Abstract
Due to their small particle size and large and modifiable surface, nanoparticles have unique advantages compared with other drug carriers. As a research focus in recent years, polyethylene glycol–polylactic acid (PEG–PLA) block copolymer and its end-group derivative nanoparticles can enhance the drug loading of hydrophobic drugs, reduce the burst effect, avoid being engulfed by phagocytes, increase the circulation time of drugs in blood, and improve bioavailability. Additionally, due to their smaller particle size and modified surface, these nanoparticles can accumulate in inflammation or target locations to enhance drug efficacy and reduce toxicity. Recent advances in PEG–PLA block copolymer nanoparticles, including the synthesis of PEG–PLA and the preparation of PEG–PLA nanoparticles, were introduced in this study, in particular the drug release and modifiable characteristics of PEG–PLA nanoparticles and their application in pharmaceutical preparations.
Keywords: PEG-PLA, block copolymer, nanoparticles, drug delivery system
Introduction
Polylactic acid (PLA) is a synthetic biodegradable polymer. In the aquatic environment, it hydrolyzes into nontoxic hydroxyl-carboxylic acid through ester bond cleavage and then is metabolized into water and carbon dioxide through a citric acid cycle. Due to its suitable biodegradability, good security, low immunity, and good mechanical strength, PLA has been approved by the US Food and Drug Administration for application in tissue engineering, medical materials, drug carriers, for example,1 and it has good prospects for peptides, proteins, vaccines, anticancer drugs, and other drug carriers. However, PLA applications are limited due to its weak hydrophilicity, excessively long degradation time, and low drug loading of polar drugs.2 On the other hand, polyethylene glycol (PEG) has many advantages, such as good hydrophilicity, flexibility, antiphagocytosis against macrophages, resistance to immunological recognition, non-combination with proteins, and biocompatibility.3–6 Through copolymerization with PEG, PLA can be improved in hydrophilicity, degradation rate,7 and crystallization,8 showing great potential for development in drug delivery. The degradation products of PEG–PLA block copolymer can enter the tricarboxylic acid cycle or be eliminated by the kidney. Thus, in low concentration the copolymer is nontoxic and not accumulative in vivo.9 The copolymerization of PLA and PEG can increase the drug loading, reduce the burst effect, and prolong the in vivo residence time of drugs and avoid them being engulfed by macrophages. The synthesis of PEG–PLA block copolymer and its end-group derivatives and the preparation of PEG–PLA nanoparticles will be discussed in this paper, in particular their drug release and modifiable characteristics and their application in pharmaceutical preparations.
Synthesis of PEG–PLA block copolymer and its end-group derivatives
Ring-opening polymerization of PEG and lactide
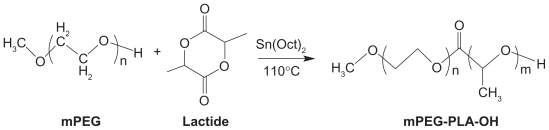
These polymers may be synthesized by ring-opening polymerization between PEG or its end-group derivatives (such as methoxy polyethylene glycol [mPEG]) and lactide (Figure 1).10 Tin salts are the commonly used catalysts, especially stannous compounds with a higher catalytic efficiency. However, due to the toxicity of these heavy metal compounds, acetic acid bismuth was used as an initiator by Kricheldorf et al.11 It was found that in the copolymerization system of l-lactide and PEG tetramer, the length of polymer chain could be controlled by changing the proportion of monomer and initiator, and copolymers with different molecular structures could be synthesized, such as A-B stellate copolymer, A-B-A triblock copolymer, multiblock copolymer, and reticular copolymer.
Figure 1.
Synthesis scheme of mPEG–PLA.10
Copyright © 2010, Elsevier Limited. Reproduced with permission from Zhang X, Li Y, Chen X, et al. Synthesis and characterization of the paclitaxel/MPEG-PLA block copolymer conjugate. Biomaterials. 2005;26(14):2121–2128.
Abbreviations: mPEG, methoxy polyethylene glycol; PLA, polylactic acid.
Anionic ring-opening polymerization
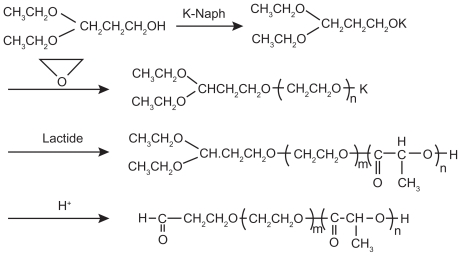
In anionic ring-opening polymerization, common catalysts include potassium alkoxide,12 sodium alkoxide,13 and butyl lithium. Otsuka et al13 synthesized 3,3-diethoxy-potassium propanol ([C2H5O]2CHCH2OK) with the initial reactants 3,3-diethoxy-propanol ([C2H5O]2CHCH2OH) and potassium naphthalene (K-Naph) and the solvent tetrahydrofuran (THF), and then synthesized α-acetal-PEG–PLA block copolymer through anionic ring-opening polymerization with ethylene oxide and lactic acid (LA) as reactants and 3,3-diethoxy-potassium propanol as an initiator (Figure 2).
Figure 2.
Synthesis scheme of acetal-ended poly(ethylene glycol)–poly(lactic acid) copolymers.13
Copyright © 2010, American Chemical Society. Reproduced with permission from Otsuka H, Nagasaki Y, Kataoka K. Surface characterization of functionalized polylactide through the coating with heterobifunctional poly(ethylene glycol)/polylactide block copolymers. Biomacromolecules. 2000;1(1):39–48.
Synthesis of PEG–PLA end-group derivatives
The physical and chemical properties of PEG–PLA copolymers can be improved by modifying the hydroxyl of the PEG end-group for better-loading hydrophobic, gene, and protein drugs.14,15 The common end-group forms of PEG–PLA derivatives include hydroformylation, propylene acylation, and amination.16 For example, Salem et al17 first linked biotin with PEG to synthesize biotin–PEG and then linked PLA with biotin–PEG to synthesize biotin–PEG–PLA polymer through ring-opening polymerization.
Preparation of PEG–PLA block copolymer nanoparticles
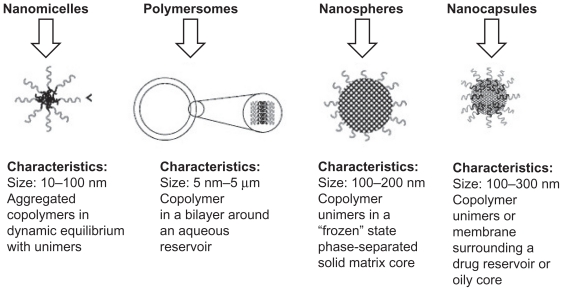
Using different compositions and preparation methods, amphiphilic block copolymers such as PEG–PLA block copolymer can be prepared into various forms of nanoparticles, including nanomicelles, polymersomes, nanospheres, and nanocapsules (Figure 3).18
Figure 3.
Classification of nanoparticle drug delivery systems and their general characteristics.18
Copyright © 2010, Elsevier Limited. Reproduced with permission from Letchford K, Burt H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: micelles, nanospheres, nanocapsules and polymersomes. Eur J Pharm Biopharm. 2007;65(3):259–269.
Preparation of PEG–PLA block copolymer nanomicelles
The method of preparation of PEG–PLA block copolymer nanomicelles mainly depends on the hydrophilicity of copolymers. The hydrophilic copolymer can form micelles by self-assembly in water. The direct dissolution method is most commonly used, ie, after being dissolved directly in water (they are able to be heated), the copolymers may form the transparent micellar solution immediately above its critical micelle concentration.19 Another is the film rehydration method, ie, copolymers and drugs are dissolved in volatile solvent to form a membrane after vaporizing solvent, and then the micelles can be formed by adding buffer solution or water as well as stirring and dissolving copolymer membranes.20 If the copolymer is insoluble in water, organic solvents may be used. The copolymer is first dissolved in the organic solvent (or water-mixed solvent), and then the organic solvent is removed by dialysis or evaporation.21
Preparation of PEG–PLA block copolymer polymersomes
Some preparation methods of liposomes, such as the injection method, film rehydration method, and ultrasonic dispersion method, can also be used to prepare copolymer polymersomes. The solvent injection method was adopted by Jain and Kumar22 to prepare amphotericin B-loaded polymersomes. In the film rehydration method, copolymers are dissolved in volatile organic solvents, the membranes form after the rotary evaporation of organic solvents, and then the copolymer polymersomes form after adding buffer solution and stirring constantly with sonication and extrusion.23
Preparation of PEG–PLA block copolymer nanospheres
The preparation methods of copolymer nanospheres include the emulsification solvent evaporation method and emulsification solvent diffusion method. The process is generally divided into two steps. The first step is emulsification, ie, the copolymer and drugs are dissolved in organic solvent and then the emulsion is formed by adding into the water phase and stirring. The second step is to remove the organic solvent in the emulsion by evaporation or dialysis.24 Venkatraman et al25 used the emulsification solvent evaporation method to prepare the PLA–PEG–PLA nanospheres. First, the copolymer was dissolved in organic solvent (acetone, THF, dimethylformamide, or dimethylacetamide) and mixed with deionized water by stirring. Then, acetone or THF was removed by evaporation, dimethylformamide or dimethylacetamide was removed by dialysis, and finally the nanospheres were obtained after freeze dehydration.
Preparation of PEG–PLA block copolymer nanocapsules
The interfacial polymerization method is commonly used to prepare PEG–PLA nanocapsules, ie, drugs and block copolymers are dissolved in water-miscible organic solvent, and then nanocapsules can be prepared by slowly dripping the mixed solvent into aqueous solution by stirring with or without surfactants.26
The release of PEG–PLA block copolymer nanoparticles
The release mechanism of PEG–PLA block copolymer nanoparticles
The release mechanism of PEG–PLA nanoparticles is similar to that of general nanoparticles, and the common mechanisms include three types: 1) adsorption and desorption of drugs on nanoparticles surface; 2) diffusion release; and 3) degradation of nanomatrix or degradation/diffusion collaborative process. 27 The drug release mainly depends on the following: 1) desorption of drugs absorbed on the surface or interface; 2) diffusion by nanoparticle matrix; 3) diffusion through the copolymer wall (nanocapsules); 4) dissolution of nanomatrix; and 5) dissolution or diffusion of bond compounds. Therefore, the process of drug release is controlled by drug diffusion and matrix degradation.28
The release mechanism for most nanoparticles can be divided into two phases: the burst release phase and controlled release phase. In the burst release phase, drugs diffuse quickly in the solvent medium due to drugs adsorbing or weak bonding onto the surface of nanoparticles with a large surface area. Because drugs are not uniformly distributed/dissolved in the matrix (hydrophobic chain), in the controlled release phase the drug release by diffusion or erosion (degradation) depends on the characteristics of the drug delivery system. The drug release mainly depends on diffusion when the rate of drug diffusion is greater than that of matrix degradation; otherwise, it mainly depends on matrix degradation when the rate of drug diffusion is less.29 Li et al21 used the volatile dialysis method with organic solvent to prepare all-trans retinoic acid-loaded nanomicelles and then analyzed the release mechanism of nanomicelles. It was found that in ensuring the integrity of nanoparticles, by using the mixture of phosphate-buffered saline (PBS) (pH 7.4) and ethanol (with the proportion of 9:1) as the release medium, the burst release occurred in the first 15 h, and then the controlled release occurred. Because nanoparticles were complete and not degraded in the above condition, the drug release mechanism was considered as diffusion after dissolution. Over 80% of drugs had been released after 5 days. Mean-while, in another method, the drug-loaded nanomicelles were incubated in PBS, and then the molecular weight was analyzed by nuclear magnetic resonance. It was found that the nanomicelles were slowly degraded. The degradation time of 27.6% of mPEG5–PLA5 and 30.1% of mPEG2–PLA16 was more than 30 days. Therefore, the release mechanism of all-trans retinoic acid-loaded mPEG–PLA nanomicelles was considered as mainly relying on drug diffusion rather than matrix degradation.
However, drug release can be induced by physical or chemical methods. Light,30 temperature,31 pH,32 power,33 magnetic field,34 and ultrasound19 have been used to control drug release from copolymer nanoparticles.
Influencing factors on drug release of PEG–PLA block copolymer nanoparticles
PEG–PLA block copolymer is an amphiphilic polymer with good stability in vivo. With good biocompatibility, the PEG hydrophilic layer can increase the solubility of insoluble drugs, effectively prevent the protein absorbed on the nanoparticle surface, make nanoparticles unrecognizable by the reticuloendothelial system as foreign bodies, and thereby show a characteristic of long circulation. Many factors may influence drug release of PEG–PLA block copolymer nanoparticles. The main factors are as follows:
Molecular weight, chain length of PEG or PLA, and PEG/PLA ratio in the polymer: The chain length of PEG and PLA can be controlled by changing the molecular weight of PEG and the concentrations of PEG and PLA. The longer the PLA chain length, the larger would be the nanoparticle size and the drug loading of hydrophobic drugs. As the PEG content and weight-average molecular weight (Mw) of PLA–PEG–PLA copolymers increased, the amount of drug release increased and the total Mw of copolymers of nanoparticles decreased. Drug release from nanoparticles could potentially be controlled by changing the content of PEG, Mw of PEG, and total Mw of copolymer.35 It was found by Yang et al36 that the longer the PLA chain length, the larger the diameter of micelles and drug-loaded micelles would be. An in vitro release test showed that the longer the PLA chain length, the greater the interaction between PLA chain and hydrophobic drug would be and the slower the drug release rate of micelles would be. In case of a low Mw of PEG, deformation occurs easily due to the small molecular chain and low flexibility. The greater the molecular weight of PEG, the longer the PEG molecular chain length will be and the more stable the structure will be. The increase of PLA block Mw in the copolymer will reduce significantly the stability of nanoparticles and even lead to condensation in the solvent.21
Preparation methods and conditions of PEG–PLA block copolymer nanoparticles: Different preparation technologies of nanoparticles will influence the crystal shape of polymer, drug distribution, and stability in the carrier materials; influence the surface morphology, particle size, and internal compactness of nanoparticles; and thus influence the rate and degree of drug release.37
Particle size of PEG–PLA block copolymer nanoparticles: The particle size of PEG–PLA block copolymer nanoparticles can be changed by adjusting PEG chain length and PEG/PLA ratio or using a different preparation method. PEG–PLA block copolymer nanoparticles with different sizes will lead to different degradation or diffusion rates of nanomatrix, resulting in differences in drug release.38
Drug loading of PEG–PLA block copolymer nanoparticles: Drug loading of PEG–PLA block copolymer nanoparticles is also an important influencing factor for drug release. The relationship between drug loading and micelle stability was studied by Huh et al,39 and it was found that due to the incorporation of hydrophobic drugs, the micellar hydrophilic–hydrophobic balance was destroyed, and the stability of micelles would be increased with drug loading decreased. When the paclitaxel loading of PEG–PLA micelles was 17.6%, the paclitaxel would be dissolved completely in sodium salicylate solution after 5 days, whereas in the case of loading capacity of 27.6%, the paclitaxel would be dissolved completely after 3 days.
In addition to the above major influencing factors, drug release will be influenced by copolymer concentration,40 pH,41 zeta potential,42 and solvents.43
Surface modification of PEG–PLA block copolymer nanoparticles
By modifying the surface of PEG–PLA block copolymer nanoparticles, various special nanoparticles may be prepared in order to increase the therapeutic effect of drugs, such as long-circulating nanoparticles, immunonanoparticles, thermosensitive nanoparticles, and pH-sensitive nanoparticles. Common materials for surface modification include three types: 1) polysaccharides such as cyclodextrin and chitosan; 2) surfactants such as polysorbate; and 3) PEG poloxamer. Due to their core shell structure, the surface of PEG–PLA block copolymer nanoparticles is often modified by folic acid, peptide, lectin, and albumin. Compared with PEG–PLA block copolymer nanoparticles with passive targeting, these modified nanoparticles can actively target special locations for enhancing drug efficacy and decreasing drug toxicity. For example, PEG–PLA nanoparticles with surface-bound lectins (biorecognitive ligands) were established by Gao et al.44 Due to abundant N-acetyl-d-glucosamine and sialic acid in the nasal cavity, wheat germ agglutinin was selected as a model lectin to bind them. The resulting nanoparticles increased significantly the uptake of drugs in the brain associated with nanoparticles through intranasal delivery, which might provide a novel effective noninvasive technique for drug delivery to the brain, particularly for biotech drugs such as peptides, proteins, and DNA. Yu et al45 synthesized the aldehyde–PEG–PLA block copolymer by ring-opening polymerization and conjugated a peptide (K237 ligand) to the aldehyde group of PEG chain by using the N-terminal PEGylation technique. The K237-conjugated paclitaxel nanoparticles could be significantly internalized by human umbilical vein endothelial cells through the K237-KDR (K237-vascular endothelial growth factor receptor) interaction. This facilitated uptake of paclitaxel led to the enhanced antiangiogenic activity, migration, and tube formation compared with cells treated with paclitaxel nanoparticles and commercial taxol. PEG–PLA block copolymer nanoparticles modified with folic acid or its salts as target materials can accumulate in cancer locations, increase drug concentration in cancer locations, and prolong the action time. Tsai et al46 prepared poly(HEMA-co-histidine)-g-PLA and folate-PEG–PLA nanomicelles. Folate for cancer-specific target was bound at the end of the polymer chain. It was found that cellular uptake of folate micelles was higher than that of non-folate micelles due to the folate-binding effect on the cell membrane. An in vivo study of folate micelles exhibited cancer targeting and effective inhibition of tumor growth.
Application of PEG–PLA block copolymer nanoparticles in pharmaceutical preparation
The PEG–PLA copolymer has the advantages of both PEG and PLA. As a drug carrier, PEG–PLA copolymer nanoparticles have some advantages, eg, 1) reducing the first-pass effect and increasing bioavailability;47 2) increasing drug loading and encapsulation efficiency;48 3) reducing particle size and burst release while improving targeting;49 4) avoiding recognition and removal by the reticuloendothelial system, thereby prolonging the circulation time of drugs in the blood and improving stability;50 and 5) good safety.9 In many studies, PEG–PLA block copolymer nanoparticles were used as carriers for vaccine, protein, and gene drugs, particularly in a sustained/controlled release drug delivery system and targeted-drug delivery system that could enhance drug efficacy and reduce drug resistance.51
Sustained and controlled release drug delivery system
PEG–PLA copolymer nanoparticles are mainly diffusion and degradation controlled release systems. Hydrophobic drugs mainly accumulate in the hydrophobic matrix. In diffusion controlled systems, drugs are dissolved or dispersed in PLA polymers, and the release rate is controlled by drug diffusion through a PLA matrix. The drugs adjacent to the membrane surface can be released smoothly, whereas drugs inside the membrane are required to be diffused to the membrane surface first and then are released successfully. In the degradation controlled system, drugs are dispersed in PLA, and the drug release rate is determined by degradation rate due to influences from PLA chain length, drug loading of nanoparticles, release medium, and other factors.
PEG–PLA and PLA nanoparticle mixture loaded with betamethasone disodium phosphate was prepared by Ishihara et al.52 Taking inflammation mice as a model, the anti-inflammatory activity was found to be partly weakened by nanoparticles. With in vitro detection on nanoparticle degradation, PEG chains on the surface of nanoparticles disappeared in a few days and then the drug was slowly released. These nanoparticles accumulated at inflammation and injury locations preferentially, and a large amount of drugs was gradually degraded from these locations with the duration of more than 14 days.
PEG–PLA nanoparticles do not change the spatial configuration of proteins, antigens, and other bioactive substances to maintain their biological activities. Meanwhile, PEG–PLA copolymer nanoparticles loaded with proteins may avoid the degradation of proteins by proteases in the blood, reduce the recognition of immune cells, and enhance stability, so that protein inactivation is not able to occur easily. Thus, they are particularly suitable as a biotech drug carrier, especially for oral controlled release delivery of proteins and enzymes.53,54 The protein drug and gene therapeutic agents encapsulated in PEG–PLA block copolymer nanoparticles for sustained/controlled release may improve therapeutic effects of drugs and the quality of life of patients, and consequently the nanoparticles have become popular carriers for proteins and gene drugs. For example, Rafat et al15 evaluated PEG–PLA microparticles for encapsulation and delivery of a transactivator of transcription-enhanced green fluorescent protein fusion (Tat-EGFP) to retinal cells. The results suggested that PEG–PLA microparticles can deliver proteins in cell culture, allowing protein internalization in as little as 1 h. In vivo, protein was shown to localize within the photoreceptor layer of the retina and persist for at least 9 weeks with no observed toxicity.
Targeted drug delivery system
PEG–PLA nanoparticles with a narrow distribution of particle size have a smaller particle size than PLA nanoparticles. Thus, they may accumulate easily in inflammation locations and then slowly release the drugs. In particular, targeting molecules can be introduced in the PEG terminal to enhance active targeting. Therefore, the combination of passive and active targeting can act effectively on lesion locations. The carboxy-PEG–PLA block copolymer was synthesized by Ueki et al55 and was used to prepare camptothecin nanoparticles. The results showed that nanoparticles can effectively improve the delivery efficiency of camptothecin to the tumor location. Wang et al56 successfully prepared combretastatin A4(CA4)-loaded nanomicelles. Arg–Gly–Asp (RGD) peptides were coupled to the surface of nanomicelles. It was concluded that RGD-targeted micelles significantly enhanced the cell uptake of encapsulated drug in angiogenic tumor endothelial cells, which also resulted in increased antiproliferative activity of antivascular agent. Additionally, a lectin-PEG–PLA nanoparticle drug delivery system was established by Gao et al.44 The retention of the biorecognitive activity of lectin after the covalent coupling procedure was confirmed by hemagglutination test. Taking coumarin as a fluorescent marker, the results showed that the nanoparticles modified by lectin could be quickly delivered into the brain by nasal administration, and the brain’s uptake of coumarin carried by lectin-functionalized nanoparticles was about two-fold in different brain tissues compared with that of coumarin incorporated in the unmodified ones. In the meantime, it has a higher application security. Due to the hydrophilic chain of PEG, it can stay longer in the nasal cavity and facilitate nanoparticles through cell transit. Moreover, after linking modifiers of special biological activity in the PEG–PLA chain end, such as peptide, amino acid, and protein,57–59 drugs in PEG–PLA nanoparticles can enter the brain through the blood–brain barrier.
Conclusions and prospects
In conclusion, with their biodegradability, good biocompatibility, and amphiphilic characteristics, PEG–PLA block copolymers can be prepared into various nanoparticles. By adjusting the content and Mw of PLA and PEG and the PEG/PLA ratio, the block copolymers can increase the drug loading and encapsulation efficiency of hydrophobic drugs, reduce particle sizes, avoid recognition by the reticuloendothelial system, and prolong blood circulation time. However, the long circulating property of PEG–PLA block copolymer nanoparticles is required to be improved further. Additionally, due to small particle sizes and large surface areas of nanoparticles with surface adsorption, the first-pass effect still exists, and blood clearance can also be accelerated. Otherwise, the synthesis of PEG–PLA block copolymer by the lactide ring-opening polymerization method is expensive and not suitable for large-scale production, whereas the polymer prepared by a direct method has a lower Mw with a wider distribution. The current studies on PEG–PLA nanoparticles are still performed in the laboratory, and there is a long way to go on how to make technologies more stable and mature and ultimately promote large-scale production for clinical application. PEG–PLA nanoparticles can be expected to provide more tools and possibilities for the clinical treatment of diseases with broad application prospects in pharmaceutical preparations.
Acknowledgment
This work was supported by grants from the Foundation of Zhejiang Science and Technology Department (2009C33005), National Natural Science Foundation of China (81001647), China Postdoctoral Science Foundation (20100471757), and National Natural Science Foundation of China (20906016).
Footnotes
Disclosure
The authors report no conflicts of interest. The authors are solely responsible for the content and writing of the article.
References
- 1.Lee WC, Li YC, Chu IM. Amphiphilic poly(D,L-lactic acid)/poly(ethylene glycol)/poly(D,L-lactic acid) nanogels for controlled release of hydrophobic drugs. Macromol Biosci. 2006;6(10):846–854. doi: 10.1002/mabi.200600101. [DOI] [PubMed] [Google Scholar]
- 2.Kulkarni RK, Pani KC, Neuman C, Leonard F. Polylactic acid for surgical implants. Arch Surg. 1966;93(5):839–843. doi: 10.1001/archsurg.1966.01330050143023. [DOI] [PubMed] [Google Scholar]
- 3.Kim K, Yu M, Zong X, et al. Control of degradation rate and hydrophilicity in electrospun non-woven poly(D,L-lactide) nanofiber scaffolds for biomedical applications. Biomaterials. 2003;24(27):4977–4985. doi: 10.1016/s0142-9612(03)00407-1. [DOI] [PubMed] [Google Scholar]
- 4.Gref R, Lück M, Quellec P, et al. Stealth’ corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf B Biointerfaces. 2000;18:3–4. 301–313. doi: 10.1016/s0927-7765(99)00156-3. [DOI] [PubMed] [Google Scholar]
- 5.Bradley AJ, Murad KL, Regan KL, Scott MD. Biophysical consequences of linker chemistry and polymer size on stealth erythrocytes: size does matter. Biochim Biophys Acta. 2002;1561(2):147–158. doi: 10.1016/s0005-2736(02)00339-5. [DOI] [PubMed] [Google Scholar]
- 6.Zhu A, Lu P, Wu H. Immobilization of poly([var epsilon]-caprolactone)-poly(ethylene oxide)-poly([var epsilon]-caprolactone) triblock copolymer on poly(lactide-co-glycolide) surface and dual biofunctional effects. Appl Surf Sci. 2007;253(6):3247–3253. [Google Scholar]
- 7.Hu DSG, Liu HJ. Effect of soft segment on degradation kinetics in polyethylene glycol/poly (L-lactide) block copolymers. Polym Bull. 1993;30(6):669–676. [Google Scholar]
- 8.Younes H, Cohn D. Phase separation in poly(ethylene glycol)/poly(lactic acid) blends. Eur Polym J. 1988;24(8):765–773. [Google Scholar]
- 9.Ignatius AA, Claes LE. In vitro biocompatibility of bioresorbable polymers: poly(L,DL-lactide) and poly(L-lactide-co-glycolide) Biomaterials. 1996;17(8):831–839. doi: 10.1016/0142-9612(96)81421-9. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Li Y, Chen X, et al. Synthesis and characterization of the paclitaxel/MPEG-PLA block copolymer conjugate. Biomaterials. 2005;26(14):2121–2128. doi: 10.1016/j.biomaterials.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 11.Kricheldorf HR, Hachmann-Thiessen H, Schwarz G. Telechelic and star-shaped poly(L-lactide)s by means of bismuth(III) acetate as initiator. Biomacromolecules. 2004;5(2):492–496. doi: 10.1021/bm030064c. [DOI] [PubMed] [Google Scholar]
- 12.Stefani M, Coudane J, Vert M. In vitro ageing and degradation of PEG-PLA diblock copolymer-based nanoparticles. Polym Degrad Stab. 2006;91(11):2554–2559. [Google Scholar]
- 13.Otsuka H, Nagasaki Y, Kataoka K. Surface characterization of functionalized polylactide through the coating with heterobifunctional poly(ethylene glycol)/polylactide block copolymers. Biomacromolecules. 2000;1(1):39–48. doi: 10.1021/bm990005s. [DOI] [PubMed] [Google Scholar]
- 14.Pulkkinen M, Pikkarainen J, Wirth T, et al. Three-step tumor targeting of paclitaxel using biotinylated PLA-PEG nanoparticles and avidin-biotin technology: formulation development and in vitro anticancer activity. Eur J Pharm Biopharm. 2008;70(1):66–74. doi: 10.1016/j.ejpb.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 15.Rafat M, Cléroux CA, Fong WG, et al. PEG-PLA microparticles for encapsulation and delivery of Tat-EGFP to retinal cells. Biomaterials. 2010;31(12):3414–3421. doi: 10.1016/j.biomaterials.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 16.Li XR, Yuan XY. Poly(ethylene glycol)-poly(lactic acid) copolymers for drug carriers. Prog Chem. 2007;19(6):973–981. [Google Scholar]
- 17.Salem AK, Cannizzaro SM, Davies MC, et al. Synthesis and characterisation of a degradable poly(lactic acid)-poly(ethylene glycol) copolymer with biotinylated end groups. Biomacromolecules. 2001;2(2):575–580. doi: 10.1021/bm010030+. [DOI] [PubMed] [Google Scholar]
- 18.Letchford K, Burt H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: micelles, nanospheres, nanocapsules and polymersomes. Eur J Pharm Biopharm. 2007;65(3):259–269. doi: 10.1016/j.ejpb.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Xia H, Wang J, Li Y. High intensity focused ultrasound-responsive release behavior of PLA-b-PEG copolymer micelles. J Control Release. 2009;139(1):31–39. doi: 10.1016/j.jconrel.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 20.Zhan C, Gu B, Xie C, Li J, Liu Y, Lu W. Cyclic RGD conjugated poly(ethylene glycol)-co-poly(lactic acid) micelle enhances paclitaxel anti-glioblastoma effect. J Control Release. 2010;143(1):136–142. doi: 10.1016/j.jconrel.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Qi XR, Maitani Y, Nagai T. PEG-PLA diblock copolymer micelle-like nanoparticles as all-trans-retinoic acid carrier: in vitro and in vivo characterizations. Nanotechnology. 2009;20(5):055106. doi: 10.1088/0957-4484/20/5/055106. [DOI] [PubMed] [Google Scholar]
- 22.Jain JP, Kumar N. Development of amphotericin B loaded polymersomes based on (PEG)3-PLA co-polymers: factors affecting size and in vitro evaluation. Eur J Pharm Sci. 2010;40(5):456–465. doi: 10.1016/j.ejps.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Li S, Byrne B, Welsh J, Palmer AF. Self-assembled poly(butadiene)-b-poly(ethylene oxide) polymersomes as paclitaxel carriers. Biotechnol Prog. 2007;23(1):278–285. doi: 10.1021/bp060208+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hammady T, Rabanel JM, Dhanikula RS, Leclair G, Hildgen P. Functionalized nanospheres loaded with anti-angiogenic drugs: cellular uptake and angiosuppressive efficacy. Eur J Pharm Biopharm. 2009;72(2):418–427. doi: 10.1016/j.ejpb.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Venkatraman SS, Jie P, Min F, Freddy BYC, Leong-Huat G. Micelle-like nanoparticles of PLA-PEG-PLA triblock copolymer as chemotherapeutic carrier. Int J Pharm. 2005;298(1):219–232. doi: 10.1016/j.ijpharm.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 26.Pereira MA, Mosqueira VC, Vilela JM, Andrade MS, Ramaldes GA, Cardoso VN. PLA-PEG nanocapsules radiolabeled with 99m Technetium-HMPAO: release properties and physicochemical characterization by atomic force microscopy and photon correlation spectroscopy. Eur J Pharm Sci. 2008;33(1):42–51. doi: 10.1016/j.ejps.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Xu B. Nano Medicine. 1st ed. Beijing: 2004. [Google Scholar]
- 28.Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release. 2001;70:1–2. 1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 29.Niwa T, Takeuchi H, Hino T, Kunou N, Kawashima Y. Preparations of biodegradable nanospheres of water-soluble and insoluble drugs with D,L-lactide/glycolide copolymer by a novel spontaneous emulsification solvent diffusion method, and the drug release behavior. J Control Release. 1993;25:1–2. 89–98. [Google Scholar]
- 30.Lee HI, Wu W, Oh JK, et al. Light-induced reversible formation of polymeric micelles. Angew Chem. 2007;119(14):2505–2509. doi: 10.1002/anie.200604278. [DOI] [PubMed] [Google Scholar]
- 31.Nakayama M, Okano T, Miyazaki T, Kohori F, Sakai K, Yokoyama M. Molecular design of biodegradable polymeric micelles for temperature-responsive drug release. J Control Release. 2006;115(1):46–56. doi: 10.1016/j.jconrel.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Bae Y, Jang WD, Nishiyama N, Fukushima S, Kataoka K. Multifunctional polymeric micelles with folate-mediated cancer cell targeting and pH-triggered drug releasing properties for active intracellular drug delivery. Mol BioSyst. 2005;1(3):242–250. doi: 10.1039/b500266d. [DOI] [PubMed] [Google Scholar]
- 33.Murdan S. Electro-responsive drug delivery from hydrogels. J Control Release. 2003;92:1–2. 1–17. doi: 10.1016/s0168-3659(03)00303-1. [DOI] [PubMed] [Google Scholar]
- 34.Hu SH, Liu TY, Liu DM, Chen SY. Controlled pulsatile drug release from a ferrogel by a high-frequency magnetic field. Macromolecules. 2007;40(19):6786–6788. [Google Scholar]
- 35.Matsumoto J, Nakada Y, Sakurai K, Nakamura T, Takahashi Y. Preparation of nanoparticles consisted of poly(L-lactide)-poly(ethylene glycol)-poly(L-lactide) and their evaluation in vitro. Int J Pharm. 1999;185(1):93–101. doi: 10.1016/s0378-5173(99)00153-2. [DOI] [PubMed] [Google Scholar]
- 36.Yang ZL, Li XR, Yang KW, Liu Y. Amphotericin B-loaded poly(ethylene glycol)-poly(lactide) micelles: preparation, freeze-drying, and in vitro release. J Biomed Mater Res A. 2008;85(2):539–546. doi: 10.1002/jbm.a.31504. [DOI] [PubMed] [Google Scholar]
- 37.Blanco E, Bey EA, Dong Y, et al. Beta-Lapachone-containing PEG-PLA polymer micelles as novel nanotherapeutics against NQO1-overexpres sing tumor cells. J Control Release. 2007;122(3):365–374. doi: 10.1016/j.jconrel.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vila A, Sánchez A, Évora C, Soriano I, McCallion O, Alonso MJ. PLA-PEG particles as nasal protein carriers: the influence of the particle size. Int J Pharm. 2005;292:1–2. 43–52. doi: 10.1016/j.ijpharm.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Huh KM, Lee SC, Cho YW, Lee J, Jeong JH, Park K. Hydrotropic polymer micelle system for delivery of paclitaxel. J Control Release. 2005;101:1–3. 59–68. doi: 10.1016/j.jconrel.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Wu X, Han Y, Mo F, Duan Y, Li S. Novel thymopentin release systems prepared from bioresorbable PLA-PEG-PLA hydrogels. Int J Pharm. 2010;386:1–2. 15–22. doi: 10.1016/j.ijpharm.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 41.Ahmed F, Pakunlu RI, Srinivas G, et al. Shrinkage of a rapidly growing tumor by drug-loaded polymersomes: pH-triggered release through copolymer degradation. Mol Pharm. 2006;3(3):340–350. doi: 10.1021/mp050103u. [DOI] [PubMed] [Google Scholar]
- 42.Sant S, Poulin S, Hildgen P. Effect of polymer architecture on surface properties, plasma protein adsorption, and cellular interactions of pegylated nanoparticles. J Biomed Mater Res A. 2008;87(4):885–895. doi: 10.1002/jbm.a.31800. [DOI] [PubMed] [Google Scholar]
- 43.Sasatsu M, Onishi H, Machida Y. In vitro and in vivo characterization of nanoparticles made of MeO-PEG amine/PLA block copolymer and PLA. Int J Pharm. 2006;317(2):167–174. doi: 10.1016/j.ijpharm.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 44.Gao X, Tao W, Lu W, et al. Lectin-conjugated PEG-PLA nanoparticles: preparation and brain delivery after intranasal administration. Biomaterials. 2006;27(18):3482–3490. doi: 10.1016/j.biomaterials.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 45.Yu DH, Lu Q, Xie J, Fang C, Chen HZ. Peptide-conjugated biodegradable nanoparticles as a carrier to target paclitaxel to tumor neovasculature. Biomaterials. 2010;31(8):2278–2292. doi: 10.1016/j.biomaterials.2009.11.047. [DOI] [PubMed] [Google Scholar]
- 46.Tsai HC, Chang WH, Lo CL, et al. Graft and diblock copolymer multifunctional micelles for cancer chemotherapy and imaging. Biomaterials. 2010;31(8):2293–2301. doi: 10.1016/j.biomaterials.2009.11.059. [DOI] [PubMed] [Google Scholar]
- 47.Jain AK, Goyal AK, Mishra N, Vaidya B, Mangal S, Vyas SP. PEG-PLA-PEG block copolymeric nanoparticles for oral immunization against hepatitis B. Int J Pharm. 2010;387:1–2. 253–262. doi: 10.1016/j.ijpharm.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 48.Wei Q, Wei W, Tian R, Wang LY, Su ZG, Ma GH. Preparation of uniform-sized PELA microspheres with high encapsulation efficiency of antigen by premix membrane emulsification. J Colloid Interface Sci. 2008;323(2):267–273. doi: 10.1016/j.jcis.2008.04.058. [DOI] [PubMed] [Google Scholar]
- 49.Lim Soo P, Cho J, Grant J, Ho E, Piquette-Miller M, Allen C. Drug release mechanism of paclitaxel from a chitosan-lipid implant system: effect of swelling, degradation and morphology. Eur J Pharm Biopharm. 2008;69(1):149–157. doi: 10.1016/j.ejpb.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 50.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:1–2. 271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 51.Molina J, Urbina J, Gref R, Brener Z, Rodrigues JM., Junior Cure of experimental Chagas’ disease by the bis-triazole DO870 incorporated into ‘stealth’ polyethyleneglycol-polylactide nanospheres. J Antimicrob Chemother. 2001;47(1):101–104. doi: 10.1093/jac/47.1.101. [DOI] [PubMed] [Google Scholar]
- 52.Ishihara T, Takahashi M, Higaki M, Mizushima Y, Mizushima T. Preparation and characterization of a nanoparticulate formulation composed of PEG-PLA and PLA as anti-inflammatory agents. Int J Pharm. 2010;385:1–2. 170–175. doi: 10.1016/j.ijpharm.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 53.Simone EA, Dziubla TD, Arguiri E, et al. Loading PEG-catalase into filamentous and spherical polymer nanocarriers. Pharm Res. 2009;26(1):250–260. doi: 10.1007/s11095-008-9744-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takeda M, Maeda T, Ishihara T, et al. Synthesis of prostaglandin E1 phosphate derivatives and their encapsulation in biodegradable nanoparticles. Pharm Res. 2009;26(7):1792–1800. doi: 10.1007/s11095-009-9891-5. [DOI] [PubMed] [Google Scholar]
- 55.Ueki K, Onishi H, Sasatsu M, Machida Y. Preparation of carboxy-PEG-PLA nanoparticles loaded with camptothecin and their body distribution in solid tumor-bearing mice. Drug Dev Res. 2009;70(7):512–519. [Google Scholar]
- 56.Wang Y, Yang T, Wang X, Wang J, Zhang X, Zhang Q. Targeted polymeric micelle system for delivery of combretastatin A4 to tumor vasculature in vitro. Pharm Res. 2010;27(9):1861–1868. doi: 10.1007/s11095-010-0184-9. [DOI] [PubMed] [Google Scholar]
- 57.Ren WH, Chang J, Yan CH, et al. Development of transferrin functionalized poly(ethylene glycol)/poly(lactic acid) amphiphilic block copolymeric micelles as a potential delivery system targeting brain glioma. J Mater Sci Mater Med. 2010;21(9):2673–2681. doi: 10.1007/s10856-010-4106-5. [DOI] [PubMed] [Google Scholar]
- 58.Hu K, Li J, Shen Y, et al. Lactoferrin-conjugated PEG-PLA nanoparticles with improved brain delivery: in vitro and in vivo evaluations. J Control Release. 2009;134(1):55–61. doi: 10.1016/j.jconrel.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 59.Parikh T, Bommana MM, Squillante E., 3rd Efficacy of surface charge in targeting pegylated nanoparticles of sulpiride to the brain. Eur J Pharm Biopharm. 2010;74(3):442–450. doi: 10.1016/j.ejpb.2009.11.001. [DOI] [PubMed] [Google Scholar]