Abstract
PURPOSE
To evaluate the possible mechanisms influencing the infiltration of CD8 T lymphocytes into the tumor epithelium of advanced serous ovarian cancers.
EXPERIMENTAL DESIGN
Immunohistochemical localization of CD8 T lymphocytes was performed on a homogeneous population of 184 high-grade, advanced-stage serous ovarian cancer tissue specimens. Microarray analysis was performed on microdissected tumor epithelium from 38 specimens to identify genes up- or down-regulated in specimens with differing numbers of tumor-infitrating CD8 T lymphocytes. Quantitative real-time polymerase chain reaction and immunohistochemistry were used to validate a candidate gene. Univariate and multivariate survival analyses were performed combining CD8 T lymphocyte number and HLA-DMB expression with standard prognostic factors.
RESULTS
Marked CD8 T lymphocyte infiltration of the tumor epithelium is associated with a 20 month improvement in median overall survival. Additionally, when combined with cytoreduction status and age, CD8 T lymphocyte status is an independent prognostic factor for survival. Microarray analysis demonstrated HLA-DMB, a component of the major histocompatibility complex (MHC) II antigen-presentation machinery, to be differentially expressed in specimens with an abundance of tumor-infiltrating CD8 T lymphocytes. This relationship was validated at both the mRNA and protein levels. As well, high HLA-DMB expression in the tumor epithelium was associated with a significant improvement in median overall survival in both univariate and multivariate analyses.
CONCLUSION
Tumor cell expression of HLA-DMB is associated with increased numbers of tumor-infiltrating CD8 T lymphocytes and both are associated with improved survival in advanced serous ovarian cancer.
INTRODUCTION
Epithelial ovarian cancer (EOC) accounts for more deaths annually than all other gynecologic cancers combined.1 Although early-stage disease has a 5-year survival that approaches 95%,2 most cases are diagnosed at an advanced stage.2 With cytoreductive surgery and chemotherapy, greater than half of such patients will achieve remission, but most will experience recurrence and ultimately die of their disease.2 Therefore, therapies complementary to standard surgery and chemotherapy are sought to improve long-term survival in advanced EOC.
The immune system has long been noted to have an anti-tumor effect.3 Tumor-infiltrating cytotoxic T lymphocytes (CD8) are associated with improved overall survival and have been described in several solid tumors including ovarian, endometrial, colon, and esophageal cancers.4,5,6,7,8 Zhang et al., analyzing CD3 T lymphocyte infiltration in 174 advanced ovarian cancers, noted improved survival in tumors with infiltrating CD3 T lymphocytes.4 Equivalently, Sato et al. demonstrated CD8 T lymphocyte infiltration in the ovarian tumor epithelium is associated with prolonged overall survival.5
Prior reports examining CD3 and/or CD8 T lymphocyte infiltrates in EOC included very heterogeneous populations, often incorporating all histological subtypes and stages of disease. Furthermore, tumor intrinsic and extrinsic factors that may be associated with increased tumor-infiltrating CD8 T lymphocytes have not been completely identified.
The present study was undertaken to better understand the molecular mechanisms underlying differences in tumor-infiltrating cytotoxic T lymphocytes. Differential transcription profiling was performed by microarray analysis on mRNA isolated from the microdissected epithelium of serous ovarian cancer. HLA-DM gene expression strongly correlated with the number of tumor-infiltrating CD8 T lymphocytes. HLA-DM protein is also directly related to antigen presentation and lymphocyte activation. The expression of HLA-DM was further evaluated. HLA-DRA was also analyzed on ovarian cancer cell lines and paraffin sections to study the possibility of an existing major histocompatibility complex (MHC) II antigen-presentation machinery in ovarian cancer cell.
METHODS
STUDY PATIENTS
184 paraffin-embedded and frozen ovarian cancer tissue samples were used. All ovarian cancer tissues were of the serous subtype, high-grade, and International Federation of Gynecology and Obstetrics (FIGO) stage IIIB-IV (advanced-stage). Tissues were collected from patients undergoing primary cytoreductive surgery for ovarian cancer at the Brigham and Women’s Hospital (Boston, MA) between 1990 and 2006. After surgery, patients received platinum-based combination chemotherapy. All patients for the survival study received paclitaxel in combination with a platinum as the bulk of the cases accessioned were after 1999, when paclitaxel was already in common usage in clinical practice. Optimal surgical cytoreduction was defined by residual tumor ≤ 1 cm in diameter. The duration of overall survival was measured from the date of diagnosis to death or censored at the date of last follow-up. Clinical data including age, cytoreduction status (optimal vs. suboptimal), and overall survival were abstracted from the medical record. All specimens and clinical data were collected under approval of the Institutional Review Board.
SAMPLE SIZE ANALYSIS
Using 0.05 as alpha value and 0.80 as desired power 160 samples are required for the multivariate Cox proportional hazards model studies.
SAMPLE COLLECTION AND PRESERVATION
All samples were collected from primary surgeries and tumor tissues were processed with standard pathology paraffinazition method to make paraffin embedded blocks. Fresh tumor tissues from the same patients were also frozen in Tissue-Tek O.C.T compound (Sakura Finetek USA, Torrance CA) and stored at −80 °C till frozen sections were made for laser microdissection.
IMMUNOHISTOCHEMISTRY
Immunolocalization of tumor-infiltrating CD8 T lymphocytes was performed (n=184) using monoclonal mouse, anti-human CD8 (clone C8/144B, 1:50 dilution, DAKO, Carpinteria, CA). Paraffin-embedded sections were deparaffinized, dehydrated, and antigen retrieval performed in Target Retrieval Solution (DAKO) with the pressure cooker at 120° Celsius for 20 minutes. Alternatively, frozen sections were fixed in methanol for 10 minutes. After blocking, sections were incubated with the primary antibody (anti-CD8) at room temperature for 90 minutes and then washed two times with 1x Tris-Buffered Saline (TBS, Boston BioProducts, Worcester, MA), and incubated with Polymer-AP (DAKO) for 30 minutes. CD8 positive signals were visualized using Fast Red (DAKO).
Immunolocalization of tumor-infiltrating CD4 T lymphocytes was performed on a subset (n=24) high-grade, advanced-stage serous ovarian cancer specimens using monoclonal mouse, anti-human CD4 (pre-diluted, Abcam Inc., Cambridge, MA). CD4 positive signals were visualized utilizing Envision G2 System/AP kit (permanent red) (DAKO).
Immunolocalization of HLA-DMB was performed on a subset (n=64) of paraffin-embedded, high-grade, advanced-stage serous ovarian cancer specimens. After deparaffinization and dehydration, antigen retrieval was performed in Target Retrieval Solution (DAKO) with the pressure cooker at 120° Celsius for 10 minutes. After blocking, tissue sections were incubated with monoclonal mouse, anti-human HLA-DMB (clone 6B3, 1:50 dilution, Novus Biologicals, Littleton, CO) at room temperature for 2 hours, washed two times, and then HLA-DMB positive signals were visualized with the Envision G2 System/AP kit (permanent red) (DAKO).
QUANTIFICATION OF IMMUNOHISTOCHEMISTRY
The number of CD8 and CD4 T lymphocytes infiltrating the tumor epithelium was determined by counting manually the number of positive cells in 4–8 high-powered fields (20x objective) from each case. The counts from each high-powered field were averaged thus creating a mean number of tumor-infiltrating CD8 or CD4 T lymphocytes per specimen. Each specimen was analyzed by a single investigator (M.C.) with random specimens being evaluated by a second investigator (S.M.) for quality control.
HLA-DMB protein expression was quantified using Image-Pro Plus 5.1.0.20 for Windows (Media Cybernetics, Bethesda, MD). One to two sections per case were analyzed quantitatively. The staining saturation was measured from 3 fixed-size areas in the tumor epithelium and one background area in the tumor stroma. The difference between each epithelial and the stromal measurement was obtained and the values averaged yielding one score for each case.
IMMUNOFLUORESCENCE
A subset of 5 high-grade, advanced-stage serous ovarian cancer specimens with high numbers of tumor-infiltrating CD8 T lymphocytes were selected for double immunofluorescent staining with rabbit, anti-human CD8 (pre-diluted, Abcam Inc.) and mouse, anti-human HLA-DR (clone LN-3, 1:50 dilution, Vision BioSystems, Novocastra, Newcastle Upon Tyne, UK). After deparaffinization, dehydration, and antigen retrieval, blocking was performed with 10% normal goat serum diluted in 1x TBS (Boston BioProducts). Next, the specimens were incubated with the first primary antibody (anti-CD8) for 60 minutes at room temperature, washed two times, and then Alexa Fluor 647 (Molecular Probes, Eugene, OR) goat, anti-rabbit IgG (1:200 dilution) was applied for 60 minutes at room temperature. After washing, the specimens were incubated with the second primary antibody (anti-HLA-DR) for 60 minutes at room temperature, washed two times, and then Alexa Fluor 546 (Molecular Probes) goat, anti-mouse IgG (1:200 dilution) was applied for 60 minutes at room temperature. Nuclear staining with 4,6-diamidino-2-phenylindole (DAPI, Molecular Probes) was performed. Fluorescent signals were visualized with a Leica IRE2 fluorescent microscope.
MICROARRAY ANALYSIS
cRNA from 38 of the high-grade, advanced-stage ovarian cancer tissue samples (training set) was prepared according to the Affymetrix Expression Analysis Technical Manual (Affymetrix, Santa Clara, CA) and hybridized to the arrays as described previously.9 Global normalization at a target value of 500 was applied to all of the arrays under consideration using GeneChip Operating Software (Affymetrix), and the normalized data were uploaded into the NCI’s Microarray Analysis Database.9 Biometrics Research Branch (BRB) ArrayTools version 3.6 was used for statistical analysis of the array data. Probe sets scored as absent (A) at α1=0.05 or marginal (M) at α2=0.065 were excluded from the analysis. In addition, only those transcripts present in >50% of the arrays and displaying a variance in the top 50th percentile were evaluated. Using these filtering criteria, 14,657 probe sets were tested by a Pearson correlation test employing a random variance model. Signal intensities across the 38 specimens were correlated with the number of CD8+ cells per 20x field. Probe sets possessing a significant correlation coefficient (p<0.01) were considered further.
QUANTITATIVE REAL-TIME POLYMERASE CHAIN REACTION
Quantitative real-time polymerase chain reaction (qRT-PCR) was performed to evaluate HLA-DMB (n=32) and HLA-DRA (n=32) mRNA expression in the microdissected high-grade, advanced-stage serous tumor epithelium of cases in the training set. qRT-PCR was performed using the 7300 Real-Time PCR System and TaqMan Gene Expression Assay probes (Applied Biosystems, Foster City, CA). For each qRT-PCR reaction, the primer of interest and cyclophilin A (an endogenous control) were multiplexed as follows: a total of 2 ul of cDNA was added to 10 ul of TaqMan Universal PCR Master Mix (Applied Biosystems), 1 ul of LR1 TaqMan primer/probe, 1 ul of cyclophilin A TaqMan primer/probe, and 6 ul of autoclaved dH20. Reactions started with a 10-minute hold at 95°C, followed by 40 cycles of denaturation at 95°C for 125 seconds, followed by annealing/extending at 60°C for one minute. The 7300 Real-Time PCR System software monitored the amplification process and determined the threshold cycle (Ct) for each reaction. Quantification was relative to one of the tumor specimens and was reported as a fold-change as determined by the ΔΔCt method as described previously.10
Additionally, in a separate validation set of 25 microdissected high-grade, advanced-stage serous ovarian cancers, qRT-PCR was performed with HLA-DMB primer/probe as described above.
STATISTICAL ANALYSIS
The Kaplan and Meier method and log-rank statistic were used to estimate survival. Hazard ratios among covariates were estimated using a multivariate Cox proportional hazards model. The Mann-Whitney U Test compared medians of continuous variables between two independent samples. The Pearson and Spearman rank correlation assessed the relationship between two continuous variables. A logistic regression model was used to predict the outcome of a dichotomous variable (CD8) from one predictor variable (HLA-DMB expression). All statistical analyses were performed with SPSS Rel 14.0.0 (2005).11 A p-value < 0.05 (two-sided test) was considered statistically significant.
RESULTS
TUMOR-INFILTRATING CD8 T LYMPHOCYTES ARE A PREDICTOR FOR IMPROVED OVERALL SURVIVAL
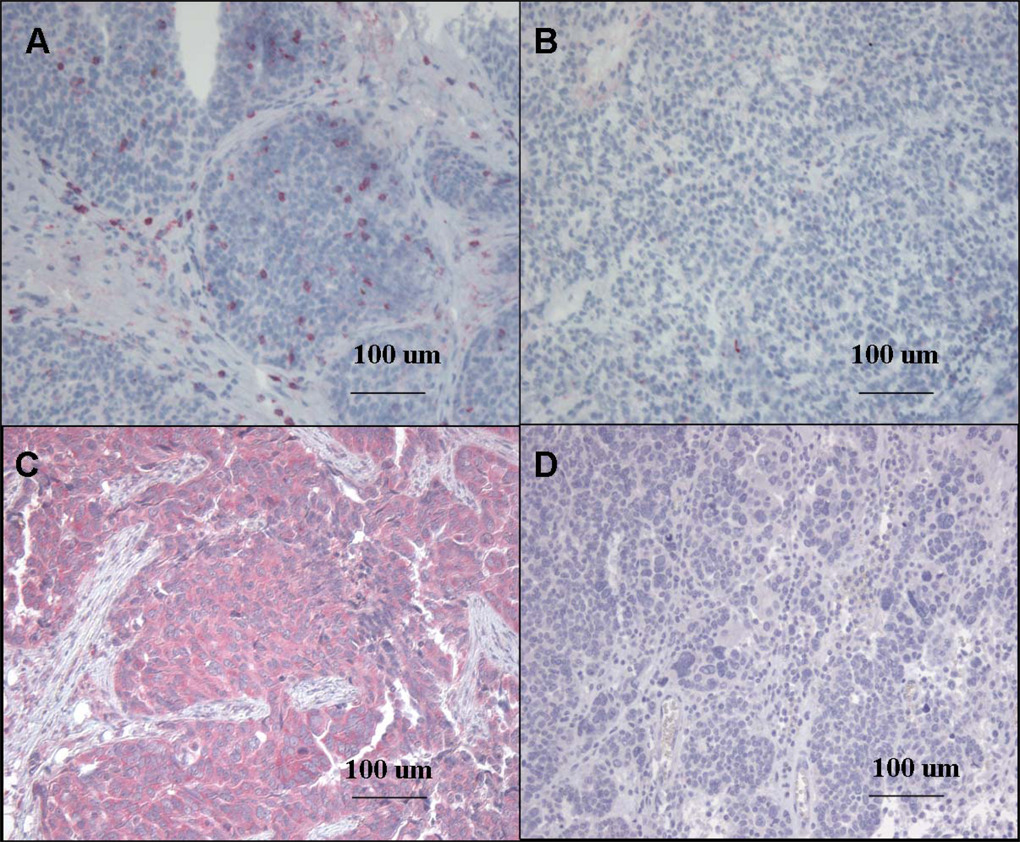
A total of 184 high-grade, advanced-stage serous ovarian cancer samples were analyzed for tumor-infiltrating CD8 T lymphocytes (Figure 1a and 1b). Cytoreduction data was available on 180 patients, 144 (80%) which were optimally cytoreduced. The median age of the patients was 59.5 years (range, 20–95).
Figure 1.
1a: High-Grade, Advanced-Stage Serous Ovarian Cancer Immunohistochemically Stained for CD8 T Lymphocytes and HLA-DMB. Marked CD8 T lymphocyte (red) infiltration in the tumor epithelium (10X)
1b: Scant CD8 T lymphocyte infiltration in the tumor epithelium (10X)
1c: High expression of HLA-DMB (red) in the tumor cell cytoplasm(10X)
1d: Low expression of HLA-DMB (10X)
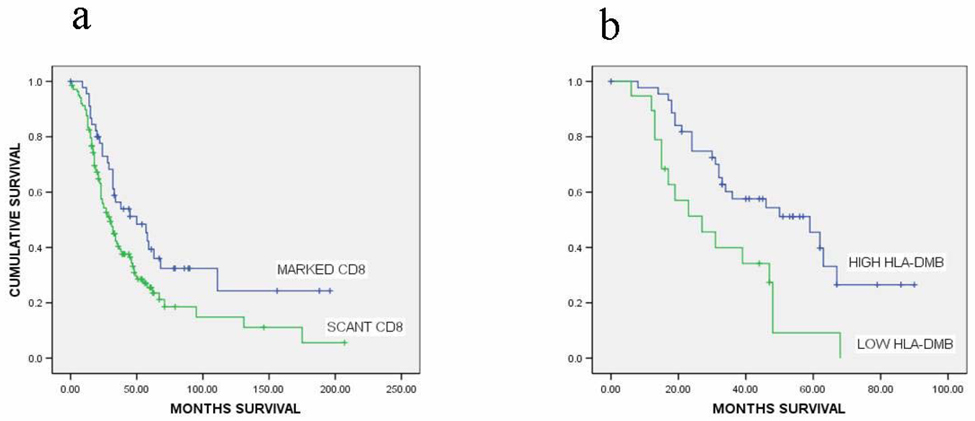
For CD8 Kaplan-Meier analysis, patients were divided into two groups (marked vs. scant CD8 T lymphocytes infiltration ) using the 75th percentile of the CD8 counts as the cutpoint. Patients with marked infiltration of the tumor epithelium with CD8 T lymphocytes (n=46) had a median overall survival of 50.0 months (95% CI, 23.9–76.1 months), while those with scant infiltration (n=138) had a median overall survival of 30.0 months (95% CI, 23.6–36.4 months, p=0.02, Figure 2a).
Figure 2.
2a: Kaplan-Meier Survival Analysis. Marked CD8 T lymphocyte infiltration in the tumor epithelium (median overall survival, 50.0 months) versus scant CD8 T lymphocyte infiltration in the tumor epithelium (median overall survival, 30.0 months), p=0.021. Cut-off for marked infiltration of CD8 T lymphocytes was the 75th percentile.
2b: High expression of HLA-DMB in the tumor epithelium (median overall survival, 59.0 months) versus low expression of HLA-DMB in the tumor epithelium (median overall survival, 27.0 months), p=0.003. Cut-off for high expression of HLA-DMB was the 33rd percentile.
In a proportional hazards model, with cytoreduction and age adopted as covariates, tumor-infiltrating CD8 T lymphocyte number was an independent prognostic factor for overall survival. This relationship held true regardless of whether CD8 T lymphocyte count was analyzed as a continuous (Table 1a) or dichotomous variable (Table 1b). The 50th, 66th, and 75th percentiles for tumor-infiltrating CD8 T lymphocyte number were analyzed separately as dichotomous variables in the proportional hazards model. The 75th percentile cutpoint for tumor-infiltrating CD8 T lymphocyte number produced the most clinically significant hazard ratio (data not shown for 50th and 66th percentiles) and thus was used in the above Kaplan-Meier analysis and Cox proportional hazards model. Additionally, the hazard ratio for the CD8 dichotomous variable was less than that of the continuous variable, implying there may be a critical threshold of CD8 T lymphocytes necessary in the tumor epithelium to meaningfully impact overall survival. Interestingly, older age was related to fewer numbers of tumor-infiltrating cytotoxic T lymphocytes, but the correlation did not reach statistical significance (Spearman’s rho=−0.130, p=0.08). Additionally, the number of tumor-infiltrating CD8 T lymphocytes was not related to achievement of optimal cytoreduction (p=0.52, Mann-Whitney U Test).
Table 1.
1a: Multivariate analysis of prognostic factors for overall survival in high-grade, advanced-stage serous ovarian cancer (CD8 number as a continuous variable).
1b: Multivariate analysis of prognostic factors for overall survival in high-grade, advanced-stage serous ovarian cancer (CD8 number as a dichotomous variable, cutpoint at 75th percentile).
1c: Multivariate analysis of prognostic factors for overall survival in high-grade, advanced-stage serous ovarian cancer (HLA-DMB expression as a dichotomous variable, cutpoint at 33rd percentile).
| VARIABLE | HAZARD RATIO |
95% CONFIDENCE INTERVAL |
P-VALUE |
|---|---|---|---|
| CD8 number as a continuous variable | |||
| AGE | 1.013 | 0.998–1.028 | 0.092 |
| OPTIMAL CYTOREDUCTION |
0.565 | 0.370–0.862 | 0.008 |
| TUMOR-INFILTRATING CD8 T LYMPHOYCYTES (CONTINUOUS) |
0.962 | 0.935–0.989 | 0.006 |
| CD8 number as a dichotomous variable, cutpoint at 75th percentile | |||
| AGE | 1.013 | 0.998–1.028 | 0.098 |
| OPTIMAL CYTOREDUCTION |
0.604 | 0.396–0.920 | 0.019 |
| TUMOR- INFILTRATING CD8 T LYMPHOCYTES (DICHOTOMOUS) |
0.577 | 0.373–0.893 | 0.014 |
| HLA-DMB expression as a dichotomous variable, cutpoint at 33rd percentile | |||
| AGE | 1.004 | 0.976–1.033 | 0.775 |
| OPTIMAL CYTOREDUCTION |
0.603 | 0.265–1.373 | 0.229 |
| HLA-DMB EXPRESSION (DICHOTOMOUS) |
0.470 | 0.222–0.996 | 0.049 |
DIFFERENTIAL EXPRESSION OF HLA-DMB mRNA IS POSITIVELY CORRELATED WITH TUMOR-INFILTRATING CD8 T LYMPHOCYTES
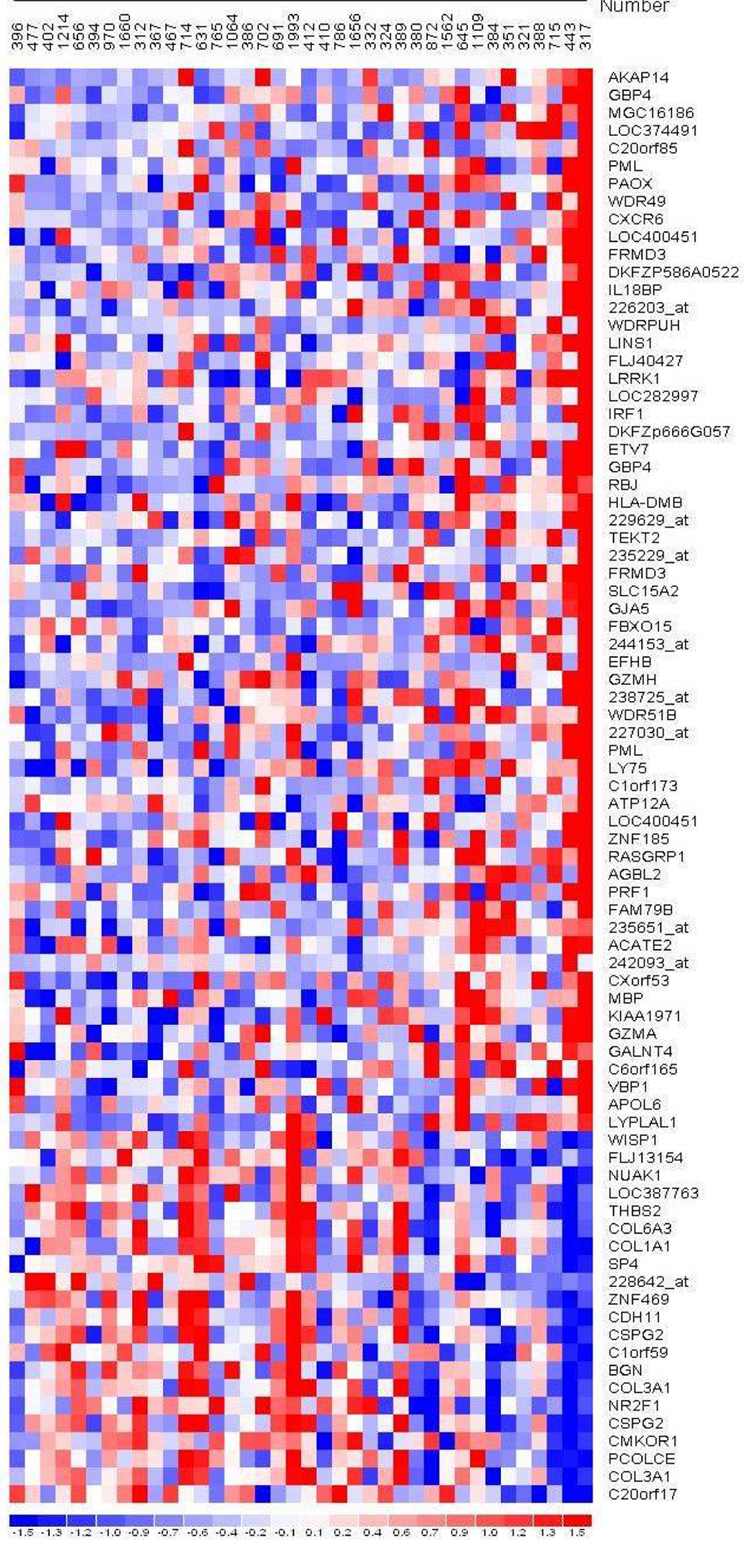
Microarray analysis of microdissected tumor epithelium (n=38) demonstrated multiple genes whose expression significantly correlated with the tumor-infiltrating CD8 T lymphocyte number (Figure 3). HLA-DMB, a gene whose protein product is involved in the major histocompatibility complex (MHC) class II antigen presentation pathway, was selected for validation. Additionally, HLA-DMB expression in the samples with greater numbers of tumor-infiltrating CD8 T lymphocytes was a finding common to microarray analysis by all normalization methods (data not shown). Differential HLA-DMB mRNA expression in the tumor epithelium (training set), evaluated by qRT-PCR, positively correlated with increased numbers of tumor-infiltrating CD8 T lymphocytes (Pearson’s rho=0.741, p<0.001). In a separate validation set of 25 microdissected high-grade, advanced-stage serous ovarian cancer specimens, HLA-DMB mRNA expression in the tumor epithelium positively correlated with tumor-infiltrating CD8 T lymphocyte number (Spearman’s rho=0.416, p=0.04).
Figure 3.
RMA Normalization, Pearson correlation p<0.01, increasing tumor-infiltrating CD8 T lymphocyte number (left to right), genes ordered according to descending correlation coefficient.
INCREASED HLA-DMB PROTEIN EXPRESSION IN THE TUMOR EPITHELIUM IS ASSOCIATED WITH INCREASED NUMBERS OF TUMOR-INFILTRATING CD8 T LYMPHOYCTES
In a separate set of 64 high-grade, advanced-stage serous ovarian cancer specimens HLA-DMB protein expression was evaluated by immunohistochemical method. HLA-DMB expression was localized to the tumor cell cytoplasm (Figure 1c,d).
Significantly greater numbers of tumor-infiltrating CD8 T lymphocytes were found in the cases with high HLA-DMB expression (p<0.001, Mann-Whitney U Test). For further statistical analysis cases were divided into high (n=45) and low (n=19) expressors of HLA-DMB at the 33rd percentile. High HLA-DMB expression in the tumor epithelium predicted greater numbers of tumor-infiltrating CD8 T lymphocytes (marked infiltration) utilizing a logistic regression model (OR 13.2, 95% CI,1.6–107.3, p=0.02).
HLA-DMB PROTEIN EXPRESSION IN THE TUMOR EPITHELIUM IS ASSO CIATED WITH IMPROVED OVERALL SURVIVAL
Utilizing Kaplan-Meier analysis, patients with high HLA-DMB expression in the tumor epithelium had a significantly greater median overall survival than patients with low expression of HLA-DMB (59.0 months (95% CI, 33.4–84.6 months) vs. 27.0 months (95% CI, 10.9–43.1 months), p=0.003, Figure 2b).
In a proportional hazards multivariate analysis with cytoreduction status and age adopted as covariates, high HLA-DMB expression was associated with an improved overall survival (HR 0.47, 95% CI 0.222–0.996, p=0.05, Table 1c).
EXPRESSION OF HLA-DMB AND INVARIANT CHAIN (Ii) mRNA IN THE TUMOR EPITHELIUM CORRELATE POSITIVELY WITH HLA-DRA mRNA EXPRESSION
After demonstrating a differential expression of HLA-DMB in advanced serous ovarian cancers with greater numbers of tumor-infiltrating CD8 T lymphocytes, we investigated the expression of other genes involved in the MHC class II antigen presentation pathway. Expression of HLA-DRA mRNA in the tumor epithelium correlated positively with HLA-DMB mRNA expression in the tumor epithelium of high-grade, advanced-stage serous ovarian cancers (Spearman’s rho=0.415, p=0.02). Additionally, a subset of cases was evaluated for Invariant chain Ii mRNA expression and its expression positively correlated with HLA-DRA mRNA expression (data not shown).
Tumor-infiltrating CD8 T lymphocyte number correlated positively with tumor-infiltrating CD4 T lymphocyte number (Spearman’s rho=0.428, p=0.04) and HLA-DRA mRNA expression (Spearman’s rho=0.353, p=0.05). However, tumor-infiltrating CD4 T lymphocyte number did not correlate with the mRNA expression level of any of the above genes.
HLA-DR IS EXPRESSED BY THE TUMOR EPITHELIAL CELLS AND ALSO CO-LOCALIZES TO CD8 T LYMPHOCYTES
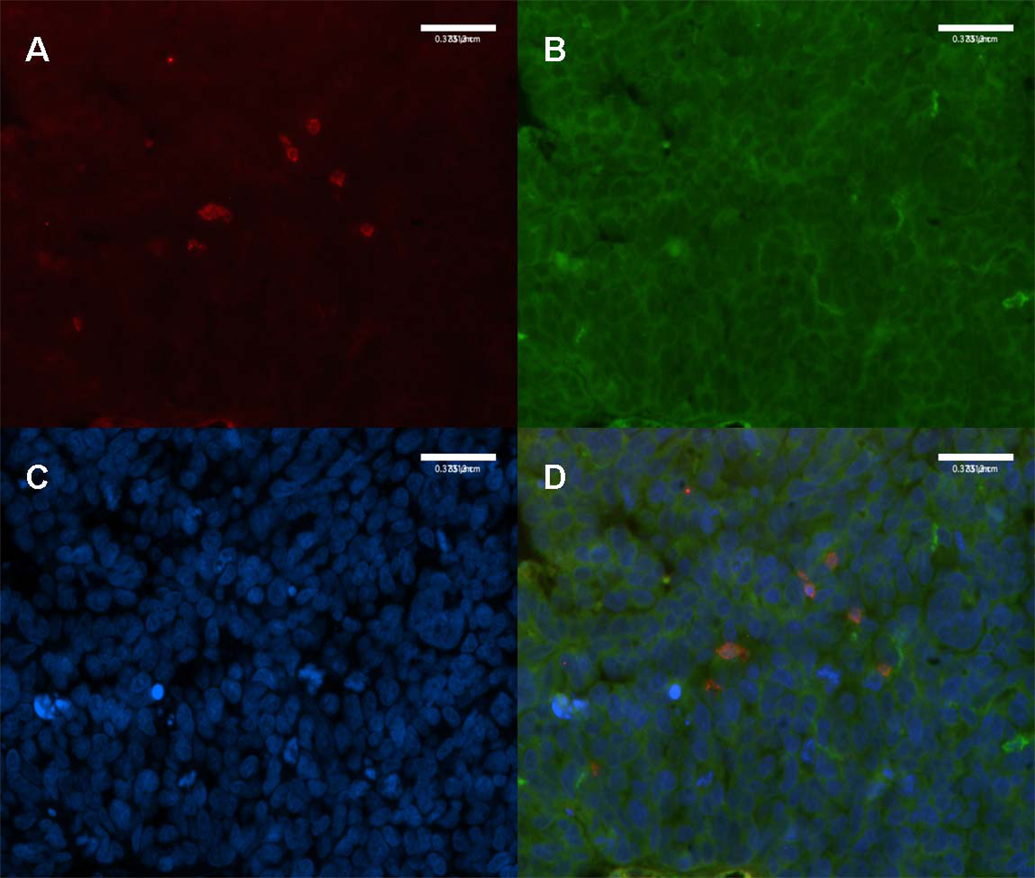
Utilizing immunofluorescence technique in a subset of 5 high-grade, advanced-stage serous ovarian cancer cases, HLA-DR was noted to be expressed by the tumor epithelial cells and localized to the cell membrane (Figure 4). Additionally, tumor-infiltrating CD8 T lymphocytes were noted to express HLA-DR, indicating their status as activated cytotoxic T lymphocytes (Figure 4).12,13
Figure 4.
4a: High-Grade, Advanced-Stage Serous Ovarian Cancer, Double-Immunofluorescence Staining for CD8 T Lymphocytes and HLA-DR. CD8 T lymphocytes (red) infiltrating the tumor epithelium. (20X)
4b: HLA-DR localized to the tumor cell membrane. (20X)
4c: Tumor cell nuclei marked with DAPI (blue). (20X)
4d: HLA-DR positive CD8 T lymphocytes (orange) indicating activated status. (20X)
DISCUSSION
The present study confirms that increased tumor-infiltrating cytotoxic T lymphocytes are associated with improved overall survival and also presents a new candidate gene which might have a role in increasing the number of the tumor infiltrating lymphocytes and therefore improve survival in serous ovarian cancer patients.
In our study, utilizing Kaplan-Meier analysis with a 75th percentile cutpoint, patients with marked numbers of tumor-infiltrating CD8 T lymphocytes had a 20 month improvement in overall survival. The improvement in overall survival held in a multivariate analysis, indicating tumor-infiltrating CD8 T lymphocytes are an independent prognostic factor in advanced-stage serous ovarian cancer.
As demonstrated in this and previous studies, the presence of tumor-infiltrating T lymphocytes, specifically cytotoxic T lymphocytes (CD8), confers an improved overall survival in many different epithelial tumors.4,5,6,7,8 However, the underlying molecular mechanisms that promote or inhibit the infiltration of cytotoxic T lymphocytes is not fully understood. In our sample of 38 microdissected advanced-stage serous ovarian cancers, HLA-DMB was positively differentially expressed in the tumor epithelium of patients with an abundance of tumor-infiltrating cytotoxic T lymphocytes. Furthermore, a significant positive correlation between the differential expression of HLA-DMB mRNA in the tumor epithelium and the number of tumor-infiltrating CD8 T lymphocytes was noted both in the training set and in a separate validation set of specimens. This relationship held true at the protein level as well, implicating ovarian cancer epithelial cells in the process of antigen presentation.
Normal adult epithelial cells are not thought to express MHC class II molecules, such as HLA-DR and HLA-DM, both of which are composed of α and β subunits. However, various epithelial tumor cells have been demonstrated to express HLA-DR molecules and their presence is associated with an improved prognosis.14,15,16 Additionally, in breast cancer, expression of HLA-DM and its cooperating molecules, HLA-DR and Ii, is associated with a helper CD4 T lymphocyte-associated response and improved survival.16 HLA-DM enhances antigen presentation by catalyzing the removal of class II-associated invariant chain peptide (CLIP) from the antigen-binding groove of HLA-DR molecules.17 CLIP is the product of cleavage of the invariant chain, which occupies and stabilizes HLA-DR molecules after their synthesis and assembly.18 By catalyzing the dissociation of CLIP from the binding groove of HLA-DR, HLA-DM allows efficient antigen peptide loading onto HLA-DR for presentation at the cell membrane to helper CD4 T lymphocytes.18,19
A robust and effective cytotoxic CD8 T lymphocyte immune response requires the aid of helper CD4 T lymphocytes.20 In vitro and in vivo experiments have demonstrated that tumor cells may be converted into efficient antigen presenting cells (APCs) by expression of MHC class II components and inhibition of the invariant chain.21 Consequently, they are able to present endogenous tumor antigen to CD4 helper T lymphocytes.21 The present study demonstrates that advanced serous ovarian cancers with an abundance of tumor-infiltrating cytotoxic CD8 T lymphocytes differentially express increased levels of HLA-DMB in the tumor epithelium. HLA-DM serves to catalytically replace CLIP (cleavage product of Ii) with antigen peptide. Hence, increased expression of HLA-DM may be functionally similar to inhibition of the invariant chain and enhance antigen peptide presentation by HLA-DR. Additionally, we found that HLA-DMB and Ii mRNA expression both correlate positively with HLA-DRA mRNA expression in the tumor epithelium and HLA-DR localizes to the tumor epithelial cell membrane. Consequently, it may be hypothesized that certain serous ovarian cancer epithelial cells, coordinately expressing these three critical components of the MHC class II antigen presentation pathway, are able to present endogenous tumor antigen to helper CD4 T lymphocytes and, by extension, activate an efficient and robust CD8 cytotoxic immune response.
However, the present study cannot define whether the differential expression of HLA-DMB by the tumor epithelial cells leads, by extension, to a marked CD8 T lymphocyte response, or vice versa. It is plausible that tumor-infiltrating CD8 T lymphocytes may induce the expression of MHC class II components, such as HLA-DM, HLA-DR, and Ii in tumor epithelial cells by the production and secretion of IFN-γ.22 Therapeutic upregulation of the IFN-γ production of T lymphocytes might increase HLA-DM, HLA-DR and Ii expression in ovarian tumor cells which might contribute to better survival in selected patients.
In summary, the present study serves to begin to elucidate the molecular mechanisms that may underlie the differential numbers of tumor-infiltrating CD8 T lymphocytes in advanced-stage serous ovarian cancer. Further study will be necessary to evaluate the potential antigen presenting capabilities of serous ovarian cancer cells.
Acknowledgement
We thank Yuxin Dai for her expert technical assistance.
This study was supported in part by Dana-Farber/Harvard Cancer Center Ovarian Cancer SPORE grant P50CA105009 and R33CA103595 from National Institute of Health, Department of Health and Human Services, Gillette Center For Women’s Cancer, Adler Foundation, Inc., Edgar Astrove Fund, the Ovarian Cancer Research Fund, Inc., the Morse Family Fund, the Natalie Pihl Fund, the Ruth N. White Research Fellowship, Friends of Dana Farber Cancer Institute, the Robert and Deborah First Fund, and the intramural research program of the National Cancer Institute.
Footnotes
This study was presented in part at the Society of Gynecologic Oncologists 38th Annual Meeting (poster), San Diego, CA March 2007
STATEMENT OF CLINICAL RELEVANCE
The present study serves to elucidate the underlying molecular mechanisms which may lead to an increase in the number of tumor-infiltrating CD8 T lymphocytes in advanced stage high-grade serous ovarian cancer from patients with improved survival rates. We showed the first time that HLA-DMB expression levels were significantly associated with CD8 T lymphocytes numbers, and HLA-DMB is a prognostic factor for patient survival. These data suggest that therapeutic upregulation of the HLA-DMB antigen presenting machinery might be a novel approach in ovarian cancer treatment. This therapeutic approach may enhance chemotherapy and particularly vaccine immunotherapy treatment by forcing cancer cells to present their own antigens, which activates the adoptive immune response machinery, increases the number of tumor-infiltrating CD8 T lymphocytes, and subsequently leads to improved survival in ovarian cancer patients.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 3.Dunn GP, Old LA, Schreiber RD. The immunobiology of caner immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 5.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kondratiev S, Sabo E, Yakirevich E, et al. Intratumoral CD8+ T lymphocytes as a prognostic factor of survival in endometrial carcinoma. Clin Cancer Res. 2004;10:4450–4456. doi: 10.1158/1078-0432.CCR-0732-3. [DOI] [PubMed] [Google Scholar]
- 7.Naito Y, Saito K, Shiiba K, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–3494. [PubMed] [Google Scholar]
- 8.Schumacher K, Haensch W, Röefzaad C, et al. Prognostic significance of activated CD8+ T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61:3932–3936. [PubMed] [Google Scholar]
- 9.Bonome T, Lee J, Park D, et al. Expression profiling of serous low malignant potential, low-grade, and high-grade tumors of the ovary. Cancer Res. 2005;65:10602–10612. doi: 10.1158/0008-5472.CAN-05-2240. [DOI] [PubMed] [Google Scholar]
- 10.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 11.SPSS for Windows. Rel. 14.0.0. Chicago: SPSS Inc.; 2005. [Google Scholar]
- 12.Salgado FJ, Lojo J, Fernandez-Alonso CM, et al. Interleukin-dependent modulation of HLA-DR expression on CD4 and CD8 activated T cells. Immunol Cell Biol. 2002;80:138–147. doi: 10.1046/j.1440-1711.2002.01055.x. [DOI] [PubMed] [Google Scholar]
- 13.Hoshino Y, Morishima T, Kimura H, et al. Antigen-driven expansion and contraction of CD8+ -activated T cells in primary EBV infection. J Immunol. 1999;163:5735–5740. [PubMed] [Google Scholar]
- 14.Lovig T, Andersen SN, Thorstensen L, et al. Strong HLA-DR expression in microsatellite stable carcinomas of the large bowel is associated with good prognosis. Br J Cancer. 2002;87:756–762. doi: 10.1038/sj.bjc.6600507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsushita K, Takenouchi T, Shimada H, et al. Strong HLA-DR antigen expression on cancer cells relates to better prognosis of colorectal cancer patients: possible involvement of c-myc suppression by interferon-γ in situ. Cancer Sci. 2006;97:57–63. doi: 10.1111/j.1349-7006.2006.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oldford SA, Robb JD, Codner D, et al. Tumor cell expression of HLA-DM associates with a Th1 profile and predicts improved survival in breast carcinoma patients. Int Immunol. 2006;18:1591–1602. doi: 10.1093/intimm/dxl092. [DOI] [PubMed] [Google Scholar]
- 17.Denzin LK, Cresswell P. HLA-DM induces CLIP dissociation from MHC class II αβ dimers and facilitates peptide loading. Cell. 1995;82:155–165. doi: 10.1016/0092-8674(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh P, Amaya M, Mellins E, et al. The structure of an intermediate in class II MHC maturation: CLIP bound to HLA-DR3. Nature. 1995;378:457–462. doi: 10.1038/378457a0. [DOI] [PubMed] [Google Scholar]
- 19.Wang R. The role of MHC class II-restricted tumor antigens and CD4+ T cells in antitumor immunity. Trends Immunol. 2001;22:269–276. doi: 10.1016/s1471-4906(01)01896-8. [DOI] [PubMed] [Google Scholar]
- 20.Bevan M. Helping the CD8+ T-cell response. Nat Rev Immunol. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 21.Humphreys R, Hillman G, von Hofe E, et al. Forcing tumor cells to present their own tumor antigens to the immune system: a necessary design for an efficient tumor immunotherapy. Cell Mol Immunol. 2004;1:180–185. [PubMed] [Google Scholar]
- 22.Pandiyan P, Hegel J, Krueger M, et al. High IFN-γ production of individual CD8 T lymphocytes is controlled by CD152 (CTLA-4) J Immunol. 2007;178:2132–2140. doi: 10.4049/jimmunol.178.4.2132. [DOI] [PubMed] [Google Scholar]