THROUGH 1979, immunosuppressive therapy for liver transplantation at our center was with azathioprine (or cyclophosphamide) and steroids, to which antilymphocyte gloublin (ALG) was usually added.1-3 Failure was more frequent than success. Nevertheless, 55 (32.4%) of the first 170 recipients treated from 1963 on in Denver survived for more than a year, and 31 (18.2%) passed or are just reaching the five-year mark. The following is an account of these pioneer patients who have become the leaders in a population of liver recipients that will be expanding rapidly with the improved immunosuppression that is available today.
CASE MATERIAL
The 170 patients were treated with orthotopic liver transplantation at the University of Colorado Health Sciences Center from March 1, 1963, through the first month of 1980.1-4 Eighty-six of the 170 patients were 18 years old or younger, and 84 were 19 years or older. All of these patients were treated with azathioprine and prednisone and most had ALG as well. Sixteen patients (OT 42 through 57) received cyclophosphamide instead of azathioprine for several months, and 21 were treated with thoracic duct drainage as an adjunct to therapy with azathioprine and prednisone.
Cyclosporine was not available for any of these patients at the time of transplantation, but in five patients (OT 56, 77, 137, 142, 164), cyclosporine was substituted for azathioprine four to 11 years after the transplant because of chronic rejection in four patients and in order to reduce the maintenance dose of prednisone for growth retardation in one patient (OT 142). Two patients (OT 56 and 137) received second transplants with cyclosporine and low-dose steroid therapy, and one patient (OT 164) returned to azathioprine after several months of cyclosporine therapy.
Patient code number (OT number), age at the time of transplant, sex, diagnosis of liver disease, survival, present status of recipient, cause of death after five years, and current immunosuppression therapy are listed in Table 1.
Table 1.
List of 33 Five-Year Survivors
| Present Immunosuppression Therapy |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| OT No. |
Age at Transplant |
Sex | Transplant Date |
Indication | Survival | Present Status/Cause of Death | Imuran (mg/d) |
Prednisone (mg/d) |
Cyclosporine (mg/d) |
| 27 | 11 yr | M | 7/15/69 | Wilson’s disease | Died at 5 yr, 362 d | Died, chronic liver failure | — | — | — |
| 33 | 3 yr | F | 1/22/70 | Biliary atresia and inci- dental hepatoma |
Alive more than 14 yr | Full-time school, normal liver function | 50.0 | 10/5* | — |
| 42 | 16 yr | M | 3/23/71 | Wilson’s disease | Alive more than 13 yr | Full-time work, normal liver function | 75.0 | — | — |
| 46 | 3 yr | M | 7/31/71 | Biliary atresia | Alive more than 13 yr | Full-time school, normal liver function | 100.0 | 20/0* | — |
| 53 | 1 yr., 8 mo | F | 2/20/72 | Alpha-1-antitrypsin de- ficiency disease |
Alive more than 12 yr | Full-time school, normal liver function | 75.0 | 7.5 | — |
| 56 | 18 yr | M | 5/15/72 2/1/84 |
Congenital hepatic fi- brosis |
Alive more than 12 yr | Retransplant for chronic rejection, full- time school. |
— | 15.0 | 600 |
| 64 | 3 yr | F | 2/18/73 | Biliary atresia | Alive more than 11 yr | Full-time school, normal liver function | 50.0 | 10.0 | — |
| 73 | 4 yr | F | 10/8/73 | Biliary atresia | Alive more than 10 yr | Full-time school, normal liver function | 25.0 | 15/0* | — |
| 77 | 16 yr | M | 2/3/74 | Chronic active hepatitis | Alive more than 10 yr | Full-time work, chronic rejection; total bilirubin, 2.5 mg/100 mL |
— | 15.0 | 800 |
| 82 | 37 yr | M | 4/9/74 | Alcoholic cirrhosis | Alive more than 10 yr | Full-time work, normal liver function | 100.0 | 15.0 | — |
| 91 | 3 yr, 2 mo | F | 10/16/74 | Biliary atresia | Alive more than 9 yr | Full-time school, normal liver function | 25.0† | 10.0 | — |
| 92 | 11 yr | M | 11/27/74 | Chronic active hepatitis | Alive more than 9 yr | Full-time school, normal liver function | 50.0 | 10/0* | — |
| 93 | 22 yr | F | 11/28/74 | Budd-Chiari syndrome | Alive more than 9 yr | Housewife, normal liver function, 2 children since transplant |
100.0 | 5.0 | — |
| 105 | 30 yr | F | 1/21/76 | Secondary biliary cirrho- sis |
Alive more than 8 yr | Housewife, normal liver function | 37.5 | 5.0 | — |
| 114 | 27 yr | F | 9/19/76 | Liver sarcoma of unde- termined cell type |
Alive more than 7 yr | Housewife, normal liver function | 100.0 | 10.0 | — |
| 115 | 43 yr | M | 10/27/76 | Chronic active hepatitis | Alive more than 7 yr | Not working, normal liver function | 50.0 | 10.0 | — |
| 117 | 15 yr | M | 11/27/76 | Alpha-1-antitrypsin de- ficiency disease |
Alive more than 7 yr | Full-time work, normal liver function | 50.0 | 20/0* | — |
| 120 | 33 yr | M | 1/8/77 | Alcoholic cirrhosis | Alive more than 7 yr | Full-time work, normal liver function | 50.0 | 15.0 | — |
| 125 | 10 mo | F | 4/6/77 | Biliary atresia | Alive more than 7 yr | Full-time school, normal liver function | 20.0 | 10/0* | — |
| 133 | 44 yr | M | 7/26/77 | Chronic active hepatitis | Alive more than 7 yr | Full-time work, normal liver function | 50.0 | 15.0 | — |
| 135 | 34 yr | M | 8/13/77 | Alcoholic cirrhosis | Alive more than 6 yr | Not working, normal liver function | 50.0 | 20.0 | — |
| 137 | 13 yr | F | 8/31/77 8/16/83 |
Chronic active hepatitis Chronic rejection |
Died at 6 yr | Dead: 1st graft, chronic rejection; died after 2nd graft, pancreatitis and sepsis |
— | — | — |
| 139 | 23 yr | F | 11/13/77 | Chronic active hepatitis | Alive more than 6 yr | Housewife, normal liver function | 75.0 | 15.0 | — |
| 140 | 1 yr, 8 mo | F | 1/4/78 | Biliary atresia | Alive more than 6 yr | Full-time school, normal liver function | 50.0 | 15/0* | — |
| 142 | 5 yr | F | 2/26/78 | Alpha-1-antitrypsin de- ficiency disease, inci- dental hepatoblas- toma |
Alive more than 6 yr | Full-time school, normal liver function | — | 10/0* | 400 |
| 144 | 39 yr | F | 4/19/78 | Chronic active hepatitis | Alive more than 6 yr | Housewife | 37.5 | 12.5 | — |
| 146 | 49 yr | F | 6/14/78 | Primary biliary cirrhosis | Alive more than 6 yr | Housewife | 100.0 | 15.0 | — |
| 150 | 15 yr | F | 7/15/78 | Secondary biliary cirrho- sis |
Alive more than 6 yr | Housewife | None for the last 2 yr | ||
| 155 | 11 yr | M | 10/6/78 | Alpha-1-antitrypsin de- ficiency disease |
Alive more than 6 yr | Full-time school, normal liver function | 37.5 | 20/0* | — |
| 164 | 33 yr | F | 5/8/79 | Chronic active hepatitis | Alive more than 5 yr | Housewife, chronic rejection; total bili- rubin, 3 mg/100 mL |
50.0 | 10.0 | — |
| 166 | 41 yr | F | 9/9/79 | Chronic active hepatitis | Alive more than 4 yr, 11 mo |
Housewife, normal liver function | 50.0 | 12.5 | — |
| 169 | 21 yr | F | 12/3/79 | Chronic active hepatitis | Alive more than 4 yr, 8 mo |
Housewife, normal liver function, 1 child since transplant |
50.0 | — | — |
| 170 | 9 yr | M | 2/3/80 | Alpha-1-antitrypsin de- ficiency disease |
Alive more than 4 yr, 6 mo |
Full-time school, normal liver function | 75.0 | 10/5* | — |
Alternate day prednisone therapy.
Cyclophosphamide.
RESULTS
Survival
Of the 170 patients, 31 lived for more than five years and two others are living and well with normal liver function between 4¾ and five years after liver transplantation as of the end of August 1984. The latter two patients are considered five-year survivors. The actuarial survival rate at five years is 19.4%, and that at ten years is 17.9% (Fig 1).
Fig 1.
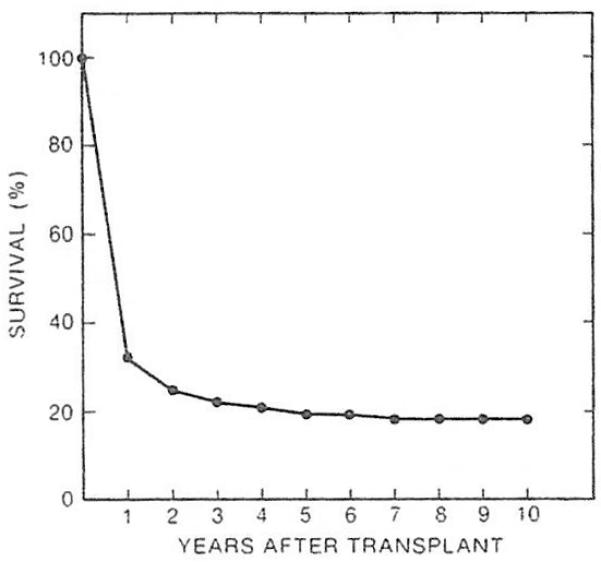
The survival curve of 170 patients who had received orthotopic liver transplantation with azathioprine, prednisone, and ALG between 1963 and 1980.
Of the 33 five-year survivors, 19 were among 86 patients between ten months and 18 years of age and 14 were among 84 patients older than 18 years at the time of transplantation. Thus, five-year survival was better in the pediatric age group (22.1%) than in the adult age group (16.7%).
The influence of preexisting hepatic disease upon five-year survival was examined and the results are summarized in Tables 2 and 3. In the pediatric age group, biliary atresia was the most common indication for liver transplantation; only seven (13.7%) of 51 children with this diagnosis survived for more than five years (Table 2). In contrast, seven (53.8% of 13 children with inborn metabolic diseases, such as Wilson’s disease and alpha-1-antitrypsin deficiency disease, lived for five or more years as well as three (23.1%) of 13 children with chronic aggressive hepatitis and cirrhosis.
Table 2.
Main Indications for Liver Transplantation and Five-Year Survival in Pediatric Patients (Age, ≤18 Years)
| Type of Indication |
No. of Patients | 5-yr Survivors |
|---|---|---|
| Biliary atresia | 51 | 7 (13.7%) |
| Inborn metabolic errors* | 13 | 7 (53.8%) |
| Chronic aggressive hepatitis | 13 | 3 (23.1%) |
| Primary liver malignancy† | 3 | 0 |
| Neonatal hepatitis | 2 | 0 |
| Congenital hepatic fibrosis | 2 | 1 (50.0%) |
| Secondary biliary cirrhosis‡ | 2 | 1 (50.0%) |
| Total | 86 | 19 (22.1 %) |
Alpha-1-antitrypsin deficiency disease, nine; Wilson’s disease, two; tyrosinemia, one; and type IV glycogen storage disease, one.
Five other patients had incidental malignancies (four hepatomas and one hepatoblastoma) in their excised livers. The principal diagnosis in these five cases was biliary atresia (three), alpha-1-antitrypsin deficiency (one), and congenital tyrosinemia (one).
Secondary to trauma or choledochal cyst (one each).
Table 3.
Main indications for Liver Transplantation and Five-Year Survival in Adult Patients (Age, ≥19 Years)
| Type of Indication |
No. of Patients |
5-yr Survivors |
|---|---|---|
| Chronic aggressive hepatitis | 33 | 7 (21.2%) |
| Alcoholic cirrhosis | 15 | 3 (20.0%) |
| Primary liver malignancy* | 15 | 1 (6.7%) |
| Sclerosing cholangitis | 7 | 0 |
| Primary biliary cirrhosis | 6 | 1 (16.7%) |
| Alpha-1-antitrypsin deficiency | 2 | 0 |
| Secondary biliary cirrhosis† | 2 | 1 (50.0%) |
| Hemochromatosis | 1 | 0 |
| Protoporphyria | 1 | 0 |
| Budd-Chiari syndrome | 1 | 1 (100.0%) |
| Acute hepatitis B | 1 | 0 |
| Total | 84 | 14 (16.7%) |
Hepatoma, seven; duct cell carcinoma (Klatskin), five; cholangiocarcinoma, one; hemangioendothelial sarcoma, one; and unclassified sarcoma, one.
One example each of possible duct hypoplasia and choledochaf cyst. Both patients had had multiple operations.
In the adult age group, seven (21.2%) of 33 patients with chronic aggressive hepatitis and cirrhosis and three (20%) of 15 patients with alcoholic cirrhosis lived more than five years after transplantation, which was slightly better than the 16.7% overall 5-year survival rate among 84 adult patients (Table 3).
A total of 23 patients (eight in the pediatric and 15 in the adult age groups) had liver malignancy at the time of transplantation. Five of the tumors were incidental to another disease and were not the principal indications for transplantation (Table 2†). Two of the five patients (OT 33 and 147) with incidental primary liver malignancies are living 14⅔ and 6½ years after transplantation; both are free of malignancy (Table 1). Only one (5.5%) of 18 patients in whom malignant tumors were the principal indication for transplantation lived for more than five years. This exceptional patient (OT 114) has no evidence of tumor recurrence nearly nine years after transplantation (Table 1).
Only three patients were known to be positive for HBsAg prior to transplantation and three more patients became positive after transplantation. None of the three patients who had positive HBsAg prior to transplantation lived five years, but all three patients (OT 77, 115, and 137) who became positive for HBsAg after transplantation lived five years or longer. Because HBsAg antigen detection was not as accurate years ago as it is now and was not performed regularly after transplantation, the true incidence and influence of HBsAg on survival could not be determined in this study.
Causes of Death After Five Years
Only two patients died after five years. A boy who was 11 years old at the time of transplantation (OT 27), died a few days before the six-year mark, of chronic liver failure. The cause of this liver failure was uncertain because the patient had undergone several reoperations for biliary duct strictures; these could not be completely corrected. In addition, medical attention for this patient was not sought at his local hospital until he became critically ill. Autopsy was not granted. It was speculated at that time that he might not have had adequate medication, if any, for several months prior to his death. During his survival period, he obtained a junior high school education and he had been looking for work (Table 1).
Another patient (OT 137) died after exactly six years. She developed chronic rejection five years after transplantation. Azathioprine was discontinued and cyclosporine therapy was begun. Despite a favorable response to this change, the graft function continued to deteriorate. She died from necrotizing pancreatitis and abdominal sepsis 15 days after retransplantation. She had finished high school and was married before her death (Table 1).
Rehabilitation: Quality of Life
Nine children who were below school age at the time of transplantation are all attending school, one (OT 33) being a college student. Of ten children who were in school before transplantation, seven graduated, and the other three are continuing their education. Of the seven who graduated, all worked except the one who died (OT 27). Two of them married.
Among the 14 adult patients, 12 are working full-time including nine housewives. Of the nine housewives, four also held full-time or partime jobs outside of the home. One woman gave birth twice to healthy babies and another woman also has had a normal baby. Two men could not keep their regular occupations because of both social and medical reasons (Table 1). Thus, all but two of the 33 five-year survivors were rehabilitated. The quality of life has been satisfactory.
CONCLUSION
Although these results are far inferior to those obtained in recent years with cyclosporine and low-dose steroid therapy, each of these 170 heroes or heroines contributed to the mass of scientific data and to the development of the surgical techniques that have become routine today. Even now, their contributions continue, and demonstrate that survival between five and ten years is more than 90%, and that rehabilitation and quality of life are excellent, allowing important community service, parenthood, and other noble human functions.
ACKNOWLEDGMENT
The authors express their greatest appreciation to the patients and families who have devoted themselves to the development of liver transplantation, and to the many physicians, nurses, and other hospital and laboratory personnel who have directly and indirectly served and cared for the patients who needed this therapy years ago before it had been standardized.
Supported by research grants from the Veterans Administration and by project grant No. AM-29961 from the National Institutes of Health.
REFERENCES
- 1.Starzl TE, Putnam CW. Experience in Hepatic Transplantation. Saunders; Philadelphia: 1969. with the assistance of. [Google Scholar]
- 2.Starzl TE, Porter KA, Putnam CW, et al. Surg Gynecol Obstet. 1976;142:487. [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE, Koep LJ, Halgrimson CG, et al. Gastroenterology. 1979;77:375. [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Hepatology. 1982;2:614. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]


