In 1899, Metchnikoff first conceived of specific therapy with antilymphocyte globulin (ALG) compounds.1 Research was carried out sporadically in Europe, mostly by Swiss immunologists. Antilymphocyte serum (ALS) was first used to prevent skin graft rejection in experimental animals by Waksman et al2 and subsequently by Woodruff and Anderson.3
ALG was first used clinically in 1966.4 Its value was promptly confirmed in a number of centers in Europe, Australia, and the United States. Yet while ALS and ALG demonstrated potent immunosuppressive qualities, they could not be standardized. Moreover, lack of predictability of response greatly limited their use.
The original antihuman ALGs were prepared after immunization of horses, rabbits, or goats with human lymphoid tissue.5 The active fraction, IgG, was then extracted and used for intramuscular or intravenous (IV) injection. These primitive ALGs often contained antibodies that reacted to contaminating blood elements as well as other nonrelevant antigens.
With the development of hybridoma technology, an important refinement of the original ALG concept became possible.6 Kung et al7 reported in 1979 the development of monoclonal antibody, ORTHOCLONE OKT3 (Ortho Pharmaceutical Corp., Raritan, NJ), using the hybridoma technology. ORTHOCLONE OKT3 is a standardizable substance with defined specificity and greater than 95% relevant protein.
ORTHOCLONE OKT3 was introduced clinically by Cosimi et al in 1981.8 Most clinical trials with ORTHOCLONE OKT3 have been for the treatment of renal rejection occurring despite immunosuppression with azathioprine and prednisone. With the expanding use of cyclosporine, the way in which ORTHOCLONE OKT3 fits in with cyclosporine-steroid therapy is an important issue. Initially, there was some speculation, based largely on hypothetical grounds, that ORTHOCLONE OKT3 might be pharmacologically incompatible with cyclosporine. That notion has been largely dispelled by practical experience. The aim of our studies was to determine the usefulness of ORTHOCLONE OKT3, in conjunction with cyclosporine and steroids.
METHODS
Sixty-two patients were treated with ORTHOCLONE OKT3, including 52 liver and ten kidney allograft recipients whose baseline immunosuppression was with cyclosporine and steroids. ORTHOCLONE OKT3 treatment was initiated in all renal transplant recipients, because rejection continued in spite of cyclosporine and high-dose steroid therapy. The initial 18 liver recipients were part of a randomized trial comparing ORTHOCLONE OKT3 and high-dose steroids, whereas the remaining 34 liver patients received ORTHOCLONE OKT3 after failing to respond to a steroid recycle. Adults were given 5 mg IV of ORTHOCLONE OKT3 daily for ten to 14 days. Small pediatric recipients were given half the adult dose; in some small infants, one fifth of the adult dose was used. Thus, doses were not administered completely on a body weight basis.
Volume overload was corrected if present. In addition, since symptoms are seen generally within the first few days of treatment, when lymphocyte destruction is greatest, premedication with hydrocortisone and diphenhydramine was given. Development of anaphylactoid reactions (pulmonary edema) is heralded by respiratory symptoms that mimic asthma. As a precaution, when patients complained of shortness of breath, even without objective signs of distress, they were moved into the intensive care area, as they may have required intubation and ventilatory support as well as resuscitation. Epinephrine administered IV was the most effective drug for severe distress.
RESULTS WITH RENAL TRANSPLANTATION
Eight of ten steroid-resistant kidney recipients responded to monoclonal treatment: four partially and four completely. Failures were in patients who had vascular (antibody-mediated) rejection. This was documented pathologically in one of two failures; in the other, there also was cellular rejection. Four patients achieved stable but abnormal function, although two eventually lost their kidney grafts. Overall, all ten patients would probably have lost their grafts; but with ORTHOCLONE OKT3, six were salvaged, four with completely normal function.
Figure 1 shows the course of a patient who started the postoperative period with an acute tubular necrosis, but began to recover with delayed diuresis. Diuresis was cut off at the onset of rejection, which could not be reversed or controlled with high-dose steroid therapy. ORTHOCLONE OKT3 therapy was initiated on day 25 posttransplant, and there was immediate reversal of rejection and recovery of renal function within a few days. Immunosuppressive therapy with cyclosporine was not reduced in this case. Steroid therapy was left at baseline level.
Fig 1.
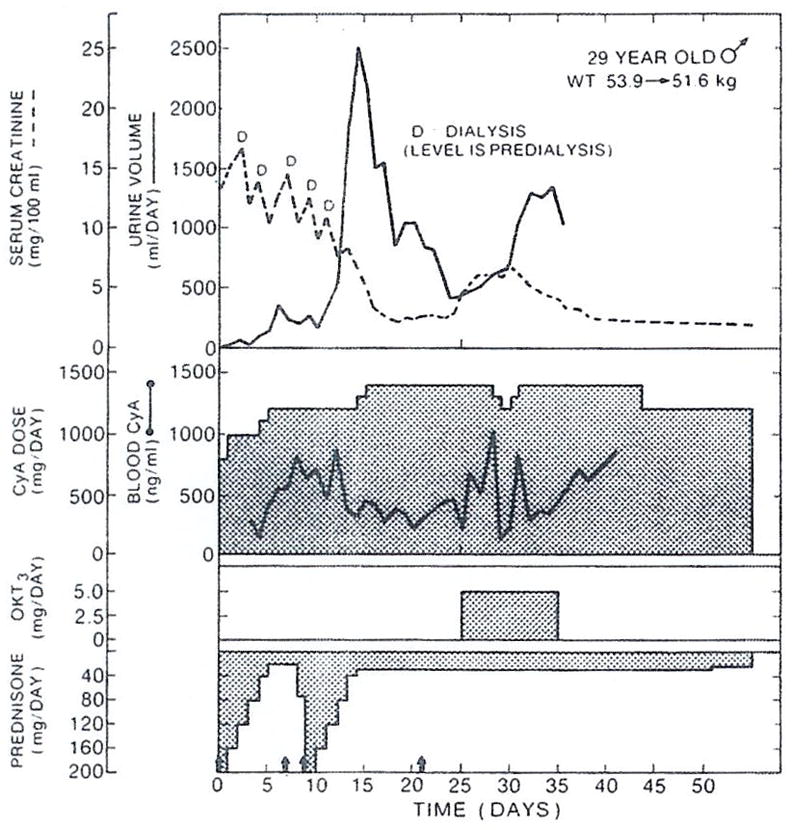
Clinical course of cadaveric kidney recipient whose graft underwent initial period of acute tubular necrosis, with beginning subsequent diuresis interrupted by rejection. Arrows = administration of 1 g methylprednisolone.
In some patients, monoclonal treatment was used as a window through which baseline immunosuppressive therapy could be increased or decreased according to what was believed optimal for maintenance; this is perhaps the key to avoiding rerejection.
Minor infections occurred during the treatment period in 40% of patients, usually a viral infection (most commonly herpes simplex reactivation). There was no mortality.
RESULTS WITH LIVER TRANSPLANTATION
Initially, a randomized trial on treatment of hepatic rejection was conducted in which ORTHOCLONE OKT3 and high-dose steroids were administered. Our conclusion was that ORTHOCLONE OKT3 was superior to steroid therapy. Of seven patients randomized to receive ORTHOCLONE OKT3, six had full recovery of hepatic function. Rescue of ORTHOCLONE OKT3 treatment failures would have been with high-dose steroid therapy; this was not required, however, as there was no recurrence of rejection in ORTHOCLONE OKT3 responders.
Eleven patients were randomized to steroid therapy; four had reversal of rejection, while seven remained resistant to steroid therapy and required rescue with ORTHOCLONE OKT3. ORTHOCLONE OKT3 was successful in reversing the rejection in six of the seven patients.
As the trial progressed, concerns were raised that randomization might not be appropriate. One possibility was that ORTHOCLONE OKT3 was being used in cases which might have responded to increased steroids. In cases of severe rejection, it was possible that ORTHOCLONE OKT3 was being withheld when it could have been life-saving, in order to adhere to the study protocol.
For these reasons, randomization was stopped in favor of the protocol described in Fig 2. Patients who entered the study had liver dysfunction of unexplained etiology (technical causes were ruled out). Workup included a percutaneous biopsy, which allowed proof of cell-mediated rejection (Fig 3A). If the biopsy demonstrated rejection, a steroid recycle was tried. This caused a response in some cases, but in the event of no response, ORTHOCLONE OKT3 therapy was given for ten to 14 days. Thus, a protocol of rescue of steroid-resistant patients was arrived at. In these patients, cellular rejection was concentrated around the portal tracts. The percutaneous biopsies allowed proof of improvement (Fig 3B). Most patients started on ORTHOCLONE OKT3 were rebiopsied after completion of therapy. The pathologic correlation of improvement with ORTHOCLONE OKT3 was virtually absolute.9
Fig 2.
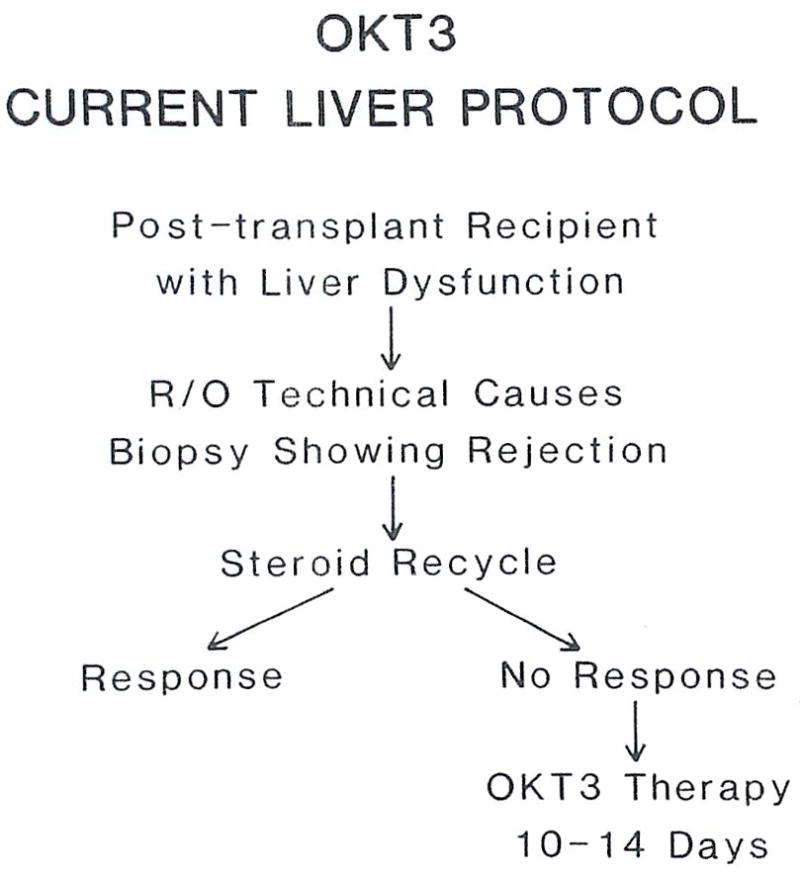
Current protocol for treatment of suspected rejection.
Fig 3.
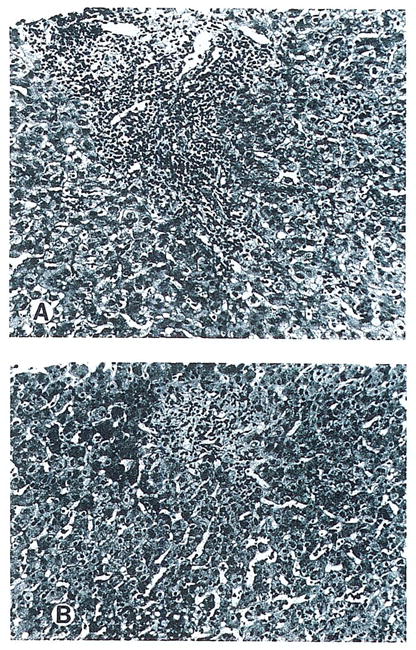
(A) Histologic findings of cellular rejection typical in group 2 liver patients consist of portal mononuclear cell infiltration with damage to bile duct epithelium and endothelium of vessels. (B) Improvement of histologic findings after ORTHOCLONE OKT3 therapy. Note marked decrease of inflammatory cells.
The results are stratified into three groups. Group 1 patients were given ORTHOCLONE OKT3 therapy during the first nine postoperative days. They had poor hepatic function in the early postoperative period. Renal failure, a common complication in patients with endstage liver disease and a complication of cyclosporine treatment, was also common. Rejection was suspected, and in 15 of the 18 patients, rejection was proven by biopsy, although not always at the exact moment ORTHOCLONE OKT3 therapy was started. Biopsies were not carried out at this time in cases in which severe liver failure led to such poor coagulation that the procedure was not safe.
Group 2 consisted of 22 patients who were between 10 and 90 days postoperative. In this group there was no real ambiguity in ascribing hepatic dysfunction to rejection, since biopsies could be obtained routinely.
Group 3 consisted of 12 patients treated beyond 90 days, in whom an element of chronic rejection may have been present; confusing perioperative factors characteristic of group 1 patients, however, were not present.
Results are summarized in Table 1. In group 1 patients in whom renal failure in combination with liver dysfunction was common, ORTHOCLONE OKT3 therapy allowed lowering of potentially nephrotoxic cyclosporine doses. An example of a patient who had poor primary hepatic graft function is shown in Fig 4. The patient’s bilirubin began at a high level and continued to rise concurrent with rapidly advancing renal failure. However, when ORTHOCLONE OKT3 therapy was started, oral cyclosporine was continued, and IV cyclosporine stopped, blood levels of cyclosporine leveled off adequately.
Table 1.
Results of ORTHOCLONE OKT3 Therapy in Liver Transplant Recipients
| Response(%) |
Long-term Graft Function* (%) | |||
|---|---|---|---|---|
| None | Partial | Full | ||
| Overall | 22 | 25 | 53 | 71 |
| Group 1 | 28 | 39 | 33 | 66 |
| Group 2 | 9 | 18 | 73 | 31 |
| Group 3 | 33 | 25 | 42 | 52 |
Follow-up 7.2 ± 1.7 mo.
Fig 4.
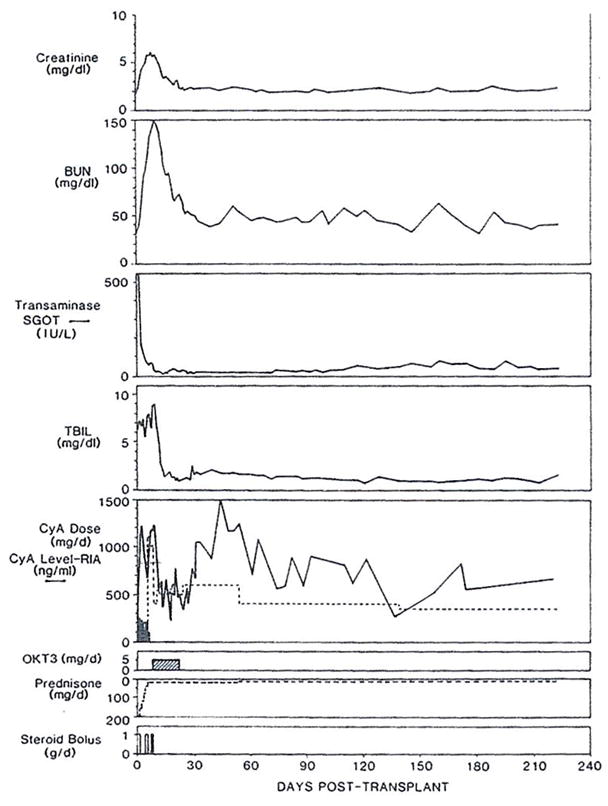
Clinical course of liver recipient whose ORTHOCLONE OKT3 course was started after nine days, in part because renal failure precluded giving therapeutic doses of cyclosporine (CyA) to treat presumed rejection. Note resolution of azotemia during ORTHOCLONE OKT3 therapy. Note cyclosporine was given at first by both IV (dark shading) and oral (no shading) routes. Abbreviation: TBIL, total bilirubin; SGOT, serum glutamic-oxaloacetic transaminase. Solid bars for steroid bolus = methylprednisolone IV; open bars = hydrocortisone IV.
Group 1 patients had the poorest record: 33% had a full response; 39% had a partial response, and approximately 30% had no response (Table 1). Yet, incongruously, the impact of ORTHOCLONE OKT3 on liver transplantation should be greatest for group 1-type patients, because this is the group that will have a high mortality rate if something decisive cannot be done, such as rescue with ORTHOCLONE OKT3 therapy.
In group 2, better than 90% of patients had reversal of rejection (Table 1). Three quarters of the patients had a full response with return of normal hepatic function.
In group 3, almost half of the patients treated for more than 3 months had a full response, even though in many there was undoubtedly an element of chronic rejection; one third had no response; the rest had a partial response (Table 1).
Morbidity and mortality are summarized in Table 2. No deaths were caused by ORTHOCLONE OKT3. One patient had an anaphylactoid reaction, which may have been caused by fluid overload. In patients with extrarenal transplants, including liver and heart recipients, dialysis may not be as effective in reducing fluid overload as in renal recipients. Overall mortality was low, considering that previous treatment with steroids had failed in these patients.
Table 2.
Morbidity and Mortality of Patients Treated With ORTHOCLONE OKT3
| Liver Recipients (%) | Kidney Recipients (%) | |
|---|---|---|
| Morbidity | ||
| Fever | 75 | 30 |
| Hemodynamic instability | 62 | 60 |
| Dyspnea | 32 | 20 |
| Chills | 28 | 70 |
| Anaphylactoid reaction | 2 | 0 |
| Infections: Major | 15 | 0 |
| Minor | 56 | 40 |
| Mortality | ||
| Overall | 23 | 0 |
| Group 1 | 33 | — |
| Group 2 | 18 | — |
| Group 3 | 17 | — |
Long-term graft function in liver recipients is shown in Table 1. Overall, 71% of these patients had a functioning graft after an average follow-up of 7 months. This is a significant improvement for a patient population which for the most part had not responded to steroid therapy.
The rate of rerejection was low in these liver recipients as it had been in our kidney recipients. In this protocol cyclosporine treatment was not discontinued during treatment with ORTHOCLONE OKT3. It is during the ORTHOCLONE OKT3 treatment period that basic immunosuppression can be adjusted. If this is done effectively to maintenance standards, incidence of later recurrent rejection will be small, and maximum potential of ORTHOCLONE OKT3 will be realized.
Serologic studies have shown that more than half of ORTHOCLONE OKT3 recipients developed antimurine antibodies during therapy. Patients with demonstrable antimurine antibodies from a first treatment were not retreated, although retreatment has been done successfully by us and by D.J. Norman (personal communication Nov, 1985). We have avoided re-treating patients who had demonstrable antimurine antibodies from a first treatment. Nevertheless, the fact that so many patients develop antibodies to the murine protein is an argument against using ORTHOCLONE OKT3 unless it is absolutely needed.
CONCLUSIONS
ORTHOCLONE OKT3 in conjunction with cyclosporine and steroids appears to be an effective immunosuppressive combination in achieving reversal of renal and hepatic rejection. Long-term graft survival without excessive recurrent rejection episodes has been achieved.
Acknowledgments
Supported by Research Grants from the Veterans Administration and Project Grant No. AM-29961 from the National Institutes of Health, Bethesda, Maryland. Dr J.J. Fung is a recipient of the American Heart Association-Western Pennsylvania Affiliate Fellowship.
These studies were carried out with the collaboration of Professor K.A. Porter of St Mary’s Hospital Medical School, London, who aided in the pathologic analyses in cooperation with University of Pittsburgh pathologists, A.J. Demetris and R. Jaffe.
References
- 1.Metchnikoff E. Ann Inst Pasture (Paris) 1899;13:737. [Google Scholar]
- 2.Waksman BH, Arbouys S, Arnason BG. J Exp Med. 1961;114:997. doi: 10.1084/jem.114.6.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woodruff MFA, Anderson NF. Ann NY Acad Sci. 1964;120:119. doi: 10.1111/j.1749-6632.1964.tb34710.x. [DOI] [PubMed] [Google Scholar]
- 4.Starzl TE, Marchioro TL, Porter KA, et al. Surg Gynecol Obstet. 1967;124:30. [PMC free article] [PubMed] [Google Scholar]
- 5.Iwasaki Y, Porter KA, Amend J, et al. Surg Gynecol Obstet. 1967;124:1. [PMC free article] [PubMed] [Google Scholar]
- 6.Kohler G, Milstein C. Nature. 1975;256:495. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 7.Kung PC, Goldstein G, Reinherz EL, et al. Science. 1979;206:347. [Google Scholar]
- 8.Cosimi AB, Burton RC, Colvin RB, et al. Transplantation. 1981;32:535. doi: 10.1097/00007890-198112000-00018. [DOI] [PubMed] [Google Scholar]
- 9.Fung JJ, Demetris AJ, Porter KA, et al. Nephron. doi: 10.1159/000184431. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


