Abstract
Sequential liver and kidney transplantation from the same donor was performed in 2 patients. The kidney in Patient 1, which was transplanted after the liver, was hyperacutely rejected and removed 6 hours later. The first liver as well as another liver transplanted 3 days later developed widespread hemorrhagic necrosis. Although the cytotoxic crossmatch of preoperative recipient serum with both donors was negative, patchy widespread IgM and C1q deposits were found in all 3 organs. In Patient 2, who had a strongly positive cytotoxic crossmatch with his donor, the liver suffered a massive but reversible injury, while the kidney never functioned. Both patients developed a coagulopathy a few minutes after liver revascularization. The kidneys in these cases had served like the canaries which miners once used to detect a hostile environment and their presence made more understandable how an indolent version of hyperacute rejection of the liver can take place.
Keywords: liver Tx, kidney Tx, hyperacute rejection
The almost immediate losses of kidneys transplanted across blood group (ABO) barriers into recipients with antigraft isoagglutinins were the first examples of humorally-mediated hyperacute rejection (1). Soon after, the prompt destruction of a kidney transplant in a patient whose serum contained cytotoxic antigraft antibodies was described by Terasaki et al. (2) and called hyperacute rejection by Kissmeyer-Nielsen et al. (3). Rejection of the human heart within a few minutes to a few hours also has been reported (4, 5, 6). However, there have been no unequivocal examples of hyper-acute rejection after clinical hepatic transplantation, supporting the widely held opinion (7–11) that the liver is resistant to this kind of antibody-mediated injury.
We present evidence, from 2 cases of combined liver and kidney transplantation, that humoral rejection of human liver grafts, analogous to hyper-acute renal rejection, can occur. However, the process develops more slowly than with the kidney – and presumably other organs – it may be reversible, and in individual cases it may be impossible to establish a direct relation to antigraft antibodies. It will be suggested also that a progressive and severe coagulopathy developing shortly after hepatic revascularization should arouse suspicion of an acute antibody reaction even if there has not been a positive cytotoxic antibody crossmatch with the donor.
Case reports
Case 1
A 61-yr-old male with a 30-yr history of ulcerative colitis had an 8-yr history of progressive jaundice, right upper quadrant pain, and fever. The diagnosis of sclerosing cholangitis was proved by cholangiography and biopsy. The physical examination was unremarkable except for deep jaundice, hepatomegaly and splenomegaly.
At admission, liver function tests included: total bilirubin 15 mg/− 100 ml (257 mmol/l), alkaline phosphatase 679 U/l (normal < 100), aspartate aminotransferase (AST) 65 U/l (normal < 34) and prothrombin time 15 seconds/11 s control. Indocyanine green retention was 77% at 20 min (normal < 5%). Hepatitis B serology was negative. Doppler study of the liver vessels showed no hepatopetal flow through the portal vein which was patent.
During 4 wk hospitalization, the serum bilirubin rose to 27.1 mg/− 100 ml (465 mmol/l), other liver chemistries deteriorated, and the patient became anuric. On 18 and 19 December 1986, orthotopic liver transplantation was performed from a 27-yr-old donor of the same blood type (A1Rh +) as the recipient. There were no major complications during the hepatectomy and during performance of the vena caval and portal vein anastomoses. When the liver allograft was revascularized with portal blood after a cold ischemia period of 5–1/2 h, it perfused well and produced bile immediately.
The recipient hepatic artery had an intimal dissection, and arterialization could not be completed for another 6–1/2 h. Eventually, a free graft of donor iliac artery was anastomosed to the infrarenal aorta of the recipient, tunneled beneath the pancreas, and anastomosed to the graft coeliac axis (Table 1). Immunosuppression in the operating room consisted of 1 g intravenous methylprednisolone before revascularization and 2 mg/kg of intravenous cyclosporine which was given as soon as the portal revascularization was completed.
Table 1.
Relation in case 1 of organ revascularization times to tissue collection and histopathologic findings
| Date and Time | Event | Comments | Liver | Kidney |
|---|---|---|---|---|
| 12/19/86–2:30 am | liver graft 1 portal vein opened | Perfused well, prompt bile production, wound dry for 30 minutes, then diffuse hemorrhage | – | – |
| 12/19/86–9 am | liver graft 1 artery opened | Liver grossly normal, wound now dry but bile production ceased | – | – |
| 12/19/86–11:30 am | liver graft 1 biopsy | after 9 hrs perfusion | Sinusoidal congestion with neutrophil clustering and fibrin deposition. Diffuse spotty acidophilic necrosis of hepatocytes and microvesicular steatosis. Slight neutrophil sludging in portal capillaries. Immune stains; light IgM and C1q in arterial and portal vein walls. | – |
| 12/19/86–7:30 pm | kidney graft revascularized | well perfused for a few minutes, then cyanotic | ||
| 12/19/86–10 pm | kidney graft biopsie | gross diagnosis hyperacute rejection 2.5 hrs after revascularization | – | Glomerular congestion with occasional neutrophil sludging and focal capillary loop thrombosis. Platelet-fibrin thrombi in two arteries. Prominent tubular vacuolization and interstitial congestion. IgM and C1q in arterial walls; same pattern as in liver. |
| 12/20/86–1:30 am | kidney graft removal | Vessels open no technical problems after 6 hours revascularization | – | Qualitatively same as biopsy findings; approximately 10–20% of glomeruli involved. Focal subintimal fibrinoid necrosis of arterioles, interlobular arteries and veins. IgM + C1q arteries and arterioles |
| 12/22/86–2 am | liver graft 1 removal | Large, mottled, geographic hemorrhage necrosis 72 hours after revascularization | Enlarged graft (1700 gm) Focal arterial necrosis and slight fibrin deposition in sinusoids. Multiple infarcts and candida superinfection in hilum. Patchy IgM and C1q in arteries. | |
| – | ||||
| 12/22/86–2 am | back table biopsy liver graft 2 | 2nd graft grossly normal, perfect donor | Normal histologic appearance | – |
| 12/22/86–4:25 am | liver graft 2 portal vein opened | perfused well, made bile immediately; arterialized 30 minutes later | – | – |
| 12/22/86–10 am | liver graft 2 biopsy | No distinctive gross features, 5.5 hrs after reperfusion | Striking change when compared to preimplantation sample. Findings descriptively similar to those seen in first graft, but quantitatively worse | – |
| 12/24/86–10 pm | died – hepatic failure; no autopsy | – | – | – |
By the time biliary tract reconstruction was started at noon on December 19, 1986 (Table 1), the initially copious bile production had ceased although the gross appearance of the liver was not obviously abnormal. The biliary tract reconstruction was with a choledochojejunostomy.
The operation did not start out being technically difficult. During the initial phases including hepatectomy, the wound was dry with good clot formation. By the time of portal revascularization, cumulative blood loss was 3 l. Diffuse hemorrhage then ensued, necessitating replacement with 20 units of red blood cells and 20 units of fresh frozen plasma over the next 5 h. By the end of the operation, blood loss was an estimated 371. Total replacement was with 50 units packed red blood cells, 50 units fresh frozen plasma, 38 units of platelets, and 30 units cryoprecipitate.
Observations on blood coagulation were made throughout. Preoperatively, the coagulation profile was not alarming in comparison to that of most liver transplant recipients (12). The prothrombin time was 1.3 times control; activated partial thromboplastin time, thrombin time, and reptilase time were 2 times control; the fibrinogen level was 420 mg%; factor II was 0.47 U/ml; factor VIII was 2.75 U/ml; euglobulin lysis time was> 2 h; fibrin degradation products were> 40 μg/ml, and platelet count was 1900/mm3. Thrombelastography (TEG) showed a minimal prolongation of reaction time suggesting deficiency of coagulation factors. This TEG profile was maintained until reperfusion of the liver graft, except that fibrin degradation products became positive and euglobulin lysis time fell to less than 60 min during the anhepatic stage while the patient was on veno-venous bypass (13).
Beginning abruptly 30 min after portal reperfusion, the TEG showed signs of inhibition of coagulation, with severely prolonged reaction time and formation of poor quality clots. The prothrombin time rose from 12.8 to 18 s and the platelet count was 150 000/mm3. Response to treatment with cryoprecipitates and platelets was minimal and transient. Fibrin monomers and fibrin degradation products were negative and euglobulin lysis time was normal. Fibrinolysis was seen on TEG on only one occasion and was not treated.
After the upper abdominal incision had been closed, a kidney from the same donor was transplanted without difficulty to the left: pelvic extra-peritoneal location (Table 1). The renal graft perfused normally at first, but it did not make urine and, within 15 min, it became cyanotic. Cortical and ureteric bleeding ceased. The renal circulation was only temporarily improved with intraarterial or periarterial papaverine or xylocaine, or by intravenous prostaglandin E. The hyper acutely rejected kidney was removed 6 h after it had been revascularized (Table 1). The renal vascular anastomoses were perfect and the vessels did not contain clots.
Postoperatively, the hepatic failure became worse with progressive elevations of serum AST, bilirubin, and prothrombin time. OKT3 therapy was added to cyclosporine and steroids at the end of the 1st postoperative day. It was obvious that a catastrophic hepatic injury had occurred.
A second liver was obtained from a 21-yr-old physiologically stable donor of A1Rh + blood type. At reoperation, the excised first liver graft which had been in place for 72 h was swollen and hard with areas of gross hemorrhagic necrosis. The second liver was revascularized with both portal venous and hepatic arterial flow after a cold ischemia time of 4–1/2 h. Initial perfusion again was good with prompt bile production, but the bile flow had already stopped by the time the biliary tract reconstruction was performed and a biopsy was obtained several hours later. Hepatic failure was unrelieved and the patient died 1–1/2 d later. Permission for autopsy was denied.
Case 2
A 12–1/4-yr-old male with cystinosis had slowly evolving end stage renal disease and at the age of 6 yr was placed on an experimental protocol, using cysteamine. Hepatic veno-occlusive disease developed with portal hypertension, splenomegaly, and pancytopenia. The patient had massive hemorrhages from esophogeal varices in May, 1982, and was submitted to a gastric devascularization procedure. Cysteamine treatment was stopped, but his liver failure became worse including ascites. Renal failure also was progressive and in January, 1986, his blood urea nitrogen (BUN) and creatinine (Cr) were 53 mg/100 ml (18.9 mmol/l) and 5.4 mg/100 ml (477.3 mmol/l) respectively.
On March 3, 1986, the patient underwent orthotopic liver transplantation and renal transplantation in that order from the same donor. On admission, the recipient was stunted with a weight (21 kg) and height below the fifth percentile. He had gross ascites and a healed transverse wound of the upper abdomen. Liver chemistries included bilirubin 0.8 mg/100 ml (15.3 mmol/l); prothrombin time 15 s; albumin 2.4 g/100 ml. AST was mildly increased. Platelet count was 67 000/mm3.
The donor, a 6-yr-old child of the same weight as the recipient, was at another hospital in Pittsburgh. The donor was stable throughout the procurement operation. Preservation as with Case 1 was with the “slush” technique after infusion with cold Euro Collins solution in situ (14). The portal circulation was restored to the transplanted liver after a cold ischemic period of 3–1/2 h including 45 min after removal from the slush solution; arterialization was completed 30 min after this. Veno-venous by-pass was used during the anhepatic phase. Intraoperative immunosuppression was with 250 mg intravenous prednisolone and 40 mg cyclosporine.
A small amount of bile was produced immediately after revascularization but this ceased within a few minutes. The operation had been made difficult up to this point by the presence of massive adhesions from the emergency operation of 1982. After reperfusion of the graft, the problem of hemostasis was compounded by a coagulopathy. Prothrombin time and partial thromboplastin times ranged between 1.5 and 2 times control and the platelet count was 30 000 and 50 000/mm3 in spite of adequate replacement with fresh frozen plasma and platelets. The child received 43 units of red cells, 34 units of fresh frozen plasma, and 36 units of platelets. Completion of the liver transplantation required 21 h, of which most was spent controlling diffuse hemorrhage.
After the upper abdominal incision was closed, a kidney from the same donor was transplanted without technical difficulty to the right pelvic extra-peritoneal site. Renal revascularization was completed 18 h after revascularization of the liver and with a cold ischemic period of 22 h (Table 2). The kidney was well perfused at first, but promptly became soft and cyanotic as if hyperacute rejection had begun. Because the cyanosis and poor perfusion could be reversed with the intra-arterial injection of papaverine and xylocaine, the renal graft was left in place. However, it never functioned. The homograft renal vessels were shown with ultrasound to be patent. Radionuclide scans showed extremely poor flow. Hemodialysis was begun 3 times/wk.
Table 2.
Tissue collections in case 2
| Date and Time | Event | Comment | Liver | Kidney |
|---|---|---|---|---|
| 3/3/86–7:15 pm | liver graft, portal vein opened | perfused well | – | – |
| 3/3/86–7:45 pm | liver graft artery opened | liver grossly normal, but little bile production | – | – |
| 3/4/86–1:45 pm | kidney graft revascularized | kidney turned cyanotic and soft | – | – |
| 3/18/86 | exploration for intestinal obstruction; hepatic and renal biopsy | Had severe hepatic pulmonary, and renal failure | * Prominent cholangiolitis with central ballooning and cholestasis with bile plugging of cholangioles. Prominent portal neutrophilic exudate. No evidence of cellular rejection. No immunoglobulins detected | Occasional glomerular neutrophils in capillary loops. Endothelial swelling with one arteriolar thrombus. Prominent isometric vacuolization of tubules with cysteine crystal deposition. Focal congestion and occasional neutrophils in peritubular capillaries. No immunoglobulins detected |
| 4/22/86 | renal biopsy done because of non-function | 7 weeks postop | – | Small shrunken glomeruli with focal capillary loop thickening and mesangial widening. Mild arteriolar thickening and arterial medial hypertrophy. Prominent interstitial fibrosis and moderate mixed inflammatory infiltrate with focal tubular damage and cysteine deposition. No immunoglobulins detected |
Other liver biopsies on March 18, 21, 25 and 28 and on April 4 were essentially the same but with slow improvement.
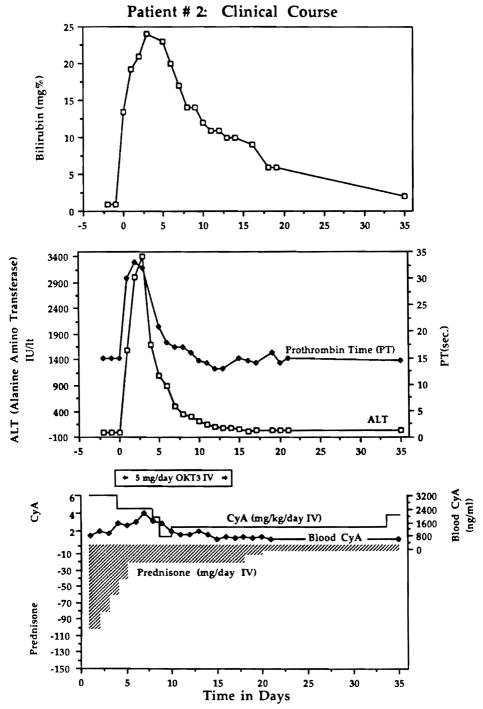
The liver also sustained a grave acute injury with serious perturbations of all of the liver functions (Fig. 1) which persisted for more than 2 wk. The child required ventilatory support for 60 d, and was in the intensive care unit for 62 d with wound infections and dehiscence, ileus from antacid inspissation, and other complications in addition to renal and hepatic failure. Eventually the liver recovered (Fig. 1).
Fig. 1.
Course of a highly sensitized child who had a liver transplantation despite a positive cytotoxic crossmatch, with very severe but reversible hepatic injury. A kidney transplanted from the same donor after the liver was in place, and while the crossmatch remained strongly positive, never functioned.
On 4 April 1987, 11 months after the combined liver-kidney transplantation, a second kidney transplantation was performed with a good result. The patient remains well with normal hepatic and renal function.
Tissue typing and serologic correlations
Patient 1 was matched with the first donor at one HLA antigen at the A locus. With the second donor, there was one A one DR locus match. The recipient’s serum was cytotoxic to less than 20% of the first donor’s lymphocytes; this was interpreted to be a negative crossmatch. No cytotoxicity was detected when the crossmatch was rechecked with a fresh serum sample after the first liver had been transplanted and just before the kidney was revascularized and hyperacutely rejected. There was also a negative crossmatch with the second donor.
Patient 2 was poorly matched with his liver-kidney donor with only one A locus antigen identity. On the day of the double organ transplantation, he had a panel reactive antibody (PRA) of 91 % and a complete kill cytotoxic crossmatch with the donor at a 1:16 titer. By absorption with pooled platelets, the PRA fell to 4%, indicating anti-HLA specificity of the antibodies.
The positive crossmatch persisted after the liver transplantation without the antibody clearing by hepatic grafts described by Fung et al. (15). The crossmatch was still strongly positive using serum collected after 10, 15, and 25 d. After many months, the PRA diminished to 2%. The kidney successfully transplanted 11 months after the double organ transplantation was from a donor with whom the patient had a negative crossmatch.
Histopathologic studies
Biopsies or other tissue samples were obtained from livers or kidneys at the time intervals after their vascularization summarized in Tables 1 and 2. The tissues were fixed in neutral buffered formalin or frozen in OCT compound for immunofluorescence. The sections were stained with hematoxylin and eosin. Kidney sections were also stained with periodic acid-Schiff, Jone’s silver stain and trichrome. Immunofluorescence and immunoperoxidase staining for IgG, IgM, IgA, C1q, C3, C4 and fibrinogen were performed using a direct technique on frozen tissue or indirect on paraffin embedded tissue (16). Electron microscopy was carried out on the kidney transplant removed from the first patient. This confirmed that there were no platelet aggregates and very few granulocytes in the vessels; there was patchy sludging of red cells.
Case 1
Both liver grafts in this patient suffered a devastating injury within a few hours after revascularization (Table 1). Before transplantation, both grafts were thought to be normal, and this was proved in the second graft by preperfusion biopsy (Fig. 2). The post-perfusion biopsies of both liver grafts had the ominous findings summarized in Table 2 (Fig. 3). In addition, the early biopsies of both livers had a light patchy but widespread deposition of IgM and C1q in the portal veins and hepatic arteries. These findings were still present in the first liver graft at 3 d (Fig. 4B). By this time, when the first liver graft was removed, the early morphologic changes had progressed to sub-massive necrosis (Fig. 4A).
Fig. 2.
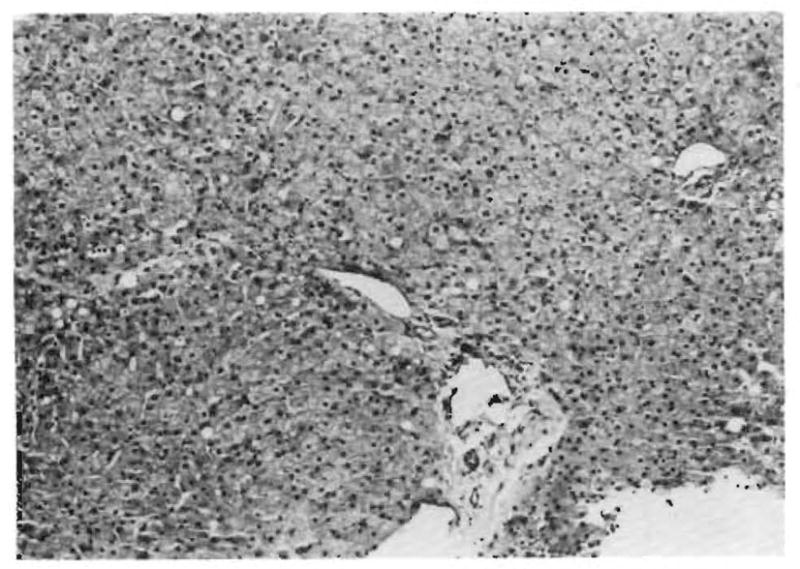
Biopsy of the normal second liver homograft before revascularization in Patient 1 (H & E, 125 ×).
Fig. 3.
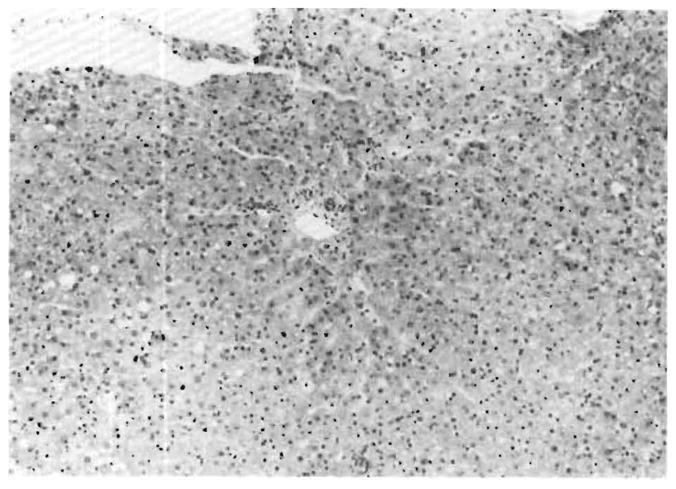
Same liver graft as in Figure 2, 5 hours after revascularization. The massive but subtle injury pattern is summarized in Table 1 (H & E, 125 ×).
Fig. 4.
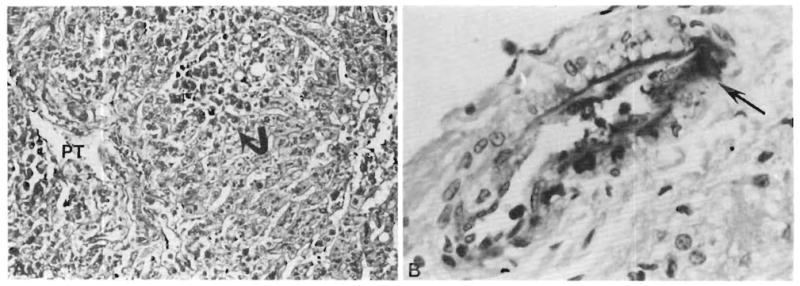
A. First failed liver allograft of Patient 1. The liver contained large areas of necrosis (arrow) 3 days after revascularization PT = portal tract; (H & E, 125 ×). B. Focal deposition of IgM (arrow) in a portal tract artery (immunoperoxidase for IgM, 450 ×).
The kidney transplanted to this patient 17 h after revascularization of the first liver graft had focal fibrinoid changes of the subintimal media of the interlobular arteries and afferent arterioles (Fig. 5A); the other findings are summarized in Table 1. The immunoglobulin deposits in the kidney were qualitatively similar to those in both livers (Fig. 5B).
Fig. 5.
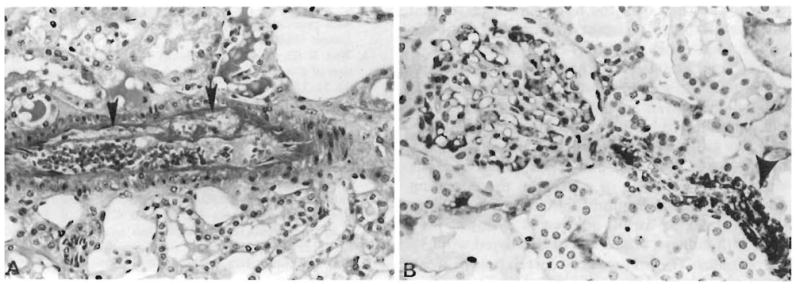
Kidney which was hyperacutely rejected in Patient 1 and removed after 6 hours. A. Fibrinoid necrosis (arrows) of interlobular artery which is crammed with red cells (H & E, 225 ×). B. Deposition of IgM in polar arteriole (arrow). A glomerulus occupies the left half of the field and has irregular staining in the capillary loops and mesangium (immunoperoxidase) for IgM, 450 ×).
Case 2
There was serious damage to the liver by the time the first biopsy was obtained 15 d postoperatively (Table 2). The findings in the kidney graft at this time and later are summarized in Table 2.
Discussion
Breaches of ABO compatibility in kidney transplantation have provided the most analyzable prototypes of hyperacute rejection since the specificities of the antigraft antibodies (the isoagglutinins) have been so obvious (1, 6). The same can be said for hearts (6). With livers, Demetris et al. (17) have demonstrated inconstant isoagglutinin fixation in the microvasculature of AB incompatible liver grafts in a collection of cases in which hemorrhagic infarction occurred five times more frequently than with ABO compatible grafts. Although the process was slower than with hyperacute rejection of kidneys, the end result was the same.
After it was realized that cytotoxic anti-lymphocyte antibodies in kidney recipients caused the same syndrome of hyperacute rejection as ABO incompatibility (2, 3), the “warm” IgG anti-T-lymphocyte variety of antibody was accepted increasingly as the responsible lymphocytotoxin (18). It has proved to be an oversimplification to view these anti-HLA antibodies as the only cause of hyperacute rejection in ABO compatible renal recipients since other antibodies have been implicated including the IgM variety (6, 19) or those attacking vascular endothelium (5). It has long been recognized (20) and recently confirmed (19) that hyperacute rejection can occur without detectable serologic evidence of preformed antibodies and with little evidence immunoglobulin deposition in the ruined kidney graft (20).
Whether or not the initiating antigen-antibody reaction is demonstrable with conventional serologic techniques, the central event of hyperacute rejection of the kidney has been thought to be occlusion of the graft microvasculature by rapidly sequestered formed blood elements including platelets as well as by clotting factors (20–22). Polymorphonuclear leukocytes infiltrate the stricken tissues contemporaneously or slightly later (23). The role of non-specific mediators of the inflammatory cascade in this seemingly irreversible pathogenesis has long been suspected (20, 24). Recently, Ito et al. (25) have correlated hyperacute renal rejection in sensitized rabbits with the appearance of the powerful inflammatory mediator, platelet activating factor (PAF). The antibody-initiated cascade in heterografts or homografts can be ameliorated with a PAF inhibitor (26, 27).
With hyperacute renal rejection, the plugging of the microvasculature with formed blood elements and clotting factors occurs so quickly after an immune event that this has been considered to be the primary pathophysiology (20, 24). Treatment with chelating agents (28) and cobra venom (29) which prevent complement activation can delay the process, but the absence of neutrophils (30) and platelets (31) cannot.
However, observations in the transplanted kidneys of our 2 patients did not appear “classic” for untreated hyperacute rejection. This suggests that a period of intense immunologically-mediated vasoconstriction could precede the mechanical closure of the microvasculature. If this vasoconstriction is not relieved spontaneously or with drugs, the kidney may be lost immediately even though most of the vascular bed is still morphologically intact as was the case in patient 1. Evidence in animal models that immunologically-mediated vasoconstriction is a primary event in accelerated or hyperacute rejection has been reported frequently (25, 32–34).
Whatever the primary events, a striking subsequent feature of hyperacute renal rejection can be the development of a consumption coagulopathy, and sometimes fibrinolysis (21, 22, 24, 35). If renal blood flow falls to zero, the exclusion of the kidney graft from the circulation eliminates the problem, but if renal blood flow is maintained by an equilibrium of clotting and fibrinolytic processes coagulopathy may develop, necessitating graft nephrectomy (24).
Hyperacute rejection of the liver has been looked for ever since recognition of the complication in kidney grafts. Twenty years ago, Drs. G. M. Williams and David Hume attributed the hemorrhagic necrosis of an orthotopic liver graft a few hours postoperatively to humoral rejection (36). Lymphocytotoxic antibodies could not be detected in the recipient’s serum and significant immunoglobulin deposits could not be found in the graft with immunofluorescence studies. However, serum collected preoperatively from the recipient as well as eluates of the liver graft at autopsy lysed cultured renal cells of the original donor.
The gross description of the liver in Hume’s child (36) was remarkably similar to the findings many years later of Knechtle et al. (37) in rats sensitized with skin grafts before orthotopic liver transplantation. The liver grafts in these rat experiments were provided only with a portal blood supply. Gubernatis et al. (38) demonstrated accelerated rejection of the fully vascularized liver in Rhesus monkeys similarly sensitized with skin grafts. Hepatic failure and complex clotting disorders in the sensitized monkeys reduced the survival from an expected mean of 26 d to 2.5 d.
Although these observations have made it clear that liver grafts can be abruptly rejected, clinical evidence accrued over the last 15 yr suggests that the liver is more resistant to humoral antibody attack than other organs (7–10). Animal experiments have demonstrated the difficulty of inducing intense enough sensitization to reduce hepatic graft survival (39, 40) or have shown that liver heterografts are rejected by heterospecific antibodies later and less violently than the heart and presumably other organs (41, 42). The fact that no one has reported the rejection of a liver on the operating table suggests that humoral rejection of the human liver, if it occurs at all, does so at a slower pace than with kidneys, which can be destroyed within a few minutes. The non-existent correlation between cytotoxic crossmatches, immunoglobulin fixation in the hepatic graft, and the outcome after clinical liver transplantation (10) has made it impossible to state unequivocally that any human liver has ever undergone hyperacute rejection.
Observations in our 2 cases have clarified the situation. Miners in the past took canaries with them to work in order to provide a warning: if the birds collapsed there were toxic fumes in the mine. The kidneys were the ‘canaries’ in our 2 liver-kidney recipients. Their immediate destruction or primary non-function identified an intensely hostile immune environment which, in Case 1, explained the loss of two consecutive livers. This kind of prompt destruction of hepatic retransplants in patients whose first liver grafts have been lost for inadequately explained reasons has been seen by us on a number of occasions as well as in other centers, causing the word-of-mouth descriptive term “liver eaters” to be applied to such recipients. In our Patient 2, the hepatic insult was grave but reversible, whereas the kidney never functioned.
It remains to be explained why the liver is less susceptible to hyperacute rejection than the kidney. If vasoconstriction is a primary or important element of hyperacute rejection, the resistance of the liver to its deadly effects could be explained by the double hepatic blood supply with its less vasoreactive portal venous system. The coagulopathy seen in both of our recipients may be characteristic of the injury pattern of this kind of liver rejection, and should trigger suspicion of this diagnosis.
Acknowledgments
Supported by Research Grants from the Veterans Administration and Project Grant No. AM 29961 from the National Institutes of Health, Bethesda, Maryland.
References
- 1.Starzl TE. Experience in renal transplantation. 37–47. WB Saunders Co; Philadelphia: 1964. pp. 249–252.pp. 317 [Google Scholar]
- 2.Terasaki PI, Marchioro TL, Starzl TE. Sero-typing of human lymphocyte antigens: Preliminary trials on long term kidney homograft survivors. Histocompatibility Testing National Acad Science National Research Council; Washington DC: 1965. pp. 83–96. [Google Scholar]
- 3.Kissmeyer-Nielsen F, Olsen S, Peterson VP, Fjeldborg O. Hyperacute rejection of kidney allografts, associated with preexisting humoral antibodies against donor cells. Lancet. 1966;2:662–5. doi: 10.1016/s0140-6736(66)92829-7. [DOI] [PubMed] [Google Scholar]
- 4.Weil R, III, Clarke DR, Iwaki Y, et al. Hyperacute rejection of a transplanted human heart. Transplantation. 1981;32:71–72. [PMC free article] [PubMed] [Google Scholar]
- 5.Brasile L, Zerbe T, Rabin B, Clarke J, Abrams A, Cerilli J. Identification of the antibody to vascular endothelial cells in patients undergoing cardiac transplantation. Transplantation. 1985;6:672–5. doi: 10.1097/00007890-198512000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Starzl TE, Tzakis A, Makowka L, et al. The definition of ABO factors in transplantation: Relation to other humoral antibody states. Transplant Proc. 1987;19:4492–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Starzl TE, Ishikawa M, Putnam CW, et al. Progress in and deterrents to orthotopic liver transplantation, with special reference to survival, resistance to hyperacute rejection, and biliary duct reconstruction. Transplant Proc. 1974;6:129–39. [PMC free article] [PubMed] [Google Scholar]
- 8.Calne RY, Williams R. Liver transplantation. Curr Probl Surg. 1979;16:3–44. doi: 10.1016/s0011-3840(79)80004-0. [DOI] [PubMed] [Google Scholar]
- 9.Iwatsuki S, Iwaki Y, Kano T, et al. Successful liver transplantation from cross-match positive donors. Transplant Proc. 1981;13:286–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon RD, Fung JJ, Markus B, et al. The antibody crossmatch in liver transplantation. Surgery. 1986;100:705–15. [PMC free article] [PubMed] [Google Scholar]
- 11.Moore SB, Wiesner RH, Perkins JD, Nagorney DM, Sterioff S, Krom RAP. A positive lymphocyte crossmatch and major histocompatibility complex mismatching do not predict early rejection of liver transplants in patients treated with cyclosporine. Transplant Proc. 1987;19:2390–1. [PubMed] [Google Scholar]
- 12.Kang YG, Martin DJ, Marquez J, et al. Intraoperative changes in blood coagulation and thrombelastographic monitoring in liver transplantation. Anesth Analg. 1985;64:888–96. [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw BW, Jr, Martin DJ, Marquez JM, et al. Venous bypass in clinical liver transplantation. Ann Surg. 1984;200:524–34. doi: 10.1097/00000658-198410000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Starzl TE, Hakala TR, Shaw BW, Jr, et al. A flexible procedure for multiple cadaveric organ procurement. Surg Gynecol Obstet. 1984;158:223–30. [PMC free article] [PubMed] [Google Scholar]
- 15.Fung JJ, Makowka L, Griffin M, Duquesnoy R, Tzakis A, Starzl T. Successful sequential liver-kidney transplantation in patients with preformed lymphocytotoxic antibodies. Clin Transplant. 1987;1:187–94. [Google Scholar]
- 16.Sinclair RA, Burns J, Dunnill MS. Immunoperoxidase staining of formalin-fixed, paraffin-embedded, human renal biopsies with a paraffin of the peroxidase-antiperoxidase (PAP) and indirect methods. Am J Clin Pathol. 1981;34:859–65. doi: 10.1136/jcp.34.8.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demetris AJ, Jaffe R, Tzakis A, et al. Antibody mediated rejection of human orthotopic liver allografts: A study of liver transplantation across ABO blood group barriers. Am J Pathol. (in press) [PMC free article] [PubMed] [Google Scholar]
- 18.Terasaki PI, Bernoco D, Park MS, Ozturk G, Iwaki Y. Microroplet Testing for HLA-A, -B, -C, and -D antigens. Am J Clin Pathol. 1978;69:103–20. doi: 10.1093/ajcp/69.2.103. [DOI] [PubMed] [Google Scholar]
- 19.Banner B, Makowka L, Demetris J, Tzakis A, Griffin M, Starzl TE. Hyperacute rejection of the kidney in patients with a negative crossmatch. Transplant Proc. 1988;20:453–9. [PMC free article] [PubMed] [Google Scholar]
- 20.Starzl TE, Lerner RA, Dixon FJ, Groth CG, Brettschneider L, Terasaki P. Shwartzman reaction after human renal homotransplantation. New Eng J Med. 1968;278:642–8. doi: 10.1056/NEJM196803212781202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simpson KM, Bunch DL, Amemiya H, et al. Humoral antibodies and coagulation mechanisms in the accelerated or hyperacute rejection of renal homografts in sensitized canine recipients. Surgery. 1970;68:77–85. [PMC free article] [PubMed] [Google Scholar]
- 22.Boehmig HJ, Giles GR, Amemiya H, et al. Hyperacute rejection of renal homografts: With particular reference to coagulation changes, humoral antibodies and formed blood elements. Transplant Proc. 1971;3:1105–17. [PMC free article] [PubMed] [Google Scholar]
- 23.Williams GM, Hume DM, Hudson RP, Jr, et al. “Hyperacute” renal-homograft rejection in man. New Eng J Med. 1968;279:611–8. doi: 10.1056/NEJM196809192791201. [DOI] [PubMed] [Google Scholar]
- 24.Starzl TE, Boehmig HJ, Amemiya H, et al. Clotting changes including disseminated intravascular coagulation, during rapid renal homograft rejection. New Eng J Med. 1970;283:383–90. doi: 10.1056/NEJM197008202830801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito S, Camussi C, Tetta C, Milrom F, Andres G. Hyperacute renal allograft rejection in the rabbit. Lab Invest. 1984;51:148–61. [PubMed] [Google Scholar]
- 26.Makowka L, Miller C, Chapchap P, et al. Prolongation of pig-to-dog renal xenograft survival by modification of the inflammatory mediator response. Ann Surg. 1987;206:482–95. doi: 10.1097/00000658-198710000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makowka L, Chapman F, Qian S, et al. The effect of FK-506 on hyperacute rejection in presensitized rats. Transplant Proc. 1987;19 (Suppl 6):79–83. [PMC free article] [PubMed] [Google Scholar]
- 28.Kux M, Boehmig HJ, Amemiya H, et al. Modification of hyperacute canine renal homograft and pig-to-dog heterograft rejection by the intra-arterial infusion of citrate. Surgery. 1971;70:103–12. [PMC free article] [PubMed] [Google Scholar]
- 29.Forbes DRC, Pinto-Blonde M, Guttman RD. The effect of anticomplementary cobra venom factor on hyperacute rat cardiac allograft rejection. Lab Invest. 1978;39:463–70. [PubMed] [Google Scholar]
- 30.Forbes RDC, Guttmann RD, Kuramochi T, Klassen J, Knack J. Nonessential role of neutrophils as mediators of hyperacute cardiac allograft rejection in the rat. Lab Invest. 1976;34:229–34. [PubMed] [Google Scholar]
- 31.Forbes RDC, Guttmann RD, Bazin H. Hyperacute rejection of cardiac allografts in a rat strain with a hereditary platelet function defect. Lab Invest. 1977;37:158–61. [PubMed] [Google Scholar]
- 32.Dempster WJ. The nature of experimental second-set kidney transplant rejection. Br J Exp Path. 1974;55:406–20. [PMC free article] [PubMed] [Google Scholar]
- 33.Busch GJ, Martins CP, Hollenberg NK, Wilson RE, Colman RW. A primate model of hypercute renal allograft rejection. Am J Path. 1975;79:31–51. [PMC free article] [PubMed] [Google Scholar]
- 34.Terada Y, Ueno A. Hyperacute renal allograft rejection in the rabbit. Transplantation. 1983;35:205–8. doi: 10.1097/00007890-198303000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Myburgh JA, Cohen I, Gecelter L, et al. Hyperacute rejection in human-kidney allografts-Shwartzman or Arthus reaction? New Eng J Med. 1969;281:131–4. doi: 10.1056/NEJM196907172810305. [DOI] [PubMed] [Google Scholar]
- 36.Hume DM, Williams GM. Cited in Starzl TE: Experience in Hepatic Transplantation. WB Saunders Co; Philadelphia: Feb 8, 1969. Personal communication; p. 269. [Google Scholar]
- 37.Knechtle S, Kolbeck PC, Tsuchimoto S, Coundouriotis A, Sanfilippo F, Bollinger RR. Hepatic transplantation into sensitized recipients. Transplantation. 1987;43:8–12. doi: 10.1097/00007890-198701000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Gubernatis G, Lauchart W, Jonker M, et al. Signs of hyperacute rejection of liver grafts in Rhesus monkeys after donor-specific presensitization. Transplant Proc. 1987;19:1082–3. [PubMed] [Google Scholar]
- 39.Kamada N, Davies HffS, Roser B. Reversal of transplantation immunity by liver grafting. Nature. 1981;292:840–2. doi: 10.1038/292840a0. [DOI] [PubMed] [Google Scholar]
- 40.Houssin D, Gugenheim J, Bellon B, et al. Absence of hyperacute rejection of liver allografts in hypersensitized rats. Transplant Proc. 1985;17:293–5. [Google Scholar]
- 41.Settaf A, Meriggi F, Van de Stadt J, et al. Delayed rejection of liver xenografts compared to heart xenografts in the rat. Transplant Proc. 1987;19:1155–7. [PubMed] [Google Scholar]
- 42.Monden M, Valdivia LA, Gotoh M, et al. Hamster-to-rat orthotopic liver xenografts. Transplantation. 1987;43:745–6. [PubMed] [Google Scholar]