Abstract
Calorie restriction (CR) is the only dietary intervention that repeatedly extends both median and maximal lifespan in a broad range of species. Although there has been considerable interest in CR and its ability to retard aging, the mechanism has remained elusive. In contrast to studies in rodent and nonmammalian systems that are now beginning to provide mechanistic insights into how CR promotes longevity, the efficacy of CR in delaying primate aging has yet to be fully demonstrated. Here we review some of the insights from CR studies in short-lived species. We describe the advantages of using the rhesus monkey as a model for human aging and detail how CR can be successfully implemented in this species. We discuss the findings from our ongoing longitudinal study and outline the effects to date of CR on rhesus monkey health. Finally, we highlight the importance of primate studies in the context of aging research and its potential to advance our understanding of human aging and health. Antioxid. Redox Signal. 14, 229–239.
Introduction
Calorie restriction (CR) offers a powerful way to explore mechanisms of aging, because it is the only environmental intervention that repeatedly and strongly increases maximum lifespan and retards aging in laboratory rodents (78). The beneficial effects depend on chronic CR without malnutrition. Although rodent models in which CR is known to retard aging have long been emphasized, there is great interest in studying aging retardation in nonmammalian species on CR-like conditions. Short-lived species are ideal for the investigation of the underlying mechanisms of CR due to the relative simplicity of the model and the relative low cost and time required to conduct longevity studies. However, there is an issue of translatability with nonmammalian systems where there are fundamental differences in aging dynamics and manifestations of aging. Even for mammals, the difference between lifespans of only a few years and lifespans measured in decades are likely to extend to multiple aspects of physiology. The rhesus monkey model is well suited to study human aging because unlike human studies, environment, dietary intake, and medical history can be fully described. Patterns of eating and sleeping behavior of rhesus monkeys mirror those of humans. Further, studies can be designed to facilitate comprehensive monitoring of subjects and strict adherence to the study protocol. Data from the University of Wisconsin study are now emerging that point to the efficacy of CR in stemming the onset of age-associated disease in rhesus monkeys, suggesting that the effect of CR in extending lifespan may also be conserved in nonhuman primates. The value of the rhesus monkey model will be more fully appreciated as we begin studies to test candidate mechanisms for aging retardation that have been identified in short-lived species. Increased understanding of the mechanistic basis for the aging process in rhesus monkey will be extremely illuminating for human aging and may be the critical turning point for novel approaches in preventative human health care.
Insights into Aging Retardation by CR in Short-Lived Species
Among the most frequently studied model organisms in CR and aging research are Saccharomyces cerevisiae (yeast), Caenorhabditis elegans (worms), Drosophila melanogaster (fruit flies), mice, and rats. The choice of species to explore the mechanisms of aging retardation by CR represents a trade-off between ease of study, cost, questions being asked, and human translatability. Insights gleaned from human studies would obviously be directly applicable to questions of human health; however, there are cost, experimental design, and ethical considerations that limit the utility of human studies to discover mechanistic insights. For many of the CR studies in nonmammalian systems it is not plausible to measure precisely how much food each individual consumes in a survival study. Nonetheless, the response to changes in nutrient conditions, the key stimulus at the core of the mechanism of CR, is likely to be highly conserved and factors identified in short-lived species may be directly relevant to primate aging. Regulation of energy metabolism is a major theme emerging from these studies. Here we briefly describe studies in several species that support a role for alterations in metabolism in the mechanisms of CR.
Yeast
Replicative aging of the yeast S. cerevisiae is associated with alterations in metabolism consistent with deregulation of glucose signaling, and this is prevented by CR as implemented by growth in a low-glucose environment (51). Further, CR is associated with a shift in metabolism toward respiration, and lifespan extension by CR requires functional mitochondria (50). The involvement of mitochondria in the response to CR is confirmed by transcriptional profiling, where 31% of genes altered by CR in wild-type yeast encode mitochondrial proteins (49). The nicotinamide adenine dinucleotide–dependent deacetylase Sir2 (50) and the metabolic pathway for regeneration of nicotinamide adenine dinucleotide have also been implicated in the mechanism of CR (1). The target of rapamycin (Tor) signaling pathway is a critical regulator of cell growth and metabolism (54). Inhibition of Tor signaling negatively impacts ribosome biogenesis, induces stress-responsive transcription, and extends replicative lifespan (46, 55). In yeast, Tor signaling has been implicated in the mechanism of lifespan extension by CR. Growth in low glucose also increases chronological lifespan, the time that cells can survive in stasis in the absence of proliferation. In this yeast aging model, CR increases respiration but decreases reactive oxygen species (ROS) production relative to oxygen consumption (4). Genetic or pharmacological inhibition of respiration in CR cultures negatively impacts survival, arguing that the shift to respiration is required for extension of chronological lifespan (70). A number of factors involved in regulation of chronological lifespan have been identified, including Sch9, glucose signaling kinase, and homologues of AKT and S6K (75), Msn2 and Msn4, stress/nutrient limitation activated kinases (27), and nutrient activated kinase Tor1 (11, 76). Inhibition of Tor by nutrient limitation or by rapamycin increases chronological lifespan in yeast in a manner that is dependent on the stress-activated transcription factors, Msn2 and Msn4 (76). It is of interest that reduced Tor signaling causes an increase in respiration and upregulates mitochondrial gene expression (11).
Worms
The nematode C. elegans has been widely used as a model organism for studying aging, and numerous single-gene mutations have been identified that extend lifespan (42). Foremost among these are genes involved in the insulin-signaling pathway (12). One of the earliest long-lived mutants identified was daf-2 (47), a mutant of the DAF-2 insulin-like receptor involved in dauer formation. In worms, CR implemented by either bacterial dilution or by growth in restricted axenic media act independently of the insulin/insulin-like growth factor-signaling pathway. Neither regimen requires the forkhead transcription factor DAF-16 (8), which is a determinant of lifespan extension by inhibition of insulin signaling. The mechanistic basis for lifespan extension by CR in worms is not well understood (42). A role for altered mitochondria energy metabolism is suggested in the mechanism of lifespan extension by bacterial reduction in the growth media because inhibitors of the electron transport chain prevent lifespan extension by this method (8).
Flies
In the fruit fly, D. melanogaster, genome-wide transcriptional analysis of pooled whole flies indicates that 23% of transcripts detected are altered with age (62). Genes involved in oxidative phosphorylation were among the most significant groups identified as being sensitive to aging with strong declines in expression observed. Further, activity of mitochondrial respiratory complexes also declines with age (58). Functional analysis of gene expression profiles point to a relationship between mitochondrial dysfunction, the stress response, and aging and suggest that muscle may be particularly sensitive to aging in Drosophila (31). In flies CR is implemented by using media in which the caloric composition is reduced. Production of ROS is reduced with CR, suggesting that mitochondrial metabolism is altered. Investigation of isolated mitochondria from flight muscle from CR animals indicates that although the mitochondrial density is not altered by CR, mitochondrial morphology and activity are significantly different (53). As in nematodes, lifespan is extended in flies by reduction of insulin signaling (17). Overexpression of the forkhead transcription factor Drosophila FOXO ortholog also extends lifespan in flies (44). Even though there is evidence to suggest that CR acts independently of FOXO transcription factors (41), factors downstream appear to be common to both insulin/insulin-like growth factor longevity pathways and lifespan extension by CR (16), in particular members of the nuclear receptor family and possibly factors influencing the TOR nutrient signaling pathway.
Mice
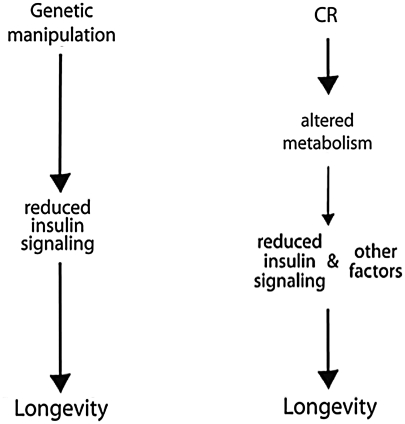
The vast majority of early work in CR involved studies in mice and rats (78). The effect of CR on average and maximal lifespan is long established in mice; however, the underlying mechanisms are not yet understood. The inverse correlation between caloric intake and maximum lifespan in mice suggests that regulators of metabolism may play an important role in the mechanism of CR (77). Successful CR involves reducing caloric intake while avoiding malnutrition, and most rodent CR studies start the diet early in life (3–16 weeks of age). Adult onset CR (12 months) also results in longer lifespan though to a lesser extent, and mice have a lower tumor incidence (77). Transcriptional profiling has revealed shifts in mitochondrial energy metabolism with CR in multiple tissues (2). CR has a striking effect on adipose tissue mass, morphology, and transcription in mice (37, 38). CR suppresses expression of over 50 genes in inflammation and promotes structural remodeling of the cytoskeleton, extracellular matrix, and vasculature (37). It is probable that reductions in systemic inflammatory tone caused by CR may underlie its ability to oppose a broad spectrum of age-associated diseases, including cancers and cardiovascular disease. Age-related changes in adiposity correlate with systemic oxidative stress in humans and mice, and in cultured adipocytes, elevation of fatty acids increased oxidative stress and cause dysregulated production of adipokines (30). The pharmacological induction of β-oxidation is currently being explored as a treatment for obesity and diabetes (10), both of which are prevented by CR. The reduction of blood glucose and enhanced insulin sensitivity in CR mammals hints at the involvement of altered insulin signaling in the mechanism of aging retardation. Genetic manipulations to reduce insulin signaling pathways have proven successful at extending maximum lifespan in other species (47); however, in mammals the effect is modest compared to CR (3). Although reduced levels of glucose and increased insulin sensitivity are hallmarks of CR in mice, monkeys, and humans, studies in nonmammalian systems indicate that CR is not mechanistically equivalent to lifespan extension by reduced insulin signaling (Fig. 1).
FIG. 1.
Calorie restriction (CR) is not mechanistically equivalent to lifespan extension by genetic manipulation leading to reduced insulin signaling. The insulin/insulin-like growth factor signaling pathway has been implicated in regulating longevity in numerous species. Transgenic, knockout, and mutant genetic variants have demonstrated that reduced signaling through this axis can increase lifespan in yeast (glucose signaling pathway), worms, flies, and mice. Studies of CR in these species reveal that there is evidence to support reduced signaling through this axis as an outcome of CR, but that the pathways for lifespan extension are not identical and that other factors distinct from insulin/insulin-like growth factor play a role in the mechanism of CR.
General insights from nonprimate CR studies
As illustrated in the examples above, CR induces an active response to altered nutrient availability that impacts mitochondrial energy metabolism and likely reflects the importance of maintenance of mitochondrial function with age (2). From an aging perspective, one of the more prominent aspects of mitochondrial function has been the generation of ROS. Oxidative damage accrues with age in multiple tissues and is attenuated in tissues from mice on CR (72). These and other observations led to the proposal of the oxidative stress theory of aging where damage arising from ROS is causative in the aging process (59). This model has been called in to question recently; numerous mouse models with transgenic overexpression or knock out of antioxidant molecules show negligible aging phenotypes despite decreased and increased oxidative damage respectively (61). Accumulating evidence suggests that ROS play a more complicated role in aging and disease vulnerability, where ROS are themselves signaling molecules (29). In this alternate view, changes in ROS signaling influence multiple aspect of cellular signaling, including the regulation of metabolism and inflammation, and the damage caused by changes in ROS production and clearance may be viewed as symptomatic rather than causative in aging.
Aging and CR studies in multiple organisms have led to the identification of a number of putative longevity factors that are currently the subject of intense research. A unifying feature of these interlinked factors is their nutrient sensitivity and their role in regulation of metabolism. Of note are (i) the Sirtuins (52), (ii) the forkhead transcription factors involved in regulation of stress and metabolism, a subset of which are regulated through insulin signaling (60), (iii) mammalian Tor (68), and (iv) peroxisome proliferator-activated receptor gamma coactivator 1α, a critical transcriptional coactivator of mitochondrial function that is responsive to changes in nutrient availability (66) and exercise (36). The conserved effect of CR on lifespan and metabolism in short-lived species suggests that nutrient sensitive metabolic regulators may also be important in primate aging and CR.
Macaca mulatta: A Highly Translational Model of Human Aging
Nonhuman primates are a vital link between basic research and clinical application in that findings from nonhuman primate studies are highly translatable to human health issues.
Genetic similarity
The rhesus monkey (M. mulatta) is the most popular nonhuman primate species for biomedical study and, with ∼93% sequence identity with the human genome, is phylogenetically very close to humans (Rhesus Genome Consortium; www.hgsc.bcm.tmc.edu/project-species-p-Rhesus Macaque.hgsc?pageLocation=Rhesus Macaque). This similarity extends to numerous aspects of their anatomy, physiology, neurology, endocrinology, immunology, and behavior.
Rate and manifestations of aging
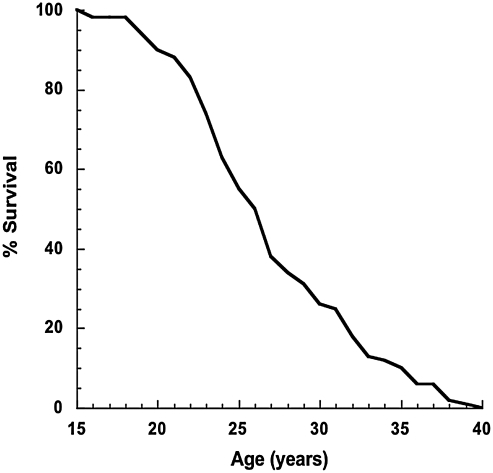
Rhesus monkeys exhibit both a realistic aging course, progressing through stages of life that are complimentary to the human life cycle, and a degree of time compression. Median lifespan of rhesus monkeys housed in captivity at the Wisconsin National Primate Research Center (WNPRC) is ∼26 years, and maximum lifespan under standard husbandry is ∼40 years of age (Fig. 2). In their late 20s, rhesus monkeys generally show signs of physical decline, such as reduced mobility, skin atrophy, facial pigmentation, and hair graying/thinning. They also develop many of the disorders common in old humans, including cancer, cataracts, osteopenia, and cardiovascular disease (74). In general, it is understood that macaques age at a rate of two and a half to three times that of humans (20). The maximum lifespan of a captive rhesus monkey appears to be 40 years compared to the maximum achieved lifespan in humans of ∼120 years. This conversion factor is appropriate in most general terms; however, care must be taken in applying this conversion factor across the lifespan for certain conditions, including menarche, adult stature, and menopause. For example, female rhesus monkeys undergo menarche at 2.5–3.5 years of age, which would convert to 7.5–10.5 human years by this generalized rule. In addition, adult stature in rhesus monkeys is reached at ∼8 years of age, which would convert to 17–24 human years. The relationship breaks down completely when considering onset of menopause between 26 and 28 years, which converts to 78–84 human years. Clearly, the rate of aging from birth to sexual maturity to old age among rhesus monkeys and humans is not parallel in this aspect. Despite this element of lifespan time compression, rhesus monkeys share many aging phenotypes with humans, are relatively long lived, and as such are the most suitable animal model in which to study aging.
FIG. 2.
Captive rhesus monkey survival curve. Percent survival in normally fed captive rhesus monkeys at the Wisconsin National Primate Research Center. Median survival is ∼26 years of age, 10% survival is ∼35 years of age, and maximum survival is ∼40 years of age (20).
Limitations of the model
As described above there are many reasons why nonhuman primates are the ideal translational model in which to study aging and interventions into the aging process. However, there are also limitations associated with the use of nonhuman primates. Aside from the obvious potential ethical and cost issues that may be associated with nonhuman primate work, there are issues involved in the evaluation of social and emotional aspects related to human aging. Further, specifically in the context of CR and aging, the tightly regulated diet of restricted animals makes evaluation of cognition and behavior challenging. While there are well-established protocols to evaluate cognition in nonhuman primates, food is used as a primary motivator. In addition to the strict limits that must be imposed on the use of food rewards in CR animals, consideration of data from cognitive and behavioral testing must include the likelihood that restricted and control animals will have differential levels of motivation. This is a limitation that can be challenging to overcome.
Implementing a CR Diet in Nonhuman Primates
The degree of lifespan extension afforded by CR is inversely proportional to caloric intake, and the earlier the diet is initiated, the more efficacious it is in extending mean and maximal lifespan. The standard CR diet in mice is generally implemented at 2 months of age when mice are sexually mature but are still less than half of their maximal body weight. The CR regimen that is most applicable to human aging is the adult onset model, where the diet is introduced at a later time point when animals are at peak mass or close to it. Ideally, therefore, a rhesus monkey study would be initiated in animals that are at least 8–10 years of age, and would continue throughout the natural lifespan. If moderate CR retards aging in a nonhuman primate species, this would be reflected in attenuated rates of change of most biological indicators of aging, increased health span and increased longevity. Below we discuss what we consider to be major considerations in study design that guide the practical aspects of conducting a CR study.
Numbers, gender, background, and origin
The considerable degree of individual genetic heterogeneity in available rhesus monkey populations is an important factor that does not arise in studies involving shorter-lived laboratory animals. Since rhesus monkey populations are not extensively inbred, there can be variation in measured parameters that can make data interpretation more challenging. However, given the degree of individual variance, trends that do emerge in aging or in response to CR are likely to be important and translatable to human aging. While the longitudinal nature of a CR and aging study will infer additional statistical power, both the innate degree of heterogeneity in rhesus monkey populations and the high degree of sexual dimorphism of this species need to be taken into account when designing rhesus monkey studies of CR. In addition, it is statistically important to include enough animals to maintain appropriate power despite subject dropout. These statistical considerations are particularly important given the long-term, involved nature of these types of studies and the associated costs involved.
Variability in dietary intake and long-term effects
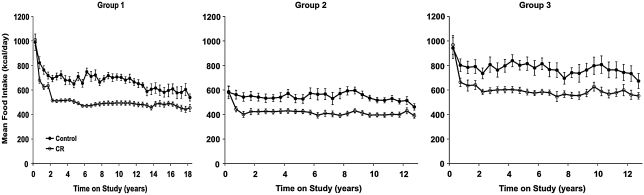
A key factor in the performance of a CR study is control over the diet. Particularly in rhesus monkeys, there is a large degree of individual variation in dietary intake that must be both accounted for and controlled for. For example, in the WNPRC CR study, ad libitum food intake levels were quantitated for each animal daily over a 3–6-month baseline period before initiation of the study. At the end of the baseline period, animals were randomly placed into either control or CR groups. For the CR animals, the individualized food allotments were then reduced by 10% per month for 3 months to reach the goal of a 30% CR (Fig. 3). This gradual approach, also an important component, ensures that the animals adjust to the lower levels of food available. A 30% reduction in caloric intake was chosen because it best balanced what was known to be effective in rodents with what was believed to be tolerable for monkeys. The actual makeup of the diet bears consideration as well. For example, should CR animals simply receive less of the same diet control animals are fed, or should the diets be altered in any way? In the WNPRC study, we chose to feed the CR and control animals a diet with similar macronutrient content (semipurified, lactalbumin, corn oil-based diet containing 15% protein, 10% fat, and 65% carbohydrates, ∼4 kcal/g). However, the CR diet is enriched by ∼30% in vitamins and minerals to prevent any micronutrient deficiencies (Table 1). Control animals have free access to food for 6–10 h per day and are maintained such that they generally have at least 20 g of food remaining each day. Food intake is quantified daily for all animals. All animals are fed once per day in the morning. Each afternoon, when food is removed and quantitated, all animals receive an allotment of food enrichment (e.g., fresh fruit) of ∼100 calories (18).
FIG. 3.
Food intake in control and CR animals. Food intake data are collected daily for each animal and averaged over a 6-month period. Food intake in CR animals is ∼30% less than their own individual baseline levels. Control animals are represented by closed circles and CR animals are represented by open circles. The drop in food intake during the first 2 years is assumed to be related to the switch from standard laboratory chow to the experimental diet. Data are presented as group mean ± standard error of the mean (SEM). All data are analyzed using a longitudinal repeated measures design as detailed previously (65).
Table 1.
Diet Composition (g/kg; Teklad, Madison, WI)
| Ingredient | Control | CR |
|---|---|---|
| Lactalbumina | 150 | 150 |
| Sucrose | 285 | 257 |
| Corn starch | 300 | 300 |
| Dextrin | 50 | 50 |
| Corn oil | 100 | 100 |
| Cellulose | 50 | 50 |
| Vitamin mixb | 10.0 | 14.3 |
| Calcium phosphate, dibasic | 21.6 | 30.9 |
| Calcium carbonate | 3.7 | 5.3 |
| Potassium citrate, monohydrate | 20.8 | 29.7 |
| Sodium chloride | 5.4 | 7.7 |
| Magnesium oxide | 2.0 | 2.9 |
| Ferric citrate | 1.2 | 1.7 |
| Manganese carbonate | 0.13 | 0.19 |
| Zinc carbonate | 0.06 | 0.09 |
| Cupric carbonate | 0.02 | 0.03 |
| Chromium potassium sulfate | 0.02 | 0.03 |
| Potassium iodate | 0.004 | 0.006 |
| Sodium selenite | 0.0004 | 0.0006 |
Protein content ∼88%–89%.
Teklad Vitain Mix #40060.
CR, calorie restriction.
Taking heterogeneity into account, ideally CR is based on individualized food intakes rather than predetermined food intake tables. This is particularly important given that the variability in food intakes that rhesus monkeys will self-select if given the opportunity. For example, within the first 6 months of the WNPRC study, as a group, the ad libitum fed control animals had a range of average daily food intakes from 570 to 1306 kcal. More recently, after 17.5 years on the study, the same animals ranged from 471 to 823 kcal. In addition, given the long-term nature of these studies, changes in self-selected food intake with advancing age need to be considered. In the WNPRC study we have chosen to deal with this eventuality by adjusting individual food allotments, in small increments (no >5% per month), to maintain animals near a previously determined ideal, healthy weight for that individual.
Influence of study environment
For controlled dietary intake experiments, animals must be singly housed and grouped in unisex rooms with other study monkeys with whom they have extensive visual and auditory contact. While not the optimal social situation for laboratory housed rhesus monkeys, for CR studies animals will ideally be housed in single cages to prevent aggression-related injuries that could impact the animal's health and aging profile, as well as to control access to food and allow collection of extremely accurate daily counts of each individual animal's food intake. While it is possible to group or pair house animals during the day and separate animals only for feeding, this will interfere with the animals' natural tendency to forage throughout the day. In accordance with nonhuman primate animal and use regulations, all animals must receive objects to enrich their home environment. The most commonly used forms of environmental enrichment for laboratory-housed rhesus monkeys include social living situations and food-based enrichment. Given the nature of CR studies, animals must be limited to nonfood types of enrichment. Ordinarily, a singly housed rhesus monkey would receive a minimum of 1 h of food-based enrichment per day; however, the calories that would be ingested from this type of enrichment would interfere with a CR study. There are other types of enrichment that these animals can receive such as auditory and visual stimuli from radios or televisions, olfactory stimuli in the form of scent-based products, and tactile devices such as cage toys that can be manipulated.
Health monitoring and intervention
The use of invasive techniques that could influence health and potentially confound the outcome is a major concern for longitudinal studies. Conversely, as animals present with various diseases and conditions, the decision must be made whether to provide care and treatment for animals with acute or chronic health conditions that would generally be treated in human medicine. This decision to intervene, as a clinician would do for a human patient, does not prevent extrapolation of the findings to predict the efficacy of a CR intervention in humans. There are several considerations that go into the treatment of animals as individual patients: (i) at what point, and if, to treat chronic conditions; (ii) whether to provide preventive treatment for conditions that may or may not occur, for example, diverticulosis, an effective treatment for this is increasing fiber intake. However, if all animals are treated with fiber before the development of the disease, does this influence the study outcomes?
Findings of the WNPRC CR Study
This project began in 1989 as a National Institute on Aging (NIA)–funded R01 involving 30 Indian-origin male rhesus monkeys between the ages of 8 and 14 years (Table 2). Subsequently, in 1994 the study was expanded to include 30 female Indian rhesus monkeys (8–14 years of age) and an additional group of 16 male Indian rhesus monkeys (6–14 years of age) (Table 2) (63). The major overarching goals of this study are to better understand the normal aging process in rhesus monkeys and to determine if a reduced calorie diet can extend the healthy period of life in addition to median and maximal lifespan in our model. To effectively address these questions, it is of utmost importance that we maintain the animals as stress free as possible. To accomplish this, we have designed our study to be as noninvasive as possible, while still allowing us to collect appropriate data for evaluation.
Table 2.
Group Ages and Body Weights at Study Onset and Current
| |
|
|
Study onset |
Current |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diet | Year | n | Age (years), mean (SEM) | Range (years) | Weight (kg), mean (SEM) | Assessment period (years) | n | Age (years), mean (SEM) | Range (years) | Weight (kg), mean (SEM) | |
| Group 1 | Control | 1989 | 15 | 9.7 (0.5) | 8.0–13.6 | 11.3 (0.5) | 18 | 5 | 27.2 (0.7) | 26.0–29.8 | 11.9 (1.4) |
| CR | 15 | 9.5 (0.5) | 7.9–14.0 | 11.3 (0.4) | 8 | 27.3 (0.7) | 26.0–31.2 | 9.8 (0.4) | |||
| Group 2 | Control | 1994 | 15 | 11.2 (0.5) | 8.7–14.6 | 7.3 (0.4) | 12.5 | 9 | 23.1 (0.5) | 21.4–26.3 | 9.1 (0.7) |
| CR | 15 | 11.3 (0.5) | 8.5–13.9 | 7.1 (0.3) | 10 | 24.4 (0.6) | 21.2–26.5 | 7.5 (0.4) | |||
| Group 3 | Control | 1994 | 8 | 10.3 (1.2) | 6.5–14.2 | 11.3 (0.7) | 12.5 | 5 | 21.9 (1.3) | 19.1–25.8 | 14.3 (1.4) |
| CR | 8 | 10.1 (1.1) | 6.6–14.2 | 11.4 (0.9) | 7 | 22.0 (1.1) | 19.1–26.7 | 10.3 (0.4) | |||
SEM, standard error of the mean.
The impact of age on circulating factors is opposed by CR
The complete blood count is a standard method routinely used to assess human health. Analysis of the quantity and distribution of blood cells provides an insight into the systemic health of the individual. Mean complete blood count values for all groups fell within age-specific normal ranges for rhesus monkeys and there was no consistent effect of CR. It is noteworthy that the number of circulating lymphocytes is thus far unaltered by CR, which, in rodents, induces a profound lymphopenia (78). Serum chemistry values for all animals have fallen with age within normal ranges for rhesus monkeys. Occasional, transient elevations in some liver enzymes are the exception to this trend. Alanine aminotransferase and aspartate aminotransferase have been elevated in both the control and CR animals; however, corresponding liver pathologies are not present. We continue to monitor these parameters, although the significance of these changes is not clear and differences have not been consistent across time.
Total plasma cholesterol concentrations, high-density lipoprotein (HDL) cholesterol, and low-density lipoprotein (LDL) cholesterol have been similar for control and CR animals, whereas plasma triglyceride (TG) levels have been significantly lower in CR animals than in controls. Further, we found that CR-induced compositional changes in LDL reduced their participation in a potentially atherogenic interaction (25). LDL particles from CR animals had a lower molecular weight and were depleted in TG and phospholipids. In addition, LDL binding with arterial proteoglycan was lower.
In contrast to cholesterol, where reduced levels with CR were detected only in very-low-density lipoprotein (VLDL), the lower plasma TG levels previously reported in CR animals reflect a decrease in TGs of all lipoprotein classes with CR. The impact of CR on lipoprotein metabolism is gender specific, with CR having a greater influence on males than on females (26). Reduced plasma lipoprotein A levels in males present an additional antiatherogenic modification of plasma lipids by CR to levels similar to those measured in CR females.
Metabonomics is a systems biology approach for quantitative analysis of the effect of a treatment on metabolites. Proton nuclear magnetic resonance metabonomic analysis detected a distinct age-induced metabonomic signature that was attenuated by long-term CR (65). The age-associated reduction in plasma levels of creatinine that was detected likely reflects differences in body composition between the control and CR groups. Branched chain amino acids are oxidized peripherally and serve as a fuel source to decrease protein degradation and to stimulate protein synthesis. We observed an age-dependent decrease in branched-chained amino acids (leucine, isoleucine, and valine) similar to what has been seen in humans (14, 67) that may indicate a reduced contribution of muscle to total body protein metabolism. Importantly, CR opposed these changes. Higher levels of other free amino acids, particularly serine, tyrosine, and glutamate, were detected in CR animals, suggesting that protein turnover rate is preferentially maintained (73). The maintenance of plasma serine, tyrosine, and glutamate along with choline and glycerylphosphorylcholine, metabolites important for neurotransmitter biosynthesis and brain function, may have relevance to the preservation of neurological function observed with CR (45).
The plasma lipoprotein profile is affected by age where we observed increased TG, decreased HDL levels, and increased VLDL. This profile is recognizable in humans as pro-atherogenic and is common to metabolic syndrome and/or diabetes. CR attenuates these age-associated changes in plasma lipoproteins, including an increase HDL. Metabonomic analysis also revealed distinct metabolic trajectories that correlated with higher insulin sensitivity, and were marked by higher levels of gluconate and acetate. These alterations are suggestive of a CR-modulated increase in metabolic flux through the pentose phosphate pathway.
CR opposes age-associated changes in body composition
Body composition is a measure of energy economy reflecting the difference between energy intake and expenditure. Alterations in body composition are directly associated with some pathologies, including obesity, sarcopenia, and osteoporosis. As such, measurement of body composition may be used as an excellent indicator of health risk.
Altered body composition is a well-established component of human aging and is detectable with imaging techniques in rhesus monkeys. With age, the ratio of fat mass to lean mass tends to increase, even with constant weight, leading to an overall increase in percent body fat. In women this is linked, in part, to postmenopausal estrogen deficiency. Importantly, in humans the size of the visceral fat depot increases with age. Rhesus monkeys show similar age-related trends (22, 43). The dramatic increase in the numbers of overweight and obese individuals in human populations has revealed that increased adiposity can precipitate diseases previously associated with age. The redistribution of fat with aging results in a high ratio of visceral to subcutaneous fat and is associated with deregulated adipose function. Increased body fat, especially visceral adiposity, is related to cardiovascular and glucoregulatory risks in human. This relationship is also observed in nonhuman primates (25, 34). Adipose dysfunction has been implicated in the development of chronic systemic inflammation, which may be a critical factor in the onset of age-associated disease. The role of adipose tissue as an endocrine organ has recently emerged with the identification of adipose secreted factors (adipokines). Adipogenic signaling influences energy balance and integrates glucose homeostasis. In addition, adipose-derived factors including adiponectin and leptin mediate many aspects of inflammation.
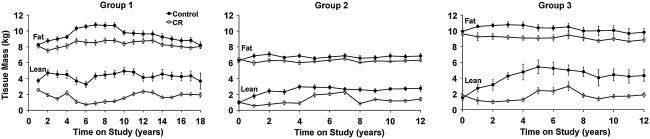
As expected, control and CR groups differ significantly in body weight attributable primarily to continued weight gain by control animals (22). Dual-energy X-ray absorptiometry (DXA) data suggest that these differences in body weight are largely attributable to differences in body fat (Fig. 4) (22, 23). Specifically, we have found a marked reduction in truncal body fat in our CR monkeys (22, 23), indicated by smaller abdominal skinfold thicknesses and decreased abdominal circumferences, in conjunction with lower total body fat (Fig. 4) and abdominal fat. In adipose tissue, metabolism and inflammation are intimately linked. The effect of CR on white adipose tissue in mice is particularly striking; adiposity is reduced, multiple metabolic pathways become activated, and expression of genes involved in inflammation is reduced (37, 38). It will be interesting to determine if these effects are also observed in adipose tissue from nonhuman primates on CR and if the same metabolic shifts are observed.
FIG. 4.
DXA-measured total body fat and lean mass in control and CR animals. Differences in fat mass between control and CR animals account for the majority of the difference in body weight between the diet groups. Control animals are represented by closed circles and CR animals are represented by open circles. Data are presented as group mean ± SEM. All data are analyzed using a longitudinal repeated measures design as detailed previously (22, 23).
Sarcopenia is delayed in skeletal muscle from CR animals
Approximately 45% of the older (>60 years) U.S. population develops sarcopenia: the loss of muscle mass that is related to disability, increased risk of injury, decreased quality of life, and increased healthcare expenditures for disability and mortality. The decrease in mass presents as reduced muscle cross-sectional area and has been attributed to a combination of muscle fiber loss and fiber atrophy. Similar to humans, rhesus monkeys lose significant skeletal muscle mass with advancing age (21). The original 30 adult males in the WNPRC study were examined over 17 years for changes in DXA-estimated skeletal muscle mass (sum of the lean mass from the arms and legs). A comparison of control and CR animals revealed that body weight adjusted skeletal muscle mass declined more rapidly in the control group than in the CR group. By ∼16 years of age both groups of animals began to show a decline in adjusted estimated skeletal muscle mass. These data demonstrate that moderate, adult-onset CR can attenuate sarcopenia in a nonhuman primate model (19). Although there appears to be a clear relationship between muscle mass and muscle strength that further translates into functionality, this has not been directly tested in our study.
Age-associated glucoregulatory dysfunction is prevented by CR
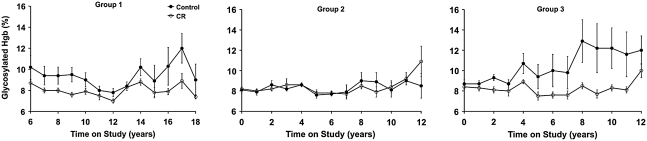
Insulin levels are potently lowered by short- and long-term CR in nonhuman primates (34). The extent to which this effect promotes health and delays aging is not clear, although the positive influence of CR on glycosylated hemoglobin values (Fig. 5) is further evidence for the positive effect of CR on long-term glucoregulatory function. Although targeted and environment-induced blunting of insulin or insulin-like signaling significantly increases lifespan in other species (35, 39), the mechanism does not appear to be equivalent to CR.
FIG. 5.
Glycosylated hemoglobin measurements in control and CR animals. Glycosylated hemoglobin is a measurement of long-term glucose control. Control animals are represented by closed circles and CR animals are represented by open circles. Data are presented as group mean ± SEM. All data are analyzed using a longitudinal repeated measures design as detailed previously (35).
An age-related decline in glycemic control, characterized by a prolonged disposal of blood glucose after an oral or intravenous glucose challenge, may underlie the elevated risk for and progression toward type 2 diabetes in humans. Several large-scale studies of humans have quantified this decline and suggest that it occurs in >40% of adults >60 years of age and that outright diabetes occurs in ∼50% of these individuals (15). Although the etiology of this age effect is multifaceted (69), an initial step appears to be a decline in the sensitivity of exogenous tissues to insulin. At this early stage, compensatory hypersecretion of insulin by pancreatic β-cells is sufficient to maintain glucose homeostasis, but ultimately, pancreatic β-cells fail to secrete insulin in sufficient quantity to compensate for peripheral insulin resistance (64). This is likely due to changes in pancreatic β-cell function, including reduced sensitivity to circulating glucose and/or decreased insulin secretory capacity (56). Age-related changes in both whole-body insulin sensitivity and pancreatic secretory capacity are conveniently estimated by applying minimal model analysis of data obtained from a frequently sampled intravenous glucose tolerance test (7). This approach allows the health of the pancreatic β-cell to be tracked longitudinally using the disposition index, which is the mathematical product of the insulin sensitivity index (SI) and the acute insulin response to a glucose challenge (27). The disposition index does not differ between control and CR monkeys in this study, as an elevated insulin secretion appears to have compensated for a decline in SI in control monkeys (34).
Impaired glycemic control has also been associated with increased adiposity. This relationship has been established in nonhuman primates (9) and in humans (71), and dietary modifications geared toward a reduction in adiposity are the first line of defense against attenuating the onset of insulin resistance in humans. Indeed, one of the most striking health benefits of CR is an increase in whole-body insulin sensitivity that parallels the loss of the amount of total body fat (34). The amount of visceral adipose tissue in particular appears to be one of the strongest predictors of whole-body insulin sensitivity, possibly due to its anatomical location, wherein liver is directly exposed to fatty acids liberated from this depot (57). Further, there is good evidence that reductions in visceral adipose tissue improve insulin sensitivity in rats (5, 6) and humans (13). The role of CR in specifically modulating the mass of this depot has not been elucidated in either nonhuman primates or humans. The intracellular accumulation of lipid in nonadipose tissues such as muscle and liver has also been implicated as a factor affecting peripheral tissue glucose uptake (24, 48), and weight loss may improve SI, in part, through depletion of TGs from peripheral tissues (40). The depletion of adipose- and peripheral tissue-derived lipids is most often measured in separate publications, and studies that relate body-wide changes in adipose depots with alterations in glycemic control in a single individual are needed.
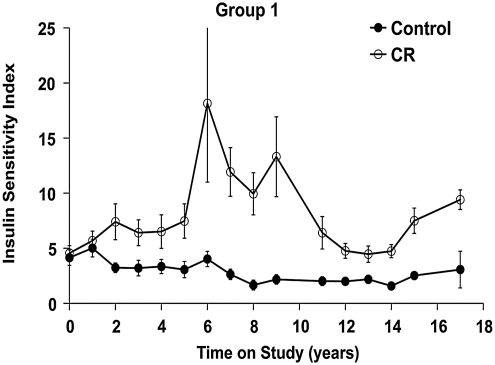
The SI has been a consistent biomarker of CR (Fig. 6). We compared SI and glucose effectiveness (KG) as estimated from three minimal models using frequently sampled intravenous glucose tolerance test data from the initial group of males, after 10 years on either the control or CR diet. We found values, levels of precision of estimation, and inter-model relationships among SI and KG to be in the ranges of those reported previously for humans and dogs (32). This suggests that the models we tested should provide valid estimates for rhesus monkeys as well. The one-compartment minimal model provides a slightly more precise estimate of SI than do version 3 of the minimal model or the two-compartment minimal model. The similarity of values and relationships among the KG model parameters suggests that under-modeling leads to inaccurate or imprecise estimates in the version 3 minimal model in rhesus monkey in a manner similar to that reported for humans and dogs (33).
FIG. 6.
Insulin sensitivity index in control and CR animals. Insulin sensitivity index values calculated by the minimal model approach for data collected by a frequently sampled intravenous glucose tolerance test. Control animals are represented by closed circles and CR animals are represented by open circles. Data are presented as group mean ± SEM. All data are analyzed using a longitudinal repeated measures design as detailed previously (35).
The effects of CR on β-cell function and SI were also analyzed using the two-compartment minimal model with stable isotope labeled glucose. We used this method because it provides similar SI values to the one-compartment model while also providing a measure of hepatic glucose production. Utilizing this model, we found that first-phase (0–10 min) insulin, but not first-phase C-peptide area under the curve, was greater among controls than CR monkeys. Second-phase (10–180 min) insulin and C-peptide are under the curve were greater in controls than CR monkeys. Even after separating out four control monkeys that were known to have hyperinsulinemia, the remaining, normal insulinemic monkeys exhibited marginally greater first-phase insulin and second-phase C-peptide, and greater second-phase insulin values than CR monkeys (32).
The longitudinal nature of this study allows for analysis of SI over many years. When SI is plotted versus total body adiposity as measured by DXA, a strong relationship is observed. In the original group of CR males, SI increased dramatically as fatness decreased, reaching a maximum of ∼15 in year 6. A gradual increase in total body adiposity in both control and CR monkeys from the original group of males began around year 10 of the study and was associated with a decrease in the group average SI (34). Currently, insulin sensitivity remains ∼2.5-fold higher in CR than in age-matched control monkeys (Fig. 6).
Outcomes and Conclusion
The possibility of slowing human aging has captured the public imagination for centuries. More recently, through advances in our understanding of the common elements of aging and the application of translatable models, the prospect of realizing this possibility is closer than ever. Elucidation of the mechanistic basis of aging retardation by CR will define avenues that may be exploited to improve health and increase longevity without maintaining a reduced-calorie diet indefinitely. One of the long-term goals of our WNPRC study of CR and aging in rhesus monkeys is provide a better understanding of the biology of aging in primates. It will be particularly important to determine whether factors identified in CR studies in short-lived species also play a role in nonhuman primates. Further development and utilization of this model will facilitate identification of important aging processes that also occur in humans.
Abbreviations Used
- CR
calorie restriction
- DXA
dual-energy X-ray absorptiometry
- HDL
high-density lipoprotein
- KG
glucose effectiveness
- LDL
low-density lipoprotein
- NIA
National Institute on Aging
- ROS
reactive oxygen species
- SEM
standard error of the mean
- SI
insulin sensitivity index
- TG
triglyceride
- Tor
target of rapamycin
- VLDL
very-low-density lipoprotein
- WNPRC
Wisconsin National Primate Research Center
Acknowledgments
The authors gratefully acknowledge the excellent technical assistance provided by S. Baum, J. Christensen, J.A. Adriansjach, C.E. Armstrong, and the Animal Care, Veterinary and Pathology Staff of the WNPRC. The authors also acknowledge support from the Institute on Aging and the Institute for Clinical and Translational Research at UW-Madison. This work was supported by grants P01 AG-11915 (NIA) and P51 RR000167 (NCRR). This research was conducted in part at a facility constructed with support from Research Facilities Improvement Program grant numbers RR15459-01 and RR020141-01 from NCRR.
References
- 1.Anderson RM. Bitterman KJ. Wood JG. Medvedik O. Sinclair DA. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature. 2003;423:181–185. doi: 10.1038/nature01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson RM. Weindruch R. Metabolic reprogramming in dietary restriction. Interdiscip Top Gerontol. 2007;35:18–38. doi: 10.1159/000096554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barger JL. Walford RL. Weindruch R. The retardation of aging by caloric restriction: its significance in the transgenic era. Exp Gerontol. 2003;38:1343–1351. doi: 10.1016/j.exger.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Barros MH. Bandy B. Tahara EB. Kowaltowski AJ. Higher respiratory activity decreases mitochondrial reactive oxygen release and increases life span in Saccharomyces cerevisiae. J Biol Chem. 2004;279:49883–49888. doi: 10.1074/jbc.M408918200. [DOI] [PubMed] [Google Scholar]
- 5.Barzilai N. Banerjee S. Hawkins M. Chen W. Rossetti L. Caloric restriction reverses hepatic insulin resistance in aging rats by decreasing visceral fat. J Clin Invest. 1998;101:1353–1361. doi: 10.1172/JCI485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barzilai N. Gupta G. Interaction between aging and syndrome X: new insights on the pathophysiology of fat distribution. Ann NY Acad Sci. 1999;892:58–72. doi: 10.1111/j.1749-6632.1999.tb07785.x. [DOI] [PubMed] [Google Scholar]
- 7.Bergman RN. Toward physiological understanding of glucose tolerance: minimal-model approach. Diabetes. 1989;38:1512–1527. doi: 10.2337/diab.38.12.1512. [DOI] [PubMed] [Google Scholar]
- 8.Bishop NA. Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature. 2007;447:545–549. doi: 10.1038/nature05904. [DOI] [PubMed] [Google Scholar]
- 9.Bodkin NL. Ortmeyer HK. Hansen BC. Diversity of insulin resistance in monkeys with normal glucose tolerance. Obes Res. 1993;1:364–370. doi: 10.1002/j.1550-8528.1993.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 10.Bogacka I. Ukropcova B. McNeil M. Gimble JM. Smith SR. Structural and functional consequences of mitochondrial biogenesis in human adipocytes in vitro. J Clin Endocrinol Metab. 2005;90:6650–6656. doi: 10.1210/jc.2005-1024. [DOI] [PubMed] [Google Scholar]
- 11.Bonawitz ND. Chatenay-Lapointe M. Pan Y. Shadel GS. Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression. Cell Metab. 2007;5:265–277. doi: 10.1016/j.cmet.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braeckman BP. Houthoofd K. Vanfleteren JR. Insulin-like signaling, metabolism, stress resistance and aging in Caenorhabditis elegans. Mech Ageing Dev. 2001;122:673–693. doi: 10.1016/s0047-6374(01)00222-6. [DOI] [PubMed] [Google Scholar]
- 13.Busetto L. Visceral obesity and the metabolic syndrome: effects of weight loss. Nutr Metab Cardiovasc Dis. 2001;11:195–204. [PubMed] [Google Scholar]
- 14.Chan YC. Suzuki M. Yamamoto S. A comparison of anthropometry, biochemical variables and plasma amino acids among centenarians, elderly and young subjects. J Am Coll Nutr. 1999;18:358–365. doi: 10.1080/07315724.1999.10718876. [DOI] [PubMed] [Google Scholar]
- 15.Chang AM. Halter JB. Aging and insulin secretion. Am J Physiol Endocrinol Metab. 2003;284:E7–E12. doi: 10.1152/ajpendo.00366.2002. [DOI] [PubMed] [Google Scholar]
- 16.Clancy DJ. Gems D. Hafen E. Leevers SJ. Partridge L. Dietary restriction in long-lived dwarf flies. Science. 2002;296:319. doi: 10.1126/science.1069366. [DOI] [PubMed] [Google Scholar]
- 17.Clancy DJ. Gems D. Harshman LG. Oldham S. Stocker H. Hafen E. Leevers SJ. Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- 18.Colman RJ. Anderson RM. Johnson SC. Kastman EK. Kosmatka KJ. Beasley TM. Allison DB. Cruzen C. Simmons HA. Kemnitz JW. Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colman RJ. Beasley TM. Allison DB. Weindruch R. Attenuation of sarcopenia by dietary restriction in rhesus monkeys. J Gerontol A Biol Sci Med Sci. 2008;63:556–559. doi: 10.1093/gerona/63.6.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colman RJ. Kemnitz J. Aging experiments using nonhuman primates. In: Yu BP, editor. Methods in Aging Research. Boca Raton: CRC Press; 1999. pp. 249–267. [Google Scholar]
- 21.Colman RJ. McKiernan SH. Aiken JM. Weindruch R. Muscle mass loss in Rhesus monkeys: age of onset. Exp Gerontol. 2005;40:573–581. doi: 10.1016/j.exger.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Colman RJ. Ramsey JJ. Roecker EB. Havighurst T. Hudson JC. Kemnitz JW. Body fat distribution with long-term dietary restriction in adult male rhesus macaques. J Gerontol A Biol Sci Med Sci. 1999;54:B283–B290. doi: 10.1093/gerona/54.7.b283. [DOI] [PubMed] [Google Scholar]
- 23.Colman RJ. Roecker EB. Ramsey JJ. Kemnitz JW. The effect of dietary restriction on body composition in adult male and female rhesus macaques. Aging. 1998;10:83–92. doi: 10.1007/BF03339642. [DOI] [PubMed] [Google Scholar]
- 24.Ebeling P. Essen-Gustavsson B. Tuominen JA. Koivisto VA. Intramuscular triglyceride content is increased in IDDM. Diabetologia. 1998;41:111–115. doi: 10.1007/s001250050875. [DOI] [PubMed] [Google Scholar]
- 25.Edwards IJ. Rudel LL. Terry JG. Kemnitz JW. Weindruch R. Cefalu WT. Caloric restriction in rhesus monkeys reduces low density lipoprotein interaction with arterial proteoglycans. J Gerontol A Biol Sci Med Sci. 1998;53:B443–B448. doi: 10.1093/gerona/53a.6.b443. [DOI] [PubMed] [Google Scholar]
- 26.Edwards IJ. Rudel LL. Terry JG. Kemnitz JW. Weindruch R. Zaccaro DJ. Cefalu WT. Caloric restriction lowers plasma lipoprotein(a) in male but not female rhesus monkeys. Exp Gerontol. 2001;36:1413–1418. doi: 10.1016/s0531-5565(01)00107-3. [DOI] [PubMed] [Google Scholar]
- 27.Eisner JR. Dumesic DA. Kemnitz JW. Abbott DH. Timing of prenatal androgen excess determines differential impairment in insulin secretion and action in adult female rhesus monkeys. J Clin Endocrinol Metab. 2000;85:1206–1210. doi: 10.1210/jcem.85.3.6453. [DOI] [PubMed] [Google Scholar]
- 28.Fabrizio P. Liou LL. Moy VN. Diaspro A. Valentine JS. Gralla EB. Longo VD. SOD2 functions downstream of Sch9 to extend longevity in yeast. Genetics. 2003;163:35–46. doi: 10.1093/genetics/163.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finkel T. Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 30.Furukawa S. Fujita T. Shimabukuro M. Iwaki M. Yamada Y. Nakajima Y. Nakayama O. Makishima M. Matsuda M. Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Girardot F. Lasbleiz C. Monnier V. Tricoire H. Specific age-related signatures in Drosophila body parts transcriptome. BMC Genomics. 2006;7:69. doi: 10.1186/1471-2164-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gresl TA. Colman RJ. Havighurst TC. Allison DB. Schoeller DA. Kemnitz JW. Dietary restriction and beta-cell sensitivity to glucose in adult male rhesus monkeys. J Gerontol A Biol Sci Med Sci. 2003;58:598–610. doi: 10.1093/gerona/58.7.b598. [DOI] [PubMed] [Google Scholar]
- 33.Gresl TA. Colman RJ. Havighurst TC. Byerley LO. Allison DB. Schoeller DA. Kemnitz JW. Insulin sensitivity and glucose effectiveness from three minimal models: effects of energy restriction and body fat in adult male rhesus monkeys. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1340–R1354. doi: 10.1152/ajpregu.00651.2002. [DOI] [PubMed] [Google Scholar]
- 34.Gresl TA. Colman RJ. Roecker EB. Havighurst T. Huang Z. Allison DB. Bergman RN. Kemnitz JW. Dietary restriction and glucose regulation in aging rhesus monkeys: a follow-up report at 8.5 years. Am J Physiol Endocrinol Metab. 2001;281:E757–E765. doi: 10.1152/ajpendo.2001.281.4.E757. [DOI] [PubMed] [Google Scholar]
- 35.Guarente L. Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- 36.Handschin C. Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454:463–469. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higami Y. Barger JL. Page GP. Allison DB. Smith SR. Prolla TA. Weindruch R. Energy restriction lowers the expression of genes linked to inflammation, the cytoskeleton, the extracellular matrix, and angiogenesis in mouse adipose tissue. J Nutr. 2006;136:343–352. doi: 10.1093/jn/136.2.343. [DOI] [PubMed] [Google Scholar]
- 38.Higami Y. Pugh TD. Page GP. Allison DB. Prolla TA. Weindruch R. Adipose tissue energy metabolism: altered gene expression profile of mice subjected to long-term caloric restriction. FASEB J. 2004;18:415–417. doi: 10.1096/fj.03-0678fje. [DOI] [PubMed] [Google Scholar]
- 39.Holzenberger M. Dupont J. Ducos B. Leneuve P. Geloen A. Even PC. Cervera P. Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 40.Houmard JA. Tanner CJ. Yu C. Cunningham PG. Pories WJ. MacDonald KG. Shulman GI. Effect of weight loss on insulin sensitivity and intramuscular long-chain fatty acyl-CoAs in morbidly obese subjects. Diabetes. 2002;51:2959–2963. doi: 10.2337/diabetes.51.10.2959. [DOI] [PubMed] [Google Scholar]
- 41.Houthoofd K. Braeckman BP. Johnson TE. Vanfleteren JR. Life extension via dietary restriction is independent of the Ins/IGF-1 signalling pathway in Caenorhabditis elegans. Exp Gerontol. 2003;38:947–954. doi: 10.1016/s0531-5565(03)00161-x. [DOI] [PubMed] [Google Scholar]
- 42.Houthoofd K. Vanfleteren JR. Public and private mechanisms of life extension in Caenorhabditis elegans. Mol Genet Genomics. 2007;277:601–617. doi: 10.1007/s00438-007-0225-1. [DOI] [PubMed] [Google Scholar]
- 43.Hudson JC. Baum ST. Frye DMD. Roecker EB. Kemnitz JW. Age and sex differences in body size and composition during rhesus monkey adulthood. Aging Clin Exp Res. 1996;8:197–204. doi: 10.1007/BF03339677. [DOI] [PubMed] [Google Scholar]
- 44.Hwangbo DS. Gersham B. Tu MP. Palmer M. Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature. 2004;429:562–566. doi: 10.1038/nature02549. [DOI] [PubMed] [Google Scholar]
- 45.Ingram DK. Young J. Mattison JA. Calorie restriction in nonhuman primates: assessing effects on brain and behavioral aging. Neurosci. 2007;145:1359–1364. doi: 10.1016/j.neuroscience.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 46.Kaeberlein M. Powers RW., 3rd Steffen KK. Westman EA. Hu D. Dang N. Kerr EO. Kirkland KT. Fields S. Kennedy BK. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- 47.Kenyon C. Chang J. Gensch E. Rudner A. Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 48.Koyama K. Chen G. Lee Y. Unger RH. Tissue triglycerides, insulin resistance, and insulin production: implications for hyperinsulinemia of obesity. Am J Physiol. 1997;273:E708–E713. doi: 10.1152/ajpendo.1997.273.4.E708. [DOI] [PubMed] [Google Scholar]
- 49.Lee YL. Lee CK. Transcriptional response according to strength of calorie restriction in Saccharomyces cerevisiae. Mol Cells. 2008;26:299–307. [PubMed] [Google Scholar]
- 50.Lin SJ. Kaeberlein M. Andalis AA. Sturtz LA. Defossez PA. Culotta VC. Fink GR. Guarente L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- 51.Lin SS. Manchester JK. Gordon JI. Enhanced gluconeogenesis and increased energy storage as hallmarks of aging in Saccharomyces cerevisiae. J Biol Chem. 2001;276:36000–36007. doi: 10.1074/jbc.M103509200. [DOI] [PubMed] [Google Scholar]
- 52.Longo VD. Kennedy BK. Sirtuins in aging and age-related disease. Cell. 2006;126:257–268. doi: 10.1016/j.cell.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 53.Magwere T. Goodall S. Skepper J. Mair W. Brand MD. Partridge L. The effect of dietary restriction on mitochondrial protein density and flight muscle mitochondrial morphology in Drosophila. J Gerontol A Biol Sci Med Sci. 2006;61:36–47. doi: 10.1093/gerona/61.1.36. [DOI] [PubMed] [Google Scholar]
- 54.Martin DE. Hall MN. The expanding TOR signaling network. Curr Opin Cell Biol. 2005;17:158–166. doi: 10.1016/j.ceb.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 55.Medvedik O. Lamming DW. Kim KD. Sinclair DA. MSN2 and MSN4 link calorie restriction and TOR to sirtuin-mediated lifespan extension in Saccharomyces cerevisiae. PLoS Biol. 2007;5:e261. doi: 10.1371/journal.pbio.0050261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meneilly GS. Ryan AS. Veldhuis JD. Elahi D. Increased disorderliness of basal insulin release, attenuated insulin secretory burst mass, and reduced ultradian rhythmicity of insulin secretion in older individuals. J Clin Endocrinol Metab. 1997;82:4088–4093. doi: 10.1210/jcem.82.12.4457. [DOI] [PubMed] [Google Scholar]
- 57.Mittelman SD. Van Citters GW. Kirkman EL. Bergman RN. Extreme insulin resistance of the central adipose depot in vivo. Diabetes. 2002;51:755–761. doi: 10.2337/diabetes.51.3.755. [DOI] [PubMed] [Google Scholar]
- 58.Morrow G. Tanguay RM. Mitochondria and ageing in Drosophila. Biotechnol J. 2008;3:728–739. doi: 10.1002/biot.200800015. [DOI] [PubMed] [Google Scholar]
- 59.Muller FL. Lustgarten MS. Jang Y. Richardson A. Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 60.Nakae J. Oki M. Cao Y. The FoxO transcription factors and metabolic regulation. FEBS Lett. 2008;582:54–67. doi: 10.1016/j.febslet.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 61.Perez VI. Bokov A. Van Remmen H. Mele J. Ran Q. Ikeno Y. Richardson A. Is the oxidative stress theory of aging dead? Biochim Biophys Acta. 2009;1790:1005–1014. doi: 10.1016/j.bbagen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pletcher SD. Macdonald SJ. Marguerie R. Certa U. Stearns SC. Goldstein DB. Partridge L. Genome-wide transcript profiles in aging and calorically restricted Drosophila melanogaster. Curr Biol. 2002;12:712–723. doi: 10.1016/s0960-9822(02)00808-4. [DOI] [PubMed] [Google Scholar]
- 63.Ramsey JJ. Colman RJ. Binkley NC. Christensen JD. Gresl TA. Kemnitz JW. Weindruch R. Dietary restriction and aging in rhesus monkeys: the University of Wisconsin study. Exp Gerontol. 2000;35:1131–1149. doi: 10.1016/s0531-5565(00)00166-2. [DOI] [PubMed] [Google Scholar]
- 64.Reaven GM. Pathophysiology of insulin resistance in human disease. Physiol Rev. 1995;75:473–486. doi: 10.1152/physrev.1995.75.3.473. [DOI] [PubMed] [Google Scholar]
- 65.Rezzi S. Martin FP. Shanmuganayagam D. Colman RJ. Nicholson JK. Weindruch R. Metabolic shifts due to long-term caloric restriction revealed in nonhuman primates. Exp Gerontol. 2009;44:356–362. doi: 10.1016/j.exger.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rodgers JT. Lerin C. Gerhart-Hines Z. Puigserver P. Metabolic adaptations through the PGC-1 alpha and SIRT1 pathways. FEBS Lett. 2008;582:46–53. doi: 10.1016/j.febslet.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rudman D. Mattson DE. Feller AG. Cotter R. Johnson RC. Fasting plasma amino acids in elderly men. Am J Clin Nutr. 1989;49:559–566. doi: 10.1093/ajcn/49.3.559. [DOI] [PubMed] [Google Scholar]
- 68.Schieke SM. Finkel T. TOR and aging: less is more. Cell Metab. 2007;5:233–235. doi: 10.1016/j.cmet.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 69.Shimokata H. Tobin JD. Muller DC. Elahi D. Coon PJ. Andres R. Studies in the distribution of body fat: I. Effects of age, sex, and obesity. J Gerontol. 1989;44:M66–M73. doi: 10.1093/geronj/44.2.m66. [DOI] [PubMed] [Google Scholar]
- 70.Smith DL., Jr. McClure JM. Matecic M. Smith JS. Calorie restriction extends the chronological lifespan of Saccharomyces cerevisiae independently of the Sirtuins. Aging Cell. 2007;6:649–662. doi: 10.1111/j.1474-9726.2007.00326.x. [DOI] [PubMed] [Google Scholar]
- 71.Smith SR. Ravussin E. Emerging paradigms for understanding fatness and diabetes risk. Curr Diab Rep. 2002;2:223–230. doi: 10.1007/s11892-002-0087-1. [DOI] [PubMed] [Google Scholar]
- 72.Sohal RS. Agarwal S. Candas M. Forster MJ. Lal H. Effect of age and caloric restriction on DNA oxidative damage in different tissues of C57BL/6 mice. Mech Ageing Dev. 1994;76:215–224. doi: 10.1016/0047-6374(94)91595-4. [DOI] [PubMed] [Google Scholar]
- 73.Tavernarakis N. Driscoll M. Caloric restriction and lifespan: a role for protein turnover? Mech Ageing Dev. 2002;123:215–229. doi: 10.1016/s0047-6374(01)00341-4. [DOI] [PubMed] [Google Scholar]
- 74.Uno H. Age-related pathology and biosenescent markers in captive rhesus macaques. Age. 1997;20:1–13. doi: 10.1007/s11357-997-0001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Urban J. Soulard A. Huber A. Lippman S. Mukhopadhyay D. Deloche O. Wanke V. Anrather D. Ammerer G. Riezman H. Broach JR. De Virgilio C. Hall MN. Loewith R. Sch9 is a major target of TORC1 in Saccharomyces cerevisiae. Mol Cell. 2007;26:663–674. doi: 10.1016/j.molcel.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 76.Wei M. Fabrizio P. Hu J. Ge H. Cheng C. Li L. Longo VD. Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9. PLoS Genet. 2008;4:e13. doi: 10.1371/journal.pgen.0040013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weindruch R. Walford RL. Fligiel S. Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116:641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- 78.Weindruch RH. Walford RL. The Retardation of Aging and Disease by Dietary Restriction. Springfield, IL: Charles C Thomas; 1988. [Google Scholar]