Abstract
The efficacy of the University of Wisconsin (UW) solution was compared with conventional Euro-Collins solution, as well as with 3 variants of a silica gel solution developed at the University of Minnesota (UM). Protection of the liver grafts with UW was superior after 24 hour preservation, although the results were inferior to those with immediate transplantation, as judged by animal survival, liver function tests, coagulation, and histopathologic parameters. The UM-III solution allowed similar animal survival as with the UW solution. Lactobionate and raffinose that are contained in both the UW and UM-III solutions were thought to be essential constituents for long-term preservation of liver grafts. The study not only establishes, under controlled circumstances, the superiority of the UW solution, but it also provides insight about the reasons for its effectiveness as well as a caution against its over exploitation.
Keywords: UW solution, liver preservation
Jamieson (1) and Kalayoglu (2) have described improved liver preservation in dogs and humans after cold infusion and “slush” refrigeration of hepatic grafts using a solution developed at the University of Wisconsin (UW). These claims have been supported by the results of a large clinical trial in our center (3–5) in which nearly 400 human liver grafts were preserved with UW solution for periods up to 35 h.
Before embarking on this clinical trial, we carried out preliminary experiments in dogs during October and November, 1987. We demonstrated that 24-h preservation of the canine liver with the UW solution could be achieved more reliably than with Euro-Collins solution, but that the results were not as good as with transplantation immediately after cooling with lactated Ringer’s solution. These inquiries have grown into a formal comparison of several solutions that are commercially available or under development for preservation of the liver or other organs.
Material and methods
Animals
Inbred beagle dogs weighing 10 to 15 kg were purchased from the University farm and were starved from the evening before the operation. Donors were slightly smaller than the recipients. Anesthesia was induced with 25 mg/kg thiopental intravenously. Maintenance of anesthesia while on mechanical ventilation was by the intravenous injection of 2 mg/kg ketamine and 0.5 mg pancuronium every 30 to 60 min. Electrocardiogram, arterial pressure, central venous pressure, and esophageal temperature were monitored.
Surgical procedures
Donor operation
Procurement of hepatic homograft and orthotopic liver transplantation in dogs were performed by the method described before (6), with minor modifications. Through a midline incision, the abdominal aorta from the diaphragm to the iliac bifurcation was isolated, leaving the celiac axis and the superior mesenteric artery intact. After incision of the fundus of the gallbladder, the common bile duct and the gastroduodenal artery were transected near the duodenum. The portal vein was cleaned off to the confluence of the superior mesenteric vein and the splenic vein into which a cannula for the infusion of cooling fluids was inserted. The distal aorta was cannulated for subsequent collection of 2 units of blood for transfusion. After ligating the superior mesenteric artery and vein, infusion and cooling of the liver was commenced through the cannula in the splenic vein with 1300 ml of cold lactated Ringer’s solution at a hydrostatic pressure of 100 cm, and the dog was exsanguinated simultaneously into the blood collection bags. At the end of the infusion, the liver was rapidly skeletonized, isolated, and removed en bloc. The liver was then placed in the basin filled with cold lactated Ringer’s solution and cleaned carefully, leaving enough vessel length for anastomoses. The liver was finally perfused on the back table with cold preservation fluids, 700 ml through the portal vein and 300 ml through the abdominal aorta, weighed, packed in a plastic bag and stored in an ice-box until transplantation.
Recipient operation
Through a midline incision, the abdominal aorta below the left renal vein was dissected for eventual arterial reconstruction. The hepatic arteries and biliary ducts of the host liver were divided and ligated high in the liver hilum. All of the hepatic ligaments were divided. Three by-pass tubings were inserted into the external jugular vein, the femoral vein and the splenic vein and the animal was placed on the veno-venous by-pass (7). The portal vein, the infra-hepatic vena cava and the supra-hepatic vena cava were cross-clamped and the host liver was removed.
The vascular anastomoses of the graft were with continuous sutures of the supra-hepatic vena cava, the portal vein, the infra-hepatic vena cava, and the abdominal aorta, in this order. After the supra-hepatic vena cava was anastomosed, the graft was flushed with 200 ml of cold lactated Ringer’s solution. The liver was revascularized immediately after the portal vein was anastomosed, which was then followed by anastomosis of the infra-hepatic vena cava. Reconstruction of the hepatic arterial flow was with an end-to-side aorta-to-aorta anastomosis. Biliary reconstruction was with cholecysto-duodenostomy, and splenectomy was carried out eventually.
Blood gas and electrolytes were frequently monitored, and abnormalities were corrected with calcium chloride, potassium chloride and sodium bicarbonate, if necessary. Low-dose dopamine was given in most cases during and after the by-pass period. Approximately 2 to 3 units electrolyte solution (Plasmalyte®) were given during the operation. The amount of blood transfusion was restricted to 5 units (2500 ml) even though bleeding continued. Three units of blood collected from a blood donor dog on the morning of operation were started at the time of graft reperfusion, and the other 2 units which had been collected from the liver donor were given later.
Animal conditions, electrolytes, blood gas and fluid maintenance were observed for the first 24 h after the transplant, but no glucose or albumin were given. One gram of cephalosporin was administered during the operation and daily thereafter for 5 d. Animals were allowed to eat from the next morning and 20 mg/kg of oral cyclosporine was given every morning.
Experimental groups
Six different preservation fluids were tested with seven experimental groups, involving 45 liver transplantations. For immediate transplantation, lactated Ringer’s solution (Group 1/LR, n= 10) and UW solution (Group 2/UW, n = 5) were used. Fluids used for 24-h preservation were Euro-Collins solution (Group 3/EC, n = 5), UW solution (Group 4/UW, n= 10), UM-I solution (Group 5/UM-I, n= 5), UM-II solution (Group 6/UM-II, n=3), and UM-III solution (Group 7/UM-III, n= 7). Lactated Ringers (Abbott Laboratories, North Chicago, IL) and Euro-Collins (Fresenius Ag, Bad Homburg, West Germany) solutions were purchased commercially, and UW solution was supplied from the DuPont Corporation, Waukegan, Illinois. All of UM solutions were sent from the University of Minnesota, Department of Surgery, MALG program, Minneapolis, Minnesota. Key contents and physiological characteristics of these fluids are listed in Table 1.
Table 1.
Characteristics and constituents of test solutions
| EC | LR | UW | UM1 | UM2 | UM3 | |
|---|---|---|---|---|---|---|
| Anions | ||||||
| Bicarbonate mM/l | 10 | |||||
| Chloride mM/l | 15 | 109 | 20 | 5 | ||
| Lactate mM/l | 28 | |||||
| Phosphate mM/l | 57.5 | 25 | 0.72 | |||
| Lactobionate mM/l | 100 | 90 | ||||
| Cations | ||||||
| Calcium mM/l | 1.5 | 0.6–1.4 | ||||
| Sodium mM/l | 10 | 130 | 30 | 146 | 146 | 1 |
| Potassium mM/l | 115 | 4 | 120 | 4.3 | 24.3 | 124 |
| Magnesium m/M/l | 5 | 4 | 7.8 | |||
| Colloids and Osmotically Active Agents | ||||||
| Hydroxy ethyl starch g/l | 50 | |||||
| Proteins g/l | 65.2 | 90 | 65 | |||
| Mannitol g/l | 3.75 | |||||
| Raffinose g/l | 17.8 | 48 | ||||
| Glucose g/l | 194 | 99 | ||||
| Others | ||||||
| Adenosine g/l | 1.34 | |||||
| Glutathione g/l | 0.922 | |||||
| Insulin Units | 100 | |||||
| Allopurinol g/l | 0.136 | |||||
| Antibiotics/Steroids | ||||||
| Ampicillin mg | 250 | 50 | ||||
| Sulfamethoxazole mg | 40 | |||||
| Trimethoprim mg | 8 | |||||
| Dexamethasone mg | 8 | |||||
| Methyl Prednisolone mg | 250 | 500 | ||||
| Osmolality (mosm/l) | 375 | 273 | 320 | 308 | 301 | |
| PH | 7.4 | 6.3 | 7.4 | 7.4 | 7.4 | 7.4 |
Evaluation
Graft weight change, occurrence of hypotension or outflow block after reperfusion, and amount of transfusion were determined during the operation. Standard liver function measurements after transplantation included aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, glucose, and prothrombin time (PT). Coagulation profiles were monitored by thromboelastogram (TEG) in most animals during the operation.
Liver biopsies for histopathologic study were taken at the beginning and at the end of the 24-h preservation, and at 3 h after graft reperfusion. Complete postmortem examinations were performed on all of animals that died. Tissues were fixed in 10% formalin and stained with Hematoxylin-Eosin. Histopathologic analyses were carried out blindly, without foreknowledge of the experimental group from which the tissues were obtained.
Statistics
Values were expressed as mean ± standard deviation (SD). Group comparisons were performed by Student’s t-test and Mann Whitney U test.
Results
Survival
Differences in animal survival after liver transplantation of all experimental groups are shown in Fig. 1. When transplantation was performed immediately after flushing with either LR (Group 1) or UW (Group 2), 80% of each group lived for more than 7 d. Two animals in Group 1 and 1 animal in Group 2 died of pulmonary edema between 12 h and 24 h post-Tx. When grafts were preserved for 24 h, only 3 animals out of 10 in the UW Group 4 (30%) and 3 animals out of 7 in the UM-III Group 7 (43%) lived for more than 7 d. In addition, all animals receiving 24-h livers preserved with EC (Group 3), UM-I (Group 5), and UM-II (Group 6) died intraoperatively or within 12 h after graft revascularization. Lethal bleeding and/or cardiac arrest were the main causes of the deaths that occurred in less than 12 h. Nine of the 12 animals that died between 12 and 24 h had pulmonary edema, reflecting the fluid resuscitation attempts in these animals which had escaped earlier death from bleeding or cardiac arrest (Table 2).
Fig. 1.
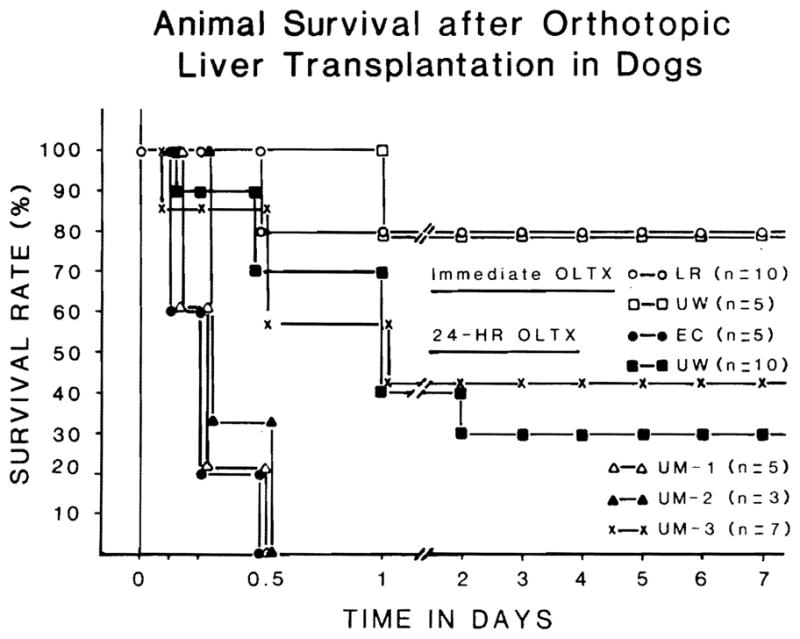
Animal survival after transplantation of liver grafts preserved with various test solutions.
Table 2.
Duration of cold ischemia time and cause of animal death after liver transplantation in dogs
| Groups | Solution | n | Cold Ischemia Time (h) | Animals Lived for more than 7 d | Cause of Animal Death (h) |
||
|---|---|---|---|---|---|---|---|
| Bleeding | Arrest | Pulmonary Edema | |||||
| 1 | LR | 10 | 1.84 ± 0.32 | 8 | 12,12 | ||
| 2 | UW | 5 | 2.28± 0.38 | 4 | 24 | ||
| 3 | EC | 5 | 25.75± 0.78 | 0 | 1.5,3,6,6,12 | ||
| 4 | UW | 10 | 25.17 ± 1.14 | 3 | 3,17 | 12,12,23,24,48 | |
| 5 | UM-1 | 5 | 25.56 ± 0.78 | 0 | 6,6,8 | 1,1 | |
| 6 | UM-2 | 3 | 24.79 ± 0.74 | 0 | 6,6,12 | ||
| 7 | UM-3 | 7 | 25.42 ± 0.74 | 3 | 10,11 | 0.5 | 18 |
Of the animals that lived for more than 7 d after the operation, 2 dogs in Group 1 (25%) and all 3 dogs in Group 4 lived for more than 3 months with normal graft function. Three animals in Group 2 (75%) and 2 animals in Group 7 (67%) are still alive in good condition after more than 2 months.
Graft weight change
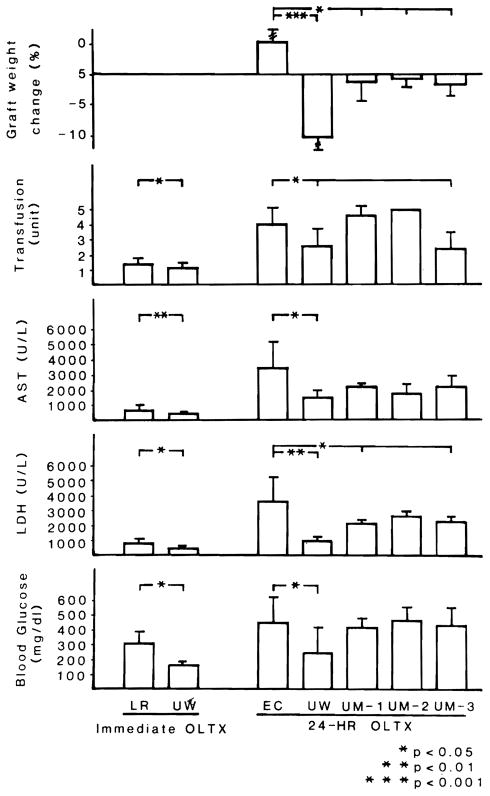
All the livers that were preserved with EC for 24 h gained weight, average 5% and ranging from 2.9% to 20% (Fig. 2). When UW was used, all grafts except one lost graft weight, averaging −10.7% and ranging from 6.6% to −16.0%. Average graft weights in 3 UM groups diminished slightly (Fig. 2).
Fig. 2.
Graft weight change, amount of blood transfusion and graft function at 1 h after graft revascularization.
Intraoperative findings
Immediate and homogeneous perfusion was invariable in Group 1 and Group 2 grafts when the portal clamp was released. There was no incidence of outflow block in these animals. Even after 24 h of preservation, re-perfusion of Group 4 grafts was as good as that in Group 1 and Group 2, but there appeared to be a moderate outflow block for the next 2 to 3 h. Disturbance of reperfusion and occurrence of outflow block was most striking in grafts from Groups 3, 5, and 6. These animals always developed profound hypotension immediately after graft revascularization with a need for rapid infusion and dopamine. Findings with Group 7 grafts were intermediate, but with less outflow block than Group 4.
Graft function
The majority of the animals that received 24-h preserved grafts died between 3 and 6 h postoperatively. Consequently, studies of graft function 1 h after graft reperfusion were the most complete for purposes of intergroup comparison (Fig. 2). Fig. 3 shows the postoperative changes of AST and total bilirubin in culled animals that lived for more than 7 d.
Fig. 3.
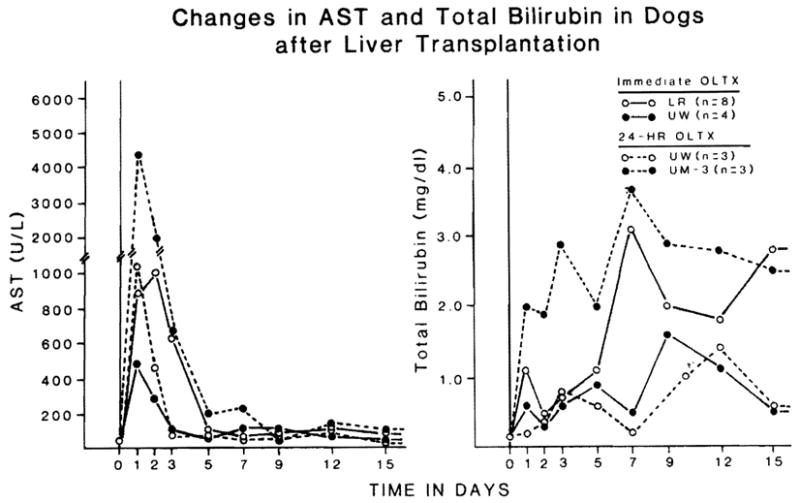
AST and total bilirubin in animals that lived for more than 7 d after liver transplantation.
Although cold ischemia time was usually less than 1 h in control groups 1 and 2, there was an elevation of liver enzymes and blood glucose (Fig. 2). These rises were slighter after the use of UW than with LR. The subsequent changes in the short ischemia dogs that lived for at least 7 d were less severe and normalized faster with UW (Group 2) than lactated Ringer (Group 1) livers (Fig. 3).
After 24 h of preservation, the liver damage 1 h after re-perfusion was worst in the EC group, intermediate in the 3 UM groups, and least in the UW group (Fig. 2). The UW animals which lived for a week had better postoperative graft function and quicker recovery than the livers preserved with UM-3 (Fig. 3).
Amount of transfusion
Quality of preserved grafts was reflected in the amount of blood given to/the animals. All of the immediate liver transplantations were accomplished with less than 2 units of blood and, of these, the UW animals required significantly less blood than those with LR (Fig. 2). In contrast, all of the animals that received 24-h grafts needed more than 2 units blood transfusion, except for 2 animals in group 4 (UW) and 1 animal in group 7 (UM-III). Blood requirement of UW and UM-3 animals was significantly smaller than the other group animals.
Coagulation
Group 1 animals showed no important changes in TEG measures and platelet counts throughout the operation, but PT at 30 min after graft revascularization was slightly but significantly prolonged (Table 3). When grafts were preserved for 24 h, coagulopathy was most striking in EC animals, with prolonged coagulation time and PT, poor clot formation and diminished platelet counts. TEG abnormalities were variable among group 4, group 5 and group 7 animals, but UW animals showed significantly better PT and higher platelet counts.
Table 3.
Differences in coagulation profiles of animals at 30 min after graft revascularization
| Variable | Immediate OLTx | 24-hr | OLTx | |||
|---|---|---|---|---|---|---|
| Control | LR | EC | UW | UM-1 | UM-3 | |
| Reaction time (min) | 5.8±2.7 | 5.5±1.7 | 13.5 ±2.1 | 10.3±4.4 | 7.5±0.7 | 6.3±3.5 |
| Coagulation time (min) | 10.0±3.3 | 9.8±3.3 | 29.5±0.7 | 18.8±6.6 | 14.0±0.0 | 16.6±5.5 |
| Clot formation (°) | 48.2±18.3 | 47.5± 13.6 | 17.5±0.5 | 30.8±7.7 | 40.0±0.0 | 38.6±6.6 |
| Prothrombin time (sec) | 7.5±0.2 | 8.5±0.4 | 26.6±13.2 | 10.6±O.6 | 18.3±2.5 | 13.1±4.1 |
| Platelet count (1000/mm3) | 238±89 | 245±143 | 91.3±46.2 | 128±29 | 47±15 | 77±45 |
Pathology
Histopathologic changes most frequently encountered in liver biopsy specimens included varying degrees of portal edema/hemorrhage, vacuolization of hepatocyte cytoplasm, neutrophilic infiltration of the sinusoids, and necrosis.
Baseline biopsies at the beginning of preservation showed no necrosis in any of these specimens. Any other alterations were minor-to-absent, with no observable differences among groups. Biopsies taken after 24 h of preservation, but before transplantation, showed increases in portal edema and hepatocyte vacuolization when compared to biopsies taken from Group 2 animals.
Another series of biopsies was taken at 3 h after transplantation. Livers preserved for 24 h before transplantation showed a greater degree of neutrophilic infiltration of sinusoids in comparison to Group 1 and Group 2 animals. In addition, areas of spotty hepatocyte necrosis were observed in all 24-h preservation Groups but not in immediate transplant groups.
Among the groups transplanted after 24-h liver preservation, little objective differences could be discerned at the 3-h post-transplant mark. Group 4 displayed somewhat less neutrophilic infiltration and contained less necrosis than Groups 3, 5, 6, or 7. Conversely, vacuolar changes were less prominent in specimens from Group 5 and 7 animals.
Discussion
Steps in the development of liver graft preservation have been few in number. However, the principles involved have been applicable for the preservation of other whole organs. The first innovation of core cooling by infusion of chilled lactated Ringer’s solution into the portal vein may have been the most important (8, 9). Before this time, survival of dogs after liver transplantation was almost never obtained, but afterwards success became almost routine (8). Earlier, it had been appreciated by cardiac surgeons that hypothermia protected ischemic tissue below the level of aortic-clamping (10), a concept which was applied by Lillehei et al. (11) with simple immersion of intestine in iced saline before its autotransplantation. The concept was quantified by Sicular and Moore (12) who reported a slowed rate of enzyme degradation in cold hepatic slices.
Hypothermia to protect human renal homografts was first accomplished with total body hypothermia of living volunteer donors (13), but before long the same teams which had learned the value of hepatic core cooling replaced this cumbersome and potentially dangerous method with infusion of chilled fluid into the kidney immediately after its removal (14). Today, core cooling is the first step in the preservation of all whole organ grafts and this is most often done in situ by some variant of the technique described by Marchioro et al. (15). This method for the continuous hypothermic perfusion of cadaveric livers and kidneys was used clinically long before the acceptance of brain death conditions. Ackerman and Snell (16) and Merkel, Jonasson and Bergan (17) popularized in situ cooling of cadaveric kidneys with cold electrolyte solutions infused into the distal aorta. An extension of this technique has allowed removal of all thoracic and abdominal organs, including the liver, without jeopardizing any of the individual organs (18).
Extension of the safe period beyond that provided by initial cooling has followed one of two prototype strategies, in research which was done mainly with kidneys and applied secondarily to livers. One approach was to provide a limited and continuous renal arterial circulation as was done by Ackerman and Barnard (19) with a perfusate that was primed with blood and oxygenated within a hyperbaric oxygen chamber. The same method with slight modifications also permitted the successful preservation of dog livers for as long as 2 d (20), and was applied clinically with remarkable success in several human cases in the pre-brain death era (21). When Belzer et al. (22) were able to eliminate the hemoglobin and hyperbaric chamber components for kidney preservation, their asanguinous perfusion technique became a worldwide standard. However, efforts to use this modification for livers were unsuccessful (23).
The alternative strategy for the preservation of kidneys, livers, and other organs has been the instillation of special solutions such as that described by Collins, Bravo-Shugarman and Terasaki (24) or the plasma-like Schalm solution (25). The original Collins solution, or modifications of it, has been used for almost 2 decades for the so-called “slush” techniques of kidney preservation. The experimental work of Benichou et al. (26) and Wall et al. (27) with the Collins and Schalm solutions preceded their first clinical use for livers in 1976 which opened up the possibility of organ sharing between cities, but within narrow time limitations. The introduction of the UW solution has been the first major development in liver preservation since then.
The superiority of the UW -solution to any of previous conventional solutions for preservation of liver and extrahepatic organs has been demonstrated in experimental test models (1, 28–32) and confirmed in clinical trials (2–5, 33). In our trials (3–5), the UW livers performed better even though they were preserved on the average for almost twice as long as the EC livers. The UW livers permitted a higher rate of graft survival, and they had a lower rate of primary non-function, hepatic artery thrombosis and retransplantation.
The present experiment, which was initially designed to evaluate the UW solution in a preclinical study, grew to a formal comparison of several solutions that are commercially available (LR and EC) or under development for kidney (UM-I), pancreas (UM-II), and liver (UM-III) preservation. The results showed that the UW solution provided superior protection of liver grafts, not only for 24-h preservation but also with immediate transplantation. The UM-III solution allowed even better animal survival after 24 h of liver preservation, although the protection as judged by graft function may not have been quite as good.
These animal studies may restore a logical perspective about the importance of storage time. The results of the clinical trials with the UW solution (2–5) have been so good that it looked as if the duration of cold ischemia was irrelevant. The controlled animal experiments have made it obvious that this is not true. In the difficult dog model, time of ischemia has been shown once more to be of the utmost importance. Transplantation of a fresh liver provided the best results either when the initial chilling was with LR solution or especially with UW solution. After preservation for 24 h, by which time in humans no deterioration in cadaveric grafts could be detected (2–5), the results in dogs had degraded to the point that only a minority of animals lived beyond the 1st post-operative wk. The same was true for the UM-III grafts. In every animal, the procedure had gone well, but most of the grafts had demonstrable dysfunction. Many of the recipients suffered from various degrees of outflow block, coagulopathy, and other manifestations of ischemic liver injury. The results were not as good as those reported by Jamieson et al. (1) who had no animal deaths due to liver failure after up to 30 h or more preservation with UW.
Whatever the limitations of cold storage may prove to be, the advantages of the UW solution are so obvious from the present study and from the clinical series (2–5), that an explanation for its efficacy will be sought. The rationale for the UW solution has been provided by Belzer and Southard (33). The UW solution contains more than 10 ingredients. Important components and their effects are: lactobionate and raffinose to prevent cell swelling, hydroxyethyl starch to support colloidal pressure, allopurinol and glutathione to inhibit oxygen free radical generation, and adenosine to enhance ATP synthesis after reperfusion (33). In our studies, the differences in the performance of the solutions tested may provide sketchy insight into the relative importance of some constituents. It is clear that an intracellular-like electrolyte solution (EC), or a hyperosmolar colloid solution (UM-I and UM-II) are not enough for long-term cold storage of the liver, even though these can be useful for extrahepatic organs (34, 35). Lactobionate and raffinose could be the most essential ingredients since they were common features of the best solutions, namely the UW solution and the UM-III preparation. The UM-III was the otherwise ineffective UM-I, supplemented only with these two ingredients. Contribution of other constituents in the UW solution for successful liver preservation is under investigation using different experimental models.
The weight gain that is common in organs preserved by past flush techniques was avoided by the use of both the UW and UM-III solutions, something previously emphasized when the UW was used for preservation by Wahlberg et al. (28). Undoubtedly, fuller understanding of the action of UW will be a touchstone for more refinements, including a renewal of interest in the continuous asanguinous perfusion techniques pioneered by the same Wisconsin research team (36).
Acknowledgments
We sincerely appreciate the help of Dr. Y. G. Kang in measuring TEG, and T. Mangan for the preparation of this manuscript.
Supported by Research Grants from the Veterans Administration, Project Grant No. OK 29961 from the National Institutes of Health, Bethesda, Maryland, and the Pathology Education and Research Foundation, Pittsburgh, Pennsylvania.
References
- 1.Jamieson NY, Sundberg R, Lindell S, et al. Successful 24-to 30-hour preservation of the canine liver: A preliminary report. Transplant Proc. 1988;20 (Suppl I):945–7. [Google Scholar]
- 2.Kalayoglu M, Sollinger WH, Stratta RJ, et al. Extended preservation of the liver for clinical transplantation. Laneet. 1988;1:617–9. doi: 10.1016/s0140-6736(88)91416-x. [DOI] [PubMed] [Google Scholar]
- 3.Todo S, Nery J, Yanaga K, et al. Extended preservation of human liver grafts with UW solution. JAMA. 1989;261:711–4. [PMC free article] [PubMed] [Google Scholar]
- 4.Todo S, Tzakis A, Starzl TE. To the Editor. Transplantation. 1988;46:925–6. [PubMed] [Google Scholar]
- 5.Starzl TE, Todo S, Tzakis A, et al. Liver transplantation: An unfinished product. Transplant Proc. 1989;21(1):2197–200. [PMC free article] [PubMed] [Google Scholar]
- 6.Todo S, Kam I, Lynch S, Starzl TE. Animal research in liver transplantation: With special reference to the dog. Semin Liver Dis. 1985;5:309–17. doi: 10.1055/s-2008-1040626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kam I, Lynch S, Todo S, et al. Low flow venovenous bypasses in small animals and pediatric patients undergoing liver replacement. Surg Gynecol Obstet. 1986;163:33–6. [PMC free article] [PubMed] [Google Scholar]
- 8.Starzl TE, Kaupp HA, Jr, Brock DR, Lazarus RE, Johnson RV. Reconstructive problems in canine liver homotransplantation with special reference to the postoperative role of hepatic venous flow. Surg Gynecol Obstet. 1960;111:733–43. [PMC free article] [PubMed] [Google Scholar]
- 9.Moore FD, Wheeler HB, Demissianos HV, et al. Experimental whole-organ transplantation of the liver and of the spleen. Ann Surg. 1960;152:374–87. [PMC free article] [PubMed] [Google Scholar]
- 10.Owens TC, Prevedel AE, Swan H. Prolonged experimental occlusion of thoracic aorta during hypothermia. Arch Surg. 1955;70:95–7. doi: 10.1001/archsurg.1955.01270070097016. [DOI] [PubMed] [Google Scholar]
- 11.Lillehei RC, Goott B, Miller FA. The physiological response of the small bowel of the dog to ischemia including prolonged in vitro preservation of the bowel with successful replacement and survival. Ann Surg. 1959;150:543–60. doi: 10.1097/00000658-195910000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sicular A, Moore FD. The postmortem survival of tissues. J Surg Res. 1961;1:16. [Google Scholar]
- 13.Starzl TE, Brittain RS, Stonington OG, Cuppinger RW, Waddell WR. Renal transplantation in identical twins. Arch Surg. 1963;865:600–7. doi: 10.1001/archsurg.1963.01310100084013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Starzl TE. Experience in Renal Transplantation. Philadelphia: W. B. Saunders Company; 1964. pp. 68–71. [Google Scholar]
- 15.Marchioro TL, Huntley RT, Waddell WR, Starzi TE. Extracorporeal perfusion for obtaining postmortem homografts. Surgery. 1963;54:900–11. [PMC free article] [PubMed] [Google Scholar]
- 16.Ackerman JR, Snell ME. Cadaveric renal transplantation. Br J Urol. 1968;40:515–21. doi: 10.1111/j.1464-410x.1968.tb11842.x. [DOI] [PubMed] [Google Scholar]
- 17.Merkel FK, Jonasson O, Bergan JJ. Procurement of cadaver donor organs: evisceration technique. Transplant Proc. 1972;4:585–9. [PubMed] [Google Scholar]
- 18.Starzl TE, Hakala T, Shaw B, et al. A flexible procedure for multiple cadaveric organ procurement. Surg Gynecol Obstet. 1984;158:223–30. [PMC free article] [PubMed] [Google Scholar]
- 19.Ackerman JR, Barnard CN. A report on the successful storage of kidneys. Br J Surg. 1966;53:525–32. doi: 10.1002/bjs.1800530608. [DOI] [PubMed] [Google Scholar]
- 20.Brettschneider L, Daloze PM, Huguet C, et al. The use of combined preservation techniques for extended storage of orthotopic liver homografts. Surg Gynecol Obstet. 1968;126:263–74. [PMC free article] [PubMed] [Google Scholar]
- 21.Starzl TE. Experience in Hepatic Transplantation. Philadelphia: W. B. Saunders Company; 1969. pp. 58–64.pp. 74–80. (with the assistance of C. W. Putman) [Google Scholar]
- 22.Belzer FO, Ashby BS, Dunphy JE. 24-hour and 72 hour preservation of canine kidneys. Lance. 1967;2:536–8. doi: 10.1016/s0140-6736(67)90498-9. [DOI] [PubMed] [Google Scholar]
- 23.Brettschneider L, Groth CG, Starzl TE. Experimental and clinical preservation of liver homografts. In: Norman JC, Folkman J, Hardison WG, Rudolf LE, Veith FJ, editors. Organ Perfusion and Preservation. New York: Appleton-Century-Crofts; 1968. pp. 271–84. [Google Scholar]
- 24.Collins GM, Bravo-Shugarman M, Terasaki PI. Kidney preservation for transportation: Initial perfusion and 30 hours ice storage. Lancet. 1969;2:1219–24. doi: 10.1016/s0140-6736(69)90753-3. [DOI] [PubMed] [Google Scholar]
- 25.Schalm SW. 1968 thesis. University of Leyden; Holland: A simple and clinically applicable method for the preservation of a liver homograft. [Google Scholar]
- 26.Benichou J, Halgrimson CG, Well R, III, Koep LJ, Starzl TE. Canine and human liver preservation for 6–18 hours by cold infusion. Transplantation. 1977;24:407–11. doi: 10.1097/00007890-197712000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wahlberg JA, Love R, Landegaard L, Southard JH, Belzer FO. 72-hour preservation of the canine pancreas. Transplantation. 1987;43:5–8. doi: 10.1097/00007890-198701000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Ploeg RJ, Goossens D, McAnulty JF, Southard JH, Belzer FO. Successful 72-hour cold storage of dog kidneys with UW solution. Transplantation. 1988;46:191–6. doi: 10.1097/00007890-198808000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Makowka L, Chapman F, Murase N, et al. Prolonged rat cardiac preservation with UW-lactobionate solution. Transplant Proc. 1989;21(1):1350–2. [PMC free article] [PubMed] [Google Scholar]
- 30.Ontell SJ, Makowka L, Ove P, Starzl TE. Improved hepatic function in the 24 hour preserved rat liver with UW-lactobionate solution and SRI 63–441. Gastroenterology. 1988;95:1617–24. doi: 10.1016/s0016-5085(88)80086-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ueda Y, Todo S, Imventarza O, et al. Canine kidney preservation with UW solution: With special reference to microvasculature protection. Transplantation. (in press) [Google Scholar]
- 32.Hoffman B, Sollinger H, Kalayoglu M, Belzer FO. Use of UW solution for kidney transplantation. Transplantation. 1988;46:338–9. [PubMed] [Google Scholar]
- 33.Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation. 1988;45:673–6. doi: 10.1097/00007890-198804000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Florack G, Sutherland DER, Heil J, Zweber B, Najarian JS. Long-term preservation of segmental pancreas autografts. Surgery. 1982;92:260–9. [PubMed] [Google Scholar]
- 35.Abouna GM, Heil JE, Sutherland DER, Najarian JS. Factors necessary for successful 48-hour preservation of pancreas grafts. Transplantation. 1988;45:270–4. doi: 10.1097/00007890-198802000-00003. [DOI] [PubMed] [Google Scholar]
- 36.McAnulty JF, Southard JH, Ploeg RJ, Belzer FO. Successful five day perfusion preservation of the canine kidney. Transplantation. 1989;47:37–41. doi: 10.1097/00007890-198901000-00009. [DOI] [PubMed] [Google Scholar]