Abstract
Expertise in medicating depression requires experience with all types of antidepressants, including several medications within each type. Likewise, electroconvulsive therapy (ECT) proficiency includes experience with each of the modern electrode placements, of which there are four. Besides traditional bilateral and right unilateral placements, ECT electrode placement includes bifrontal and left anterior right temporal (LART) placements. In comparing antidepressant drugs, clinical trials have proven few differences of statistical significance, and useful proven differences are still more unusual. Analogously, few differences have been proven between ECT electrode placements, and many reported differences can be accounted for by large differences in electrical stimulus dosage. Still, the absence of proven differences does not show that there are no useful variations. This paper reviews the meaningful differences that are generally appreciated from clinical experience and biomedical principles for ECT electrode placement as well as antidepressant drugs.
Introduction
The virtues of electroconvulsive therapy (ECT) are plainly seen by physicians who examine severely ill, melancholic, catatonic, or psychotic depressive patients before and after an ECT course. The usefulness, efficacy, and safety of ECT are documented,1 but several aspects of the ECT method—particularly the selection of electrode placement—are subject to varying opinions and habits. Specifically, electrode placement varies markedly among hospitals.2 It is appropriate that deciding between unilateral and bilateral ECT is not obvious because no single method is always best, and the essential issue is how to match the electrode placement to the individual patient.
In the absence of decisive study evidence, clinical practice operates from professional experience and biomedical principles. Systematic clinical comparisons of antidepressants have found few statistically significant differences in therapeutic benefit. Clinically useful differences are still more unusual, and no medication has proven best for all depressions. Differences among tricyclic antidepressants, such as desipramine and doxepin, are primarily appreciated from clinical experience and pharmacological concepts rather than clinical trial results— likewise among serotonin reuptake inhibitors, such as fluoxetine and paroxetine.
Few differences among the four modern ECT electrode placements have been proven. When study comparisons showed a large difference, the placement with lower efficacy (and side effects) was generally used at much weaker stimulus dosage. The main theme in this report is that ECT expertise includes experience with each of the modern electrode placements (Figures 1–4). In support of this, neurobiology and data on electrode placement efficacy and side effects will be reviewed.
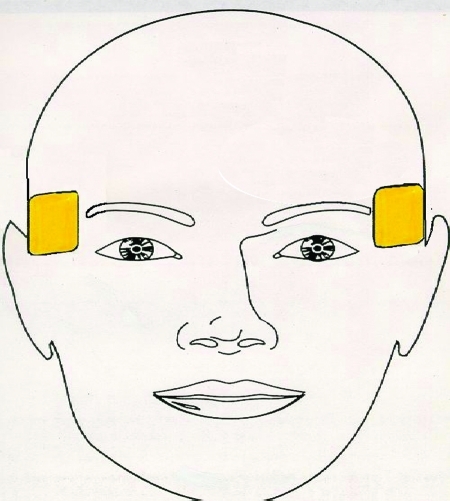
Figure 1.
Right Unilateral Placement
Figure 2.
Bitemporal Placement
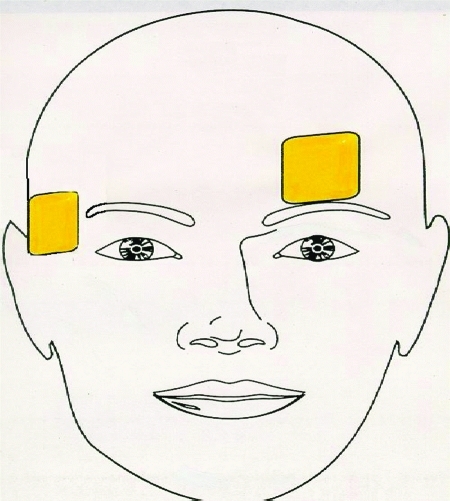
Figure 3.
Left Anterior Right Temporal (LART) Placement
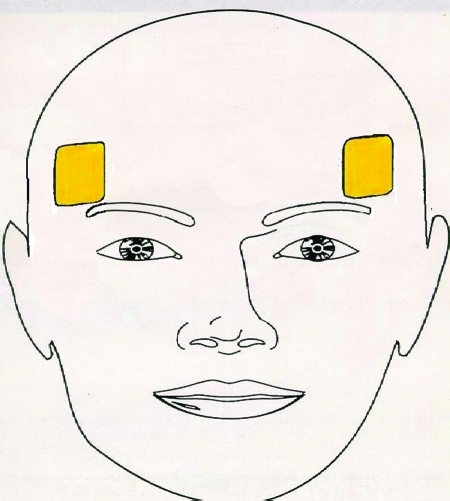
Figure 4.
Bifrontal Placement
Efficacy Goals
The objective of ECT is clinical improvement by electrically inducing a well-generalized seizure. In ECT study groups, the best results reported are 1) maximum clinical improvement and total course in a median of 6 to 8 sessions; 2) remission in at least 80 percent of patients; and 3) stable response so that relapse within 4 to 6 weeks occurs in fewer than 20 percent of remitting patients. The quality of remission should be essentially complete, setting aside symptoms of comorbid disorders with depressive complaints, e.g., anxiety disorders, personality disorders, physical illness, and alcohol and substance abuse disorders. Relapse that occurs beyond six weeks post-ECT is apparently related to post-ECT prophylaxis rather than the acute ECT course. Consistently hitting these targets for speed, remission rate, and stability essentially defines the meaning of desirability in efficacy for an ECT method. Study reports will be considered relative to these expectations.
Electrical underdosage is a basic reason for not hitting these efficacy targets. Any electrode placement can be underdosed so its effectiveness is weak. Low effectiveness from underdosing has been reported for traditional bilateral placement,3,4 sometimes called bifrontotemporal or bitemporal, right unilateral,5,6 and bifrontal placements.7 A study that underdoses a placement does not represent good clinical practice and so does not fairly examine clinical use of the placement. Some study reports found low effectiveness for one or more treatment methods but did not consider how the low effectiveness can be accounted for by the low stimulus doses used; these studies are reviewed later in this article. Mild or occasional underdosing increases the number of ECT sessions needed for remission, and strict underdosing also decreases the remission rate.
On one hand, lower stimulus dosage produces less cognitive effect, regardless of electrode placement.1 On the other hand, overdosing can negate the cognitive advantages of any placement. A method for dose adjustment is available.8 Physiologically, greater electrical dosage induces more neuronal depolarization, i.e., more seizure foci. This should produce stronger seizure generalization through the brain, which is better quality ECT. When the two stimulus electrodes are more widely separated, the volume of seizure foci between them is larger and the minimum charge that induces a seizure is higher. In bitemporal ECT the electrodes are further apart than in right unilateral ECT and the minimum seizure-inducing charge is 30 to 40-percent higher. Conversely, it is easier to underdose right unilateral than bitemporal ECT because a seizure can be induced at lower electrical dosage.
Cognitive Effects Implications
Besides adverse cognitive effects from illness or medications, patients can experience basically two kinds of cognitive side effects from ECT: gradual cumulative disorientation and acute-onset delirium. Fortunately, the latter is unusual. Although these temporary adverse effects should be milder than the pre-ECT impairment by the illness under treatment, they do require attention.
Gradual cumulative disorientation is typically first noticeable after 3 to 4 ECT sessions. It accumulates with additional ECT sessions, as the MMSE9 scores fall.10 It is generally largest with bitemporal electrode placement and high stimulus dose but varies greatly among patients, from negligible to substantial. It persists variably and up to four weeks in elderly patients. Although temporary, it can require nursing support for self-care and obscure bonafide therapeutic response, as well as a postponement in discharge. Because of these and the value people place on the privacy of their health problems even in the hospital, alternatives to bitemporal ECT should be considered.
Gradual cognitive dysfunction does not indicate adverse neuronal effects. Rather, detailed neurochemical and anatomical evidence show an absence of neuronal injury from ECT.11,12 The cause of gradual disorientation might be the opposite, which is that ECT induces the appearance of new neurons (neurogenesis) in the hippocampus, a brain structure crucial to memory.13 Ironically, this hippocampal rejuvenation might obstruct memory function until the new cells are integrated into the neural system.
Acute-onset delirium from ECT is a marked confusion after a particular treatment. It can occur after the first ECT or after several and it can be mistaken for catatonia.14 When such delirium occurs, non-convulsive status epilepticus should be considered; although uncommon, its severity is a reason to routinely monitor the EEG for seizure termination. Presumably, some concurrent neurological illnesses (e.g., cerebrovascular disease) are a risk for marked confusion.
For some patients. cognitive side effects seem unavoidable regardless of ECT method. In one series of depressed elderly patients, 5 of the 34 (15%) patients receiving unilateral ECT and 1 of the 29 (3%) patients receiving bitemporal ECT experienced severe disorientation.15 There is no reason for greater disorientation from unilateral ECT; it should have occurred in these particular patients with any placement. The high 10-percent overall incidence of severe disorientation in this study suggests that it is more likely to occur in elderly patients.
Neurobiology: Bitemporal and Unilateral
The brain regions of highest electrical current density have the most intense seizure activity, so they experienced the largest and longest interruption of brain function. Accordingly, it makes sense to avoid placing stimulus electrodes near brain structures associated with basic self-care and orientation. Wider separation of the two stimulus electrodes increases the amount of brain that experiences intense seizure activity, and likewise for higher electrical doses. Essentially by definition higher electrical doses distribute seizure activity more widely through the brain.
Besides specific location, electrode symmetry should influence ECT side effects. Both hemispheres contribute to many basic brain functions (e.g., speech, memory, cognition) although somewhat differently.16,17 One side can mitigate a deficit in the other, and bilateral lesions can be far more toxic.18 Accordingly, unilateral and other asymmetric electrode placements should interfere less with cognitive behavior than symmetric placements, such as bitemporal.
The bitemporal electrode placement, with one electrode on the flat of each temple, has the largest volume of brain geometrically between the two electrodes of the four placements. This presumably underlies the associated high levels of seizure generalization,19 efficacy, and side effects. In contrast, regular unilateral ECT20 has lower geometrical brain volume, seizure generalization, efficacy, and side effects. One electrode is located over the flat of the right temple. The other is just to the right of the vertex, the highest point of the skull; it is sometimes moved two inches posterior, which should increase its clinical effects.
Clinical Effects: Bitemporal vs. Unilateral
Most electrode placement studies have compared bitemporal with unilateral ECT. The longstanding consensus is that bitemporal placement provokes more cognitive side effects while unilateral is less effective, with fewer remissions and more relapse.1,5,21 There are two shortcomings in methods that undermine many ECT studies: electrical underdosing and concurrent anxiety disorders. Underdosing is not appropriate, and the associated results simply do not apply to proper treatment. Because concurrent anxiety disorder raises most depression rating scores (e.g., Hamilton Depression Rating Scale [HAM-D]) and is common in severely depressed patients (e.g., melancholia causes post-traumatic stress disorder) it lowers measured remission rates and increases relapse rates. In turn this obscures actual efficacy. Similarly, anxiety disorders can produce a symptom group meeting criteria for major depression but never responding to ECT (i.e., atypical depression). Such patients are not usually excluded from ECT studies, which may dilute efficacy results.
When unilateral ECT is administered at low stimulus dosage (e.g., 85mC average) its response rate is particularly low, specifically 17 percent vs. 65 percent for bitemporal ECT.5 The response rate increased to 30 to 45 percent at 130 to 175mC average dose.5,21 Similarly, the largest difference in side effects between unilateral and bitemporal ECT occurs at low stimulus doses.21 Conversely, differences between placements fade at high unilateral stimulus doses of 378mC or more for both desirable and undesirable effects.22–24 Remission rates were 65 to 80 percent in this high dose range.
The following analogy illustrates how using bitemporal or high-dose (near 400mC average) unilateral placement for every ECT patient is not prudent, despite higher remission rates: Giving every patient experiencing acute manic episode concurrent full doses of lithium, valproate, and an antipsychotic drug should produce a higher remission rate than lithium or valproate alone. However, this is generally an unnecessarily forceful first trial and will generate unnecessary side effects. Selectivity instead of overtreating is a clinical art.
Another clinical art in ECT is identifying patients who should respond well to an electrode placement with lower side effects than bitemporal ECT. Presumably, low-dose unilateral placement is mildest; it brings remission in a third of patients who would respond to bitemporal ECT.5 No studies have directly determined how to identify patients who respond to low-dose unilateral ECT. Indirectly, however, patients who show higher peak heart rate during ECT seizure respond more rapidly25 to left-anterior right-temporal (LART) ECT; this presumably applies to all placements. Because EEG measurements can reflect seizure intensity, they too might help identify patients who will respond to low stimulus doses.
When nonresponse and relapse risk simply must be minimized, as with seriously suicidal patients, bitemporal ECT seems desirable. When the risk of troublesome side effects is high but relapse is not life-threatening, a different placement might be preferable, unless it had failed for that patient. An example is a elderly patient cachectic from depressive anorexia.
Neurobiology: Bifrontal and Lart
Two bilateral placements besides bitemporal have shown high efficacy with little disorientation: bifrontal and LART. Bifrontal electrodes are located 2.5cm anterior to bitemporal sites and are likewise symmetrical. Bifrontal and bitemporal placements overlap about 50 percent because the electrodes are about 5cm wide. In LART placement the left electrode is 5cm anterior to the left bitemporal site; the right-sided site is the same as for bitemporal. For LART and bifrontal placements the geometrical volume between electrodes is about equal, and so is the expectation for efficacy at similar dosage. This volume is about 75 percent that of bitemporal placement.
The neurobiology particular to the LART placement is primarily its asymmetry and the fully anterior location of the left electrode. It should interfere less with cognitive behavior than symmetrical placements do. Its entirely anterior location separates it from the temporal lobe and the dorsolateral prefrontal cortex (DLPFC). The DLPFC assembles into short-term memory sets of environmental information for problem solving and self-care, and the anterior temporal lobe also participates in memory. Accordingly, although LART and bifrontal placements have the same interelectrode distance, they should differ in side effects.
Electrode placement near skull sutures might influence clinical effects; the placements differ in such proximity in a manner consistent with side effects. Electrical resistance at skull sutures and foramina is much lower than at unperforated bone. If an electrode is above a suture, stimulus current density will be particularly high in brain regions near the inside of the suture, inducing particularly intense seizure and function disruption; this might be similar to raising the dose. Two skull sutures criss-cross the bitemporal site, forming an “X” under it. The bifrontal site includes one suture, and the left anterior LART site has none.
Clinical Effects: Bifrontal
In comparing bifrontal, bitemporal, and unilateral placements, Lawson, et al.6,26 minimized stimulus doses, 148mC for bitemporal, 164mC for bifrontal, and 107mC for unilateral ECT on average. Their courses of bifrontal, bitemporal and unilateral ECT averaged 10.3, 11.5, and 15.7 sessions, respectively. These stimulus doses were too low and these ECT courses were too lengthy for the methods and results to apply to expected clinical treatment. Some depression scores were marginally lower with bifrontal than bitemporal ECT, but the bifrontal stimulus dose was higher. Unilateral was less effective than bifrontal and bitemporal placements, but its stimulus dose was much lower. For side effects, only verbal memory immediately after ECT showed better scores with bifrontal than bitemporal placement, but this difference is not specifically from electrode placement. This is because the post-ECT difference between bifrontal and bitemporal groups persisted for three months (i.e., longer than attributable to electrode placement at low dosage). Moreover, there was no pre-ECT baseline measurement. Taking the three-month measurement as the baseline leaves no side effect advantage for bifrontal ECT.
The efficacy of bifrontal was stated as higher than bitemporal ECT but the dose was higher and the long course of 10 to 12 ECTs for bifrontal does not show desirable efficacy. The results are further undermined by the peculiarity that bitemporal and low-dose unilateral ECT showed equal efficacy immediately after six ECTs and one week after the final ECT. A simple explanation for the peculiarities and long courses is that all placements were underdosed, as described by the method that the stimulus dose for all placements was at seizure threshold, that is, minimized. Accordingly, these studies do not correspond to clinical practice.
Comparison of higher dose bifrontal and bitemporal ECT revealed statistically significant but small side effect advantages for bifrontal placement.27 The average stimulus dose was near 250mC. The average course length was under six ECTs for both placements. The post-ECT MMSE averaged 28.1 with bifrontal (i.e., intact) and 25.7 with bitemporal. However, the standard deviation of the bitemporal MMSE was 2.5 times that for the bifrontal MMSE (p<.0001). Accordingly, the differences are largely accounted for by a few patients, and they reported that elderly patients had lower post-ECT MMSE scores. Apparently, the cognitive advantage of bifrontal over bitemporal ECT applies primarily to elderly patients, the same patients who tend to receive the highest stimulus doses because dosing needs increase with age.
The results of Heikman, et al.,7 illustrate how effects from underdosing can dominate electrode placement effects, despite requiring that motor seizure duration exceed 25 seconds. The average course of low-dose bifrontal ECT (average 120mC) was 12 ECTs versus 7 for high dose unilateral ECT (252mC) and 8 for low-dose unilateral ECT (126mC). However, low-dose unilateral was not clearly more effective than bifrontal because post-treatment Hamilton depression ratings averaged 15 and 10, respectively. Post-ECT MMSE scores were 25, 28, and 26 for high-dose unilateral, low-dose unilateral, and bifrontal, respectively. The results indicate the importance of avoiding underdosing, but have no implications for electrode placement.
Clinical Effects: Lart Placement
In an open trial of 10 female patients (9 severely ill, 1 moderately ill) the post-ECT MMSE score with LART placement was 28.4, substantially above the pre-ECT score of 11. This high MMSE score occurred although the stimulus dose was relatively high, 3.5 to 5 mC per year of age. The median treatment course was 7 sessions and the average treatment course was 8.5 sessions long. All patients achieved remission, and 90 percent sustained remission on 6 to 10 week follow-up.28 Five of these patients received ECT for drug-resistant mania, explaining the longer course that a few patients received. In a double-blinded pilot study of eight subjects, those receiving LART trended to greater efficacy and lower side effects (both right- and left-hemisphere) than bitemporal ECT; the groups were too small for statistics.29 In an open trial of LART placement in 24 patients, 88 percent achieved remission and 100 percent showed response,25 with an average course length of 6.8 sessions and a median of 6. The stimulus dose was 2.5mC per year of age at the beginning of the course and was raised to 5mC per year, usually at the sixth ECT. LART placement is used regularly in many hospitals in the US and in Russia. According to a recent survey in Russia,30 LART is used in 21.3 percent of institutions providing ECT and in 10.1 percent of all treatment sessions, while d'Elia's unilateral placement is used in 12.8 percent of institutions and 6.0 percent of treatment sessions, and bifrontal placement in 4.3 percent and 3.7 percent, respectively. This survey showed some hospitals using LART placement in 80 percent of ECTs.
How to Try Other Placements
Your understanding of clinical benefits and side effects comes from your own direct observations. If your patients have experienced troublesome side effects or disappointing efficacy with bitemporal or unilateral ECT, you have a reason to try LART and bifrontal placements. Additionally, it is important to regulate the stimulus dose along the course of treatment, because about half of patients show clear need for increasing dose. Accordingly, the Benchmark Method to individually adjust doses along the ECT course is worth considering.8 As “quality assurance monitoring” for each ECT course, you might record general outcome (e.g., remission, partial improvement, none), post-ECT MMSE score, number of ECTs, and electrode placement. As long as you aim to provide each patient with safe, effective treatment and you do not assign the placement randomly, you are conducting clinical practice, not research, just as when you first try a new antidepressant.
Rozhnov's Study
A unique yet logical approach to ECT electrode placement was introduced by V. A. Rozhnov31 in the former USSR. Rozhnov used a different electrode placement at each ECT session, giving a course of 12 ECTs to 11 patients he diagnosed with schizophrenia. He placed electrodes at locations corresponding to the numbers shown in Figure 5, obtaining seizures with each placement. Number 1 reflects the first session, number 2 the second session, and so on. From sessions 1 to 11 the electrodes were relocated progressively further back on the head. The final session was midline anterior to posterior. He reported that four patients achieved full remission, one showed large improvement, two some improvement, and four were unchanged. He used sinewave current but reported that no patient experienced harm from it. Although this does not demonstrate the absence of side effects, it suggests a generally benign nature of ECT regardless of electrode placement.
Figure 5.
Rozhnov's sequence of electrode placements
Rozhnov changed the placement each time expecting that avoiding repetition of the current through the same cortical structures would minimize side effects. The implication is that the use of the same electrode placement at every ECT session increases side effects. We might have seen fewer side effects if we rotated each ECT patient among the four different electrode placements, bitemporal, bifrontal, unilateral, and LART.
Stimulus Dosing
Electrode placement influences stimulus dosing. Dosing is the same for the three bilateral placements (bitemporal, bifrontal, LART). At the first ECT a typical good dose charge is 2.5mC per year of patient age; this equals setting “% Energy” to half the age. This was validated for the first ECT with stimuli of 900mA current and 1msec pulsewidth32 and for the first through fourth ECT sessions at stimuli of 900mA and 0.5msec pulsewidth.33 At 800mA current the half-age method underdoses the stimulus,34 and a more likely good dose is 4mC per year of age; this is the same as setting “expected joules” to 80 percent of patient age. For unilateral electrode placement a good stimulus dose should be twice these (e.g., 5mC per year of age at 900mA, and 8mC per year at 800mA).
These initial doses should avoid overdosage. In most (but not all) cases the stimulus dose needs to be increased along the course because ECT has anticonvulsant effects. Typically after the first four treatments the dose is gradually increased by 50 to 75mC each session to an eventual charge dose 60 to 100 percent above the initial dose, which is 4 to 5mC per age year for bilateral ECT at 900mA. Setting the stimulus on the basis of the weakest possible ECT, the seizure threshold, is not internally consistent;35 moreover, it is too easy to misrepresent seizure threshold measurement as ECT treatment and bill for it.
A more complete and individual approach to adjustment along the ECT course is the “Benchmark Method.” In this, ECT treatment quality is assessed according to physiological events during the ECT seizure, including peak heart rate, motor seizure duration, and various measures of EEG intensity. At the first ECT benchmark, targets are taken for peak heart rate and several measures of EEG intensity. Development of weakness in any one of these or motor seizure under 18 seconds suggests increasing the stimulus dose by 50 to 75mC. This method is detailed in a free-access Internet publication.8
In summation, there is no substantive controversy about ECT electrode placement. Rather, the ECT practitioner should match the placement to the patient's individual circumstances, and should consider obtaining clinical experience with all four placements.
Contributor Information
Conrad M. Swartz, Dr. Swartz is from the Southern Illinois University, Springfield, Illinois.
Alexander I. Nelson, Dr. Nelson is from the Russian University of Peoples Friendship, Moscow, Russia..
References
- 1.UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: A systematic review and meta-analysis. Lancet. 2003;361(9360):799–808. doi: 10.1016/S0140-6736(03)12705-5. [DOI] [PubMed] [Google Scholar]
- 2.Prudic J, Olfson M, Marcus SC, et al. Effectiveness of electroconvulsive therapy in community settings. Biol Psychiatry. 2004;55:301–12. doi: 10.1016/j.biopsych.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Robin A, de Tissera S. A double-blind controlled comparison of the therapeutic effects of low and high energy electroconvulsive therapies. Br J Psychiatry. 1982;141:357–66. doi: 10.1192/bjp.141.4.357. [DOI] [PubMed] [Google Scholar]
- 4.Christensen P, Hedemand E. EEG and EMG monitored electroconvulsive therapy. Psychopharmacol Bull. 1983;19:20–22. [PubMed] [Google Scholar]
- 5.Sackeim HA, Prudic J, Devanand DP, et al. Effects of stimulus intensity and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. N Engl J Med. 1993;328:839–46. doi: 10.1056/NEJM199303253281204. [DOI] [PubMed] [Google Scholar]
- 6.Letemendia FJ, Delva NJ, Rodenburg M, et al. Therapeutic advantage of bifrontal electrode placement in ECT. Psychol Med. 1993;23:349–360. doi: 10.1017/s0033291700028452. [DOI] [PubMed] [Google Scholar]
- 7.Heikman P, Kalska H, Katila H, et al. Right unilateral and bifrontal electroconvulsive therapy in the treatment of depression: A preliminary study. J ECT. 2002;18:26–30. doi: 10.1097/00124509-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Swartz CM. ECT Dosing by the Benchmark Method. German J Psychiatry. 2002;5(1):1–4 (www.gjpsy.uni-goettingen.de). [Google Scholar]
- 9.Folstein MR, Folstein SE, McHugh PR. Mini-mental state: A practical method of grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 10.Calev A, Cohen R, Tubi N, et al. Disorientation and bilateral moderately suprathreshold titrated ECT. Convuls Ther. 1991;7:99–110. [PubMed] [Google Scholar]
- 11.Coffey CE, Weiner RD, Djang WT, et al. Brain anatomic effects of electroconvulsive therapy. A prospective magnetic resonance imaging study. Arch Gen Psychiatry. 1991;48:1013–21. doi: 10.1001/archpsyc.1991.01810350053008. [DOI] [PubMed] [Google Scholar]
- 12.Zachrisson OC, Balldin J, Ekman R, et al. No evident neuronal damage after electroconvulsive therapy. Psychiatry Res. 2000;96:157–65. doi: 10.1016/s0165-1781(00)00202-x. [DOI] [PubMed] [Google Scholar]
- 13.Ende G, Braus DF, Walter S, et al. The hippocampus in patients treated with electroconvulsive therapy: a proton magnetic resonance spectroscopic imaging study. Arch Gen Psychiatry. 2000;57:937–43. doi: 10.1001/archpsyc.57.10.937. [DOI] [PubMed] [Google Scholar]
- 14.Swartz CM. Delirium or catatonic disorder due to general medical condition. J ECT. 2002;18:167–8. doi: 10.1097/00124509-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Coffey CE, Figiel GS, Djang WT, et al. Leukoencephalopathy in elderly depressed patients referred for ECT. Biol Psychiatry. 1988;24:143–61. doi: 10.1016/0006-3223(88)90270-3. [DOI] [PubMed] [Google Scholar]
- 16.Luria AR, Simernitskaya EG. Interhemispheral relations and functions of the right hemisphere. 1975;1(3):411–6. Report I. About functional interaction of brain hemispheres in organization of verbal-mnesic functions. Fiziol Cheloveka [Russia] [Google Scholar]
- 17.Bragina NN, Dobrokhotova TA. Human Functional Asymmetries. Moscow, Russia: Meditsina; 1981. [Google Scholar]
- 18.Lilly R, Cummings JL, Benson F, Frankel M. The human Kluver-Bucy Syndrome. Neurology. 1983;33:1141–5. doi: 10.1212/wnl.33.9.1141. [DOI] [PubMed] [Google Scholar]
- 19.Swartz CM, Larson G. Generalization of the effects of unilateral and bilateral ECT. Am J Psychiatry. 1986;143:1040–1. doi: 10.1176/ajp.143.8.1040. [DOI] [PubMed] [Google Scholar]
- 20.d'Elia G. Unilateral electroconvulsive therapy. Acta Psychiatr Scand. 1980;215(Suppl):1–98. [PubMed] [Google Scholar]
- 21.Sackeim HA, Prudic J, Devanand DP, et al. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch Gen Psychiatry. 2000;57:425–34. doi: 10.1001/archpsyc.57.5.425. [DOI] [PubMed] [Google Scholar]
- 22.Abrams R, Swartz CM, Vedak C. Antidepressant effects of high-dose right unilateral ECT. Arch Gen Psychiatry. 1991;48:746–8. doi: 10.1001/archpsyc.1991.01810320070010. [DOI] [PubMed] [Google Scholar]
- 23.McCall WV, Reboussin DM, Weiner RD, Sackeim HA. Titrated moderately suprathreshold vs fixed high-dose right unilateral electroconvulsive therapy. Arch Gen Psychiatry. 2000;57:438–44. doi: 10.1001/archpsyc.57.5.438. [DOI] [PubMed] [Google Scholar]
- 24.McCall VW, Dunn A, Rosenquist PB, Hughes D. Markedly suprathreshold right unilateral ECT versus minimally suprathreshold bilateral ECT: Antidepressant and memory effects. J ECT. 2002;18:126–9. doi: 10.1097/00124509-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Swartz CM. Physiological response to ECT stimulus dose. Psychiatry Res. 2000;97:229–35. doi: 10.1016/s0165-1781(00)00234-1. [DOI] [PubMed] [Google Scholar]
- 26.Lawson JS, Inglis J, Delva NJ, et al. Electrode placement in ECT: Cognitive effects. Psychologic Med. 1990;20:335–44. doi: 10.1017/s0033291700017645. [DOI] [PubMed] [Google Scholar]
- 27.Bailine SH, Rifkin A, Kayne E, et al. Comparison of bifrontal and bitemporal ECT for major depression. Am J Psychiatry. 2000;157:121–3. doi: 10.1176/ajp.157.1.121. [DOI] [PubMed] [Google Scholar]
- 28.Swartz CM. Asymmetric bilateral right frontotemporal left frontal stimulus electrode placement. Neuropsychobiology. 1994;29(4):174–8. doi: 10.1159/000119083. [DOI] [PubMed] [Google Scholar]
- 29.Swartz CM, Evans CM. Beyond bitemporal and right unilateral electrode placements. Psychiatr Ann. 1996;26:705–8. [Google Scholar]
- 30.Nelson AI. A national survey of electroconvulsive therapy use in the Russian Federation. Submitted for publication: January, 2005. [DOI] [PubMed]
- 31.Rozhnov VA. Electroconvulsive therapy with the changing electrode position. 1951;VII:365–71. Kirghiz State Medical Institute Proceedings, Frunze. [Google Scholar]
- 32.Petrides G, Fink M. The “half-age” stimulation strategy for ECT dosing. Convulsive Ther. 1996;12:138–46. [PubMed] [Google Scholar]
- 33.Swartz CM, Manly DT. Efficiency of the stimulus characteristics of ECT. Am J Psychiatry. 2000;157:1504–6. doi: 10.1176/appi.ajp.157.9.1504. [DOI] [PubMed] [Google Scholar]
- 34.Chanpattana W. Seizure threshold in electroconvulsive therapy: Effect of instrument titration schedule. German J Psychiatry. 2001;4(3):51–6. Available at: www.gjpsy.uni-goettingen.de. [Google Scholar]
- 35.Swartz CM. Stimulus dosing in electroconvulsive therapy and the threshold multiple method. J ECT. 2001;17:87–90. doi: 10.1097/00124509-200106000-00001. [DOI] [PubMed] [Google Scholar]