestational age, corticosteroids, and birth on expression of prostanoid EP receptor genes in lamb and baboon ductus arteriosus. J Cardiovasc Pharmacol. 2001;37:697–704. doi: 10.1097/00005344-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Schmitz T, Cox LA, Li C, et al. Prostaglandin E2 receptor expression in fetal baboon lung at 0.7 gestation after betamethasone exposure. Pediatr Res. 2007;61:421–426. doi: 10.1203/pdr.0b013e318030d141. [DOI] [PubMed] [Google Scholar]
- 23.Bezugla Y, Kolada A, Kamionka S, Bernard B, Scheibe R, Dieter P. COX-1 and COX-2 contribute differentially to the LPS-induced release of PGE2 and TxA2 in liver macrophages. Prostaglandins Other Lipid Mediat. 2006;79:93–100. doi: 10.1016/j.prostaglandins.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Chandras C, Harris TE, Bernal AL, Abayasekara DR, Michael AE. PTGER1 and PTGER2 receptors mediate regulation of progesterone synthesis and type 1 11beta-hydroxysteroid dehydrogenase activity by prostaglandin E2 in human granulosa lutein cells. J Endocrinol. 2007;194:595–602. doi: 10.1677/JOE-07-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]