Abstract
We report that the stress axis–regulated exon (STREX)-containing calcium-activated big potassium (BKCa) channel splice variant expression and physiology are regulated in part by cytoplasmic splicing and intron retention. NextGen sequencing of the mRNA complement of pooled hippocampal dendrite samples found intron 17a (i17a), the intron immediately preceding STREX, in the BKCa mRNA. Further molecular analyses of i17a revealed that the majority of i17a-containing BKCa channel mRNAs associate with STREX. i17a siRNA treatment followed by STREX protein immunocytochemistry demonstrated both reduced levels and altered subcellular distribution of STREX-containing BKCa channel protein. Selective reduction of i17a-BKCa or STREX-BKCa mRNAs induced similar changes in the burst firing properties of hippocampal neurons. Collectively, these data show that STREX splice variant regulation via cytoplasmic splicing and intron retention helps generate STREX-dependent BKCa current diversity in hippocampal neurons.
The functional importance of alternative splicing in neurons is well established (1). Although the bulk of RNA splicing occurs in the nucleus, hippocampal dendrites have the capacity to splice RNA outside the nucleus (2). The target molecules for local extranuclear splicing are cytoplasmic mRNAs that contain some intronic sequences, known as cytoplasmic intron-containing transcripts. Functionally, these partially processed mRNAs have been implicated in the regulation of essential cellular functions, including platelet clotting (3) and neural excitability (4). Here we report a noteworthy role for cytoplasmic intron-containing transcripts. Hippocampal neurons use intron retention in concert with cytoplasmic splicing to expand the diversity of calcium-activated big potassium (BKCa) channel mRNA splice variants and their consequent protein products.
A single gene, KCNMA1, encodes the pore-forming α-subunit of BKCa channels (5). Structural diversity in endogenous populations of BKCa channels is generated in part by robust tissue-specific splicing (6) of the KCNMA1 mRNA. Several alternative splice sites and alternative exons have been identified in the KCNMA1 gene (7). The stress axis–regulated exon (STREX) is by far the most well-characterized alternative exon in the KCNMA1 gene. Inclusion of this exon alters the BKCa channel properties in response to key intracellular signals, including calcium influx (8) and PKA/cAMP signaling (9). Given the functional implications of STREX, we focused our KCNMA1 intron retention studies around this exon.
We recently reported that intron-containing BKCa channel mRNAs contribute to the subcellular distribution of BKCa channel protein and burst-firing properties of hippocampal neurons (4). Here, by analyzing the alternative splicing patterns at the STREX splice site, we show that cytoplasmic transcripts retaining the intron 17a sequence (referred to as i17a-containing BKCa mRNAs) regulate STREX splice variant expression and physiology. Thus, intron retention plays a role in regulating the complexity of BKCa channel mRNA splice variants, proteins, and currents in hippocampal neurons.
Results
STREX Region Is a “Hot Spot” for Retained Introns.
Alternative splicing in the STREX region of the BKCa channel gene has been studied extensively (7). We used deep sequencing of dendrites (n = 4) and somas (n = 5) of hippocampal neurons to screen the STREX splice site region for retained introns. Pooled (∼300–700) dendrites or individual cell somas were harvested, subjected to aRNA amplification (10), and used as template to generate libraries for Illumina deep-sequencing analysis. The sequencing runs of hippocampal dendrites generated an average of 11.3 million sequence reads of 36–50 bases long. Accepting up to two mismatches, 45.5% of the reads mapped to the rat genome. These dendritic sequencing results are consistent with those of previous mammalian sequencing efforts (11).
The sequence-specific read coverage alignment was performed using Bowtie (12) version 10.0.2 with the default parameters on the rat genome version 3.4 sequence (13). Genome-unique reads overlapping KCNMA1 gene features were identified using the RefSeq gene annotation. The sequence alignments from the dendritic samples to the KCNMA1 gene produced two intronic regions upstream of the STREX exon, intron 16 (i16) and intron 17a (i17a), with multiple-sequence hits (Fig. 1 A and B). We previously reported the presence of i16 in hippocampal dendrites (4); thus, the presence of i16 in the Illumina deep-sequencing reads is consistent with our previous BKCa channel intron analysis. The i17a sequence immediately precedes STREX, and this intron contains the regulatory sequence elements that direct activity-dependent alternative splicing of STREX (14). This verifies the presence of STREX region introns in hippocampal dendrites.
Fig. 1.
Identifying retained introns in the STREX region. (A) Schematic of the KCNMA1 gene structure preceding the STREX splice site. Constitutive exons are designated e16 and e17. The alternatively spliced exon is designated e17a. The black arrows represent Illumina sequencing hits for each exon and intron. The numbers above the arrow represent the number of the Illumina sequencing hits for each exon and intron (dendrite samples, n = 4; soma samples, n = 5). (B) We looked for mate pairs whose ends uniquely align to nonrepetitive KCNMA1 intronic regions but whose insert length was unusually long. These “long-distance mate pair alignments” produced three unique sequence reads that span exon–intron boundaries in the STREX region. Two upstream exons, e16 and e17, uniquely aligned as long distance mate pairs with i17a, as shown in the splicing diagram. (C) Hippocampal cDNA samples were screened by RT-PCR for i17a-containing BKCa channel mRNAs. (Left) A representative gel. (Right) The structural identity of the PCR products. (D and E) Quantitative PCR technique, MALDI-TOF MS base extension, was used to determine the repertoire of STREX splice variants and i17a-containing BKCa channel mRNAs in hippocampal neurons. The chart in D shows the abundances and exonic identities of the exon- only STREX splice variants (n = 3). The chart in E shows the number and exon identities of the i17a-containing BKCa channel transcripts at the STREX splice site (n = 3).
i17a Associates with STREX in BKCa Channel mRNA.
We used RT-PCR cloning to examine whether i17a-containing BKCa mRNAs contain STREX. The PCR primers were placed in i17a and e19, and hippocampal cDNA was used as a template. BLAST search analysis verified that the structural identity of the i17a PCR products corresponded to i17a, STREX, e18, and e19 (n = 40; Fig. 1C). The exonic borders between STREX and e18 and between e18 and e19 were all perfectly matched to their reported exon–exon junctions. These results demonstrate the presence of STREX in i17a-containing BKCa channel mRNAs and, more importantly, provide contiguous sequence information for this retained intron. The correct downstream splicing junctions within the i17a-STREX–containing BKCa channel mRNAs show that the PCR products were not derived from genomic DNA or nuclear premRNAs. The absence of clones without STREX shows that i17a-STREX–containing BKCa channel mRNAs are spliced in a highly restrictive manner. Further sequence analysis of the i17a sequence revealed that all reading frames contained multiple stop codons. Thus, without additional cytoplasmic splicing, the direct translation of transcripts with i17a-STREX–containing BKCa mRNAs would generate significantly truncated BKCa channel proteins that lacked several key functional domains, including the STREX domain.
Multiple STREX-Containing Splice Variants Are Present in the Hippocampus.
We used a quantitative PCR approach, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) base extension, to identify the repertoire of splice variants at the STREX splice site in hippocampal neurons. Hippocampal cDNA was amplified with PCR primers in exon 16 (forward) and exon 18 (reverse) to capture the complete BKCa channel splice variant population. Next, a single extension primer was used to assay the PCR products (Fig. 1D). Using this approach, we first determined the levels and structural identities of the BKCa channel mRNAs that contain only exons. Because the STREX site contains two alternatively spliced exons, e17a and e17b (STREX), this site has the potential to generate four distinct transcripts (Fig. 1D). Hippocampal neurons generated all four transcripts, but the most abundant BKCa channel mRNA was the one without alternative exons, the e17-e18 transcript (75%; Fig. 1D). Within the population of transcripts with alternatively spliced exons, all three BKCa channel splice variants were present at detectable levels (Fig. 1D). These findings are similar to the levels of STREX BKCa channel splice variants detected by real-time PCR in the mouse hippocampus (15).
Only One STREX-Containing Splice Variant Is Linked to i17a.
We used the same quantitative PCR approach to determine the levels and exonic identities of i17a-containing transcripts at the STREX splice site. This site has the potential to generate four distinct i17a-containing BKCa channel mRNAs (Fig. 1E). By far the most abundant i17a-containing BKCa channel mRNA in hippocampal neurons was i17a-STREX-e18 (3.1%; Fig. 1E). Within the STREX mRNA population, i17a containing STREX mRNAs accounted for 20% of all transcripts (Fig. 1 D and E). These results are in agreement with our RT-PCR cloning results, in which only the i17a-STREX–containing BKCa channel splice variant was detected (Fig. 1C). In summary, hippocampal neurons generated two distinct classes of BKCa channel splice variants with STREX: two splice variants from the “exon-only” class (Fig. 1D) and one splice variant from the “retained intron” class (Fig. 1E). The specificity of the retained intron class implies that i17a-STREX–containing mRNAs are biologically important transcripts for hippocampal neurons.
In Situ Hybridization Analysis of i17a-Containing BKCa mRNA.
We visualized the subcellular distribution of i17a-containing BKCa channel mRNAs within hippocampal neurons by in situ hybridization (ISH). We used two short (30 nucleotides) biotin-labeled i17a oligonucleotide probes to analyze the subcellular localization patterns of i17a-containing BKCa channel mRNAs (Fig. 2 A and B). We designed both the sense and antisense biotin-labeled i17a oligonucleotide probes based on our i17a cloning and sequencing data (Fig. 1). The i17a antisense probe ISH signal was detectable within several subcellular regions; the signal extended from the cell body out into the proximal and distal dendrites (Fig. 2A). The finding of the i17a ISH signal within the dendrites is consistent with our detection of i17a in the Illumina sequencing results (Fig. 1). As a control, we performed ISH with the i17a sense probe; as expected, this probe did not show a detectable ISH signal (Fig. 2B). These findings provide independent morphological confirmation of the presence of i17a sequences in both primry and secondary hippocampal dendrites.
Fig. 2.
ISH analysis of i17a-containing BKCa channel mRNAs in hippocampal neurons. (A and B) Photomicrographs showing ISH of cultured hippocampal neurons with i17a antisense (A) and i17a sense (B) probes (n = 3). (Insets) Photomicrograph MAP-2–positive regions for each. Biotin-labeled oligo probes (30 nucleotides long) were used for detection. Qdot 605 streptavidin conjugates were used for visualization. (Scale bars: 20 μm.) (C) Schematic showing the STREX region BKCa channel mRNA targets for each siRNA treatment. (D) Summary plot of the average fluorescence (%) for an i17a ISH signal for the indicated condition: si16, 100 ± 3.97, n = 9; si17a, 34.96 ± 9.73, n = 10; siSTX, 35.05 ± 6.52, n = 10. Data are mean ± SEM. (Scale bar: 25 μm.)
RNA Interference Selectively Reduces i17a-Containing BKCa Channel mRNAs.
We used the combination of RNA interference and ISH to selectively alter and monitor changes in the levels of BKCa channel splice variants in hippocampal neurons. Using our splice variant linkage data (Fig. 1), we designed siRNAs to selectively target two distinct populations of BKCa channel splice variants: STREX-containing and STREX-lacking. siRNAs directed against i17a (si17a) or STREX (siSTX) sequences were used to target the STREX-containing splice variant population (Fig. 2C). Identifying target sequences for the STREX-lacking population was not as straightforward. All BKCa channel mRNAs, including i17a and STREX, associate with constitutive exons (e.g., e18 and e19; Fig. 1) in the STREX region. Thus, finding target siRNA sequences for the STREX-lacking population required the use of another STREX region intron-containing BKCa splice variant. Given our earlier finding that i16 is not associated with the STREX exon (4), we used siRNAs directed against i16 (si16) sequences to target the STREX-lacking splice variant population (Fig. 2C). For each siRNA treatment, we used two nonoverlapping siRNAs to selectively reduce the levels of their respective target sequences. As a control, we used MAP-2 staining to identify dendritic processes and verify healthy cellular morphology of siRNA-treated neurons. The i17a antisense ISH signal was dramatically reduced by siRNA treatments targeting the STREX-containing population (si17 and siSTX) compared with siRNA treatments targeting the STREX-lacking population (si16). We quantified this reduction by measuring the i17a ISH signal from several individual neurons. We found no difference in the i17a ISH signals of si17a-and siSTX-treated neurons (Fig. 2D); however, the i17a ISH signal was significantly reduced (by ∼50%) in si17-and siSTX-treated neurons compared with si16-treated neurons (Fig. 2D). These results verify that the i17a and STREX BKCa channel mRNA sequences are indeed linked together in the cytoplasm of hippocampal neurons.
STREX Splice Variant Proteins Are Present in Multiple Subcellular Compartments.
The i17a-STREX BKCa channel mRNA linkage offers a unique opportunity to analyze the involvement of an intron in the selection of expressed exons. To analyze this direct molecular linkage at the protein level, we developed antibodies specific to the STREX region of the BKCa channel protein. We verified the specificity of the STREX antibody by ELISA screening and competitive Western blot analysis (Fig. S1). We first used our STREX antibodies to characterize the normal STREX BKCa channel expression patterns in hippocampal neurons by dual-label immunostaining with MAP-2. STREX BKCa channel protein staining was detectable throughout the cell bodies and dendrites of hippocampal neurons. The signal was most intense in the cell soma and proximal dendrites, but was evident well into the distal dendrites (Fig. 3A, Lower). These STREX BKCa channel protein distribution data agree with the results of previous studies showing similar subcellular distributions for BKCa channel proteins in neurons using pan-specific (i.e., all splice variants) antibodies (4, 16, 17).
Fig. 3.
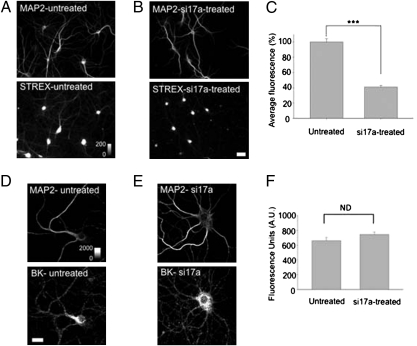
i17a-containing BKCa channel RNAs contribute to STREX BKCa channel proteins in hippocampal neurons. (A and B) Photomicrographs showing MAP2 (Upper) and STREX (Lower) immunostaining in untreated (A) and si17a-treated (B) hippocampal neurons. To quantify the STREX BKCa channel proteins in untreated and si17a-treated neurons, we selected the dendritic regions of the neurons as identified by MAP-2 staining and quantified the intensity of STREX fluorescence. (C) Summary plot of the normalized fluorescence pixel average STREX fluorescence for the indicated condition. Untreated, 99.99 ± 4.28, n = 12; si17a-treated, 40.95 ± 2.21, n = 12. ***P < 0.001. These data were statistically analyzed using the Student t test. (Scale bar: 25 μm.) (D and E) Total BKCa channel expression in hippocampal neurons. Photomicrographs showing MAP2 (Upper) and BKCa channel protein (Lower) immunostaining in untreated (D) and si17a-treated (E) hippocampal neurons. (F) Summary plot of the average BKCA fluorescence for the indicated conditions.
i17a-STREX–Containing mRNAs Generate STREX BKCa Channel Proteins.
By combining RNA interference with STREX-specific immunostaining, we next asked whether the i17a-STREX–containing BKCa channel mRNAs contribute to the STREX BKCa channel protein population in hippocampal neurons. We used MAP-2 staining to identify dendritic processes and verify normal cellular morphology of the si17-treated neurons. Although MAP-2 staining was statistically similar in the si17-treated and untreated neurons (Fig. 3 A and B, Upper), STREX-specific staining was greatly reduced (by ∼60%) in the si17-treated neurons (Fig. 3 A and B, Lower, and quantified in Fig. 3C). The STREX signal reduction was most prominent in the dendrites compared with the cell soma region (Fig. 3 A and B, Lower). In contrast to the STREX-specific staining, we found no detectable difference in total BK channel protein staining in the si17-treated neurons compared with the untreated neurons (Fig. 3 D and E, and quantified in Fig. 3F). The si17a sensitivity demonstrates that i17a-STREX–containing BKCa channel mRNAs contribute to STREX BKCa channel proteins in hippocampal neurons. Retained introns in BKCa channel mRNAs might allow for the regulation of splice variants, just as different 3′-UTRs regulate BKCa channel splice variants (18). Most notably, the restricted splicing linkages of i17a allow for the selective regulation of only one STREX-containing BKCa channel mRNA, the i17a-STREX-e18 splice variant.
Functional Analysis of BKCa Channel Splice Variants.
One well-known intrinsic property of hippocampal neurons is their ability to fire bursts of action potentials in response to a depolarizing current (19, 20). Because BKCa channels play a significant role in burst firing, we used this property as our quantitative output to assess the functional contributions of individual BKCa splice variants. We used siRNA treatments to selectively manipulate the levels of three different BKCa channel splice variants: i17a, STREX, and i16. Functional analyses of BK splice variants were previously performed in transfected continuous cell lines. Here, in contrast, we performed these analyses on the endogenous transcripts in primary hippocampal neurons, in an effort to evaluate their influence in a native neuronal environment. We used current clamp recordings following the siRNA treatment to evaluate the functional contributions of each splice variant in hippocampal burst firing. Three additional conditions (untreated, mock, and control siRNA) served as controls for these experiments. All of the treatments resulted in similar resting membrane potentials, input resistances, action potential heights, and action potential thresholds (Fig. S2 A, B, and C), indicating that the neurons have comparable membrane properties under each experimental condition. Representative traces for each indicated condition are shown in Fig. 4A and Fig. S2D.
Fig. 4.
Retained introns contribute to the burst-firing properties of hippocampal neurons. Hippocampal neurons were transfected with pools of siRNAs directed against indicated targets and current-clamped at −80 mV, and brief current injections (500 ms) were applied to evoke a train of action potentials. (A) Representative traces showing the initial action potentials (100 msec of the 500-ms current injection) from the 250-pA current injection for each indicated condition. (Scale bars: 10 mV and 10 msec.) (B) Summary plot of the average fAHP for the indicated conditions. (C) Summary plot of the average action potential half-width for the indicated conditions. (D) Summary plot of the average number of action potentials for each indicated condition. Data are mean ± SEM. The data were statistically analyzed using the Student t test. Black bars represent 250-pA current injection; red bars, 200-pA current injection. *P < 0.05.
Reducing i17a and STREX mRNA Levels Decreases Fast Afterhyperpolarization.
BK channels regulate the fast afterhyperpolarization (fAHP) phase, which plays a role in controlling the frequency and duration of action potentials during burst firing (19–21). Although the fAHP is a well-characterized BK channel–specific property of hippocampal neuron burst firing, the contributions of individual BKCa channel splice variants are unknown. We found varying changes in the first-spike fAHP among the treatments. The si16-RNA treatment and pharmacologic blocking of several BKCa channel currents with iberiotoxin (IBX) did not significantly decrease the first-spike fAHP compared with controls under all conditions (Fig. 4B). These results show that the si16-BKCa channels and IBX-sensitive channels do not make a major contribution to the first-spike fAHP under our recording conditions. In contrast, the STREX-specific siRNA treatments (si17a and siSTX) significantly decreased the first-spike fAHP compared with all control conditions (Fig. 4B). These results are consistent with those from previous studies using pharmacologic treatments that reduce multiple BKCa channel currents (19–21). The significant reduction in the first-spike fAHP by the si17a and siSTX treatments demonstrates that the STREX splice variant makes a significant contribution to the fAHP. The direct molecular connection between the two sequences and similar fAHP phenotypes indicates a shared and important biological role for i17a- and STREX-containing BKCa channel mRNAs in the burst-firing physiology of hippocampal neurons.
Action Potential Width Is Augmented by siRNA-i17a Treatments.
During burst firing in hippocampal neurons, spike accommodation occurs in part because of BKCa channel inactivation during the burst (21). The increases in the first-spike half-width differed among the BKCa channel treatments. The siSTX or and IBX treatment did not significantly increase the first-spike half-width compared with control conditions (Fig. 4C). These results indicate that siSTX BKCa channels and IBX-sensitive channels do not make major contribution to the half-width of the first action potential under our recording conditions. This was not the case for the intron-directed siRNA treatments, however. Both the si17a and si16 treatments generated significantly increased first-spike half-width compared with all of the control conditions (Fig. 4C). Previous studies have reported similar results by pharmacologically blocking BKCa channels with compounds that do not discriminate between individual BKCa channel splice variants (19, 20). This suggests that under endogenous burst firing conditions in hippocampal neurons, the BKCa channels that arise from these variants inactivate more slowly. This property would help maintain smaller half-widths during the initiation of burst firing and thus ultimately prolong the burst length.
Burst Firing Is Significantly Diminished by i17a and STREX mRNA Reduction.
The detected alterations in the properties of the first action potential by the BKCa channel siRNA treatments predicts a decrease in the number of action potentials during the burst under these recording conditions. Indeed, we found a significantly reduced number of action potentials in all BKCa channel siRNA-treated neurons compared with control conditions. si17a treatment generated a greater decrease in number of action potentials compared with either si16 or siSTX treatment (Fig. 4D). This condition also consistently generated the greatest changes in the fAHP and action potential half-width, so the finding that that this condition had the fewest number of action potentials of the BKCa channel–treated group was not unexpected. IBX treatment did not significantly decrease the number of action potentials compared with all control conditions (Fig. 4D). IBX-sensitive channels also did not alter the fAHP or action potential half-width, consistent with the results of i17 knockdown studies. Under our recording conditions, the IBX-sensitive class of BKCa channels did not greatly influence BKCa channel burst firing. Collectively, these physiology results demonstrate that altering individual BKCa mRNAs can disrupt burst firing and subsequently terminate typical burst firing prematurely.
Discussion
Neurons frequently use alternative splicing to generate structural and functional diversity within discrete mRNA and protein populations. Our knowledge of the events that regulate the process of alternative splicing is now expanding beyond key RNA sequences and RNA-binding proteins; for example, a recent study has demonstrated that chromatin structure plays a role in directing alternative splicing (22). Here we show that cytoplasmic intron retention directs the inclusion of the STREX exon to a subset of BKCa mRNAs. This mechanism of coupling intron retention to an alternative exon contributes to structural and functional diversity within local populations of BKCa channel mRNAs and proteins (model in Fig. S4). Our findings define an additional feature of KCNMA1 gene regulation and emphasize the value of such a cytoplasmic splicing system for linking intron sequences to altered BKCa channel function.
Key Splicing Elements Are Present in i17a-STREX BKCa Channel mRNAs.
The i17a sequence contains the well-documented activity-dependent alternative splicing sequences known as calcium-responsive RNA elements (CaRRE). In neurons, these sequences are typically regulated by calcium entry through voltage-gated calcium channels and the activation of the calcium/calmodulin-dependent protein kinase (CaMK) pathway. This pathway has been shown to mediate calcium-dependent alternative splicing in BKCa channel (STREX) and NMDA receptor mRNAs (NMDARI subunit) in neurons (14, 23). Retention of i17a in BKCa channel mRNAs directly links the CaRRE sequences and STREX together throughout the cytoplasm of hippocampal neurons. Apart from their role in nucleus, the CaRRE sequences also may play a regulatory role in the cytoplasmic splicing of i17a-STREX BKCa channel mRNAs.
Local Splicing Generates STREX Proteins From i17a-Containing mRNAs.
In addition to the CaRRE sequences, a second notable feature of i17a BKCa channel mRNAs is that every reading frame within the i17a sequence contains multiple stop codons, suggesting that i17a sequences must be spliced out to generate a full-length BKCa channel protein with the STREX epitopes. Direct translation of the i17a-STREX BKCa channel mRNA would produce a truncated BKCa channel protein that our STREX antibodies could not recognize. Thus, RNA splicing in the cytoplasm of hippocampal neurons must occur for full-length STREX BKCa channel proteins to arise from i17a-STREX BKCa channel mRNAs.
i17a–STREX Association Generates Functional Changes Within BKCa Channel Populations.
By generating a selective BKCa channel splice variant specific antibody, here we report the previously undescribed subcellular distribution of the STREX BKCa channel splice variant. This exon is the most well-studied BKCa channel splice variant, due to its involvement in eliciting multiple functional effects. Previous studies using heterologous expression systems have shown that inclusion of the STREX motif alters BKCa channel properties in response to calcium influx (8) and PKA/cAMP signaling (9, 24). The STREX-specific alterations in BKCa channel physiology, coupled with the direct association of i17a to STREX, allow hippocampal neurons to use intron retention and cytoplasmic splicing to modify their membrane properties. The presence of the STREX BKCa channel proteins and i17a throughout the dendritic processes gives hippocampal neurons the capacity to locally modify their BKCa channel currents and membrane properties via the i17a–STREX association.
Intron Retention Helps Create Local BKCa Channel Diversity.
To function properly, all neurons require precise subcellular localization of their mRNAs and proteins. The manner in which distinct subcellular populations of ion channels are generated and maintained within a neuron remains incompletely understood. We have shown that the direct association of i17a and STREX permits the selective regulation of only one STREX-containing BKCa channel splice variant—the e17-STREX-e18 protein—by i17a. siRNAs targeted against i17a dramatically reduce the levels of STREX BKCa channel proteins. This reduction is most prominent in the mid to distal dendritic processes compared with the cell bodies and proximal dendritic regions. These findings suggest two key points regarding BKCa channel protein localization within hippocampal neurons. First, the mid to distal dendritic STREX BKCa channel proteins predominately arise from i17a-STREX BKCa channel mRNAs. Second, our quantitative PCR results show that i17a generates only one STREX-containing BKCa mRNA; thus, the primary STREX splice variant protein within the mid to distal dendrites is likely the e17-STREX-e18 variant, not the e17-e17a-STREX-e18 variant. Unfortunately, neither BKCa channel blockers nor STREX antibodies can discriminate between the two STREX-containing BKCa channel proteins e17-STREX-e18 and e17-e17a-STREX-e18. More study is needed to further characterize the functional contributions of each STREX-containing variant.
BKCa channels can be divided into two pharmacologic classes: type I (fast-gated, IBX-sensitive) and type II (slow-gated, IBX-resistant) (25). BKCa channels containing the BKCa beta4 subunit belong to the latter class (26–28). In heterologous expression systems, the expression of the STREX form of BKCa channels and their association with the BKCa beta4 subunit further modifies the gating properties of BKCa channels (29). We found that in hippocampal neurons, the BKCa beta4 subunit protein is highly expressed in the cell bodies and proximal dendrites compared with the distal dendrites (Fig S3). This is similar to the STREX BKCa channel pattern of expression. Within a given neuron, different subcellular regions contain different levels of type I and type II STREX BKCa channels. The action potential–generating region in the cell body contains mainly the type II STREX-beta4-BKCa complex. This result supports our findings showing that IBX treatment does not significantly alter the fAHP, action potential half-width, or number of action potentials in the burst (Fig. 4 B, C, and D). In contrast, the postsynaptic sites in the distal dendrites contain a higher proportion of the type I STREX BKCa channels. Local RNA processing of i17a-STREX–containing BKCa channel mRNAs, coupled with the beta4 subunit association, gives hippocampal neurons the ability to carefully modify their BKCa channel currents.
Burst Firing Requires STREX-Containing BKCa Channel mRNAs.
By systematically reducing the levels of one splice variant in the BKCa channel mRNA population with siRNA, we have shown that typical hippocampal burst firing requires the presence of two distinct classes of BKCa channel mRNAs: the retained-intron class and the exon-only class. The potent effect of the retained-intron class is unexpected. The robust physiological effect of the retained-intron class–targeted siRNA treatment strongly suggests that a large number of functional BKCa channels are indeed generated from intron-containing BKCa channel mRNAs. The rapid conversion of the retained-intron class into the exon-only class could dramatically underestimate the amount of cytoplasmically localized retained-intron class mRNAs. These data highlight the functional significance of both intron retention and alternative splicing to the typical physiology of hippocampal neurons.
Perhaps even more important than transcriptional regulation, posttranscriptional events determine mRNA-specific splicing patterns (i.e., exon utilization), subcellular localization patterns, and RNA stabilities in neurons. The complexity of this posttranscriptional regulation is ever growing. The chromatin structure has been implicated in the control of alternative splicing (22), and large RNP complexes have been shown to coordinately regulate a sizeable pool of mRNAs via oskar mRNA during Drosophila development (30). The coupling of intron retention and exon selection for the BK channel suggests that the cells require a method for rapidly selecting particular functional protein domains. Because the retained-intron mRNA is localized throughout the dendrite and cell soma, it is positioned so that it can be spliced and translated wherever the stimulatory signal is received (likely an external signal). The existence of such a regulatory response suggests that there is significant parsimony in the use of cellular resources, or that the presence of particular exon motif–containing proteins may have an important effect on cell signaling. Cells attempt to limit their expression of these species by encrypting the protein sequence in pre-mRNAs that are rapidly spliced and translated as needed at the sites where their protein function is required.
Methods
Hippocampal Cell Harvesting and Culturing.
Timed pregnant rats were purchased from The Jackson Laboratory. On embryonic day 19, the embryos were harvested, and neurons were isolated and cultured (31). All procedures were approved by the University of Pennsylvania's Institutional Animal Care Committee.
Illumina Sequence Alignment.
Samples of hippocampal dendrites (300–700 per sample) were collected and subjected to aRNA amplification. A 100-ng sample from the final aRNA amplication reaction was submitted for library generation and IIIumina sequencing.
Hippocampal Neuron RT-PCR Analysis.
Hippocampal RNA was isolated by treating cultured neurons with TRIzol (Invitrogen). cDNA was synthesized from the samples and used as a PCR template with the Advantage2 PCR system (BD Biosciences).
MALDI-TOF MS and Quantitative KCNMA1 Splice Variant Detection.
MALDI-TOF MS analysis of BKCa channel PCR amplicons were performed as described previously (4). See SI Methods for more details.
ISH Using Cultured Neurons.
Antisense biotin-labeled oligo probes were obtained from IDT Technologies. Primary neurons (∼14 d) were fixed, permeabilized, and hybridized as described previously (4). See SI Methods for more details.
Immunostaining.
The neurons were permeabilized with 0.3% Triton X-100 and blocked at room temperature for 60 min in 10% normal goat serum, 1× PBS, and 0.1% Tween 20. The primary and secondary antibodies were diluted in the blocking solution. Washing was done with 1× PBS with 0.1% Tween 20.
siRNA Treatments.
Cultured primary rat hippocampal neurons were transfected in DharmaFect 3 (Dharmacon) at 7–9 d after plating (SI Methods). The cultures were maintained at 37 °C with 5% CO2 for 72 h.
Whole-Cell Recordings.
The bathing solution consisted of 140 mM NaCl, 3 mM KCl, 1 mM CaCl2, 1 mM Mg Cl2, and 10 mM Hepes, adjusted to a pH of 7.4 with NaOH. The internal solution consisted of 120 mM potassium gluconate, 20 mM KCl, 10 mM Hepes, 0.1 mM EGTA, 2 mM MgCl2, 2 mM ATP, and 0.25 mM GTP, adjusted to a pH of 7.4 with KOH.
Supplementary Material
Acknowledgments
We thank J. Saunders for the use of his electrophysiology equipment and M. Maronski for help with cell culture. This work was funded in part by National Institutes of Health Grants MH088849 and AG09900 and Director's Pioneer Award DP004117.
Footnotes
Conflict of interest statement: C.R.C. and R.M. are employees of Sequenom, Inc, whose technology was used in this work.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015264107/-/DCSupplemental.
References
- 1.Lipscombe D. Neuronal proteins custom designed by alternative splicing. Curr Opin Neurobiol. 2005;15:358–363. doi: 10.1016/j.conb.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Glanzer J, et al. RNA splicing capability of live neuronal dendrites. Proc Natl Acad Sci USA. 2005;102:16859–16864. doi: 10.1073/pnas.0503783102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denis MM, et al. Escaping the nuclear confines: Signal-dependent pre-mRNA splicing in anucleate platelets. Cell. 2005;122:379–391. doi: 10.1016/j.cell.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell TJ, et al. Cytoplasmic BK(Ca) channel intron–containing mRNAs contribute to the intrinsic excitability of hippocampal neurons. Proc Natl Acad Sci USA. 2008;105:1901–1906. doi: 10.1073/pnas.0711796105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkinson NS, Robertson GA, Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science. 1991;253:551–555. doi: 10.1126/science.1857984. [DOI] [PubMed] [Google Scholar]
- 6.Tseng-Crank J, et al. Cloning, expression, and distribution of functionally distinct Ca(2+)-activated K+ channel isoforms from human brain. Neuron. 1994;13:1315–1330. doi: 10.1016/0896-6273(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 7.Shipston MJ. Alternative splicing of potassium channels: A dynamic switch of cellular excitability. Trends Cell Biol. 2001;11:353–358. doi: 10.1016/s0962-8924(01)02068-2. [DOI] [PubMed] [Google Scholar]
- 8.Xie J, McCobb DP. Control of alternative splicing of potassium channels by stress hormones. Science. 1998;280:443–446. doi: 10.1126/science.280.5362.443. [DOI] [PubMed] [Google Scholar]
- 9.Tian L, et al. Distinct stoichiometry of BKCa channel tetramer phosphorylation specifies channel activation and inhibition by cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 2004;101:11897–11902. doi: 10.1073/pnas.0402590101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eberwine J, et al. Analysis of gene expression in single live neurons. Proc Natl Acad Sci USA. 1992;89:3010–3014. doi: 10.1073/pnas.89.7.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang ET, et al. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456:470–476. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:1–10. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibbs RA, et al. Rat Genome Sequencing Project Consortium (2004) Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature. 428:493–521. doi: 10.1038/nature02426. [DOI] [PubMed] [Google Scholar]
- 14.Xie J, Black DL. A CaMK IV–responsive RNA element mediates depolarization-induced alternative splicing of ion channels. Nature. 2001;410:936–939. doi: 10.1038/35073593. [DOI] [PubMed] [Google Scholar]
- 15.MacDonald SH, Ruth P, Knaus HG, Shipston MJ. Increased large conductance calcium-activated potassium (BK) channel expression accompanied by STREX variant down-regulation in the developing mouse CNS. BMC Dev Biol. 2006;6:1–11. doi: 10.1186/1471-213X-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu H, et al. Presynaptic Ca2+-activated K+ channels in glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. J Neurosci. 2001;21:9585–9597. doi: 10.1523/JNEUROSCI.21-24-09585.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Misonou H, et al. Immunolocalization of the Ca2+-activated K+ channel Slo1 in axons and nerve terminals of mammalian brain and cultured neurons. J Comp Neurol. 2006;496:289–302. doi: 10.1002/cne.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pietrzykowski AZ, et al. Posttranscriptional regulation of BK channel splice variant stability by miR-9 underlies neuroadaptation to alcohol. Neuron. 2008;59:274–287. doi: 10.1016/j.neuron.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Storm JF. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol. 1987;385:733–759. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lancaster B, Nicoll RA. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J Physiol. 1987;389:187–203. doi: 10.1113/jphysiol.1987.sp016653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shao LR, Halvorsrud R, Borg-Graham L, Storm JF. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J Physiol. 1999;521:135–146. doi: 10.1111/j.1469-7793.1999.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luco RF, et al. Regulation of alternative splicing by histone modifications. Science. 2010;327:996–1000. doi: 10.1126/science.1184208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie J, Jan C, Stoilov P, Park J, Black DL. A consensus CaMK IV–responsive RNA sequence mediates regulation of alternative exons in neurons. RNA. 2005;11:1825–1834. doi: 10.1261/rna.2171205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian L, Hammond MS, Florance H, Antoni FA, Shipston MJ. Alternative splicing determines sensitivity of murine calcium-activated potassium channels to glucocorticoids. J Physiol. 2001;537:57–68. doi: 10.1111/j.1469-7793.2001.0057k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reinhart PH, Chung S, Levitan IB. A family of calcium-dependent potassium channels from rat brain. Neuron. 1989;2:1031–1041. doi: 10.1016/0896-6273(89)90227-4. [DOI] [PubMed] [Google Scholar]
- 26.Meera P, Wallner M, Toro L. A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc Natl Acad Sci USA. 2000;97:5562–5567. doi: 10.1073/pnas.100118597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Behrens R, et al. hKCNMB3 and hKCNMB4, cloning and characterization of two members of the large-conductance calcium-activated potassium channel beta subunit family. FEBS Lett. 2000;474:99–106. doi: 10.1016/s0014-5793(00)01584-2. [DOI] [PubMed] [Google Scholar]
- 28.Brenner R, et al. BK channel beta4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat Neurosci. 2005;8:1752–1759. doi: 10.1038/nn1573. [DOI] [PubMed] [Google Scholar]
- 29.Petrik D, Brenner R. Regulation of STREX exon large conductance, calcium-activated potassium channels by the beta4 accessory subunit. Neuroscience. 2007;149:789–803. doi: 10.1016/j.neuroscience.2007.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reveal B, et al. BREs mediate both repression and activation of oskar mRNA translation and act in trans. Dev Cell. 2010;18:496–502. doi: 10.1016/j.devcel.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilcox KS, Buchhalter J, Dichter MA. Properties of inhibitory and excitatory synapses between hippocampal neurons in very-low-density cultures. Synapse. 1994;18:128–151. doi: 10.1002/syn.890180206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.